Abstract
Metastasis and recurrence are the challenges of cancer therapy. Recently, mounting evidence has suggested that cancer stem cells (CSCs) and epithelial-mesenchymal transition (EMT) are critical factors in tumor metastasis and recurrence. The oncogene, Bmi-1, promotes the development of hematologic malignancies and many solid tumors. The aim of the present study was to elucidate the mechanisms through which Bmi-1 promotes the invasion and migration of colon CSCs (CCSCs) using the HCT116 colon cancer cell line. Sphere formation medium and magnetic-activated cell sorting were used to enrich and screen the CCSCs. CD133 and CD44 were regarded as markers of CCSCs and they were found to be co-expressed in the HCT116 colon cancer cell line. Colony formation assay, cell proliferation assay and viability assay using the Cell Counting Kit-8, and transplantation assay using nude mice injected with CCSCs were used to examine the CCSCs. The CD133+CD44+ HCT116 cells exhibited greater cloning efficiency, an enhanced proliferative ability, increased cell viability and stronger tumorigenicity; these cells were used as the CCSCs for subsequent experiments. In addition, the invasive and migratory abilities of the CD133+CD44+ HCT116 cells were markedly decreased when Bmi-1 was silenced by small interfering RNA (siRNA). The results of RT-qPCR and western blot analysis suggested that Bmi-1 had a negative effect on E-cadherin expression. On the whole, our findings suggest that Bmi-1 promotes the invasion and migration of CCSCs through the downregulation of E-cadherin, possibly by inducing EMT. Our findings thus indicate that Bmi-1 may be a novel therapeutic target for the treatment of colon cancer.
Keywords: Bmi-1, HCT116 cells, colon cancer stem cells, E-cadherin, metastasis
Introduction
Colorectal cancer (cancer of the colon and/or rectum) is the second leading cause of cancer-related mortality in the US according to the National Comprehensive Cancer Network (NCCN) in 2015 (1). It has been reported that in 15–25% of patients with colorectal cancer, hepatic metastasis has already occurred prior to diagnosis and metastasis occurs in more than half of the patients with this disease. Although the 5-year survival rate of patients with colon cancer following radical surgery is often >50%, this decreases to <12% with the occurrence of metastasis (2,3). Metastasis has thus become the key obstacle to the effective treatment of colon cancer and remains a big challenge for clinicians due to the wide disparities in survival rates.
The cancer stem cell (CSC) theory is a new theory which has appeared in recent years as regards the occurrence, development, metastasis and recurrence of tumors. CSCs are defined as a subpopulation of cancer cells which have the characteristics of self-renewal, differentiation abilities, metastatic potential and the ability to resist conventional chemoradiotherapeutics (4–6). The discovery of markers of CSCs has facilitated the screening and studying of a number of types of cancer, including leukemia, brain cancer, breast cancer, abdominal cancer and cancers of the reproductive system (7–15). CD133 and CD44 are considered markers of the surface membrane of cells and have been used to identify colon CSCs (CCSCs) (11–13) and have been recently reported to be co-expressed in colon cancer with hepatic metastases (16).
Epithelial-mesenchymal transition (EMT) is considered to occur during cancer invasion and migration, or tumor progression. In this process, epithelial cells lose their epithelial characteristics and adopt a mesenchymal-like appearance or characteristics (17,18). The downregulation or loss of E-cadherin, a transmenbrane protein important for cell-cell junctions, is treated as the hallmark of EMT (19). Since CSCs and EMT both play significant roles in cancer development, understanding the link between them may enhance our knowledge of the pathogenesis and mechanisms responsible for metastasis in cancer.
The polycomb group (PcG) of proteins are a family of transcriptional repressors that orchestrate alterations in chromatin structure to regulate gene activity (20,21). A number of PcG proteins have been confirmed to be altered in human cancers, such as Bmi-1 (22–24). Bmi-1 is known as transcriptional repressor targeting the Ink4a/Arf gene locus, and has also been described as an oncogene in many solid tumors, playing a critical role in the maintenance of CSCs (25). However, the role of Bmi-1 in the functions CCSCs has rarely been reported, at least to the best of our knowledge.
In our previous study (26), we found that the expression of Bmi-1 in colon cancer tissues closely correlated with the clinical stage, invasion depth and metastatic ability of the tumors. In the present study, we screened and identified CCSCs using the HCT116 colon cancer cell line. We also wished to determine the role that Bmi-1 plays in CCSCs and to elucidate the underlying mechanisms. It would be of clinical and therapeutic significance to provide a novel target for colon cancer therapy.
Materials and methods
Cell line and cell culture
The HCT116 colon cancer cell line was obtained from the Cell Bank of the Committee on Type Culture Collection of the Chinese Academic of Science (CCTCC; Shanghai, China). The cells were cultured in RPMI-1640 (Corning Inc., Corning, New York, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL, Grand Island, NY, USA)at 37°C with 5% CO2. The culture conditions for the HCT116 cells to form tumor spheres in suspension were as previously described (27–29). The sphere formation medium (SFM) used was RPMI-1640 supplemented with 20 ng/ml basic fibroblast growth factor (bFGF) and 20 ng/ml epidermal growth factor (EGF) (both from PeproTech, Inc., Rocky Hill, NJ, USA), 2% B27 (1:50 dilution; Gibco-BRL), and 0.4% bovine serum albumin (BSA; Invitrogen Life Technologies, Carlsbad, CA, USA). Enzymatically dissociated single cells were diluted to a density of 2×104/ml and gradually replaced with SFM after plating into 24-well plates. The cells were cultured in an incubator at 37°C with 5% CO2.
Flow cytometry
The HCT116 cells cultured in SFM were washed twice with phosphate-buffered saline (PBS; Corning, Inc.) and resuspended in PBS at a density of 1×107 cells/100 µl. The dissolved cells were stained using anti-human CD133-PE antibody (1:10 dilution; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) [no need for cells after magnetic-activated cell sorting (MACS)], and anti-human/mouse CD44 antibody (1:50 dilution; eBioscience, Inc., San Diego, CA, USA), followed by incubation for 20 min on ice and subsequent washing with PBS again twice. The respective isotype controls were used at the same concentrations according to the manufacturer's instructions (eBioscience, Inc.). The cells were analyzed on a flow cytometer (FACSVerse; BD Biosciences, Franklin Lakes, NJ, USA) at the Sun Yat-Sen Memorial Hospital of the Sun Yat-Sen University (Guangzhou, China).
MACS
The HCT116 cells cultured in SFM were resuspended in PBS with 2% FBS to a total volume of 1 ml at a density of 1×108 cells/ml in a 12×75 mm polystyrene tube to properly fit into the magnet (EasySep; STEMCELL Technologies, Inc.). This was followed by the addition of 100 µl anti-human CD32 (FcγRII) blocker, 50 µl of anti-human CD133-PE, 100 µl of PE selection cocktail and 50 µl of magnetic nanoparticles in turn according to the manufacturer's instructions (EasySep). The cell suspension was then brought to a total volume of 2.5 ml by the addition of PBS with 2% FBS (Step A). The tube was then placed into the magnet for 5 min and the supernatant fraction was poured off (Step B). Steps A and B were repeated twice. The magnetically labeled cells remained inside the tube and held by the magnetic field of the magnet. The cells in the tube were then used flow cytometric analysis or other assays.
Colony formation assay
In total, numbers of 50/100/200 CD133+CD44+ and CD133−CD44− HCT116 cells were seeded in each well in 6-well plates. The medium was discarded when colonies were observed by the naked eye. The colonies were fixed in methanol and stained with 10% Giemsa (Teaching and Research Section of Pathology of Shantou Medical College, Shantou, China). A microscope (Leica DMI, Leica Microsystems Inc., Buffalo Grove, IL, USA) was used to confirm that the colonies were made up of >50 cells and to measure the diameters.
