Abstract
Nonterminal blood sample collection of sufficient volume and quality for research is complicated in mice due to their small size and anatomy. Large (>100 μL) nonterminal volumes of unhemolyzed or unclotted blood currently are typically collected from the retroorbital sinus or submandibular plexus. We developed a third method—submental blood collection—which is similar in execution to the submandibular method but with minor changes in animal restraint and collection location. Compared with other techniques, submental collection is easier to perform due to the direct visibility of the target vessels, which are located in a sparsely furred region. Compared with the submandibular method, the submental method did not differ regarding weight change and clotting score but significantly decreased hemolysis and increased the overall number of high-quality samples. The submental method was performed with smaller lancets for the majority of the bleeds, yet resulted in fewer repeat collection attempts, fewer insufficient samples, and less extraneous blood loss and was qualitatively less traumatic. Compared with the retroorbital technique, the submental method was similar regarding weight change but decreased hemolysis, clotting, and the number of overall high-quality samples; however the retroorbital method resulted in significantly fewer incidents of insufficient sample collection. Extraneous blood loss was roughly equivalent between the submental and retroorbital methods. We conclude that the submental method is an acceptable venipuncture technique for obtaining large, nonterminal volumes of blood from mice.
Blood collection is one of the most common and important techniques of in-vivo research. However, blood collection in mice, the most common research animal, is complicated by their small size and anatomy. The challenge is obtaining high-quality blood samples without adversely affecting the animal's physiology and wellbeing. The largest volume that can be collected humanely is nonetheless a very small volume from the perspective of the bioanalytical scientist. Hemolysis and clotting unintentionally and negatively affect sample quality and data.16,28 When large volumes (100 μL per sample) of unhemolyzed or unclotted blood are needed at a nonterminal time point, retroorbital and submandibular bleeding are the 2 most commonly referenced techniques.
Retroorbital blood collection involves inserting a small-diameter glass tube (typically a narrow-mouthed Pasteur pipette or a microcapillary tube) into the venous sinus behind the eyeball and exerting pressure to penetrate the sinus. The literature sometimes refers to the retroorbital venous sinus as a retrobulbar venous plexus or periorbital sinus, but the correct term is a venous sinus rather than a venous plexus, as is often incorrectly cited in the literature.27 The use of the retroorbital method is not universally acceptable.2,5,12,13,17,21,22,29-31 In general, when performed incorrectly, the retroorbital method has potential to have more animal welfare complications than other mouse phlebotomy methods. Trauma from the method is thought to be somewhat higher than the 1% to 2% reported with other methods5,30 but may go undetected due to the location deep within the orbit.2,5,17,29 Many institutions require general anesthesia followed by topical anesthetics when retroorbital bleeds are performed.2,5,13,17,29 The introduction of anesthetics may not be compatible with every research study and may pose a risk to some animal models.4,9 Retroorbital bleeding without the use of anesthesia is often discouraged—but not strictly banned—in the United States, although some institutions allow this technique to be performed without pain relief when sufficient and scientific justification is provided. Therefore, finding an alternative to retroorbital bleeding is desirable.
Blood collection from the submandibular region can also yield large-volume, high-quality blood samples.10,26 This method is commonly referenced as superficial temporal vein or facial vein blood collection in regard to the specific vessel being targeted or as submandibular blood collection in regard to the anatomic region from which it is collected. The method is sometimes erroneously referred to as submandibular vein collection, but this is likely the result of incorrect nomenclature being attributed to one or more of the target vessels.11 The superficial temporal, maxillary, linguofacial, and facial veins are all potential targets, given the variations among practitioners in restraint and lancet penetration techniques. Therefore, we refer to the method as ‘submandibular blood collection’ throughout this article. Although the submandibular method has been proposed as a humane alternative to the retroorbital method,10 scientific proof is scarce. To date, we have identified 3 studies directly comparing animal welfare impacts. Two found the submandibular method to be more stressful,23,26 and one found that it resulted in excessive hemorrhaging.14 In general, we found the submandibular approach difficult to train and consistently collect. This technique involves blind insertion of the lancet into an area covered with fur, and the target vessel may shift due to animal movement, differences in restraint techniques, or due to individual animal anatomy. Difficulties experienced during a study can result in multiple collection attempts or blood samples of insufficient volume or poor quality. In addition, we observed that the submandibular method occasionally results in animal lethargy or hemorrhage prior to or after sample collection. Similar observations have been reported elsewhere.8,14,25
We identified a third approach—submental collection—that similarly enables the collection of larger nonterminal blood volumes in mice. The submental technique is similar to the submandibular method but with slight modifications in sampling location and animal restraint. By accessing the mouse vasculature under the chin—that is, the submental region—we can target blood vessels that are visible under the skin in a sparsely furred area. There are few muscles and no major glands in this region. We term this method ‘submental blood collection’ in regard to the anatomic region in which it is performed rather than a specific submental vein, given that multiple veins are likely to be involved when performing venipuncture in this area. We found that the submental approach is easy to learn and frequently can be performed by using a smaller phlebotomy lancet than might be indicated19 for submandibular collections from similarly sized mice. Submental venipuncture does not appear to result in arterial hemorrhage or the lethargy seen with the submandibular method. The maximal blood volume obtainable with the submental method appears to be somewhat less than that from either a retroorbital or a submandibular blood collection, but it is nonetheless generally easy to obtain approximately 10% of a mouse's circulating blood volume. The current study was conducted because, prior to widely recommending this new approach at our facility, we had to demonstrate that the submental technique was neutral to or better than the retroorbital and submandibular methods, from the viewpoints of animal welfare and research. Terminal blood collection methods (for example, cardiocentesis) and those used for smaller blood volumes (for example, saphenous vein and tail vessel) were not considered as part of this study.
