Abstract
Corneal alkali burns (CAB) are characterized by injury-induced inflammation, fibrosis and neovascularization (NV), and may lead to blindness. This review evaluates the current knowledge of the molecular mechanisms responsible for CAB. The processes of cytokine production, chemotaxis, inflammatory responses, immune response, cell signal transduction, matrix metalloproteinase production and vascular factors in CAB are discussed. Previous evidence indicates that peroxisome proliferator-activated receptor γ (PPAR-γ) agonists suppress immune responses, inflammation, corneal fibrosis and NV. This review also discusses the role of PPAR-γ as an anti-inflammatory, anti-fibrotic and anti-angiogenic agent in the treatment of CAB, as well as the potential role of PPAR-γ in the pathological process of CAB. There have been numerous studies evaluating the clinical profiles of CAB, and the aim of this systematic review was to summarize the evidence regarding the treatment of CAB with PPAR-γ agonists.
Keywords: corneal alkali burn, corneal neovascularization, inflammation, peroxisome proliferator-activated receptor-γ, agonists
1. Introduction
The cornea is the protective ocular surface, and is transparent to enable the transmission of light. Chemical burns can damage this barrier (1), and in addition to corneal injury and eyelid burns, are risk factors for ocular complications, including ulcers, scars and neovascularization (NV) (2,3). Several potential interventional strategies, including limbal stem cells, amniotic membranes and corneal transplantations have been demonstrated to have some success in clinical outcomes. There are numerous risk factors and molecular markers for the progression of ocular chemical burns. Improvements in the knowledge of the novel biomarkers associated with the inflammation, angiogenesis and fibrosis of ocular chemical injuries have contributed to the development of novel therapeutics. Chemical burns can be divided into alkali and acid burns, with corneal alkali burns (CAB) frequently resulting in a greater severity of injury (4). Peroxisome proliferator-activated receptor (PPAR) controls the regulation of genes through the activation of nuclear receptors, and plays a role in the control of a variety of inflammatory, angiogenic and fibrotic physiological processes (5). This reviews covers the key aspects associated with biomarker research into the pathological process of CAB, and analyzes the potential therapeutic role of PPAR agonists in the treatment of CAB. The processes of cytokine production, chemotaxis, inflammatory and immune responses, signal transduction, matrix metalloproteinase (MMP) production and vascular factors in CAB are summarized, and the potential application of PPAR agonists as treatments to control lesion severity in CAB are also discussed.
2. Conventional CAB treatment
Stem cells potentiate regeneration due to their ability to differentiate into multiple cell lineages. The most common sources of stem cells for clinical use are embryonic, adult and induced (6). Surface transplantation and subsequent keratoplasty can result in good visual function following ocular injury (7). Limbal stem cell grafts with amniotic membrane transplantation or simple limbal epithelial transplantation may additionally be used to restore vision and reduce symptoms in cases with limbal stem cell deficiency following chemical burns (8–12). Cultivated oral mucosal epithelial transplantation has been indicated to enable the complete epithelialization of persistent corneal epithelial defects, and stabilize the ocular surface in patients with severe ocular surface disease (13). The Boston keratoprosthesis type I is an effective artificial cornea and aids in the recovery from advanced ocular surface disease, and has been shown to result in a significant increase in eyesight (14). Additionally, Boston keratoprosthesis implantation may reduce the risk of post-keratoplasty complications by the wearing of contact lenses (15). Alternatively, a large tectonic corneoscleral lamellar graft represents a good treatment method (16). These methods can treat a selection of clinical applications and present some benefits; however, they require further study.
3. Limitations of traditional therapeutic strategies
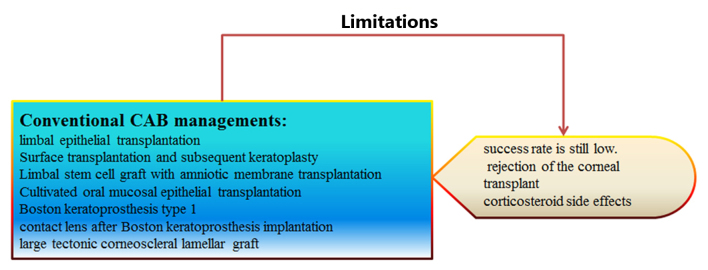
Scarring has been attributed to the proliferation of inflammatory cells and fibroblasts during burn wound healing. The irregular remodeling of matrix structures may lead to scar formation. Stem cell immunomodulation has been indicated to address pathological scarring (17–19). Limbal transplantation is a standard procedure to restore ocular surface disorders and, considering the shortage of corneal donors, is a viable alternative treatment strategy; however, the success rate remains low (20). Additionally, the separation and purification rates of limbal cells and the efficiency of migration require further investigation, and the therapeutic efficacy and safety of limbal transplantation should be clarified (21). Furthermore, the rejection rate of keratoplasty is high in cases of CAB, and the number of suitable donor corneas available is not sufficient to meet the demands (22,23). Corticosteriods are the predominant current treatment, and treat the inflammation associated with corneal NV (CNV); however, they can result in side-effects, such as cataracts and increased intraocular pressure (24). Further studies are required to understand stem cell applications targeting NV and the inflammatory and fibrotic processes associated with CAB (Fig. 1).
