Abstract
Issues
Patient-centered care (PCC) is increasingly accepted as an integral component of good health care, including addiction medicine. However, its implementation has been controversial in people with alcohol use disorders.
Approach
A systematic search strategy was devised to find completed randomized controlled trials enrolling adults (>18 years) with alcohol use disorders. Studies had to use a PCC approach such that they should have been individualized, respectful to the patients’ own goals, and empowering. Studies until September 2015 were searched using PubMed, Scopus, the Cochrane Library, PsychINFO, and Web of Knowledge.
Key findings
In total, 40 studies enrolling 16,020 patients met the inclusion criteria. Assessment revealed two main categories of study: psychosocial (n=35 based on motivational interviewing) and pharmacological (n=5 based on an as needed dosing regimen). Psychosocial interventions were further classified according to the presence or absence of an active comparator. When no active comparator was present, studies were classified according to the number of sessions (≥1). Results from single sessions of motivational interviewing showed no clear benefit on alcohol consumption outcomes, with few studies indicating benefit of PCC versus control. Although the results for studies of multiple sessions of counseling were also mixed, many did show a significant benefit of the PCC intervention. By contrast, studies consistently demonstrated a benefit of pharmacologically supported PCC interventions, with most of the differences reaching statistical significance.
Implications
PCC-based interventions may be beneficial for reducing alcohol consumption in people with alcohol use disorders.
Keywords: psychosocial intervention, pharmacological intervention, motivational interviewing, as-needed
Introduction
The Institute of Medicine has included patient-centered care (PCC) as one of the major aims in care quality and defines it as “providing care that is respectful of and responsive to individual patient preferences, needs, and values, and ensuring that patient values guide all clinical decisions”.1 Although not a new phenomenon, it has recently attracted renewed attention.2,3 PCC advocates for a shift from disease-oriented to patient-oriented medicine. Doctors should no longer be authoritative figures who make all the relevant decisions. Instead, they must engage in a shared decision-making model where patients are acknowledged to be experts with regard to their own symptoms and values and where they are recognized as unique and diverse. In such a model, the responsibility is shared between a patient and a physician, and the physician’s key role is to strengthen the patient’s capabilities to handle his or her part of responsibility.4
Application of PCC in the field of mental disorders remains a controversial issue. It has long been argued that patients with psychiatric disorders are vulnerable to impediments in decision making,5–8 and a paternalistic approach has been the preferred norm in the field.9,10 Conversely, a number of studies indicate that patients engaged in the decision-making process show greater satisfaction and collaboration, with greater efficacy of treatment.11,12
While considering the field of alcohol use disorders, it is also likely that ideological bias and stigma have exacerbated the paternalistic approach. The harmful use of alcohol is one of the world’s leading health risks and is the leading risk factor for death of people aged 15–49 years.13 However, patients with alcohol use disorders often receive a lower quality of health care than those with other chronic conditions; many dependent patients go without treatment, and even when they are treated, pharmacotherapy is underutilized.14–16 Abstinence has been the prevailing goal, usually irrespective of patients’ own aims or desire.17 Crucially, patients do not always view abstinence as an acceptable, desirable, or realistic treatment goal, and there is an increasing debate about the possibility and the convenience of broadening treatment goals in accordance with a PCC model.18 For example, this could mean establishing reduction in heavy drinking as a possible objective for some patients.18–20 Reduction of alcohol consumption has been shown to reduce the annual and lifetime risk of alcohol-related death, and it could attract patients who are currently not inclined to seek treatment or do not accept abstinence as a treatment goal.20–22 Indeed studies show that patients with alcohol use disorders are more likely to achieve self-set goals (eg, reduction or abstinence), rather than goals that are imposed on them.23,24
Several treatment options (psychosocial and pharmacological) are available for people with alcohol use disorders, but no single therapy has been proven to be more effective than another. PCC and shared decision making are considered especially appropriate when outcomes of the different treatments are similar and when an active role of the patient is needed.25 Thus, some experts consider alcohol use disorders as potentially a suitable situation to use a PCC approach.2 Although some of the components of PCC might have been previously tested for the treatment of alcohol use disorders, for example, in the form of individually tailored feedback and treatment, these have not been systematically assessed in a cohesive manner. The aim of this review was to systematically assess the efficacy of interventions based on a PCC health care approach, both pharmacological and psychosocial, for the management of alcohol use disorders.
Methods
This systematic review was conducted in accordance with the principles recommended by the Cochrane Handbook for Systematic Reviews of Interventions.26 The Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidance was followed for the reporting of procedures; its checklist can be seen in Supplementary materials.
Definition of PCC
Although patient centeredness is not an easy concept to define in a concise manner, a previous systematic review operationalized it under four defining attributes: holistic, individualized, respectful, and empowering.27 Many studies might be considered as PCC, but they might not use this exact expression, or any of the attributes of PCC previously reported. Thus, in order to conduct an appropriately sensitive electronic search, we predefined several adjectives and expressions to cover the four attributes. For example, alternative terms for “empowering” included “patient involvement”, “patient perspective”, “shared decision-making”, and “patient decision”, and alternative terms for “individualized” included “tailored”, “personalized”, and “customized”.
Data sources and searches
The following databases were searched: PubMed, Scopus (which contains EMBASE), the Cochrane Library, PsychINFO, and the Web of Knowledge. The search strategies for PubMed, Scopus, and the Cochrane Library are listed in Supplementary materials. The searches were run until September 2015. Additional hand searches of the reference lists of included randomized controlled trial and relevant systematic reviews were conducted. Finally, the following clinical trial registries were also searched for relevant studies: ClinicalTrials.gov, ISRCTN Register, UK Clinical Trials Gateway, and metaRegister of Controlled Trials.
Study selection
Individual (not cluster) randomized controlled trials enrolling adults (≥18 years) with alcohol use disorders (including hazardous or harmful drinking, alcohol dependence, or any other alcohol use disorder) were included. All the studies had to use a PCC approach such that they should have been individualized, respectful to the patients’ own goals, and empowering. Computerized interventions were not included in this review.
As described earlier, although interventions might not have been described with these same adjectives, they were fully reviewed to check whether they met the criteria (ie, the description of the intervention was individually assessed to determine whether it could be considered PCC). Studies could use any standardized outcome regarding alcohol consumption (eg, heavy drinking days, grams of alcohol, days of abstinence, percentage of patients drinking below recommended limits on validated screening tools). Only publications in English were considered.
To homogenize the review, studies of patient populations with psychiatric comorbidities were excluded from this review as were studies including populations with relevant and differential psychological variables (eg, mandated or incarcerated patients and pregnant women). Studies conducted in the inpatient setting and short-term studies with <3 months of follow-up were also excluded. Studies using cover stories where patients did not know the real intention of the intervention were also excluded, as this is clearly contrary to the concept of PCC. Finally, any comparator was eligible as long as it was not PCC based.
Data extraction
PB and AG independently screened all the studies for inclusion. Disagreements were resolved by discussion when possible. If not, a third person was consulted. Data were extracted by PB and independently checked by AG. The extracted data consisted of participant characteristics, setting, study methods, intervention characteristics, comparators, outcomes, and results.
Quality assessment
PB and AG independently evaluated the quality of the studies. Following the Cochrane guidelines and the methods used in a recent systematic review undertaken by Mdege et al,28 a domain-based approach was used. The following criteria were applied: power calculation, adequacy of randomization, allocation concealment, adjustment for covariates in the analysis, blinding of participants when possible, blinding of outcome assessors, explanation of dropouts, and use of intention-to-treat analysis.
Data synthesis
Considerable heterogeneity existed between the studies, mainly in terms of reported outcomes, how the outcomes were defined and reported, and the duration of the studies. Given the different methodologies that were employed, the studies were grouped according to whether they primarily assessed a pharmacological or a psychosocial intervention. In the psychosocial group, a further grouping was made according to the number of sessions received, categorizing studies between 1 or >1 sessions.
A meta-analysis was conducted on the basis of our findings, by trying to build an outcome construct based on construct validity. However, given the significant methodological issues involved, the analysis was deemed inappropriate and a narrative synthesis was instead conducted.
Results
Literature search
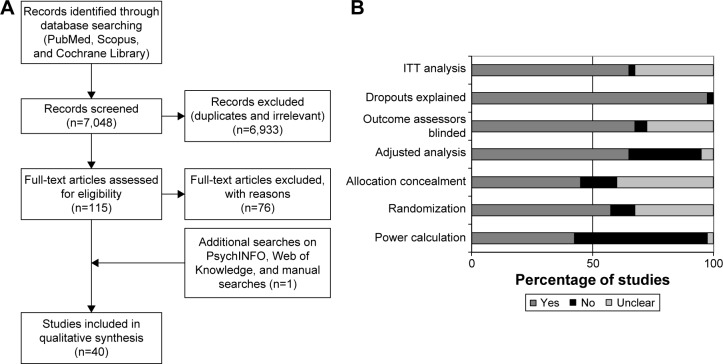
A total of 7,048 records were screened through the search strategy, and 115 full-text articles were assessed for eligibility. An additional reference was identified by hand searching, and eventually, after exclusion criteria were applied (reasons for exclusion of full-text papers are available in the Supplementary materials), a total of 40 studies were included in the systematic review.29–68 Figure 1A depicts the flow diagram of the study.
Figure 1.
(A) Study flow chart and (B) quality assessment.
Abbreviation: ITT, intention-to-treat.
Study characteristics
The 40 studies included in this review involved a total of 16,020 enrolled patients. Sample size in each study ranged from 54 to 987 patients. An initial assessment of the studies revealed two groups according to the main treatment evaluated: psychosocial or pharmacological, with 35 evaluating psychosocial interventions and five evaluating primarily pharmacological interventions.
All psychosocial interventions that met the inclusion criteria in this review were based on motivational interviewing (MI) principles, which might be considered as the cornerstone of patient-centered psychosocial interventions nowadays. MI is defined as “a directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence”.69 Compared to nondirective counseling, it is more focused and goal directed. The examination and resolution of ambivalence is its central purpose, and the counselor is intentionally directive in pursuing this goal.
Regarding pharmacological interventions, all those fulfilling the inclusion criteria were based on the “as needed” concept. The “as needed” [or pro re nata] treatment paradigm is a well-understood medical concept, where the patient takes the medication in response to individual circumstances and not on a scheduled basis. Although it has been a standard practice for many years in asthma, diabetic, and allergy care, it represents a paradigm shift in the way that pharmacotherapy is used in the management of alcohol use disorders.70
For further grouping studies, psychosocial interventions based on MI and with no active comparator (defined as receiving no further intervention or receiving only information, either orally or written materials) were divided according to the number of sessions they contained (1 or >1), while the studies containing an active comparator (like cognitive behavioral therapy [CBT], for example) were included in a separate category. Thus, four categories were created to report the results of the systematic review: single-session PCC with no active comparator, more than one session PCC with no active comparator, PCC with active comparator, and patient-centered pharmacological interventions.