Cell Counting Kit-8 (CCK-8) assay for the analysis of cell proliferation and cell viability
One hundred microliters of suspension (RPMI-1640 only, CD133+CD44+ and CD133−CD44− HCT116 cells) was respectively plated in 96-well plates and cultured for approximately 4 h until the cells had completely attached to the bottom of the wells. The cells were cultured at a population of 1,000, 2,000, 4,000 and 8,000 cells per well. Subsequently, 10 µl of 2-(2-methoxyl-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8; (Dojindo Molecular Technologies Inc., Kumamoto, Japan) were added to each well followed by incubation at 37°C for approximately 4 h. The absorption (OD value) was read at 450 nm using a spectrophotometer (Multiskan GO; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The OD values were corrected after the value of the control (cells cultured in RPMI-1640 only) was subtracted. To examine cell viability, 8,000 cells were used from each group, and the experiment was repeated 30 times. Cell proliferation and viability assays were performed by CCK-8 assay as previously described (30–34).
Tumor transplantation assay using BALB/c-nu/nu mice
CD133+CD44+ and CD133−CD44− HCT116 cells were trypsinized, pelleted and resuspended in RPMI-1640 with Matrigel (1:10 dilution; BD Biosciences). The mice (license no. SCXK 2011–0029; Sun Yat-Sen University; n=42; age, 28–35 days; weight, 12–13 g) were randomly divided into 7 groups by the breeder. The mice were first divided into 3 main groups (CD133+CD44+, CD133−CD44− and NaCl solution). The CD133+CD44+ and CD133−CD44− groups were further respectively divided into 3 subgroups (2,000, 20,000 and 200,000 cells). Thus, there were 7 subgroups, with 6 mice in each group. Only the mice in the CD133+CD44+ main group formed tumors. Two hundred microliters of cell suspension (2,000/20,000/200,000 CD133+CD44+ or CD133−CD44− cells) or NaCl solution were then injected subcutaneously into the BALB/C-nu/nu mice. Tumor volumes and the body weight of the mice were measured at regular time intervals using an electronic balance. After 4 weeks, the mice were administered an intraperitoneal anesthesia with 4% chloral hydrate (400 mg/kg), and sacrificed by cervical dislocation and the tumors were removed. Following removal, the tumors were stored in formalin and were sent to Google Biological Technology Co., Ltd. (Wuhan, China) for processing (the tumors were fixed in 10% neutral buffered formalin, and then subjected to conventional methods of dehydration, paraffin-embedding and H&E staining). All animal experiments were conducted in accordance with the protocol of the Institutional Animal Care and Use Committee of Sun Yat-Sen University (IACUC-DB-15-1210; Guangzhou, China). and following the approval of the Research Ethics Committee of Guangdong General Hospital, Guangdong Academy of Medical Sciences (no. GDREC 2015268A).
Transfection with small interfering RNA (siRNA) targeting Bmi-1 (Bmi-1-siRNA)
The sequences of the siRNAs used to suppress Bmi-1 were as follows: forward, 5′-GCGGUAACCACCAAUCUUCdTdT-3′ and reverse, 3′-dTd TCGCCAUUGGUGGUUAGAAG-5′, which targeted the sequence of GCGGTAACCACCAATCTTC. The control siRNA sequence was custom ordered and provided by Shanghai SBO Medical Biotechnology Co., Ltd. (Shanghai, China). The CD133+CD44+ HCT116 cells were transfected with 100 nM of siRNA using SunBio Trans-EZ (SBO) according instructions provided by the manufacturer. Cells only transfected with reagent (normal cells) were used as negative controls.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from thye cultured cells using TRIzol reagent [Takara Biotechnology (Dalian) Co., Ltd., Dalian, China] and 1.0 µg of total RNA was used for cDNA synthesis using the PrimeScript RT reagent Master mix (Takara Biotechnology (Dalian) Co., Ltd.). Quantitative (real-time) PCR (qPCR) was performed using SYBR Premix Ex Taq II (Tli RNaseH Plus) (Takara Biotechnology (Dalian) Co., Ltd.) with an ABI PRISM 7500 Fast Real-Time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with the following program: 95°C for 30 sec, 95°C for 5 sec, 60°C for 1 min, and 95°C for 30 sec, for 40 cycles. The results were analyzed using the 2−∆∆CT method. β-actin gene expression was measured as an endogenous control. Experiments were carried out in technical triplicates and were repeated at least twice independently. Primers were custom ordered (Boshang Biotechnology Co., Ltd, Shanghai, China) with the following sequences: Bmi-1 forward, 5′-TCTGGGAGTGACAAGG-3′ and reverse, 5′-AAACAAGAAGAGGTGGA-3′; E-cadherin forward, 5′-TGCCCAGAAAATGAAAAAGG-3′ and reverse, 5′-GTGTATGTGGCAATGCGTTC-3′; β-actin forward, 5′-GCCAACACAGTGCTGTCTG-3′ and reverse, 5′-TACTCCTGCTTGCTGATCCA-3′.
Western blot analysis
The cells were lysed in lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 0.1% NP-40, 0.5% sodium deoxycholate). The protein concentration of the lysate was quantitated using the BSA method. Equal amounts of lysate were loaded and separated by SDS-polyacrylamide gels, and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Inc.). The membranes were blocked with 5% non-fat milk powder in TBS for 1 h and probed with primary antibodies against Bmi-1 (D20B7) rabbit monoclonal antibody (mAb) (#6964, 1:1,000 dilution) and E-cadherin (4A2) mouse mAb (#14472, 1:1,000 dilution) (both from Cell Signalling Technology, Inc., Danvers, MA, USA), and GAPDH (KC-5G4, 1:8,000 dilution; Kangchen Biotech, Inc., Shanghai, China). After washing with TBS-T, the membranes were incubated with secondary antibodies (1:6,000 dilution, A21020, HRP goat anti-rabbit; A21010, HRP goat anti-mouse; Abbkine, Redlands, CA, USA) and visualized using chemiluminescence with ImageQuant LAS 500 software (GE Healthcare Life Sciences, Buckinghamshire, UK).
Wound healing assay
The cells (5×105/well) were plated in 6-well plates and cultured until they reached confluence. A diametric scratch was created using a pipette tip and washed with PBS 3 times. The cells were photographed under a microscope (Leica DMI1, Leica Microsystems Inc.) in several pre-marked spots as 0 h. Images were then acquired at 24 h in the same spots for comparison. The scratch width was measured and the migration rates of each group cells were compared on average using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Transwell migration assay
A Matrigel matrix (BD Biosciences) was used at a working concentration of 300 µg/ml; the cells were plated in 24-well plates at 100 µl/well and cultured in an incubator for 1 h before the Falcon cell culture inserts (Corning, Inc.) were added. The cells were resuspended in RPMI-1640 at a concentration of 1×105/ml. The upper chamber was loaded with 100 µl of cell suspension and the lower chamber was loaded with 500 µl of RPMI-1640 with 20% FBS. Following incubation for 24 h at 37°C with 5% CO2, the filter was fixed with methanol and stained with 10% Giemsa. The cells on the upper side of the filter were wiped off using a cotton swab. The cells that had migrated to the undersurface of the membrane were counted under a microscope (Leica DMI1, Leica Microsystems Inc.). Nine microscopic fields (×100 magnification) were randomly selected to count the cells. Each assay was carried out in triplicate.
Statistical analyses
All data are presented as the means ± standard error of the mean (SEM). Statistical significance of differences between mean values was assessed by the Student's t-test for unpaired data. Comparisons of data between multiple groups were performed using analysis of variance (ANOVA). A value of P<0.05 was considered to indicate a statistically significant differene.
