Abstract
Autophagy is an evolutionarily conserved process in which cytoplasmic proteins and organelles are degraded and recycled for reuse. There are numerous reports on the role of autophagy in cell growth and death; however, the role of autophagy in methylmercury (MeHg)-induced neurotoxicity has yet to be identified. We studied the role of autophagy in MeHg-induced neurotoxicity in astrocytes. MeHg reduced astrocytic viability in a concentration- and time-dependent manner, and induced apoptosis. Pharmacological inhibition of autophagy with 3-methyladenine (3-MA) or chloroquine (CQ), as well as the silencing of the autophagy-related protein 5 (Atg5), increased MeHg-induced cytotoxicity and the ratio of apoptotic astrocytes. Conversely, Rapamycin, an autophagy inducer, along with as N-acetyl-L-cysteine (NAC), a precursor of reduced glutathione (GSH), decreased MeHg-induced toxicity and the ratio of apoptotic astrocytes. These results indicated that MeHg-induced neurotoxicity was reduced, at least in part, through the activation of autophagy. Accordingly, modulation of autophagy may offer a new avenue for attenuating MeHg-induced neurotoxicity.
Introduction
Methylmercury (MeHg) is an established toxicant with significant adverse effects on human health (Wormser et al. 2012). It damages the nervous system in human and experimental animals (Castoldi et al. 2001; Choi 1989; Farina et al. 2011; Ni et al. 2012; Sanfeliu et al. 2001). MeHg readily crosses the blood-brain barrier (BBB) and accumulates in the brain (Hong et al. 2012a; Hong et al. 2012b). Although most studies on MeHg-induced CNS damage focus on neuronal toxicity (Hong et al. 2012b; Tamm et al. 2006), astrocytes play a key role in mediating this toxicity (Aschner et al. 2000). MeHg preferentially accumulates in astrocytes and inhibits glutamate uptake by these cells (Aschner et al. 2000; Liu et al. 2013b; Shanker et al. 2001; Shanker and Aschner 2001), leading to dysregulation of excitatory amino acid homeostasis, excessive synaptic glutamate concentration and excitotoxicity (Aschner et al. 1993; Deng et al. 2014; Liu et al. 2014). In addition, MeHg induces reactive oxygen species (ROS) production in neonatal rat cerebral astrocyte cultures (Dreiem and Seegal 2007; Wagner et al. 2010) and in brain slices (Wagner et al. 2010), an effect that is reversed by treatment with antioxidants (Allen et al. 2001; Allen et al. 2002; Shanker and Aschner 2003).
Autophagy is an evolutionarily conserved catabolic pathway that is responsible for the bulk of proteolysis in normal cells, leading to the degradation of proteins and organelles, especially in stressed cells (Cherra et al. 2010; Rami 2009). Nevertheless, activation of the autophagic pathway above a certain threshold may result in cellular dysfunction and cell death (Mader et al. 2012; Shen et al. 2012). Autophagy commences with the formation of double-membrane vesicles, referred to as autophagosomes (autophagic vacuoles in mammalian cells), that sequester cytoplasm and organelles. The autophagosomes fuse with lysosomes, forming autophagolysosomes, resulting in the degradation of their contents into basic molecules, and recycling for re-usage (Benbrook and Long 2012; Cuervo et al. 2005; Essick and Sam 2010).
Autophagy contributes to the turnover of cytoplasmic components (Cuervo et al. 2005; Larsen and Sulzer 2002) and mediates normal development and differentiation in response to environmental stimuli (Jones et al. 2013; Milisav et al. 2012). It has been shown that excessive metal exposures can also induce autophagy both in vitro and in vivo (Chargui et al. 2011; Guo et al. 2010; Harhaji-Trajkovic et al. 2009; Yang et al. 2014). For example, cadmium (Cd) has been shown to induce autophagy in skin epidermal cells (Son et al. 2011) and in vascular endothelial cells (Dong et al. 2009), while inorganic mercury (Hg) has been shown to induce autophagic cell death in rat hepatocytes and glioma cells (Chatterjee et al. 2012). In addition, oxidative stress-induced autophagy has been shown to be involved in apoptosis in vivo (Duan et al. 2013) and in vitro (Ranjan et al. 2014). However, autophagic cell death appears to be independent of apoptosis in transformed cancer cells (Chen and Gibson 2008). The precise relationship between autophagy and apoptosis has yet therefore to be fully understood.
Here, we examine the role of autophagy in MeHg-induced neurotoxicity in primary rat astrocytes and decipher its underlying toxic mechanisms, thereby identifying potential novel targets for neuroprotection against MeHg.
Materials and Methods
Reagents and antibodies
For western blot analysis, the polyclonal antibodies to LC3B, Beclin 1, P62/SQSTM1 and cleaved caspase 3 were obtained from Cell Signaling Technology (Beverly, MA, USA). beta-Actin and horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (CA, USA). 3-methyladenine (3-MA), Chloroquine (CQ) and acridine orange (AO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Rapamycin was purchased from Gene Operation, Inc. (Michigan, USA), and the Annexin V FITC/PI apoptosis detection kit was obtained from Multisciences (Hangzhou, China).
Cell culture and treatments
Primary rat astrocytes cultures were prepared as follows: The cerebral hemispheres of neonatal rats were removed and the meninges dissected away. The basal ganglia and midbrain were excised, and the remaining cortical tissue was dissociated with trypsin. Cells were grown in Dulbecco's modified Eagle medium (DMEM; Hyclone), supplemented with 10% fetal bovine serum (Gibco). Cultures were maintained in an environment of 5% CO2 at 37°C, with fresh medium exchanged twice weekly. Cultures were treated with MeHg after 2 weeks in vitro, when cells had reached ~95% confluency. Astrocyte cultures used for western blot analysis and apoptosis detection assays were grown in 6-well plates, whereas those used for cell viability and cytotoxicity assays were grown in 96-well plates.
Measurement of cell viability and cytotoxicity
Primary astrocytes were treated for 6 hours with MeHg at 0, 1, 2, 5, or 10 µM. These concentrations were selected based on calculation by Shapiro and Chan (2008) and Aschner (2012) and they are physiologically relevant to human in vivo exposures (Choi et al. 1978; Takeuchi 1985; Aschner 2012). A lactate dehydrogenase (LDH) cytotoxicity detection kit (Nanjing Jiancheng, Nanjing, China) was used as a surrogate measure of cell death. The culture supernatant was transferred into 96-well plates and LDH activity in the culture supernatant analyzed. Absorbance was analyzed at 490 nm, according to the manufacturer's protocol.
Cell viability was determined by the MTT 3-(4, 5-dimethylthiazol-2-yl-2, 5-diphenyl-tetrazolium bromide) assay. After treatment with MeHg, MTT was added (final concentration 0.5 mg/ml) and cultures incubated for 3 hours. Tetrazolium formed in viable cells was released by the addition of dimethyl sulfoxide (DMSO), and optical density was determined with a microplate reader (BIO-TEK Instruments, Inc.) at 490 nm.
Assessment of apoptosis by flow cytometry
To quantify apoptotic cells, astrocytes were treated with MeHg (5 µM for 6 hours), with or without pretreatment with several autophagy regulators (3-MA, CQ, rapamycin or atg5 siRNA). Next, flow cytometric analysis was performed with the Annexin V FITC/PI apoptosis detection kit, according to the manufacturer's instructions, and analyzed by flow cytometery with BD Accuri C6 software (Becton Dickinson). Both Annexin V+/PI+ cells and Annexin V+/PI− cells were considered as apoptotic cells (Fu et al. 2008).
Detection of acidic vesicular organelles with acridine orange staining
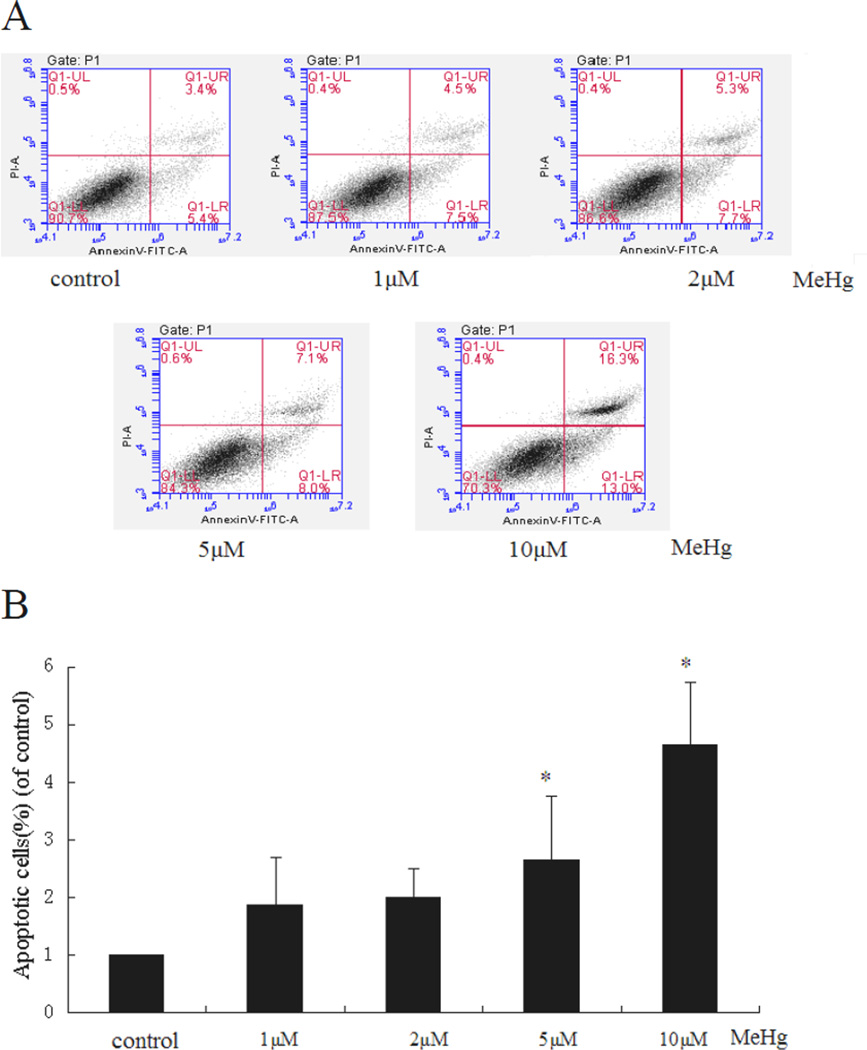
The volume of the cellular acidic compartment, a marker of autophagy, was visualized with the lysosomotropic agent, acridine orange (AO) (Kanzawa et al. 2003; Tasdemir et al. 2008). AO freely crosses biological membranes and accumulates in acidic compartments, where it can be visualized as bright red fluorescence. Astrocytes were treated for 6 hours with MeHg (5µM) followed by 15 min incubation with 1 µg/ml AO. The cells were washed 3 times with PBS before observation with a fluorescent microscope (Zeiss Axi Observer).
Western blot analysis
Western blot analysis was performed as previously described. Briefly, to prepare whole-protein lysates, cells were washed 3 times with PBS, harvested and lysed in a radio-immunoprecipitation assay (RIPA) lysis buffer, then incubated on ice for 10 min and centrifuged at 12,000 g for 15 min. Protein concentration was determined with bicinchoninic acid (BCA). Equal amounts of protein were subjected to SDS polyacrylamide gels, transferred to PVDF (polyvinylidene difluoride) membranes and incubated overnight at 4°C with primary antibody (LC3 B, Beclin 1, p62 and beta-actin). The membranes were incubated with secondary antibodies. The corresponding bands were detected using the MiniChemi Mini Size Chemiluminescent Imaging System (Beijing Sage Creation Science Co. Ltd, Beijing, China). Results were expressed as percentages of the control group.
siRNA interference
The small interfering RNAs (siRNAs) for Atg5 were designed and purchased from Guangzhou Ribobio Co., Ltd (Guangzhou, China). The sequences of the three siRNAs were as follows: (5'-GACGGATUUCCAACGUGCUU dTdT-3' and 5'-AAGCACGUUGGAAUCCGUC dTdT-3'), (5'-GAAGGUUAUGAGACAAGAA dTdT-3' and 5'-UUCUUGUCUCAUAACCUUC dTdT-3'), and (5'-GAGGCUCACUUUAUGUCAU dTdT-3' and 5'-AUGACAUAAAGUGAGCCUC dTdT-3'). Astrocytes were seeded in 24-well plates and, at ~60% confluence, transfected with 80 nM siRNA with Lipofectamine 2000 in Opti-MEM medium (Invitrogen), according to the manufacturer's protocol. The transfected cells were cultured for an additional 48 hours and validated for efficacy of silencing by Western blot analysis.