Quality assessment
The results of quality assessment of the included studies are shown in Supplementary materials. Overall, less than half (42.5%) of the studies were deemed as adequately powered, 57.5% had an adequate randomization, and 45% adequate allocation concealment. Around two-thirds (65%) used adjusted analysis and 67.5% reported blinding of the outcome assessors, and all but one study reported adequate information on the patients who dropped-out. Most (65%) of the studies stated clearly an intention-to-treat analysis (Figure 1B). In psychosocial intervention studies, it was not considered possible to blind participants to the delivered intervention; therefore, participant blinding was only assessed in pharmacological intervention studies, which were all deemed as adequate on this item.
Efficacy of PCC
Single-session PCC with no active comparator
Seventeen studies were included in this subgroup (Table 1).29–45 Globally, they failed to show a clear benefit of the PCC intervention over the control groups.
Table 1.
Single-session patient-centered counseling – no active comparator
| Study | Intervention | Patients | Follow-up duration | Outcomes reported | Key findings |
|---|---|---|---|---|---|
| Bazargan-Hejazi et al29 2005, USA | 1. BI 2. Usual treatment in the ED |
• N=295 • ED attendants with CAGE score ≥1 |
3 months | • Changes in AUDIT category from baseline to follow-up ○ Low risk: 0–6 ○ At-risk/moderate risk: 7–18 ○ High risk: 19–40 |
• 48% reduced their AUDIT risk category in the intervention group vs 38% in the control group. Differences were not statistically significant • In the subgroup of moderate risk, 34% of the intervention group had a risk reduction vs 13% in the control group (P=0.0099) |
| Bischof et al30 2008, Germany | 1. Full care: personalized feedback at baseline + MI + BCC on the phone at baseline, 1, 3, and 6 months in 30-minute sessions 2. Stepped care: if no improvement reported, then patients received personalized feedback at baseline + MI + BCC (as above) 3. Control: booklet on health behavior |
• N=408 • Subjects attending 81 general practices in North Germany • AUDIT score ≥5, or LAST score ≥2 • Individuals who screened positive were then assessed to identify AD, AA, AR, and HED with the lifetime version of the M-CIDI |
12 months | • Average daily alcohol consumption • HED |
Grams of alcohol per day: • Intervention groups reduced from 46.9 (49.3) and 49.0 (51.3) to 35.7 (48.1); control group reduced from 41.0 (50.3) to 34.9 (48.9). Differences not significant (P=0.883) Percent binge drinkers at follow-up: • For alcohol-dependent patients: intervention group 54.5%; control group 50.0% (P=0.694) • For patients with alcohol abuse or at risk drinking: intervention group 25.0%; control group 41.3% (P=0.039) • For HED only patients: intervention group 32.9%; control group 27.5% (P=0.672) |
| Brown et al31 2010, Canada | 1. One 30-minute brief MI session, adapted for DWI offenders 2. Control |
• N=92 • Adult drivers convicted of at least 2 DWI offences within the last 15 years • AUDIT score >8 • Residence within a 100 km radius of Montreal • No current DWI intervention involvement |
12 months | • Percent of risky drinking days in the last 180 days (defined as ≥42 g of alcohol for males) and ≥2 standard drinks (≥28 g of alcohol) for females | • Group × time interaction not statistically significant • No differences between groups at either time • Significant reduction from 6 to 12 months in risky drinking days for intervention group (mean reduction (SD) =6.1 (20.2) to 12.2 (25.0) for intervention group; 8.5 (22.4) to 7.5 (22.7); P<0.02) • Greater reduction of MCV for intervention group at 3 months in MCV (mean (SD) =9.6 (34.3) vs 1.75 (23.9); P<0.005) |
| Carey et al32 2006, USA | 1. TLFB + BMI enhanced with decisional balance 2. BMI enhanced with decisional balance 3. TLFB + basic BMI 4. Basic BMI 5. TLFB 6. Control: initial assessment only 7. Control: brochure |
• N=615 • Adult university students reporting >1 episode of heavy drinking in a week, or four heavy drinking episodes in the last month |
12 months | • Drinks/week • Drinks/drinking day • Heavy drinking frequency |
• Reduction in all drinking outcomes in all groups (significance not reported) • Between groups effect sizes at 1 month were medium for intervention vs control group in drinks per drinking day, peak BAC and RAPI (0.41; 0.44 and 0.52) • At 12 months also medium for intervention vs control group in peak BAC and RAPI (0.57 and 0.50) • All other outcomes resulted in small effect sizes |
| Chang et al33 2011, USA | 1. BI 2. Control |
• Outpatient women with hypertension, diabetes, osteoporosis, or infertility • T-ACE alcohol screen-positive; and/or typically consuming ≥7 drinks a week or ≥2 drinks at a time |
12 months | • Drinks per drinking day • Percent drinking days • Number of binge episodes, with binges defined as ≥4 drinks per occasion |
Drinks per drinking day: • Intervention group reduced from 2.1 (1.4) to 2.0 (2.0); control group reduced from 2.2 (1.5) to 1.9 (1.6). Differences not significant (P=0.60) Percent drinking days: • Intervention group reduced from 26% to 22%; control group reduced from 21% to 20%. Differences not significant (P=0.52) Binge episodes: • Intervention group reduced from 7.4 (24) to 6.0 (21); control group reduced from 5.7 (17) to 3.8 (13). Differences not significant (P=0.17) |
| Cherpitel et al34 2010, Poland | 1. 20 minutes of BNI 2. Assessment only 3. Screening only |
• N=446 • Patients attending the ED • Positive on any one of the four RAPS4 items during the last year, or reported ≥11 standard drinks per week for men (≥6 for women), or reported ≥4 drinks on a typical drinking day for men (≥3 for women) in the last year |
12 months | • Percent at risk drinking • Drinking days per week • Drinks per drinking day • Maximum drinks per occasion last month |
At risk drinking: • Intervention group reduced from 87.5% to 63.8%; control groups reduced from 87.4% and 88.7% to 54.0% and 64.9%, respectively. No significant differences between groups. Within group differences significant for all groups (P<0.01) Drinking days per week: • Intervention group reduced from 2.5 (0.2) to 1.8 (0.2); control groups reduced from 2.3 (0.2) and 2.3 (0.2) to 2.0 (0.2) and 2.1 (0.2), respectively. Differences between groups not significant. Within group differences only statistically significant for intervention group (P<0.01) Drinks per drinking day: • Intervention group reduced from 5.6 (0.4) to 4.1 (0.4); control groups reduced from 5.0 (0.4) and 5.3 (0.4) to 3.5 (0.3) and 4.2 (0.4), respectively. Within group differences significant for all groups (P<0.01). No differences between groups Maximum drinks per occasion last month: • Intervention group reduced from 9.3 (0.8) to 7.4 (0.6); control groups reduced from 6.7 (0.5) and 7.8 (0.5) to 6.1 (0.6) and 7.7 (0.6), respectively. No differences between groups. Within group differences only significant for intervention group (P<0.05) |
| Daeppenet al35 2007, Switzerland | 1. BAI (15-minute session) 2. Control with assessment 3. Control with no assessment |
• N=987 • Patients admitted to the ED for an injury • Hazardous drinking, defined as men aged <65 years who drank >14 drinks per week or five drinks on a single occasion in the past 30 days, or men aged >65 years and women who drank >7 drinks per week or four drinks on a single occasion |
12 months | • Percentage of patients changing from high- to low-risk drinking |
Drinks per week among binge drinkers: • Intervention group baseline to follow-up difference −1.5 (13.2), control group 0.8 (10.8). Differences statistically significant (P=0.004) Binge drinking occasions per month among binge drinkers: • Intervention group baseline to follow-up difference −1.5 (3.4), control group −0.8 (3.2). Differences statistically significant (P=0.04) |
| Daeppenet al36 2011, Switzerland | 1. BMI (15–45 minutes) 2. Control |
• N=271 • 20-year old men entering military service who were binge drinkers (typical drinking episodes of 60 g pure alcohol at least once a month) |
6 months | • Typical number of drinks per week (standard drink containing ~10 g of pure alcohol) • Typical number of binge drinking episodes per month • AUDIT score • Number of drinks per occasion • Number of drinking days per week |
Hazardous drinkers: • Intervention group reduced from 100% to 64.4%, control groups reduced from 100% to 66.0% and 63.0%, respectively. Differences not significant (P=0.71) Binge drinking episodes last month: • Intervention group reduced from 4.2 (6.1) to 3.7 (5.8); control groups reduced from 4.0 (6.2) and 3.7 (6.1) to 3.6 (6.1) and 3.6 (6.4), respectively. Differences not significant (P=0.46) Number of drinks in last 7 days: • Intervention group reduced from 13.4 (12.8) to 10.3 (11.3); control groups reduced from 13.3 (14.7) to 11.1 (11.9) and 10.9 (14.2). Differences not significant (P=0.65) AUDIT score: • Intervention group reduced from 9.7 (5.2) to 7.8 (5.0), control groups reduced from 8.7 (4.1) to 7.2 (4.3) and 7.3 (4.7). Differences not significant (P=0.27) Number of drinks per occasion: • Intervention group reduced from 4.0 (2.6) to 3.7 (2.9), control groups reduced from 3.8 (2.4) and 3.7 (2.8) to 3.5 (2.6) and 3.4 (2.6). Differences not significant (P=0.28) Number of days drinking per week: • Intervention group reduced from 3.5 (2.3) to 3.0 (2.2), control groups reduced from 3.5 (2.4) and 3.6 (2.3) to 3.2 (2.4) and 3.1 (2.4). Differences not significant (P=0.52) |
| D’Onofrio et al37 2008, USA | 1. BNI 2. BNI + booster 3. Standard care |
• N=494 • Patients presenting to an ED |
12 months | • Number of standard drinks per week • Number of binge episodes (>4 drinks for women and >5 drinks for men) in the past 30 days • Percent of participants in each treatment condition who exceeded NIAAA low-risk drinking limits in the past month |
Drinks per week: • Intervention group reduced from 13.6 (11.6) to 9.8 (14.3); control group reduced from 12.4 (8.7) to 9.8 (10.9). Differences not significant Binge episodes last month: • Intervention group reduced from 6.0 (6.8) to 4.0 (6.7); control group reduced from 5.4 (5.4) to 3.9 (6.2). Differences not significant Proportion of patients over NIAAA guidelines (with previous outcomes combined): • Intervention group reduced from 99.2% to 62.0%; control group reduced from 98.0% to 65.4%. Differences not significant |