Results
Enrichment and screening of CCSCs by the use of SFM and MACS
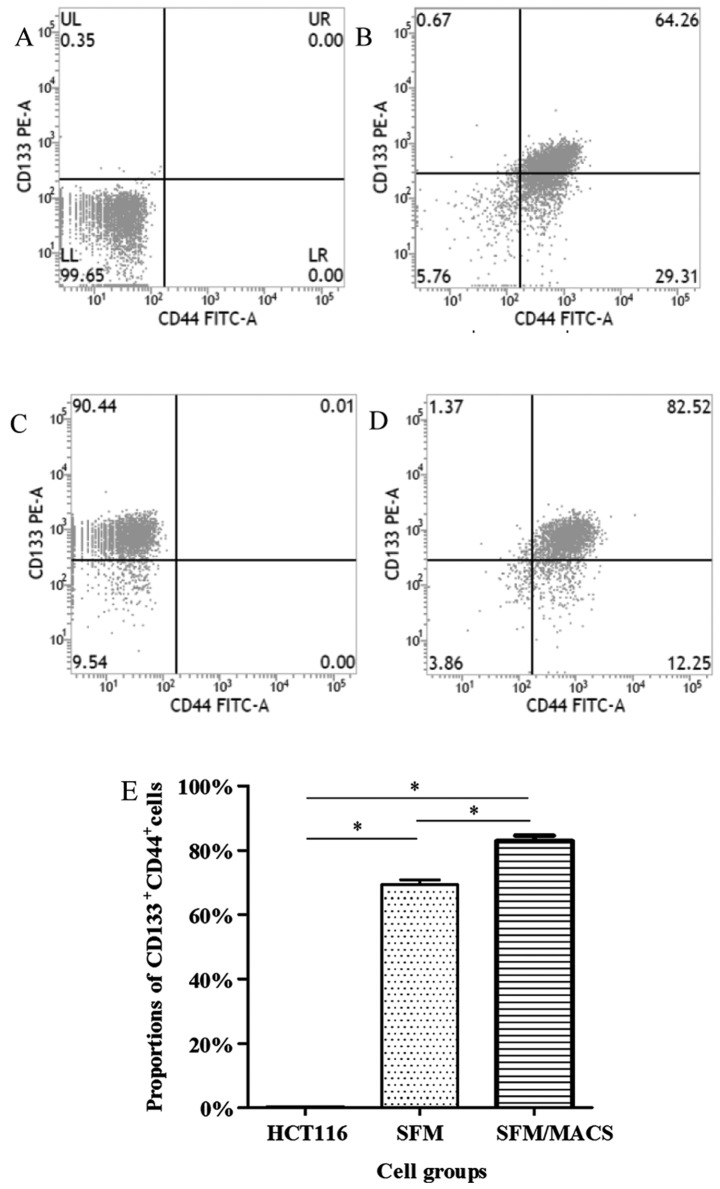
The surface markers, CD133 and CD44, have been widely used for the selection and isolation of CCSCs from colon cancer cells (11–13). In our study, the CD133+CD44+ subpopulation of HCT116 cells before the experiment only accounted for <1.00%; thus, these cells were defined as CD133−CD44− cells. Following culture in SFM and subsequent MACS, we found that the proportion of CD133+CD44+ cells greatly increased and these cells were defined as CCSCs (Fig. 1). Furthermore, it seemed that the proportion of CD133+CD44+ cells depended on the amount of CD133+ cells. In addition, there was no significant difference in the frequency of the CD133+ and CD44+ subpopulation between the CCSCs transfected with the control siRNA or these transfected with Bmi-1-siRNA (data not shown).
Figure 1.
Proportions of CD133+CD44+ cells as analyzed by flow cytometry. (A) The proportion of CD133+CD44+ cells was originally <1.00% in the HCT116 cells. (B) This increased to 69.27±1.56% following culture in sphere formation medium (SFM); (C) This increased even further to 90.40±0.85% (for CD133+ cells) with magnetic-activated cell sorting (MACS); and (D) to 82.91±1.60% with MACS. (E) Quantitative analysis of the results. The difference between the3 groups (HCT116 cells before culture with SFM and MACS, cells cultured in SFM, and cells after MACS) was significant, *P<0.05.
Colony-forming ability of CCSCs in vitro
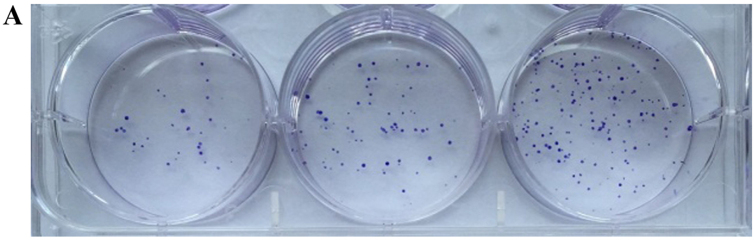
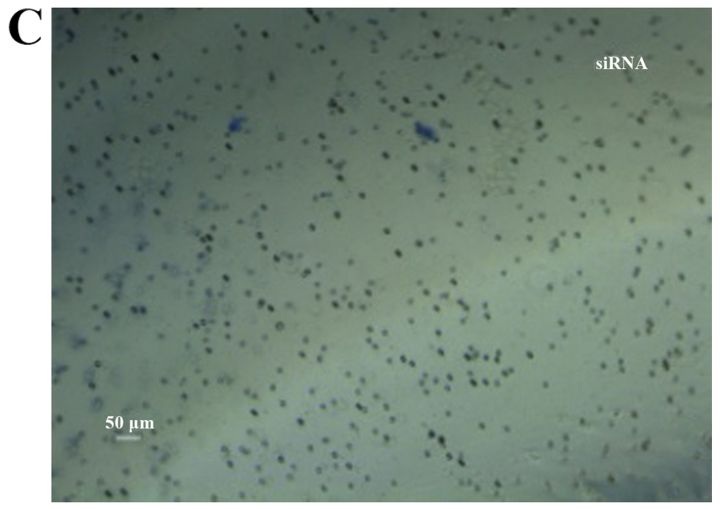
Colony formation assay in vitro was used to identify the CSCs, which reflected the self-renewal and differentiation abilities of the CSCs. Six-well plates seeded with cells were photographed following culture for 1 week and the cloning efficiency of the CD133+CD44+ cells was markeldy higher than that of the CD133−CD44− cells (Fig. 2). The biggest and smallest colonies were almost 10.0 and 5.00 µm in diameter as observed under a microscope (×100 magnification) after being stained with 10% Giemsa.
Figure 2.
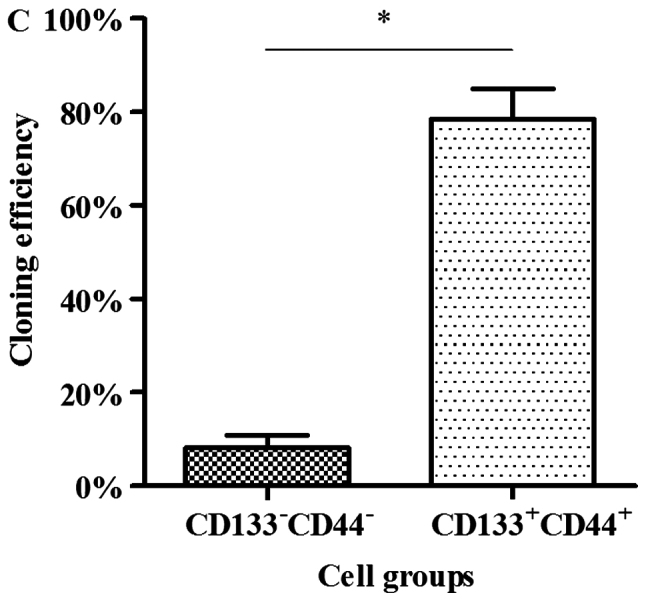
Colonies of cells following staining with 10% Giemsa by the naked eye and a microscope. (A) Cloning efficiency of CD133+CD44+ cells was 78.44±6.54% on average; (B) cloning efficiency of CD133−CD44− cells was 8.22±2.68% on average. (C) Quantitative analysis of the results. The difference between the 2 groups was significant, *P<0.05.
