Abstract
For bilateral cochlear implant (CI) patients, electrodes that receive the same frequency allocation often stimulate locations in the left and right ear that do not yield the same perceived pitch, resulting in a pitch mismatch. This pitch mismatch may be related to degraded binaural abilities. Pitch mismatches have been found for some bilateral CI users and the goal of this study was to determine whether pitch mismatches are prevalent in bilateral CI patients, including those with extensive experience with bilateral CIs. To investigate this possibility, pitch matching was conducted with 16 bilateral CI patients. For 14 of the 16 participants, there was a significant difference between those electrodes in the left and right ear that yielded the same pitch and those that received the same frequency allocation in the participant’s clinical map. The results suggest that pitch mismatches are prevalent with bilateral CI users. The results also indicated that pitch mismatches persist even with extended bilateral CI experience. Such mismatches may reduce the benefits patients receive from bilateral CIs.
Keywords: pitch matching, bilateral cochlear implants, current steering, perceptual misalignment
Cochlear implant (CI) users often show improved speech perception and localization with two CIs compared with one alone (Dunn, Tyler, Oakley, Gantz, & Noble, 2008; Eapen, Buss, Adunka, Pillsbury, & Buchman, 2009; van Hoesel, 2004). However, their binaural benefits are less than those found with normal-hearing listeners (Kerber & Seeber, 2012; Loizou et al., 2009). The benefits may be suboptimal because, despite differences in electrode array insertion depth and neural survival for the left and right ear (Aschendorff et al., 2005; Fayad, Linthicum, Otto, Galey, & House, 1991; Marsh et al., 1993; Reiss, Lowder, Karsten, Turner, & Gantz, 2011; Svirsky, Fitzgerald, Sagi, & Glassman, 2015), these patients’ clinical maps traditionally route a given frequency region in the input to the same numbered electrode in the left and right array. This may result in a situation where electrodes that receive the same frequency allocation stimulate different regions of the two cochleas. This mismatch may yield different pitches and may also reduce the patient’s binaural benefits. For example, sensitivity to two binaural cues for localization, interaural time differences (ITDs) and interaural level differences (ILDs), can both be degraded by place or pitch mismatches (Francart & Wouters, 2007; Poon, Eddington, Noel, & Colburn, 2009; van Hoesel, 2004), as can speech perception in noise (Yoon, Liu, & Fu, 2011; Yoon, Shin, & Fu, 2013). Additionally, the signals from the two ears may not fuse together when large mismatches occur (Aronoff, Shayman, Prasad, Suneel, & Stelmach, 2015; Kan, Stoelb, Litovsky, & Goupell, 2013; Staisloff, Lee, & Aronoff, 2016). This suggests that having a proper pitch alignment across the two arrays could be beneficial in providing maximal benefits for bilateral CI users.
For CI users, pitch mismatches could potentially result from differences in place of stimulation (i.e., place pitch) or differences in pulse rates (i.e., temporal pitch). In terms of place pitch, in general, the more apical the stimulation location, the lower the pitch (e.g., Eddington, Dobelle, Brackmann, Mladejovsky, & Parkin, 1978; Landsberger, Mertens, Punte, & Van De Heyning, 2014). In terms of temporal pitch, for certain ranges of pulse rates, the slower the pulse rate, the lower the pitch (Eddington et al., 1978; Landsberger, Vermeire, Claes, Van Rompaey, & Van de Heyning, 2016; Shannon, 1983; Tong et al., 1979; Zeng, 2002). Although it is possible to have pitch differences between the two ears based on either place of stimulation or pulse rate, clinical pulse rates, which are generally above 900 Hz, are typically too high to yield discriminable temporal pitch. Temporal pitch is generally discriminable for pulse rates below approximately 300 Hz (Kong, Deeks, Axon, & Carlyon, 2009; Shannon, 1983; Zeng, 2002), although some CI users are able to discriminate temporal pitch up to approximately 900 Hz (Kong & Carlyon, 2010). Thus, pitch mismatches based on different pulse rates in the two ears are unlikely with clinical pulse rates.