| Emmen et al38 2005, the Netherlands | 1. Dutch Motivational Drinker’s Check-Up 2. Control |
• N=123 • Patients from a general internal medicine outpatient department • Positive answer to one of three questions: 1) Have you ever felt the need to cut down on your drinking? 2) Do you ever drink to forget your worries? 3) Do close relatives ever worry or complain about your drinking? |
6 months | • Change in self-reported alcohol consumption in units/day • Change in CDT |
• Overall, patients reduced their alcohol consumption over time from 3.9 (2.42) units/day to 3.11 (2.29) units/day; P<0.001 • Intervention group reduced from 4.16 (2.15) to 0.81 (2.0), control group from 3.70 (2.67) to 0.84 (2.61). Differences not significant • No significant differences in the reduction of % CDT values |
| Gaume et al39 2011, Switzerland | 1. BMI 2. Control |
• N=446 • Swiss army conscripts, who were heavy episodic users, defined as having ≥1 episode per month of ≥6 drinks on a single occasion |
6 months | • Number of standard drinks (~10 g of pure alcohol) per week and number of heavy drinking episodes (≥6 drinks on a single occasion) per month |
Among heavy episodic users: Drinks per week: • Baseline to 6-month difference −0.4 (13.1) for the intervention group; 0.7 (19.1) for the control group. Differences not statistically significant (P=0.90) Heavy drinking episodes per month: • Baseline to 6-month difference −0.7 (3.2) for the intervention group; −0.8 (3.8) for the control group. Differences not statistically significant (P=0.61) |
| Hansen et al40 2012, Denmark | 1. BMI (10 minutes) and a brief telephone booster session 4 weeks later 2. Control |
• N=772 • Patients attending a health examination, after completing an online survey • Weekly alcohol consumption above the Danish National Board of Health limits (14 drinks =168 g of alcohol for women, 21 drinks =252 g for men) • Dependent drinkers included |
12 months | • Number of drinks per week | • The difference in the number of drinks per week between intervention and control group in change over time was 1.0 favoring intervention • Difference not significant (95% CI: −2.15 to 0.23; P=0.114) |
| Lee et al41 2011, USA | 1. CAMI 2. MI (1.5 hours) |
• N=54 • Hazardous drinking (≥5/4 drinks/occasion for men/women or ≥14/7 drinks/week for men/women) • Hispanic nationality |
6 months | • Heavy drinking days/month • Drinking days/month • DrInC |
• Significant within groups decline for both groups on drinking days/month; heavy drinking days/month, and DrInC (numbers not reported), with no between groups differences at 6 months • At 2 months, CAMI had a greater reduction than MI in drinking days/month • Trend favoring CAMI (PCC) in reduction of heavy drinking days (P=0.08) • Significant time by treatment interaction on the DrInC Impulse scale at 6 months favoring CAMI |
| Murphy et al42 2001, USA | 1. Basics: MI (50 minutes) 2. Education: 30-minutes of video watching and individual discussion 3. Control: assessment only |
• N=54 • University undergraduate students on the upper third of the screening sample in terms of drinks per week, as measured by the DDQ • Endorsed ≥2 alcohol-related problems on the RAPI |
9 months | • Drinks per week • Binge drinking days per week, drinking days per week, RAPI score, ADS score |
Drinks per week: • The intervention group reduced from 22.38 (12.04) to 16.63 (9.29); the control groups reduced from 21.29 (10.06) and 20.76 (10.75) to 15.72 (7.75) and 18.46 (14.17), respectively Drinking days per week: • The intervention group reduced from 3.90 (1.35) to 3.17 (1.21); the control groups reduced from 3.75 (1.45) and 3.92 (1.46) to 3.37 (1.14) and 3.89 (1.93), respectively Binge drinking days per week: • The intervention group reduced from 2.57 (1.38) to 1.87 (1.11); the control groups reduced from 2.67 (1.05) and 2.44 (1.23) to 1.90 (1.33) and 1.93 (1.54), respectively • Not significant multivariate effect of group (P=0.22) |
| Murphy et al43 2004, USA | 1. PDF + plus 30–50 minute MI session 2. PDF alone |
• N=54 • College students who consumed at least 13 drinks per week and endorsed one or more alcohol-related problems in the past month on the RAPI |
6 months | • Drinks per week • Frequency of heavy drinking in the past month |
• In general, both the groups showed moderate drinking reductions • Within-group effect size across the three drinking measures was 0.48 for intervention group (PDF + MI) and 0.42 for control group (PDF alone) • No significant differences between groups |
| Senft et al44 1997, USA | 1. Intervention: delivered in two parts: a 30-second message from the primary care clinician and a 15-minute MI session 2. Control: usual care |
• N=524 | 12 months | • Drinking amount in units • Usual drinking amount per occasion • Health care utilization |
At 12 months: • Intervention patients reported fewer drinking days per week (2.7 vs 3.1; P=0.04) than controls, but similar numbers of standard drinks (157 vs 179; P=0.13) and drinks per drinking day (3.6 vs 3.3; P=0.20) • Intervention patients were more likely than controls to report drinking within daily recommended limits (≤3 for men, ≤2 for women) (80% vs 73%; P=0.07) • No significant differences in other drinking outcomes (percent abstinent, frequency of drinking ≥6 drinks per drinking occasion, estimated peak blood alcohol concentration), or use of medical care in the year following intervention |
| Soderstrom et al45 2007, USA | 1. Personalized MI 2. BIA |
• N=497 • Patients attending a trauma center • Any positive response to an item of the CAGE, or • Drinking >2 times per week with total weekly drinking of ≥8 drinks for women and ≥15 for men, or • Drinking 2–4 times a month with typical daily consumption of ≥4 drinks for women and ≥5 drinks for men, or • Drinking ≥6 drinks on one occasion, weekly, daily, or almost daily |
12 months | • Number of drinks within the past 90 days • Number of binges within the last 90 days |
• Within group significant declines in number of binge episodes during the last 90 days and number of drinks during the last 90 days • No statistical significance between groups (final numbers not reported) |
Abbreviations: AA, alcohol abuse; AD, alcohol dependence; ADS, Alcohol Dependence Scale; AR, at-risk drinking; AUDIT, alcohol use disorders identification test; BAC, blood alcohol content; BAI, brief alcohol intervention; BCC, behavioral change counseling; BI, brief intervention; BIA, brief intervention and advice; BMI, brief motivational interviewing; BNI, brief negotiated interview; CAGE, Cut down, Annoyed, Guilty, Eye-opener; CAMI, culturally adapted motivational intervention; CDT, carbohydrate deficient transferrin; DrInC, Drinker Inventory of Consequences; DWI, driving while intoxicated; ED, emergency department; HED, heavy episodic drinking; LAST, Luebeck alcohol dependence and abuse screening test; M-CIDI, Munich composite international diagnostic interview; MCV, mean cell volume; MI, motivational interviewing; NIAAA, National Institute on Alcohol Abuse and Alcoholism; PCC, patient-centered care; PDF, personalized drinking feedback; RAPI, Rutgers alcohol problem index; SD, standard deviation; TLFB, time line follow back.
Amount and frequency of alcohol consumption
Seven studies reported on the number of drinks per week at study end,35–37,40,42–44 with only Daeppen et al36 reporting a statistically significant difference from baseline to follow-up (−1.5 vs 0.8; P=0.004) favoring the PCC group.
Bischof et al reported a nonsignificant difference in grams of alcohol per day between groups,30 Emmen et al reported a nonsignificant difference in drinks per day,38 and Soderstrom et al reported on the number of drinks in the last 90 days, with no differences between groups.45
Five studies reported the number of drinks per drinking day. Four could not find significant differences,33–35,44 while Carey et al only reported a small effect size, with no significance value.32
Four studies reported on drinking days per week,34,35,42,44 none of them reaching statistical significance between groups. Similarly, Lee et al reported on drinking days/month, with no differences between groups.41 Chang et al reported no differences in the percentage of drinking days between groups,33 and Senft et al44 reported no differences in the percentages of abstinent patients.
Hazardous and heavy drinking
Seven studies reported on the number of binge episodes per month.33,35–37,39,41,43 Only Daeppen et al (2011) reported a statistically significant difference favoring the PCC group (baseline to follow-up difference −1.5 vs −0.8, P=0.04).36
Murphy et al reported the number of binges per week and Soderstrom et al reported the number of binges in the last 90 days, both of them showing no significant differences between groups.43,45 Brown et al (2007) reported a significant difference only for men in the reduction in number of risky drinking days (30% vs 12.9%, significance not reported),31 and Senft et al found no differences in the frequency of risky drinking.44
Three studies reported the percentage of heavy drinkers at study end. Two could not find any significant difference,35,37 while the remaining study by Bischof et al reported a statistically significant difference only in the subgroup of patients with alcohol abuse (25% vs 41.3%; P=0.039), with no difference in the alcohol-dependent or heavy episodic drinking-only subgroups.30 Cherpitel et al reported a non-significant difference in the percentage of at risk drinkers between groups or in the maximum number of drinks per occasion.34 Carey et al reported a medium effect size in peak blood alcohol favoring the PCC group (significance not reported),32 while Senft et al reported no significant difference in this outcome.44
Scores
Bazargan-Hejazi et al reported a nonsignificant difference in the percentage of patients changing their drinking risk status according to the Alcohol Use Disorders Identification Test (AUDIT) score.29 In the subgroup of moderate risk (scores 7–18), the PCC group had a larger reduction (34% vs 13%; P=0.0099). Daeppen et al reported a nonsignificant difference between groups regarding the change in the AUDIT score.35
More than one session PCC with no active comparator
Fifteen studies were included in this subgroup (Table 2).46–60 Taken together, data show mixed results, with some studies reporting significant differences between groups, whereas others do not.
Table 2.