Proliferative ability and viability of CCSCs in vitro
Cell proliferative ability and cell viability assays were performed using CCK-8 assay as previously described (30–34). The distinction between the 2 groups of cells was most conspicuously reflected with 8,000 cells after different gradients of cell populations were designed according to the manufacturer's instructions (Fig. 3A). Subsequently, another 8,000 cells from each group were cultured as above to compare cell viability, and the assay was repeated 30 times. The CD133+CD44+ cells exhibited a greater viability compared with the CD133−CD44− cells (Fig. 3B).
Figure 3.
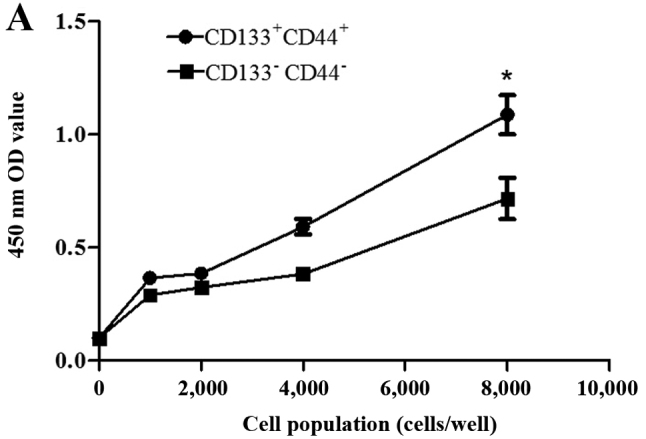
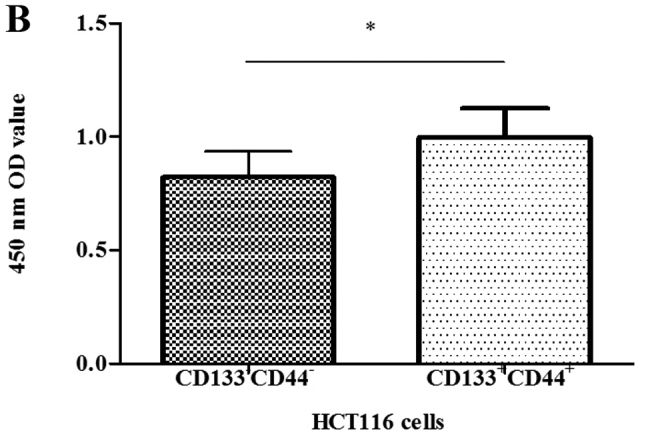
Cell proliferation curves and comparison of the viability of CD133+CD44+ and CD133−CD44− HCT116 cells using the Cell Counting Kit-8 (CCK-8). (A) The distinction was most conspicuously reflected with 8,000 cells in each group: 1.09±0.09 and 0.72±0.09; (B) Values of 8,000 cells in each group: 1.00±0.13 and 0.82±0.11, repeated 30 times. *P<0.05.
Tumor transplantation assay using BALB/c-nu/nu mice in vivo
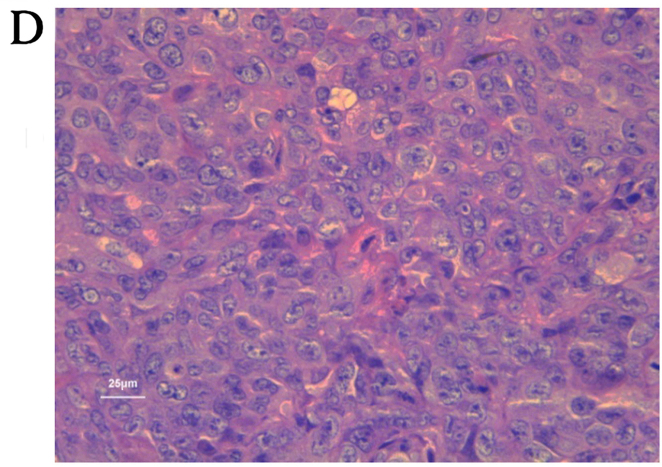
We used a tumor transplantation assay to confirm that the CCSCs had a stronger tumorigenicity in vivo, which is also considered to be an important characteristic of CSCs. At 4 weeks after the injection, the BALB/c-nu/nu mice were sacrificed and the tumors were removed. The body weights of the mice injected with the CD133+CD44+ cells were markedly lower than those of the mice injected with the CD133−CD44−cells or with NaCl (Fig. 4A). Only the mice injected with the CD133+CD44+ cells developed tumors (100%), which were concentration-dependent (Fig. 4B). The shortest tumor formation time was approximately 10 days with the injection of 200,000 cells and the tumor growth curves in the mice in the CD133+CD44+-injected group are shown in Fig. 4C. All tumors underwent routine pathological section examinations to confirm that the assay was successful (Fig. 4D).
Figure 4.
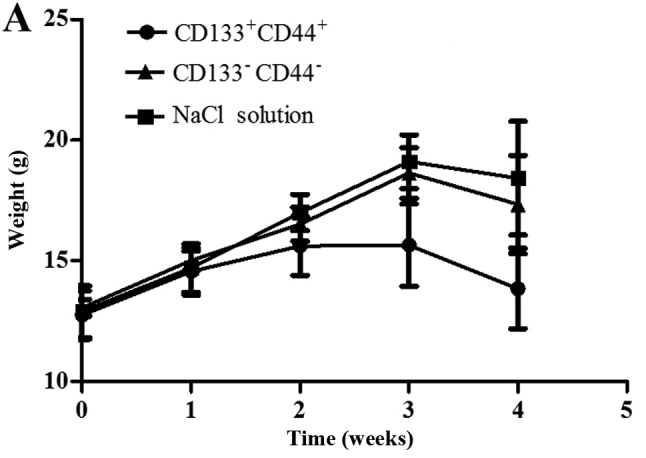
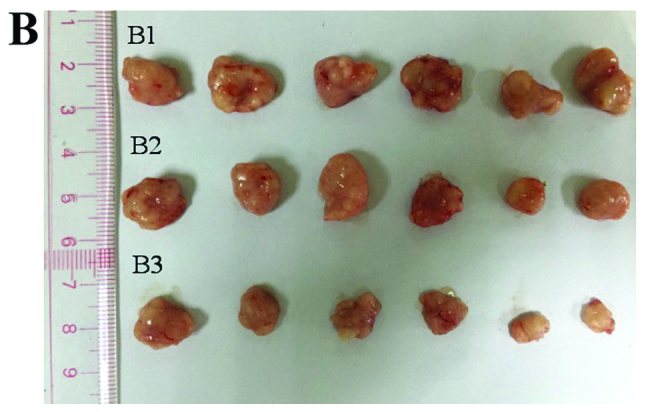
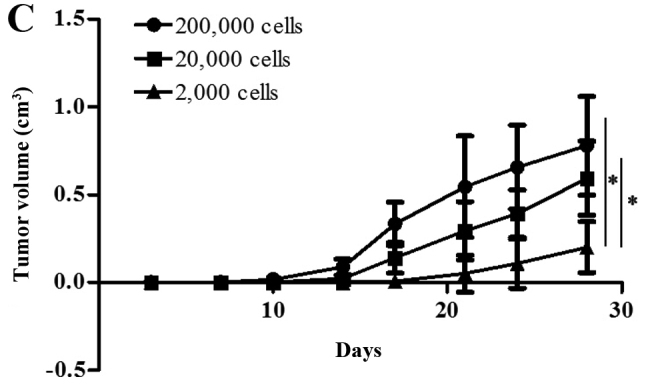
Tumor transplantation assay using BALB/c-nu/nu mice. (A) The body weights of the mice injected with NaCl, or the CD133−CD44− cells or CD133+CD44+ cells were measured over a period of 4 weeks. (B) Tumors of the mice in the CD133+CD44+ group injected with various concentrations of the cells (B1, 200,000 cells; B2, 20,000 cells; B3, 2,000 cells) were measured using a ruler (cm). (C) Tumor growth curves of the mice injected with various concentrations of CD133+CD44+ cells (200,000, 20,000 or 2,000 cells); *P<0.05. (D) Pathological section examination stained with hematoxylin and eosin (H&E) under a microscope (×400 magnification).