Materials and Methods
The present study examined the submental blood collection method by comparing it with the retroorbital and submandibular methods. Phlebotomists collected approximately 150 μL of blood through serial sampling conducted at 2-wk intervals, for a total of 3 collections per animal. Large mice were used to better tolerate repeated high-volume blood draws. Because we sought to evaluate the methods as commonly performed in the research setting, retroorbital blood collections were performed under general anesthesia, whereas submental and submandibular collections were performed without anesthesia. In addition, we recruited our most skilled phlebotomist for each method, on the basis of personal preference and evaluation of proficiency. All phlebotomists received training on sample handling as part of their jobs. Prior to the start of the study, the phlebotomists collaborated to ensure that their sample-handling techniques were consistent with each other's.
Animals.
Female CD1 mice (age, 12 to 13 wk) were obtained from a commercial vendor (Harlan, Indianapolis, IN) and were housed until each weighed more than 30 g (age, 23 wk). Large mice were used to better tolerate the effects of repeated large-volume blood draws. Mice were group-housed at a density of 5 per cage on arrival. Cage mates were assigned to study groups without randomization (n = 15 per group, submental, submandibular, and retroorbital). Animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals15 at an AAALAC-accredited facility in sterile ventilated microisolation housing on corncob bedding and with enrichment items. All research protocols were approved by the IACUC. Animals had unlimited access to pelleted feed (Harlan 2920X, Madison, WI) and reverse-osmosis–purified water through an automatic watering system. Mice were maintained on a 12:12-h light:dark cycle in rooms kept at approximately 72 ± 2 °F (22.2 ± 1.1 °C) and 30% to 70% humidity in an SPF facility as determined by quarterly sentinel surveillance. Sentinel mice were negative for respiratory and enteric bacterial pathogens, Helicobacter spp., Mycoplasma pulmonis, Sendai virus, murine norovirus, mouse hepatitis virus, pneumonia virus of mice, minute virus of mice, mouse parvovirus types 1 through 4, reovirus 1 and 3, mouse rotavirus, ectromelia virus, lymphocytic choriomeningitis virus, polyoma virus, K virus, mouse adenovirus types 1 and 2, mouse cytomegalovirus, lactate dehydrogenase-elevating virus, mouse thymic virus, Hantaan virus, Seoul virus, Theiler murine encephalomyelitis virus, endoparasites, and ectoparasites.
Supplies.
Equipment included 4- and 5-mm Goldenrod animal lancets (Medipoint, Mineola, NY), uncoated 50-μL Microcaps microcapillary tubes (Drummond, Broomall, PA), K2EDTA-coated Microtainer collection vials (BD, Franklin Lakes, NJ), facility-sterilized 4×4-in. Curity all-purpose medical sponges (Covidien, Mansfield, MA), and Isoflo isoflurane (Abbot Laboratories, Abbot Park, IL).
Study overview.
Phlebotomists attempted to collect approximately 150 μL of blood from each mouse, for a total of 3 collections and with a 2-wk recovery period between collections. For the submental and submandibular groups, phlebotomists were allowed to perform no more than 2 attempts per collection, which were restricted to the right side (collection 1), the left side (collection 2), and the right side (collection 3). For the retroorbital group, the phlebotomist was allowed to make only one entry into the orbit of the right eye (collection 1), left eye (collection 2), and right eye (collection 3). Collections from the 3 groups occurred on the same day at approximately the same time of day (± 1 h). Submental and submandibular collections were performed in the animal husbandry room under a laminar downdraft hood. Retroorbital collections were performed in a procedure room directly across the hall from the animal husbandry room, in a biosafety cabinet, which was necessary because of the isoflurane anesthesia.