Figure 1.
Conventional corneal alkali burns (CAB) managements and their limitations.
4. Topical CAB therapies from bench to clinic
A number of topical therapeutics against NV or inflammation associated with CAB are under investigation (25). Some of these potential topical therapeutics under investigation are aloe vera, prospero homeobox 1 short interfering RNA, Rho-associated protein kinase inhibitors (AMA0526), 0.5% ketorolac tromethamine, keratinocyte growth factor-2, omentum, protein phosphatase magnesium dependent-1 and melatonin, and may potentially be used for the treatment of CAB in clinical practice (26–33). Subconjunctival bevacizumab injection may be considered as a secondary treatment for CNV caused by chemical injuries that are not responsive to conventional steroid therapy (34,35). These topical therapies may be effective treatments for severe cases of CAB, although further studies may be required to fully determine this.
5. PPAR-γ and the healing process of CAB
PPARs belong to a nuclear receptor superfamily that includes steroid, thyroid hormone, vitamin D and retinoid receptors. PPAR-γ is activated by transcription factors and plays an important role in the regulation of cell proliferation and inflammation (36,37). PPAR suppresses inflammatory cytokines, proteolytic enzymes, adhesion molecules, chemotactic and atherogenic factors (38–40). Transforming growth factor (TGF) β1 has been shown to transdifferentiate keratocytes to myofibroblasts involved in the repair of the corneal epithelium, and stromal and corneal scar formation in CAB, by regulating monocytes, macrophages, vascular endothelial growth factor (VEGF), neutrophils and monocyte/macrophage chemotactic protein-1 (32,41). In a previous study, the expression of the PPAR-γ gene was shown to induce anti-inflammatory and anti-fibrogenic responses in an alkali-burned mouse cornea. Additionally, PPAR-γ gene expression suppressed TGFβ1 and MMP expression in macrophages, indicating a potentially effective strategy for the treatment of CAB (3). PPAR-γ expression has been reported to increase with the infiltration of numerous inflammatory cells in the pathological process of CAB. As previously demonstrated, treatment with an ophthalmic solution of a PPAR-γ agonist suppressed the expression levels of interleukin (IL)-1β, IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), TGFβ1 and VEGF-A in corneal inflammation induced by an alkali burn. An ophthalmic solution of the PPAR-γ agonist may provide a novel treatment strategy with useful clinical applications for corneal inflammation and wound healing (42). Burns induce the activation of an inflammatory cascade and wound progression. The PPAR-γ agonsist, rosiglitazone, reduces the percentage of unburned skin interspaces that progress to full necrosis in a rat model and prevent burn-induced organ damage. Therefore, the PPAR-γ agonists hold potential for clinical application (36,43). In this review, the potential role of PPAR-γ agonists in the treatment of CAB and the underlying molecular mechanisms are discussed.
6. Potential role of PPAR agonists in the treatment of CAB
PPAR-γ ligands are divided into endogenous (9, 13 and 15-hydroxyoctadecadienoic acid) and synthetic (pioglitazone, troglitazone, rosiglitazone, liglitazone and TZD18) compounds (44). PPAR isoforms (PPAR-γ, PPAR-α and PPAR-β/δ) have been shown to exhibit anti-inflammatory and immunomodulatory properties. PPARs may represent a novel target in the treatment of inflammatory and vascular diseases (45). Pioglitazone may inhibit corneal fibroblast migration and reduce corneal fibroblast-induced collagen contraction in the corneal wound healing process (46). Previous studies have supported the anti-inflammtory, anti-angiogenic and anti-fibrotic functions of PPAR-γ.