More than one session patient-centered counseling – no active comparator
| Study | Intervention | Patients | Follow-up times | Outcomes reported | Key findings |
|---|---|---|---|---|---|
| Aalto et al46 2000, Finland | 1. BI sessions at 0, 2, 6, 12, 18, 24, and 30 months (10–20 minutes based on FRAMES) 2. BI sessions at 0, 12, and 24 months 3. Control: patients were advised to reduce drinking and contact GP in the event of health problems |
• N=118 • Female patients consecutively attending four primary care health clinics and one occupational health care clinic • Heavy drinker with self-reported alcohol consumption ≥190 g of absolute ethanol per week • And/or ≥2 affirmative answers on CAGE |
36 months | • Drinking amount per week • Drinking frequency per week • Drinking amount per occasion • CDT • GGT |
• No differences between or within groups at 3 years in alcohol consumption variables • Significant reductions within all groups in MCV • GGT decreased in intervention groups, while not in the control group (difference not statistically significant) |
| Aalto et al47 2001, Finland | 1. BI sessions at 0, 2, 6, 12, 18, 24, and 30 months (10–20 minutes based on FRAMES) 2. BI sessions at 0, 12, and 24 months 3. Control: patients were advised to reduce drinking and contact GP in the event of health problems |
• N=296 • Male patients • Heavy drinker with self-reported alcohol consumption ≥280 g of absolute ethanol per week • And/or ≥3 affirmative answers on CAGE |
36 months | • Drinking amount per week • Drinking frequency per week • Drinking amount per occasion • CDT • GGT |
Grams of alcohol per week: • Intervention groups decreased from 270 (251) and 284 (262) to 272 (302) and 290 (273), respectively; control group decreased from 308 (337) to 338 (371). Differences not significant Drinking frequency per week: • Intervention groups increased from 2.0 (1.6) and 2.4 (1.6) to 2.0 (1.8) and 2.6 (1.8), respectively; control group increased from 2.3 (1.8) to 2.4 (1.9). Differences not significant Usual drinking amount per occasion in grams: • Intervention groups decreased from 154 (86) and 131 (80) to 151 (89) and 125 (76), respectively. Control group increased from 130 (83) to 137 (82). Differences not significant Significant decreases within groups for all groups in MCV: • Intervention groups from 94.5 (4.2) and 94.2 (4.2) to 93.7 (4.1) and 93.2 (4.2); control group from 94.5 (4.0) to 93.2 (3.9) |
| Allen et al48 2011, Russia | 1. MI up to four sessions 2. Control |
• N=441 • Male patients aged 25–54 years recruited from a longitudinal observational study (the Izhevsk Family Study II) • Criteria for hazardous and harmful drinking were: ○ Zapoi in the last year (behavior resulting in ≥2 days of continuous drunkenness) ○ Drinking surrogates (nonbeverage alcohols) in the last year ○ Hangover and/or excessive drunkenness and/or going to sleep clothed due to being drunk twice or more per week on average over the past year ○ Weekly consumption of 250 mL or more of ethanol (from beverages) over the past year |
3 months | • Self-report of hazardous and harmful drinking |
Hazardous drinking over previous month: • Intervention group decreased from 70.5% to 47.5%, control group reduced from 73.8% to 54.0%. Differences not significant (AOR 0.64, 95% CI 0.39–1.06) |
| Beich et al49 2007, Denmark | 1. BI (10-minute session and follow-up session with GP) 2. Control – no intervention |
• N=906 • Listed patients aged 18–64 years, scheduled to see their GP • AUDIT score ≥8 |
12–14 months | • Drinking frequency • Drinking diary • Binge drinking • AUDIT score • Compliance |
Units of alcohol per week: • Intervention group increased from 12.8 (8.7) to 13.5 (11.1); control group increased from 12.9 (9.0) to 13.6 (11.7) • Differences not significant |
| Brown et al50 2007, USA | 1. Male intervention MI (six sessions) 2. Male control 3. Female intervention MI (six sessions) 4. Female control |
• N=897 • Patients (21–59 years) who exceeded recommendations on low-risk drinking • DSM-IV criteria for alcohol abuse or dependence • No alcohol treatment in the past 3 months • Men who exceeded 56 standard drinks in 28 days and men who had >4 standard drinks in any 1 day • Women who exceeded 44 drinks in 28 days and women who had >3 drinks in any 1 day |
3 months | • Number of drinks per month • Risky drinking days per month |
Men in the intervention group: • 17.3% decrease in 28-day alcohol consumption in standard drinks (from 69.4 to 57.4; P<0.001) • 30% decrease in number of risky drinking days (from 6.2 to 4.3; P<0.001) Men in the control group: • 12.9% decrease in 28-day alcohol consumption in standard drinks (from 82.1 to 71.5; P=0.002) • 8.3% decrease in number of risky drinking days (from 7.2 to 6.6; P=0.009) • Decreases in the intervention group significantly larger Women in the intervention group: • 15.7% decrease in 28-day alcohol consumption in standard drinks (from 50.4 to 42.5; P<0.001) • 20.7% decrease in number of risky drinking days (from 5.8 to 4.6; P<0.001) Women in the control group: • 13.5% decrease in 28-day alcohol consumption in standard drinks (P<0.001) • 15.4% decrease in number of risky drinking days (P<0.001) • No differences between groups |
| Curry et al51 2003, USA | 1. BI 2. Control |
• N=307 • Outpatients visiting their primary care provider • Consuming an average of ≥2 alcoholic drinks per day in the past month (chronic drinking) • ≥2 episodes of binge drinking (defined as consuming ≥5 drinks on a single occasion) in the past month • ≥1 episode of driving after consuming ≥3 drinks |
12 months | • Prevalence of at-risk drinking practices and weekly alcohol consumption |
Any at risk drinking pattern reported at 12 months: • Intervention group 57%, control group 43% (P=0.003) Drinks per week: • Intervention group reduced from 14.93 (0.82) to 9.3; control group reduced from 13.56 (0.83) to 9.5. Differences not statistically significant (P=0.40) |
| D’Onofrio et al52 2012, USA | 1. BNI 2. BNI + booster phone call at 1 month 3. Standard care (screening with health questionnaire) |
• N=889 | 12 months | • Past 7-day alcohol consumption • Number of binge-drinking episodes in the past 28 days (>4 drinks per occasion for men and >3 drinks per occasion for women) |
Mean number of drinks in the past 7 days: • Intervention groups reduced from 20.4 (18.8–22.0) and 19.8 (18.3–21.4) to 13.0 (10.5–15.5) and 14.3 (11.9–16.8); control group reduced from 20.9 (18.7–23.2) to 17.6 (14.1–21.2). Differences statistically significant (P=0.045) Mean number of binge days in past 28 days: • Intervention groups reduced from 7.5 (6.8–8.2) and 7.2 (6.5–7.9) to 4.7 (3.9–5.6) and 5.1 (4.2–5.9); control group reduced from 7.2 (6.2–8.2) to 5.8 (4.6–7.0) • Differences statistically significant (P=0.03) |
| Hermansson et al53 2010, Sweden | 1. Comprehensive intervention: patients were offered ≤3 sessions (BI + TLFB + drinking diary over 4 weeks) 2. BI (one 15-minute session) 3. Control: no feedback on screening or verbal counseling |
• N=194 • Adults presenting to an ED |
12 months | • Change in percentage of patients with AUDIT positive |
Change in AUDIT status: • Overall, 51.3% of patients tested positive at baseline, and 22.8% at follow-up (−56% reduction, P<0.0001) • No significant differences between groups intervention groups reduced 1.55 (2.47) and 1.11 (1.95) points, respectively; controls reduced 1.11 (2.12) points (P=0.57) |
| Longabaugh et al54 2001, USA | 1. BI (40 minutes in ED) + booster MI session 2. BI (single 40-minute session) 3. Standard care |
• N=539 • Adults attending an ED • Assessed as a hazardous or harmful drinker; defined by one of three criteria ○ Breath alcohol positive (BAC >0.003 mg/dL) ○ Reported having ingested alcohol in the 6 hours prior to their injury ○ AUDIT score ≥8 |
12 months | • Number of heavy drinking days • Negative consequences from drinking (DrInC) • Alcohol-related injuries (IBC-R) |
Number of heavy drinking days: • BI with booster session had a mean of 1.68 (1.15), BI of 1.72 (1.23) and standard care 1.70 (1.09) • Differences between groups not significant DrInC inventory: • BI with booster had the lowest score, with 2.24 (0.082). BI was 2.40 (0.078) and standard care was 2.52 (0.076) • Differences between groups statistically significant (P=0.005) |
| Maisto et al55 2001, USA | 1. MET (30–45 minutes session + two booster sessions) 2. BI (1×10–15 minutes session) 3. Standard care |
• N=301 • Adults attending a primary care clinic • AUDIT score ≥8 • Or ≥16 standard (0.6 oz ethanol) drinks per week for men and ≥12 drinks for women |
12 months | • Number of drinks in past 30 days • Drinks per drinking days, past 30 days • Number of days in the past month in which patients had between 1–6 drinks |
Days abstinent: • Motivational enhancement therapy group showed the greatest reduction in the difference between baseline to 12-month follow-up: −3.58 (−5.57, −1.58). BI reduced −2.54 (−4.56, −0.53), and standard care −1.16 (−2.67, −0.34) • Regression models showed no significant differences between either MET or BI vs SC Number of drinks: • Motivational enhancement therapy group showed a reduction in the difference between baseline to 12-month follow-up of 22 drinks (11.65, 32.32). BI reduced 33.20 (18.21, 48.19), and standard care reduced 14.24 (4.21, 24.26) • Regression models showed no significant differences between MET vs SC. BI showed a regression coefficient of −1.27 (P<0.05) Drinks per drinking day: • Motivational enhancement therapy group showed a reduction in the difference between baseline to 12-month follow-up of 1.30 (0.64, 1.96). BI reduced 1.55 (0.79, 2.32), and standard care 1.48 (0.85, 2.11) • Regression models showed no significant differences between either MET or BI vs SC |
| Mello et al56 2013, USA | 1. BMI 2. Standard care |
• N=285 • Patients attending the ED • ≥14 drinks/week for male subjects, ≥7 drinks/week for female subjects • Or ≥5 drinks/occasion for males, ≥4 drinks/occasion for females |
12 months | • Changes in AUDIT-C • Alcohol-related injuries • DrInC score |
AUDIT-C score AUDIT-1: • The intervention group decreased from 2.76 (0.89) to 2.33 (0.94); the control group decreased from 2.74 (0.83) to 2.31 (1.07). Differences at 12 months not statistically significant (P=0.87) AUDIT-2: • The intervention group decreased from 1.53 (1.14) to 1.15 (1.03); the control group decreased from 1.59 (1.12) to 1.16 (1.14). Differences at 12 months not statistically significant (P=0.0.97) AUDIT-3: • The intervention group decreased from 1.84 (1.16) to 1.41 (1.13); the control group decreased from 1.88 (1.01) to 1.51 (1.16). Differences at 12 months not statistically significant (P=0.52) Alcohol-related injuries: • Significant decrease favoring the intervention group (difference between baseline and 12-month: −0.33 (0.74) vs −0.16 (0.60); P=0.04) DrInC score: • Intervention group reduced from 3.54 (1.77) to 2.54 (1.72); the control group reduced from 3.38 (1.85) to 2.65 (1.86). Differences between baseline and 12-month score were −1.00 (1.50) for the intervention group and −0.74 (1.70) for the control group • Differences not statistically significant (P=0.09) |
| Monti et al57 2007, USA | 1. MI (30–40 minutes) + 2× telephone booster sessions 2. Feedback only |
• N=198 • Adults attending a level 1 trauma center • Blood alcohol concentration >0.01% • Reported drinking alcohol in the 6 hours prior to the event that caused their visit • Or AUDIT score ≥8 |
12 months | • Number of days drinking • Number of heavy drinking days • Average drinks per week |
Number of days drinking in the last month: • The intervention group reduced from 8.27 (6.35) to 4.52 (5.70); the control group reduced from 7.31 (6.27) to 6.54 (6.24). Differences statistically significant in the treatment × time interaction (P<0.001) Number of heavy drinking days in the last month: • The intervention group reduced from 5.49 (5.94) to 2.72 (4.70); the control group reduced from 4.01 (4.48) to 3.53 (4.28). Differences statistically significant in the treatment × time interaction (P<0.01) Number of drinks per week in the last month: • The intervention group reduced from 13.07 (11.59) to 6.10 (8.33); the control group reduced from 10.77 (10.73) to 8.83 (9.67). Differences statistically significant in the treatment × time interaction (P<0.01) • No differences in RAPI scores at 12 months |
| Noknoy et al58 2010, Thailand | 1. MET (3×15-minute sessions) 2. Control: assessment only |
• N=117 • Consecutive primary care attenders • AUDIT score ≥8 • Patients with alcohol dependence excluded |
6 months | • Amount of alcohol consumption during previous week • Binge drinking episodes during previous week • GGT levels |
Drinks per drinking day in the last week: • The intervention group reduced from 5.19 (4.30) to 2.26 (2.70); the control group reduced from 4.31 (4.23) to 4.02 (4.00). Difference at 6 months statistically significant between groups (P=0.018) Drinks per week in the last week: • The intervention group reduced from 13.27 (15.40) to 4.72 (8.34); the control group reduced from 10.55 (16.96) to 11.24 (17.74). Difference at 6 months statistically significant between groups (P=0.04) Episodes of binge drinking in the last week: • The intervention group reduced from 1.00 (1.49) to 0.45 (1.38); the control group reduced from 0.88 (1.54) to 0.32 (0.72). Difference at 6 months not statistically significant between groups (P=0.139) |
| Sellman et al59 2001, New Zealand | 1. MET (four sessions) 2. NDRL (four sessions) 3. Control: received feedback at initial session |
• N=122 • Alcohol-dependent patients (according to DSM-IV criteria) of a community alcohol service |
6 months | • Abstinence • Heavy drinking (drinking ≥10 standard drinks ≥6 times in the 6-month follow-up period) |
• Abstinence: no differences between groups (MET 11.9%, NDRL 10%, control 7.5%; P=0.51) • Heavy drinking: MET 42.9%, NDRL 62.5%, control 65%; differences between groups significant (P=0.04) |
| Sommers et al60 2013, USA | 1. BI (2×20-minute sessions + one face to face in ED + one telephone visit) 2. Contact control group (20-minute assessment interview) 3. No-contact control group: patients were asked for contact information only and were not interviewed |
• N=476 • Young drivers (18–44 years) at a level I trauma center • Positive on two risky driving and two hazardous drinking items in a 3-minute screening |
12 months | • Self-reported risky driving behaviors • Alcohol consumption |
6 months period: • Absolute risk reduction for maximum number of drinks within a 6-hour period for intervention relative to control group of 0.81 (95% CI, 0.67–0.97). Intervention group decreased two drinks, control only one • Odds ratio for drinking >4 drinks in a day at 6 months for intervention relative to control group of 0.41 (95% CI, 0.17–0.98) • Significance lost at 12-month follow-up |
Notes: AUDIT-1, -2, or -3 refers to the first, second, or third AUDIT question; AUDIT-C is the short version of AUDIT.