Pitch mismatches have been found for bilateral CI patients (Hu & Dietz, 2015; Kan et al., 2013; Reiss et al., 2011; Svirsky et al., 2015). Bimodal patients and patients with single-sided deafness and a CI in one ear presumably have a large mismatch between the stimulation location and the assigned frequency allocation (Landsberger, Svrakic, Roland, & Svirsky, 2015). For these patients, mismatches between the electric and acoustic ear are also prevalent (Reiss et al., 2015; Vermeire et al., 2015).
Despite factors that may result in pitch mismatches, CI users may have the capability of adapting over time, yielding increasingly similar pitches for electrodes assigned the same frequency allocation in the two ears. For example, Reiss et al. (2011) found that, for a patient with a 10-mm array in one ear and a 24-mm array in the other ear, pitch misalignment was dramatically reduced by over 10 mm with adaptation. Although adaptation was not complete for this individual, the magnitude of the adaptation was large (see also Svirsky et al., 2015). Such a large magnitude of adaptation may be sufficient to correct the presumably smaller pitch misalignments for bilateral CI patients with the same length array in both ears. As such, it is possible that pitch mismatches may be rare for experienced bilateral CI users.
Because previous studies have compared pitch matches at relatively few locations and with few participants, the goal of this study was to use a relatively large sample size to determine whether pitch misalignments are prevalent or rarely occur, with a particular emphasis on CI users with at least 2 years of bilateral CI experience.
Methods
Participants
Pitch matching data were collected from 16 participants as part of ongoing experiments. For 11 participants (C03, C14, I01, I02, I03, I05, I06, I10, I11, I14, and I26), the data were collected as a preliminary step in ongoing studies examining different methods for creating bilateral maps (e.g., Aronoff, Stelmach, Padilla, & Landsberger, 2016). For I04, I07, I09, I13, and I15, the data were collected as part of an ongoing longitudinal study investigating new bilateral users. All participants had Advanced Bionics CII or HiRes 90 K implants. Their bilateral CI experience ranged from 0 days to 12 years (see Table 1).
Table 1.
Participant Characteristics Ordered by Bilateral Implant Experience.
| Subject | Age | Gender | Hearing loss onseta | Cause | Array type | Implant experience |
|---|---|---|---|---|---|---|
| I15 | 46 | Female | 6 months old | Measles | HiFocus Mid-Scala (L) HiFocus 1J (R) | 0 days (L) 10 years (R) |
| I07 | 53 | Male | 30 years old | Familial | HiFocus Mid-Scala (L & R) | 1 week (L) 1 week (R) |
| I09 | 56 | Male | 27 years old (L) 43 years old (R) | Unknown | HiFocus Mid-Scala (L & R) | 1 year (L) 2 weeks (R) |
| I04 | 57 | Female | 36 years old | Progressive autoimmune | HiFocus Mid-Scala (L) HiFocus 1J (R) | 1 month (L) 1 years (R) |
| I13 | 33 | Male | 3 years old | High fever/viral | HiFocus Mid-Scala (L & R) | 6 months (L) 1 year (R) |
| I10 | 49 | Female | 29 years old | Autoimmune | HiFocus Mid-Scala (L & R) | 1 year (L) 2 years (R) |
| I06 | 56 | Female | 36 years old | Genetic—Maternal | HiFocus Mid-Scala (L) HiFocus 1J (R) | 1 year (L) 3 years (R) |
| I11 | 67 | Male | 9–10 years old (L) 57 years old (R) | Sudden, unknown | HiFocus Mid-Scala (L) HiFocus 1J (R) | 2 years (L) 10 years (R) |
| I02 | 60 | Female | 2 years old | Meningitis | HiFocus 1J (L & R) | 2 years (L) 5 years (R) |
| I14 | 65 | Male | <25 years old | Ménière’s, progressive | HiFocus Mid-Scala (L) HiFocus Helix (R) | 2 years (L) 3 years (R) |
| I26 | 45 | Female | Birth | Hereditary | HiFocus Mid-Scala (L & R) | 2 years (L) 2 years (R) |
| C03 | 57 | Female | 29 years old | Hereditary | HiFocus 1J (L & R) | 7 years (L) 4 years (R) |
| C14 | 48 | Male | 4.5 months | Maternal rubella | HiFocus 1J (L & R) | 4 years (L) 8 years (R) |
| I01 | 62 | Female | Birth | Unknown | HiFocus 1J (L & R) | 8 years (L) 5 years (R) |
| I03 | 70 | Female | Birth | Unknown | HiFocus 1J (L & R) | 12 years (L) 7 years (R) |
| I05 | 56 | Male | 5 years old | Unknown (injury or genetic) | HiFocus 1J (L & R) | 12 years (L) 12 years (R) |
Note. L = left ear; R = right ear.
aCalculated based on self-reported time of first-noticed hearing loss.