PPAR-γ, cytokines and cellular immunity
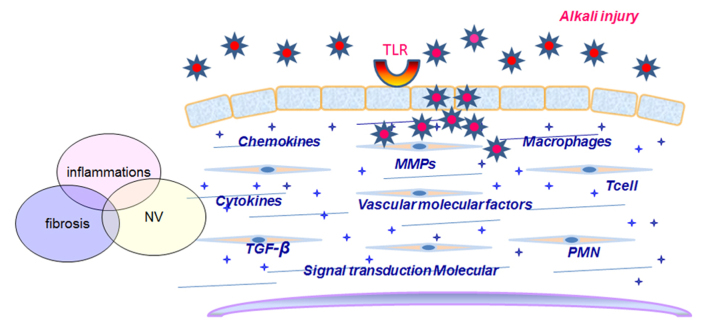
Toll-like receptors (TLRs) play key roles in innate immune responses. PPAR-γ gene silencing affects genes involved in the innate immune process (47). Injury primes the innate immune system for enhanced TLR-2- and TLR-4-mediated responses, and suggests that increasing TLR activity may contribute to the progression of systemic inflammation following severe injury (48). Previously, Th1-activated macrophages were considered a key cellular defense against intracellular pathogens. However, more recently, Th2-activated macrophages have been indicated to be involved in repair and tissue regeneration via the modulation of PPARs in immunological inflammation, and this may lead to new therapeutic approaches (49–51). Dendritic cells (DCs) from burned skin notably express low levels of human leukocyte antigen-antigen D related and TLR-4 immediately following cell isolation. In the post-burn period, the ability of skin DCs to respond to bacterial stimuli is impaired. These alterations in DCs may contribute to impaired host defenses against bacteria, leading to post-burn infection (52). Burns are associated with γδ T-cell activation at the injury site, which initiates the infiltration of the wound with large numbers of αβ T-cells that may facilitate the transition from the inflammatory to the proliferative phase of healing (53). Burns and TLRs are associated with the induction of the innate immune system, with a greater number of TLR-2-induced Kupffer cells (KCs) and macrophage inflammatory protein (MIP)-1β production post-injury, whereas the levels of IL-6, IL-10 and MIP-1β and the number of KCs are greater following TLR-4-induced activation following burns. TLR-mediated inflammatory responses have been reported to be augmented post-burn by the induction of inflammatory mediators (54). TLR-5 is normally present on the superficial cells of the conjunctival epithelium, and may be upregulated following chemical burns (55). TLR activates the innate immune system to recognize antigens and induce the production of inflammatory cytokines and chemokines (56,57). The TLR-related genes, heat-shock 70kDa protein (HSP)A1A, Harvey rat sarcoma viral oncogene homolog, mitogen-activated protein kinase (MAPK) kinase 3, Toll interacting protein, v-rel avian reticuloendotheliosis viral oncogene homolog A, FBJ murine osteosarcoma viral oncogene homolog and TLR-1 have been observed to be reduced in the primary epidermal keratinocytes of patients with severe burns, and restoring the expression of these genes may improve clinical outcomes (58). High levels of cytokines promote collagen degradation, the apoptosis of keratinocytes and vascular compromise. Local inflammation induced by severe burns can clear cellular debris, protect against microbial agents and induce cell growth and proliferation (58,59). The reduction of the activation and recruitment of macrophages may be a potential therapeutic strategy for the corneal scarring of alkali-burned ocular surfaces (60). Agonists of TLR-4, 1/2 and -5 suppress the activity of PPAR-α and PPAR-γ in astrocytes (61). PPAR-β/δ expression is regulated in TLR agonist-stimulated astrocytes via the regulation of the pro-inflammatory genes. p38, MAP2K1/2, MAPK2/3 and c-Jun N-terminal kinase (JNK) (62). The PPAR-α agonist, WY14643, has been shown to significantly reduce amylase, lipase and myeloperoxidase activity, and IL-6, intercellular adhesion molecule-1, and TLR-2 and 4 levels (63). PPAR-γ inhibits interferon (IFN)-β production in TLR3- and 4-stimulated macrophages by preventing interferon regulatory factor 3 binding to the IFN-β promoter (64). Treatment with rosiglitazone was previously shown to result in higher levels of PPARγ and a reduction in serum inflammatory cytokine levels, and the levels of TLR2/4 and nuclear factor-κB (NF-κB) activity in aortic tissues. These biological functions of rosiglitazone in P. gingivalis-accelerated atherosclerosis were shown to be dependent upon the inhibition of the inflammatory response and the TLR/NF-κB signaling pathway (65). PPAR-γ and TGF-β can enhance regulatory T cell (Treg) generation, providing a potential therapeutic strategy for the treatment of inflammatory and autoimmune diseases (66). PPAR-γ restores the abnormal immune gene expression of p38MAPK, activating transcription factor-2, MAPK-activated protein kinase 2 and HSP27 in T-cell mediated immune responses in vivo (67). Cell types in the innate and adaptive immune system, including neutrophils, macrophages, mast cells, B cells and T cells, have all been implicated to play a role in burn-induced immunology (68). Burn injury disrupts the immune system, resulting in the marked suppression of the immune response. The mononuclear phagocyte system (MPS) is a critical component of the innate immune response, and is able to initiate an adaptive immune response. Severe burns inhibits the functions of DCs, monocytes and macrophages. The MPS in the pathophysiology of severe burns will guarantee a more rational immunotherapy for patients with severe burns (69). These results collectively suggest that PPAR-α, -γ and -β/δ are likely mediators of TLR activation in transducing inflammation in CAB pathologies; however, the relative immune mechanisms require clarification. The molecular mechanisms of CAB are summarized in Fig. 2.