Abbreviations: AOR, adjusted odds ratio; AUDIT, Alcohol Use Disorders Identification Test; BAC, blood alcohol content; BI, brief intervention; BMI, brief motivational interviewing; BNI, brief negotiated interview; CAGE, Cut down, Annoyed, Guilty, Eye-opener; CDT, carbohydrate deficient transferrin; CI, confidence interval; DrInC, Drinker Inventory of Consequences; DSM-IV, Diagnostic and statistical manual of mental disorders, fourth edition; FRAMES, Feedback, Responsibility, Advice, Menu, Empathy, Self-efficacy; ED, emergency department; GGT, gamma glutamil transpeptidase; GP, general practitioner; IBC-R, Injury behavior check-list; MCV, mean cell volume; MET, motivational enhancement therapy; MI, motivational interviewing; NDRL, nondirective reflective listening; RAPI, Rutgers alcohol problem index; SC, standard care; TLFB, time line follow back.
Amount and frequency of alcohol consumption
Nine studies reported data regarding the amount of alcohol consumption. Five studies reported it in the form of units of alcohol per week.49,51,52,57,58 Two failed to show statistically significant differences between groups,49,51 while the other three reported significant differences for the intervention groups.52,57,58 The amount of alcohol consumption in the two intervention groups in D’Onofrio et al decreased from 20.4 and 19.8 to 13.0 and 14.3, respectively, while that in the control group ranged from 20.9 to 17.6 (P=0.045).52 The reductions in Monti et al for the intervention and the control group were 13.07–6.10 and 10.77–8.83, respectively (P<0.01 in the treatment × time interaction).57 Noknoy et al reported decreases from 13.27 to 4.72 and from 10.55 to 11.24 for the intervention and control group, respectively (P=0.04).58 Maisto et al reported significant decreases for brief interventions but not for MI in the number of drinks in the past month.55
Two studies reported on the amount of grams of alcohol per week,46,47 with no significant differences between groups at study end. These two studies also reported on the number of drinks per drinking day, again failing to reach any significant difference between groups. Noknoy et al also reported on this outcome, finding a statistically significant difference at 6 months follow-up favoring the PCC group (2.26 vs 4.02; P=0.018).58
Finally, five studies reported on the number of days drinking. Three of them failed to find any significant difference,46,47,55 whereas Monti et al reported a significant difference in the time × treatment interaction favoring the PCC group (4.52 vs 6.54 in the last month; P<0.001).57 Sellman et al reported no difference regarding the percentage of abstinent patients.59
Hazardous and heavy drinking
Ten studies reported outcomes related to heavy or hazardous drinking. Curry et al reported a 19% difference between groups in the proportion of patients reporting any at risk drinking pattern, favoring the PCC group (42% vs 61%; P=0.003).51 Allen et al failed to show any significant difference on the same outcome.48 Sellman et al reported a significant decrease in the percentage of heavy drinking days in the past 6 months favoring PCC (42.9% vs 65%).59
Sommers et al and Beich et al reported on the relative risk of heavy drinking between groups. Although differences favored the PCC group, they were not statistically significant at study end.49,60
Three studies reported on the number of risky drinking days in the last month. Brown et al did not find any significant difference,50 whereas the other two reported statistically significant differences favoring the PCC groups. D’Onofrio et al reported a decrease from 7.5 and 7.2 to 4.7 and 5.1 versus a decrease from 7.2 to 5.8 (P=0.03).52 Monti et al reported a statistically significant difference in the treatment × time interaction (4.52 versus 6.54; P<0.001).57 Longabaugh et al found no significant difference in the number of heavy drinking days between groups.54
Noknoy et al reported on the number of binge episodes in the last week, with no significant differences between groups,58 and Sommers et al reported on the maximum units of alcohol in a 6-hour period, with no significant differences between groups at study end.60
Alcohol scores
Two studies reported no significant changes in AUDIT or AUDIT-C scores between groups.53,56 Hermansson et al reported a significant decrease for the whole sample in the AUDIT score.53
PCC – active comparator
Three studies were included in this subgroup (Table 3).61–63 Patient-centered counseling strategies, mainly through MI, where compared against CBT and 12 steps facilitation in one study,61 against CBT in another,63 and against social behavioral network therapy in the other.62 Taken together, data from these studies suggest that all these counseling strategies are effective in the treatment of alcohol use disorders, with no significant differences between any of them.
Table 3.
Patient-centered counseling – active comparator
| Study | Intervention | Patients | Follow-up times | Outcomes reported | Key findings |
|---|---|---|---|---|---|
| Project MATCH61 1997, USA | 12 weeks therapy: 1. MET 2. CBT 3. 12-step facilitation |
• N=648 • Outpatients with a current DSM III-R diagnosis of alcohol abuse or dependence; active drinking for the 3 months before enrollment |
15 months | • % days abstinent in last month • Drinks per drinking day in last month |
• No consistent and clinically meaningful differences in efficacy between treatments • In the outpatient arm, there was a tendency for CBT patients to have a slightly higher rate of drinking days over time than the other two groups |
| Shakeshaft et al63 2002, Australia | 1. One or multiple BI sessions (<90 minutes) based on FRAMES 2. Face-to-face counseling for six consecutive weekly sessions, organized around specific themes |
• N=295 • Patients attending a free, community-based, drug and alcohol counseling service • At least one of four inclusion criteria needed: 1. To be attending for their own concerns with alcohol 2. Consumption of ≥280 g (males) or 140 g (females) of alcohol per week 3. Consumption of ≥60 g of alcohol on one occasion at least weekly or in the 7 days prior to completing the questionnaire 4. AUDIT score ≥8 |
6 months | • Weekly alcohol consumption • Binge alcohol consumption • Alcohol-related problems (APQ) • AUDIT score • Cost-effectiveness |
• Intervention group reduced weekly consumption from 32.7 to 24.9 drinks (P<0.01); and binge occasions last 30 days 20.9 to 15.4 (P<0.01) • No statistical significance when compared to control group on both outcomes (P=0.78 and P=0.98) |
| UKATT Research Team62 2005, UK | 1. MET 3×50 minutes sessions over 8–12 weeks 2. SBNT: 8×50-minute sessions over 8–12 weeks |
• N=742 • Patients who would normally receive an offer of treatment from British treatment sites for alcohol problems |
12 months | • Number of drinks per drinking day • Percent of days abstinent • Alcohol dependence, measured by the Leeds dependence questionnaire • Alcohol-related problems over the past 3 months, measured by the alcohol problems questionnaire and GGT levels |
• Improvement for the whole sample in all drinking outcomes at 3 and 12 months (significance not reported) • No significant difference in percentage of days abstinent (MET 45.4% vs SBNT 46.6; difference −1.19; 95% CI [−4.50 to 6.88]) • No significant difference in number of drinks per drinking day (MET 18.7 vs SBNT 19.8; difference 1.14; 95% CI [−0.95 to 3.22]) |
Abbreviations: BI, brief intervention; BNI, brief negotiated interview; CBT, cognitive behavioral therapy; CI, confidence interval; DSM-III, Diagnostic and statistical manual of mental disorders, third edition; FRAMES, Feedback, Responsibility, Advice, Menu, Empathy, Self-efficacy; GGT, gamma glutamil transpeptidase; MET, motivational enhancement therapy; SBNT, social behavioral network therapy; UKATT, UK Alcohol Treatment Trial.
Amount and frequency of alcohol consumption
The three studies failed to report a significant difference between groups while measuring outcomes related to alcohol consumption. Two reported on number of drinks per drinking day, with no significant differences between groups, although globally the whole study sample improved significantly (in the UK Alcohol Treatment Trial study, eg, the reduction was from 26.8 to 19.2).61,62 The same two studies reported also on percentage of days abstinent, with the same general improvement, with no group significant differences (again, in the UK Alcohol Treatment Trial study, it changed from 29.5% to 46.0% for the whole sample). The study by Shakeshaft et al reported a significant within PCC group decrease in the number of drinks per week (from 32.7 to 24.9; P<0.01), with no statistically significant differences when compared to CBT.63
Hazardous and heavy drinking
Two studies reported on measures related to heavy drinking.61,63 Project MATCH (Matching Alcoholism Treatments to Client Heterogeneity) reported a statistical superiority of 12 steps facilitation against CBT and motivational enhancement therapy in the survival analysis in relapse to heavy drinking (defined as three consecutive days of >5 drinks per day for men and >3 drinks per day for women), where 53% of 12 steps facilitation patients did not relapse, and 49% and 48% in the motivational enhancement therapy and CBT groups did not relapse.61 Shakeshaft et al reported a significant within PCC group decrease in heavy drinking episodes in the last 30 days (from 20.9 to 15.4; P<0.01). Again, no statistically significant differences were noted between groups.63
Patient-centered pharmacological interventions
Five studies were classified in this group (Table 4).64–68 They all shared the same strategy: targeted or as-needed medication. Three used nalmefene as the study medication, and two used naltrexone. Overall, data clearly suggest a benefit of the PCC intervention, when compared with the control groups, in terms of reduction in alcohol consumption and heavy drinking, with most of the differences reaching statistical significance.
