Abstract
Background
Exposure to carbon dioxide (CO2) gas as a killing method is aversive and exposure to high concentrations is likely to be painful. Bradycardia during exposure to CO2 is associated with nociception and pain. However, it is unclear if bradycardia occurs before loss of consciousness as definitions of loss of consciousness vary in the literature. The objectives of this study were to explore the relationship between recumbency, loss of righting reflex (LORR) and a quiescent electromyograph as measures of loss of consciousness, and identify the onset of bradycardia in relation to these measures. Our primary hypothesis was that CO2 exposure would result in bradycardia, which would precede LORR.
Methods
Thirty-two adult, female Sprague-Dawley rats were instrumented with a telemetry device and randomly assigned to one of four killing methods (concentrations of 100% CO2, CO2 (70%)/O2 (30%), isoflurane (5%) and intraperitoneal pentobarbital (200 mg/kg). Time to achieve recumbency, LORR, quiescent electromyograph, isoelectric electrocorticograph, heart rate and apnea were recorded.
Results
The general order of progression was recumbency, LORR, quiescent electromyograph, isoelectric electrocorticograph and apnea. Recumbency preceded LORR in the majority of animals (CO2; 7/8, CO2/O2; 8/8, isoflurane; 5/8, pentobarbital; 4/8). Bradycardia occurred before recumbency in the CO2 (p = 0.0002) and CO2/O2 (p = 0.005) groups, with a 50% reduction in heart rate compared to baseline. The slowest (time to apnea) and least consistent killing methods were CO2/O2 (1180 ± 658.1s) and pentobarbital (875 [239 to 4680]s).
Conclusion
Bradycardia, and consequently nociception and pain, occurs before loss of consciousness during CO2 exposure. Pentobarbital displayed an unexpected lack of consistency, questioning its classification as an acceptable euthanasia method in rats.
Introduction
The majority of laboratory rodents used in biomedical research are killed upon project completion. Ideally, the killing process is a “good death” (euthanasia), free from pain and distress. [1,2] The most recent Canadian Council on Animal Care (CCAC) and American Veterinary Medical Association (AVMA) euthanasia guidelines are broadly similar in their classification of killing methods. [1,2] Both guidelines consider CO2 to be “conditionally acceptable”/“acceptable with conditions” while overdose with intravenous or intra-peritoneal (IP) barbiturate is considered an acceptable method. In contrast, overdose with an inhalational anaesthetic agent (followed by a second method to ensure death after loss of consciousness) is considered acceptable by the CCAC and acceptable with conditions by the AVMA. Death from an overdose of inhalational anaesthetic alone, without switching to a secondary method after loss of consciousness, is slow and therefore impractical. [1,2]
Overdose with carbon dioxide (CO2) gas is a common killing method but exposure to low concentrations (< 20%) is aversive to rats and mice. [3–5] Despite this, CO2 remains popular as it is rapidly acting, simple to use, familiar, has a low risk of harm associated with human exposure and is effective for groups of animals. Exposure to the volatile anaesthetic agent, isoflurane (ISO), offers a refinement over CO2 by reducing, but not preventing, aversion in rats. [3,6] A less explored alternative, a mixture of CO2 and oxygen (CO2/O2) has been associated with fewer signs of distress during exposure than CO2 alone, though results have been conflicting. [7–9]
When CO2 is employed, a gradual fill technique with displacement rates of between 10–30% of the chamber volume per minute (cv/min) are recommended to avoid pain resulting from exposure to high concentrations of CO2 (>50%) prior to loss of consciousness. [1,2] The evidence for pain is from the human literature, with self-reports of nasal irritation and pain beginning at CO2 concentrations of > 35%. [10,11] Exposure to similar concentrations has been shown to activate nociceptors in rats [12–16] and result in reflex bradycardia. [17–19] Therefore, the observation of bradycardia during exposure to CO2 may serve as an indicator of nociception and potentially pain in rats. [20] If so, the timing of bradycardia in relation to loss of consciousness is critical to evaluating the presence of nociception or pain. However, there is confusion in the literature in how loss of consciousness is identified in rodents, leading to conflicting reports of the occurrence of bradycardia before or after loss of consciousness. [20,21] There is currently no consensus over how to identify loss of consciousness in rats, with some studies relying on cessation of movement or recumbency. [20–24] This contrasts with experimental evidence suggesting that the appropriate surrogate measure of unconsciousness is loss of the righting reflex (LORR). [25]
Using 3 treatment groups, CO2, CO2/O2 and isoflurane, the aims of this study were: 1. to compare three putative measures of loss of consciousness (recumbency, LORR and a quiescent electromyograph [EMG]) and examine the relationship of each to the presence of bradycardia and 2. to investigate the relationship between an isoelectric electrocorticograph (ECoG) and apnea as indicators of impending death. We hypothesised that bradycardia would precede the loss of righting reflex, indicating the possibility of pain prior to loss of consciousness and that the appearance of an isoelectric ECoG would be closely related to apnea. After initiating the project, a fourth treatment group, IP sodium pentobarbital (PB), was added as it was felt this would serve as a criterion standard for comparison.
Materials and Methods
Animals
Experiments were performed at the University of Calgary following approval by the University of Calgary Health Science Animal Care Committee (protocol AC11-0044), which operates under the auspices of the CCAC.
A sample size calculation for the primary outcome of bradycardia (decrease in heart rate of 100 bpm) indicated a sample size of 7 animals (beta of 0.8, alpha of 0.05, mean difference of 100 bpm, SD of 75). Thirty-two female Sprague-Dawley rats (Health Science Centre Animal Resource Centre, Calgary, Alberta, Canada) without previous exposure to anaesthesia or CO2 gas and weighing between 250 to 500 grams were used. Animals were housed in a 12h:12h light cycle (lights on at 0700h) and were group housed prior to instrumentation and singly housed afterwards (to avoid cage mates interfering with surgical incisions), in micro-isolator rat cages (48 x 27 x 20cm [Ancare Corp., Worcester, MA, USA]). Fresh water and food (Prolab 2500 Rodent 5p14, Lab diet, PMI Nutrition International, St Louis MO, USA) were available ad libitium. Plastic tubing (PVC pipe, provided by the Health Science Animal Resource Centre, Calgary, AB, Canada) wood shavings (Aspen chip, NEPCO, Warrensburg, NY, USA) and Nestlets (Nestlets nesting material, Ancare, Bellmore, New York, USA) were provided for bedding and enrichment. All experiments were performed between 1000h and 1600h.