Statistical analysis
Results were expressed as means ± SD. Statistical analysis was performed by a one-way analysis of variance (ANOVA), following appropriate transformation to normalized data and equalized variance where necessary. All assays were repeated at least three times in at least three independently derived astrocyte cultures. A p value < 0.05 was considered statistically significant.
Results
Cytotoxic effects of MeHg in astrocytes
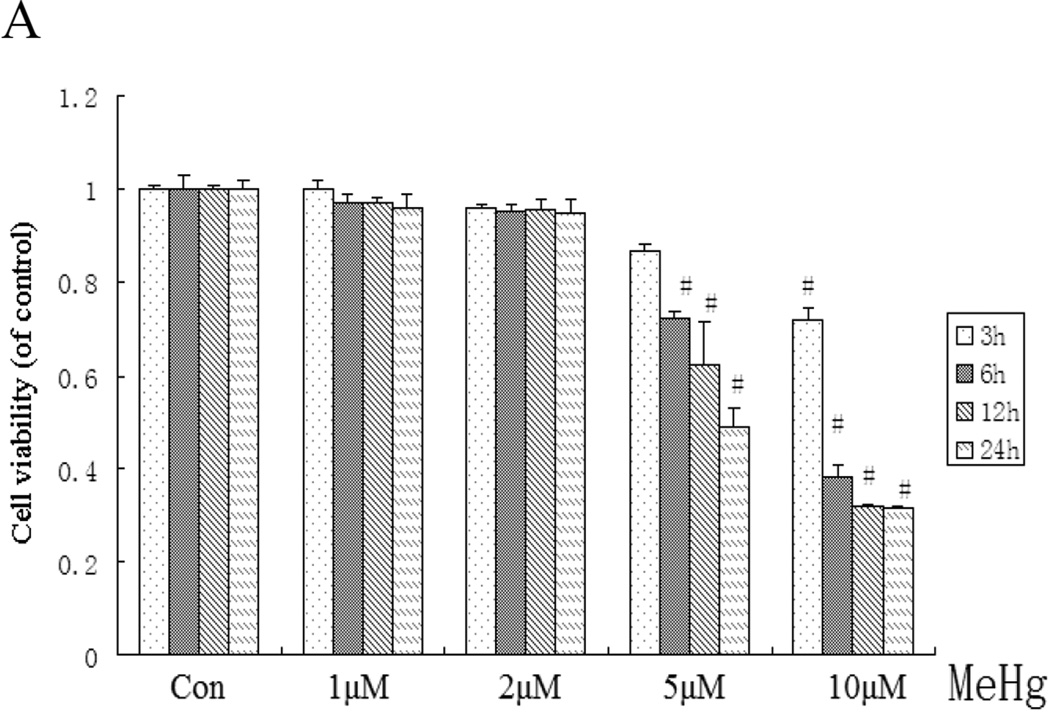
Astrocytes were treated with MeHg (0, 2, 5, or 10 µM) over various time intervals (from 3 to 24 hours), followed by cell viability determination. MeHg reduced cell viability in a concentration- and time-dependent manner (Figure 1A). When the cells were treated with 5 µM MeHg for 6 hours, cell viability was significantly reduced compared to controls (P<0.05). LDH release (Figure 1B) was increased in a concentration-dependent manner, and was significantly elevated compared to controls (P<0.05) in astrocytes treated with 5 µM MeHg for 6 hours.
Figure 1.
Effect of MeHg on cell viability and cytotoxicity in rat primary astrocytes. Astrocytes were treated with various concentrations (1–10µM) of MeHg for 6 hours. Next, cell viability and the cytotoxicity were assessed with the MTT assay (A) and LDH detection kits (B). Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Dunnett test. Values represent means ± SD. *:P < 0.05 compared to the control group.
MeHg activates apoptosis in astrocytes
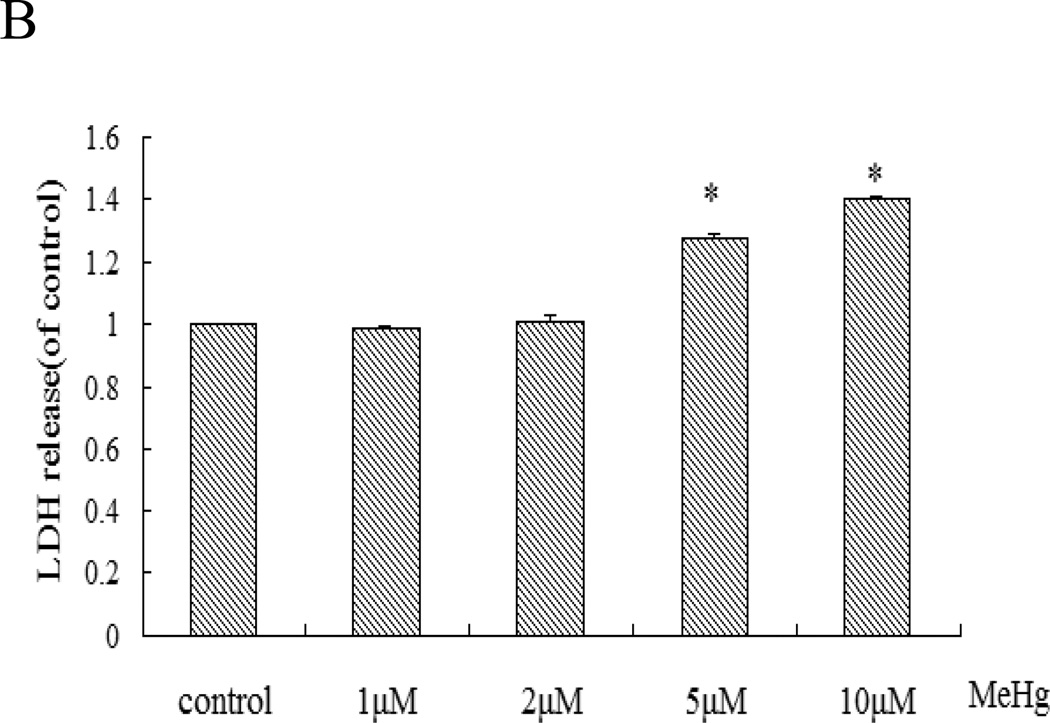
To determine the MeHg-induced cell death modality, we assessed apoptosis with the Annexin V FITC/PI apoptosis detection kit. The ratio of apoptotic astrocytes induced by MeHg was significantly increased (1, 5, 10 µM) in a concentration-dependent manner (Figure 2). Notably, cleaved caspase-3 was also detected in these cells (Figure 3), consistent with MeHg-induced apoptosis.
Figure 2.
Flow cytometric analysis of apoptotic cells upon MeHg treatment. Astrocytes were treated for 6 hours with MeHg (5µM) followed by detection of cell apoptosis with the Annexin V FITC/PI apoptosis detection kit. Data shown are representative of three independent experiments. A: Representative flow cytometry analysis of Annexin V-PI staining in rat primary astrocytes upon MeHg treatment. Cells located in the lower right quadrant (PI-negative/Annexin V-positive) were designated as early apoptotic. Cells in the lower left quadrant (PI-negative/Annexin V-negative) were considered as viable, and cells in the upper quadrants (PI-positive) were considered as late apoptotic or necrotic. B: Quantitative analysis of flow cytometry. Results were expressed as percentage of total number of cells counted. Statistical analysis was carried out by one-way ANOVA followed by Dunnett test. Values are means ± SD.*: P<0.05 compared with the control group.
Figure 3.
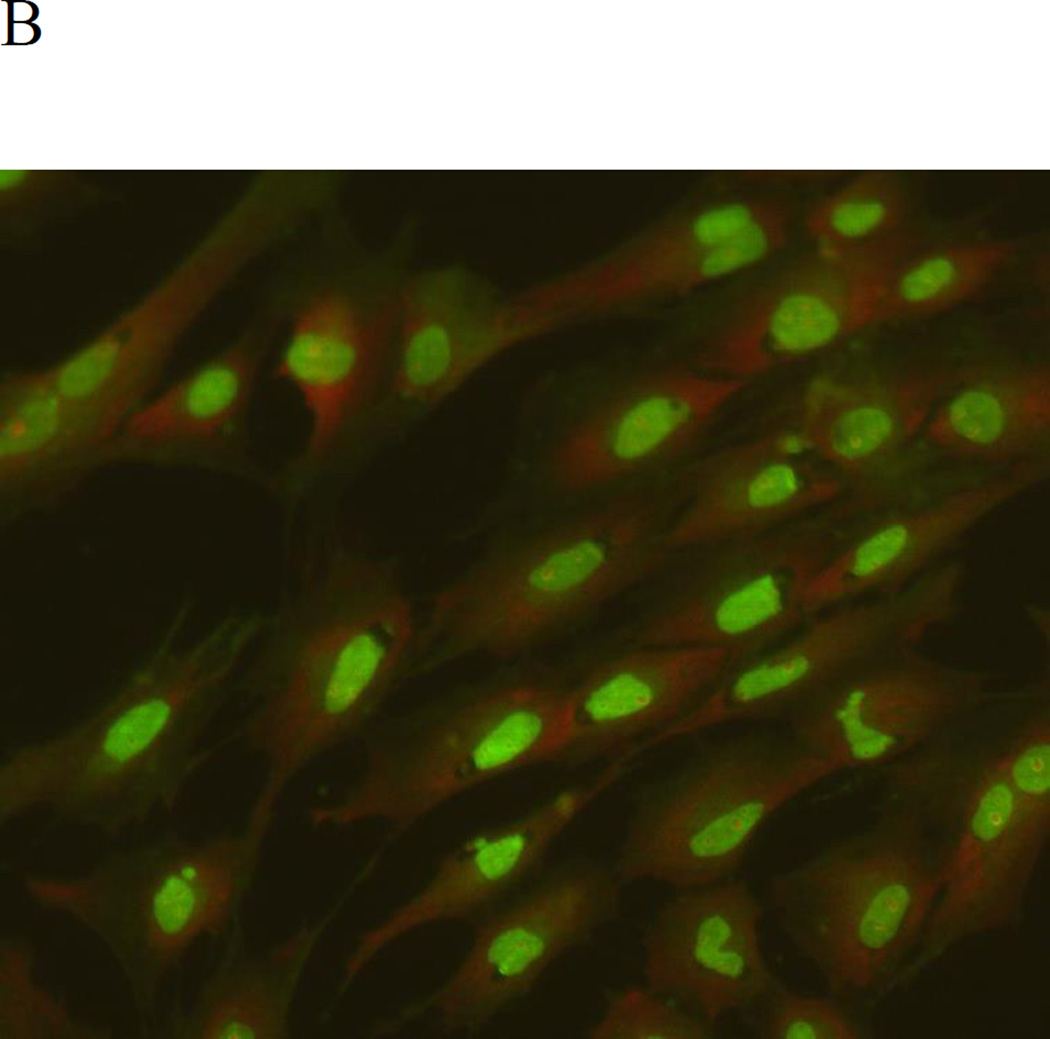
Effect of MeHg on the formation of AVOs in primary rat astrocytes. Cells were treated for 6 hours with MeHg (5µM), stained with the lysosomotropic agent acridine orange (AO), and examined by fluorescence microscopy (200×). A: Control, B: Treatment with 5µM MeHg for 6 hours.
