Abstract
Aspiculuris tetraptera continues to be a problem in rodent vivaria, in part due to difficulties in parasite detection. Although PCR testing is highly sensitive, it is expensive and does not always provide immediate results. Consequently, many institutions rely on passive fecal flotation as a quick inhouse exam for diagnosing A. tetraptera infections. To increase the sensitivity of this test, we examined multiple parameters to determine the optimal test protocol. A 30-min soaking period prior to fecal flotation for 15 min allowed fecal pellets to soften and facilitated efficient egg isolation. We also evaluated the effect of time of day, sample size, age, sex, and housing status on egg isolation. No evidence of cyclical egg shedding was found, and although larger fecal sample sizes did not result in more eggs isolated, their use reduced the incidence of false-negative exams. The most eggs were isolated from 8- and 12-wk-old mice, and as mice aged, the number of eggs isolated declined. Overall, neither sex nor housing status influenced the number of eggs isolated. Finally, examination of multiple diagnostic tests (fecal flotation exam, direct examination of cecal and colonic contents, and fecal PCR) revealed that no single test was definitive, thus indicating that multiple tests might be required to successfully screen mice with low pinworm burdens. These findings provide guidance regarding sample selection, collection, and processing to efficiently detect A. tetraptera.
Aspiculuris tetraptera, the most commonly isolated pinworm to naturally infect laboratory rodents, continues to persist in laboratory colonies, in part due to difficulties with detection.26 Detection proves challenging because traditional dirty-bedding sentinels are poor indicators of colony pinworm infection status.10 Newer applications, such as PCR analysis of samples from the rack exhaust plenum, are proving increasingly useful in the rapid screening of large colonies for parasites.10,13,23 However, although PCR is an exceptionally sensitive test, it is expensive and requires days to weeks for results when an outside diagnostic lab is used. Consequently, many institutions rely on fecal flotation as an initial screening exam, because it is relatively inexpensive and technically simple, with a rapid turn-around time. However, fecal flotation exam is a less sensitive test than is PCR analysis.10 After observing that the results from our inhouse passive fecal flotation exams did not correlate with positive PCR tests, we decided to systematically evaluate the passive fecal flotation exam to improve diagnostic efficiency.
Efficient methods to detect and eradicate A. tetraptera are necessary, given the pinworm's potential effect on research. Despite rarely causing overt clinical disease, pinworms elicit a Th2 response from the host and thus have the potential to alter immunology studies.22,24,26 In addition, common treatments for pinworms, which include ivermectin, benzimidazoles, and piperazine compounds,14 have been described to affect research results or induce toxicity in mice at therapeutic doses.6,8,19,27,29,34
A detailed understanding of the pinworm life cycle is necessary to improve diagnostic methods. A. tetraptera has a direct life cycle, with mice becoming infected after ingesting embryonated eggs.33 The eggs hatch, and the larvae develop in the crypts of the middle colon before moving to the proximal colon as adult worms.14 Female A. tetraptera adults have a 45 to 50 d lifespan and produce eggs multiple times during their reproductive life, unlike Syphacia obvelata pinworms, which produce eggs only once.15 A. tetraptera is the only rodent pinworm that is readily detectable through fecal flotation, because adult female worms shed eggs in the mucous covering of rodent fecal pellets.14 A. tetraptera eggs require approximately 5 to 8 d to embryonate, and the prepatent period is 21 to 25 d.1,10,33 Male mice are more susceptible to heavy pinworm burdens, but both sexes gradually develop resistance to experimental infection as they age.4,9,11,20,30,31 Strain-related differences in susceptibility to infection have been reported.9,17
The current study had 2 main objectives. First, we investigated protocols for sample collection and processing to determine the optimal method for detection of A. tetraptera by using a simple, quick fecal flotation exam. In light of previous experience, we hypothesized that more eggs would be isolated if the fecal pellets were allowed to soak prior to flotation and if a longer flotation period was used. We also postulated that increasing the fecal pellet sample size would correlate with increased eggs isolated through fecal flotation exam. In addition, we sought to determine the optimal time of day to collect feces for flotation testing, hypothesizing that more eggs would be shed in fecal pellets passed overnight compared with fecal pellets passed during the day. Second, we aimed to characterize the effect of age, sex, and housing status of mice on the isolation of A. tetraptera eggs to provide guidance on which cages are most likely to yield eggs in a suspected colony outbreak. We hypothesized that more eggs would be isolated from younger mice and that fecal samples from male mice would yield more eggs. We further hypothesized the singly housed mice would lose their A. tetraptera infections first, followed by breeding pairs and group-housed mice. Collectively, these studies yielded insights regarding an optimal fecal flotation protocol and ideal sample collection characteristics to maximize test sensitivity.
Materials and Methods
Study animals.