Figure 2.
The molecular mechanisms associated with corneal alkali burns (CAB).
Cytokines and cellular immunity
As an anti-TNF-α monoclonal antibody, topical infliximab has been reported to significantly reduce corneal perforation, leukocyte infiltration, cluster of differentiation (CD)45+ cell infiltration and fibrosis in the eyelids. The topical application of infliximab may be useful in the treatment of ocular diseases (70). Topically applied IL-1 receptor antagonist (IL-1ra) may suppress corneal inflammation and promote recovery following CAB. All cytokine/chemokine levels, in particular IL-6 and IL-10, have been shown to be significantly reduced in IL-1ra-treated eyes, with the opposite effect observed in IL-1ra knockout mice (71–74). The treatment of inflammation with minimal infiltrating cells and normal levels of IL-1α and IL-1β may accelerate the healing of CAB (75). A reduction in IL-6 and TGF-β1 expression has been indicated to protect the cornea from chemical damage (76). In addition, the inhibition of inflammation and NV has been reported to play a significant role in preventing corneal angiogenesis and inflammation in alkali-burned corneal beds, which results in higher allograft survival rates (77). Furthermore, in a CAB model, the infiltrated polymorphonuclear leukocytes and the mRNA expression of VEGF receptor 1 and 2, basic fibroblast growth factor, IL-1β, IL-6, MMP-2, -9 and -13, in addition to the protein expression levels of VEGFR2, IL-1β, IL-6 and MMP-2 and -9, were upregulated in the corneas. The suppression of CNV, inflammatory cytokines and MMPs aids in reducing the damage associated with CAB (78). Human peripheral blood mononuclear cells and inflammatory cytokines can be stimulated by chemically injured keratocytes. MMP-9 and macrophage migration inhibitory factor levels have been reported to be higher in burn injury (79). CD4 and CD44 (memory) CD8 T cells have been found to be significantly increased, in addition to TLR-4, post-burn injury, and functional T cell responses have additionally been demonstrated. Complex adaptive immune responses have been reported in burn injury (80); however, this differs in the process of CAB. IFN-γ and CD4 were not detected in rat corneas following alkali burns, indicating that cytokines were induced in the cornea by burn injury without a specific immunological stimulus (81). To inhibit excessive inflammatory damage, particular anti-inflammatory agents may be applied for the treatment of alkali burns. PPAR-γ agonists are a good candidate for anti-inflammatory activity in preventing TNF-α damage (82). Pioglitazone therapy has been demonstrated to suppress the mRNA levels of the inflammatory cytokines monocyte, MCP-1, IL-1 and IL-6, produced by macrophages in the cerebral arteries (83). PPAR-γ represents an appealing strategy for decreasing inflammation and improving the healing of chronic injuries, and PPAR-γ in inflammatory cells may be a potential therapeutic target (84,85). Pioglitazone has been shown to exert anti-inflammatory effects on acute gouty arthritis by inhibiting the expression of TNF-α and IFN-γ (86). Notably, there is anti-inflammatory therapeutic potential for the treatment of Alzheimer's disease, dental implants and lipid inflammation processes through the PPAR-γ pathway (47,87,88). PPAR-γ modulates macrophage and T cell-mediated inflammation. Reductions in the levels of PPAR-γ in T cells have been shown to result in an increased expression of adhesion molecules and pro-inflammatory cytokines (IL-6 and IL-1β), and to modulate Treg recruitment (89). Thus, PPAR-γ agonists are effective in controlling inflammation-related damage and inhibiting cytokines and chemokines, suggesting their therapeutic potential in the treatment of CAB.
PPAR-γ and NV
Pathological conditions including infection, trauma and loss of the limbal stem cell barrier can lead to CNV formation, from the limbal area to the vascular cornea (90). NV is mediated by cellular and molecular factors, such as VEGF and pigment epithelium-derived factor (PEDF), which play roles in the development of NV (91). Corneal transparency is essential for maintaining good visual acuity, and NV in CAB forms the basis of multiple visual pathologies that may result in blindness. However, CNV formations respond poorly to current therapies. Therefore, potential anti-angiogenic topical treatments against CNV resulting from alkali burns have been investigated in in vitro studies and clinical trials (25,92–95). The suppression of VEGF and placental growth factor levels in the cornea in a mouse model of alkali burns was observed to significantly inhibit NV growth and the regression of established vessels (96). PPAR-γ agonists are potent inhibitors of NV and show potential for the treatment of inflammatory vasculoproliferative diseases (97–100). Rosiglitazone has been shown to protect vascular endothelial cells by reducing the expression of the chemerin receptor, ChemR23 (101). Thiazolidinediones (TZDs) inhibit retinal and choroidal NV by suppressing tube formation in human umbilical vein endothelial cells (HUVECs). In addition, TZDs may inhibit VEGF induced non-inflammatory NV in vivo (102). PEDF is a potent anti-angiogenic factor and can induce endothelial cell apoptosis, and can inhibit angiogenesis by augmenting PPAR-γ expression in ischemic heart tissue (103). Therefore, PPAR-γ may be a useful target in the prevention and treatment of vascular inflammatory diseases.