MeHg activates autophagy in astrocytes
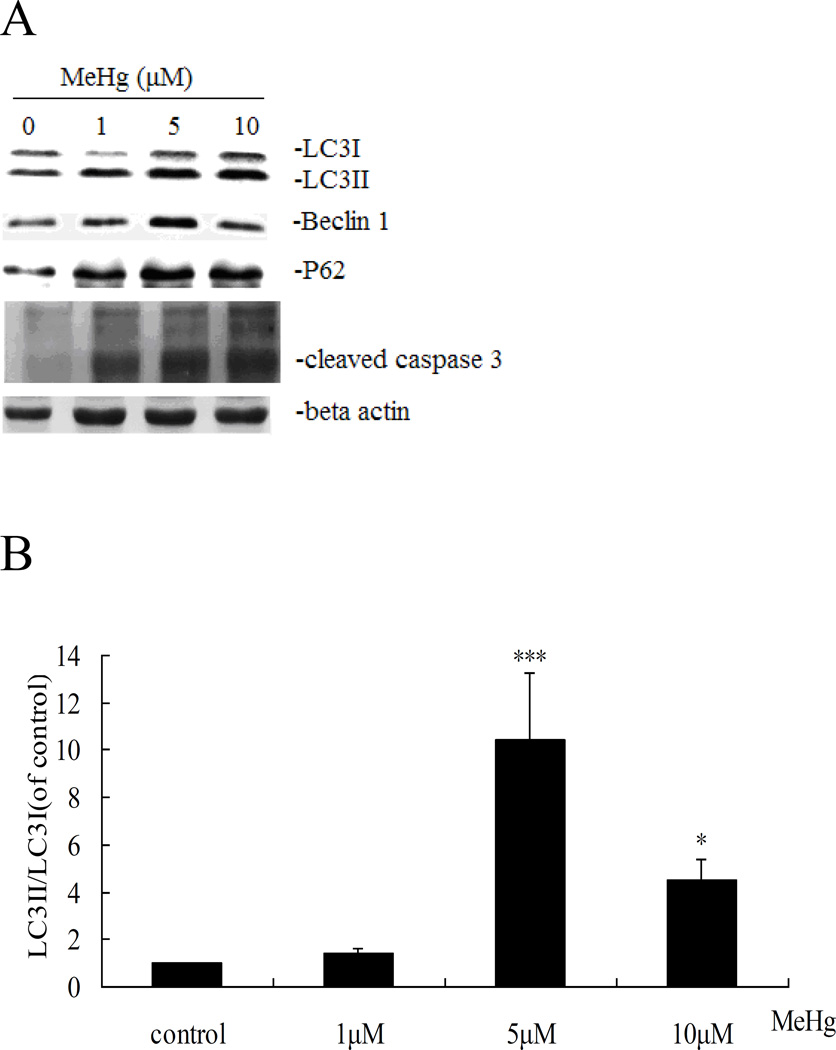
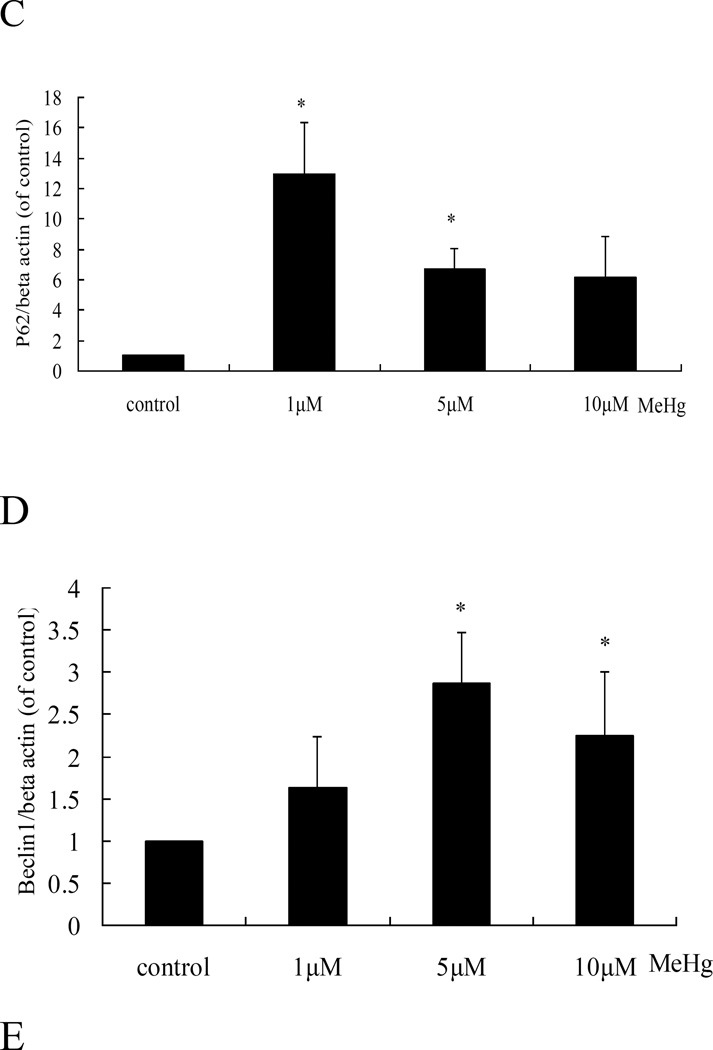
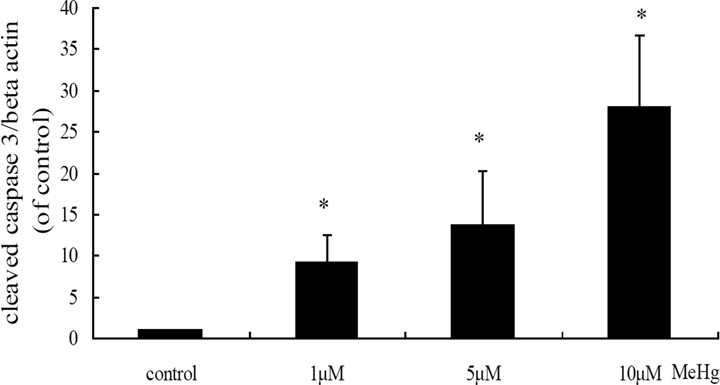
Next, we investigated whether autophagy could be induced in astrocytes upon treatment with MeHg. As shown in figure 3, autophagosomes (red dots) were observed in MeHg treated cells, while untreated cells showed fewer autophagosomes. The conversion of LC3-I into LC3-II and the expression of Beclin 1 showed a similar pattern (Figure 4), with significant increases in the expression of LC3-II and Beclin1 in response to 1 µM MeHg, and a sharp decrease in expression at 5 µM or 10 µM MeHg. Unexpectedly, P62, which is normally considered as the substrate of autophagy and should decrease when autophagy is activated, showed an analogous trend for increased LC3-II.
Figure 4.
Autophagy-related protein expression in MeHg-treated astrocytes. A: Cells were treated for 6 hours with MeHg (0µM, 1µM, 5µM or 10µM). The cytoplasmic protein fraction was analyzed by western blotting. β-actin was taken as internal control. B–E: Densitometric analyses of LC3B, Beclin 1, P62, and cleaved caspase 3 in MeHg-treated astrocytes. Relative expression was expressed as a percentage of β-actin, which was used for loading controls across the various lanes. The figures are representative of one of three independent experiments. Statistical analysis was carried out by one-way ANOVA followed by Dunnett test. Values represent means ± SD.*: P<0.05 compared to the control group.
Suppression of autophagy enhances MeHg-induced cytotoxicity in astrocytes
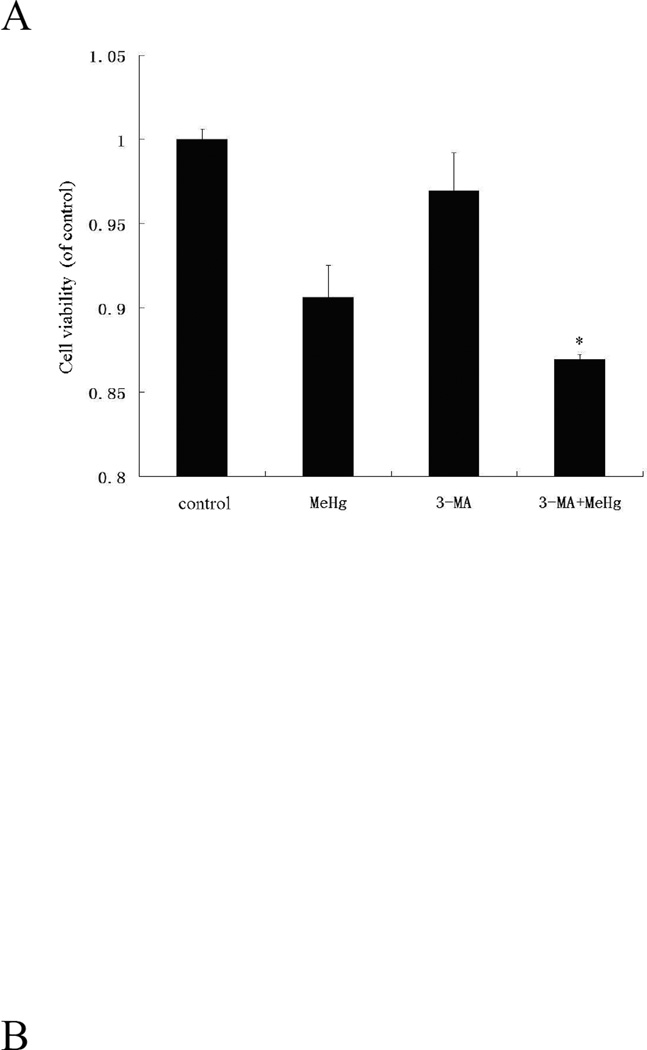
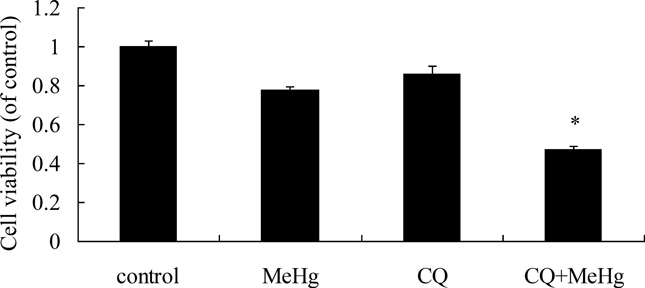
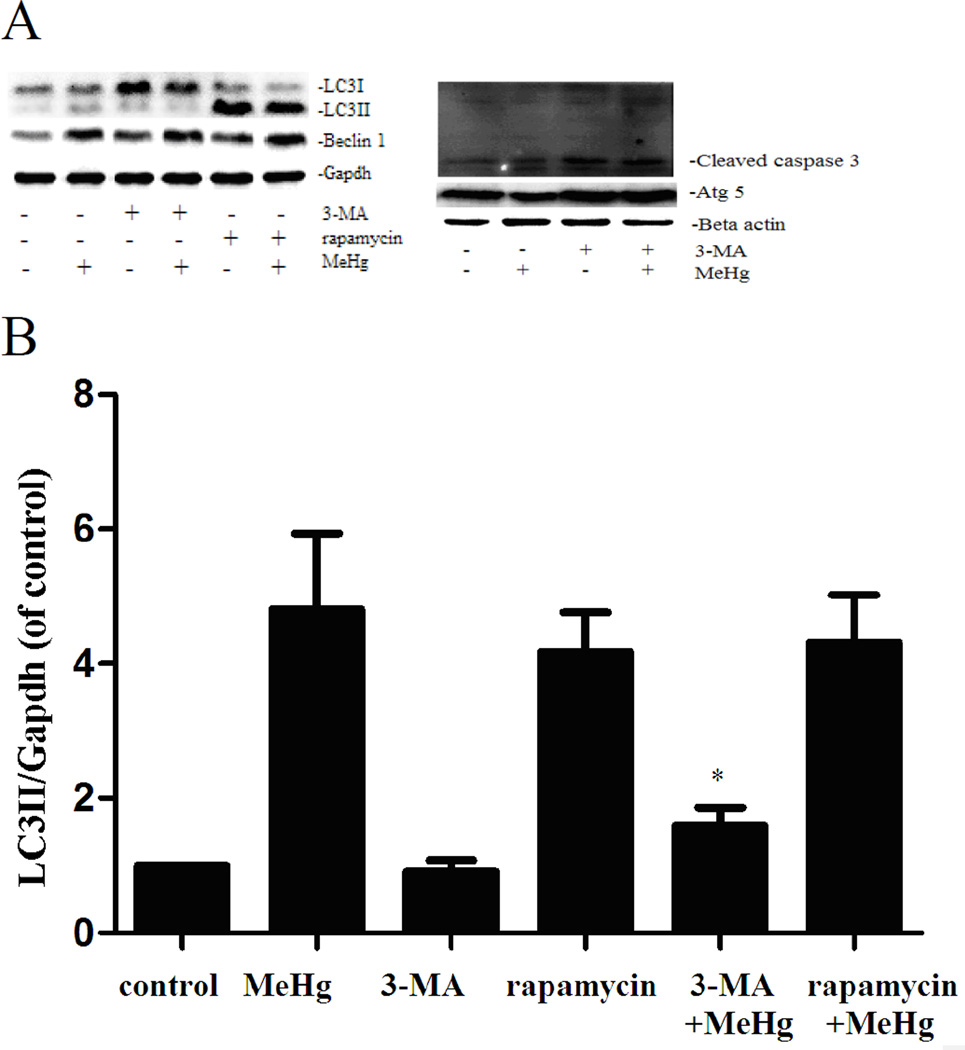
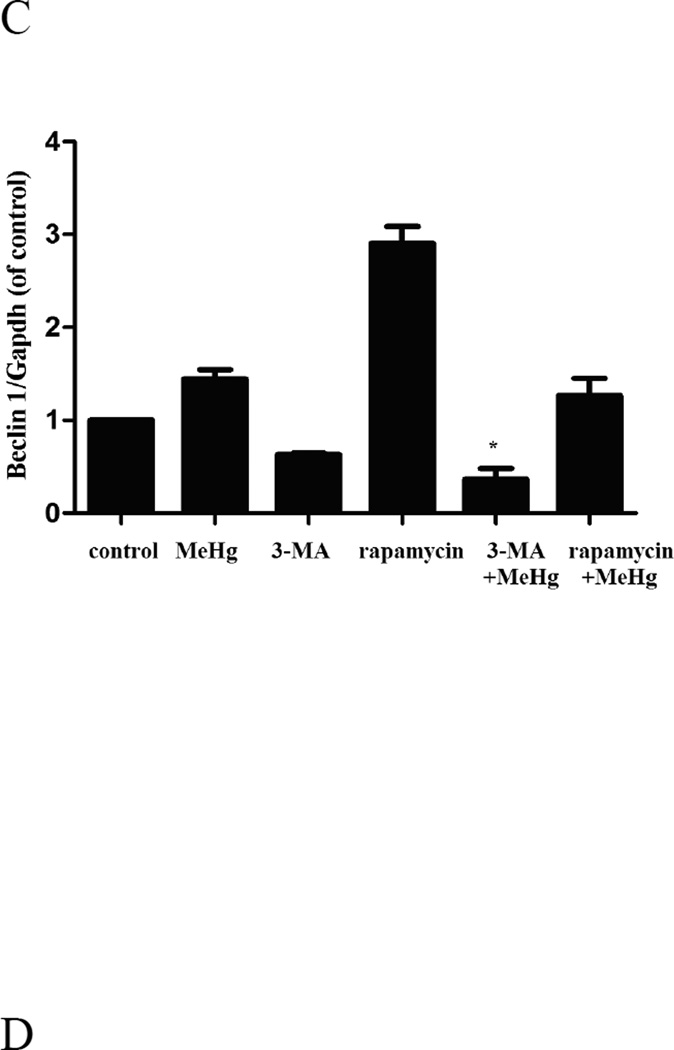
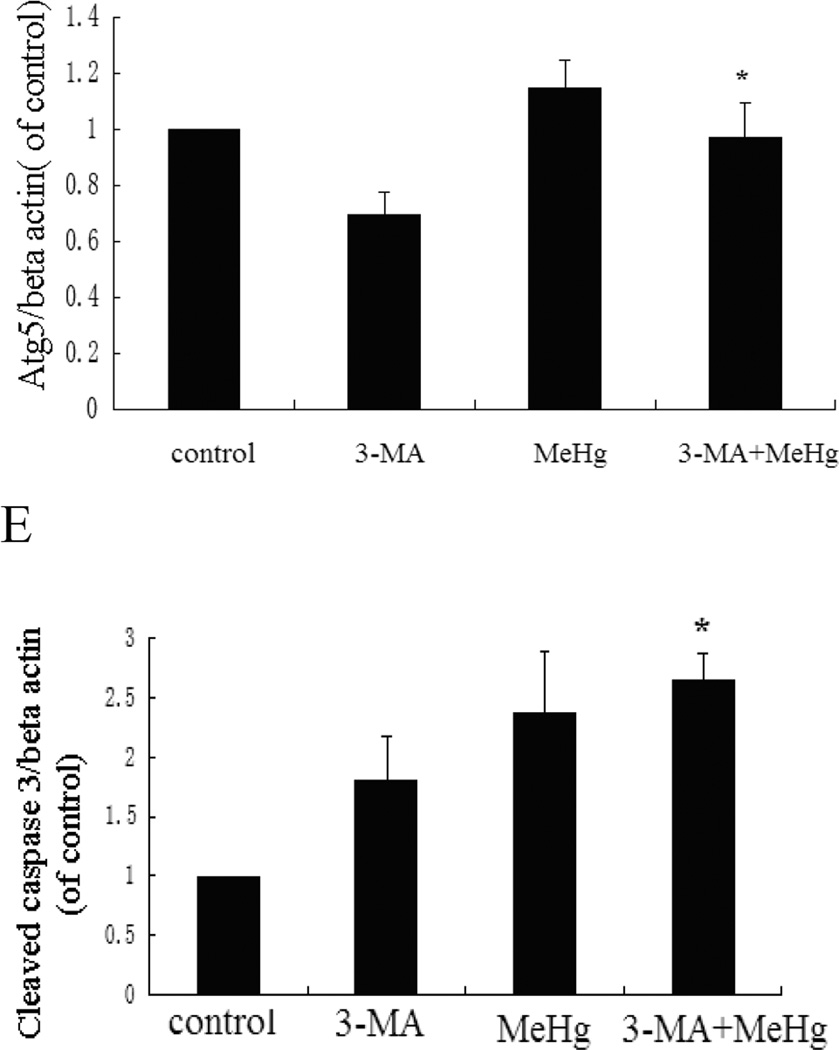
To investigate the role of autophagy in MeHg-induced neurotoxicity, astrocytes were pretreated with 3-methyadenine (3-MA) (2 mM for 12 hours) or chloroquine (CQ) (50 µM for 6 hours), followed by treatment with MeHg (5 µM for 6 hours). 3-MA is a class III phosphatidylinositol-3 kinase (PI3K) inhibitor which specifically inhibits formation of autophagsome (Wu et al. 2013). CQ is clinically used as an antimalarial drug, which suppresses fusion of autophagsome with lysosomes (Palmeira-Dos-Santos et al. 2014). Cell viability was determined with the MTT assay and autophagy markers were detected by western blot analysis. As shown in Figure 5, the viability of astrocytes pretreated with 3-MA or CQ prior to MeHg treatment was significantly decreased (P<0.05) compared to cells treated with MeHg alone. The level of LC3II and Beclin1 significantly decreased (P<0.05) upon pretreatment with 3-MA and CQ (Figure 6). However, the