Abstract
Ozone is a highly reactive environmental toxicant that can react with the double bonds of lipids in pulmonary surfactant. This study was undertaken to investigate the proinflammatory properties of the major lipid-ozone product in pulmonary surfactant, 1-palmitoyl-2-(9’-oxo-nonanoyl)-glycerophosphocholine (16:0/9al-PC), with respect to eicosanoid production. A dose-dependent increase in the formation of 5-lipoxygenase (5-LO) products was observed in murine resident peritoneal macrophages (RPM) and alveolar macrophages (AM) upon treatment with 16:0/9al-PC. In contrast, the production of cyclooxygenase (COX) derived eicosanoids did not change from basal levels in the presence of 16:0/9al-PC. When 16:0/9al-PC and the TLR2 ligand, zymosan, were added to RPM or AM, an enhancement of 5-LO product formation along with a concomitant decrease in COX product formation was observed. Neither intracellular calcium levels or arachidonic acid release were influenced by the addition of 16:0/9al-PC to RPM. Results from mitogen-activated protein kinase (MAPK) inhibitor studies and direct measurement of phosphorylation of MAPKs revealed that 16:0/9al-PC activates the p38 MAPK pathway in RPM, which results in activation of 5-LO. Our results indicate that 16:0/9al-PC has a profound effect on the eicosanoid pathway, which may have implications in inflammatory pulmonary disease states where eicosanoids have been shown to play a role.
TABLE OF CONTENTS GRAPHIC
INTRODUCTION
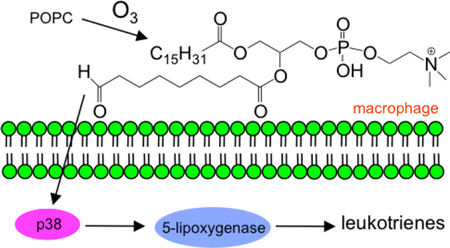
Ozone is an environmental toxicant produced from photochemical reactions between nitrogen oxides and volatile organic compounds. Pulmonary surfactant is the initial barrier that inhaled ozone encounters in the lung and is therefore one of the primary targets of ozone.1 Due to the high reactivity of ozone with double bonds2 and the high concentration of lipids in the pulmonary surfactant,3 it has been suggested that lipid ozonation products are responsible for mediating many of the deleterious effects observed due to ozone exposure. Upon exposure of pulmonary surfactant to ozone, the major lipid-ozone product observed is 1-palmitoyl-2-(9’-oxo-nonanoyl)-glycerophosphocholine (16:0/9al-PC) 4,5 derived from PC lipids containing oleate or palmitoleate at the sn-2 position that are sufficiently present in pulmonary surfactant.6 This oxidized phospholipid product is considered a toxic compound that initiates inflammatory events by stimulating the release of proinflammatory cytokines and chemokines7 and that results in impairment of the innate immune function of macrophages.8
Eicosanoids are proinflammatory lipid mediators that have been implicated to play a role in the pathogenesis of chronic inflammatory pulmonary disease states, such as asthma.9 Specifically cysteinyl leukotrienes, prostaglandin D2, and thromboxane A2 exhibit potent bronchoconstrictive behavior and leukotriene B4 (LTB4) is a potent chemoattractant that plays a critical role in attracting neutrophils and eosinophils into the airways during inflammatory events. Eicosanoid production is initiated by the release of arachidonic acid (AA) from membrane phospholipids by a calcium dependent cytosolic phospholipase A2 (cPLA2α).10 The free AA can be metabolized into bioactive lipid mediators by two predominant pathways. One of these pathways is the cyclooxygenase (COX) pathway that catalyzes the initial transformation of AA into prostaglandin H2 (PGH2) by the constitutive COX-1 or the inducible COX-2.11 This PGH2 intermediate is unstable and quickly converted by specific synthases to prostaglandins (PGE2, PGD2, PGF2α) or thromboxanes (TXA2, TXB2). Another metabolic pathway that can result in bioactive lipid mediators is the leukotriene pathway where AA is converted to leukotriene A4 (LTA4) by the action of 5-lipoxygenase (5-LO) and 5-LO-activating protein (FLAP).12 LTA4 is then converted into either LTB4 by LTA4 hydrolase or leukotriene C4 (LTC4) by LTC4 synthase. The expression of 5-LO is tightly regulated and is only actively expressed in a limited number of cells, specifically neutrophils, eosinophils, monocytes, macrophages, mast cells, and basophils.13 In resting cells, 5-LO is a soluble enzyme. Upon cell stimulation, elevation of intracellular calcium levels can occur that causes translocation of 5-LO to the nuclear membrane, which is a key step in initiating leukotriene synthesis.14 Additionally, in some cells 5-LO can be activated by stimulation of the p38 mitogen-activated protein kinase (MAPK) or extracellular signal-regulated protein kinase 1 and 2 (ERK1/2) pathway, which results in translocation of 5-LO to the nuclear membrane without a concomitant increase in intracellular calcium.15–18
A limited number of studies have explored the correlation between oxidized phospholipids and eicosanoid production. Specifically, in cultured human bronchial epithelial cells 16:0/9al-PC activated phospholipase A2, C, and D and enhanced PGE2 release.7,19 These two studies indicated that the generation and release of eicosanoids observed in lung inflammation and injury may be mediated in part by ozonized lipids, however this premise has not been comprehensively investigated. In the current study we focused on the proinflammatory nature of ozonized lipid products with respect to eicosanoid production. Specifically, the major lipid-ozone product, 16:0/9al-PC, was used for most of these experiments. The in vitro exposure of murine resident peritoneal macrophages (RPM) and alveolar macrophages (AM) to 16:0/9al-PC resulted in the activation of the 5-LO pathway with no effect on COX product formation. Additionally, the effect of 16:0/9al-PC on eicosanoid production was examined in the presence of zymosan, a TLR2 ligand, and an enhancement of 5-LO product formation was observed with a concomitant decrease in COX product formation. We demonstrate through use of MAPK inhibitors and direct measurement of phosphorylation of MAPK pathways that 16:0/9al-PC activates the p38 MAPK pathway and thereby activates 5-LO in RPM. These results reported herein reveal another mechanism by which lipid-ozone products act in a proinflammatory manner in the lung and may have implications in the exacerbation of pulmonary inflammatory diseases where eicosanoids have been demonstrated to have pathophysiological importance.
METHODS
Materials
Female 8–12 week old wild type mice (C57BL/6J) were purchased from Jackson Laboratory (Bar Harbor, ME). The Institutional Animal Care and Use Committee at the University of Colorado Denver approved all animal experiments. All of the lipid standards, including 16:0/9al-PC, 1-palmitoyl-2-(5'-oxo-valeroyl)-sn-glycero-3-phosphocholine (16:0/5al-PC), 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine (16:0/9COOH-PC), 1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine (16:0/5COOH-PC), POPC, and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) were purchased from Avanti Polar Lipids (Alabaster, AL). Deuterated eicosanoid standards (d4-LTB4, d8-5-HETE, d4-PGE2, d4-TXB2, d5-LTC4, and d8-AA) were purchased from Cayman Chemical (Ann Arbor, MI). The MEK/ERK inhibitor (U0126), p38 MAPK inhibitor (SB202190), and c-Jun N-terminal kinase (JNK) inhibitor (SP600125) were from EMD Millipore (Billerica, MA) and solubilized into stock solutions in DMSO at a concentration of 10 mM. The β-actin antibody and phospho-specific antibodies to ERK1/2 and p38 were obtained from Cell Signaling Technology (Danvers, MA). Halt phosphatase and protease inhibitor mixtures and the BCA protein assay kit were from Thermo Scientific (Rockford, IL). All solvents used were HPLC or Optima grade and were purchased from Fischer Scientific (Fair Lawn, NJ). Indo-1 AM was purchased from Invitrogen (Eugene, OR). Other chemicals used in this study were obtained from Sigma Aldrich (St. Louis, MO). The human bronchoalveolar lavage fluid (hBALF) at a concentration of 25 µM phospholipid was a gift from Dr. Dennis Voelker.
Preparation of lipids and stimuli for experiment
A stock zymosan solution was prepared for these experiments as described previously.20 Before each experiment, the stock solution of zymosan was passed through a 25G needle 15–20 times in order to decluster the particles. Ozone was generated by sending oxygen (1 l/min) through a high voltage source and ozonolysis of POPG was carried out by dissolving POPG in HBSS with Ca2+/Mg2+ and bubbling ozone through the solution for 3 min. After ozonolysis, the ozonized POPG (ozPOPG) products were isolated using a modified Bligh Dyer extraction21 and characterized using electrospray mass spectrometry (see below). Prior to the addition to cells, oxidized PC standards, POPC and ozPOPG were taken to dryness under a stream of N2 and resuspended in HBSS with Ca2+/Mg2+. In addition, some experiments were performed where 16:0/9al-PC was directly resuspended in hBALF. The lipid solution was vortexed vigorously and sonicated for 5 minutes in a bath sonicator (Avanti Polar Lipids, Alabaster, AL).