PPAR-γ and fibrosis
Corneal fibrosis can result in visual impairment and blindness. Alkali burned corneas were observed to exhibit obvious interfibrillar distances with greater levels of the fibrotic marker α-smooth muscle actin (αSMA) (104). The TGFβ-induced differentiation of corneal fibroblasts to myofibroblasts could be prevented (105). The level of inflammation and scarring/fibrosis has been observed to increase during healing in injured tissue in a model of CAB. The prognosis of CAB is dependent upon ocular surface inflammation, and the scarring and fibrosis of the cornea and eyelid (70,106). PPAR-γ possess strong anti-fibrotic properties in the cornea and several other types of tissue, with PPAR-γ ligands blocking αSMA induction (107). A number of studies have demonstrated that treatment with ophthalmic solutions of PPAR-γ agonists reduced the fibrotic reaction in the early phase post-CAB and in additional fibrotic pathologies (106,108,109).
PPAR-γ agonists and cell signal transduction
PPAR-γ is an important modulator of lipid metabolism during inflammation, via the inhibition of the expression of proinflammatory molecules (110). NF-κB is activated and translocates to the nucleus where it controls the expression of a large number of target genes, which are involved in the regulation of inflammation and innate and adaptive immune responses (111). Telomeric repeat binding factor was discovered as a modulator that regulates NF-κB signaling. The inhibition of repeat binding factor may lead to the design of specific inhibitors of NF-κB for the treatment of ocular injuries (112). The effects of SN50, an inhibitor of NF-κB, were reported to be dependent on TNF-α/JNK signaling in a mouse model of CAB, with the topical application of SN50 shown to be effective in treating CAB (113). PPAR-γ has been indicated to be the predominant pathway involved in the inhibition of IL-1β-induced inflammation [nitric oxide and prostaglandin E2 production, in addition to inducible nitric oxide synthase and cyclooxygenase 2 (COX-2) expression, NF-κB and MAPK activation (114).
PPAR-γ agonists and chemokines
TC14012 [a chemokine (C-X-C motif) receptor 4 (CXCR4) antagonist and CXCR7 agonist] has been reported to initially enhance alkali burn-induced CNV, then reduce CNV in later stages. In addition to CXCR4, CXCR7 has been implicated in the pathogenesis of CNV (115). Granulocyte-colony stimulating factor (G-CSF) post-traumatic gene expression activates innate immune responses and suppresses adaptive immune responses. The G-CSF signal transducer and activator of transcription axis has been indicated to be a key protective mechanism post-injury in reducing the risk of infection (116). PPAR-γ reduces the expression levels of pro-inflammatory chemokines, including chemokine (C-C motif) ligand 20, CXC ligand (CXCL)2, CXCL3 and chemokine (C-X3-C motif) ligand 1 (CX3CL1) in colon tissues. It has been shown that increasing the transcriptional activity of PPAR-γ can modulate inflammatory signaling pathways, suggesting a novel target for therapeutic agents (117). The investigation of inflammatory markers in vascular disorders reveals augmented levels of circulating cytokines and chemokines among carriers of classic risk factors for atherosclerosis. Dysregulation of the PPAR signaling pathway may explain the association of IL-8/12 and very low density lipoprotein (VLDL)-c in the promotion of dysglycemia (118). The PPAR signaling pathway was shown to be important in the modulation of inflammatory factors, including MCP-1, TNF-α, IL-1 and IL-6, COX-2, nicotinamide adenine dinucleotide phosphate, protein kinase C, vascular cell adhesion molecule-1, NF-κB and monocyte expressions in HUVECs. The inhibition of the PPAR pathway in endothelial inflammation suggests a potential role of PPAR agonists in the treatment of vascular inflammation (119,120). Rosiglitazone has been shown to suppress angiogenesis by downregulating the expression of CXCR4 in a dose-, time- and PPAR-γ-dependent manner (121). Regulating the expression of MCP-1 and activating the 5′ AMP-activated protein kinase-sirtuin 1-PPAR signaling pathway may be a novel therapeutic agent for atherosclerosis (122). PPAR-γ has been indicated to regulate hypoxia/reoxygenation-stimulated IL-8 production in U937 cells (123). PPAR-γ serves an inhibitory role in hepatic injury by downregulating the local expression of proinflammatory cytokines, chemokines and adhesion molecules following reperfusion (124,125).