CCSCs exhibit decreased invasive and migratory abilities after the silencing of Bmi-1
The oncogene Bmi-1 plays a critical role in the maintenance of CSCs (25) and we found that it was expressed in the HCT116 colon cancer cell line. Therefore, we investigated whether Bmi-1 affects the metastatic potential of CCSCs by gene silencing, using siRNA transfection and then confirmed our findings by RT-qPCR and western blot analysis (Fig. 5). By performing wound healing and Transwell migration assays, we found that the CCSCs exhibited decreased invasive and migratory abilities, which are often representative of metastatic potential, after the silencing of Bmi-1 (Figs. 6 and 7).
Figure 5.
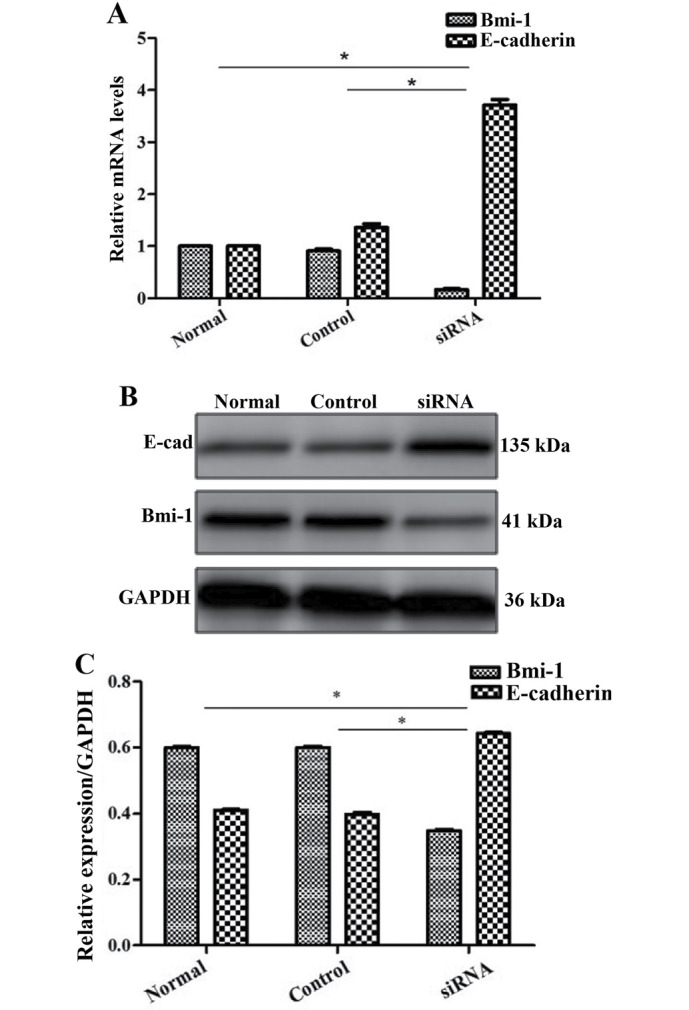
Expression of Bmi-1 and E-cadherin following transfection of colon cancer stem cells (CCSCs) with Bmi-siRNA. (A) RT-qPCR evaluation; (B and C) western blot analysis and evaluation. *P<0.05 compared to the control siRNA-transfected cells.
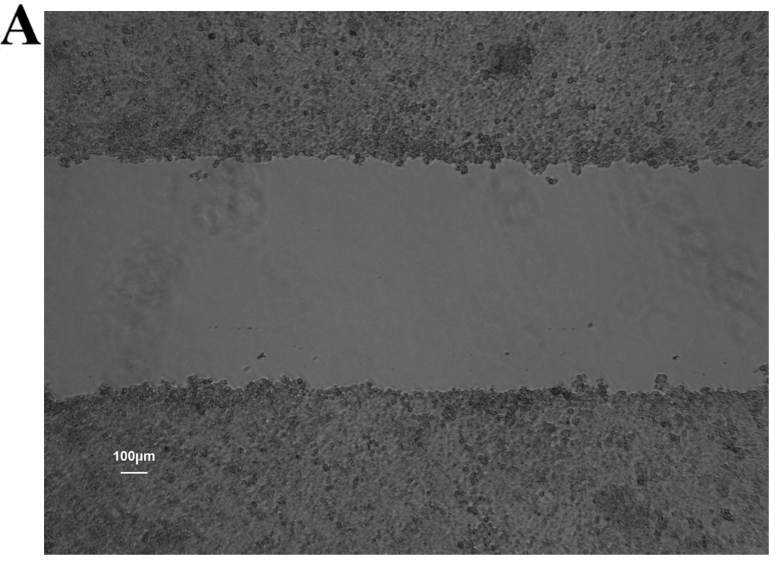
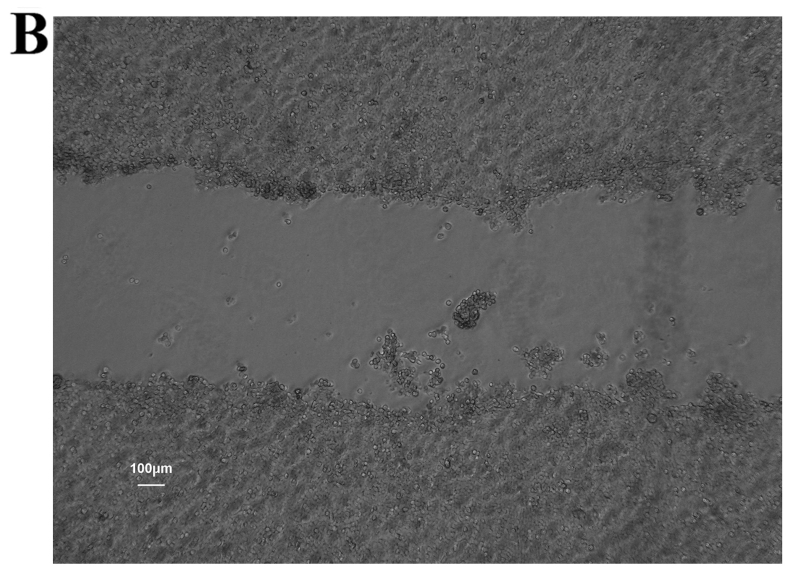
Figure 6.
Wound healing ability of colon cancer stem cells (CCSCs) seeded in 6-well plates and cultured for 24 h. (A) A diametric scratch using a pipette tip was made at 0 h; (B) CCSCs treated with transfection reagent after 24 h; (C) CCSCs transfected with control small interfering RNA (siRNA) and transfection reagent after 24 h; (D) CCSCs transfected with Bmi-1-siRNA and transfection reagent after 24 h; 24 h average migration rates of (B–D) were: 5.25±0.70, 5.14±0.54 and 3.93±0.38 µm/h, respectively (shown in Fig. 7D).