Study mice were mixed-background B6.129 mice donated from a research colony after detection of A. tetraptera. All mice used in this study were offspring of a single breeding pair and were naturally infected by means of their parents prior to weaning. Transmission was verified through fecal flotation exams or PCR analysis (Charles River Laboratories International, Wilmington, MA). Soiled-bedding sentinel testing indicated that mice were negative for mouse hepatitis virus, mouse minute virus, mouse parvovirus types 1 and 2, Theiler mouse encephalomyelitis virus, epizootic diarrhea of infant mice, Sendai virus, pneumonia virus of mice, reovirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse adenovirus types 1 and 2, mouse cytomegalovirus, Mycoplasma pulmonis, fur mites, and S. obvelata. Mice were maintained in a quarantine facility within IVC (Allentown Caging Equipment, Allentown, PA) containing autoclaved corncob bedding with a cotton enrichment square. Mice were provided unrestricted access to autoclaved rodent chow (2018SX, Harlan Teklad, Indianapolis, IN) and filtered reverse-osmosis–treated water through an automated in-cage watering system (Rees Scientific, Trenton, NJ). Cages were changed every 2 wk in a dedicated biosafety cabinet. The room was maintained on a 14:10-h light:dark cycle and at 22.8 ± 2 °C, with relative humidity of 30% to 50%. All experimental procedures were approved by the Johns Hopkins University Animal Care and Use Committee, the program is accredited by AAALAC, and procedures were consistent with the Guide for the Care and Use of Laboratory Animals.16
Fecal flotation.
All samples collected for studies focused on the refinement of sample collection and fecal flotation exam techniques were from cages known to be positive for A. tetraptera; therefore, negative test results from these cages were considered to be false negatives. Fecal pellets were collected from mouse cages and placed in conical tubes. An effort was made to collect fecal pellets representing the various sizes and desiccation levels of fecal pellets within each cage. The number of pellets collected and size of tubes varied according to the experimental conditions (Figure 1). An approximately half-tube volume of zinc sulfate solution (Centaur, Olathe, KS) was added, the tube was shaken, and the fecal pellets were allowed to soak as indicated by the experimental conditions. The sample was then mixed with a wooden stir stick and vortexed (VWR, Radnor, PA) for 10 s. Care was taken to ensure that the fecal sample was well incorporated into the flotation medium. Additional zinc sulfate solution was added to form a convex meniscus at the top of the tube. A coverslip (Fisher Scientific, Pittsburgh, PA) was placed over the tube, and samples were left to sit for the prescribed flotation period. Coverslips were then transferred to glass slides (Fisher Scientific), and eggs were counted by using a 4× or 10× microscope objective. Fecal flotation exams used randomized samples, and the person reading the slides (AEG) was blinded to the sample origin during completion of the exam.
Figure 1.
Summary of study parameters.
Fecal flotation protocol optimization.
A flotation period of 15 min (our existing protocol) was compared with one of 60 min. For each trial (n = 28), 2 sets of 10 fecal pellets were collected, the samples were each placed in 5-mL conical tubes, and eggs were presoaked for 30 min prior to mixing (test group 1, Figure 1).
To evaluate the need for a soaking period prior to flotation, 2 sets of 20 fecal pellets were collected for 10 trials, and samples were each placed in a 15-mL conical tube. One tube was allowed to sit for 30 min, whereas the other tube was immediately processed. Samples were then evaluated by using a 15 min flotation period, as indicated by the first study (test group 2, Figure 1).
Fecal sample size optimization.
To determine the optimal number of fecal pellets required to detect an infection with A. tetraptera, we collected samples of 2, 5, or 10 fecal pellets at a single time point from each cage of mice (n = 15). To provide sufficient zinc sulfate solution for the different numbers of fecal pellets, 2 pellets were placed in a 2 mL conical tube and 5 or 10 pellets were placed in a 5-mL conical tube. According to results from the first 2 studies, fecal samples were allowed to soak for 30 min prior to flotation for 15 min (test group 3, Figure 1).
Determination of ideal fecal collection time.
Ten cages of 6- to 8-wk-old, same-sex mice were housed in groups of 2 or 3 in cages without bedding. Each day for 5 d, the entire fecal output was collected from each cage at 3 time points: 0800 to 1200, 1200 to 1600, and 1600 to 0800. At each collection time point, cages were wiped clean of urine to ensure that all mice remained dry, and cages were changed as necessary. Fecal samples were placed in 50-mL conical tubes and allowed to soak for 30 min prior to a 15 min flotation period (test group 4, Figure 1).
Evaluation of age, housing status, and sex of mice on A. tetraptera burden.
Infected mice were weaned at 3 wk and either housed singly (5 male mice, 5 female mice), as breeding pairs (n = 5 pairs), or in same-sex groups of 2 or 3 mice (5 cages with male mice, 5 cages with female mice). At 8, 12, 16, 20, and 24 wk of age, mice were placed from 1600 to 0800 in cages without bedding, and the entire fecal output was collected. The fecal samples were placed in 50 mL conical tubes and processed as previously described. When the fecal flotation yielded no eggs, a second sample was collected in the same manner within 1 wk of the first sample. If the second sample yielded no eggs, 10 fecal pellets were submitted for PCR analysis (Charles River Laboratories).