Treatment groups
Animals were block randomized (www.random.org) to one of three killing methods (n = 8 per group): CO2 (Praxair, Calgary, AB, Canada); exposure to 100% CO2 at a fill rate of 20% cv/min, isoflurane group; 5% isoflurane carried in oxygen at a fill rate of 20% cv/min until LORR, followed by stopping isoflurane administration and switching to 100% CO2 (30% cv/min), and CO2/O2; exposure to a mixture of 70% carbon dioxide and 30% oxygen at a fill rate of 20% cv/min. A fourth group of 8 animals, assigned to receive IP PB; (200 mg/kg, 240 mg/ml, Euthanyl, Bimedia MTC, Cambridge, ON, Canada), was added after beginning the study.
Telemetry instrumentation
Each rat was implanted with a radio transmitter (4ET-S2 Radio Transmitter Data Sciences International, St Paul, MN, USA) placed subcutaneously lateral to midline on the dorsum with leads for EMG, electrocardiography (ECG) and ECoG tunnelled subcutaneously to the central trapezius muscle of the neck (EMG), pectoral muscles (ECG) and skull (ECoG leads [+ 2 mm from Bregma, + 2 mm from midline], [- 2 mm from Bregma, - 2 mm from midline], [- 2 mm from Bregma, - 2 mm from midline] and reference [-3 mm from Lambda, 0 mm from midline]). Leads were sutured (ECG, EMG) or glued (ECoG, dental acrylic) in place. Surgery for instrumentation was facilitated with general anaesthesia as follows. General anaesthesia was induced with isoflurane (5%) carried in oxygen (1 L/min), with rats placed singly in a perspex chamber (3L volume). Following LORR the rat was moved to the surgical area and isoflurane (1.5–2%) delivered through a nose cone. Surgical sites were clipped and aseptically prepared and pre-emptive analgesia given. All animals received 0.1 ml (2 mg) of 2% lidocaine (diluted in 0.8 ml saline) as incisional line blocks, enrofloxican (50 mg/kg SC, 25 mg/ml, Baytril, Bayer, Toronto, ON, Canada), saline (4 ml, NaCl 0.9%, Baxter Corporation, Mississauga, Ontario, CA), buprenorphine 0.05 mg/kg SC, every 8 hours (0.3 mg/ml Vetergesic, Champion Alstoe Animal Health, Whitby, ON, Canada) and meloxicam 1 mg/kg SC, every 24 hours (Metacam, Boehringer Ingelheim, Burlington, ON, Canada). Analgesics were continued for a minimum of 24 hours following surgery and pain assessed regularly (every 6–8 hours) by monitoring activity, posture, grooming and body weight. Antibiotics were continued for two days following the surgery. A minimum of 7 days passed before the experimental day.
Experiment
For the experiment, animals were placed singly in a customised perspex chamber (25.5 (l) x 10 (w) x 12 (h) cm). The chamber had ports for gas entry and exit located on the short sides at opposite ends. The following physiological parameters were collected using commercial software (Data quest Advanced Research Technology version 4.3, Data Sciences International St. Paul, MN, USA): ECoG, EMG and ECG. The ECoG and EMG signals were sampled at 500 Hz with a 0–100 Hz bandpass filter. The ECG signal was sampled at 1000 Hz with a 0–250 Hz bandpass filter. Baseline data were recorded over five minutes during exposure to room air. In the IP PB group, injections were given following baseline recording and the animal immediately returned to the recording chamber. A two-person injection technique was used, with one person holding the rat in dorsal recumbency with the head slightly lower than the pelvis (approximately 30 degrees to horizontal) and the other person giving the injection (through a 20G 1 inch needle, injectate volume calculated from body mass) in the right caudal abdominal quadrant approximately 0.5 cm from midline and at the level of the coxofemoral joint. Aspiration was performed before injection to minimise risk of perforating a hollow viscus.
Times were recorded from baseline (beginning inhaled agent or completing IP injection) to each of the following events: recumbency, LORR, quiescent EMG, isoelectric ECoG and apnea.
Recumbency was defined as the moment when an animal’s body and head were in full contact with the chamber floor. The LORR was determined by manually tilting the chamber to place the animal on its back, assessing its ability to right itself. The onset of recumbency triggered the first assessment of LORR. LORR was confirmed if a rat could be turned on to its back for at least 10s. If LORR occurred at the first test, the same time was given for recumbency and LORR. When an animal was able to right itself, the LORR was re-tested once recumbency had been regained. Observations of recumbency and LORR were performed by a single observer. An isoelectic ECoG was identified by off-line visual inspection of the ECoG and defined as a waveform amplitude within ± 0.025 mV, similar to the definition in humans (Fig 1). [26]
Fig 1. A representative example of the onset of an isoelectric electrocorticograph (ISOEL), occurring after loss of the righting reflex (LORR).
A quiescent EMG was determined by off-line visual inspection of the EMG and defined as a waveform amplitude < ± 0.01 mV.
Heart rates were averaged over the 10 seconds immediately preceding each of the following times: end of baseline and occurrence of recumbency, LORR, isoelectric ECoG and apnea. Each rat was kept in the chamber until cardiac asystole was observed on the ECG. Death was confirmed by digital palpation of the thorax to confirm absence of a heart beat.
Statistical analyses
Data were blinded and analysed with commercial software (Prism v7.0a, GraphPad Software Inc., La Jolla, CA, USA). Data were assessed for normality with a Shapiro-Wilk normality test. The number of animals becoming recumbent before LORR was evaluated with a Sign test. Differences between groups (time to event data) were compared with one-way ANOVA with a Tukey’s post hoc test. Differences within groups (time to event data) were compared with one-way ANOVA for repeated measures (a Greenhouse-Geisser correction was applied for violations of sphericity) and a Sidak’s post hoc test. Heart rate data were analysed for differences within groups with a one-way ANOVA for repeated measures (a Greenhouse-Geisser correction was applied for violations of sphericity) and a Dunnett’s post hoc test (comparison to baseline values). Where there was a significant change in heart rate between baseline and recumbency or LORR, a one-way ANOVA with Sidak’s post hoc test was used to compare heart rates between groups at these two time points. Pentobarbital data were handled separately and compared with the CO2 treatment group with either a Mann-Whitney test or unpaired t test, depending on distribution of the data. Coefficient of variation was calculated to provide an indication of data variability. A value of p < 0.05 was considered significant and 95% confidence intervals of the mean difference (95% CI) presented where available.