PPAR-γ agonists and MMPs
Keratocytes are able to directly degrade type I collagen and create stromal spaces, promoting CNV through VEGF induced MMP-13 expression (126). The inhibition of alkali burn-induced CNV in mice may be possible via reductions in the production of the angiogenic factors, inflammatory cytokines and MMPs involved in the angiogenic response (78,127,128). MMP-12 may disintegrate certain components of the extracellular matrix (ECM) released following severe alkali burn, which may be involved in ECM remodeling (129). Inhibiting alkali burn-induced CNV by accelerating corneal wound healing and by reducing the production of angiogenic factors, inflammatory cytokines and MMPs may be a potential therapeutic strategy (29,125,130–133). PPAR-γ agonists are able to affect proliferation, differentiation, apoptosis and inflammation in different cell types. PPAR-γ ligands were able to inhibit K562 and HL-60 cell adhesion to ECM proteins by inhibiting the expression of MMP-2 and -9 (134). PPAR-γ agonists have been shown to inhibit macrophage infiltration, the expression of TNF-α and MMP-9 in aortic tissue, thus may be used as anti-inflammatory agents in cardiovascular fields (135). Degradation of the epithelial basement membrane in burned cornea in vivo was reversed by an MMP inhibitor (136), additionally; MMP inhibitors have been shown to block the progression of alkali burns to ulceration (137). These data may indicate that PPAR-γ agonists are a potential strategy for preventing CAB progression.
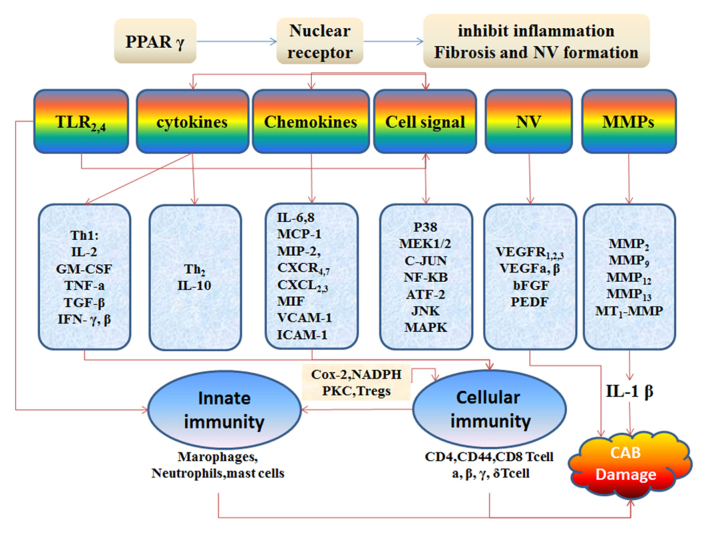
Taken together, the evidence suggests that PPAR-γ may lessen NV, inflammation and scarring. However, additional studies are necessary to evaluate the potential therapeutic effects of PPAR-γ in ocular NV, tissue inflammation and the resultant fibrosis following burn injury (Fig. 3).
Figure 3.
The molecular mechanisms responsible for the inhibitory effects of peroxisome proliferator activated receptor γ (PPAR-γ) agonists on corneal alkali burns (CAB).
Acknowledgments
The present study was supported by the Natural Science Foundation of China (grant no. 81300727) and Jilin University Basic Scientific Research Operating Expenses Fund (Research Fund of the Bethune B Plan of Jilin University; grant no. 2012230).