Table 4.
Patient-centered pharmacological interventions
| Study | Intervention | Patients | Follow-up times | Outcomes reported | Key findings |
|---|---|---|---|---|---|
| Gual et al65 2013, Southern urope | 1. Nalmefene: as-needed dosing of nalmefene 18 mg, 1–2 h before anticipated drinking. Patients also received BRENDA 2. Control: placebo and BRENDA |
• N=718 • Adults with a primary diagnosis of alcohol dependence |
24 weeks | • Heavy drinking days • Total alcohol consumption (g/day) |
Mean number of heavy drinking days per month: • The nalmefene group decreased from 19.8 (6.8) to 6.6 (8.9), the placebo group decreased from 18.3 (7.0) to 7.5 (9.2). • The group difference at month 6 was −1.7 days/month favoring nalmefene (95% CI −3.1 to −0.4; P=0.012) Total alcohol consumption in grams per day: • The nalmefene group decreased from 93 (46) to 30 (36), the placebo group decreased from 89 (48) to 33 (38) • The group difference at month 6 was −5.0 g/day favoring nalmefene (95% CI −10.6 to 0.7; P=0.08) |
| Heinala et al67 2001, Finland | 1. Coping + naltrexone: coping group therapy at weeks 1, 2, 5, and 12 +50 mg of daily naltrexone for 12 weeks, then targeted naltrexone for 20 weeks 2. Coping + placebo 3. Supportive + naltrexone: supportive therapy at weeks 1, 2, 5, and 12 (emphasis on abstinence) +50 mg of daily naltrexone for 12 weeks, then targeted naltrexone for 20 weeks 4. Supportive + placebo |
• N=121 • DSM IV diagnosis of alcohol dependence • Average consumption in the last 30 days of ≥5 drinks per day |
32 weeks | • Relapse to heavy drinking (defined as ≥5 drinks on one occasion, having ≥5 drinking occasions in 1 week, or arriving at a visit intoxicated) |
Grams per week (with SD) in the last 8 weeks of the study: • Coping + naltrexone 231 (40); coping + placebo 354 (62); supportive + naltrexone 357 (81); supportive-placebo 326 (80), coping + naltrexone significantly better than the rest of the groups, P=0.05) • Differences not significant prior to this point, with coping + naltrexone tending to do better • Overall comparison in survival analysis significant, with coping + naltrexone doing better in preventing relapses to heavy drinking. In the coping group, naltrexone was better than placebo (P=0.008). Naltrexone did better in the coping group compared to the supportive group (P=0.041). |
| Karhuvaara et al66 2007, Finland | 1. Nalmefene: as-needed dosing patients also received limited elements of BRENDA 2. Control: placebo and limited elements of BRENDA |
• N=403 • ≥18 heavy drinking days and no more than 14 consecutive abstinence days during the last 12 weeks • Sober at inclusion visit and with no withdrawal symptoms |
28 weeks | • Decrease in heavy drinking days per month (≥5 drinks/day in man, ≥4 in women) |
Heavy drinking days per month: • The nalmefene group decreased from 15.5 (6.9) in the first month to 8.8 (7.3) in the last month; the control group reduced from 16.2 (6.9) to 10.6 (8.3). Differences between groups statistically significant (P=0.0065) Heavy drinking days during the study period of 28 weeks: • The nalmefene group had 58.2, the control group had 86.1 (risk of heavy drinking 32.4% smaller in the nalmefene group [95% CI 14.2%–46.8%; P=0.0013]) Drinks per week: • The nalmefene group reduced from 43.2 (22.4) in the first month to 23.2 (20.8) in the last month; the control group reduced from 45.0 (23.3) to 28.5 (23.7). Differences between groups statistically significant (P=0.0018) Drinks per drinking day: • The nalmefene group reduced from 9.6 (4.8) in the first month to 6.3 (3.9) in the last month; the control group reduced from 9.5 (4.0) to 7.3 (3.7). Differences between groups statistically significant (P=0.0134) |
| Kranzler et al68 2009, USA | 1. Targeted naltrexone 2. Targeted placebo 3. Daily naltrexone 4. Daily placebo |
• N=163 • Average weekly alcohol consumption ≥24 standard drinks for men and ≥18 for women |
12 weeks | • Number of standard drinks per day |
Mean number of drinks consumed per day: • Targeted naltrexone group drank 16.5% less than other groups. Differences not statistically significant. Exact numbers not reported • Among men, at week 12 the targeted naltrexone group drank less than the other groups (P=0.027; exact numbers not reported) |
| Mann et al64 2013, Northern Europe | 1. Nalmefene: as-needed dosing of nalmefene 18 mg, 1–2 hours before anticipated drinking. Patients also received BRENDA 2. Control: placebo and BRENDA |
• N=598 • Adults with a primary diagnosis of alcohol dependence |
24 weeks | • Heavy drinking days • Total alcohol consumption (g/day) |
Mean number of heavy drinking days per month: • Nalmefene group decreased from 19 to 8, placebo group decreased from 20 to 11. Adjusted change from baseline to month 6 was −11.2 (0.6) for nalmefene, −8.9 (0.6) for placebo. Group difference of −2.3 days (95% CI −3.8 to –0.8) statistically significant (P=0.0021) Total alcohol consumption in grams per day: • The nalmefene group decreased from 84 to 33, the placebo group decreased from 85 to 45 • Adjusted change from baseline to month 6 was −50.7 (2.4) for nalmefene, −39.7 (2.2) for placebo. Group difference of −11.0 g/day (95% CI −16.8 to −5.1) statistically significant (P=0.0003) |
Abbreviations: BRENDA, Biopsychosocial evaluation, Report to the patient on assessment, Empathic understanding of the patient’s situation, Needs collaboratively identified by the patient and treatment provider, Direct advice to the patient on how to meet those needs, Assess reaction of the patient to advice and adjust as necessary for best care; DSM-IV, Diagnostic and statistical manual of mental disorders, fourth edition; SD, standard deviation.
Amount and frequency of alcohol consumption
Four of the five studies in this group reported statistically significant reductions in the intervention group when compared with the control. Mann et al found a significant reduction in grams per day for the nalmefene group (−50.7 vs −39.7).64 Heinala et al reported a significant difference in grams/week when comparing the coping-naltrexone group with the rest combined (231 vs 354, 357, and 326; P=0.05).67 Karhuvaara et al reported statistically significant differences in both drinks per week and drinks per drinking day favoring the targeted nalmefene group (drinks per week in the last month: 23.2 vs 28.5, P=0.0018; drinks per drinking day in the last month: 6.3 vs 7.3, P=0.0134).66 Kranzler et al reported the targeted naltrexone group drinking 16.5% less than the other groups, although differences were not statistically significant.68 However, among men at week 12, the differences did reach significance (P=0.027). The remaining study by Gual et al reported a baseline to 6-month difference of −5.0 g/day favoring the PCC group, but it did not reach statistical significance (95% CI −10.6 to 0.7; P=0.08).65
Hazardous and heavy drinking
The four studies assessing this outcome (Heinala et al, 2001, Karhuvaara et al, 2007, Mann et al, 2013, and Gual et al, 2013) reported statistically significant differences in heavy drinking favoring the PCC groups.64–67 The 6-month differences in heavy drinking days per month reported by Gual et al and Mann et al were −1.7 days/month (95% CI −3.1 to 0.4; P=0.012) and −2.3 days/month (P=0.0021), respectively, both favoring the nalmefene group.64,65 In the study by Heinala et al, the targeted naltrexone group combined with nonabstinenceoriented group therapy did better than the others in survival analysis for preventing relapse to heavy drinking, reaching statistical significance (exact numbers not reported).67 Karhuvaara et al reported a statistically significant decrease in heavy drinking days per month favoring the targeted nalmefene group (from 15.5 to 8.8 vs 16.2 to 10.6; P=0.0065).66
Discussion
In this paper, we systematically reviewed the efficacy of interventions based on a PCC health care approach for the management of alcohol use disorders. When reviewing the studies identified by our search, it was realized that all PCC trials selected for the review could easily be categorized into two groups: psychosocial interventions and pharmacological supported interventions, and hence, this grouping was adopted. Psychosocial interventions could then be classified further into single sessions of PCC and multiple sessions of PCC.
Regarding psychosocial interventions, our results are in line with previous systematic reviews conducted specifically for MI, which, as mentioned earlier, was the cornerstone of PCC psychosocial interventions found in this review.28,71 Findings within the categories of trials on PCC interventions based on MI appeared mixed. If differences in alcohol consumption emerged between intervention and control groups, they were usually not significant if participants attended one counseling session only. The proportion of studies suggesting that PCC is more effective than control interventions increased if participants took part in several counseling sessions; that is, the number of counseling sessions seems to moderate the effectiveness of PCC. This is in line with the finding of a previous systematic review which concluded that PCC interventions of more than one session work by first increasing the patients’ readiness to change during the first session, and then effecting reduction in alcohol consumption in the follow-up sessions.28
Regarding pharmacologically supported PCC interventions, based on the as needed approach, an effect seemed to emerge from our review. However, the effect cannot be attributed fully to pharmacological elements, since studies in this group also had a psychosocial component, and therefore interactions between the two should be taken into account when interpreting the findings. Considering the fact that only a very small percentage of patients with alcohol use disorders (<10%) receive treatment,72,73 this finding has significant implications for current health care. All the studies of pharmacological interventions were included in this review on the basis of their “as needed” use. This paradigm has been controversial in addiction medicine for many years, with many believing that medications for alcohol use disorders need to be taken on a supervised, strict basis. In this respect, a recent analysis of clinical trial data for nalmefene concluded that people with alcohol dependence are able to adhere to an as needed regimen.68 The data indicate that medication intake varies according to drinking patterns with some patients taking the medication daily and others taking medication at tailored intervals. In their recent editorial, Bradley and Kivlahan2 suggested that pharmacological interventions might help bring the concepts of PCC into the alcohol field. By understanding that effective treatments are available, health care professionals are better able to offer people with alcohol use disorders various evidence-based options, including medications and psychosocial support, to achieve recovery.
A key difficulty in conducting this review was the definition and operationalization of PCC as a concept that translates into alcohol use disorders. PCC is not a binary concept (present or absent), and the potential alcohol interventions had to be judged along a continuum of PCC interventions. Despite this fact, we had to simplify and finally dichotomize interventions as PCC or not PCC. For example, the efficacy of pharmacologically supported interventions was considered a key component of this research. Although medications are not PCC (they are just chemical compounds), as needed use allows them to be prescribed in accordance with the principles of PCC. Conversely, computerized interventions were not included in this review. Although one might argue that they can also be considered PCC depending on how they are structured and conducted, they are ultimately based on a predefined number of algorithms and options, thus making it difficult to truly individualize the intervention according to the needs of each patient.