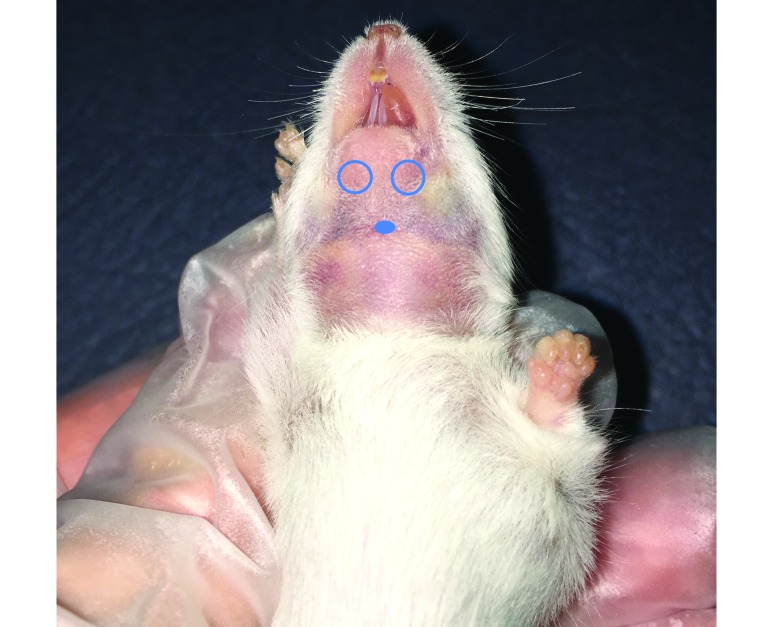
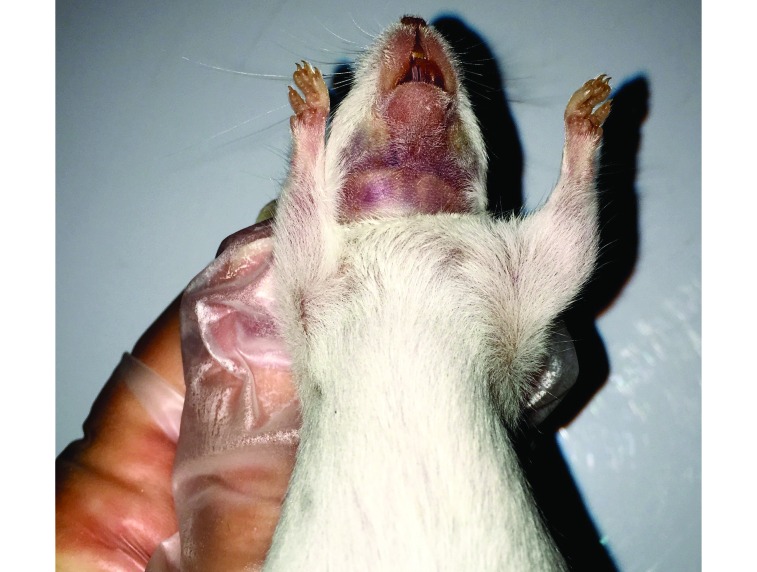
The submental venipuncture method was performed by first restraining the animal by the scruff near the ears so that its head was immobilized and tilted back to expose the submental region, using a grip sufficient to draw back any lose skin or fatty tissue from the access site yet not so tight as to restrict breathing or blood flow to the region. In most mice, a dark area was visible where the facial and submental veins converge (Figure 1) and could be directly targeted. When not readily visible, this area was located by moving slightly rostrolateral from a group of hairs located on the midline of the throat and finding a slightly softer spot in the tissue just medial to the facial vein. As an alternative target, a visible portion of the facial vein could have been targeted directly (Figure 2); in our experience, this yields a slightly smaller sample volume than does targeting the area of vascular convergence and therfore was not practiced in this study. A 4- or 5-mm mouse phlebotomy lancet (as chosen by the phlebotomist) was used to pierce the animal. The lancet was inserted and withdrawn in a smooth, firm fashion. Blood was allowed to drip freely into the collection vial without manipulation of the puncture site. After collection, each mouse was placed in a gauze-lined recovery cage until all bleeding had stopped. Wound compression was not used on any animal in this group because bleeding from the submental region stops rapidly once the animal is released from restraint and resumes a normal head position. However, if any animal had bled excessively (more than 1 drop of blood in the recovery cage), wound compression would have been applied.
Figure 1.
The darker areas indicate vessels under the skin, and the black dot indicates the location of the hair or fur whorl. Circled areas indicate the approximate sites where the facial and submental veins converge. Neck shaved for photography.
Figure 2.
The anterior facial veins are visible. Neck shaved for photography.
The submandibular venipuncture method10 was performed by first restraining the animal by the scruff near the shoulder blades so that its head was immobilized and the loose skin around the face was retracted sufficiently but not excessively. A 4- or 5-mm mouse phlebotomy lancet (as chosen by the phlebotomist) was used to pierce the submandibular region of the mouse along the posterior edge of the mandible. We believe that the superficial temporal and maxillary veins were the most commonly achieved targets for this venipuncture, but we acknowledge that some of the mice might have been bled from the linguofacial or facial veins. The lancet was inserted and withdrawn from the animal in a smooth, firm fashion. Blood was allowed to drip freely into the collection vial without manipulation of the puncture site. Gauze compression was applied after collection, when needed, to help stop blood loss. Each mouse was then placed in a gauze-lined recovery cage until all bleeding had stopped, with additional compression when needed.
The retroorbital venous sinus blood collection method2,13 was performed by first anesthetizing each mouse with 5% isoflurane delivered in an induction chamber at a rate of 2 L/min, with a duration of exposure sufficient to ensure that the animal would remain unconscious throughout the procedure. The anesthetized mouse was then held by the scruff so that its head was pointing down, with the skin was pulled away from the eyeball and the eyeball gently protruding from the socket. A microcapillary tube was inserted into the ventrocaudal corner of the eye at an approximate 45° angle and was gently rotated and pressed forward until blood was visualized flowing through the microcapillary tube. At this point, the mouse and tube were inverted so that blood could drip freely from the end of the microcapillary tube into the collection vial. When approximately 100 μL of blood had collected in the vial, the microcapillary tube was removed from the retroorbital venous sinus, and the remaining blood was dispensed to the collection vial by capillary action. Gauze was used gently to stop any blood loss. Each mouse was then placed in the gauze-lined recovery cage until fully recovered from the anesthesia. Prior to being returned to the home cage, each mouse was observed for behavioral changes that might indicate injury or trauma to the eye (squinting, pawing at the eye).