References
- 1.Leong YY, Tong L. Barrier function in the ocular surface: From conventional paradigms to new opportunities. Ocul Surf. 2015;13:103–109. doi: 10.1016/j.jtos.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Cabalag MS, Wasiak J, Syed Q, Paul E, Hall AJ, Cleland H. Early and late complications of ocular burn injuries. J Plast Reconstr Aesthet Surg. 2015;68:356–361. doi: 10.1016/j.bjps.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Saika S, Yamanaka O, Okada Y, Miyamoto T, Kitano A, Flanders KC, Ohnishi Y, Nakajima Y, Kao WW, Ikeda K. Effect of overexpression of PPARgamma on the healing process of corneal alkali burn in mice. Am J Physiol Cell Physiol. 2007;293:C75–C86. doi: 10.1152/ajpcell.00332.2006. [DOI] [PubMed] [Google Scholar]
- 4.Pargament JM, Armenia J, Nerad JA. Physical and chemical injuries to eyes and eyelids. Clin Dermatol. 2015;33:234–237. doi: 10.1016/j.clindermatol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Gu H, Hu N. Role of Peroxisome Proliferator-Activated Receptor γ in Ocular Diseases. J Ophthalmol. 2015;2015:275435. doi: 10.1155/2015/275435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu CC, Peng CH, Hung KH, Lee YY, Lin TC, Jang SF, Liu JH, Chen YT, Woung LC, Wang CY, et al. Stem cell therapy for corneal regeneration medicine and contemporary nanomedicine for corneal disorders. Cell Transplant. 2015;24:1915–1930. doi: 10.3727/096368914X685744. [DOI] [PubMed] [Google Scholar]
- 7.Mittal V, Jain R, Mittal R, Vashist U, Narang P. Successful management of severe unilateral chemical burns in children using simple limbal epithelial transplantation (SLET) Br J Ophthalmol. 2015;2015:307179. doi: 10.1136/bjophthalmol-2015-307179. [DOI] [PubMed] [Google Scholar]
- 8.Movahedan A, Genereux BM, Darvish-Zargar M, Shah KJ, Holland EJ. Long-term management of severe ocular surface injury due to methamphetamine production accidents. Cornea. 2015;34:433–437. doi: 10.1097/ICO.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 9.Kafle PA, Singh SK, Sarkar I, Surin L. Amniotic membrane transplantation with and without limbal stem cell transplantation in chemical eye injury. Nepal J Ophthalmol. 2015;7:52–55. doi: 10.3126/nepjoph.v7i1.13168. [DOI] [PubMed] [Google Scholar]
- 10.Scholz SL, Thomasen H, Hestermann K, Dekowski D, Steuhl KP, Meller D. Long-term results of autologous transplantation of limbal epithelium cultivated ex vivo for limbal stem cell deficiency. Ophthalmologe. 2016;113:321–329. doi: 10.1007/s00347-015-0110-y. In German. [DOI] [PubMed] [Google Scholar]
- 11.Almaliotis D, Koliakos G, Papakonstantinou E, Komnenou A, Thomas A, Petrakis S, Nakos I, Gounari E, Karampatakis V. Mesenchymal stem cells improve healing of the cornea after alkali injury. Graefes Arch Clin Exp Ophthalmol. 2015;253:1121–1135. doi: 10.1007/s00417-015-3042-y. [DOI] [PubMed] [Google Scholar]
- 12.Holan V, Trosan P, Cejka C, Javorkova E, Zajicova A, Hermankova B, Chudickova M, Cejkova J. Comparative Study of the Therapeutic Potential of Mesenchymal Stem Cells and Limbal Epithelial Stem Cells for Ocular Surface Reconstruction. Stem Cells Transl Med. 2015;4:1052–1063. doi: 10.5966/sctm.2015-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sotozono C, Inatomi T, Nakamura T, Koizumi N, Yokoi N, Ueta M, Matsuyama K, Kaneda H, Fukushima M, Kinoshita S. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol. 2014;92:e447–e453. doi: 10.1111/aos.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudnisky CJ, Belin MW, Guo R, Ciolino JB. Boston Type 1 Keratoprosthesis Study Group: Visual Acuity Outcomes of the Boston Keratoprosthesis Type 1: Multicenter Study Results. Am J Ophthalmol. 2016;162:89–98. doi: 10.1016/j.ajo.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kammerdiener LL, Speiser JL, Aquavella JV, Harissi-Dagher M, Dohlman CH, Chodosh J, Ciolino JB. Protective effect of soft contact lenses after Boston keratoprosthesis. Br J Ophthalmol. 2016;100:549–552. doi: 10.1136/bjophthalmol-2014-306396. [DOI] [PubMed] [Google Scholar]
- 16.Iyer G, Srinivasan B, Rishi E, Rishi P, Agarwal S, Subramanian N. Large lamellar corneoscleral grafts: Tectonic role in initial management of severe ocular chemical injuries. Eur J Ophthalmol. 2016;26:12–17. doi: 10.5301/ejo.5000631. [DOI] [PubMed] [Google Scholar]
- 17.Prockop DJ. Inflammation, fibrosis, and modulation of the process by mesenchymal stem/stromal cells. Matrix Biol. 2016;51:7–13. doi: 10.1016/j.matbio.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi Y, Jiang D, Sindrilaru A, Stegemann A, Schatz S, Treiber N, Rojewski M, Schrezenmeier H, Vander Beken S, Wlaschek M, et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J Invest Dermatol. 2014;134:526–537. doi: 10.1038/jid.2013.328. [DOI] [PubMed] [Google Scholar]