level of the LC3-binding protein P62, a specific substrate for autophagy, was increased. In contrast to this finding, autophagy was previously reported to be accompanied by reduction in P62 levels (Larsen et al. 2010). Notwithstanding this exception (see discussion below), our findings were consistent with MeHg-induced inhibition of autophagy and increased cytotoxicity. Cleaved caspase 3, an apoptotic protein marker, showed the same trend (Figure 6). These results confirmed that the induction of autophagy protected astrocytes from MeHg-induced cytotoxicity.
Figure 5.
Effect of 3-methyladenine (3-MA) and Chloroquine (CQ) on MeHg-induced cytotoxicity. Astrocytes were pretreated with 3-MA (2 mM for 12 hours) or CQ (50 µM for 6 hours), followed by treatment with MeHg (5µM) for 6 hours. Cell viability was determined with the MTT assay A: Treatment with 3-MA; B: Treatment with CQ; Results shown are the means of three independent experiments; Statistical analysis was carried out by one-way ANOVA followed by Dunnett test. *: P<0.05 compared with the MeHg-treated group.
Figure 6.
Expression of LC3B, Beclin 1, Atg5 and cleaved caspase 3 in astrocytes upon pretreatment with 3-MA or CQ. A: Cells were treated for 6 hours with 5µM MeHg with or without of pretreatment with 3-methyladenine (3-MA) (2 mM for 12 hours), Chloroquine (CQ) (50 µM for 6 hours) or rapamycin (1µM for 12 hours). Proteins probed by western blot analysis. β-actin and GAPDH served as loading controls. B–E: Densitometric analyses of LC3B, Beclin 1, Atg5, and Cleaved caspase 3 in astrocytes. Statistical analysis was carried out by one-way ANOVA followed by Dunnett test. Figures are representative of one of three independent experiments. Values are means ± SD. *: P<0.05 compared with the MeHg-treated group.
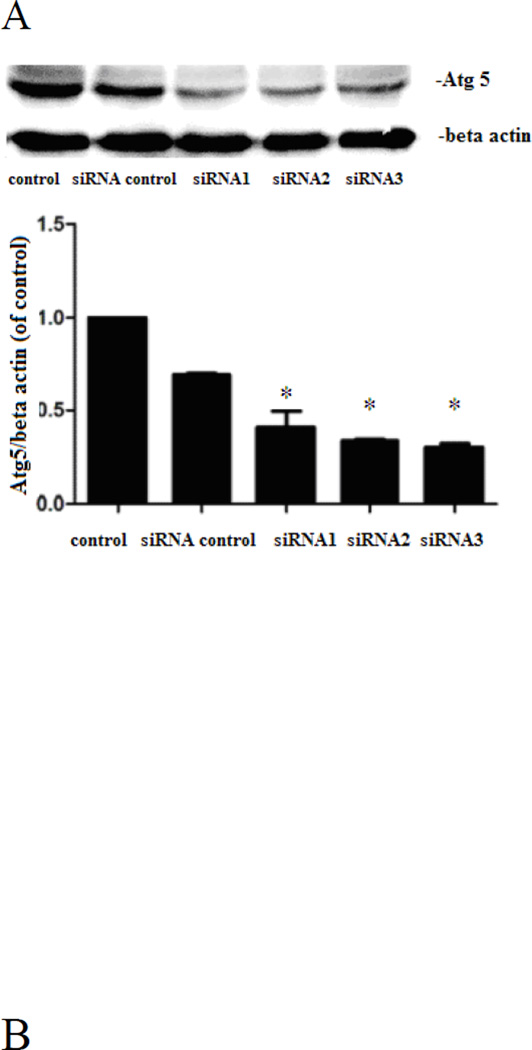
Moreover, siRNA targeting Atg5 significantly suppressed (P<0.05) the expression of Atg5 in astrocytes (Figure 8A). Transfection with negative control siRNA did not affect cell viability, whereas the MeHg cytotoxicity in Atg-5-silenced cells was significantly (P<0.05) increased compared to the negative control (Figure 8B).
Figure 8.
Expression of autophagy related protein 5(Atg5). A: Atg5 targeted siRNA suppresses the expression of Atg5. Astrocytes were transfected for 48 hours with siRNA targeting Atg5, followed by western blot analysis. B: Interference with Atg5 significantly decreased astrocytic viability upon treatment with MeHg (5µM for 6hours). Cell viability was determined with the MTT assay. Statistical analysis was carried out by one-way ANOVA followed by Dunnett test. *: P<0.05, compared with siRNA control group. #: P<0.05 compared with the MeHg-treated group.