Electric Stimulation
Stimulation was controlled with the Bionic Ear Data Collection System (BEDCS version 1.17) or HRStream (version 1.0.2). Both systems allow equivalent control over stimulation parameters. Participants were stimulated with pulse trains consisting of approximately 32 µs phase durations and a pulse rate of approximately 976 pulses per second. These parameters were chosen based on the other ongoing experiments in which the individuals were involved. Although the pulse rate used was below those used in their clinical processors (typically 1,856 pulses per second), both the rate used for the current data and that used in the clinical maps were above those that should provide discriminable temporal pitch (e.g., Kong & Carlyon, 2010) and within the range for other clinical processors.
To minimize confounding loudness differences for the stimuli presented in the pitch-matching task, electrodes were loudness balanced within and across arrays. The signal was loudness balanced at a most comfortable loudness level within each array by presenting groups of four adjacent electrodes in sequence using 500-ms pulse trains with a 1-s interstimulus interval. Electrode 1 was the reference electrode for the first group, and stimulation on that electrode was presented at the most comfortable level. The stimulation level was adjusted by the experimenter for any electrode that the participant indicated was perceived as louder or softer than the level of the first electrode. This adjustment was typically done in 1–2 µA steps. After all electrodes in that group were loudness balanced, a new group of four adjacent electrodes was chosen with the first electrode from the new group being the same as the last electrode from the previous group (e.g., Group 1: Electrodes 1–4; Group 2: Electrodes 4–7).
When loudness balancing across arrays, stimulation consisted of 500-ms pulse trains with an interstimulus period of approximately 500 ms. Stimulation alternated between the left and the right ear. Loudness balancing across ears was conducted by either having the subjects use a mouse to increase or decrease the stimulation level of the target by 0.5, 1, or 1.5 dB or by having the experimenter adjust the loudness of the target stimulation based on the subjects’ report of the loudness of the target stimulation compared with the reference stimulation. (These differences were dependent on the particular experiment in which the subject was involved.) The reference stimulus was set at the most comfortable level. Loudness balancing across arrays was conducted for electrode 10 and the stimulation levels for all electrodes were subsequently globally adjusted by shifting the stimulation level by the same percentage of the dynamic range for all electrodes.
Pitch-Matching Procedures
Pitch matching was conducted using 500-ms pulse trains delivered to the left and right ear sequentially with an approximately 500-ms interstimulus interval. For pitch matching, the reference ear was usually the left ear. Place of stimulation could be adjusted in the target ear in 0.1 electrode steps using current steered virtual channels (e.g., Firszt, Koch, Downing, & Litvak, 2007; Landsberger & Srinivasan, 2009) by turning a knob (Powermate, Griffin Technology) or clicking on arrows using the mouse. To minimize bias based on the initial target location, the starting stimulation location for the target was randomly selected for each reference. This process of stimulus presentation followed by a subject-directed change in the place of stimulation continued until the left and right ear stimulation was perceived as having the same pitch. Pitch matches were obtained for at least 22 unique reference locations, typically separated 0.5 to 1 electrode apart. For most participants, each reference location was used only once. However, for C03, 6 of the 33 reference locations were measured twice and for I02, 4 of the 34 reference locations were measured twice. In both cases, the repeated measures yielded similar results, with an average test–retest difference in terms of the pitch-matched stimulation site of 0.3 electrodes for C03 and 1.2 electrodes for I02. The procedures were approved by the St. Vincent Medical Center institutional review board (affiliated with the House Ear Institute) and the institutional review board for the University of Illinois at Urbana-Champaign.
Data Analysis
The pitch-matching data were fit with a least trimmed squares regression (a robust regression measure) and the 95% confidence interval for the fit was calculated using a bootstrap method. This was done by generating a series of bootstrap distributions, each created by randomly sampling with replacement from a participants’ pitch-matching data such that there were the same number of data points in the bootstrap distribution as in the original distribution. Five hundred and ninety-nine bootstrap distributions were generated, and a linear fit was calculated for each bootstrap distribution. Based on that linear fit, each of the 16 reference electrodes (the number used in the Advanced Bionics arrays used by the participants) was paired with a stimulation location in the opposite ear based on the following equation:
| (1) |
where P is the paired location, α is slope, R is the reference, and I is the intercept derived from the linear fit.