Results
Data from the inhalational treatment groups were normally distributed. In the IP PB group heart rate data were normally distributed whereas time data were not. No animals recovered consciousness or a heart beat upon removal from the test chamber after observing asystole on the ECG.
Recumbency precedes loss of righting reflex
Recumbency preceded LORR in 7/8 animals in the CO2 group (p = 0.035), 8/8 animals in the CO2/O2 group (p = 0.004) and 5/8 animals in the ISO group (p = 0.36). The time from recumbency to LORR ranged from 21.2–49.1 seconds (Table 1) but did not differ significantly for each group (CO2; p = 0.30; 95%CI [-57.0, 14.5], CO2/O2; p = 0.16; 95%CI [-115.0, 16.7] and ISO; p = 0.61; 95%CI [-82.0, 34.2]).
Table 1. Time (seconds) between events for each treatment group.
| Treatment group | Baseline—recumbency | Baseline—LORR | Baseline—quiescent EMG | Baseline—isoelectric EEG | Baseline—apnea |
|---|---|---|---|---|---|
| CO2 | 115.3 ± 31.2 | 136.5 ± 53.0a 164.9 ± 54.1 | 193.3 ± 83.2a,bb | 239.3 ± 73.0bb | |
| Isoflurane | 137.3 ± 24.0 | 161.4 ± 54.6aa 184.5 ± 55.4 | 236.0 ± 63.4aa,bbb | 434.1 ± 99.7bbb | |
| CO2/O2 | 119.8 ± 26.3 | 168.9 ± 66.9a,bbb | 226.6 ± 107.6a | 338.5 ± 63.2bbb,c | 1180.0 ± 658.1c |
Time (seconds) between events for each treatment group. CO2, carbon dioxide. CO2/O2, carbon dioxide/ oxygen. Main effects were significant for each group (CO2; p = 0.0007, isoflurane; p < 0.0001, CO2/O2; p = 0.004) Same superscript letter denotes significant difference between time points within a group: single letter; p < 0.05, two letters; p ≤ 0.01, three letters; p ≤ 0.001. Statistical comparisons were restricted to: recumbency vs. loss of righting reflex (LORR), LORR vs. quiescent electromyograph (EMG), LORR vs. isoelectric electrocorticograph (ECoG), isoelectric ECoG vs. apnea. Results of between group comparisons are presented in the text. Data are mean ± SD.
There were no significant differences between inhalational treatment groups for the time from baseline to recumbency (main effect; p = 0.26, CO2 vs. ISO, p = 0.26, 95%CI [-56.4, 12.4]; CO2 vs CO2/O2, p = 0.94, 95% CI [-38.9, 29.9]; ISO vs CO2/O2, p = 0.42, 95% CI [-16.9, 51.9], Table 1). Similarly, there were no significant differences between inhalational treatment groups from baseline to LORR (main effect; p = 0.52, CO2 vs. ISO, p = 0.68, 95%CI [-98.6, 48.8]; CO2 vs CO2/O2, p = 0.52, 95% CI [-106.1, 41.3]; ISO vs CO2/O2, p = 0.96, 95% CI [-81.2, 66.2]).
LORR preceded EMG quiescence in all animals in the CO2/O2 treatment group and the delay between these events was significant (mean difference 57.8 seconds, Table 1). The majority of animals in the CO2 (7/8) and ISO (6/8) groups exhibited EMG quiescence prior to LORR, though the shorter mean time differences was not significantly different within each group (Table 1). There were no significant differences between inhalational treatment groups from LORR to a quiescent EMG (main effect; p = 0.12, CO2 vs. ISO, p = 0.95, 95%CI [-37.9, 48.4]; CO2 vs CO2/O2, p = 0.22, 95% CI [-72.5, 13.8]; ISO vs CO2/O2, p = 0.13, 95% CI [-77.8, 8.5]).
PB did not differ significantly from the CO2 group in the time elapsed between baseline and recumbency (p = 0.43) or baseline and LORR (p = 0.12, Table 2), with recumbency preceding LORR in 4/8 animals. However, in contrast to the inhalational treatment groups, EMG quiescence preceded LORR in 7/8 animals. This early onset of EMG quiescence was significantly faster than the CO2 group (p = 0.004).
Table 2. Recorded times for recumbency, loss of righting reflex (LORR) and electromyography (EMG) quiescence in the pentobarbital treatment group.
| Time points | median (range) | mean ± SD |
|---|---|---|
| Baseline to recumbency | 130.0 (40.0, 445.0) | 174.6 ± 125.4 |
| Baseline to LORR | 165 (50.0, 181.0) | 272.1 ± 204.8 |
| Baseline to quiescent EMG | 157 (25.0, 583.0) | 259.0 ± 201.0 |
Recorded times for recumbency, loss of righting reflex (LORR) and electromyography (EMG) quiescence in the pentobarbital treatment group. Statistical comparisons with the CO2 treatment group were performed with median (range) data; mean ± SD are provided for completeness.
Bradycardia precedes both recumbency and loss of righting reflex
Heart rates did not differ between treatment groups at baseline (main effect; p = 0.10, CO2 vs. CO2/O2, p = 0.58, 95%CI [-27.9, 64.9]; CO2 vs. isoflurane, p = 0.44, 95% CI [-69.5, 23.3]; CO2/O2 vs. isoflurane, p = 0.08, 95%CI [-4.8, 88.0]; CO2 vs. PB, p = 0.52, 95% CI [-26.2, 49.4]), with average values ranging from 396 to 438 beats per minute (Fig 2 and Table 3).
Fig 2. Heart rates in the carbon dioxide (circles) and carbon dioxide-oxygen (triangles) treatment groups decrease significantly compared to the isoflurane group (squares) at recumbency (RECUMB, **** p < 0.0001, both comparisons) and loss of the righting reflex (LORR, **** p < 0.0001, both comparisons).
At LORR, heart rates are significantly increased in the carbon dioxide-oxygen group compared with the carbon dioxide group (†† p = 0.008). ISOEL, isoelectric electrocorticograph. Data are mean ± SEM.