Any difficulties or complications were noted at the time of collection. The size of the phlebotomy lancet used and incidence of repeat attempts were noted for the submental and submandibular groups. All mice were observed daily throughout the study for signs of injury or distress. Animals were weighed prior to phlebotomy and at 24 and 48 (± 2) h after phlebotomy. Sample volumes were calculated by weighing the collection vials before and after sample collection and then using the conversion factor: 1.06 g blood = 1 mL blood.1 Extraneous blood loss was assessed indirectly by calculating the weight of any residual blood, beyond that collected in the sample vials. To this end, a quantity of gauze was weighed and used to line the surfaces of the collection field and a recovery cage, and a portion was placed at-hand for use in wound compression or blotting up blood. The gauze was situated in a manner that would ensure any blood not collected in the sample would be caught by the gauze. Used phlebotomy lancets were wiped with gauze before being discarded, and the microcapillary tubes used for the retroorbital bleeds were weighed before and after collection. To avoid contaminating the gauze with urine, the urinary bladders of the mice were expressed away from the collection field prior to beginning the collection.
After the final phlebotomy was complete, a veterinarian performed a postmortem examination of the collection sites in mice comprising the submental and submandibular groups. Approximately half of the animals were examined 2 d after the final blood collection, and the remaining mice were evaluated 7 d after the final phlebotomy. Mice were euthanized via CO2 asphyxiation followed by cervical dislocation. The skin was dissected away from the collection site, and the amount of tissue damage was assessed visually. Mice were scored as having no gross visible tissue damage (score, 1); a trace mark at the collection site but no hematoma (score, 2); or a hematoma covering the collection site (score, 3).
Samples were assessed for hemolysis and clotting by submitting the blood samples to be processed in our clinical pathology lab and scored by a pathologist. For the hemolysis assessment, a microhematocrit tube was prepared, and blood samples were scored visually using a sample hemoglobin scale with representative values at 0 mg/dL (score, none), 40 mg/dL (score, 1+) 80 and 120 mg/dL (score, 2+), 160 mg/dL (score, 3+), 200 and 320 mg/dL (score, 4+) and 360+ (score, abnormal). For the clotting assessment, a whole-blood smear was prepared, and the degree of clotting was based on the number of clots found at the peripheral or ‘feathered’ edge of the smear according a scale of none, rare (1 clot), few (2 to 10 clots), and many (more than 10 clots).
Statistical analysis.
A P value of less than 0.05 was used as the benchmark for statistical significance in all analyses. Analysis of weight change, hemolysis, and clotting were performed using SAS (SAS Institute, Cary, NC). Comparison between methods for missed collections, overall sample quality, and repeat bleed attempts was performed by using the 2-tailed Fisher exact test (Excel, Microsoft, Seattle, WA).
For the analysis of weight change, each individual mouse's change in weight first was averaged over the 3 phlebotomy sessions. A repeated-measures ANOVA model was used to assess differences between methods. The model included factors of phlebotomy method, day of collection, and their interaction. The submental method was compared with the submandibular and retroorbital blood collection methods at both the overall level (all 3 blood collections combined) and on individual collection days. The Dunnett multiple comparison adjustment was applied in each set of comparisons.
For analyses of hemolysis and clotting, sample volumes of less than half that targeted (that is, less than 75 μL) were designated as ‘missed collections’ and excluded; such low volumes are strongly suggestive of difficulties during collection. Many of these samples were extremely low in volume or were extremely poor in quality. In biomedical studies requiring large volumes of high-quality blood, such samples would typically be discarded and another collection attempted or the mouse skipped for that collection. Remaining scores were first converted into numerical scores on a scale from 0 to 5 (hemolysis) and 0 to 3 (clotting). Scores were then averaged over the 3 phlebotomy sessions per mouse. An ANOVA model was used for evaluating the effect of the blood collection methods. The submental method was compared with the submandibular and retroorbital methods by using the Dunnett multiple-comparison adjustment.
Samples of sufficient volume with little to no hemolysis and little to no clotting are highly desirable from a biomedical standpoint. Overall sample quality was designated as ‘good’ for samples where: sample volume, 75 μL; hemolysis score, none or 1+; and clotting score, none or rare. Sample quality was designated as ‘variable’ when the volume exceeded 75 μL, but hemolysis or clotting did not meet the criteria designating good-quality samples.
Results
According to daily observation of the mice, no technique-related complications or distress occurred in the submental and submandibular groups. The retroorbital group included 2 mice that exhibited minor inflammation at the corner of the eyelid where the microcapillary tube had been inserted, but no tearing or squinting, for a few days after collection. This minor inflammation resolved without medical intervention within a few days.