Murine RPM and AM isolation and stimulation
Murine RPM20 and AM22 were isolated as previously described, plated on tissue culture treated 48-well plates at a density of 0.75×106 cells per well (RPM) and 0.5 ×106 cells per well (AM), and incubated at 37°C in a 5% CO2 incubator for 2h. Nonadherent cells were removed after the 2h incubation by rinsing twice with 0.5 ml calcium- and magnesium-free HBSS. To the adherent cells, HBSS with Ca2+/Mg2+, various amounts of oxidized lipid (as indicated in the text and figure legends), and zymosan (25 particles/cell) were added to achieve a final volume of 200 µl per well. These cells were incubated in a 37°C in a 5% CO2 incubator for 1h. In certain cases, the total cells isolated from the peritoneal cavity (macrophages and lymphocytes; ~50/50) were treated in suspension (1×106 cells/ml in HBSS with Ca2+/Mg2+) with oxidized lipid and zymosan at 37°C for one hour. For certain experiments, MAPK pathway inhibitors, including SB202190, U0126, and SP600125, were added at a concentration of 10 µM to RPM 30 min prior to oxidized lipids and the appropriate vehicle controls were used. The reactions were terminated by the addition of ice cold methanol (200 µl) containing 1 ng each of internal standards d4-LTB4, d4-PGE2, d4-TXB2 and d8-5-HETE and 2 ng each of d5-LTC4 and d8-AA. In order to assess toxicity, a trypan blue exclusion assay was performed and 88% of the RPM were alive after 1 hour of treatment with 37.5 µM 16:0/9al-PC, which was similar to the cell viability results of RAW 264.7 cells exposed to 16:0/9al-PC.8
Eicosanoid extraction and analysis by reverse phase HPLC and ESI-MS/MS
Samples were diluted with water to a concentration of 10% methanol and then extracted using a solid phase extraction cartridge (Strata-X 33µ Polymeric Reversed Phase, 60 mg/ml; Phenomenex, Torrance, CA). The eluate was dried down under N2 and resuspended in 20 µl HPLC solvent B (acetonitrile/methanol, 65/35, v/v) and 40 µl HPLC solvent A (8.3 mM acetic acid adjusted to pH 5.7 with ammonium hydroxide). Half of each sample was injected onto a C18 HPLC column (Kinetex, 50×2.1 mm, 5µ, Phenomenex, Torrance, CA) and eluted with a linear gradient from 25%B to 75%B in 8 minutes, from 75%B to 98%B in 1 minute and followed by an isocratic hold at 98%B for 5 minutes. Detection of eicosanoids eluting from the column was accomplished using an AB Sciex 5500 triple quadrupole linear ion trap hybrid mass spectrometer in negative ion mode by multiple reaction monitoring (MRM). The specific MRM transitions used for monitoring eicosanoids of interest were m/z 319→115 for 5-HETE, m/z 327→116 for d8-5-HETE, m/z 335→195 for LTB4 and 6-trans-LTB4 isomers, m/z 339→197 for d4-LTB4, m/z 624→272 for LTC4, m/z 629→272 for d5-LTC4, m/z 351→271 for PGE2, m/z 355→275 for d4-PGE2, m/z 353→193 for PGF2α, m/z 369→169 for TXB2, m/z 373→173 for d4-TXB2, m/z 369→169 for 6-keto-PGF1α, m/z 303→259 for AA, and m/z 311→267 for d8-AA. Quantitation was performed using standard isotope dilution with a standard curve for each analyte ranging from 30 pg to 30 ng.23
ESI-MS of ozPOPG
Ozonized POPG was infused into an AB Sciex 3000 triple quadrupole mass spectrometer at a flow rate of 10 µl/min. The concentration of the sample was approximately 500 nM in methanol:acetonitrile:water (60:20:20) with 1 mM ammonium acetate. The relevant experimental parameters in the negative ion mode for both full scan and collision-induced dissociation experiments were an electrospray voltage of −4800 V and a declustering potential of −65 V. The collision-induced dissociation mass spectra of the ozPOPG products were acquired with a collisional offset of 40 V.
Calcium flux
Peritoneal cavity cells (RPM and lymphocytes; ~50/50) at a concentration of 10×106/ml in HBSS with 0.5% fatty acid-free BSA were loaded with 2 µM indo-1 AM for 30 min at 37°C. Cells were rinsed two times and resuspended in HBSS buffer with 0.05% fatty acid-free BSA and kept on ice until flow cytometry was performed. For the calcium assay, the fatty acid-free BSA was removed from 1×106 cells by centrifugation and the cells were resuspended in 0.5 ml HBSS buffer. Just before the assay, propidium iodide (to monitor permeabilization) was added at a final concentration of 1 µg/ml. Intracellular calcium changes were monitored by the change in Indo2/Indo1 fluorescence (LSR II, Becton Dickinson, Franklin Lakes, NJ) following stimulation with 16:0/9al-PC (7.5–75 µM final concentration). Additionally, the positive controls used in this study were PAF (100 nM final concentration) and ATP (100 µM final concentration).24,25 All results shown are representative of three independent experiments.
Immunoblotting of phosphorylated MAPKs
After incubation with appropriate stimulus as described above, RPM were rinsed twice with ice cold PBS and scraped into an ice cold modified RIPA buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 1 mM EGTA, and 1 mM EDTA) containing protease and phosphatase inhibitors. The cell lysates were incubated on ice for 15 minutes and centrifuged at 11,000 × g for 10 min at 4°C. The protein content of each sample was determined using a BCA assay. After the addition of Laemmli buffer, the cell lysates were boiled for 5 min and lysates containing equal amounts of protein (12 µg) were separated by 10% SDS-PAGE. After transfer to a nitrocellulose membrane, samples were incubated in blocking buffer (20 mM Tris HCl, pH 7.6, 137 mM NaCl, 0.05% Tween with 5% nonfat milk) for 1h and then incubated overnight with phospho-specific antibodies against ERK1/2 and p38 MAPK. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibody (1:5000) for 1h at room temperature. The membrane was reblotted with an anti-β-actin antibody as an internal control. The immunoreactive proteins were detected using the Amersham Biosciences ECL system (GE Healthcare).
Statistical analysis
The data is expressed as the mean ± standard error of the mean from independent experiments. All the p-values were calculated using an unpaired, two-tailed t-test (GraphPad Software, San Diego, CA).
RESULTS
5-LO is activated by 16:0/9al-PC in RPM and AM
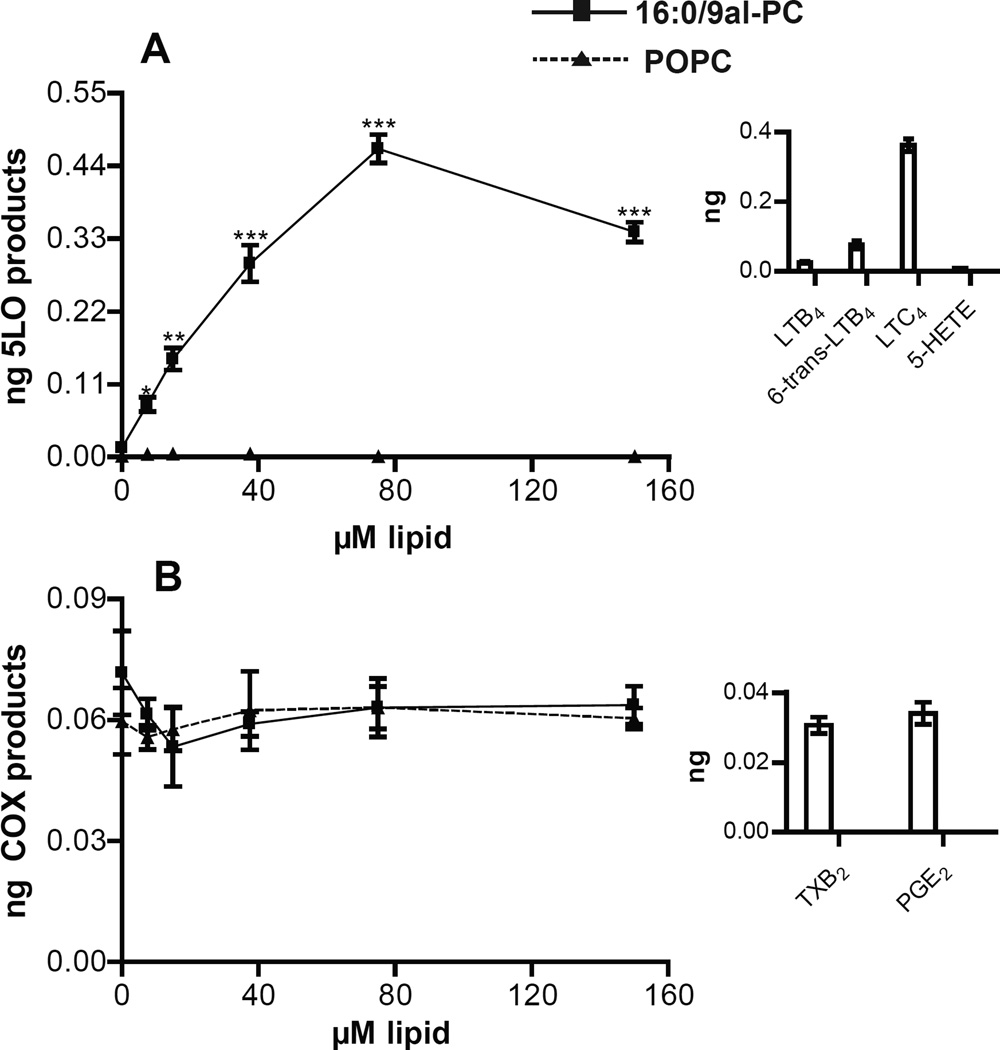
Incubation of RPM with 16:0/9al-PC (0–150 µM) for one hour resulted in the production of 5-LO-derived eicosanoids (LTC4, 6-trans-LTB4, LTB4, and 5-HETE) (Figure 1A) with LTC4 being the most abundant leukotriene product (Figure 1A inset). The production of 5-LO-derived eicosanoids initiated by 16:0/9al-PC in RPM was dose dependent with a significant production of leukotrienes observed even at the lowest dose of 16:0/9al-PC (7.5 µM), while the addition of POPC (0–150 µM) to RPM did not result in the production of leukotrienes (Figure 1A). In contrast, the dose response of the production of COX-derived eicosanoids (TXB2 and PGE2) in RPM for one hour revealed that the levels of COX-derived eicosanoids did not change from basal levels in the presence of either 16:0/9al-PC or POPC (Figure 1B). The AA released from RPM after the 1h incubation with 16:0/9al-PC or POPC was also measured and it was determined that the levels of AA do not change upon treatment of RPM with either lipid (Figure S1). Additionally, the 16:0/9al-PC standard (37.5 µM) was added to RPM in hBALF, which contains both lipids and protein, in order to mimic the environment that these lipids would experience in the lung. The 5-LO pathway was activated in RPM by 16:0/9al-PC in the presence of hBALF, however the amount of 5-LO products detected was half that observed when 16:0/9al-PC was added to RPM in HBSS (Table 1). Furthermore, other oxidized lipids (37.5 µM) including, 16:0/9COOH-PC, 16:0/5al-PC, 16:0/5COOH-PC, and ozPOPG, which is a mixture of 16:0/9al-PG and 16:0/9COOH-PG (Figure S2), were incubated with RPM for 1h and each of these different oxidized lipids resulted in production of 5-LO metabolites (Table 1). The amount of 5-LO metabolites observed when these oxidized lipids (16:0/9COOH-PC, 16:0/5al-PC, 16:0/5COOH-PC, and ozPOPG) were incubated with RPM was less than that observed for 16:0/9al-PC, but significantly above the POPC negative control. The effect of 16:0/9al-PC on AM was also examined and it was found that the level of 5-LO-derived eicosanoid products observed in AM was similar to that observed in RPM in the presence of 16:0/9al-PC, while the COX-derived eicosanoids remained at basal levels (Table 1).