The 95% confidence interval was separately calculated for each reference electrode by rank ordering the pitch-matched target locations for each electrode and selecting the 14th smallest and 585th largest values (i.e., 95% of the predicted pitch matches fell between those two values). The magnitude of the confidence intervals is indicative of the variability in a participant’s pitch-matching data, with larger confidence intervals indicating greater variability. The 95% confidence interval was compared to the isoline (the line that would indicate matches based on the patients’ clinical frequency allocation tables). If the confidence interval did not overlap the isoline for a given reference electrode, it indicated that the pitch-matching data significantly deviated from the isoline for that reference location.
Results
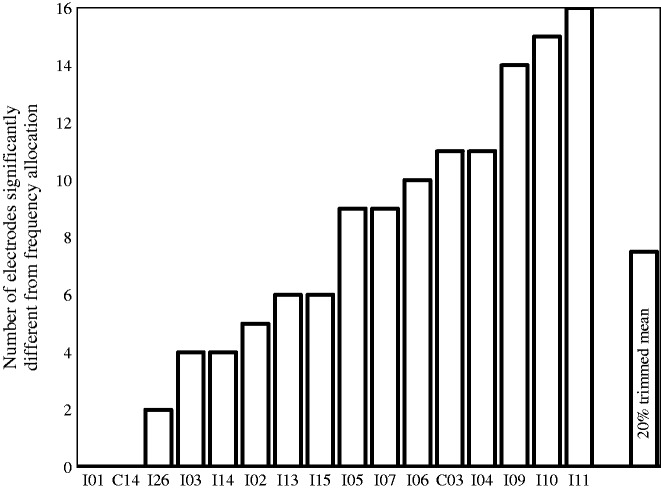
The results showed that for 14 of the 16 participants, the pitch-matching data significantly deviated from what would be predicted by the frequency allocations for at least one electrode (20% trimmed mean: 7.5 electrodes significantly deviated from the pairing predicted by the frequency allocation, see Figure 1; individual pitch-matching data are presented in Figure 2). This indicates that a portion of the clinically paired electrodes were not perceived as pitch matched for the majority of the participants. Results were similar when pitch matches were compared with matches predicted by electrode numbers, which often, but not always, corresponded with frequency allocation. Importantly, these significant mismatches occurred both with those with minimal bilateral CI experience and those with extensive bilateral CI experience. The number of significant mismatches is likely a conservative estimate of mismatches, given the large variance and thus large confidence intervals for some participants, which reduces power in the analysis. The difference between electrodes paired based on the linear fit of the pitch-matching data and those predicted by frequency allocations was calculated based on the following equations:
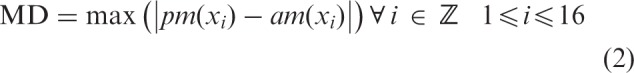
where MD is maximum difference, xi is an individual electrode, pm is pitch matched to xi and am is allocation matched to xi.
| (3) |
where AD is average difference, xi is an individual electrode, pm is pitch matched to xi, am is allocation matched to xi and N = 16 (i.e., the number of electrodes for the devices the participants had).
Figure 1.
Number of electrodes that significantly deviate from the clinical pairings for each participant.
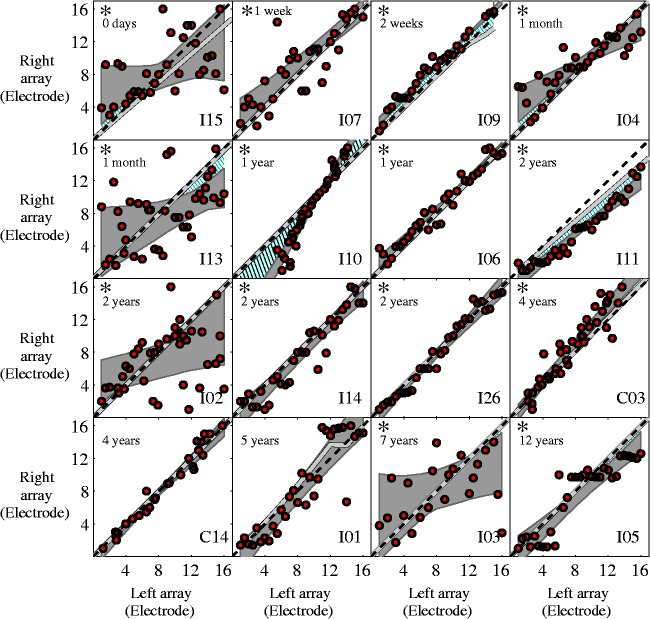
Figure 2.