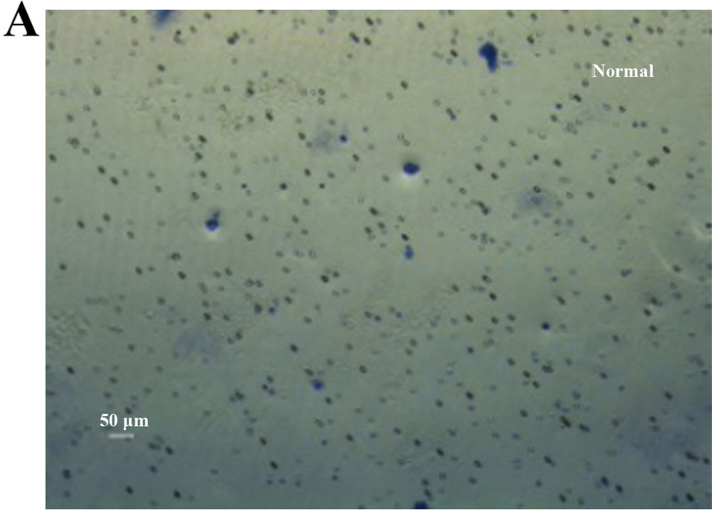
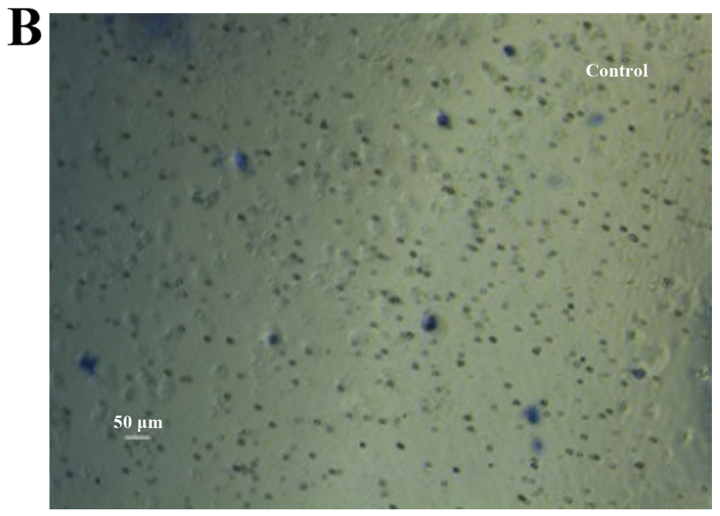
Figure 7.
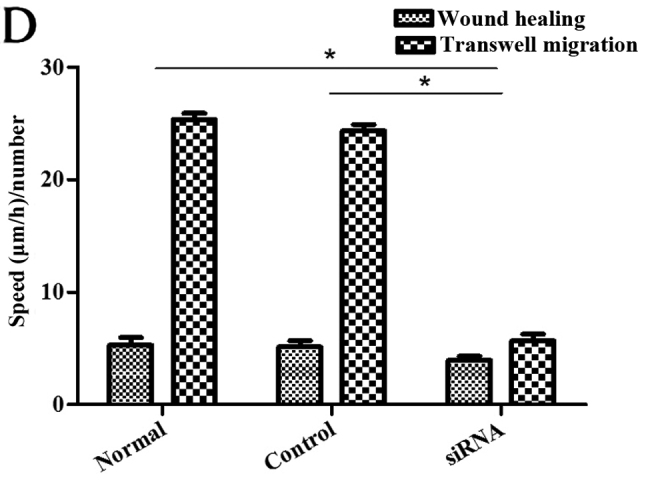
Cell numbers in the under surface of Transwell membrane after 24 h. (A) Colon cancer stem cells (CCSCs) treated with transfection reagent after 24 h; (B) CCSCs transfected with control small interfering RNA (siRNA) and transfection reagent after 24 h; (C) CCSCs transfected with Bmi-1-siRNA and transfection reagent after 24 h; (D) cell numbers in the undersurface of membrane of (A–C) were: 25.33±0.58, 24.33±0.58 and 5.67±0.58 as observed under a microscope (×100 magnification) after staining with 10% Giemsa, respectively. *P<0.05 compared to siRNA-transfected group.
We further explored the possible mechanisms responsible for the effects Bmi-1 on CCSCs. Since EMT occurs during cancer metastasis and interacts with CSCs (17,18), we focused on E-cadherin (a hallmark of EMT; the loss of E-cadherin is indicative of EMT) as a target protein. We found that Bmi-1 had a negative impact on E-cadherin. In the cells not transfected with Bmi-1-siRNA, the expression of E-cadherin was low. However, after the silencing of Bmi-1, E-cadherin expression markedly increased, as shown by RT-qPCR and western blot analysis (Fig. 5), which may indicate that Bmi-1 promotes the invasion and migration of CCSCs through the downregulation of E-cadherin, possibly by inducing EMT. The silencing of Bmi-1 increases E-cadhein expression, thus inhibiting EMT.
Discussion
The morbidity associated with colorectal cancer is ranked 3rd among malignant tumors in recent years on the basis of the treatment guidelines of early colorectal cancer in China published in 2015 (3). The prognosis of patients with colorectal cancer is closely related to early diagnosis and the 5-year survival rate decreases to <12% at the advanced stage of the disease (2,3). The incidence and metastasis associated with colorectal cancer are major concerns and obstacles to effective treatment in both Western and Eastern countries. It is thus crucial that further research be carried out to identify methods with which to prevent the development and metastasis of colon cancer.
The isolation and acquisition of CSCs is a major achievement in basic and clinical medicine. SFM and MACS can be used for the enrichment and selection of CSCs. Functional experiments in vitro and in vivo are often performed for the identification of CSCs (35–37). In this study, we found that the CD133+CD44+ HCT116 cells had a greater cloning efficiency, an enhanced proliferative ability and increased viability, as well as a stronger tumorigenicity; therefore, they were used as CCSCs for subsequent experiments. The successful separation and identification of CCSCs in ours and other studies strongly supports the CSC theory in colon cancer.
CD133 and CD44 were discovered as important surface markers of CCSCs (11–13). It is recommended that the screening and identification of CSCs be performed with more than one marker. Different markers of cells may represent different functions and may prove helpful to the understanding of the overall features. For instance, CD133 may be associated with cloning efficiency and proliferative ability, while CD44 may be related to metastasis and survival prediction (16). It has been reported that other markers of CCSCs include membrane proteins, such as EpCAM (39), Lgr5 (40–42), CD24 (43), CD26 (44,45), CD29 (46) and CD166 (38,47); cytosolic enzymes, such as ALDH1 (48,49); transcription factors, such as as Oct4 (50), Sox2 (51), Ascl2 (52–54) and Hes1 (55,56); and even the Wnt (57) and Notch (55) signaling pathways. Different markers may reflect different functions of CCSCs from diverse perspectives and provide more targets for study and therapy. However, the critical one and interconnections among them have not yet been clearly elaborated.
The discovery and eradication of CSCs hold promise in cancer therapy, as well as genes acting on CSCs and the regulatory mechanisms. The epigenetic regulator, Bmi-1, is considered to play essential roles in the self-renewal and propagation of normal cells and CSCs (25). We found that CCSCs exhibited a decreased invasive and migratory abilities after Bmi-1 was silenced by siRNA. This suggests that Bmi-1 is a positive regulator of cell invasion and migration in colon cancer and that the downregulation of Bmi-1 may help to prevent metastasis or the progression of colon cancer. Since the gene targeted therapy of cancer has important theoretical significance and clinical application prospects, the knockdown of Bmi-1 is expected to become supplementary treatment for colon cancer.