Table 3. Heart rates (beats per minute, averaged over 10 seconds immediately before each event) recorded at different events in treatment groups.
| CO2 | CO2/O2 | Isoflurane | PB | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 414.6 ± 39.7 | 396.1 ± 34.6 | 437.7 ± 36.0 | 426.2 ± 30.2 | ||||
| Recumbency | 173.0 ± 74.6 | p = 0.0002 (158.1, 325.2) | 193.0 ± 100.9 | p = 0.005 (78.4, 327.9) | 418.6 ± 31.1 | p = 0.48 (-23.3, 61.4) | 403.6 ± 45.5 | p = 0.58 (-35.0, 80.3) |
| LORR | 119.9 ± 57.6 | p = 0.0001 (255.5, 334.0) | 233.9 ± 119.3 | p = 0.03 (20.5, 303.8) | 425.4 ± 19.7 | p = 0.71 (-25.2, 49.8) | 399.1 ± 37.4 | p = 0.32 (-21.4, 75.7) |
| ECoG | 135.4 ± 49.2 | p = 0.0001 (207.4, 351.0) | 267.3 ± 80.6 | p = 0.01 (31.4, 226.1) | 351.3 ± 103.5 | p = 0.21 (-43.1, 216.0) | 320.1 ± 56.0 | p = 0.01 (26.0, 186.3) |
| Apnea | 91.6 ± 23.1 | p = 0.0001 (262.8, 383.3) | 124.8 ± 49.7 | p = 0.0001 (202.3, 340.3) | 190.6 ± 100.6 | p = 0.0007 (136.1, 358.1) | 249.2 ± 34.7 | p = 0.0001 (137.5, 216.7) |
Heart rates (beats per minute, averaged over 10 seconds immediately before each event) recorded at different events in treatment groups. PB = pentobarbital. LORR = loss of righting reflex. ECoG = isoelectric electrocorticograph. p values represent within group comparisons to baseline. Main effects were significantly different for all groups (p < 0.0001, all cases). Figures in parentheses are the 95% confidence intervals for the mean difference between baseline and event being compared. See text for results of between group comparisons. Data are mean ± SD.PB = pentobarbital. LORR = loss of righting reflex. ECoG = isoelectric electrocorticograph.
Bradycardia prior to loss of the righting reflex only occurred in the CO2 and CO2/O2 groups (Fig 2 and Table 3) with an average decrease of 58.3% and 51.3%, respectively. In the isoflurane and PB treatment groups, bradycardia appeared at or after the onset of an isoelectric ECoG (Table 3). At recumbency, the bradycardia observed in the CO2 and CO2/O2 groups was significantly lower than the isoflurane group (main effect; p < 0.0001, isoflurane vs. CO2, 95% CI [-342.4, -148.8]; isoflurane vs CO2/O2, 95% CI [-322.4, -128.8]; p < 0.0001 both comparisons, Fig 2). There was no significant difference between CO2 and CO2/O2 groups at recumbency (p = 0.85, 95% CI [-116.8, 76.8]) but heart rate was significantly higher (approximately double) in the CO2/O2 group at the LORR (p = 0.008, 95% CI [-202.3, -25.7], Fig 2). Both CO2 and CO2/O2 groups had significantly lower rates than the isoflurane group (isoflurane vs. CO2, 95% CI [-393.8, -217.2]; isoflurane vs CO2/O2, 95% CI [-279.8, -103.2]; p < 0.0001 both comparisons). Heart rates in all groups converged at the point of apnea (Table 3).
Isoelectric ECoG occurs after loss of righting reflex and precedes apnea
An isoelectric ECoG occurred after LORR in all animals, representing an increasing depth of anaesthesia (Fig 3A). The onset of an isoelectric ECoG was shortest in the CO2 group (Table 1). This was not significantly different from the isoflurane group (main effect; p = 0.0002, p = 0.73, 95% CI [-76.6, 40.9]) and occurred sooner than in the CO2/O2 group (169.6 ± 50.2 seconds, p = 0.0002, 95% CI [-171.6, -54.1]). Onset of an isoelectric ECoG was also earlier in the isoflurane group compared with the CO2/O2 group (p = 0.002, 95% CI [-153.8, -36.3]). The PB group did not differ from the CO2 group, but exhibited considerable data variability (p = 0.06, 101 [25.0 to 2342.0] seconds).
Fig 3. Time periods during which differences between treatment groups emerged.
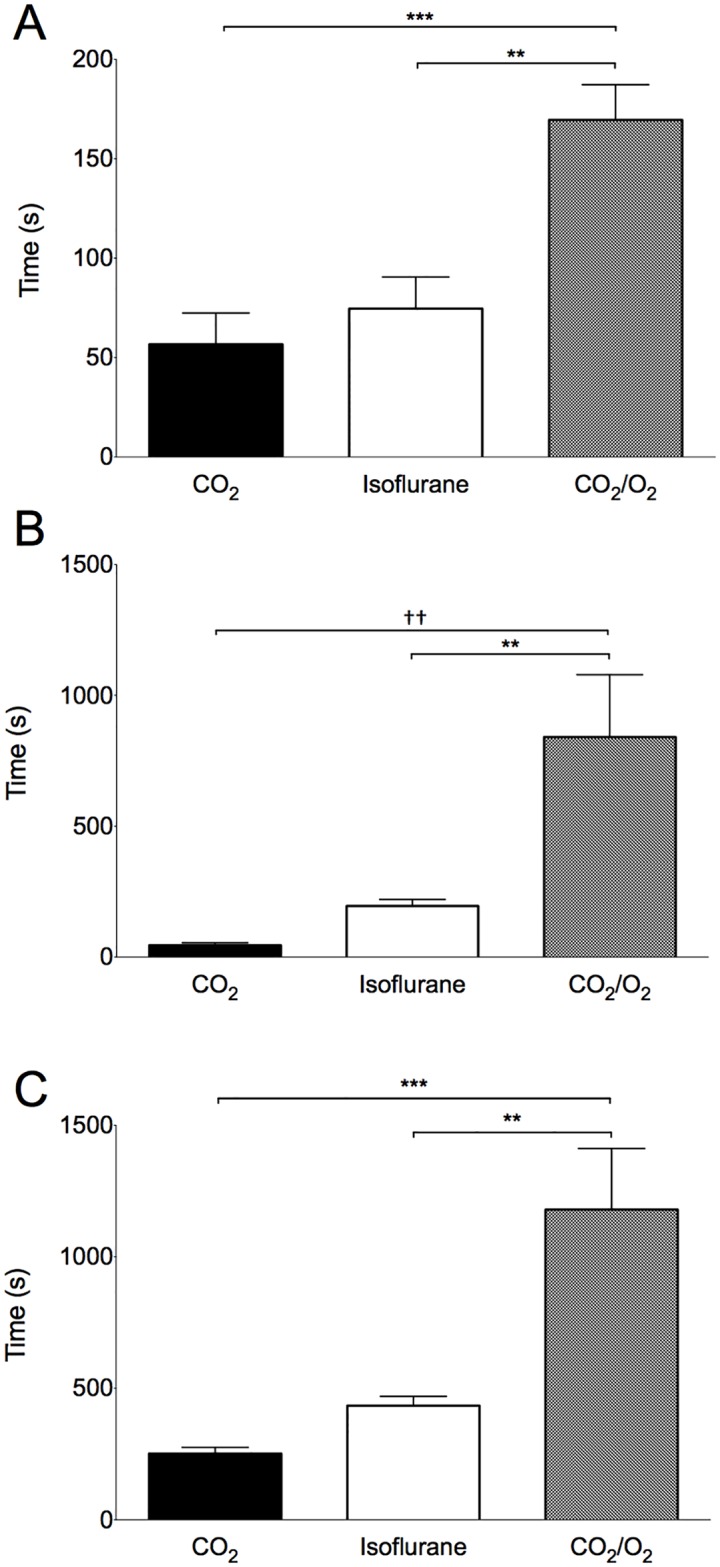
A: Time from loss of the righting reflex until an isoelectric electrocorticograph. *** p = 0.0002, ** p = 0.002. B: Time from an isoelectric electrocorticograph until apnea. †† p = 0.002, ** p = 0.01. C: Time from baseline until apnea. ** p = 0.003, *** p = 0.0003. CO2, carbon dioxide. CO2/O2, carbon dioxide/oxygen. Data are mean ± SEM.