Figure 1. 16:0/9al-PC activates the 5-LO pathway in resident peritoneal macrophages.
Dose response of the production of A) 5-LO products (LTC4, 6-trans-LTB4, LTB4, and 5-HETE) and B) COX products (PGE2 and TXB2) with the addition of 16:0/9al-PC (closed squares) and POPC (closed triangles) to RPM for 1h at 37°C. The insets show the amounts of each individual 5-LO metabolite (LTC4, 6-trans-LTB4, LTB4, and 5-HETE) and COX metabolite (PGE2 and TXB2) produced with 75 µM 16:0/9al-PC treatment. After incubation, the eicosanoids (LTC4, 6-trans-LTB4, LTB4, 5-HETE, PGE2, and TXB2) were analyzed by LC-MS/MS and quantified using standard isotope dilution. Results shown are averages ± SEM (n=3) from three independent experiments. * p< 0.05; ** p<0.01; *** p<0.0001.
Table 1.
Quantitation of eicosanoid production in murine resident peritoneal macrophages (RPM) and alveolar macrophages (AM) after the addition of oxidized lipid (37.5 µM) for 3 min followed by the addition of zymosan for 1h at 37°C. Results shown are averages ± SEM (n=3).
| Total 5-LO products# (ng/106 cells) |
Total COX products## (ng/106 cells) |
|||
|---|---|---|---|---|
| no zymosan | with zymosan | no zymosan | with zymosan | |
| RPM | ||||
| no lipid | <0.03 | 7.35±0.84 | 0.03±0.01 | 2.49±0.33 |
| POPC | <0.03ns | 7.16±0.62ns | 0.03±0.01ns | 2.36±0.25ns |
| 16:0/9al-PC | 0.29±0.03*** | 19.4±2** | 0.06±0.01ns | 0.63±0.08*** |
| hBALF | <0.03 | 2.92±0.34 | 0.04±0.01ns | 1.32±0.12 |
| 16:0/9al-PC + hBALF | 0.15±0.03** | 5.78±0.51** | 0.05±0.02ns | 0.41±0.06*** |
| 16:0/9COOH-PC | 0.11±0.01** | 11.4±1.1*** | 0.06±0.02ns | 1.27±0.06** |
| 16:0/5al-PC | 0.20±0.02*** | 14.5±1.3*** | 0.05±0.02ns | 0.73±0.07** |
| 16:0/5COOH-PC | 0.08±0.01** | 6.79±0.44*** | 0.04±0.01ns | 1.32±0.09** |
| ozPOPG | 0.21±0.04*** | 17.6±1.9*** | 0.05±0.02ns | 1.02±0.14** |
| AM | ||||
| no lipid | <0.03 | 1.27±0.11 | <0.03 | 0.14±0.03 |
| POPC | <0.03ns | 1.19±0.08ns | <0.03ns | 0.16±0.03ns |
| 16:0/9al-PC | 0.25±0.04** | 2.78±0.14** | <0.03ns | 0.07±0.01** |
total 5-LO products – 6-trans-LTB4, LTB4, LTC4, and 5-HETE
total COX products – PGE2 and TXB2
hBALF – human bronchoalveolar lavage fluid
p>0.05,
p<0.05;
p<0.01;
p<0.0001 compared to the appropriate no lipid control in same column
Eicosanoid synthesis is influenced by pretreatment with 16:0/9al-PC in zymosan stimulated RPM and AM
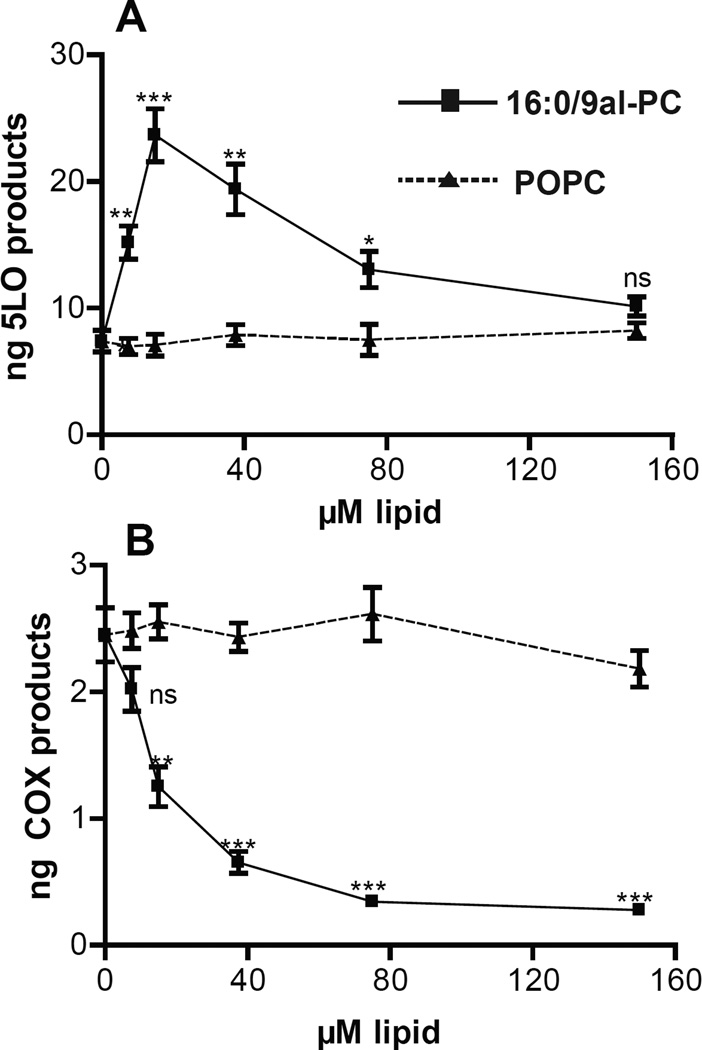
RPM produce TXB2 and PGE2 as well as LTC4 and low levels of 5-HETE and LTB4 when stimulated with zymosan.26,27 The total 5-LO products observed in RPM exposed to zymosan for 1h were 7.35±0.84 ng, while the total COX products present in the sample were 2.49±0.33 ng (Table 1). The production of 6-keto-PGF1α was observed in these studies but was not quantitated, however the trends observed were consistent with that observed for PGE2 and TXB2. Increased production of 5-LO-derived eicosanoids was observed with 16:0/9al-PC (7.5–75 µM) pretreatment 3 minutes before the addition of zymosan compared to no lipid pretreatment (0 µM) or to pretreatment with POPC (7.5–75 µM) (Figure 2A). Pretreatment of RPM with POPC (7.5–150 µM) 3 min prior to zymosan addition did not alter the production of 5-LO-derived eicosanoids compared to no lipid pretreatment (0 µM) (Figure 2A). The optimal effect of pretreatment of RPM with 16:0/9al-PC prior to zymosan addition was achieved at 15 µM with approximately 3.2 times more total 5-LO products (23.7±2.1 ng) compared to no lipid or POPC pretreatment (Figure 2A). Furthermore, significant enhancement in the production of 5-LO-derived eicosanoids in zymosan treated RPM was observed at doses of 16:0/9al-PC as low as 7.5 µM (Figure 2A). In contrast, a dose dependent decrease of COX-derived eicosanoids was observed with 16:0/9al-PC pretreatment 3 minutes before the addition of zymosan to RPM compared to both no lipid and POPC pretreatment (Figure 2B). Pretreatment of RPM with POPC (7.5–150 µM) 3 min prior to zymosan addition did not alter the production of COX-derived eicosanoids compared to no lipid pretreatment (0 µM) (Figure 2B). The greatest effect of 3 minute pretreatment with 16:0/9al-PC was observed at the highest dose studied with approximately 8.5 times less total COX products (0.29±0.03 ng) for 16:0/9al-PC (Figure 2B) compared to zymosan alone or a 3 minute POPC pretreatment. A significant reduction of COX-derived eicosanoids produced by zymosan stimulation in RPM was observed at doses of 16:0/9al-PC as low as 15 µM (Figure 2B). AA release was also measured in the supernatants of RPM pretreated with 16:0/9al-PC for 3 min before the addition of zymosan for 1h and the AA levels did not significantly change compared to no lipid pretreatment or a 3 min POPC lipid pretreatment (Figure S3). Additionally, 16:0/9al-PC was added to RPM either before, after, or at the same time as zymosan and the effect on eicosanoid production was established. Whether 16:0/9al-PC was added to RPM up to 10 minutes before, at the same time or up to 10 min after the zymosan stimulus, a significant increase in the production of 5-LO-derived eicosanoids and a decrease in production of COX-derived eicosanoids was observed (Figure S4). The most dramatic effects were detected when RPM were pretreated for 10 min with 16:0/9al-PC before zymosan addition, however the pretreatment time that resulted in maximal 5-LO activation was not determined.