In addition, we found E-cadherin was upregulated when Bmi-1 was silenced, while vimentin and N-cadherin were downregulated (although we could not make a statistical conclusion as they were weakly expressed in CCSCs originally; data not shown). This may indicate that Bmi-1 functions through the downregulation of E-cadhein, possibly by inducing EMT. This has been previously demonstrated in nasopharyngeal (58), breast (59,60), melanoma (61), endometrial (62), prostate (63), and bladder (64) cancers. E-cadherin is a transmenbrane protein important for cell-cell junctions, it suppresses tumorigenesis and the metastasis of cancers, and its downregulation or loss is regarded as a hallmark of EMT. The downregulation or loss of E-cadherin on the surface leads to the shedding of many types of cancer cells from the tumor mass, which is the precondition of cancer invasion and metastasis (65,66). The molecular mechanisms responsible for cancer metastasis, regarded as a promising target for cancer chemotherapy, are currently receiving increased attention. Bmi-1 was considered to play an important role in the pathogenesis of nasopharyngeal cancer by inducing EMT, partly by targeting the tumor suppressor, PTEN, thus activating the PI3K/Akt pathway (58). It was also demonstrated that Bmi-1 induced cell invasion with the activation of the Akt pathway in breast cancer cells (60). Alternatively, it was shown that Bmi-1 acted negatively on PTEN and E-cadherin in colorectal cancer from a histological perspective by Liao et al (67). Therefore, we speculate there is a strong possibility that Bmi-1 promotes the invasion and migration of CCSCs through the downregulation of E-cadherin and the induction of EMT, and through the PI3K/Akt pathway. However, the crosstalk between different pathways for expanding the cellular communication signaling network should be also given several considerations. In this study, we put forward the hypothesis of the role of Bmi-1 and EMT in CCSCs for the first time, to the best of our knowledge. Namely, the eradication of CCSCs, and the blocking of EMT and the downregulation of Bmi-1 may together prevent the metastasis of colon cancer at an early stage and may thus improve the 5-year survival rate when applied clinically.
In conclusion, our study demonstrates that CD133+CD44+ HCT116 cells can be used as CCSCs for subsequent medical studies. Bmi-1 promotes the invasion and migration of CCSCs through the downregulation of E-cadherin, possibly by inducing EMT. This finding may provide a new target for colon cancer therapy. To this end, it would be of great interest for us to further investigate the signaling pathways and regulatory mechanisms of the interaction between Bmi-1 and EMT in colon cancer.
Acknowledgments
We sincerely thank other colleagues in our laboratory for their active help in this study. This study was supported by a grant from the Natural Science Foundation of Guangdong Province (2014A030313543).
References
- 1.Provenzale D, Jasperson K, Ahnen DJ, Aslanian H, Bray T, Cannon JA, David DS, Early DS, Erwin D, Ford JM, et al. Colorectal cancer screening, version 1.2015. J Natl Compr Canc Netw. 2015;13:959–968. doi: 10.6004/jnccn.2015.0116. [DOI] [PubMed] [Google Scholar]
- 2.Gastrointestinal surgery group. Colorectal and anal surgery group. Colorectal cancer Specialized Committee Guidelines for diagnosis and comprehensive treatment of colorectal cancer liver metastases (V 2013) Zhonghua wei chang wai ke za zhi. 2013;16:780–788. In Chinese. [Google Scholar]
- 3.Association of Digestive Endoscopy, Cancer endoscopy Specialized Committee Guidelines for early colorectal cancer screening and endoscopic diagnosis and treatment in China (2014) Nat Med J China. 2015;95:2235–2252. In Chinese. [Google Scholar]
- 4.Polyak K, Hahn WC. Roots and stems: stem cells in cancer. Nat Med. 2006;12:296–300. doi: 10.1038/nm1379. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Tiede B, Massagué J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 6.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 8.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 9.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 12.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 13.Chu P, Clanton DJ, Snipas TS, Lee J, Mitchell E, Nguyen ML, Hare E, Peach RJ. Characterization of a subpopulation of colon cancer cells with stem cell-like properties. Int J Cancer. 2009;124:1312–1321. doi: 10.1002/ijc.24061. [DOI] [PubMed] [Google Scholar]
- 14.Laganà AS, Colonese F, Colonese E, Sofo V, Salmeri FM, Granese R, Chiofalo B, Ciancimino L, Triolo O. Cytogenetic analysis of epithelial ovarian cancer's stem cells: an overview on new diagnostic and therapeutic perspectives. Eur J Gynaecol Oncol. 2015;36:495–505. [PubMed] [Google Scholar]
- 15.López J, Valdez-Morales FJ, Benítez-Bribiesca L, Cerbón M, Carrancá AG. Normal and cancer stem cells of the human female reproductive system. Reprod Biol Endocrinol. 2013;11:53. doi: 10.1186/1477-7827-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jing F, Kim HJ, Kim CH, Kim YJ, Lee JH, Kim HR. Colon cancer stem cell markers CD44 and CD133 in patients with colorectal cancer and synchronous hepatic metastases. Int J Oncol. 2015;46:1582–1588. doi: 10.3892/ijo.2015.2844. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 18.Birchmeier W, Birchmeier C. Epithelial-mesenchymal transitions in development and tumor progression. EXS. 1995;74:1–15. doi: 10.1007/978-3-0348-9070-0_1. [DOI] [PubMed] [Google Scholar]
- 19.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs JJ, van Lohuizen M. Polycomb repression: from cellular memory to cellular proliferation and cancer. Biochim Biophys Acta. 2002;1602:151–161. doi: 10.1016/s0304-419x(02)00052-5. [DOI] [PubMed] [Google Scholar]
- 21.Piunti A, Pasini D. Epigenetic factors in cancer development: polycomb group proteins. Future Oncol. 2011;7:57–75. doi: 10.2217/fon.10.157. [DOI] [PubMed] [Google Scholar]
- 22.Honig A, Weidler C, Häusler S, Krockenberger M, Buchholz S, Köster F, Segerer SE, Dietl J, Engel JB. Overexpression of polycomb protein BMI-1 in human specimens of breast, ovarian, endometrial and cervical cancer. Anticancer Res. 2010;30:1559–1664. [PubMed] [Google Scholar]
- 23.Vékony H, Raaphorst FM, Otte AP, van Lohuizen M, Leemans CR. High expression of Polycomb group protein EZH2 predicts poor survival in salivary gland adenoid cystic carcinoma. J Clin Pathol. 2008;61:744–749. doi: 10.1136/jcp.2007.054262. [DOI] [PubMed] [Google Scholar]
- 24.Silva J, García JM, Peña C, García V, Domínguez G, Suárez D, Camacho FI, Espinosa R, Provencio M, España P, Bonilla F. Implication of polycomb members Bmi-1, Mel-18, and Hpc-2 in the regulation of p16INK4a, p14ARF, h-TERT, and c-Myc expression in primary breast carcinomas. Clin Cancer Res. 2006;12:6929–6936. doi: 10.1158/1078-0432.CCR-06-0788. [DOI] [PubMed] [Google Scholar]
- 25.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu XH, Sha WH, Lin F, Cen RY, Liao SY. The correlation between genetic expressions of Bmi-1 and clinicopathological parameters of colorectal cancer. Modern Digestion & Intervention. 2011;16:1–5. In Chinese. [Google Scholar]
- 27.Yang J, Liu J, Lyu X, Fei S. Resveratrol inhibits cell proliferation and up-regulates MICA/B expression in human colon cancer stem cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31:889–893. In Chinese. [PubMed] [Google Scholar]
- 28.Xiong B, Ma L, Hu X, Zhang C, Cheng Y. Characterization of side population cells isolated from the colon cancer cell line SW480. Int J Oncol. 2014;45:1175–1183. doi: 10.3892/ijo.2014.2498. [DOI] [PubMed] [Google Scholar]