Mice were euthanized by using carbon dioxide asphyxiation after the final (week 24) fecal sample collection. When cages had continued to yield positive fecal flotation exams through 20 wk, 10 fecal pellets were obtained from cages at necropsy and submitted for PCR analysis. The cecum and colon were isolated and placed in a culture dish. The intestines were incised longitudinally, and luminal contents were separated from the mucosal surface. Warm saline was added, and the sample was allowed to sit for a minimum of 10 min. Contents were then examined under a dissecting microscope for adult pinworms. Mice were considered negative for A. tetraptera when the results of the week-24 fecal flotation exam, PCR analysis, and direct exams were all negative.
Statistical analysis.
Data were analyzed by using a commercial statistical software package (PRISM version 6.0, GraphPad Software, San Diego, CA). Statistical tests included Mann–Whitney and Kruskal–Wallis tests for non-normally distributed data, with Dunn posthoc tests as appropriate, and 2-way ANOVA with Tukey posthoc tests. P values less than 0.05 were considered significant.
Results
Optimization of fecal flotation protocol.
Preliminary observations had suggested that increasing the flotation period (that is, the period cover-slipped tubes are allowed to rest before microscopy) results in more effective recovery of eggs. We compared a flotation period of 15 min, our existing protocol (test group 1A; Figure 1), with one of 60 min (test group 1B). The number of eggs isolated did not differ (Mann–Whitney P = 0.46) between 15 and 60 min of flotation. Three false-negative results were obtained during the 60-min fecal flotation exam, but only one false negative occurred with the 15 min exam, yielding sensitivities of 89.3% and 96.4%, respectively (Table 1). Therefore, to maximize test efficiency, a 15 min flotation period was used for all subsequent studies.
Table 1.
Incidence of false-negative results for A. tetraptera eggs
| Study | Test group | No. of false-negative tests/total tests | Sensitivity |
| Duration of fecal flotation | 1A | 1/28 | 96.4% |
| 1B | 3/28 | 89.3% | |
| Soaking period | 2A | 0/10 | 100.0% |
| 2B | 0/10 | 100.0% | |
| Fecal sample size | 3A | 6/15 | 60.0% |
| 3B | 1/15 | 93.3% | |
| 3C | 2/15 | 86.7% | |
| Fecal collection time | 4A | 15/50 | 70.0% |
| 4B | 18/50 | 64.0% | |
| 4C | 0/50 | 100.0% |
See Figure 1 for definition of test groups.
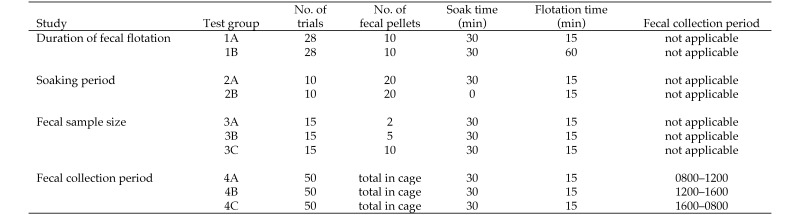
A. tetraptera eggs are excreted in a mucous layer that covers the fecal pellets.14 Our current protocol requires soaking the fecal pellets in zinc sulfate solution to soften the pellets and facilitate mechanical breakdown. To determine whether this step was necessary, we compared the number of eggs isolated both with (test group 2A, Figure 1) and without (test group 2B) soaking in zinc sulfate solution for 30 min prior to mechanical breakdown. More eggs were isolated from soaked samples (median, 34.5 eggs) compared with nonsoaked samples (median, 9.5 eggs; Mann–Whitney P = 0.0084; Figure 2).
Figure 2.
Prior to flotation exam, fecal pellets were either soaked for 30 min (soaked) or processed immediately (nonsoaked), and the eggs isolated were counted (n = 10 trials per group; solid horizontal lines indicate the median values for each group of data points; Mann–Whitney, P = 0.0084).
Optimization of fecal sample size.
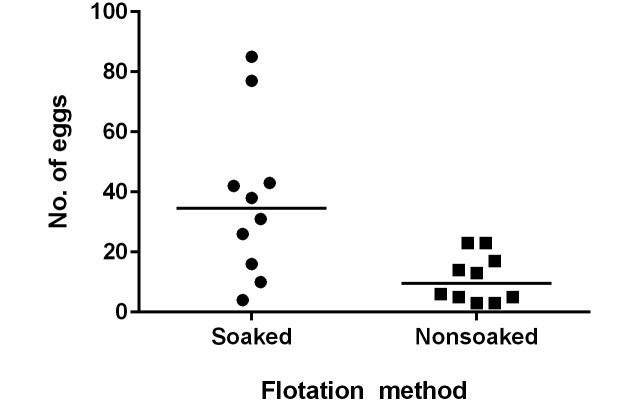
To determine the optimal number of fecal pellets required to detect an infection with A. tetraptera, the number of eggs isolated from 2 (test group 3A), 5 (test group 3B), or 10 (test group 3C) fecal pellets was compared. No correlation was found between the total number of eggs isolated via fecal flotation exam and number of fecal pellets analyzed (Kruskal–Wallis P = 0.28; Figure 3). Furthermore, there were false negative tests in all group sizes, indicating sample collection of up to 10 fecal pellets can still yield false-negative results (Table 1). However, when considering cumulative results from all 3 samples taken simultaneously from each cage (17 pellets total) all cages revealed at least one positive result.