Figure 2. 16:0/9al-PC influences eicosanoid production in zymosan treated resident peritoneal macrophages.
Dose response of the production of A) 5-LO products (LTC4, 6-trans-LTB4, LTB4, and 5-HETE) and B) COX products (PGE2 and TXB2) with the addition of 16:0/9al-PC (closed squares) and POPC (closed triangles) for 3 min followed by the addition of zymosan (25 particles per cell) to resident peritoneal macrophages for 1h at 37°C. After incubation, the eicosanoids (LTC4, 6-trans-LTB4, LTB4, 5-HETE, PGE2, and TXB2) were analyzed by LC-MS/MS and quantified using standard isotope dilution. Results shown are averages ± SEM (n=3) from three independent experiments. * p< 0.05; ** p<0.01; *** p<0.0001.
When RPM were pretreated for 3 min with 16:0/9al-PC in hBALF and subsequently stimulated with zymosan for 1h, the 5-LO derived eicosanoid products were increased and the COX-derived eicosanoids were decreased compared to zymosan alone in hBALF (Table 1). The addition of zymosan or 16:0/9al-PC and zymosan in hBALF decreased eicosanoid production compared to addition of these stimuli in buffer (Table 1). Likewise, when other oxidized lipids (16:0/9COOH-PC, 16:0/5al-PC, 16:0/5COOH-PC, and ozPOPG) were used to pretreat RPM before zymosan addition, an increased production of 5-LO-derived eicosanoids with a concomitant decrease in COX-derived eicosanoids was observed (Table 1). The effect of pretreatment with 16:0/9al-PC before zymosan on the eicosanoid production was also examined in AM. The production of 5-LO-derived eicosanoids was enhanced in AM when pretreated with 16:0/9al-PC for 3 min before the addition of zymosan, while the COX-derived eicosanoids were decreased which was similar to what was observed in RPM (Table 1).
Intracellular Ca2+ levels are not influenced by 16:0/9al-PC in RPM
Since calcium plays a crucial role in the activation of enzymes in the leukotriene synthesis pathway, the effect of 16:0/9al-PC on intracellular calcium levels was studied in cells isolated from the peritoneal cavity (lymphocytes and macrophages) loaded with indo-1 AM along with simultaneous staining with propidium iodide to detect permeabilization of the plasma membrane. PAF (100 nM) or ATP (100 µM) were added to indo-1 AM loaded cells and in both cases a receptor mediated calcium response was observed along with no membrane permeabilization (Figure S5). In contrast, when 16:0/9al-PC (7.5–75 µM final concentration) was added to indo-1 AM loaded cells, a calcium response was not elicited (Figure S5) and these doses did not cause membrane permeabilization (data not shown). Therefore, it was concluded that modulation of calcium signaling was not the cause of the activation of the 5-LO pathway in cells by 16:0/9al-PC. Additionally, the eicosanoids produced by the incubation of the total cells isolated from the peritoneal cavity in suspension with oxidized lipid were analyzed and the trends were identical to those observed in adherent macrophages (data not shown).
Effect of 16:0/9al-PC on the phosphorylation of MAPK
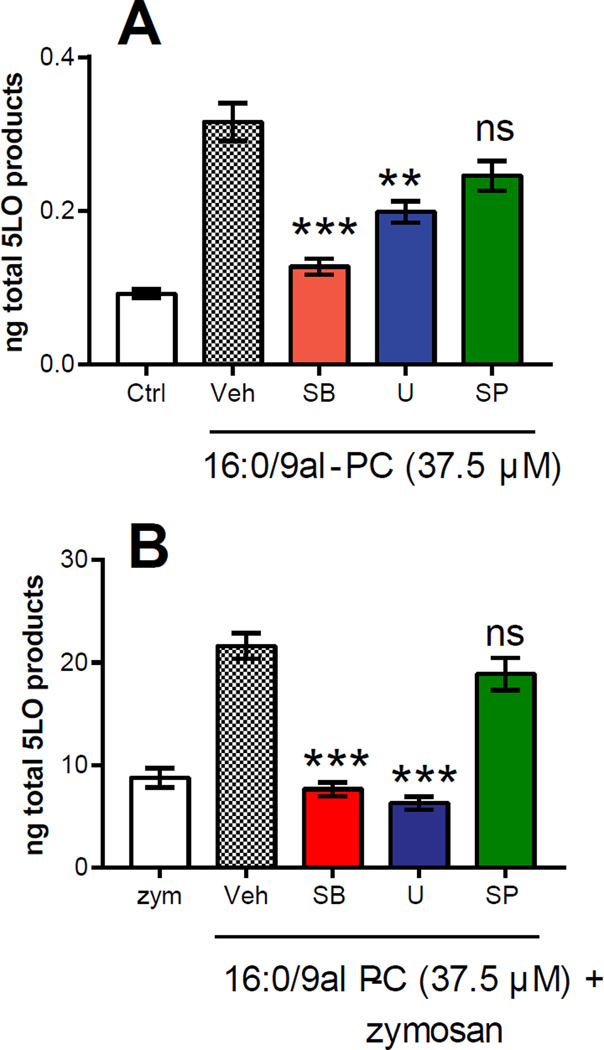
The effect of 16:0/9al-PC on phosphorylation of MAPKs, which is an important step in 5-LO activation, was examined using MAPK pathway inhibitors. As shown in Figure 3A, 5-LO eicosanoid production in RPM by 16:0/9al-PC after 1h was significantly reduced by 60% in the presence of a p38 MAPK inhibitor (SB202190) and by 37% with a MEK/ERK inhibitor (U0126). However, the JNK inhibitor (SP600125) did not have a significant effect on 5-LO eicosanoid production in RPM by 16:0/9al-PC (Figure 3A). While both the p38 MAPK and MEK/ERK inhibitors did result in reduced levels of 5-LO-derived eicosanoids in the presence of 16:0/9al-PC, neither inhibitor completely returned the total 5-LO product levels to basal levels. Additionally, the production of 5-LO-derived eicosanoids in RPM in the presence of both 16:0/9al-PC and zymosan for 1h was significantly reduced by 65% in the presence of the p38 MAPK inhibitor and by 71% with the MEK/ERK inhibitor (Figure 3B). Individually each of these inhibitors reduced the levels of 5-LO derived eicosanoids to that observed in RPM stimulated with zymosan alone (Figure 3B). The presence of the JNK inhibitor did not affect the production of 5-LO eicosanoids in RPM in the presence of both 16:0/9al-PC and zymosan (Figure 3B). This data suggested a possible role of p38 MAPK and ERK1/2 in the activation of the 5-LO pathway by 16:0/9al-PC in RPM.
Figure 3. Effect of MAPK inhibitors on the production of 5-LO products in resident peritoneal macrophages.
RPM were pre-incubated with the MAPK inhibitors (10 µM), SB202190 (SB; p38 MAPK inhibitor), U0126 (U; MEK/ERK inhibitor), and SP600125 (SP; JNK inhibitor), or vehicle control for 30 min and then stimulated with A) 16:0/9al-PC (37.5 µM) and B) 16:0/9al-PC (37.5 µM) and zymosan (25 particles/cell) for 1h. After incubation, the 5-LO-derived eicosanoids (LTC4, 6-trans-LTB4, LTB4, and 5-HETE) were analyzed by LC-MS/MS and quantified using standard isotope dilution. Results shown are averages ± SEM (n=3) from three independent experiments. * p< 0.05; ** p<0.01; *** p<0.0001.