Activation of autophagy protects astrocytes from MeHg-induced cytotoxicity
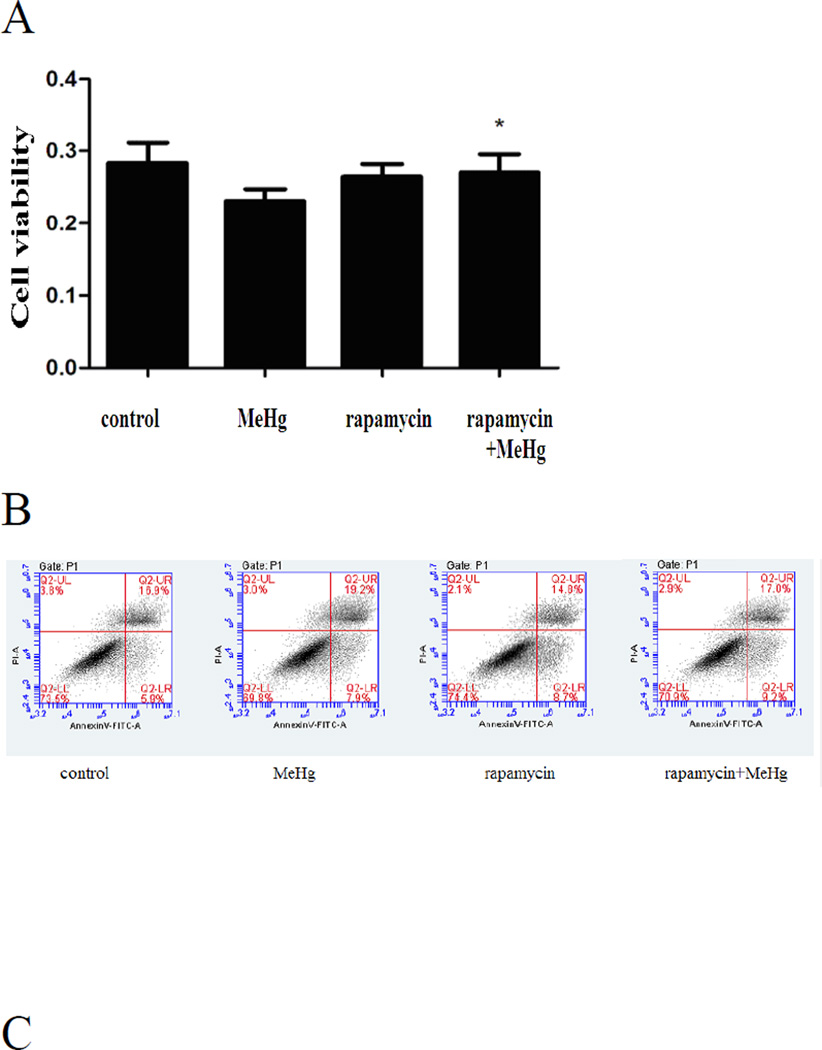
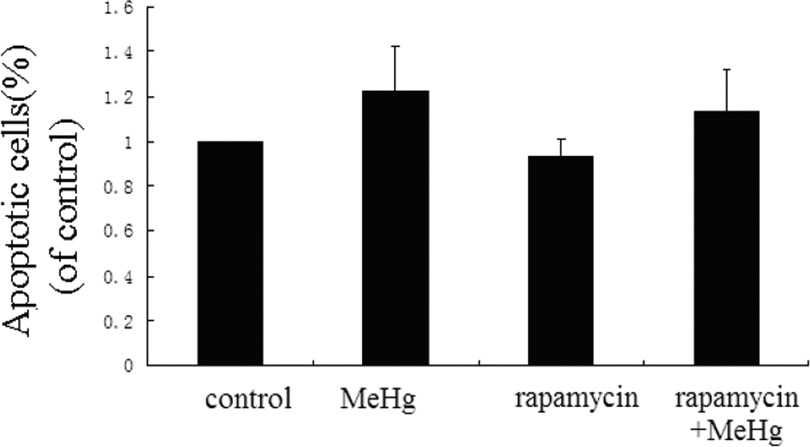
Astrocytes were treated for 6 hours with 5µM MeHg after pretreatment with Rapamycin (1µM for 12 hours). Rapamycin is an inhibitor of the mTOR signaling pathway and is frequently used to induce autophagy (Thoreen and Sabatini 2009). As shown in the Figure 6, the conversion of LC3-I to LC3-II and the level of Beclin 1 were significantly increased upon pretreatment with Rapamycin compared to astrocytes treated with MeHg alone (P<0.05). In contrast to the results of pretreatment with 3-MA or CQ, the viability of astrocytes pretreated with Rapamycin was significantly increased (P<0.05) when compared to cells treated with MeHg alone (Figure 9A).
Figure 9.
Activation of autophagy protects astrocytes from MeHg-induced cytotoxicity. Astrocytes were treated with 5µM MeHg for 6 hours in the pre-presence or absence of rapamycin (1µM for 12hours). Apoptotic cells were detected with the Annexin V FITC/PI apoptosis detection kit. Astrocytic viability was measured with the MTT assay (A). B: Representative flow cytometry analysis of Annexin V-PI staining in MeHg treated astrocytes with or without the pretreatment of Rapamycin. C: Quantitative analysis of flow cytometry. Results expressed as percentage of total number of cells counted. Statistical analysis was carried out by one-way ANOVA followed by Dunnett test. Data shown are representative of three independent experiments. *: P<0.05 compared with the MeHg-treated group.
Autophagy delays apoptosis in MeHg-treated astrocytes
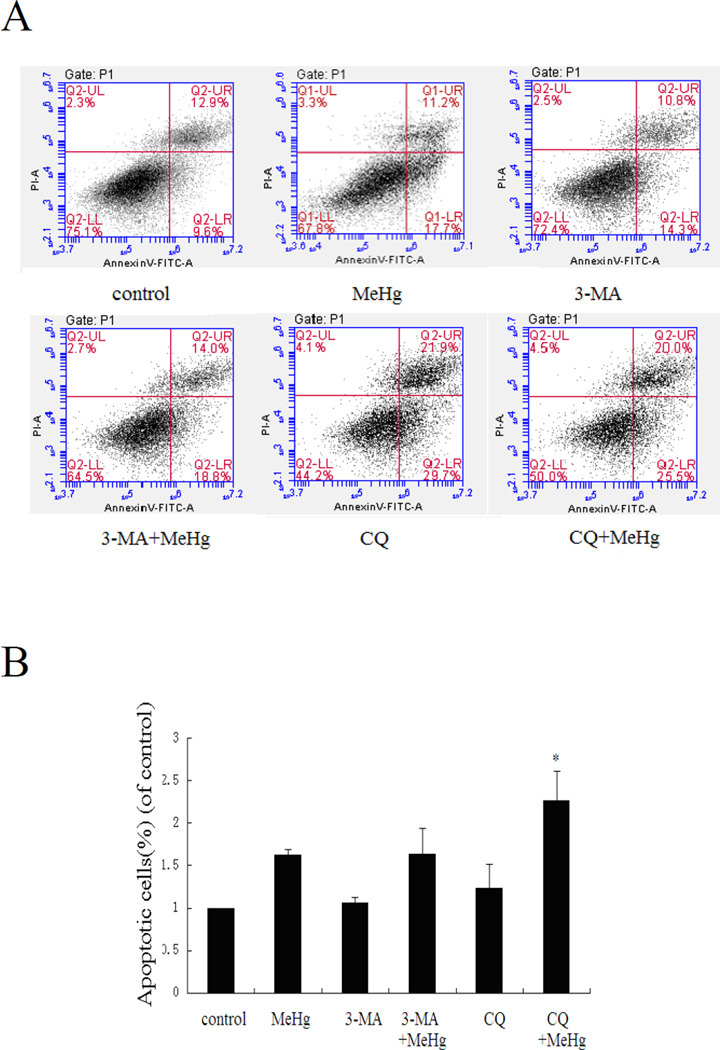
To determine whether MeHg induced toxicity was accompanied by increased apoptosis, astrocytes were subjected to flow cytometry with the Annexin V FITC/PI apoptosis detection kit. As shown in figure 7A, upon pretreatment with 3-MA or CQ, the ratio of apoptotic cells significantly increased (P<0.05) compared to astrocytes treated with MeHg alone. The ratio of apoptotic cells in Rapamycin pre-treated astrocytes significantly decreased compared to astrocytes treated with MeHg alone (P<0.05) (Figure 9B). These results indicate that induced autophagy attenuated apoptosis in MeHg-treated cells.
Figure 7.
Suppression of autophagy increases the ratio of apoptotic astrocytes. Astrocytes were treatment with MeHg (5µM for 6 hours), with or without pretreatment with 3-methyladenine (3-MA) (2 mM for 12 hours), Chloroquine (CQ) (50 µM for 6 hours). Apoptotic cells were detected with the Annexin V FITC/PI apoptosis detection kit. A: Representative flow cytometry analysis of Annexin V-PI staining in MeHg treated astrocytes with or without the pretreatment of 3-MA or CQ. Cells located in the lower right quadrant (PI-negative/Annexin V-positive) were designated early apoptotic. Cells in the lower left quadrant (PI-negative/Annexin V-negative) were considered viable, and cells in the upper quadrants (PI-positive) were considered late apoptotic or necrotic. B: Quantitative analysis of flow cytometry. Results expressed as percentage of total number of cells counted. Statistical analysis was carried out by one-way ANOVA followed by Dunnett test. Data shown are representative of three independent experiments. *: P<0.05 compared with the MeHg-treated group.