Individual pitch-matching data showing that, for most participants, pitch-matched electrode pairs significantly deviate from the isoline for at least part of the array. Panels are organized from shortest bilateral CI experience (top left) to longest bilateral CI experience (bottom right). The duration of bilateral CI experience is indicated in the top left corner of each panel. Circles indicate one pitch-matching trial. The dashed line represents the matches based on electrode numbers in the left and right processor. The gray line represents the matches based on frequency allocations in the left and right processor. The gray area indicates the 95% confidence interval for a linear fit of the pitch-matching data. The blue hatched area highlights the regions where there is a significant deviation from the frequency allocation. Asterisks in the upper left corner of a plot indicate that there was a significant deviation from clinically paired electrodes for at least part of the array for that participant.
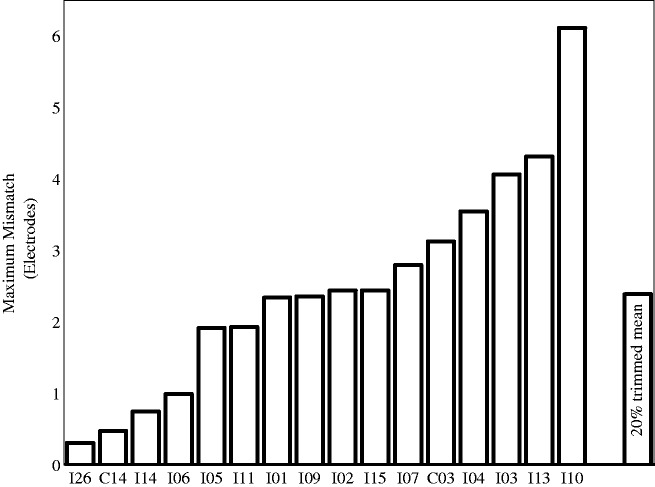
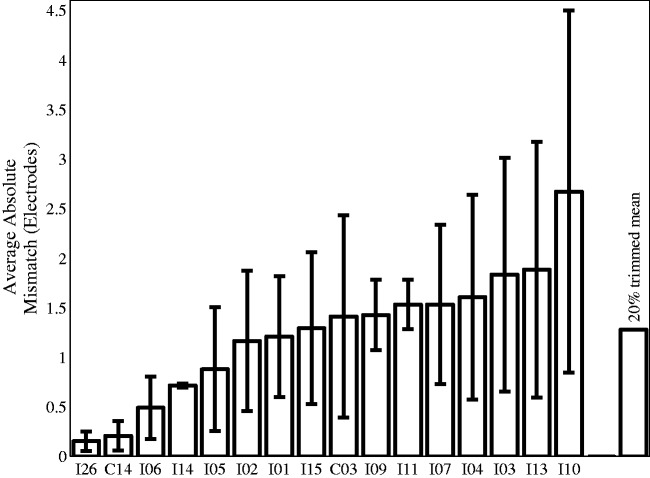
These analyses indicated that the 20% trimmed mean for the maximum difference was 2.4 electrodes and the average difference between the pitch-matching data and the frequency allocation predictions was 1.3 electrodes (see Figures 3 and 4). This corresponds to 2.5 and 1.3 mm, respectively.
Figure 3.
Maximum magnitude of the difference between the pitch-matched electrode pairs and the clinical pairings for each participant.
Figure 4.
Average magnitude and standard deviation of the difference between the pitch-matched electrode pairs and the clinical pairings for each participant.