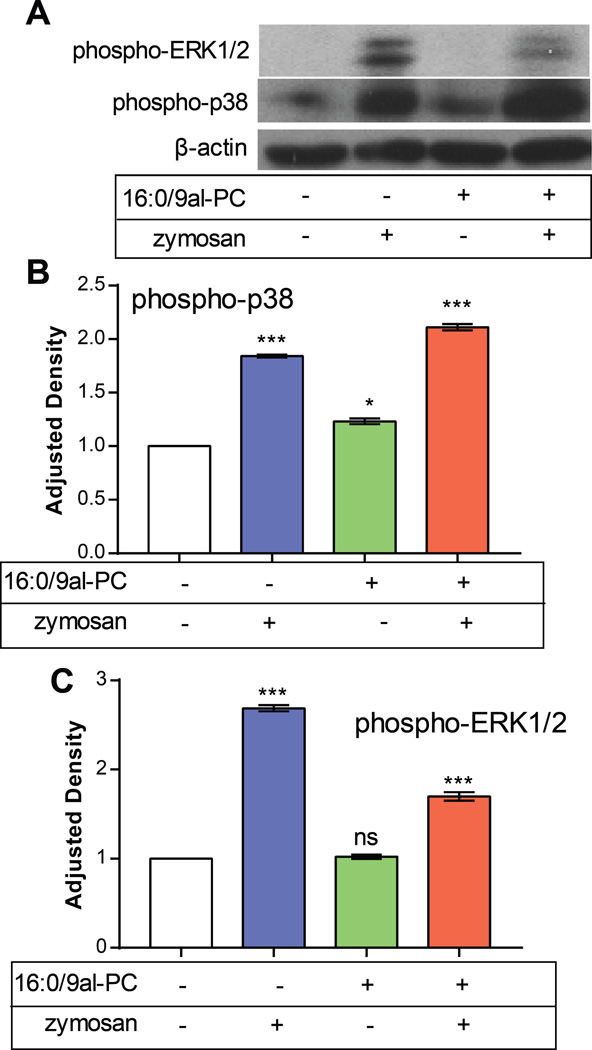
The activation of p38 MAPK and ERK1/2 pathways was examined using Western blots with antibodies to phosphorylated forms of p38 MAPK and ERK1/2. These particular experiments were performed after incubation of RPM with stimuli for 30 min since phospho-p38 and phospho-ERK1/2 signals were maximal at this time (data not shown). The same trends in 5-LO production as described above at 1h were observed at 30 min (Figure S6). This Western blot data demonstrated the phosphorylation of both p38 and ERK1/2 after the addition zymosan alone or 16:0/9al-PC and zymosan in RPM after 30 min. (Figure 4). However, after stimulation of RPM with 16:0/9al-PC phosphorylation of p38 MAPK was observed whereas activation of the ERK1/2 was not detected (Figure 4). This data suggests that phosphorylation of the p38 MAPK pathway mediates activation of the 5-LO pathway by16:0/9al-PC in RPM.
Figure 4. 16:0/9al-PC induces phosphorylation of p38 in resident peritoneal macrophages.
RPM were treated with zymosan (25 particles/cell), 16:0/9al-PC (37.5 µM), or 16:0/9al-PC (37.5 µM) and zymosan (25 particles/cell) for 30 min. A) Cell lysates were collected and analyzed by Western blotting with antibodies to phosphorylated forms of p38 and ERK1/2 and β-actin to normalize for equal loading. The data in this figure are representative of 3 independent experiments. The optical densities measured for the control samples were set as 100% and the results shown for B) phospho-p38 and C) phospho-ERK1/2 are averages ± SEM (n=3) from three independent experiments. * p< 0.05; ** p<0.01; *** p<0.0001.
DISCUSSION
Due to the limited aqueous stability and the chemical reactivity of ozone it has been suggested that pulmonary surfactant serves as the primary target of inhaled ozone and is the most likely site for formation of ozonized products.1 In order for 16:0/9al-PC to be produced in appreciable amounts, the precursor lipids must be present in the pulmonary surfactant in significant amounts. Human pulmonary surfactant contains 90% lipid with the phospholipid content in the range of 40–70 mM.3 Phosphatidylcholine (PC) lipids are the most prevalent class of lipids in pulmonary surfactant and are present at roughly 80% of the total phospholipid.28 By far, the two most abundant phospholipids in pulmonary surfactant that will react with ozone are POPC and palmitoyl-palmitoleoyl-PC (PPoPC), which are present at 20% of the total PC (7–11 mM) in pulmonary surfactant.29 Both of these PC lipids have palmitic acid esterified to the sn-1 position of the glycerol backbone and a monounsaturated fatty acid with the double bond at C9 esterifed at the sn-2 position of the glycerol backbone. Upon exposure to ozone, POPC and PPoPC both result in the formation of 16:0/9al-PC as the major product.30 The second most abundant phospholipid class in pulmonary surfactant is phosphatidylglycerol (PG) lipids, which comprises 10% of the total phospholipid, with POPG as the predominant molecular species.29 Similar to the reaction of POPC and PPoPC with ozone, the prevalent product observed upon reaction of POPG with ozone is 16:0/9al-PG (Figure S2). Even though the reported levels of oxidized PC lipids in lung surfactant after ozone exposure or cigarette smoke are in the nM concentration range,48 most studies that have probed the biological effects of oxidized phospholipids in various biological systems have used concentrations in the µM range similar to this study.31–36 This lipid concentration can be justified because many inhaled agents, such as ozone, initiate oxidative stress and could result in relatively high concentrations of oxidized lipids locally.37 In order to achieve concentrations of oxidized PC at µM levels in pulmonary surfactant less than 0.5% of the total precursor PC lipids (POPC and PPoPC) would need to be converted to active product and accumulate in the lung. Additionally, the critical micellar concentration of 16:0/9al-PC has been measured at 22 µM and considerable activation of the 5-LO pathway was observed below this concentration (Figures 1 and 2).38 Furthermore, the toxicity of 16:0/9al-PC was addressed in this study using a trypan blue exclusion assay and it was found that 88% of the macrophages were alive after treatment with the oxidized lipid for one hour, which correlates with a previous study which found that the viability of macrophages was not affected by treatment with 16:0/9al-PC (up to 40 µM).8
Previously, it has been proposed that lipid-ozone products found in surfactant exposed to ozone can act as mediators of ozone toxicity in the lung since they are small, diffusible, and relatively stable.2 Alveolar macrophages are also present in the pulmonary surfactant and provide the first line of defense against inhaled antigens and play a key role in regulating inflammation in the lung. Additionally, it has been found that after ozone inhalation, the number of macrophages in the surfactant is increased.39 It is therefore a reasonable hypothesis that the oxidized lipid products would primarily encounter alveolar macrophages and therefore the effects of oxidized lipids were examined in macrophages. In the current study mouse RPM were used for most of the experiments because the yield of AM from bronchoalveolar lavage is relatively low (~0.5×106/mouse) and many mice would have had to be sacrificed in order to complete the experiments described in this manuscript. Additionally, it was preferred to use primary cells since these cells have an endogenous distribution of phospholipids compared to immortalized cell lines that are typically severely depleted in PUFAs. The effect of oxidized lipids on the eicosanoid pathway was examined because as described below a limited number of studies have indicated activation of the eicosanoid pathway by either ozone or oxidized phospholipid products in cultured cell lines relevant to the lung, but the effect of oxidized phospholipid products on the eicosanoid pathway of macrophages has not been previously described.
Previously studies have reported that a wide variety of eicosanoids (PGE2, PGD2, PGF2α, TXB2, LTB4, HETEs, and LTC4) are increased in the bronchoalveolar lavage fluid following ozone exposure.40–46 Reports also have been published on the production of eicosanoids (TXB2, PGE2, cysteinyl leukotrienes, and LTB4) upon the exposure of airway and tracheal epithelial cells to ozone.47,48 Other studies have shown that ozone exposure increases eicosanoid production upon activation of the eicosanoid pathway by calcium ionophore in AM and airway segments.44,49 More relevant to the work reported herein, the effects of ozonized lipid products on activation of the eicosanoid pathway have been examined to some extent. Arachidonic acid release by BEAS-2B cells and PGE2 production in primary human bronchial epithelial cells were observed in the presence of the ozonized lipid product, 16:0/9al-PC.7,19 Additionally, the ozonolysis products of membrane fatty acids, unsaturated C3-, C6-, and C9-aldehydes released from phospholipids upon ozone exposure, induced the release of AA and the subsequent formation of PGE2, PGF2α, and 15-HETE in human airway epithelial cells.50 A common theme in these studies is that the mechanism of how ozone or ozonized lipid products exert their effect on the eicosanoid pathway remains unknown.