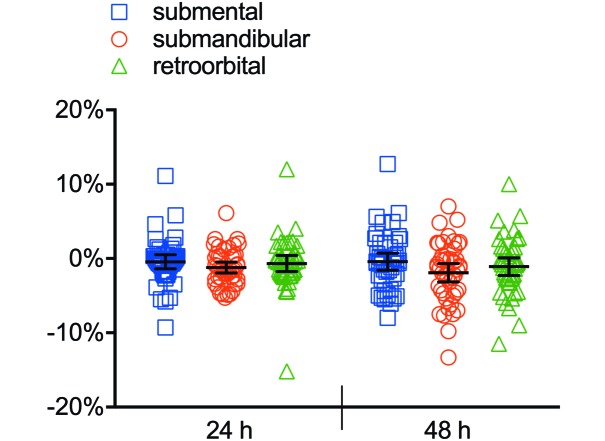
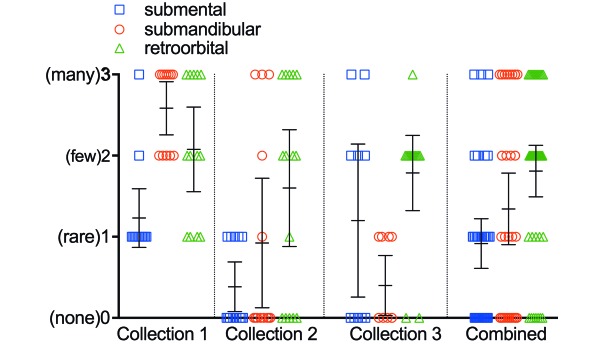
The average postcollection weight changes at 24 and at 48 h was –0.4% and –0.4% for the submental group, –1.2% and –1.9% for the submandibular method, and –0.6% and –1.0% for the retroorbital group, respectively, calculated as a combined average for all 3 bleeds in all 45 animals (Figure 3). Differences in weight change between groups were not statistically significant. The combined extraneous blood loss across all 3 collections, as measured by the weight of residual blood on the collection materials, was 0.61 g for the submental group, 1.87 g for the submandibular technique, and 0.88 g for the retroorbital process (Table 1). The submental method incurred an average hemolysis score of 0.28, which was significantly lower than those for the submandibular method (1.09, P < 0.0001) and retroorbital method (1.55, P < 0.0001) (Figure 4). The submental method incurred an average clotting score of 0.92, which did not differ from that of the submandibular method (1.34, P = 0.0507) but was lower than that for the retroorbital method (1.81, P = 0.0002; Figure 5). The submental method yielded more good-quality compared with variable-quality samples than did either other method (submental compared with submandibular, P = 0.0142; submental compared with retroorbital, P < 0.0001). The submental method also yielded more good-quality samples overall as compared with variable-quality samples + incidents of missed collection (submental compared with submandibular, P = 0.0344; submental compared with retroorbital, P < 0.0001). However, the retroorbital method yielded fewer missed collections than did the submental technique (P = 0.0496; Table 2).
Figure 3.
Weight change after phlebotomy for all 3 blood-collection methods (pooled samples). No significant difference was found between methods. Bar, 95% confidence interval.
Table 1.
Extraneous blood loss
| Blood collection number |
||||
| 1 | 2 | 3 | Combined | |
| Submental | 0.23 | 0.18 | 0.20 | 0.61 |
| Submandibular | 0.57 | 0.92 | 0.38 | 1.87 |
| Retroorbital | 0.43 | 0.26 | 0.19 | 0.88 |
Data are presented as total weight (in grams) of blood residue on collection materials for all mice.
Figure 4.
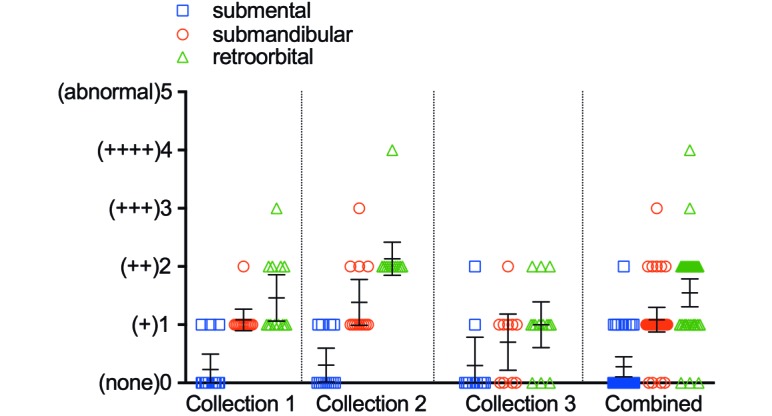
Average hemolysis score. For blood collections combined: submental compared with submandibular, P < 0.0001; submental compared with retroorbital, P < 0.0001. Statistical significance was not calculated for individual collections. Bar, 95% confidence interval.
Figure 5.
Average clotting score. For blood collections combined: submental compared with submandibular, P = 0.0507; submental compared with retroorbital, P = 0.0002. Statistical significance was not calculated for individual collections. Bar, 95% confidence interval.
Table 2.
Sample quality and missed collections
| Submental | Submandibular | Retroorbital | |
| Good sample | 28a,b | 17a | 5b |
| Variable sample | 8 | 18 | 37 |
| Missed collection | 9c | 10 | 2c |
The total number of blood collections was 45 for both the submental and submandibular methods and 44 for the retroorbital technique. A good sample had a volume of 75 μL or more, a hemolysis score of N or +, and a clotting score of none or rare. A missed collection was one with a volume of less than 75 μL.
P = .0142 versus ‘Variable sample’, P = 0.0344 versus (‘Variable sample’ + ‘Missed collection)
P < 0.0001 versus ‘Variable sample’ and P <0.0001 versus (‘Variable sample’ + ‘Missed collection’)
P = 0.0496 versus (‘Good sample’ + ‘Variable sample’)
Lancet size differed between the submental and submandibular methods (P < 0.0001), although this outcome was driven by phlebotomist preference. The number of repeat attempts did not differ between the submental and submandibular methods (Table 3). At 2 d after the final phlebotomy, the submentally bled mice were judged to have subjectively less severe injury at the access site, but no animals from either group exhibited grossly visible tissue damage at 7 d afterward (Table 4). In addition, 3 mice in the submental group and 5 in the submandibular group in which the final phlebotomy resulted in a missed collection showed no grossly visible tissue damage at 2 d thereafter.