Apnea occurred after an isoelectric ECoG in all cases (Fig 3B). This period was shortest for the CO2 group (Table 1) and was significantly faster compared with the CO2/O2 group (main effect; p = 0.0002, p = 0.002, 95% CI [-1288, 302.6]), but not the isoflurane group (p = 0.72, 95% CI [-644.7, 340.4]). This time course was also shorter in the isoflurane compared with the CO2/O2 group (p = 0.009, 95% CI [-1136.0, 150.5]). The PB group did not differ from the CO2 group, but again displayed large data variability (287.5 [4.0 to 4200.0 seconds], p = 0.07).
Time to apnea
The time course for the entire observation period (from baseline until apnea) was fastest in the CO2 and ISO groups (main effect; p = 0.0002, Fig 3C and Table 1). The time to apnea in the CO2 group (239.3 ± 73.0 seconds) was approximately half that of the ISO group (434.1 ± 99.7 seconds), though there was no significant difference between these groups (p = 0.61, 95% CI [-669.0, 304.0]). The source of the increased time to apnea in the ISO group resulted from a four fold increase in average time between isoelectric ECoG and apnea compared to the CO2 group (Fig 3B and Table 1). Both CO2 and isoflurane treatment groups reached apnea faster than the CO2/O2 group (vs. CO2, p = 0.0003, 95% CI [-1415.0, -441.0]; vs. ISO, p = 0.003, 95% CI [-1232.0, -259.0]). Time to apnea was faster in the CO2 group than the PB group (p = 0.005, 875 [239 to 4680] seconds). The most consistent killing methods, with the lowest coefficients of variation, were CO2 (26.9%) and ISO (23.0%), followed by CO2/O2 (55.8%) and PB (114.1%). In the PB treatment group, three rats contributed to substantial variability in the data set, as a result of suspected misinjection.
Discussion
In evaluating euthanasia methods the AVMA Guidelines for the Euthanasia of Animals include assessment of the following criteria: the “time required to induce loss of consciousness”, “reliability” and the “ability to induce loss of consciousness and death with a minimum of pain and distress”. [1] Our data provide insight on the time to loss of consciousness and reliability of the studied methods, allowing comment on the potential for pain and distress.
We have shown that: 1. Recumbency was identified before LORR, indicating that recumbency is not an accurate indicator of loss of consciousness, 2. bradycardia occurs in response to exposure to carbon dioxide gas both with and without supplemental oxygen and that bradycardia precedes LORR, 3. euthanasia with a gradual fill carbon dioxide technique is the fastest of the methods studied to achieve apnea but the time to LORR did not differ between carbon dioxide and isoflurane. The addition of supplemental oxygen during carbon dioxide euthanasia substantially increases time to apnea and 4. considerable variability is associated with both CO2/O2 and IP PB methods, questioning the classification of IP PB as an acceptable euthanasia method. [1,2]
There is a strong positive correlation between LORR in rodents and unconsciousness in humans, suggesting that LORR is an appropriate proxy for loss of consciousness in rats. [25] The onset of LORR equates to a light plane of anaesthesia, insufficient to prevent movement in response to a noxious stimulus, approximating MACawake in humans, where MAC is the minimum alveolar concentration of an inhalational anaesthetic agent which prevents gross, purposeful movement in response to a supramaximal noxious stimulus in an individual (or 50% of a study population). [27] And MACawake is the lower concentration of anaesthetic, approximately 50% of MAC, when an individual (or 50% of a study population) cannot provide a verbal response to a command. [28] For this reason, we chose LORR as our endpoint to indicate unconsciousness, accepting that it is, by definition, an estimate. However, we felt, as supported by presented data, that recumbency is an imprecise indicator of unconsciousness. Using recumbency as an outcome underestimates loss of consciousness and consequently the period of time during which pain may be perceived.
Recumbency preceded LORR in the majority of animals studied and was significant for the CO2 and CO2/O2 groups, though the time difference within groups was not statistically significant. This suggests that previous investigations which used recumbency as a proxy for loss of consciousness underestimated the speed to reach loss of consciousness. [20–23,29] This is a likely explanation for the return of movement observed in one study, where absence of movement was used as a proxy for loss of consciousness and as the trigger for switching from isoflurane to 100% CO2, to complete the killing process. [24] As the time between initiation of the killing process and unconsciousness is a critical period when pain or distress may be perceived, the reliance on recumbency has implications for the assessment of welfare of killing methods. In this study, the mean time to achieve recumbency in the CO2 group of 115 seconds, is similar to that previously reported where gradual fill techniques were used. [14,20,21,23] The observed lack of significant difference between time to recumbency and time to LORR reflects the relatively small mean differences between these time points in the context of data variability. Importantly, this should not detract from the finding that a recumbent state was observed before LORR, spanning 20–50 seconds, during which time pain may be perceived.