Figure 3.
The eggs isolated during fecal flotation from sample sizes of 2, 5, or 10 fecal pellets were counted. Larger sample size did not result in the isolation of more eggs (n = 15 trials per group; solid horizontal lines indicate median values for each group of data points; Kruskal–Wallis, P = 0.28).
Determination of ideal period for fecal collection.
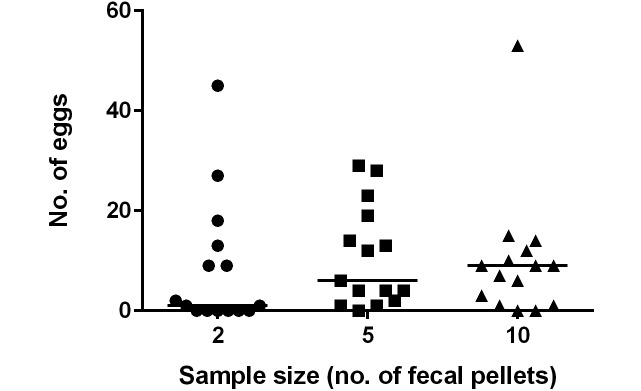
Previous reports indicate that more pinworm eggs are shed in the feces overnight.25 Therefore, we examined the time period during which feces yielded the most eggs: 0800 to 1200 (test group 4A, Figure 1), 1200 to 1600 (test group 4B), or 1600 to 0800 (test group 4C). The overnight time period (that is, 1600 to 0800) was the most effective period for diagnosis, with the most eggs isolated and no negative results obtained (Kruskal–Wallis P < 0.0001; Figure 4). In contrast, 15 samples (30%) collected between 0800 and 1200 and 18 samples (36%) collected between 1200 and 1600 did not yield eggs (Table 1). Combining the results from the 0800–1200 and 1200–1600 periods for the same day and cage reduced the number of samples that did not yield eggs to 7 (14%). Although the overnight collection period resulted in more eggs observed, the collection period was 16 h as compared with 4 h. When the nighttime results were divided by 4 to approximate the daytime collection periods, there was no statistical difference in the number of eggs isolated from fecal samples during 4 h periods in the morning, afternoon, or overnight (Kruskal–Wallis P = 0.069; data not shown).
Figure 4.
The number of eggs isolated by fecal flotation was evaluated in fecal pellets collected from 1600 to 0800, 0800 to 1200, or 1200 to 1600 (n = 50 cages per group; solid horizontal lines indicate median values for each group of data points; Kruskal–Wallis, P < 0.0001).
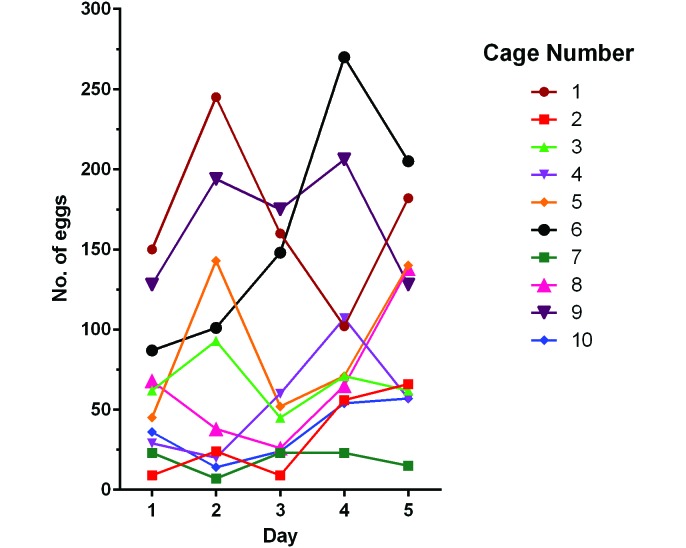
In addition, egg yield did not differ between male and female mice (Mann–Whitney P = 0.90; data not shown). The number of eggs isolated daily from group-housed mice varied markedly over the 5-d test period, but no cyclic pattern of egg excretion was observed (Figure 5). In light of this finding, samples during the subsequent age study were collected only once every 4 wk from 1600 to 0800.
Figure 5.
Variability in egg excretion was examined over the course of 5 d by using individual cages of group-housed mice (cage nos. 1 through 5 contained male mice, and cage nos. 6 through 10 contained female mice). Each line represents a different cage.
Effects of age, housing status, and sex on A. tetraptera burden.