The considerable heterogeneity of the studies included made it impractical to perform any meta-analyses of the data, and so we were limited to narrative descriptions. As discussed by the authors of other systematic reviews of alcohol interventions, this will remain a barrier until consensus is reached in the preferred methods for measuring alcohol consumption.28,71,74 It is therefore important to note that the European Medicines Agency now recommends total alcohol consumption and the number of heavy drinking days as suitable outcomes for studies of alcohol reduction as well as the continued abstinence rate for studies where abstinence is the stated goal. It further suggests that total alcohol consumption and heavy drinking days can also be used to assess the impact of an abstinence-based intervention when patients have a relapse (or lapse or slip).17 It is hoped that such guidance will aid the future standardization of studies.
Other potential limitations of our review include the broad definition of alcohol use disorders (hazardous or harmful drinking, alcohol dependence, or any other alcohol use disorder) and the consequent inclusion of patients with and without a diagnosis of alcohol dependence. It may be that patients with dependence require longer periods of interventions than those without dependence, and this factor might have contributed to some of the findings, especially for PCC psychosocial interventions. To try and homogenize the review, we did not include studies of patients with psychiatric comorbidities or with relevant and differential characteristics (eg, mandated or incarcerated patients and pregnant women). Although necessary for the purposes of this review, application of these exclusion criteria resulted in the exclusion of a number of potentially interesting studies. For example, one large long-term study with nalmefene75 was excluded because it included patients taking antipsychotics or antidepressants for current psychiatric comorbidities. Also, it is important to remark that an important share of alcohol patients do suffer from negative emotional and affective states that might indeed be produced by alcohol itself. Therefore, this exclusion might have oversimplified a complex reality such as the one of alcohol use disorders. Finally, the external validity of our findings is handicapped by the fact that male patients were more than twice as frequent as females in all the categories.
Conclusion
The limitations of the review, as well as the mixed results found in some of the categories investigated, prevent firm conclusions to be drawn. Single-session studies did not appear to show a clear benefit, multiple-session studies showed mixed results, and active comparator studies did not report significant differences while measuring outcomes related to alcohol consumption. Although pharmacological studies were found the most robustly effective, the shorter follow-up periods and the concomitant presence of psychosocial components in the studies prevent a full and clear attribution to be done. However, since we believe that PCC is increasingly accepted as a central tenet of high-quality health care, and some of the results of this review suggest PCC could indeed be an appropriate strategy for alcohol use disorders, there is an urgent need for additional research evidence on the effectiveness of PCC-based alcohol interventions.
Acknowledgments
H Lundbeck A/S funded this systematic review. The authors were solely responsible for the collection, analysis, and interpretation of data and in the writing of the report. There was no payment for the writing of the report, and the decision to submit the report for publication also rested with the authors. The authors thank Anita Chadha-Patel (ACP Clinical Communications Ltd. funded by H Lundbeck A/S) for editorial assistance (English language editing and referencing).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Institute of Medicine Committee on Quality of Health Care in America . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 2.Bradley KA, Kivlahan DR. Bringing patient-centered care to patients with alcohol use disorders. JAMA. 2014;311:1861–1862. doi: 10.1001/jama.2014.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70(4):351–379. doi: 10.1177/1077558712465774. [DOI] [PubMed] [Google Scholar]
- 4.Lusk JM, Fater K. A concept analysis of patient-centered care. Nurs Forum. 2013;48(2):89–98. doi: 10.1111/nuf.12019. [DOI] [PubMed] [Google Scholar]
- 5.Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357:1834–1840. doi: 10.1056/NEJMcp074045. [DOI] [PubMed] [Google Scholar]
- 6.Roberts LW. Informed consent and the capacity for voluntarism. Am J Psychiatry. 2002;159:705–712. doi: 10.1176/appi.ajp.159.5.705. [DOI] [PubMed] [Google Scholar]
- 7.Coverdale JH, Chervenak FA, McCullough LB, Bayer T. Ethically justified clinically comprehensive guidelines for the management of the depressed pregnant patient. Am J Obstet Gynecol. 1996;174:169–173. doi: 10.1016/s0002-9378(96)70390-3. [DOI] [PubMed] [Google Scholar]
- 8.McCullough LB, Coverdale JH, Chervenak FA. Ethical challenges of decision making with pregnant patients who have schizophrenia. Am J Obstet Gynecol. 2002;187:696–702. doi: 10.1067/mob.2002.125767. [DOI] [PubMed] [Google Scholar]
- 9.Goossensen A, Zijlstra P, Koopmanschap M. Measuring shared decision making processes in psychiatry: skills versus patient satisfaction. Patient Educ Couns. 2007;67:50–56. doi: 10.1016/j.pec.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Goss C, Moretti F, Mazzi MA, Del Piccolo L, Rimondini M, Zimmermann C. Involving patients in decisions during psychiatric consultations. Br J Psychiatry. 2008;193:416–421. doi: 10.1192/bjp.bp.107.048728. [DOI] [PubMed] [Google Scholar]
- 11.Cox K, Stevenson F, Britten N, Dundar Y. A systematic review of communication between patients and health care professionals about medicine-taking and prescribing King’s College London, GKT Concordance Unit, Guys’ King’s and St Thomas’ School of Medicine. 2003. [Accessed December 1, 2015]. Available from: www.medicines-partnership.org.
- 12.Swanson KA, Bastani R, Rubenstein LV, Meredith LS, Ford DE. Effect of mental health care and shared decision making on patient satisfaction in a community sample of patients with depression. Med Care Res Rev. 2007;64:416–430. doi: 10.1177/1077558707299479. [DOI] [PubMed] [Google Scholar]
- 13.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson P, Braddick F, Reynolds J, Gual A. Alcohol policy in Europe: Evidence from AMPHORA. 2nd ed. The AMPHORA project. 2013. [Accessed December 1, 2015]. Available from: http://amphoraproject.net/view.php?id_cont=45.
- 15.Anderson P, Wojnar M, Jakubczyk A, et al. Managing alcohol problems in general practice in Europe: results from the European ODHIN survey of general practitioners. Alcohol Alcohol. 2014;49:531–539. doi: 10.1093/alcalc/agu043. [DOI] [PubMed] [Google Scholar]
- 16.Rehm J, Anderson P, Barry J, et al. Prevalence of and potential influencing factors for alcohol dependence in Europe. Eur Addict Res. 2015;21:6–18. doi: 10.1159/000365284. [DOI] [PubMed] [Google Scholar]
- 17.EMA Guideline on the development of medicinal products for the treatment of alcohol dependence. EMA/CHMP/EWP/20097/2008 (previously EMEA/CHMP/EWP/20097/2008) 2010. [Accessed December 1, 2015]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/03/WC500074898.pdf.
- 18.Gastfriend DR, Garbutt JC, Pettinati HM, Forman RF. Reduction in heavy drinking as a treatment outcome in alcohol dependence. J Subst Abuse Treat. 2007;33:71–80. doi: 10.1016/j.jsat.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Craig M, Annis HM, Bornet AR, MacDonald KR. Random assignment to abstinence and controlled drinking: evaluation of a cognitive-behavioral program for problem drinkers. J Consult Clin Psychol. 1984;52:390–403. doi: 10.1037//0022-006x.52.3.390. [DOI] [PubMed] [Google Scholar]
- 20.Ambrogne JA. Reduced-risk drinking as a treatment goal: what clinicians need to know. J Subst Abuse Treat. 2002;22:45–53. doi: 10.1016/s0740-5472(01)00210-0. [DOI] [PubMed] [Google Scholar]
- 21.Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luquiens A, Reynaud M, Aubin HJ. Is controlled drinking an acceptable goal in the treatment of alcohol dependence? A survey of French alcohol specialists. Alcohol Alcohol. 2011;46:586–591. doi: 10.1093/alcalc/agr083. [DOI] [PubMed] [Google Scholar]
- 23.Al-Otaiba Z, Worden BL, McCrady BS, Epstein EE. Accounting for self-selected drinking goals in the assessment of treatment outcome. Psychol Addict Behav. 2008;22:439–443. doi: 10.1037/0893-164X.22.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodgins DC, Leigh G, Milne R, Gerrish R. Drinking goal selection in behavioral self-management treatment of chronic alcoholics. Addict Behav. 1997;22:247–255. doi: 10.1016/s0306-4603(96)00013-5. [DOI] [PubMed] [Google Scholar]
- 25.Barry MJ, Edgman-Levitan S. Shared decision making – pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 26.Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0.2011. 2011. [Google Scholar]
- 27.McMillan SS, Kendall E, Sav A, et al. Patient-centered approaches to health care: a systematic review of randomized controlled trials. Med Care Res Rev. 2013;70:567–596. doi: 10.1177/1077558713496318. [DOI] [PubMed] [Google Scholar]
- 28.Mdege ND, Fayter D, Watson JM, Stirk L, Sowden A, Godfrey C. Interventions for reducing alcohol consumption among general hospital inpatient heavy alcohol users: a systematic review. Drug Alcohol Depend. 2013;131:1–22. doi: 10.1016/j.drugalcdep.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Bazargan-Hejazi S, Bing E, Bazargan M, et al. Evaluation of a brief intervention in an inner-city emergency department. Ann Emerg Med. 2005;46:67–76. doi: 10.1016/j.annemergmed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Bischof G, Grothues JM, Reinhardt S, Meyer C, John U, Rumpf HJ. Evaluation of a telephone-based stepped care intervention for alcohol-related disorders: a randomized controlled trial. Drug Alcohol Depend. 2008;93:244–251. doi: 10.1016/j.drugalcdep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Brown TG, Dongier M, Ouimet MC, et al. Brief motivational interviewing for DWI recidivists who abuse alcohol and are not participating in DWI intervention: a randomized controlled trial. Alcohol Clin Exp Res. 2010;34:292–301. doi: 10.1111/j.1530-0277.2009.01092.x. [DOI] [PubMed] [Google Scholar]