In the current studies, it was found that 16:0/9al-PC, as well as other oxidized phospholipids (Table 1), activates the 5-LO pathway in RPM (Figure 1). The first step in the leukotriene pathway is release of arachidonic acid from a glycerophospholipid via the action of cPLA2α and AA is transformed into LTA4, which is a precursor of LTB4 and LTC4, through the action of 5-LO/FLAP.10,12 A key step in both the cPLA2α and 5-LO enzymatic mechanism is the translocation of the enzyme to intracellular membranes. Elevation of intracellular calcium levels drives the translocation of both cPLA2α and 5-LO to intracellular membranes and is a key step in the enzymatic mechanisms of both enzymes.12 Therefore, the effect of 16:0/9al-PC on AA release and intracellular calcium levels was examined. Even though AA release by oxidized phospholipids in various types of cultured cells has been described,19,51 our results do not indicate a measurable increase in AA levels in RPM treated with 16:0/9al-PC (Figure S1). However, it is possible that 16:0/9al-PC does increase the amount of free AA at a local subcellular region, but could not be observed since we measured the total free AA in the macrophage. Next, the influence of 16:0/9al-PC on intracellular calcium levels was probed using the fluorescent indicator, indo-1 AM. Previously, both PAF and lyso-PC have been shown to elevate intracellular calcium levels in thioglycollate elicited peritoneal macrophages,25 oxLDL has been shown to increase intracellular calcium concentrations in RPM,52 and oxPAPC has been shown to induce elevation of calcium levels in human endothelial cells.32 However, a calcium response was not elicited when 16:0/9al-PC was added to indo-1 AM loaded cells isolated from the peritoneal cavity, which leads to the conclusion that modulation of calcium signaling was not the cause of the activation of the 5-LO pathway observed in these studies.
In addition to calcium-mediated activation, 5-LO can be regulated through protein phosphorylation. Activation of the 5-LO pathway can occur by phosphorylation at Ser271 by a p38 MAPK dependent pathway or at Ser663 by an ERK2 dependent pathway.15,16 Depending on the cell type, the p38 MAPK or ERK pathway can stimulate 5-LO activity under conditions that do not lead to increases in intracellular calcium levels and result in translocation to the nuclear membrane.17,18 In contrast, phosphorylation of Ser523 by PKA results in the inhibition 5-LO activity.53 Previous studies have demonstrated that oxidized phospholipids, specifically oxPAPC, have the ability to activate a number of protein kinase pathways.32,54,55 Additionally, it has been found that activation of p38 MAPK and ERK1/2 pathways occurs in mouse macrophages upon exposure to the membrane fatty acid oxidation products, 4-hydroxynonenal and acrolein, which results in 5-LO activation and production of leukotrienes.56–58
The effect of 16:0/9al-PC in RPM on the MAPK pathways involved in 5-LO activation was assessed using a specific p38 MAPK inhibitor (SB202190), a specific MEK/ERK inhibitor (U0126), and the non-specific JNK inhibitor (SP600125).59,60 It was found that both the p38 MAPK and MEK/ERK inhibitors significantly reduced 5-LO-derived eicosanoid production in RPM with 16:0/9al-PC (Figure 3). The JNK inhibitor did not have an effect on 5-LO production by in RPM treated with 16:0/9al-PC. In parallel, it was also found that 16:0/9al-PC induced the production of phospho-p38 in RPM, but not phospho-ERK1/2 (Figure 4). MAPK pathway inhibitors were also used in RPM treated with 16:0/9al-PC and zymosan. The amount of 5-LO products observed in RPM treated with 16:0/9al-PC and zymosan in the presence of the p38 MAPK and MEK/ERK inhibitors was reduced to levels observed with zymosan treatment (Figure 3B). The Western blot data indicated that phosphorylation of both p38 and ERK1/2 occurred in RPM when zymosan was used as a stimulus, which has been observed previously.61 The levels of phospho-p38 were increased and the levels of phospho-ERK1/2 were decreased in RPM treated with both 16:0/9al-PC and zymosan compared to zymosan alone (Figure 4). The phospho-p38 data correlates well with the amount of 5-LO products observed in 16:0/9al-PC (0.26±0.03 ng), zymosan (2.61±0.14 ng), and 16:0/9al-PC with zymosan (5.78±0.26 ng) in RPM (Figure S6). However, the phospho-ERK1/2 does not reflect the trend observed in the 5-LO product formation. These results indicated that 16:0/9al-PC plays a role in the regulation of 5-LO activity and therefore leukotriene production via the p38 MAPK pathway in RPM. Additionally, this data correlates with previously published results that indicate that MAPK pathway activation enhances 5-LO activity and product formation in the presence of an additional stimulus, like zymosan,15 and with the evidence that intracellular calcium levels do not need to be elevated for 5-LO activation to occur.17,18
In a previous study with RAW 264.7 cells, activation of the p38 MAPK pathway was not observed after treatment with 16:0/9al-PC for 48h.8 The discrepancy of our current results with this previous study is most likely explained by the different incubation times with 16:0/9al-PC in these studies. In the current study, a time course was performed and it was found that maximal phospho-p38 and phospho-ERK1/2 signals were observed after exposure of RPM to 16:0/9al-PC for 30 min and that after 60 min the phosphorylation of both p38 MAPK and ERK1/2 had decreased by almost half. Therefore, it is not surprising that phosphorylation of the p38 MAPK was not observed in the previous study where cells were exposed to 16:0/9al-PC for 48h.
Another unexpected finding in the current studies was observation of an enhanced production of 5-LO derived eicosanoids with a concomitant decrease in the production of COX derived eicosanoids in RPM in the presence of 16:0/9al-PC and zymosan (Figure 2), which is unique and not previously described. Previously, studies with COX-1−/− and COX-2 mice suggested that PGE2 production formed in the response of RPM to zymosan occurs through COX-1 with very little contribution from COX-2.62 COX-1 is expressed constitutively, and is broadly distributed, and the main regulator of this enzyme is the availability of substrate, AA (11). This enzyme is located on the endoplasmic reticulum, the Golgi apparatus, and the associated nuclear envelope.63 It is possible that the level of free AA at the nuclear membrane, where 5-LO pathway enzymes are located, is increased in RPM treated with 16:0/9al-PC which could account for the increase of 5-LO products. Conversely, the decrease in COX products could reflect that the local concentration of free AA is not increased at the endoplasmic reticulum and the Golgi apparatus, where COX is located.
A limited number of experiments were completed with AM using a single concentration of 16:0/9al-PC (37.5 µM) and it was determined that the response of AM to 16:0/9al-PC was similar to that observed in RPM with activation of the 5-LO pathway that resulted in the production of 5-LO metabolites (Table 1). Given that AM are quiescent cells and generally do not lead to a robust production of leukotrienes,64 this finding is quite remarkable and indicates that when oxidized lipids are present in lung surfactant that the resident alveolar macrophages can be activated to synthesize 5-LO products in the absence of any other stimulus. Additionally, a synergistic effect on 5-LO-derived eicosanoid production was observed in AM treated with 16:0/9al-PC and zymosan (Table 1). While the total amount of 5-LO products was greater in RPM compared to AM, the general trends observed in both of these cells was similar. This could have implications in inflammatory pulmonary disease where it has long been established that 5-LO products play a contributory role in these diseases because these studies indicate that in the presence of oxidized lipid that AM become considerably more responsive. Additionally, this scenario is quite relevant in vivo as pulmonary pathogens will most likely be encountered in the lung in the presence of inhaled toxicants and oxidized phospholipids.
In summary, this work focused on the unexpected finding that 16:0/9al-PC derived from reaction of ozone with lung surfactant phospholipids, both independently and in the presence of zymosan, profoundly influences eicosanoid production in both peritoneal and alveolar macrophages. For the first time, it was demonstrated that oxidized phospholipids cause an enhancement of the 5-LO pathway coupled to a concomitant inhibition of the COX pathway. From these studies, the mechanism of the influence of oxidized phospholipids on the regulation of 5-LO activity and therefore leukotriene production occurs through the p38 MAPK pathway in RPM. These results reported herein provide another mechanism by which oxidized phospholipids, formed by the reaction of surfactant phospholipids with ozone, act in a proinflammatory fashion in the lung and may play a role in exacerbating pulmonary inflammatory diseases, such as COPD and asthma.
Supplementary Material
Acknowledgments
We thank Dr. Dennis R. Voelker for providing the human BALF, Dr. S. Courtney Frasch for facilitating the development of the calcium assays, and Dr. Christina C. Leslie and Heejung Lee for performing the phospho-ERK1/2 and phospho-p38 assays.
FUNDING SOURCES
This work was supported by grants from the National Institutes of Health (HL117798 and HL03430).