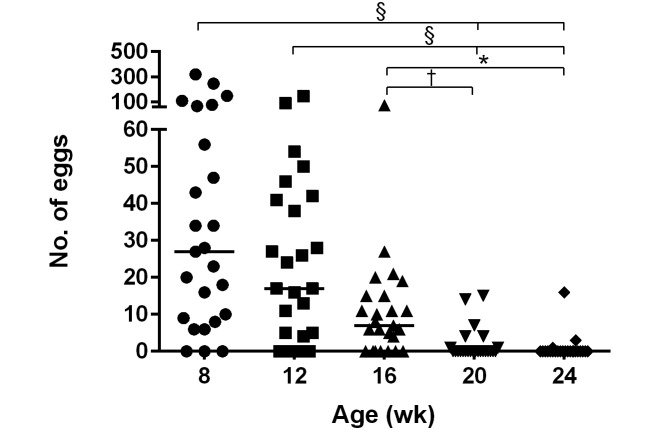
We evaluated singly housed, same-sex group-housed, and breeding pairs of mice in regard to the number of eggs isolated at 8, 12, 16, 20, and 24 wk of age. Over time, the number of eggs isolated from all cages of mice decreased, regardless of housing situation, with the highest yields from 8- and 12-wk-old mice (Kruskal–Wallis P < 0.0001; Dunn multiple-comparison posthoc: week 8 compared with 20, 8 compared with 24, 12 compared with 20, and 12 compared with 24, P < 0.0001; week 16 compared with 20, P < 0.05; and week 16 compared with 24, P < 0.01; Figure 6).
Figure 6.
Eggs isolated by fecal flotation of samples collected from 8-, 12-, 16-, 20-, and 24-wk-old mice were counted. More eggs were isolated from 8- and 12-wk-old mice compared with older mice (n = 25 cages at each time point; solid horizontal lines indicate median values for each group of data points; Kruskal–Wallis with Dunn multiple comparison posthoc; *, P < 0.05; †, P < 0.01; §, P < 0.0001).
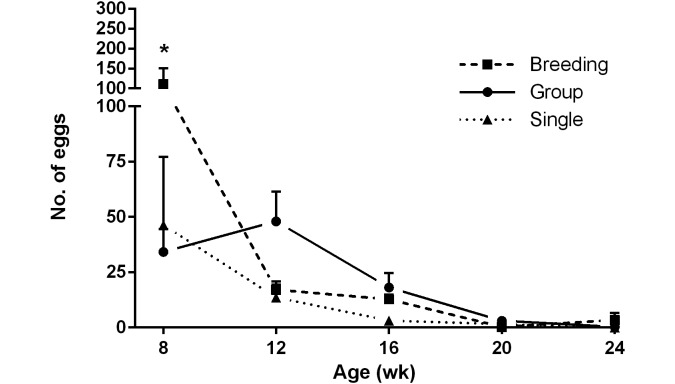
At only one time point, when mice were 8 wk of age, was there a significant difference between housing groups, with more eggs isolated from breeding mice compared with singly or group-housed mice (2-way ANOVA P < 0.05; Tukey multiple-comparison posthoc: group-housed compared with breeding, P = 0.0007; singly housed compared with breeding, P = 0.0053; Figure 7). There was no significant effect of sex, regardless of age, on the number of eggs isolated (data not shown).
Figure 7.
Eggs isolated by fecal flotation of samples collected from 8-, 12-, 16-, 20-, and 24-wk-old mice housed as breeders (Breeding), in groups (Group), and singly (Single) were evaluated. Over time, fewer eggs were isolated from all mice, regardless of housing status, as they cleared their infections (n = 5 to 10 cages per time point per housing group; data presented as mean ± SEM; *, P < 0.05, Tukey multiple-comparison posthoc).
Prior to necropsy at 24 wk of age, 18 cages of mice had 2 consecutive negative fecal flotation exams and were PCR negative. However, prior to that point, there were some discrepancies. Two cages were negative on the initial fecal flotation exam, but were positive on the second. These samples were from a cage of group-housed female mice and a singly housed male mouse at 16 wk of age. At the next time point (4 wk later), both cages were negative by both fecal flotation and PCR analysis. In addition, 3 cages that had 2 successive negative fecal flotation exams were found to be PCR positive. At the next time point (4 wk later), all 3 cages were found to be negative by both fecal flotation and PCR analysis.
When they were 24 wk old, we necropsied the 48 mice that had been used to evaluate the effects of age, housing status, and sex on A. tetraptera egg isolation. We evaluated infection status through fecal flotation, direct intestinal exam, and PCR analysis of fecal samples. Only 1 of the 25 cages of mice was positive by all 3 tests (Table 2), whereas 18 cages were negative on all 3 tests and the mice were presumed to have cleared the infection. The remaining 6 cages, including 2 cages that had been negative at 20 wk by both fecal flotation exam and PCR analysis, contained mice with conflicting test results (Table 2).
Table 2.
Results of pinworm tests that had at least one positive exam for A. tetrapteraat 24 wk
| Cage | Fecal flotation exam | Direct intestinal exam | Fecal PCR analysis |
| 1 | + | — | — |
| 2 | + | + | — |
| 3 | — | + | + |
| 4 | + | — | + |
| 5 | — | + | + |
| 6 | — | — | + |
| 7 | + | + | + |
Only one cage (no. 7) yielded positive results for all 3 exams.