Table 3.
Lancet size and repeat attempts
| Submental (n = 45) | Submandibular (n = 45) | |
| 4-mm lancet used | 35a | 0a |
| 5-mm lancet used | 10a | 45a |
| Repeat attempts | 10b | 19b |
Data are presented as the number of mice affected.
P < 0.0001
P = 0.0702
Table 4.
Postmortem evaluation of access site
| Submental | Submandibular | |
| 2 d after phlebotomy | ||
| No gross injury noted | 4 | 0 |
| Trace access mark, no hematoma | 3 | 4 |
| Hematoma over access site | 0 | 1 |
| 7 d after phlebotomy | ||
| No gross injury noted | 5 | 5 |
| Trace access mark, no hematoma | 0 | 0 |
| Hematoma over access site | 0 | 0 |
A total of 5 mice were accessed per method, except for the 2-d submental time point (n = 7). Data presented as the number of mice affected. Three mice from the submental group and 5 from the submandibular group had ‘missed collections’ on the final day of phlebotomy; these animals also were assessed at 2 d after phlebotomy and had no grossly visible injury.
Discussion
Compared with the submandibular method, the submental method of blood collection from mice did not result in adverse animal welfare outcomes. This result may reflect the relative ease with which the method can be performed due to the ease of finding the target vessels. The small phlebotomy lancet used for most of the submental collections presumably limited the amount of extraneous blood loss and trauma to the collection site. However, in our experience, the method appears less likely to result in hemorrhaging and staunches more rapidly than the submandibular method, which also limited the amount of extraneous blood loss regardless of lancet size used. Despite the decreased lancet size, the submental method resulted in fewer repeat attempts and a similar frequency of missed collection. The current study might be criticized for allowing the phlebotomist's preference to drive the selection of lancet size. The phlebotomy lancets used for the submandibular collections were in line with the manufacturer's recommendations,19 based on the age range of the mice (2 to 6 mo). The animals used in the study were of a large stock approaching the end of the recommended age range (23 wk). We believe that the difference in lancet size is more likely to be linked to inherent differences in the way the 2 techniques are accomplished. The extraneous blood loss incurred in the submandibular group (1.87 g) is concerning given that most institutions allow the withdrawal of no more than 10% of the circulating blood volume every 2 to 4 wk.2,5,13,18,22,29 As a general guideline, the total circulating blood volume ranges from approximately 55 to 80 mL/kg in mice,1,5,21,29 such that a 30-g adult mouse has a total blood volume of 1.7 to 2.4 mL (55 to 80 mL/kg × 0.03 kg = 1.7 to 2.4 mL). Therefore, the maximal amount of blood available for removal during a nonterminal bleed is 170 to 240 μL (10% of 1.7 to 2.4 mL). Using the conversion factor of 1 mL blood = 1.06 g,1 we estimate an average of 39 μL extraneous blood loss per mouse per submandibular collection (1.87 g of blood residue / 1.06 g/mL = 1.76 mL blood; 1.76 mL/45 collections = 0.039 mL [that is, 39 μL] per mouse per collection). For the submental method, we estimate an average of only 13 μL of extraneous blood loss per mouse per collection (0.61 g blood residue / 1.06 g/mL = 0.57 mL blood; 0.57 mL/45 collections = 0.013 mL per mouse per collection). The choice of a smaller lancet did not correspond to more repeat attempts or missed collections. Sample hemolysis scores were lower for the submental compared with submandibular method. Although less trauma at the collection site may have influenced this outcome, the fur over the submandibular region might have increased the associated hemolysis, given that RBC might shear as they slide against the fur. The overall number of good-quality samples, characterized by sufficient volume and limited hemolysis and clotting, were significantly higher for the submental method. We primarily attribute this result to the ease with which the submental method is performed, thus fewer collection difficulties resulted in better samples.
The submental method was similar to the retroorbital method when the animal welfare outcome was evaluated on the basis of weight change or extraneous blood loss. However, it cannot be concluded that gaining vascular access in the submental region or the need for the occasional repeat attempts needed when using this method is less traumatic to the mouse. Future studies incorporating stress responses or a histologic assessment of the retroorbital region would help to answer this question. The submental method resulted in significantly lower hemolysis and clotting scores and provided more good-quality samples, therefore, when sample quality is of primary concern, the submental method might be a better choice than the retroorbital technique. However the retroorbital method resulted in fewer missed collections and might therefore be the preferred choice when obtaining high volumes of blood from all mice in a study is particularly important. To this point, we would argue that if sample quality is unimportant, there are less invasive methods of obtaining samples than the retroorbital method (i.e. tail vessel).