ABBREVIATIONS
- 16:0/9al-PC
1-palmitoyl-2-(9’-oxo-nonanoyl)-glycerophosphocholine
- 5-LO
5-lipoxygenase
- COX
cyclooxygenase
- AA
arachidonic acid
- cPLA2
cytosolic phospholipase A2
- LTB4
leukotriene B4
- PGH2
prostaglandin H2
- FLAP
5-lipoxygenase activating protein
- LTC4
leukotriene C4
- PGE2
prostaglandin E2
- TXB2
thromboxane B2
- RPM
resident peritoneal macrophage
- AM
alveolar macrophage
- 16:0/9COOH-PC
1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine
- 16:0/5al-PC
1-palmitoyl-2-(5'-oxo-valeroyl)-sn-glycero-3-phosphocholine
- 16:0/5COOH-PC
1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine
- POPG
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol
- hBALF
human bronchoalveolar lavage fluid
- ozPOPG
ozonized 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol
- PGF2α
prostaglandin F2α
- PC
phosphatidylcholine
- PPoPC
1-palmitoyl-2-palmitoleoyl-sn-glycero-3-phosphocholine
- PG
phosphatidylglycerol
- oxPAPC
oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- ERK1/2
extracellular signal-regulated protein kinases 1 and 2
Footnotes
SUPPORTING INFORMATION
Dose response of AA release by 16:0/9al-PC in RPM, Fig. S1; mass spectrum of ozPOPG products, Fig. S2; dose response of AA release by 16:0/9al-PC and zymosan in RPM, Fig. S3; time course of 16:0/9al-PC activation of RPM prior to addition of zymosan, Fig. S4; calcium flux by 16:0/9al-PC in cells isolated from the peritoneal cavity, Fig. S5; production of eicosanoids in RPM with 16:0/9al-PC and zymosan for 30 min, Fig. S6. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Pryor WA. How far does ozone penetrate into the pulmonary air/tissue boundary before it reacts? Free Radic. Biol. Med. 1992;12:83–88. doi: 10.1016/0891-5849(92)90060-t. [DOI] [PubMed] [Google Scholar]
- 2.Pryor WA, Squadrito GL, Friedman M. The cascade mechanism to explain ozone toxicity: the role of lipid ozonation products. Free Radic. Biol. Med. 1995;19:935–941. doi: 10.1016/0891-5849(95)02033-7. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JF, Jobe AH. Surfactant and the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1993;147:218–233. doi: 10.1164/ajrccm/147.1.218. [DOI] [PubMed] [Google Scholar]
- 4.Uhlson C, Harrison K, Allen CB, Ahmad S, White CW, Murphy RC. Oxidized phospholipids derived from ozone-treated lung surfactant extract reduce macrophage and epithelial cell viability. Chem. Res. Toxicol. 2002;15:896–906. doi: 10.1021/tx010183i. [DOI] [PubMed] [Google Scholar]
- 5.Almstrand A-C, Voelker D, Murphy RC. Identification of oxidized phospholipids in bronchoalveolar lavage exposed to low ozone levels using multivariate analysis. Anal. Biochem. 2015;474:50–58. doi: 10.1016/j.ab.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postle AD, Heeley EL, Wilton DC. A comparison of the molecular species compositions of mammalian lung surfactant phospholipids. Comp. Biochem. Physiol, Part A Mol. Integr. Physiol. 2001;129:65–73. doi: 10.1016/s1095-6433(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 7.Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Induction of inflammatory mediators in human airway epithelial cells by lipid ozonation products. Am. J. Respir. Crit. Care Med. 1999;160:1934–1942. doi: 10.1164/ajrccm.160.6.9902025. [DOI] [PubMed] [Google Scholar]
- 8.Kimura T, Shibata Y, Yamauchi K, Igarashi A, Inoue S, Abe S, Fujita K, Uosaki Y, Kubota I. Oxidized phospholipid, 1-palmitoyl-2-(9’-oxo-nonanoyl)-glycerophosphocholine (PON-GPC), produced in the lung due to cigarette smoking, impairs immune function in macrophages. Lung. 2012;190:169–182. doi: 10.1007/s00408-011-9331-2. [DOI] [PubMed] [Google Scholar]
- 9.Wenzel SE. Arachidonic acid metabolites: mediators of inflammation in asthma. Pharmacotherapy. 1997;17:3S–12S. [PubMed] [Google Scholar]
- 10.Leslie CC. Regulation of arachidonic acid availability for eicosanoid production. Biochem. Cell Biol. 2004;82:1–17. doi: 10.1139/o03-080. [DOI] [PubMed] [Google Scholar]
- 11.Smith WL, Urade Y, Jakobsson P-J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 2011;111:5821–5865. doi: 10.1021/cr2002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy RC, Gijón MA. Biosynthesis and metabolism of leukotrienes. Biochem. J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 13.Peters-Golden M, Brock TG. 5-lipoxygenase and FLAP. Prostaglandins Leukot. Essent. Fatty Acids. 2003;69:99–109. doi: 10.1016/s0952-3278(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 14.Flamand N, Lefebvre J, Surette ME, Picard S, Borgeat P. Arachidonic acid regulates the translocation of 5-lipoxygenase to the nuclear membranes in human neutrophils. J. Biol. Chem. 2006;281:129–136. doi: 10.1074/jbc.M506513200. [DOI] [PubMed] [Google Scholar]
- 15.Werz O, Klemm J, Samuelsson B, Rådmark O. 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5261–5266. doi: 10.1073/pnas.050588997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werz O, Bürkert E, Fischer L, Szellas D, Dishart D, Samuelsson B, Rådmark O, Steinhilber D. Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes. FASEB J. 2002;16:1441–1443. doi: 10.1096/fj.01-0909fje. [DOI] [PubMed] [Google Scholar]
- 17.Werz O, Bürkert E, Samuelsson B, Rådmark O, Steinhilber D. Activation of 5-lipoxygenase by cell stress is calcium independent in human polymorphonuclear leukocytes. Blood. 2002;99:1044–1052. doi: 10.1182/blood.v99.3.1044. [DOI] [PubMed] [Google Scholar]
- 18.Bürkert E, Szellas D, Rådmark O, Steinhilber D, Werz O. Cell type-dependent activation of 5-lipoxygenase by arachidonic acid. J. Leukoc. Biol. 2003;73:191–200. doi: 10.1189/jlb.0702354. [DOI] [PubMed] [Google Scholar]
- 19.Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Lipid ozonation products activate phospholipases A2, C, and D. Toxicol. Appl. Pharmacol. 1998;150:338–349. doi: 10.1006/taap.1998.8418. [DOI] [PubMed] [Google Scholar]
- 20.Qiu ZH, de Carvalho MS, Leslie CC. Regulation of phospholipase A2 activation by phosphorylation in mouse peritoneal macrophages. J. Biol. Chem. 1993;268:24506–324513. [PubMed] [Google Scholar]
- 21.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 22.Bosio CM, Dow SW. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 2005;175:6792–6801. doi: 10.4049/jimmunol.175.10.6792. [DOI] [PubMed] [Google Scholar]
- 23.Hall LM, Murphy RC. Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J. Am. Soc. Mass Spectrom. 1998;9:527–532. doi: 10.1016/S1044-0305(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 24.Través PG, Pimentel-Santillana M, Carrasquero LMG, Pérez-Sen R, Delicado EG, Luque A, Izquierdo M, Martín-Sanz P, Miras-Portugal MT, Boscá L. Selective impairment of P2Y signaling by prostaglandin E2 in macrophages: implications for Ca2+-dependent responses. J. Immunol. 2013;190:4226–4235. doi: 10.4049/jimmunol.1203029. [DOI] [PubMed] [Google Scholar]
- 25.Ogita T, Tanaka Y, Nakaoka T, Matsuoka R, Kira Y, Nakamura M, Shimizu T, Fujita T. Lysophosphatidylcholine transduces Ca2+ signaling via the platelet-activating factor receptor in macrophages. Am. J. Physiol. 1997;272:H17–H24. doi: 10.1152/ajpheart.1997.272.1.H17. [DOI] [PubMed] [Google Scholar]
- 26.Rouzer CA, Scott WA, Cohn ZA, Blackburn P, Manning JM. Mouse peritoneal macrophages release leukotriene C in response to a phagocytic stimulus. Proc. Natl. Acad. Sci. U.S.A. 1980;77:4928–4932. doi: 10.1073/pnas.77.8.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouzer CA, Scott WA, Kempe J, Cohn ZA. Prostaglandin synthesis by macrophages requires a specific receptor-ligand interaction. Proc. Natl. Acad. Sci. U.S.A. 1980;77:4279–4282. doi: 10.1073/pnas.77.7.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veldhuizen R, Nag K, Orgeig S, Possmayer F. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta. 1998;1408:90–108. doi: 10.1016/s0925-4439(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 29.Wright SM, Hockey PM, Enhorning G, Strong P, Reid KB, Holgate ST, Djukanovic R, Postle AD. Altered airway surfactant phospholipid composition and reduced lung function in asthma. J. Appl. Physiol. 2000;89:1283–1292. doi: 10.1152/jappl.2000.89.4.1283. [DOI] [PubMed] [Google Scholar]
- 30.Santrock J, Gorski RA, O’Gara JF. Products and mechanism of the reaction of ozone with phospholipids in unilamellar phospholipid vesicles. Chem. Res. Toxicol. 1992;5:134–141. doi: 10.1021/tx00025a023. [DOI] [PubMed] [Google Scholar]
- 31.Thimmulappa RK, Gang X, Kim J-H, Sussan TE, Witztum JL, Biswal S. Oxidized phospholipids impair pulmonary antibacterial defenses: evidence in mice exposed to cigarette smoke. Biochem. Biophys. Res. Commun. 2012;426:253–259. doi: 10.1016/j.bbrc.2012.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bochkov VN, Mechtcheriakova D, Lucerna M, Huber J, Malli R, Graier WF, Hofer E, Binder BR, Leitinger N. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 2002;99:199–206. doi: 10.1182/blood.v99.1.199. [DOI] [PubMed] [Google Scholar]