NAC attenuates cytotoxicity and autophagy in MeHg-treated astrocytes
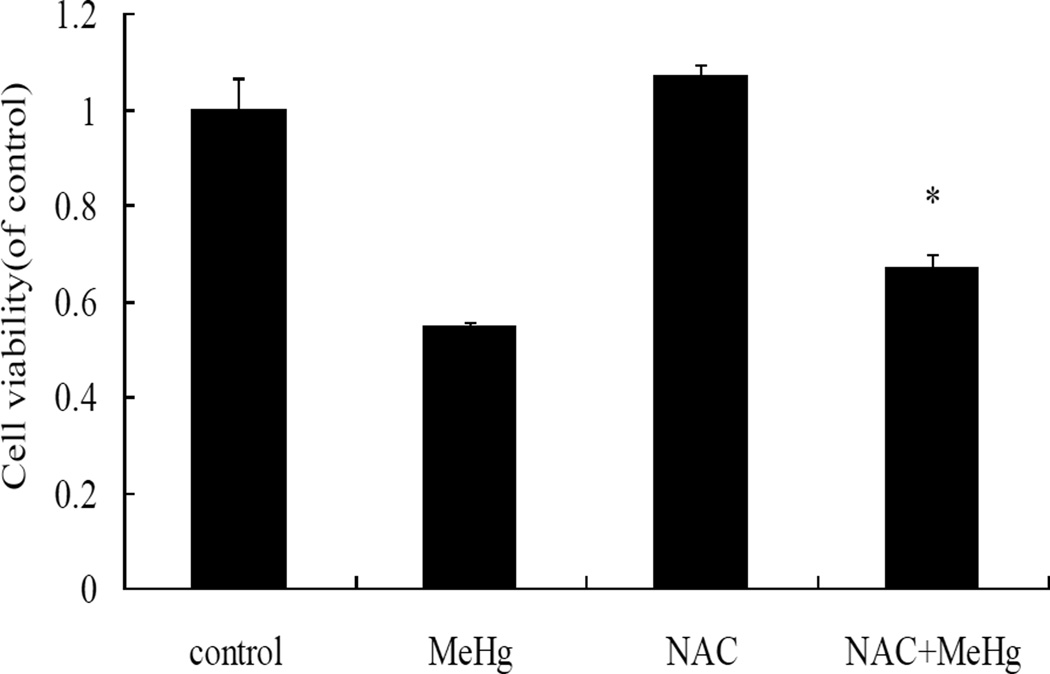
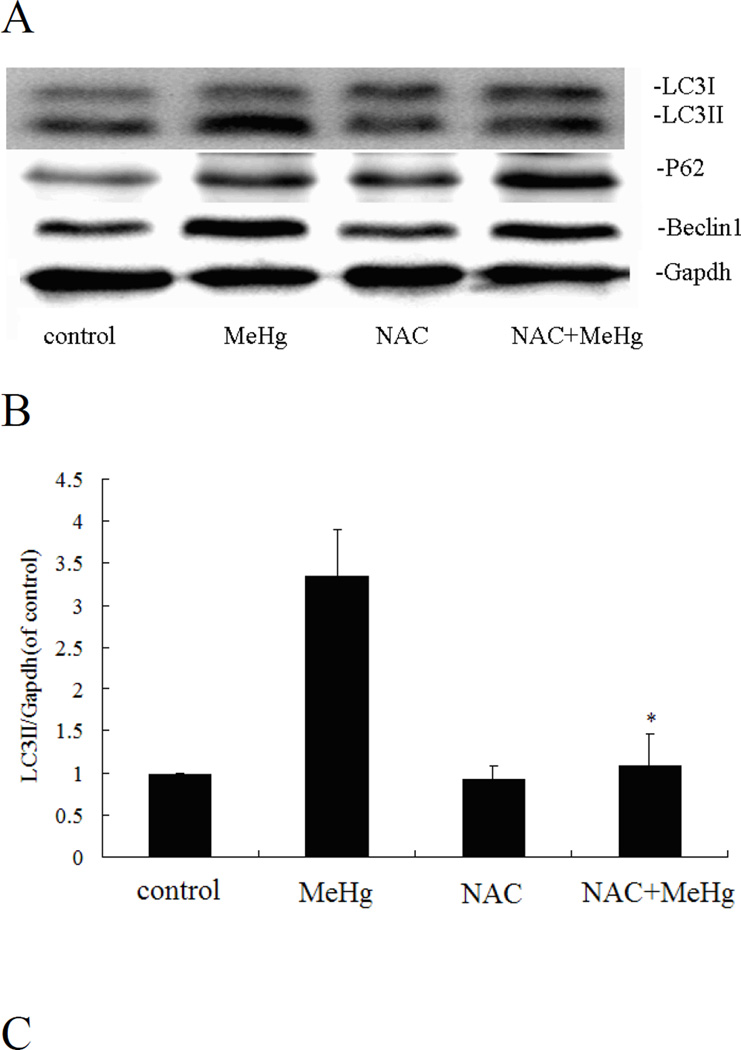
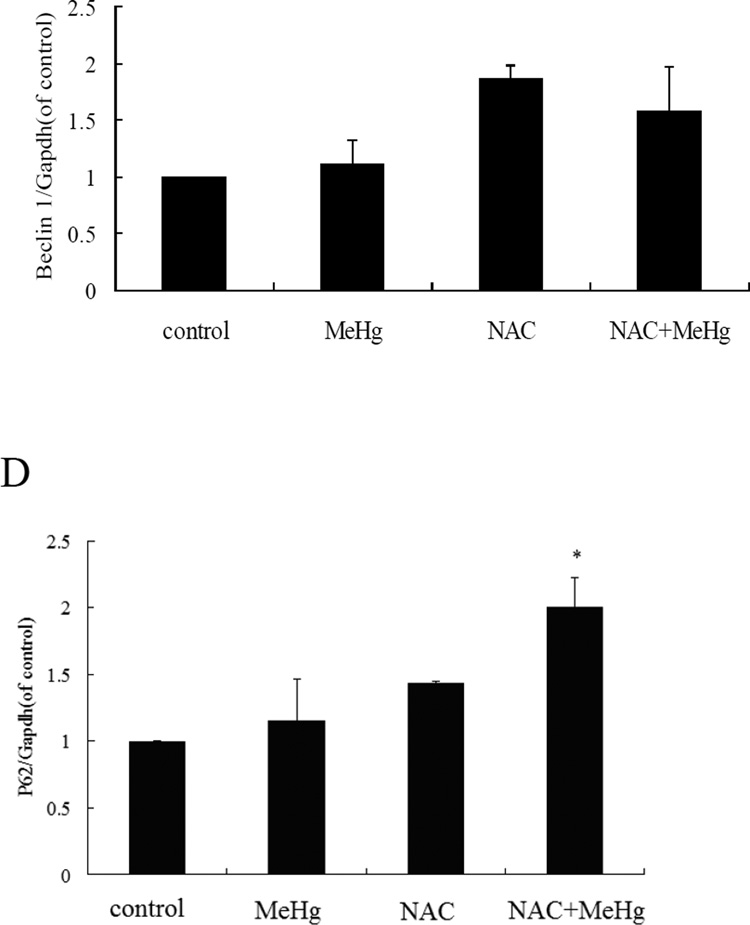
MeHg-induced cytotoxicity in astrocytes was attenuated by pretreatment with N-acetyl-L-cysteine (NAC) (5 mM for 4 hours) followed by MeHg (5µM for 6 hours) treatment (Figure 10). Furthermore, pre-treatment with NAC followed by MeHg treatment significantly (P<0.05) decreased the level of the autophagy-related proteins, LC3, Beclin1 and P62, compared to treatment with MeHg alone (Figure 11). These results indicated that NAC confered protection from MeHg-induced cytotoxicity, concomitant with a reduction in MeHg-induced autophagy.
Figure 10.
N-acetylcysteine protects astrocytes from MeHg. The cells were treated with MeHg (5µM, 6hours) in the presence or absence of pretreatment with NAC (5 mM for 4 hours). Astrocytic viability was measured with the MTT assay. Data shown are representative of three independent experiments. Statistical analysis was carried out by one-way ANOVA followed by Dunnett test. *: P<0.05, compared with the MeHg-treated group.
Figure 11.
N-acetylcysteine attenuates autophagy in astrocytes. A: Cells were treated for 6 hours with 5µM MeHg in the presence or absence of pretreatment with NAC (5 mM for 4 hours), followed by western blot analysis. GAPDH served as a loading control. B–D: Densitometric analyses of LC3B, Beclin 1, p62 in astrocytes. Statistical analysis was carried out by one-way ANOVA followed by Dunnett test. The figures are representative of one of three independent experiments. The figures are representative of one of three independent experiments. Values are means ± SD. *: P<0.05 compared with the MeHg-treated group.
Discussion
In this study, we tested whether MeHg could induce autophagy in rat primary astrocytes. The results establish that MeHg treatment decreases the viability of primary astrocytes cultures in a concentration- and time-dependent manner, with induction of both autophagy and apoptosis. Unregulated autophagy protects rat primary astrocytes from MeHg-induced cell death. The MTT assay (Figure 5) confirmed that pharmacological inhibition of autophagy with 3-MA or CQ as well as genetic inhibition of autophagy with Atg5 siRNA exacerbates MeHg’s neurotoxicity, while activation of autophagy by Rapamycin prevents cell death (Figure 9). Notably, NAC can inhibit the activation of autophagy by MeHg. These results indicate that autophagy plays an important role in MeHg-induced neurotoxicity.
This is the first study to establish significance of MeHg-induced autophagy in the nervous system, the tissue most susceptible to this metal. Autophagy has been recognized as an essential pathway to degrade and recycle damaged proteins and/or organelles and also serves as a survival response to stress or triggering mechanisms of toxic actions (Orrenius et al., 2013). The fact that autophagy in neonatal rat primary astrocytes is activated by MeHg is thus not surprising. Induction of both autophagy and apoptosis by MeHg has been described in human neural stem cells via the AKT-mTOR pathway and caspase-dependent apoptosis (Chang et al. 2013). In addition, inorganic mercury has been shown to induce autophagy in rat hepatocytes treated in vitro (Chatterjee et al. 2012, 2013). Other metals, such as cadmium, chromium, selenium, and copper have also been shown to induce autophagy and apoptosis (Laha et al. 2014; Pourahmad and O’Brien 2001; Wang et al. 2013; Yin et al. 2011). Since all metals are known to reduce cellular redox via oxidative stress, the role of ROS (superoxide ion, hydrogen peroxide, and hydroxyl radical) in metal-induced autophagy warrants further exploration.
NAC has been widely used as an anti-oxidant and a donor of sulfur group for reduced GSH production. Our findings established that NAC attenuated MeHg-induced cytotoxicity and autophagy (Fig. 11), corroborating that oxidative stress plays critical role in MeHg neurotoxicity (Farina et al., 2011a, b). Inhibition of autophagy by specific autophagic inhibitors and genetic RNA interference exacerbate MeHg cytotoxicity, and in contrast, pre-induction of autophagy partially abrogate MeHg cytotoxicity in astrocytes. Thus, it may be concluded that activation of autophagy by MeHg is a pro-survival response that may be attributed to ROS overproduction. Our findings provide additional evidence for its underlying mechanisms of toxicity, suggesting that oxidative stress is a common pathway in metal ion-induced autophagy.
Autophagy and apoptosis are not mutually exclusive phenomena. The crosstalk between autophagy and apoptosis reflects a complex relationship. Within the context of MeHg exposure, we find that this organometallic compound induces both autophagy (Figure 2) and apoptosis (Figure 4). Inhibition of autophagy exaggerated MeHg-induced toxicity. The flow cytometry results (Figure 7) confirmed this hypothesis by demonstrating that enhanced autophagy protects astrocytes from MeHg-induced toxicity. Several previous reports showed that inhibition of apoptosis causes autophagy, and inhibition of autophagy triggers apoptosis. Low concentrations of cadmium (Cd) induced autophagy in vascular endothelial cells, and as a consequence, apoptosis was inhibited (Wang et al. 2009). Chang and colleagues (2011) demonstrated a significant increase in autophagy in A549 cells stably expressing elevated BECN1, concomitant with a decrease in the levels of apoptosis-related protein. The present study suggests that MeHg-induced autophagy may delay the occurrence of apoptosis in astrocytes, consistent with previous reports on autophagy-delayed apoptosis under stress conditions (Liou et al. 2014; Liu et al. 2013a; Son et al. 2011).
We found that P62 protein levels showed a similar change to LC3II (Fig 4). P62 has a UBL binding sequence (UBS) domain through which it can mediate sequestration of ubiqutinated proteins into autophagosomes (Seto et al. 2013; Zhang et al. 2013). It is commonly degraded with the cargo, and its levels are regulated by the Nrf2 pathway (Jain et al. 2010), the Ras/MAPK pathway (Galavotti et al. 2013) or the JNK/c-Jun pathway (Puissant and Auberger 2010). Though degradation of p62 serves as a marker of autophagy activation, our findings somewhat unexpectedly showed that p62 increases with activation of autophagy. This may be due to MeHg-induced inhibition of p62 by lysosomal disruption (Daré et al, 2001; Tofighi et al. 2011). p62 has multiple domains to regulate cellular events (Bitto et al, 2014; Wang et al. 2014), and may trigger nuclear factor-κB (NF-κB) activity to promote cell survival responses (Chen et al. 2014). Thus, the significance of enhanced p62 protein by MeHg needs further study.
In summary, we demonstrate that MeHg activates pro-survival autophagy via ROS production and that induction of autophagy protects against MeHg neurotoxicity. Our findings provide a putative, novel target for therapeutic intervention to protect against MeHg-induced toxicity.
Acknowledgments
This work was supported in part by the Natural Science Foundation of Jiangsu Province (No. BK 20040061) and the Natural Science Foundation of China (No. 30872139, 81273124, 31100964). MA was supported in part by R01 ES07331 and ES020852 from the National Institute of Environmental Health Sciences (NIEHS).