To determine whether the pitch misalignment for clinically paired electrodes was related to length of bilateral CI use, length of bilateral CI use was compared, using a correlation analysis, with the maximum and average magnitude of the distance between their pitch-matching fit and the pairing based on frequency allocation as well as the number of electrode pairs significantly different from the pairings based on frequency allocation. The results indicated that there was no significant relationship for either of those correlations (p > .1 for both analyses).
Discussion
The results indicate that, for the majority of the participants, pitch matches deviated from matches based on the patients’ clinical frequency allocation table for at least part of the array, suggesting that pitch mismatches are prevalent for bilateral CI users. Because larger pitch-matching variability yields greater confidence interval magnitudes, the prevalence of mismatches found in this study may underestimate the true prevalence. One caveat is that the Advanced Bionics 1J array, which many of the participants used, has higher insertion depth variability than other arrays (Landsberger et al., 2015), and this may have increased the probability that the left and right arrays had different insertion depths, resulting in more pitch misalignments.
In terms of length of bilateral CI experience, mismatches occurred for both individuals with virtually no bilateral CI experience and individuals with many years of bilateral CI experience. This suggests that experience is not sufficient to alleviate mismatches. It is not possible without longitudinal data to determine whether mismatches were reduced with experience. However, previous research indicates that CI users have the capability of adapting over time, at least when there are large initial mismatches (e.g., Reiss et al., 2011; Svirsky et al., 2015). The current results may indicate that pitch-matching adaptation does not occur for bilateral CI patients with modest initial pitch mismatches, possibly because of broad pitch fusion (Reiss, Ito, Eggleston, & Wozny, 2014). Alternatively pitch-matching adaptation may occur but require more time than provided in this dataset or adaptation may be limited, possibly by the innate tonotopic organization of the cochlea.
The results from I09 suggest that the tonotopic organization of the cochlea may have a larger influence than the frequency allocations. I09, who had 2 weeks of bilateral experience, had a medial electrode disabled on the left array, resulting in a nonlinear relationship between the frequency allocations in the left and right ear as shown by the gray line in Figure 2. Despite this nonlinearity, his pitch-matching data were well modeled with a linear fit (see Figure 2). Additionally, the electrodes to the left and right of the disabled electrode were pitch matched to locations separated by two electrodes, consistent with the physical separation of the electrodes in the left ear but not the frequency allocation. This suggests, at least with limited experience, that the physical locations of the electrodes may play a large role in which stimulation locations perceptually match across ears, although additional experience may have yielded results more consistent with the frequency allocation. I01 also had a medial electrode disabled in one ear. However, given the variability in her pitch matching for basal electrodes, it is difficult to determine whether the physical electrode location matters more than the frequency allocation for that participant.
There are various potential methods for determining the optimal alignment including ones based on perceived pitch matches between ears (Aronoff et al., 2016; Goupell, Stoelb, Kan, & Litovsky, 2013; Kan et al., 2013), ITD sensitivity (Hu & Dietz, 2015; Long, Eddington, Colburn, & Rabinowitz, 2003; Poon et al., 2009), ILD sensitivity (Long et al., 2003), and the binaural interaction component (Hu & Dietz, 2015). Although there is some variability across these different methods, pitch matches are typically within approximately one electrode of the optimal paired location based on these different measures (Hu & Dietz, 2015; Poon et al., 2009). In comparison, the largest mismatch between the electrodes paired based on frequency allocations and those based on pitch matches was typically twice as large. This suggests that differences between the various methods of optimally pairing electrodes are smaller than the misalignments that would occur when using typical clinical frequency allocations.
Aligning the two arrays based on pitch can yield important improvements in performance. For example, it can improve lateralization abilities (Goupell et al., 2013; Kan et al., 2013), ILD sensitivity (Francart & Wouters, 2007; Long et al., 2003), ITD sensitivity (Long et al., 2003; Poon et al., 2009), and the ability to fuse sounds from the two ears into a single auditory object (Aronoff et al., 2015; Kan et al., 2013). Although attempts are rarely made clinically to perceptually align the two arrays, presumably based on the assumption that adaptation will correct for those mismatches over time, the current data strongly suggest that adaptation is not sufficient to correct for mismatches.
Conclusions
The results from this study suggest that pitch mismatches with clinically paired electrodes are likely to be very prevalent for bilateral CI users. This is true even for those with extensive bilateral CI experience, indicating that, while users may adapt to mismatches, that adaptation is, at best, partial. Such mismatches may reduce the benefits patients receive from bilateral CIs.