Discussion
The first objective of this study was to determine the optimal protocols for sample collection and the passive fecal flotation exam to detect A. tetraptera eggs. Refinement of the passive fecal flotation exam is critical, given that this test is prone to false-negative results. Passive fecal flotation uses a hypertonic solution with a specific gravity greater than that of the parasite eggs, such that the less-dense eggs float to the surface.2 We used a zinc sulfate solution that has a specific gravity of 1.18 and is the recommended flotation solution for the isolation of helminth eggs by means of passive fecal flotation.2 Previous observations in our lab suggested that using longer flotation periods would yield more eggs. However, others have stated that exceeding the recommended 15-min flotation might lead to egg distortion due to the hypertonicity of the zinc sulfate solution.2 The current study found no statistical difference in the number of eggs isolated after flotation of 15 or 60 min. Furthermore, we noted no malformed or distorted eggs due to osmotic damage throughout this study, suggesting that neither increased flotation times nor a preflotation soak in zinc sulfate solution impaired egg structure. Mouse fecal pellets can be dry and very compact, and we routinely soak the pellets in the zinc sulfate solution prior to the 15-min flotation to facilitate mechanical breakdown and release of eggs into the flotation solution. This study confirmed that soaking samples prior to mechanical mixing yielded more eggs.
One limitation of our study was that we did not evaluate centrifugal flotation, which is thought to be more sensitive than passive flotation, given that centrifugal flotation uses gravitational forces to accelerate the movement of eggs to the top of the solution.2 However, centrifugal flotation requires additional time and equipment, and we wanted to focus on a simple test that could be used easily at any institution. Our general findings regarding the effects of sample size, fecal collection time period, sex, age, and housing status likely are applicable to all methods of egg isolation.
Historically, our protocol for sample collection has called for using 1 or 2 fecal pellets per mouse or cage. However, because eggs might be excreted intermittently, we suspected that collecting more fecal pellets per cage might result in more eggs isolated and fewer false-negative exams. We found that larger sample sizes did not necessarily result in greater numbers of eggs isolated (Figure 3), and all sample sizes (2, 5, and 10 fecal pellets) generated false-negative results. These findings suggest that the number of eggs excreted varies greatly between fecal pellets. However, pooling the results from all 17 of the pellets collected at the same time from each cage resulted in no false-negative results. Given these findings, we suggest collecting at least 20 fecal pellets per cage when investigating a suspected infection.
We also wanted to determine the best time at which to collect fecal samples when evaluating a colony for a suspected pinworm infection. Egg release has been reported to be greatest right before dawn and in the afternoon.5,25 However, these previous studies evaluated only small cohorts of individual mice. We chose to conduct a similar study, with modification of the sampling time and a larger cohort of group-housed mice to better represent conditions during an outbreak. We chose longer collection periods, during morning, afternoon, and overnight, that might easily be adapted to a caretaker's schedule. Typically, animal care staff do not collect samples between 1600 and 0800; therefore, we collected all of the pellets excreted during that time period as a single sample to reflect normal practices. We found there was no statistical difference in the number of eggs collected between the 3 time periods once the longer overnight collection period was accounted for. However, because we did not subdivide the overnight sample into shorter periods, we cannot rule out the possibility that the majority of nighttime eggs were excreted just before dawn, as observed previously.25 Differences between previous and our current studies may have been due to housing status, because earlier studies recorded the fecal output of a singly housed mouse. Variability between group-housed mice might have masked individual fluctuations in egg excretion. Another factor may have been mode of transmission; our model used natural transmission of pinworms, whereas previous studies used oral gavage of eggs. Even if the mice had been inoculated with a specific number of embryonated eggs, the number of resulting adult worms likely would vary between mice. Given that collecting the entire fecal output from a cage of mice during a single daytime 4-h period carries a high probability of false-negative results, we suggest that, when feasible, collecting the entire overnight fecal output maximizes test sensitivity.
Cyclical shedding of eggs has been reported to occur in S. muris21 and S. obvelata,7 but this pattern was not observed in our study. However, in contrast to Syphacia species, A. tetraptera adult worms have a long life cycle, and multiple generations might be present within a single mouse.28 Given that adult female worms have been found to shed 17 eggs each day and usually shed eggs many times during their reproductive lifespan, it is logical that there would not be a distinct cyclical shedding pattern.25
To our knowledge, the current study is the first to characterize the effects of age, sex, and housing status in a mouse colony modeling natural infection with A. tetraptera. We found that the number of eggs isolated from mice peaked at 8 and 12 wk of age and then subsequently declined. These findings are consistent with previous observations that infecting older mice leads to reduced A. tetraptera burdens.20,31 Interestingly, 3 of the singly housed mice in our study were negative according to fecal flotation exams and PCR analysis at the 8-wk time point despite being born to infected parents. Previous studies have established that immune expulsion is a characteristic of infections with A. tetraptera,3,14,22 and 76% of our immunocompetent mice had cleared their infections by 6 mo in the current study. Fecal flotation exam findings typically correlated with results of PCR analyses. However, 3 cages of mice were found to be PCR positive after negative fecal flotation exams, but were negative on both exams 4 wk later. These findings suggest low pinworm burdens may be difficult to detect by using fecal flotation exams. Housing status did not prove to be an important factor regarding the number of eggs isolated from mice over time. Only at the 8-wk time point were significantly more eggs collected from the breeding mice compared with singly or group-housed mice. We suspect that this finding reflects an altered immune status during pregnancy, but it might also be due to natural variation in infection. In contrast to a previous study which found that male mice harbored greater A. tetraptera burdens than did female mice,31 we found no difference in egg counts between sexes, similar to findings reported for S. obvelata.31,32 Because we investigated only a single mouse strain, we cannot rule out the possibility that susceptibility and response to A. tetraptera varies between strains.