References
- Allen JW, Mutkus LA, Aschner M. Methylmercury-mediated inhibition of 3H-D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res. 2001;902(1):92–100. doi: 10.1016/s0006-8993(01)02375-7. doi:S0006-8993(01)02375-7 [pii] [DOI] [PubMed] [Google Scholar]
- Allen JW, Shanker G, Tan KH, Aschner M. The consequences of methylmercury exposure on interactive functions between astrocytes and neurons. Neurotoxicology. 2002;23(6):755–759. doi: 10.1016/S0161-813X(01)00076-6. doi:S0161-813X(01)00076-6 [pii]10.1016/S0161-813X(01)00076-6. [DOI] [PubMed] [Google Scholar]
- Aki T, Funakoshi T, Unuma K, Uemura K. Impairment of autophagy: from hereditary disorder to drug intoxication. Toxicology. 2013;311(3):205–215. doi: 10.1016/j.tox.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Aschner M. Considerations on methylmercury (MeHg) treatments in in vitro studies. Neurotoxicology. 2012;33(3):512–513. doi: 10.1016/j.neuro.2012.05.002. doi: 10.1016/j.neuro.2012.05.002[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Du YL, Gannon M, Kimelberg HK. Methylmercury-induced alterations in excitatory amino acid transport in rat primary astrocyte cultures. Brain Res. 1993;602(2):181–186. doi: 10.1016/0006-8993(93)90680-l. doi:0006-8993(93)90680-L [pii] [DOI] [PubMed] [Google Scholar]
- Aschner M, Yao CP, Allen JW, Tan KH. Methylmercury alters glutamate transport in astrocytes. Neurochem Int. 2000;37(2–3):199–206. doi: 10.1016/s0197-0186(00)00023-1. doi:S0197-0186(00)00023-1 [pii] [DOI] [PubMed] [Google Scholar]
- Ballatori N, Lieberman MW, Wang W. N-Acetylcysteine as an antidote in methylmercury poisoning. Environ Health Perspect. 1998;106(5):267–271. doi: 10.1289/ehp.98106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook DM, Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp Oncol. 2012;34(3):286–297. doi:3596 [pii] [PubMed] [Google Scholar]
- Bitto A1, Lerner CA, Nacarelli T, Crowe E, Torres C, Sell C. p62/SQSTM1 at the interface of aging, autophagy, and disease. Age (Dordr) 2014 Feb 21; doi: 10.1007/s11357-014-9626-3. 2014 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman L, Lundekvam BF, Lægreid T, et al. Mercury in human brain, blood, muscle and toenails in relation to exposure: an autopsy study. Environ Health. 2007;6:30. doi: 10.1186/1476-069X-6-30. doi: 10.1186/1476-069X-6-30[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoldi AF, Coccini T, Ceccatelli S, Manzo L. Neurotoxicity and molecular effects of methylmercury. Brain Res Bull. 2001;55(2):197–203. doi: 10.1016/s0361-9230(01)00458-0. doi:S0361-9230(01)00458-0 [pii] [DOI] [PubMed] [Google Scholar]
- Chang JW, Choi H, Cotman SL, Jung YK. Lithium rescues the impaired autophagy process in CbCln3(Deltaex7/8/Deltaex7/8) cerebellar cells and reduces neuronal vulnerability to cell death via IMPase inhibition. J Neurochem. 2011;116(4):659–668. doi: 10.1111/j.1471-4159.2010.07158.x. doi:10.1111/j.1471-4159.2010.07158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Lee HJ, Kang B, et al. Methylmercury induces caspase-dependent apoptosis and autophagy in human neural stem cells. J Toxicol Sci. 2013;38(6):823–831. doi: 10.2131/jts.38.823. doi:DN/JST.JSTAGE/jts/38.823 [pii] [DOI] [PubMed] [Google Scholar]
- Chargui A, Zekri S, Jacquillet G, et al. Cadmium-induced autophagy in rat kidney: an early biomarker of subtoxic exposure. Toxicol Sci. 2011;121(1):31–42. doi: 10.1093/toxsci/kfr031. doi:10.1093/toxsci/kfr031kfr031 [pii] [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Ray A, Mukherjee S, Agarwal S, Kundu R, Bhattacharya S. Low concentration of mercury induces autophagic cell death in rat hepatocytes. Toxicol Ind Health. 2012:1–10. doi: 10.1177/0748233712462442. doi:0748233712462442 [pii]10.1177/0748233712462442. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Nandi P, Mukherjee S, Chattopadhyay A, Bhattacharya S. Regulation of autophagy in rat hepatocytes treated in vitro with low concentration of mercury. Toxicol Environ Chem. 95(3):504–514. [Google Scholar]
- Chen Y, Gibson SB. Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy. 2008;4(2):246–248. doi: 10.4161/auto.5432. doi:5432 [pii] [DOI] [PubMed] [Google Scholar]
- Chen Z, Fu Q, Shen B, Huang X, Wang K, He P, Li F, Zhang F, Shen H. Enhanced p62 expression triggers concomitant autophagy and apoptosis in a rat chronic spinal cord compression model. Mol Med Rep. 2014;9(6):2091–2096. doi: 10.3892/mmr.2014.2124. [DOI] [PubMed] [Google Scholar]
- Cherra SJ, 3rd, Dagda RK, Chu CT. Review: autophagy and neurodegeneration: survival at a cost? Neuropathol Appl Neurobiol. 2010;36(2):125–132. doi: 10.1111/j.1365-2990.2010.01062.x. doi:10.1111/j.1365-2990.2010.01062.xNAN1062 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH. The effects of methylmercury on the developing brain. Prog Neurobiol. 1989;32(6):447–470. doi: 10.1016/0301-0082(89)90018-x. doi:0301-0082(89)90018-X [pii] [DOI] [PubMed] [Google Scholar]
- Choi BH, Lapham LW, Amin-Zaki L, Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: a major effect of methylmercury poisoning in utero. J Neuropathol Exp Neurol. 1978;37(6):719–733. doi: 10.1097/00005072-197811000-00001. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining "clean" cells. Autophagy. 2005;1(3):131–140. doi: 10.4161/auto.1.3.2017. doi:2017 [pii] [DOI] [PubMed] [Google Scholar]
- Cui Q, Tashiro S-i, Onodera S, Minami M, Ikejima T. Autophagy preceded apoptosis in oridonin-treated human breast cancer MCF-7 cells. Biological and Pharmaceutical Bulletin. 2007;30(5):859–XXX. doi: 10.1248/bpb.30.859. [DOI] [PubMed] [Google Scholar]
- Daré E1, Li W, Zhivotovsky B, Yuan X, Ceccatelli S. Methylmercury and H(2)O(2) provoke lysosomal damage in human astrocytoma D384 cells followed by apoptosis. Free Radic Biol Med. 2001;30(12):1347–1356. doi: 10.1016/s0891-5849(01)00526-3. [DOI] [PubMed] [Google Scholar]
- Deng Y, Xu Z, Xu B, et al. Exploring cross-talk between oxidative damage and excitotoxicity and the effects of riluzole in the rat cortex after exposure to methylmercury. Neurotox Res. 2014;26(1):40–51. doi: 10.1007/s12640-013-9448-6. [DOI] [PubMed] [Google Scholar]
- Dong Z, Wang L, Xu J, et al. Promotion of autophagy and inhibition of apoptosis by low concentrations of cadmium in vascular endothelial cells. Toxicol In Vitro. 2009;23(1):105–110. doi: 10.1016/j.tiv.2008.11.003. doi:10.1016/j.tiv.2008.11.003S0887-2333(08)00273-7 [pii] [DOI] [PubMed] [Google Scholar]
- Dreiem A, Seegal RF. Methylmercury-induced changes in mitochondrial function in striatal synaptosomes are calcium-dependent and ROS-independent. Neurotoxicology. 2007;28(4):720–726. doi: 10.1016/j.neuro.2007.03.004. doi:S0161-813X(07)00052-6 [pii]10.1016/j.neuro.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan WJ, Liu FL, He RR, et al. Autophagy is involved in the effects of resveratrol on prevention of splenocyte apoptosis caused by oxidative stress in restrained mice. Mol Nutr Food Res. 2013;57(7):1145–1157. doi: 10.1002/mnfr.201200662. [DOI] [PubMed] [Google Scholar]
- Essick EE, Sam F. Oxidative stress and autophagy in cardiac disease, neurological disorders, aging and cancer. Oxid Med Cell Longev. 2010;3(3):168–177. doi: 10.4161/oxim.3.3.2. doi:10.4161/oxim.3.3.1210612106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Rocha JB, Aschner M. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci. 2011a;89(15–16):555–563. doi: 10.1016/j.lfs.2011.05.019. doi:10.1016/j.lfs.2011.05.019S0024-3205(11)00265-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Aschner M, Rocha JB. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol. 2011b;256(3):405–417. doi: 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Dou J, Li W, et al. Mecamylamine prevents neuronal apoptosis induced by glutamate and low potassium via differential anticholinergic-independent mechanisms. Neuropharmacology. 2008;54(4):755–765. doi: 10.1016/j.neuropharm.2007.12.003. doi:10.1016/j.neuropharm.2007.12.003S0028-3908(07)00396-6 [pii] [DOI] [PubMed] [Google Scholar]
- Galavotti S, Bartesaghi S, Faccenda D, et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene. 2013;32(6):699–712. doi: 10.1038/onc.2012.111. [DOI] [PubMed] [Google Scholar]
- Guo WJ, Ye SS, Cao N, Huang J, Gao J, Chen QY. ROS-mediated autophagy was involved in cancer cell death induced by novel copper(II) complex. Exp Toxicol Pathol. 2010;62(5):577–582. doi: 10.1016/j.etp.2009.08.001. doi:10.1016/j.etp.2009.08.001S0940-2993(09)00230-9 [pii] [DOI] [PubMed] [Google Scholar]
- Harhaji-Trajkovic L, Vilimanovich U, Kravic-Stevovic T, Bumbasirevic V, Trajkovic V. AMPK-mediated autophagy inhibits apoptosis in cisplatin-treated tumour cells. J Cell Mol Med. 2009;13(9B):3644–3654. doi: 10.1111/j.1582-4934.2009.00663.x. doi:10.1111/j.1582-4934.2009.00663.xJCMM663 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong MY, Gao JL, Cui JZ, et al. Effect of c-Jun NH(2)-terminal kinase-mediated p53 expression on neuron autophagy following traumatic brain injury in rats. Chin Med J (Engl) 2012a;125(11):2019–2024. [PubMed] [Google Scholar]
- Hong YS, Hunter S, Clayton LA, Rifkin E, Bouwer EJ. Assessment of mercury and selenium concentrations in captive bottlenose dolphin's (Tursiops truncatus) diet fish, blood, and tissue. Sci Total Environ. 2012b;414:220–226. doi: 10.1016/j.scitotenv.2011.11.021. doi:10.1016/j.scitotenv.2011.11.021S0048-9697(11)01286-1 [pii] [DOI] [PubMed] [Google Scholar]
- Jain A, Lamark T, Sjøttem E, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. Journal of biological chemistry. 2010;285(29):22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Mills KH, Harris J. Autophagy and inflammatory diseases. Immunol Cell Biol. 2013;91(3):250–258. doi: 10.1038/icb.2012.82. doi:10.1038/icb.2012.82icb201282 [pii] [DOI] [PubMed] [Google Scholar]
- Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9(12):1004–1010. doi: 10.1038/nrm2527. doi:10.1038/nrm2529nrm2529 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha D, Pramanik A, Maity J, et al. Interplay between autophagy and apoptosis mediated by copper oxide nanoparticles in human breast cancer cells MCF7. Biochim Biophys Acta. 2014;1840(1):1–9. doi: 10.1016/j.bbagen.2013.08.011. doi:10.1016/j.bbagen.2013.08.011S0304-4165(13)00355-3 [pii] [DOI] [PubMed] [Google Scholar]
- Larsen KB, Lamark T, Overvatn A, Harneshaug I, Johansen T, Bjorkoy G. A reporter cell system to monitor autophagy based on p62/SQSTM1. Autophagy. 2010;6(6):784–793. doi: 10.4161/auto.6.6.12510. doi:12510 [pii] [DOI] [PubMed] [Google Scholar]
- Larsen KE, Sulzer D. Autophagy in neurons: a review. Histol Histopathol. 2002;17(3):897–908. doi: 10.14670/HH-17.897. [DOI] [PubMed] [Google Scholar]
- Lee JY, Hwang GW, Naganuma A. Rip1 enhances methylmercury toxicity through production of reactive oxygen species (ROS) in budding yeast. J Toxicol Sci. 2009;34(6):715–717. doi: 10.2131/jts.34.715. doi:JST.JSTAGE/jts/34.715 [pii] [DOI] [PubMed] [Google Scholar]