The success rates of the submental (and submandibular) methods might be improved by allowing phlebotomists to bleed from whichever side of the mouse they preferred, particularly during a repeat attempt after missing on the first side. The design of our study did not accommodate this option. In addition, allowing a phlebotomist to make more than 2 attempts with the submental method likely would increase the success rate, although such a practice would diminish the rationale for choosing the method over retroorbital collections on the basis of humane reasons. Hemolysis in the retroorbital samples might reflect the shearing of RBC as they slide against the side of the microcapillary tube, and clotting may be caused by progression through the microcapillary tube, thus slowing the distribution of the sample in the anticoagulant-coated collection vial. Some phlebotomists use anticoagulant-coated microcapillary tubes to collect retroorbital blood samples. Because we were concerned with the introduction of anticoagulant into the mice, anticoagulant-coated tubes are not used for retroorbital bleeds at our facility and were not included in our current study design. At least one study has shown that the retroorbital method can produce high-quality blood samples in terms of minimal sample hemolysis and clotting, although the submandibular method was not included among the methods considered.3 Fundamentally, it may be that skill with a particular technique drives both sample quality and animal welfare, and these hypotheses are potential directions for future research.
It cannot be excluded that the variation in differences observed in the study are solely attributable to the performance by the phlebotomists. In our experience, methods that are easier to learn and execute tend to be practiced more often, thus driving proficiency with the method. We did not have anyone on staff who was equally competent in and had equal preference for all 3 methods. Although some studies have used the same phlebotomist in an attempt to standardize this variable, claims that any single person is equally competent at all methods should also be viewed with skepticism. It cannot be excluded that personal bias influenced the data, given that the study was not blinded. Animal weights, extraneous blood loss, and clotting scores are somewhat less likely to be influenced due to the nature of the assessments, whereas hemolysis scores and postmortem assessment of injury would be somewhat more vulnerable to bias; future studies could better control for these factors. Furthermore the anesthesia used for the retroorbital group might have confounded the comparison. If the retroorbital bleeds had been performed without anesthesia, we presume the method would have been more traumatic to the mice, resulting in more poor-quality samples due to animal movement. Conversely, if the submental and submandibular methods had been performed with anesthesia, we presume the collections would have been easier and may have resulted in better study outcomes. Future research studies could investigate these possibilities, particularly in light of how the techniques typically are performed at other institutions.
As a general rule, variations in individual skill with a particular technique can have a substantial effect on animal welfare, experimental outcome, and reproducibility.2,3,5-7,12,13,18,30 A wide variety of blood parameters are influenced by the collection site.3,7,20,24 Therefore, the adoption and promotion of a standard venipuncture method that is (in general) easy to learn and perform must certainly have a positive effect through obtaining data of improved quality and consistency. As a result of our study, the submental blood collection method was determined to be an acceptable venipuncture method for collecting large, nonterminal volumes of blood from mice. In practice, the submental technique has become the preferred method at our facility due to its relative ease of implementation, improved animal wellbeing compared with the submandibular method, and ability to yield high-quality blood samples. Additional technical and scientific staff members have become competent in the technique and have used it in many research studies. Prior to adoption of the submental method we often experienced reluctance among investigators to move away from the retroorbital method. Difficulties experienced with the submandibular method led to complaints about sample quality; therefore, many investigators felt that the retroorbital method was the only way to obtain blood samples of sufficient volume and quality for their studies. The ease of training and execution of the submental method has positively influenced our investigators’ interest in and commitment to change their previous venipuncture approach. Since adoption of the submental method, the retroorbital method has been phased out of many research protocols. Submandibular blood collection is not performed frequently in our facility, given that personnel generally prefer to learn and implement the submental method.
Acknowledgments
We thank Emily Frazier and Derek Masse for their technical expertise in assisting with the blood collections for this study. We also appreciate Linda Cherepow's and Keith Bailey's assistance in anatomical identification of vessels of interest.
References
- 1.Bernstein SE. [Internet]. 1966. Physiological characteristics, chapter 16. In: Green EL. Biology of the laboratory mouse, 2nd ed. [Cited 4 June 2014]. Available at http://www.informatics.jax.org/greenbook [Google Scholar]
- 2.BVA/FRAME/RSPCA/UFAW Joint Working Group on Refinement 1993. Removal of blood from laboratory mammals and birds. Lab Anim 27:1–22. [DOI] [PubMed] [Google Scholar]
- 3.Christensen SD, Mikkelsen LF, Fels JJ, Bodvarsdottir TB, Hansen AK. 2009. Quality of plasma sampled by different methods for multiple blood sampling in mice. Lab Anim 43:65–71. [DOI] [PubMed] [Google Scholar]
- 4.Deckardt K, Weber I, Kaspers U, Hellwig J, Tennekes H, van Ravenzwaay B. 2007. The effects of inhalation anesthetics on common clinical pathology parameters in laboratory rats. Food Chem Toxicol 45:1709–1718. [DOI] [PubMed] [Google Scholar]
- 5.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C, European Federation of Pharmaceutical Industries Association and European Centre for the Vaidation of Alternative Methods 2001. A good-practice guide to administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21:15–23. [DOI] [PubMed] [Google Scholar]
- 6.Everds NE, Snyder PW, Bailey KL, Bolon B, Creasy DM, Foley GL, Rosol TJ, Sellers T. 2013. Interpreting stress responses during routine toxicity studies: a review of the biology, impact, and assessment. Toxicol Pathol 41:560–614. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez I, Pena A, Del Teso N, Perez V, Rodiquez-Cuesta J. 2010. Clinical biochemistry parameters in C57Bl/6J mice after blood collection from the submandibular vein and retroorbital plexus. J Am Assoc Lab Anim Sci 49:202–206. [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes N, Brayton C, Grindle S, Shepherd S, Tyler B, Guarnieri M. 2010. Morbidity and mortality associated with serial bleeding from the superficial temporal vein in mice. Lab Anim (NY) 39:236–240. [DOI] [PubMed] [Google Scholar]
- 9.Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. 2012. Mice anesthesia, analgesia, and care. Part II: anesthetic considerations in preclinical imaging studies. ILAR J 53:E70–E81. [DOI] [PubMed] [Google Scholar]
- 10.Golde WT, Gollobin P, Rodriquez LL. 2005. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 34:39–43. [DOI] [PubMed] [Google Scholar]
- 11.Grindle S. [Internet]. Mouse phlebotomy: a comparison of superficial temporal vein and retroorbital venous plexus collection methods. [Cited 4 June 2015]. Available at http://www.medipoint.com/html/mouse_phlebotomy.html.