Acknowledgment
We thank Roozbeh Soleymani for providing mathematical expertise.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by a grant from the National Organization for Hearing Research, and NIDCD grants T32DC009975, R01-DC12152, R01-DC-001526, R01-DC004993, R03-DC-010064, and R03-DC-013380.
References
- Aronoff J. M., Shayman C., Prasad A., Suneel D., Stelmach J. (2015) Unilateral spectral and temporal compression reduces binaural fusion for normal hearing listeners with cochlear implant simulations. Hearing Research 320: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff J. M., Stelmach J., Padilla M., Landsberger D. M. (2016) Interleaved processors improve cochlear implant patients’ spectral resolution. Ear and Hearing 37(2): e85–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschendorff A., Kubalek R., Turowski B., Zanella F., Hochmuth A., Schumacher M., Laszig R. (2005) Quality control after cochlear implant surgery by means of rotational tomography. Otology & Neurotology 26(1): 34–37. [DOI] [PubMed] [Google Scholar]
- Dunn C. C., Tyler R. S., Oakley S., Gantz B. J., Noble W. (2008) Comparison of speech recognition and localization performance in bilateral and unilateral cochlear implant users matched on duration of deafness and age at implantation. Ear and Hearing 29(3): 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen R. J., Buss E., Adunka M. C., Pillsbury H. C., 3rd, Buchman C. A. (2009) Hearing-in-noise benefits after bilateral simultaneous cochlear implantation continue to improve 4 years after implantation. Otology & Neurotology 30(2): 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddington D. K., Dobelle W. H., Brackmann D. E., Mladejovsky M. G., Parkin J. (1978) Place and periodicity pitch by stimulation of multiple scala tympani electrodes in deaf volunteers. Transactions of the American Society for Artificial Internal Organs 24: 1–5. [PubMed] [Google Scholar]
- Fayad J., Linthicum F. H., Jr, Otto S. R., Galey F. R., House W. F. (1991) Cochlear implants: Histopathologic findings related to performance in 16 human temporal bones. The Annals of Otology Rhinology and Laryngology 100(10): 807–811. [DOI] [PubMed] [Google Scholar]
- Firszt J. B., Koch D. B., Downing M., Litvak L. (2007) Current steering creates additional pitch percepts in adult cochlear implant recipients. Otology & Neurotology 28(5): 629–636. [DOI] [PubMed] [Google Scholar]
- Francart T., Wouters J. (2007) Perception of across-frequency interaural level differences. The Journal of the Acoustical Society of America 122(5): 2826–2831. [DOI] [PubMed] [Google Scholar]
- Goupell M. J., Stoelb C., Kan A., Litovsky R. Y. (2013) Effect of mismatched place-of-stimulation on the salience of binaural cues in conditions that simulate bilateral cochlear-implant listening. The Journal of the Acoustical Society of America 133(4): 2272–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Dietz M. (2015) Comparison of interaural electrode pairing methods for bilateral cochlear implants. Trends in Hearing 19: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan A., Stoelb C., Litovsky R. Y., Goupell M. J. (2013) Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear-implant users. The Journal of the Acoustical Society of America 134(4): 2923–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerber S., Seeber B. U. (2012) Sound localization in noise by normal-hearing listeners and cochlear implant users. Ear and Hearing 33(4): 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. Y., Carlyon R. P. (2010) Temporal pitch perception at high rates in cochlear implants. The Journal of the Acoustical Society of America 127(5): 3114–3123. [DOI] [PubMed] [Google Scholar]
- Kong Y. Y., Deeks J. M., Axon P. R., Carlyon R. P. (2009) Limits of temporal pitch in cochlear implants. The Journal of the Acoustical Society of America 125(3): 1649–1657. [DOI] [PubMed] [Google Scholar]