- 33.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 34.Walton KA, Cole AL, Yeh M, Subbanagounder G, Krutzik SR, Modlin RL, Lucas RM, Nakai J, Smart EJ, Vora DK, Berliner JA. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler. Thromb. Vasc. Biol. 2003;23:1197–1203. doi: 10.1161/01.ATV.0000079340.80744.B8. [DOI] [PubMed] [Google Scholar]
- 35.Halasiddappa LM, Koefeler H, Futerman AH, Hermetter A. Oxidized phospholipids induce ceramide accumulation in RAW 264.7 macrophages: role of ceramide synthases. PLoS ONE. 2013;8:e70002. doi: 10.1371/journal.pone.0070002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stemmer U, Dunai ZA, Koller D, Pürstinger G, Zenzmaier E, Deigner HP, Aflaki E, Kratky D, Hermetter A. Toxicity of oxidized phospholipids in cultured macrophages. Lipids Health Dis. 2012;11:110. doi: 10.1186/1476-511X-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Postlethwait EM. Scavenger receptors clear the air. J Clin Invest. 2007;117:601–604. doi: 10.1172/JCI31549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Code C, Mahalka AK, Bry K, Kinnunen PKJ. Activation of phospholipase A2 by 1-palmitoyl-2-(9’-oxo-nonanoyl)-sn-glycero-3-phosphocholine in vitro. Biochim. Biophys. Acta. 2010;1798:1593–1600. doi: 10.1016/j.bbamem.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Hotchkiss JA, Harkema JR, Kirkpatrick DT, Henderson RF. Response of rat alveolar macrophages to ozone: quantitative assessment of population size, morphology, and proliferation following acute exposure. Exp. Lung Res. 1989;15:1–16. doi: 10.3109/01902148909069605. [DOI] [PubMed] [Google Scholar]
- 40.Cooper PR, Mesaros AC, Zhang J, Christmas P, Stark CM, Douaidy K, Mittelman MA, Soberman RJ, Blair IA, Panettieri RA. 20-HETE mediates ozone-induced, neutrophil-independent airway hyper-responsiveness in mice. PLoS ONE. 2010;5:e10235. doi: 10.1371/journal.pone.0010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakano H, Aizawa H, Matsumoto K, Fukuyama S, Inoue H, Hara N. Cyclooxygenase-2 participates in the late phase of airway hyperresponsiveness after ozone exposure in guinea pigs. Eur. J. Pharmacol. 2000;403:267–275. doi: 10.1016/s0014-2999(00)00524-0. [DOI] [PubMed] [Google Scholar]
- 42.van Hoof HJ, Zijlstra FJ, Voss HP, Garrelds IM, Dormans JA, van Bree L, Bast A. The effect of ozone exposure on the release of eicosanoids in guinea-pig BAL fluid in relation to cellular damage and inflammation. Mediators Inflamm. 1997;6:355–361. doi: 10.1080/09629359791497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devlin RB, McDonnell WF, Becker S, Madden MC, McGee MP, Perez R, Hatch G, House DE, Koren HS. Time-dependent changes of inflammatory mediators in the lungs of humans exposed to 0.4 ppm ozone for 2 hr: a comparison of mediators found in bronchoalveolar lavage fluid 1 and 18 hr after exposure. Toxicol. Appl. Pharmacol. 1996;138:176–185. doi: 10.1006/taap.1996.0111. [DOI] [PubMed] [Google Scholar]
- 44.Coffey MJ, Wheeler CS, Gross KB, Eschenbacher WL, Sporn PH, Peters-Golden M. Increased 5-lipoxygenase metabolism in the lungs of human subjects exposed to ozone. Toxicology. 1996;114:187–197. doi: 10.1016/s0300-483x(96)03487-7. [DOI] [PubMed] [Google Scholar]
- 45.Chen C, Arjomandi M, Balmes J, Tager I, Holland N. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ. Health Perspect. 2007;115:1732–1737. doi: 10.1289/ehp.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlesinger RB, Driscoll KE, Gunnison AF, Zelikoff JT. Pulmonary arachidonic acid metabolism following acute exposures to ozone and nitrogen dioxide. J Toxicol Environ Health. 1990;31:275–290. doi: 10.1080/15287399009531456. [DOI] [PubMed] [Google Scholar]
- 47.McKinnon KP, Madden MC, Noah TL, Devlin RB. In vitro ozone exposure increases release of arachidonic acid products from a human bronchial epithelial cell line. Toxicol. Appl. Pharmacol. 1993;118:215–223. doi: 10.1006/taap.1993.1027. [DOI] [PubMed] [Google Scholar]
- 48.Leikauf GD, Driscoll KE, Wey HE. Ozone-induced augmentation of eicosanoid metabolism in epithelial cells from bovine trachea. Am. Rev. Respir. Dis. 1988;137:435–442. doi: 10.1164/ajrccm/137.2.435. [DOI] [PubMed] [Google Scholar]
- 49.Szarek JL, Valentovic MA. Release of prostaglandin E2 and leukotriene C4/D4 from airway segments isolated from rats after exposure to ozone for 20 months. Toxicology. 1995;100:111–119. doi: 10.1016/0300-483x(95)03070-v. [DOI] [PubMed] [Google Scholar]
- 50.Leikauf GD, Zhao Q, Zhou S, Santrock J. Ozonolysis products of membrane fatty acids activate eicosanoid metabolism in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 1993;9:594–602. doi: 10.1165/ajrcmb/9.6.594. [DOI] [PubMed] [Google Scholar]
- 51.Huber J, Fürnkranz A, Bochkov VN, Patricia MK, Lee H, Hedrick CC, Berliner JA, Binder BR, Leitinger N. Specific monocyte adhesion to endothelial cells induced by oxidized phospholipids involves activation of cPLA2 and lipoxygenase. J. Lipid Res. 2006;47:1054–1062. doi: 10.1194/jlr.M500555-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Matsumura T, Sakai M, Kobori S, Biwa T, Takemura T, Matsuda H, Hakamata H, Horiuchi S, Shichiri M. Two intracellular signaling pathways for activation of protein kinase C are involved in oxidized low-density lipoprotein-induced macrophage growth. Arterioscler. Thromb. Vasc. Biol. 1997;17:3013–3020. doi: 10.1161/01.atv.17.11.3013. [DOI] [PubMed] [Google Scholar]
- 53.Flamand N, Surette ME, Picard S, Bourgoin S, Borgeat P. Cyclic AMP-mediated inhibition of 5-lipoxygenase translocation and leukotriene biosynthesis in human neutrophils. Mol. Pharmacol. 2002;62:250–256. doi: 10.1124/mol.62.2.250. [DOI] [PubMed] [Google Scholar]
- 54.Zimman A, Mouillesseaux KP, Le T, Gharavi NM, Ryvkin A, Graeber TG, Chen TT, Watson AD, Berliner JA. Vascular endothelial growth factor receptor 2 plays a role in the activation of aortic endothelial cells by oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 2007;27:332–338. doi: 10.1161/01.ATV.0000252842.57585.df. [DOI] [PubMed] [Google Scholar]
- 55.Krönke G, Bochkov VN, Huber J, Gruber F, Blüml S, Fürnkranz A, Kadl A, Binder BR, Leitinger N. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J. Biol. Chem. 2003;278:51006–51014. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 56.Kim CE, Lee SJ, Seo KW, Park HM, Yun JW, Bae JU, Bae SS, Kim CD. Acrolein increases 5-lipoxygenase expression in murine macrophages through activation of ERK pathway. Toxicol. Appl. Pharmacol. 2010;245:76–82. doi: 10.1016/j.taap.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Yun MR, Im DS, Lee SJ, Park HM, Bae SS, Lee WS, Kim CD. 4-Hydroxynonenal enhances CD36 expression on murine macrophages via p38 MAPK-mediated activation of 5-lipoxygenase. Free Radic. Biol. Med. 2009;46:692–698. doi: 10.1016/j.freeradbiomed.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 58.Lee SJ, Kim CE, Yun MR, Seo KW, Park HM, Yun JW, Shin HK, Bae SS, Kim CD. 4-Hydroxynonenal enhances MMP-9 production in murine macrophages via 5-lipoxygenase-mediated activation of ERK and p38 MAPK. Toxicol. Appl. Pharmacol. 2010;242:191–198. doi: 10.1016/j.taap.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Cohen P. Protein kinases--the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 60.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem. J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gijón MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC. Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that Do and Do not mobilize calcium. Novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J. Biol. Chem. 2000;275:20146–20156. doi: 10.1074/jbc.M908941199. [DOI] [PubMed] [Google Scholar]
- 62.Rouzer CA, Tranguch S, Wang H, Zhang H, Dey SK, Marnett LJ. Zymosan-induced glycerylprostaglandin and prostaglandin synthesis in resident peritoneal macrophages: roles of cyclo-oxygenase-1 and −2. Biochem J. 2006;399:91–99. doi: 10.1042/BJ20060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song I, Smith WL. C-terminal Ser/Pro-Thr-Glu-Leu tetrapeptides of prostaglandin endoperoxide H synthases-1 and −2 target the enzymes to the endoplasmic reticulum. Arch. Biochem. Biophys. 1996;334:67–72. doi: 10.1006/abbi.1996.0430. [DOI] [PubMed] [Google Scholar]
- 64.Lambrecht BN. Alveolar macrophage in the driver’s seat. Immunity. 2006;24:366–368. doi: 10.1016/j.immuni.2006.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.