- 29.Feng Y, Dai X, Li X, Wang H, Liu J, Zhang J, Du Y, Xia L. EGF signalling pathway regulates colon cancer stem cell proliferation and apoptosis. Cell Prolif. 2012;45:413–419. doi: 10.1111/j.1365-2184.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou M, Lu Y, Yuan L, Zheng L, Liu Y, Hong M, Zhang C, Li X. Preliminary screening of downstream proteins of Sox2 and role of Sox2 in colonic cancer cell migration and invasion. Nan Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:1594–1600. In Chinese. [PubMed] [Google Scholar]
- 31.Zhang M, Cui F, Lu S, Lu H, Xue Y, Wang J, Chen J, Zhao S, Ma S, Zhang Y, et al. Developmental pluripotency-associated 4: a novel predictor for prognosis and a potential therapeutic target for colon cancer. J Exp Clin Cancer Res. 2015;34:60. doi: 10.1186/s13046-015-0176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie X, Zhao Y, Ma CY, Xu XM, Zhang YQ, Wang CG, Jin J, Shen X, Gao JL, Li N, et al. Dimethyl fumarate induces necroptosis in colon cancer cells through GSH depletion/ROS increase/MAPKs activation pathway. Br J Pharmacol. 2015;172:3929–3943. doi: 10.1111/bph.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin YU, Wu T, Yao Q, Zi S, Cui L, Yang M, Li J. LGR5 promotes the proliferation of colorectal cancer cells via the Wnt/β-catenin signaling pathway. Oncol Lett. 2015;9:2859–2863. doi: 10.3892/ol.2015.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng HX, Wu WQ, Yang DM, Jing R, Li J, Zhou FL, Jin YF, Wang SY, Chu YM. Role of B7-H4 siRNA in proliferation, migration, and invasion of LOVO colorectal carcinoma cell line. BioMed Res Int. 2015;2015:326981. doi: 10.1155/2015/326981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng L, Qiu L, Yang XT, Zhou YH, Du J, Wang HY, Sun JH, Yang C, Jiang JX. Isolation of lung multipotent stem cells using a novel microfluidic magnetic activated cell sorting system. Cell Biol Int. 2015;39:1348–1353. doi: 10.1002/cbin.10513. [DOI] [PubMed] [Google Scholar]
- 36.Xue ZX, Zheng JH, Zheng ZQ, Cai JL, Ye XH, Wang C, Sun WJ, Zhou X, Lu MD, Li PH, Cai ZZ. Latexin inhibits the proliferation of CD133+ miapaca-2 pancreatic cancer stem-like cells. World J Surg Oncol. 2014;12:1–11. doi: 10.1186/1477-7819-12-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang DG, Jiang AG, Lu HY, Zhang LX, Gao XY. Isolation, cultivation and identification of human lung adenocarcinoma stem cells. Oncol Lett. 2015;9:47–54. doi: 10.3892/ol.2014.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin TG, Powell AE, Davies PS, Silk AD, Dismuke AD, Anderson EC, Swain JR, Wong MH. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology. 2010;139:2072–2082. doi: 10.1053/j.gastro.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Femia AP, Dolara P, Salvadori M, Caderni G. Expression of LGR-5, MSI-1 and DCAMKL-1, putative stem cell markers, in the early phases of 1,2-dimethylhydrazine-induced rat colon carcinogenesis: correlation with nuclear β-catenin. BMC Cancer. 2013;13:48. doi: 10.1186/1471-2407-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 42.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 43.Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fric P, Sovová V, Sloncová E, Lojda Z, Jirásek A, Cermák J. Different expression of some molecular markers in sporadic cancer of the left and right colon. Eur J Cancer Prev. 2000;9:265–268. doi: 10.1097/00008469-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto K, Beauchamp RD, Whitehead RH. Identification and isolation of candidate human colonic clonogenic cells based on cell surface integrin expression. Gastroenterology. 2002;123:1941–1948. doi: 10.1053/gast.2002.37065. [DOI] [PubMed] [Google Scholar]
- 47.Lugli A, Iezzi G, Hostettler I, Muraro MG, Mele V, Tornillo L, Carafa V, Spagnoli G, Terracciano L, Zlobec I. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. 2010;103:382–390. doi: 10.1038/sj.bjc.6605762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 51.Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, Miki C, Kusunoki M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009;16:3488–3498. doi: 10.1245/s10434-009-0617-z. [DOI] [PubMed] [Google Scholar]
- 52.Ziskin JL, Dunlap D, Yaylaoglu M, Fodor IK, Forrest WF, Patel R, Ge N, Hutchins GG, Pine JK, Quirke P, et al. In situ validation of an intestinal stem cell signature in colorectal cancer. Gut. 2013;62:1012–1023. doi: 10.1136/gutjnl-2011-301195. [DOI] [PubMed] [Google Scholar]
- 53.Zhu R, Yang Y, Tian Y, Bai J, Zhang X, Li X, Peng Z, He Y, Chen L, Pan Q, et al. Ascl2 knockdown results in tumor growth arrest by miRNA-302b-related inhibition of colon cancer progenitor cells. PLoS One. 2012;7:e32170. doi: 10.1371/journal.pone.0032170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jubb AM, Chalasani S, Frantz GD, Smits R, Grabsch HI, Kavi V, Maughan NJ, Hillan KJ, Quirke P, Koeppen H. Achaete-scute like 2 (ascl2) is a target of Wnt signalling and is upregulated in intestinal neoplasia. Oncogene. 2006;25:3445–3457. doi: 10.1038/sj.onc.1209382. [DOI] [PubMed] [Google Scholar]
- 55.Reedijk M, Odorcic S, Zhang H, Chetty R, Tennert C, Dickson BC, Lockwood G, Gallinger S, Egan SE. Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol. 2008;33:1223–1229. doi: 10.3892/ijo_00000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao F, Zhang Y, Wang S, Liu Y, Zheng L, Yang J, Huang W, Ye Y, Luo W, Xiao D. Hes1 is involved in the self-renewal and tumourigenicity of stem-like cancer cells in colon cancer. Sci Rep. 2014;4:3963. doi: 10.1038/srep03963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 58.Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu LJ, Kong QL, Xu LH, Zhang X, Liu WL, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009;119:3626–3636. doi: 10.1172/JCI39374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H, Song F, Chen X, Li Y, Fan J, Wu X. Bmi-1 regulates epithelial-to-mesenchymal transition to promote migration and invasion of breast cancer cells. Int J Clin Exp Pathol. 2014;7:3057–3064. [PMC free article] [PubMed] [Google Scholar]
- 60.Guo BH, Feng Y, Zhang R, Xu LH, Li MZ, Kung HF, Song LB, Zeng MS. Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Mol Cancer. 2011;10:10. doi: 10.1186/1476-4598-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu S, Tetzlaff MT, Cui R, Xu X. miR-200c inhibits melanoma progression and drug resistance through down-regulation of BMI-1. Am J Pathol. 2012;181:1823–1835. doi: 10.1016/j.ajpath.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong P, Kaneuchi M, Watari H, Hamada J, Sudo S, Ju J, Sakuragi N. MicroRNA-194 inhibits epithelial to mesenchymal transition of endometrial cancer cells by targeting oncogene BMI-1. Mol Cancer. 2011;10:99. doi: 10.1186/1476-4598-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nanta R, Kumar D, Meeker D, Rodova M, Van Veldhuizen PJ, Shankar S, Srivastava RK. NVP-LDE-225 (Erismodegib) inhibits epithelial-mesenchymal transition and human prostate cancer stem cell growth in NOD/SCID IL2Rγ null mice by regulating Bmi-1 and microRNA-128. Oncogenesis. 2013;2:e42. doi: 10.1038/oncsis.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu L, Qiu M, Tan G, Liang Z, Qin Y, Chen L, Chen H, Liu J. miR-200c inhibits invasion, migration and proliferation of bladder cancer cells through down-regulation of BMI-1 and E2F3. J Transl Med. 2014;12:305. doi: 10.1186/s12967-014-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dass SD, Cheah PL, Ong DB, Teoh KH, Looi LM. E-cadherin downregulation at the infiltrating tumour front is associated with histological grade and stage in colorectal carcinoma of Malaysians. Malays J Pathol. 2015;37:19–24. [PubMed] [Google Scholar]
- 66.Le Bras GF, Taubenslag KJ, Andl CD. The regulation of cell-cell adhesion during epithelial-mesenchymal transition, motility and tumor progression. Cell Adh Migr. 2012;6:365–373. doi: 10.4161/cam.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao WT, Cui YM, Ding YQ. Expression and Significance of Bmi-1 PTEN and E-Cadherin in Colorectal Cancer. Chin J Clin Oncol. 2012;39:559–563. In Chinese. [Google Scholar]