- Landsberger D. M., Mertens G., Punte A. K., Van De Heyning P. (2014) Perceptual changes in place of stimulation with long cochlear implant electrode arrays. The Journal of the Acoustical Society of America 135(2): EL75–EL81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger D. M., Srinivasan A. G. (2009) Virtual channel discrimination is improved by current focusing in cochlear implant recipients. Hearing Research 254(1–2): 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger D. M., Svrakic M., Roland J. T., Jr., Svirsky M. (2015) The Relationship between insertion angles, default frequency allocations, and spiral ganglion place pitch in cochlear implants. Ear and Hearing 36(5): e207–e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger D. M., Vermeire K., Claes A., Van Rompaey V., Van de Heyning P. (2016) Qualities of single electrode stimulation as a function of rate and place of stimulation with a cochlear implant. Ear and Hearing 37(3): e149–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizou, P. C., Hu, Y., Litovsky, R., Yu, G., Peters, R., Lake, J., & Roland, P. (2009). Speech recognition by bilateral cochlear implant users in a cocktail-party setting. The Journal of the Acoustical Society of America, 125(1), 372–383. [DOI] [PMC free article] [PubMed]
- Long C. J., Eddington D. K., Colburn H. S., Rabinowitz W. M. (2003) Binaural sensitivity as a function of interaural electrode position with a bilateral cochlear implant user. The Journal of the Acoustical Society of America 114(3): 1565–1574. [DOI] [PubMed] [Google Scholar]
- Marsh, M. A., Xu, J., Blamey, P. J., Whitford, L. A., Xu, S. A., Silverman, J. M., & Clark, G. M. (1993). Radiologic evaluation of multichannel intracochlear implant insertion depth. The American Journal of Otology, 14(4), 386–391. [PubMed]
- Poon B. B., Eddington D. K., Noel V., Colburn H. S. (2009) Sensitivity to interaural time difference with bilateral cochlear implants: Development over time and effect of interaural electrode spacing. The Journal of the Acoustical Society of America 126(2): 806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss L. A., Ito R. A., Eggleston J. L., Liao S., Becker J. J., Lakin C. E., McMenomey S. O. (2015) Pitch adaptation patterns in bimodal cochlear implant users: Over time and after experience. Ear and Hearing 36(2): e23–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss L. A., Ito R. A., Eggleston J. L., Wozny D. R. (2014) Abnormal binaural spectral integration in cochlear implant users. Journal of the Association forResearch in Otolaryngology 15(2): 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss L. A., Lowder M. W., Karsten S. A., Turner C. W., Gantz B. J. (2011) Effects of extreme tonotopic mismatches between bilateral cochlear implants on electric pitch perception: A case study. Ear and Hearing 32(4): 536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R. V. (1983) Multichannel electrical stimulation of the auditory nerve in man. I. Basic psychophysics. Hearing Research 11(2): 157–189. [DOI] [PubMed] [Google Scholar]
- Staisloff H. E., Lee D. H., Aronoff J. M. (2016) Perceptually aligning apical frequency regions leads to more binaural fusion of speech in a cochlear implant simulation. Hearing Research 337: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svirsky M. A., Fitzgerald M. B., Sagi E., Glassman E. K. (2015) Bilateral cochlear implants with large asymmetries in electrode insertion depth: Implications for the study of auditory plasticity. Acta Otolaryngologica 135(4): 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, Y. C., Black, R. C., Clark, G. M., Forster, I. C., Millar, J. B., O Loughlin, B. J., & Patrick, J. F. (1979). A preliminary report on a multiple-channel cochlear implant operation. The Journal of Laryngology and Otology, 93(7), 679–695. [DOI] [PubMed]
- van Hoesel R. J. (2004) Exploring the benefits of bilateral cochlear implants. Audiology & Neurootology 9(4): 234–246. [DOI] [PubMed] [Google Scholar]
- Vermeire, K., Landsberger, D. M., Van de Heyning, P. H., Voormolen, M., Kleine Punte, A., Schatzer, R., & Zierhofer, C. (2015). Frequency-place map for electrical stimulation in cochlear implants: Change over time. hearing Research, 326, 8–14. [DOI] [PMC free article] [PubMed]
- Yoon Y. S., Liu A., Fu Q. J. (2011) Binaural benefit for speech recognition with spectral mismatch across ears in simulated electric hearing. The Journal of the Acoustical Society of America 130(2): EL94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y. S., Shin Y. R., Fu Q. J. (2013) Binaural benefit with and without a bilateral spectral mismatch in acoustic simulations of cochlear implant processing. Ear and Hearing 34(3): 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F. G. (2002) Temporal pitch in electric hearing. Hearing Research 174(1–2): 101–106. [DOI] [PubMed] [Google Scholar]