- 32.Carey KB, Carey MP, Maisto SA, Henson JM. Brief motivational interventions for heavy college drinkers: a randomized controlled trial. J Consult Clin Psychol. 2006;74:943–954. doi: 10.1037/0022-006X.74.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang G, Fisher ND, Hornstein MD, et al. Brief intervention for women with risky drinking and medical diagnoses: a randomized controlled trial. J Subst Abuse Treat. 2011;41:105–114. doi: 10.1016/j.jsat.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cherpitel CJ, Korcha RA, Moskalewicz J, Swiatkiewicz G, Ye Y, Bond J. Screening, brief intervention, and referral to treatment (SBIRT): 12-month outcomes of a randomized controlled clinical trial in a Polish emergency department. Alcohol Clin Exp Res. 2010;34:1922–1928. doi: 10.1111/j.1530-0277.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daeppen J-B, Gaume J, Bady P, et al. Brief alcohol intervention and alcohol assessment do not influence alcohol use in injured patients treated in the emergency department: a randomized controlled clinical trial. Addiction. 2007;102:1224–1233. doi: 10.1111/j.1360-0443.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- 36.Daeppen JB, Bertholet N, Gaume J, Fortini C, Faouzi M, Gmel G. Efficacy of brief motivational intervention in reducing binge drinking in young men: a randomized controlled trial. Drug Alcohol Depend. 2011;113:69–75. doi: 10.1016/j.drugalcdep.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 37.D’Onofrio G, Pantalon MV, Degutis LC, et al. Brief intervention for hazardous and harmful drinkers in the emergency department. Ann Emerg Med. 2008;51:742–750.e742. doi: 10.1016/j.annemergmed.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emmen MJ, Schippers GM, Wollersheim H, Bleijenberg G. Adding psychologist’s intervention to physicians’ advice to problem drinkers in the outpatient clinic. Alcohol Alcohol. 2005;40:219–226. doi: 10.1093/alcalc/agh137. [DOI] [PubMed] [Google Scholar]
- 39.Gaume J, Gmel G, Faouzi M, Bertholet N, Daeppen J-B. Is brief motivational intervention effective in reducing alcohol use among young men voluntarily receiving it? A randomized controlled trial. Alcohol Clin Exp Res. 2011;35:1822–1830. doi: 10.1111/j.1530-0277.2011.01526.x. [DOI] [PubMed] [Google Scholar]
- 40.Hansen AB, Becker U, Nielsen AS, Gronbaek M, Tolstrup JS. Brief alcohol intervention by newly trained workers versus leaflets: comparison of effect in older heavy drinkers identified in a population health examination survey: a randomized controlled trial. Alcohol Alcohol. 2012;47:25–32. doi: 10.1093/alcalc/agr140. [DOI] [PubMed] [Google Scholar]
- 41.Lee CS, López SR, Hernández L, et al. A cultural adaptation of motivational interviewing to address heavy drinking among Hispanics. Cultur Divers Ethnic Minor Psychol. 2011;17(3):317–324. doi: 10.1037/a0024035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy JG, Duchnick JJ, Vuchinich RE, et al. Relative efficacy of a brief motivational intervention for college student drinkers. Psychol Addict Behav. 2001;15:373–379. doi: 10.1037//0893-164x.15.4.373. [DOI] [PubMed] [Google Scholar]
- 43.Murphy JG, Benson TA, Vuchinich RE, et al. A comparison of personalized feedback for college student drinkers delivered with and without a motivational interview. J Stud Alcohol. 2004;65:200–203. doi: 10.15288/jsa.2004.65.200. [DOI] [PubMed] [Google Scholar]
- 44.Senft RA, Polen MR, Freeborn DK, Hollis JF. Brief intervention in a primary care setting for hazardous drinkers. Am J Prev Med. 1997;13:464–470. [PubMed] [Google Scholar]
- 45.Soderstrom CA, DiClemente CC, Dischinger PC, et al. A controlled trial of brief intervention versus brief advice for at-risk drinking trauma center patients. J Trauma. 2007;62:1102. doi: 10.1097/TA.0b013e31804bdb26. [DOI] [PubMed] [Google Scholar]
- 46.Aalto M, Saksanen R, Laine P, et al. Brief intervention for female heavy drinkers in routine general practice: a 3-year randomized, controlled study. Alcohol Clin Exp Res. 2000;24:1680–1686. [PubMed] [Google Scholar]
- 47.Aalto M, Seppa K, Mattila P, et al. Brief intervention for male heavy drinkers in routine general practice: a three-year randomized controlled study. Alcohol Alcohol. 2001;36:224–230. doi: 10.1093/alcalc/36.3.224. [DOI] [PubMed] [Google Scholar]
- 48.Allen E, Polikina O, Saburova L, et al. The efficacy of a brief intervention in reducing hazardous drinking in working age men in Russia: the HIM (Health for Izhevsk men) individually randomised parallel group exploratory trial. Trials. 2011;12:238. doi: 10.1186/1745-6215-12-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beich A, Gannik D, Saelan H, Thorsen T. Screening and brief intervention targeting risky drinkers in Danish general practice – a pragmatic controlled trial. Alcohol Alcohol. 2007;42:593–603. doi: 10.1093/alcalc/agm063. [DOI] [PubMed] [Google Scholar]
- 50.Brown RL, Saunders LA, Bobula JA, Mundt MP, Koch PE. Randomized-controlled trial of a telephone and mail intervention for alcohol use disorders: three-month drinking outcomes. Alcohol Clin Exp Res. 2007;31:1372–1379. doi: 10.1111/j.1530-0277.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 51.Curry SJ, Ludman EJ, Grothaus LC, Donovan D, Kim E. A randomized trial of a brief primary-care-based intervention for reducing at-risk drinking practices. Health Psychol. 2003;22:156–165. [PubMed] [Google Scholar]
- 52.D’Onofrio G, Fiellin DA, Pantalon MV, et al. A brief intervention reduces hazardous and harmful drinking in emergency department patients. Ann Emerg Med. 2012;60:181–192. doi: 10.1016/j.annemergmed.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hermansson U, Helander A, Brandt L, Huss A, Rönnberg S. Screening and brief intervention for risky alcohol consumption in the workplace: results of a 1-year randomized controlled study. Alcohol Alcohol. 2010;45:252–257. doi: 10.1093/alcalc/agq021. [DOI] [PubMed] [Google Scholar]
- 54.Longabaugh R, Woolard RE, Nirenberg TD, et al. Evaluating the effects of a brief motivational intervention for injured drinkers in the emergency department. J Stud Alcohol. 2001;62:806–816. doi: 10.15288/jsa.2001.62.806. [DOI] [PubMed] [Google Scholar]
- 55.Maisto SA, Conigliaro J, McNeil M, Kraemer K, Conigliaro RL, Kelley ME. Effects of two types of brief intervention and readiness to change on alcohol use in hazardous drinkers. J Stud Alcohol. 2001;62:605–614. doi: 10.15288/jsa.2001.62.605. [DOI] [PubMed] [Google Scholar]
- 56.Mello MJ, Baird J, Nirenberg TD, Lee C, Woolard R, Longabaugh R. DIAL: a randomised trial of a telephone brief intervention for alcohol. Inj Prev. 2013;19:44–48. doi: 10.1136/injuryprev-2012-040334. [DOI] [PubMed] [Google Scholar]
- 57.Monti PM, Barnett NP, Colby SM, et al. Motivational interviewing versus feedback only in emergency care for young adult problem drinking. Addiction. 2007;102:1234–1243. doi: 10.1111/j.1360-0443.2007.01878.x. [DOI] [PubMed] [Google Scholar]
- 58.Noknoy S, Rangsin R, Saengcharnchai P, Tantibhaedhyangkul U, McCambridge J. RCT of effectiveness of motivational enhancement therapy delivered by nurses for hazardous drinkers in primary care units in Thailand. Alcohol Alcohol. 2010;45:263–270. doi: 10.1093/alcalc/agq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sellman JD, Sullivan PF, Dore GM, Adamson SJ, MacEwan I. A randomized controlled trial of motivational enhancement therapy (MET) for mild to moderate alcohol dependence. J Stud Alcohol. 2001;62:389–396. doi: 10.15288/jsa.2001.62.389. [DOI] [PubMed] [Google Scholar]
- 60.Sommers MS, Lyons MS, Fargo JD, et al. Emergency department-based brief intervention to reduce risky driving and hazardous/harmful drinking in young adults: a randomized controlled trial. Alcohol Clin Exp Res. 2013;37:1753–1762. doi: 10.1111/acer.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Project MATCH secondary a priori hypotheses. Project MATCH Research Group. Addiction. 1997;92:1671–1698. [PubMed] [Google Scholar]
- 62.Team UR Effectiveness of treatment for alcohol problems: findings of the randomised UK alcohol treatment trial (UKATT) BMJ. 2005;331:541. doi: 10.1136/bmj.331.7516.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shakeshaft AP, Bowman JA, Burrows S, Doran CM, Sanson-Fisher RW. Community-based alcohol counselling: a randomized clinical trial. Addiction. 2002;97:1449–1463. doi: 10.1046/j.1360-0443.2002.00199.x. [DOI] [PubMed] [Google Scholar]
- 64.Mann K, Bladstrom A, Torup L, Gual A, van den Brink W. Extending the treatment options in alcohol dependence: a randomized controlled study of as-needed nalmefene. Biol Psychiatry. 2013;73:706–713. doi: 10.1016/j.biopsych.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 65.Gual A, He Y, Torup L, van den Brink W, Mann K, the ESG A randomised, double-blind, placebo-controlled, efficacy study of nalmefene, as-needed use, in patients with alcohol dependence. Eur Neuropsychopharmacol. 2013;23:1432–1423. doi: 10.1016/j.euroneuro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Karhuvaara S, Simojoki K, Virta A, et al. Targeted nalmefene with simple medical management in the treatment of heavy drinkers: a randomized double-blind placebo-controlled multicenter study. Alcohol Clin Exp Res. 2007;31:1179–1187. doi: 10.1111/j.1530-0277.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- 67.Heinala P, Alho H, Kiianmaa K, Lonnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: a factorial double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2001;21:287–292. doi: 10.1097/00004714-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Kranzler HR, Tennen H, Armeli S, et al. Targeted naltrexone for problem drinkers. J Clin Psychopharmacol. 2009;29:350–357. doi: 10.1097/JCP.0b013e3181ac5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psycoth. 1995;23:325–334. [Google Scholar]
- 70.Sinclair J, Chick J, Sorensen P, Kiefer F, Batel P, Gual A. Can alcohol dependent patients adhere to an ‘as-needed’ medication regimen? Eur Addict Res. 2014;20:209–217. doi: 10.1159/000357865. [DOI] [PubMed] [Google Scholar]
- 71.Foxcroft DR, Coombes L, Wood S, Allen D, Almeida Santimano NM. Motivational interviewing for alcohol misuse in young adults. Cochrane Database Syst Rev. 2014;8:CD007025. doi: 10.1002/14651858.CD007025.pub2. [DOI] [PubMed] [Google Scholar]
- 72.World Health Organization Alcohol in the European Union: Consumption, Harm and Policy Approaches 2012. 2012. [Accessed December 1, 2015]. Available from: http://www.euro.who.int/__data/assets/pdf_file/0003/160680/e96457.pdf.
- 73.Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. Bull World Health Organ. 2004;82:858–866. [PMC free article] [PubMed] [Google Scholar]
- 74.McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011:CD005191. doi: 10.1002/14651858.CD005191.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van den Brink W, Sorensen P, Torup L, Mann K, Gual A, the SSG Long-term efficacy, tolerability and safety of nalmefene as-needed in patients with alcohol dependence: a 1-year, randomised controlled study. J Psychopharmacol. 2014;28:733–744. doi: 10.1177/0269881114527362. [DOI] [PubMed] [Google Scholar]