- 12.Heimann M, Kasermann HP, Pfister R, Roth DR, Burki K. 2009. Blood collection from the sublingual vein in mice and hamsters: a suitable alternative to retrobulbar technique that provides large volumes and minimizes tissue damage. Lab Anim 43:255–260. [DOI] [PubMed] [Google Scholar]
- 13.Hoff J. 2000. Methods of blood collection in the mouse. Lab Anim (NY) 29:47–53. [Google Scholar]
- 14.Holmberg H, Kiersgaard MK, Mikkelsen LF, Tranholm M. 2011. Impact of blood sampling technique on blood quality and animal welfare in haemophilic mice. Lab Anim 45:114–120. [DOI] [PubMed] [Google Scholar]
- 15.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 16.Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. 2006. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med 44:311–316. [DOI] [PubMed] [Google Scholar]
- 17.Luzzi M, Skoumbourdis E, Baumans V, Conte A, Sherwin C, Kerwin A, Lang T, Morton D, Barley J, Moreau E, Weilenmann RF, Reinhardt V. 2005. Collecting blood from rodents: a discussion by the laboratory animal refinement and enrichment forum. Animal technology and welfare: journal of the Institute of Animal Technology. 4:99–102. [Google Scholar]
- 18.McGuill MW, Rowan AN. 1989. Biological effects of blood loss: implications for sampling volumes and techniques. ILAR J 31:5–20. [Google Scholar]
- 19.MedIpoint [Internet]. Animal Lancets: Point Lengths. [Cited 3 March 2016]. Available at: http://www.medipoint.com/html/animal_lancets.html.
- 20.Nemzek JA, Bolgos GL, Williams BA, Remick DG. 2001. Differences in normal values for murine white blood cell counts and other hematologic parameters based on sampling site. Inflamm Res 50:523–527. [DOI] [PubMed] [Google Scholar]
- 21.National Centre for the Replacement Refinement and Reduction of Animals in Research [Internet]. Mouse: decision tree for blood sampling. [Cited 30 May 2015]. Available at: https://www.nc3rs.org.uk/mouse-decision-tree-blood-sampling.
- 22.Parasuraman S, Raveendran R, Kesavan R. 2010. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother 1:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai PP, Schlichtig A, Ziegler E, Ernst H, Haberstroh J, Stelzer HD, Hackbarth H. 2015. Effects of different blood collection methods on indicators of welfare in mice. Lab Anim (NY) 44:301–310. [DOI] [PubMed] [Google Scholar]
- 24.Rogers IT, Holder DJ, McPherson HE, Acker WR, Brown EG, Washington MV, Motzel SL, Klein HJ. 1999. Influence of blood collection sites of plasma glucose and insulin concentration in conscious c57bl/6 mice. Contemp Top Lab Anim Sci 38:25–28. [PubMed] [Google Scholar]
- 25.Teilmann AC, Kalliokoski O, Sørensen DB, Hau J, Abelson KSP. 2014. Manual versus automated blood sampling: impact of repeated blood sampling on stress parameters in male NMRI mice. Lab Anim 48:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teilmann AC, Madsen AN, Holst B, Hau J, Rozell B, Abelson KSP. [Internet]. 2014. Physiologic and pathologic impact of blood sampling by retro-bulbar sinus puncture and facial vein phlebotomy in laboratory mice. [Cited 4 June 2015]. Available at: http://www.plosone.org
- 27.Timm KI. 1980. Periorbital bleeding technique for the mouse, hamster, and rat—anatomical considerations. Synapse 13:14–15. [Google Scholar]
- 28.Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, Sanda M, Sorbara L, Stass S, Wang W, Brenner DE. 2009. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res 8:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Department of Health and Human Services—National Institute of Health [Internet]. Guidelines for survival bleeding of mice and rats. [Cited 4 June 2015]. Available at http://oacu.od.nih.gov/ARAC.
- 30.van Herck H, Baumans V, Brandt CJWM, Hesp APM, Sturkenboom JH, van Lith HA, van Tintelen G, Beynen AC. 1998. Orbital sinus blood sampling in rats as performed by different animal technologists: the influence of technique and expertise. Lab Anim 32:377–386. [DOI] [PubMed] [Google Scholar]
- 31.van Herck H, Baumans V, Stafleu FR, Beynen AC. 1992. A questionnaire-based inventory of the orbital puncture method in the Netherlands. Scand J Lab Anim Sci 19:189–196. [Google Scholar]