One aim of this study was to provide guidance regarding the selection of cages most likely to be infected with A. tetraptera during a suspected outbreak. Our results suggest that cages containing 8- to 12-wk-old mice should be prioritized, and neither housing status nor sex are significant factors to consider. We observed variability in egg shedding in a single cage; cages found to have no eggs during one fecal flotation exam were positive during a subsequent exam within the same week. Therefore, it is prudent to collect samples at several times during a week to increase the sensitivity of the fecal flotation exam.
We planned to compare PCR, direct examination of intestinal contents for adult worms, and fecal flotation exam results at the final 24-wk time point. However, 18 of the 25 cages in the study were negative by all 3 exams, indicating that the mice had cleared the infection by 24 wk. Therefore, future studies should use younger mice to ensure a sufficient sample size of A. tetraptera-infected mice. Despite this situation, we did have several interesting observations. Direct examination of cecal and colonic contents is considered to be the ‘gold standard’ for the diagnosis of A. tetraptera.14,18 However, 2 samples were negative for adult worms by direct exam, despite positive PCR analysis or fecal flotation exams (Table 2). In addition, we found one mouse that contained an adult worm but was PCR negative, as has been noted in a previous study.10 Interestingly, we also found that mice group-housed in the same cage varied in infection status, as determined by the presence of adult worms. This finding supports a previous study that suggested testing one mouse per cage is insufficient when screening for A. tetraptera infection.12
In conclusion, our study suggests improvements to the protocol for collecting and processing fecal samples for the isolation of A. tetraptera by using passive fecal flotation exam. Soaking the fecal pellets for 30 min prior to a 15-min flotation period increases the number of eggs isolated and yields maximal test sensitivity. A larger sample size per cage of at least 20 pellets likely will reduce false-negative results, as is an overnight collection of the entire fecal output. Time of day is not a factor when collecting fecal samples for isolation of A. tetraptera, and sex and housing status do not significantly influence the isolation of A. tetraptera eggs. When selecting cages of mice to test during a suspected outbreak of A. tetraptera, priority should be given to younger mice, given that older mice may have cleared the pinworms or have very low worm burdens. In older mice with low worm burdens, additional tests to passive fecal flotation, such as PCR analysis and direct exam of cecal and colonic contents, may be required to assure negative status.
Acknowledgments
This study was supported by Research Animal Resources at the Johns Hopkins University School of Medicine. We thank Theresa Meade for study design consultation and both Mary Archer and Mary Gayles for husbandry support.
References
- 1.Anya AO. 1966. Experimental studies on the physiology of hatching of Aspiculuris tetraptera Schulz (Oxyuridea:Nematoda). Parasitology 56:733–744. [DOI] [PubMed] [Google Scholar]
- 2.Baker DG. 2007. Flynn's parasites of laboratory animals, 2nd ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 3.Behnke JM. 1974. Proceedings: the effect of hydrocortisone upon infection with Aspiculuris tetraptera in laboratory mice. Parasitology 69:xviii. [PubMed] [Google Scholar]
- 4.Behnke JM. 1975. Immune expulsion of the nematode Aspiculuris tetraptera from mice given primary and challenge infections. Int J Parasitol 5:511–515. [DOI] [PubMed] [Google Scholar]
- 5.Bunte R, Nolan R. 2006. Searching for Aspiculuris tetraptera: lessons learned. J Am Assoc Lab Anim Sci 45:86. [Google Scholar]
- 6.Cai Y, Zhou J, Webb DC. 2009. Treatment of mice with fenbendazole attenuates allergic airways inflammation and Th2 cytokine production in a model of asthma. Immunol Cell Biol 87:623–629. [DOI] [PubMed] [Google Scholar]
- 7.Clifford CB, Watson J. 2008. Old enemies, still with us after all these years. ILAR J 49:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis JA, Paylor R, McDonald MP, Libbey M, Ligler A, Bryant K, Crawley JN. 1999. Behavioral effects of ivermectin in mice. Lab Anim Sci 49:288–296. [PubMed] [Google Scholar]