Moody et al. (2015) suggested a more conservative indicator of unconsciousness, an absent pedal withdrawal reflex. [30] This undoubtedly reduces the risk that an animal may be conscious during exposure to a noxious stimulus, a valid consideration when deciding to expose an animal to such a stimulus (e.g. high concentration CO2, surgery). However, the literature suggests that movement can occur when an animal (or person) is unconscious as the concentration of anaesthetic required to induce loss of consciousness is lower than that required to abolish movement. [28,31–33] Therefore, an absent spinal reflex (pedal withdrawal) reflects a greater depth of anaesthesia than that required for loss of consciousness. However, this is an important end point when the consequences of possible consciousness during noxious stimulation are unacceptable.
Residual muscle activity beyond loss of consciousness was reflected in the time to achieve a quiescent EMG exceeding that required for LORR. Hewett et al (1993) observed increased muscle tonicity during exposure to high concentrations (>90%, pre-fill) of CO2 and spontaneous muscle activity can continue after death. [21,34] Together, this indicates that appearance of a quiescent EMG is an insensitive indicator of unconsciousness.
An isoelectric ECoG represents depressed cortical function, beyond that typically observed with therapeutic doses of anaesthetic and analgesic drugs. [35] However, the presence of an isoelectric ECoG alone is insufficient to confirm death. [36–38] Our results show that the time between onset of the isoelectric EEG and apnea varied considerably between treatment groups, taking up to 14 minutes in the CO2/O2 group in contrast to approximately 3 minutes in the isoflurane group and approximately 45 seconds in the CO2 group. The prolonged time to achieve an isoelectric ECoG in the isoflurane and CO2/O2 treatment groups suggests that providing O2 may delay its onset and the time to apnea.
The potential benefit of using a mixture of CO2 and O2 for euthanasia is controversial. [7–9] Coenen et al. (1995) reported that the combination of oxygen and carbon dioxide, delivered at a high chamber fill rate (188% cv/min, 2:1 CO2:O2 ratio) prevented gasping when compared with carbon dioxide alone. [7] In contrast, Iwarsson and Rehbinder (1993) observed laboured breathing and “uneasiness” during exposure to a chamber pre-filled with carbon dioxide (80%) and oxygen (20%). [8] The combination of CO2 and O2 has a modest effect on reducing aversion to the gas mixture in comparison to CO2 alone. [9] These studies also reported a prolonged time to death with CO2/O2 compared with CO2 alone despite the rapid rate of exposure. This slowing of the killing process reflects our observations that, when compared with CO2 alone, the time from LORR to apnea was 10 times longer in the CO2/O2 group. Up to the point of LORR there was no significant difference between these two groups.
Given the conflicting reports of behaviours associated with respiratory distress, a prudent response to available evidence which takes in to account the AVMA guidelines for evaluating killing methods is to avoid the addition of O2 to CO2. [1]
In humans, nasal exposure to CO2 concentrations of approximately 35% are reported as moderately irritating, with irritation increasing as CO2 concentrations increase. [10,11] At similar concentrations, conjunctival and corneal exposure to CO2 result in stinging and burning sensations [39,40] and this broadly corresponds to nociceptor activation in rats beginning at CO2 concentrations between 25–50%. [12,15,16] In humans, the onset of pain (nasal and ocular) begins at CO2 concentrations of approximately 40%, slightly (< 10%) above that resulting in nociceptor activation. [13,14,41]
Exposure of the nasal mucosa to CO2 at concentrations associated with irritation and pain in humans results in a reflex bradycardia in rats, mediated through the vagal nerve via baro- and chemoreflexes. [17,19] This is believed to serve as a protective reflex during inhalation of noxious substances by conserving oxygen consumption. [17] It is unclear if bradycardia occurs in humans when exposed to CO2 concentrations resulting in irritation or pain as studies typically rely on self-reports of sensations. [10,11,13,14,39,40] In the few human studies in which heart rates were reported, most applied low CO2 concentrations (≤ 10%), exposure to which resulted in tachycardia. [42–44] Tachycardia was also reported in a study using a CO2 concentration of 30%. [45] In rats, the appearance of bradycardia at CO2 concentrations resulting in increased nociceptor activation in this species and self-reports of irritation/pain in humans makes it a relatively simple readout of irritation and pain. [12,15–17,19]
Our finding that bradycardia occurs prior to LORR contrasts with those of Hawkins et al. (2006), when bradycardia was observed approximately 120 seconds after recumbency. [20] Similar to our findings, three studies that recorded recumbency, but not LORR, observed bradycardia near the onset of recumbency. [7,21,23] Furthermore, the gas flow rates used (14, 17.3 and 22% cv/min) and measurement of chamber CO2 indicated that bradycardia occurred at a concentration of CO2 lower (approximately 33–35%) than the 100% reported by Yavari et al. (1996). [19] Yavari et al. did test lower concentrations of CO2 (10, 25 and 50%) but did not present their results beyond stating that cardiorespiratory responses (including bradycardia) were only “significant” during exposure to 100% CO2 and initiated “somewhere between [CO2 concentrations of] 50 and 100%”. [19] Unfortunately, we did not record CO2 concentration in our testing chamber, but an estimated concentration of 33–35% is likely given the similarities in chamber fill rates and time to recumbency reported by others. [21,23]
The variability observed in the PB group at each phase was considerably worse than expected and suspected to result from misinjection. Unfortunately, necropsy examinations were not performed and the PB solution used did not include a coloured dye. Intraperitoneal misinjection has been previously documented in rats, reporting rates of 6–20% by trained, experienced personnel. [46–48] There are several potential sites for inadvertent placement of the injectate, including intra-abdominal fat, the abdominal wall, subcutaneous space, retroperitoneal space and viscera. [46–48] Strategies to reduce misinjection rates include using a two person injection technique (as in this study), however, the efficacy of these strategies is largely unproven.
Though the incidence of misinjection could not be determined in our study, the high coefficient of variation and wide variability observed for the total observation period (baseline to apnea) raises the index of suspicion that misinjection occurred. Concerningly, the time to recumbency and LORR did not differ significantly compared to the CO2 group, with the delay to apnea occurring after these end points. This highlights the importance of confirming death. [1,2]
The observed variability when using IP PB suggests that its current classification as an “acceptable” method needs re-evaluation to account for route of administration. [1,2]
This study had several limitations. We were unable to determine an accurate time of death as animals were left undisturbed in the test chamber until all cardiac electrical activity had ceased. It is highly likely that pulseless electrical activity would have been present, which without concurrent arterial blood pressure recording, prevents accurate determination of death. Consequently, apnea was used as the study end-point. The time between apnea and loss of pulsatile blood flow was previously reported as approximately one minute using a 22% cv/min gradual fill technique with 100% CO2. [21] The time from baseline to apnea in the isoflurane group could have been shortened by increasing the flow rate of CO2 gas after LORR occurred. In doing so, it is likely that the time to produce apnea would have been closer to that of the CO2 group. This study was not designed to explore the cause(s) of the inconsistent results seen in the PB group. Further work is necessary to determine if intra-peritoneal overdose with PB can be improved. Animals included in the PB group were not randomised to treatment as this group was added after beginning the study. This raises the possibility of an inadvertent systematic bias. Our results are limited to the strain and sex studied.