- Liou JS, Wu YC, Yen WY, et al. Inhibition of autophagy enhances DNA damage-induced apoptosis by disrupting CHK1-dependent S phase arrest. Toxicol Appl Pharmacol. 2014 doi: 10.1016/j.taap.2014.04.028. doi:S0041-008X(14)00185-9 [pii]10.1016/j.taap.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Liu AJ, Wang SH, Chen KC, et al. Evodiamine, a plant alkaloid, induces calcium/JNK-mediated autophagy and calcium/mitochondria-mediated apoptosis in human glioblastoma cells. Chem Biol Interact. 2013a;205(1):20–28. doi: 10.1016/j.cbi.2013.06.004. doi:10.1016/j.cbi.2013.06.004S0009-2797(13)00134-8 [pii] [DOI] [PubMed] [Google Scholar]
- Liu W, Xu Z, Deng Y, Xu B, Wei Y, Yang T. Protective effects of memantine against methylmercury-induced glutamate dyshomeostasis and oxidative stress in rat cerebral cortex. Neurotox Res. 2013b;24(3):320–337. doi: 10.1007/s12640-013-9386-3. [DOI] [PubMed] [Google Scholar]
- Liu W, Xu Z, Deng Y, et al. Excitotoxicity and oxidative damages induced by methylmercury in rat cerebral cortex and the protective effects of tea polyphenols. Environ Toxicol. 2014;29(3):269–283. doi: 10.1002/tox.21755. [DOI] [PubMed] [Google Scholar]
- Mader BJ, Pivtoraiko VN, Flippo HM, et al. Rotenone inhibits autophagic flux prior to inducing cell death. ACS Chem Neurosci. 2012;3(12):1063–1072. doi: 10.1021/cn300145z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Wang M, Sun G, et al. Attenuation of Abeta-induced parallel autophagic and apoptotic cell death by gypenoside XVII through the estrogen receptor-dependent activation of Nrf2/ARE pathways. Toxicol Appl Pharmacol. 2014 doi: 10.1016/j.taap.2014.03.026. doi:S0041-008X(14)00130-6 [pii]10.1016/j.taap.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Milisav I, Poljsak B, Suput D. Adaptive response, evidence of cross-resistance and its potential clinical use. Int J Mol Sci. 2012;13(9):10771–10806. doi: 10.3390/ijms130910771. doi:10.3390/ijms130910771ijms-13-10771 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Li X, Rocha JB, Farina M, Aschner M. Glia and methylmercury neurotoxicity. J Toxicol Environ Health A. 2012;75(16–17):1091–1101. doi: 10.1080/15287394.2012.697840. doi:10.1080/15287394.2012.697840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Kaminskyy VO, Zhivotovsky B. Autophagy in toxicology: cause or consequence? Annu Rev Pharmacol Toxicol. 2013;53:275–297. doi: 10.1146/annurev-pharmtox-011112-140210. [DOI] [PubMed] [Google Scholar]
- Palmeira-Dos-Santos C, Pereira GJ, Barbosa CM, Jurkiewicz A, Smaili SS, Bincoletto C. Comparative study of autophagy inhibition by 3MA and CQ on Cytarabine-induced death of leukaemia cells. J Cancer Res Clin Oncol. 2014 doi: 10.1007/s00432-014-1640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick L. Mercury toxicity and antioxidants: Part 1: role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity. Altern Med Rev. 2002;7(6):456–471. [PubMed] [Google Scholar]
- Pizent A, Tariba B, Zivkovic T. Reproductive toxicity of metals in men. Arh Hig Rada Toksikol. 2012;63(Suppl 1):35–46. doi: 10.2478/10004-1254-63-2012-2151. doi:10.2478/10004-1254-63-2012-2151K496323N0Q141222 [pii] [DOI] [PubMed] [Google Scholar]
- Pourahmad J, O’Brien PJ. Biological reactive intermediates that mediate chromium (VI) toxicity Biological Reactive Intermediates VI. Springer; 2001. pp. 203–207. [DOI] [PubMed] [Google Scholar]
- Puissant A, Auberger P. AMPK- and p62/SQSTM1-dependent autophagy mediate resveratrol-induced cell death in chronic myelogenous leukemia. Autophagy. 2010;6(5):655–657. doi: 10.4161/auto.6.5.12126. doi:10.4161/auto.6.5.12126 12126 [pii] [DOI] [PubMed] [Google Scholar]
- Rami A. Review: autophagy in neurodegeneration: firefighter and/or incendiarist? Neuropathol Appl Neurobiol. 2009;35(5):449–461. doi: 10.1111/j.1365-2990.2009.01034.x. doi:10.1111/j.1365-2990.2009.01034.xNAN1034 [pii] [DOI] [PubMed] [Google Scholar]
- Ranjan K, Sharma A, Surolia A, Pathak C. Regulation of HA14-1 mediated oxidative stress, toxic response, and autophagy by curcumin to enhance apoptotic activity in human embryonic kidney cells. Biofactors. 2014;40(1):157–169. doi: 10.1002/biof.1098. [DOI] [PubMed] [Google Scholar]
- Sanfeliu C, Sebastia J, Ki SU. Methylmercury neurotoxicity in cultures of human neurons, astrocytes, neuroblastoma cells. Neurotoxicology. 2001;22(3):317–327. doi: 10.1016/s0161-813x(01)00015-8. doi:S0161813X01000158 [pii] [DOI] [PubMed] [Google Scholar]
- Seto S, Tsujimura K, Horii T, Koide Y. Autophagy adaptor protein p62/SQSTM1 and autophagy-related gene Atg5 mediate autophagosome formation in response to Mycobacterium tuberculosis infection in dendritic cells. PLoS One. 2013;8(12):e86017. doi: 10.1371/journal.pone.0086017. doi:10.1371/journal.pone.0086017PONE-D-13-43593 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker G, Allen JW, Mutkus LA, Aschner M. Methylmercury inhibits cysteine uptake in cultured primary astrocytes, but not in neurons. Brain Res. 2001;914(1–2):159–165. doi: 10.1016/s0006-8993(01)02791-3. doi:S0006-8993(01)02791-3 [pii] [DOI] [PubMed] [Google Scholar]
- Shanker G, Aschner M. Identification and characterization of uptake systems for cystine and cysteine in cultured astrocytes and neurons: evidence for methylmercury-targeted disruption of astrocyte transport. J Neurosci Res. 2001;66(5):998–1002. doi: 10.1002/jnr.10066. doi:10.1002/jnr.10066 [pii] [DOI] [PubMed] [Google Scholar]
- Shanker G, Aschner M. Methylmercury-induced reactive oxygen species formation in neonatal cerebral astrocytic cultures is attenuated by antioxidants. Brain Res Mol Brain Res. 2003;110(1):85–91. doi: 10.1016/s0169-328x(02)00642-3. doi:S0169328X02006423 [pii] [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Chan HM. Characterization of demethylation of methylmercury in cultured astrocytes. Chemosphere. 2008;74(1):112–118. doi: 10.1016/j.chemosphere.2008.09.019. doi: 10.1016/j.chemosphere.2008.09.019[pii] [DOI] [PubMed] [Google Scholar]
- Shen S, Kepp O, Kroemer G. The end of autophagic cell death? Autophagy. 2012;8(1):1–3. doi: 10.4161/auto.8.1.16618. doi:10.4161/auto.8.1.1661816618 [pii] [DOI] [PubMed] [Google Scholar]
- Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther. 2012;18(3):250–260. doi: 10.1111/j.1755-5949.2012.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YO, Wang X, Hitron JA, et al. Cadmium induces autophagy through ROS-dependent activation of the LKB1-AMPK signaling in skin epidermal cells. Toxicol Appl Pharmacol. 2011;255(3):287–296. doi: 10.1016/j.taap.2011.06.024. doi:10.1016/j.taap.2011.06.024S0041-008X(11)00257-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18(2):321–336. doi: 10.1016/0891-5849(94)00159-h. doi:089158499400159H [pii] [DOI] [PubMed] [Google Scholar]
- Takeuchi T. Human effects of methylmercury as an environmental neurotoxicant. In: Blum K, Manzo L, editors. Neurotoxicology. New York: Marcel Dekker; 1985. pp. 345–367. [Google Scholar]
- Tamm C, Duckworth J, Hermanson O, Ceccatelli S. High susceptibility of neural stem cells to methylmercury toxicity: effects on cell survival and neuronal differentiation. J Neurochem. 2006;97(1):69–78. doi: 10.1111/j.1471-4159.2006.03718.x. doi:JNC3718 [pii]10.1111/j.1471-4159.2006.03718.x. [DOI] [PubMed] [Google Scholar]
- Tasdemir E, Galluzzi L, Maiuri MC, et al. Methods for assessing autophagy and autophagic cell death. Methods Mol Biol. 2008;445:29–76. doi: 10.1007/978-1-59745-157-4_3. [DOI] [PubMed] [Google Scholar]
- Tofighi R1, Johansson C, Goldoni M, Ibrahim WN, Gogvadze V, Mutti A, Ceccatelli S. Hippocampal neurons exposed to the environmental contaminants methylmercury and polychlorinated biphenyls undergo cell death via parallel activation of calpains and lysosomal proteases. Neurotox Res. 2011;19(1):183–194. doi: 10.1007/s12640-010-9159-1. [DOI] [PubMed] [Google Scholar]
- Wagner C, Vargas AP, Roos DH, et al. Comparative study of quercetin and its two glycoside derivatives quercitrin and rutin against methylmercury (MeHg)-induced ROS production in rat brain slices. Arch Toxicol. 2010;84(2):89–97. doi: 10.1007/s00204-009-0482-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Cano M, Handa JT. p62 provides dual cytoprotection against oxidative stress in the retinal pigment epithelium. Biochim Biophys Acta. 2014;1843(7):1248–1258. doi: 10.1016/j.bbamcr.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhu J, Zhang K, et al. Induction of cytoprotective autophagy in PC-12 cells by cadmium. Biochem Biophys Res Commun. 2013;438(1):186–192. doi: 10.1016/j.bbrc.2013.07.050. doi:10.1016/j.bbrc.2013.07.050S0006-291X(13)01201-1 [pii] [DOI] [PubMed] [Google Scholar]
- Wang SH, Shih YL, Kuo TC, Ko WC, Shih CM. Cadmium toxicity toward autophagy through ROS-activated GSK-3beta in mesangial cells. Toxicol Sci. 2009;108(1):124–131. doi: 10.1093/toxsci/kfn266. doi:10.1093/toxsci/kfn266kfn266 [pii] [DOI] [PubMed] [Google Scholar]
- Wei M, Duan D, Liu Y, Wang Z, Li Z. Autophagy may protect MC3T3-E1 cells from fluoride-induced apoptosis. Mol Med Rep. 2014;9(6):2309–2315. doi: 10.3892/mmr.2014.2079. doi:10.3892/mmr.2014.2079[pii] [DOI] [PubMed] [Google Scholar]
- Wormser U, Brodsky B, Milatovic D, et al. Protective effect of a novel peptide against methylmercury-induced toxicity in rat primary astrocytes. Neurotoxicology. 2012;33(4):763–768. doi: 10.1016/j.neuro.2011.12.004. doi:10.1016/j.neuro.2011.12.004S0161-813X(11)00213-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang X, Guo H, et al. Synthesis and screening of 3-MA derivatives for autophagy inhibitors. Autophagy. 2013;9(4):595–603. doi: 10.4161/auto.23641. doi:10.4161/auto.2364123641 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Hu N, Jiang S, et al. Heavy metal scavenger metallothionein attenuates ER stress-induced myocardial contractile anomalies: role of autophagy. Toxicol Lett. 2014;225(3):333–341. doi: 10.1016/j.toxlet.2013.12.024. doi:10.1016/j.toxlet.2013.12.024S0378-4274(14)00019-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Lee E, Ni M, et al. Methylmercury-induced alterations in astrocyte functions are attenuated by ebselen. Neurotoxicology. 2011;32(3):291–299. doi: 10.1016/j.neuro.2011.01.004. doi:10.1016/j.neuro.2011.01.004S0161-813X(11)00007-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Yang YJ, Wang H, et al. Autophagy activation: a novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via AMP-activated protein kinase/mammalian target of rapamycin pathway. Stem Cells Dev. 2012;21(8):1321–1332. doi: 10.1089/scd.2011.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YB, Gong JL, Xing TY, Zheng SP, Ding W. Autophagy protein p62/SQSTM1 is involved in HAMLET-induced cell death by modulating apotosis in U87MG cells. Cell Death Dis. 2013;4:e550. doi: 10.1038/cddis.2013.77. doi:10.1038/cddis.2013.77cddis201377 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]