- 9.Derothe JM, Loubes C, Orth A, Renaud F, Moulia C. 1997. Comparison between patterns of pinworm infection (Aspiculuris tetraptera) in wild and laboratory strains of mice, Mus musculus. Int J Parasitol 27:645–651. [DOI] [PubMed] [Google Scholar]
- 10.Dole VS, Zaias J, Kyricopoulos-Cleasby DM, Banu LA, Waterman LL, Sanders K, Henderson KS. 2011. Comparison of traditional and PCR methods during screening for and confirmation of Aspiculuris tetraptera in a mouse facility. J Am Assoc Lab Anim Sci 50:904–909. [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton GJ. 1972. Intestinal helminths in inbred strains of mice. Lab Anim Sci 22:850–853. [PubMed] [Google Scholar]
- 12.Eguiluz C, Viguera E, Perez J. 2001. Modification of the anal tape method for detection of pinworms in rodents. Lab Anim (NY) 30:54–55. [DOI] [PubMed] [Google Scholar]
- 13.Feldman SH, Bowman SG. 2007. Molecular phylogeny of the pinworms of mice, rats, and rabbits and its use to develop molecular beacon assays for the detection of pinworms in mice. Lab Anim (NY) 36:43–50. [DOI] [PubMed] [Google Scholar]
- 14.Fox JG, Anderson LC, Otto G, Pritchett-Corning KR, Whary MT. 2015. Laboratory animal medicine, 3rd ed. San Diego (CA): Elsevier. [Google Scholar]
- 15.Hoag WG. 1961. Oxyuriasis in laboratory mouse colonies. Am J Vet Res 22:150–153. [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 17.King VM, Cosgrove GE. 1963. Intestinal helminths in various strains of laboratory mice. Lab Anim Care 13:46–48. [PubMed] [Google Scholar]
- 18.Klement P, Augustine JM, Delaney KH, Klement G, Weitz JI. 1996. An oral ivermectin regimen that eradicates pinworms (Syphacia spp.) in laboratory rats and mice. Lab Anim Sci 46:286–290. [PubMed] [Google Scholar]
- 19.Landin AM, Frasca D, Zaias J, Van der Put E, Riley RL, Altman NH, Blomberg BB. 2009. Effects of fenbendazole on the murine humoral immune system. J Am Assoc Lab Anim Sci 48:251–257. [PMC free article] [PubMed] [Google Scholar]
- 20.Mathies AW., Jr 1959. Certain aspects of the host–parasite relationship of Aspiculuris tetraptera, a mouse pinworm. I. Host specificity and age resistance. Exp Parasitol 8:31–38. [DOI] [PubMed] [Google Scholar]
- 21.Meade TM, Watson J. 2014. Characterization of rat pinworm (Syphacia muris) epidemiology as a means to increase detection and elimination. J Am Assoc Lab Anim Sci 53:661–667. [PMC free article] [PubMed] [Google Scholar]
- 22.Michels C, Goyal P, Nieuwenhuizen N, Brombacher F. 2006. Infection with Syphacia obvelata (pinworm) induces protective Th2 immune responses and influences ovalbumin-induced allergic reactions. Infect Immun 74:5926–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parel JD, Galula JU, Ooi HK. 2008. Characterization of rDNA sequences from Syphacia obvelata, Syphacia muris, and Aspiculuris tetraptera and development of a PCR-based method for identification. Vet Parasitol 153:379–383. [DOI] [PubMed] [Google Scholar]
- 24.Percy DH, Barthold SW. 2007. Pathology of laboratory rodents and rabbits, 3rd ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 25.Phillipson RF. 1974. Intermittent egg release by Aspiculuris tetraptera in mice. Parasitology 69:207–213. [DOI] [PubMed] [Google Scholar]
- 26.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173. [DOI] [PubMed] [Google Scholar]
- 27.Ramp AA, Hall C, Orian JM. 2010. Strain-related effects of fenbendazole treatment on murine experimental autoimmune encephalomyelitis. Lab Anim 44:271–273. [DOI] [PubMed] [Google Scholar]
- 28.Scott ME, Gibbs HC. 1986. Long-term population dynamics of pinworms (Syphacia obvelata and Aspiculuris tetraptera) in mice. J Parasitol 72:652–662. [PubMed] [Google Scholar]
- 29.Skopets B, Wilson RP, Griffith JW, Lang CM. 1996. Ivermectin toxicity in young mice. Lab Anim Sci 46:111–112. [PubMed] [Google Scholar]
- 30.Stahl W. 1966. Experimental aspiculuriasis. II. Effects of concurrent helminth infection. Exp Parasitol 18:116–123. [DOI] [PubMed] [Google Scholar]
- 31.Stahl W. 1966. Experimental aspiculuriasis. I. Resistance to superinfection. Exp Parasitol 18:109–115. [DOI] [PubMed] [Google Scholar]
- 32.Stewart PW, Chapes SK. 2003. Role of major histocompatibility complex class II in resistance of mice to naturally acquired infection with Syphacia obvelata. Comp Med 53:70–74. [PubMed] [Google Scholar]
- 33.Taffs LF. 1976. Pinworm infections in laboratory rodents: a review. Lab Anim 10:1–13. [DOI] [PubMed] [Google Scholar]
- 34.Toth LA, Oberbeck C, Straign CM, Frazier S, Rehg JE. 2000. Toxicity evaluation of prophylactic treatments for mites and pinworms in mice. Contemp Top Lab Anim Sci 39:18 –21. [PubMed]