Conclusions
The onset of recumbency is an inaccurate indicator of loss of consciousness in rats exposed to CO2, CO2/O2 and isoflurane, underestimating the presence of pain while conscious. Consequently, we recommend using LORR as measure of loss of consciousness. The appearance of bradycardia before LORR, at concentrations of CO2 resulting in nociceptor activation in rats and irritation and pain in humans, raises concerns that currently recommended chamber displacement rates for CO2 do not prevent irritation and pain.
Overdose with intraperitoneal PB was an inconsistent killing method and its classification as an acceptable euthanasia method should be revisited.
Acknowledgments
The authors wish to thank Dr Aleks Krajacic and Dr Deb De Rantere for technical assistance, Dr Darrel Florence for insightful discussions during project planning and Dr Grace Kwong for statistical advice.
Data Availability
All data are available from the Harvard Dataverse website: http://dx.doi.org/10.7910/DVN/DWME60.
Funding Statement
Natural Sciences and Engineering Research Council of Canada Discovery Grant (424022-2013), awarded to DP. Margaret Gunn Endowment for Animal Research (University of Calgary), awarded to DP.
References
- 1.Association AVM (2013)American Veterinary Medical Association. AVMA guidelines for the euthanasia of animals: 2013 edition. Available: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf [Google Scholar]
- 2.Charbonneau R, Niel L, Olfert E, von Keyserlingk M, Griffin G (2010). CCAC guidelines on: euthanasia of animals used in science. 2010.) Available:http://www.ccac.ca/en_/standards/guidelines [Google Scholar]
- 3.Leach MC, Bowell VA, Allan TF, Morton DB (2002). Aversion to gaseous euthanasia agents in rats and mice. Comp Med. 2002);52: 249–257. [PubMed] [Google Scholar]
- 4.Niel L, Weary DM (2007). Rats avoid exposure to carbon dioxide and argon. Appl Anim Behav Sci. 2007);107: 100–109. [Google Scholar]
- 5.Niel L, Stewart SA, Weary DM (2008). Effect of flow rate on aversion to gradual-fill carbon dioxide exposure in rats. Appl Anim Behav Sci. 2008;109: 77–84. [Google Scholar]
- 6.Makowska IJ, Weary DM (2009). Rat aversion to induction with inhalant anaesthetics. Appl Anim Behav Sci. 2009);119: 229–235. [Google Scholar]
- 7.Coenen AM, Drinkenburg WH, Hoenderken R, van Luijtelaar EL (1995). Carbon dioxide euthanasia in rats: oxygen supplementation minimizes signs of agitation and asphyxia. Lab Anim. 1995;29: 262–268. [DOI] [PubMed] [Google Scholar]
- 8.Iwarsson K, Rehbinder C (1993). A study of different euthanasia techniques in guinea pigs, rats, and mice. Animal response and postmortem findings. Scand J Lab Anim Sci. 1993;20: 191–205. [Google Scholar]
- 9.Kirkden RD, Niel L, Stewart SA, Weary DM (2008). Gas killing of rats: the effect of supplemental oxygen on aversion to carbon dioxide. Anim Welfare. 2008);17: 79–87. [Google Scholar]
- 10.Shusterman D, Avila PC (2003). Real-time monitoring of nasal mucosal pH during carbon dioxide stimulation: implications for stimulus dynamics. Chem Senses. 2003);28: 595–601. [DOI] [PubMed] [Google Scholar]
- 11.Wise PM, Wysocki CJ, Radil T (2003). Time-intensity ratings of nasal irritation from carbon dioxide. Chem Senses. 2003);28: 751–760. [DOI] [PubMed] [Google Scholar]
- 12.Anton F, Peppel P, Euchner I, Handwerker HO (1991). Controlled noxious chemical stimulation: responses of rat trigeminal brainstem neurones to CO2 pulses applied to the nasal mucosa. Neurosci Lett. 1991);123: 208–211. [DOI] [PubMed] [Google Scholar]
- 13.Anton F, Euchner I, Handwerker HO (1992). Psychophysical examination of pain induced by defined CO2 pulses applied to the nasal mucosa. Pain. 1992);49: 53–60. [DOI] [PubMed] [Google Scholar]
- 14.Danneman PJ, Stein S, Walshaw SO (1997). Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci. 1997);47: 376–385. [PubMed] [Google Scholar]
- 15.Peppel P, Anton F (1993). Responses of rat medullary dorsal horn neurons following intranasal noxious chemical stimulation: effects of stimulus intensity, duration, and interstimulus interval. J Neurophysiol. 1993);70: 2260–2275. [DOI] [PubMed] [Google Scholar]
- 16.Thurauf N, Friedel I, Hummel C, Kobal G (1991). The mucosal potential elicited by noxious chemical stimuli with CO2 in rats: is it a peripheral nociceptive event? Neurosci Lett. 1991);128: 297–300. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, Cheng ZB, Nosaka S (1999). Inhibition of baroreflex vagal bradycardia by nasal stimulation in rats. Am J Physiol. 1999);276: H176–84. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Majima Y (2004). Target site of inhibition of baroreflex vagal bradycardia by nasal stimulation. Brain Res. 2004);1009: 137–146. [DOI] [PubMed] [Google Scholar]
- 19.Yavari P, McCulloch PF, Panneton WM (1996). Trigeminally-mediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J Auton Nerv Syst. 1996);61: 195–200. [DOI] [PubMed] [Google Scholar]
- 20.Hawkins P, Playle L, Golledge H, Leach MC, Banzett R, Coenen A et al. (2006) Newcastle consensus meeting on carbon dioxide euthanasia of laboratory animals. 2006) Available: http://nc3rs.org.uk/euthanasia via the Internet. [Google Scholar]
- 21.Smith W, Harrap SB (1997). Behavioural and cardiovascular responses of rats to euthanasia using carbon dioxide gas. Lab Anim. 1997);31: 337–346. [DOI] [PubMed] [Google Scholar]
- 22.Correia R, Pereira A, Gabriel J, Antunes L (2015). Anaesthesia induction in small mammal’s using an instrumented anaesthetic chamber.) IEEE 4th Portugese BioEngineering Meeting. 2015.
- 23.Niel L, Weary DM (2006). Beahvioural responses of rats to gradual-fill carbon dioxide euthanasia and reduced oxygen concentrations. Appl Anim Behav Sci. 2006);100: 295–308. [Google Scholar]
- 24.Valentine H, Williams WO, Maurer KJ (2012). Sedation or inhalant anesthesia before euthanasia with CO2 does not reduce behavioral or physiologic signs of pain and stress in mice. J Am Assoc Lab Anim Sci. 2012);51: 50–57. [PMC free article] [PubMed] [Google Scholar]
- 25.Franks NP (2008). General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008);9: 370–386. [DOI] [PubMed] [Google Scholar]
- 26.Azabou E, Fischer C, Mauguiere F, Vaugier I, Annane D, Sharshar T et al. (2016) Prospective Cohort Study Evaluating the Prognostic Value of Simple EEG Parameters in Postanoxic Coma. Clin EEG Neurosci. 2016);47: 75–82. [DOI] [PubMed] [Google Scholar]
- 27.Eger EIn, Saidman LJ, Brandstater B (1965). Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology. 1965);26: 756–763. [DOI] [PubMed] [Google Scholar]
- 28.Stoelting RK, Longnecker DE, Eger EI (1970). Minimum alveolar concentrations in man on awakening from methoxyflurane, halothane, ether and fluroxene anesthesia: MAC awake. Anesthesiology. 1970);33: 5–9. [DOI] [PubMed] [Google Scholar]
- 29.Boivin GP, Bottomley MA, Dudley ES, Schiml PA, Wyatt CN, Grobe N (2016). Physiological, behavioral, and histological responses of male C57BL/6N mice to different CO2 chamber replacement rates. J Am Assoc Lab Anim Sci. 2016;55: 451–463. [PMC free article] [PubMed] [Google Scholar]
- 30.Moody CM, Makowska IJ, Weary DM (2015). Testing three measures of mouse insensibility following induction with isoflurane or carbon dioxide gas for a more humane euthanasia. Appl Anim Behav Sci. 2015);163: 183–187. [Google Scholar]
- 31.Antognini JF, Schwartz K (1993). Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993);79: 1244–1249. [DOI] [PubMed] [Google Scholar]
- 32.Antognini JF, Carstens E, Atherley R (2002). Does the immobilizing effect of thiopental in brain exceed that of halothane? Anesthesiology. 2002);96: 980–986. [DOI] [PubMed] [Google Scholar]
- 33.Antognini JF, Barter L, Carstens E (2005). Overview movement as an index of anesthetic depth in humans and experimental animals. Comp Med. 2005);55: 413–418. [PubMed] [Google Scholar]
- 34.Hewett TA, Kovacs MS, Artwohl JE, Bennett BT (1993). A comparison of euthanasia methods in rats, using carbon dioxide in prefilled and fixed flow rate filled chambers. Lab Anim Sci. 1993;43: 579–582. [PubMed] [Google Scholar]
- 35.Powner DJ (1976). Drug-associated isoelectric EEGs. A hazard in brain-death certification. JAMA. 1976);236: 1123. [PubMed] [Google Scholar]
- 36.Citerio G, Crippa IA, Bronco A, Vargiolu A, Smith M (2014). Variability in brain death determination in europe: looking for a solution. Neurocrit Care. 2014);21: 376–382. [DOI] [PubMed] [Google Scholar]
- 37.Kimura J, Gerber HW, McCormick WF (1968). The isoelectric electroencephalogram. Significance in establishing death in patients maintained on mechanical respirators. Arch Intern Med. 1968);121: 511–517. [DOI] [PubMed] [Google Scholar]
- 38.Shemie SD, Hornby L, Baker A, Teitelbaum J, Torrance S, Young K et al. (2014) International guideline development for the determination of death. Intensive Care Med. 2014);40: 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Gallar J, Pozo MA, Baeza M, Belmonte C (1995). CO2 stimulation of the cornea: a comparison between human sensation and nerve activity in polymodal nociceptive afferents of the cat. Eur J Neurosci. 1995);7: 1154–1163. [DOI] [PubMed] [Google Scholar]
- 40.Feng Y, Simpson TL (2003). Nociceptive sensation and sensitivity evoked from human cornea and conjunctiva stimulated by CO2. Invest Ophthalmol Vis Sci. 2003);44: 529–532. [DOI] [PubMed] [Google Scholar]
- 41.Thurauf N, Gunther M, Pauli E, Kobal G (2002). Sensitivity of the negative mucosal potential to the trigeminal target stimulus CO(2). Brain Res. 2002);942: 79–86. [DOI] [PubMed] [Google Scholar]
- 42.Dripps RD and Comroe JH (1947). The respiratory and circulatory response of normal man to inhalation of 7.6 and 10.4 per cent CO2 with a comparison of maximal ventilation produced by severe muscular exercise, inhalation of CO2 ad maximal voluntary hyperventilation. Am J Physiol—Legacy Content. 1947);149: 43–51. [DOI] [PubMed] [Google Scholar]
- 43.Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B et al. (2001) Brain responses associated with consciousness of breathlessness (air hunger). Proc Natl Acad Sci USA. 2001);98: 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregory GA, Eger EI, Smith NT, Cullen BF (1974). The cardiovascular effects of carbon dioxide in man awake and during diethyl ether anesthesia. Anesthesiology. 1974);40: 301–304. [DOI] [PubMed] [Google Scholar]
- 45.McArdle L (1959). Electrocardiographic studies during the inhalation of 30 percent carbon dioxide in man. Br J Anaesth. 1959);31: 142–151. [DOI] [PubMed] [Google Scholar]
- 46.Ballard T (2009). Intraperitoneal route of administration—how accurate is this technique. Animal Technology and Welfare. 2009);8: 17–18. [Google Scholar]
- 47.Coria-Avila GA, Gavrila AM, Menard S, Ismail N, Pfaus JG (2007). Cecum location in rats and the implications for intraperitoneal injections. Lab Anim (NY). 2007);36: 25–30. [DOI] [PubMed] [Google Scholar]
- 48.Lewis RE, Kunz AL, Bell RE (1966). Error of intraperitoneal injections in rats. Lab Anim Care. 1966);16: 505–509. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the Harvard Dataverse website: http://dx.doi.org/10.7910/DVN/DWME60.