- 19.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreira PB, Magalhães RS, Pereira NC, Oliveira LA, Sousa LB. Limbal transplantation at a tertiary hospital in Brazil: A retrospective study. Arq Bras Oftalmol. 2015;78:207–211. doi: 10.5935/0004-2749.20150054. [DOI] [PubMed] [Google Scholar]
- 21.Schimke MM, Marozin S, Lepperdinger G. Patient-Specific age: The other side of the coin in advanced mesenchymal stem cell therapy. Front Physiol. 2015;6:362. doi: 10.3389/fphys.2015.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamm V, Hara H, Mammen A, Dhaliwal D, Cooper DK. Corneal blindness and xenotransplantation. Xenotransplantation. 2014;21:99–114. doi: 10.1111/xen.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heindl LM, Cursiefen C. Split-cornea transplantation-a novel concept to reduce corneal donor shortage. Klin Monbl Augenheilkd. 2012;229:608–614. doi: 10.1055/s-0031-1299494. In German. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Zhou Q, Hanus J, Anderson C, Zhang H, Dellinger M, Brekken R, Wang S. Inhibition of multiple pathogenic pathways by histone deacetylase inhibitor SAHA in a corneal alkali-burn injury model. Mol Pharm. 2013;10:307–318. doi: 10.1021/mp300445a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakunowicz-Łazarczyk A, Urban B. Assessment of therapeutic options for reducing alkali burn-induced corneal neovascularization and inflammation. Adv Med Sci. 2016;61:101–112. doi: 10.1016/j.advms.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Atiba A, Wasfy T, Abdo W, Ghoneim A, Kamal T, Shukry M. Aloe vera gel facilitates re-epithelialization of corneal alkali burn in normal and diabetic rats. Clin Ophthalmol. 2015;9:2019–2026. doi: 10.2147/OPTH.S90778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rho CR, Choi JS, Seo M, Lee SK, Joo CK. Inhibition of lymphangiogenesis and hemangiogenesis in corneal inflammation by subconjunctival Prox1 siRNA injection in rats. Invest Ophthalmol Vis Sci. 2015;56:5871–5879. doi: 10.1167/iovs.14-14433. [DOI] [PubMed] [Google Scholar]
- 28.Sijnave D, Van Bergen T, Castermans K, Kindt N, Vandewalle E, Stassen JM, Moons L, Stalmans I. Inhibition of Rho-associated kinase prevents pathological wound healing and neovascularization after corneal trauma. Cornea. 2015;34:1120–1129. doi: 10.1097/ICO.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 29.Lima TB, Ribeiro AP, Conceição LF, Bandarra M, Manrique WG, Laus JL. Ketorolac eye drops reduce inflammation and delay re-epithelization in response to corneal alkali burn in rabbits, without affecting iNOS or MMP-9. Arq Bras Oftalmol. 2015;78:67–72. doi: 10.5935/0004-2749.20150019. [DOI] [PubMed] [Google Scholar]
- 30.Cai J, Dou G, Zheng L, Yang T, Jia X, Tang L, Huang Y, Wu W, Li X, Wang X. Pharmacokinetics of topically applied recombinant human keratinocyte growth factor-2 in alkali-burned and intact rabbit eye. Exp Eye Res. 2015;136:93–99. doi: 10.1016/j.exer.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Shadmani A, Kazemi K, Khalili MR, Eghtedari M. Omental transposition in treatment of severe ocular surface alkaline burn: An experimental study. Med Hypothesis Discov Innov Ophthalmol. 2014;3:57–61. [PMC free article] [PubMed] [Google Scholar]
- 32.Dvashi Z, Sar Shalom H, Shohat M, Ben-Meir D, Ferber S, Satchi-Fainaro R, Ashery-Padan R, Rosner M, Solomon AS, Lavi S. Protein phosphatase magnesium dependent 1A governs the wound healing-inflammation-angiogenesis cross talk on injury. Am J Pathol. 2014;184:2936–2950. doi: 10.1016/j.ajpath.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Crooke A, Guzman-Aranguez A, Mediero A, Alarma-Estrany P, Carracedo G, Pelaez T, Peral A, Pintor J. Effect of melatonin and analogues on corneal wound healing: Involvement of Mt2 melatonin receptor. Curr Eye Res. 2015;40:56–65. doi: 10.3109/02713683.2014.914540. [DOI] [PubMed] [Google Scholar]
- 34.Iannetti L, Abbouda A, Fabiani C, Zito R, Campanella M. Treatment of corneal neovascularization in ocular chemical injury with an off-label use of subconjunctival bevacizumab: A case report. J Med Case Reports. 2013;7:199. doi: 10.1186/1752-1947-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozdemir O, Altintas O, Altintas L, Ozkan B, Akdag C, Yüksel N. Comparison of the effects of subconjunctival and topical anti-VEGF therapy (bevacizumab) on experimental corneal neovascularization. Arq Bras Oftalmol. 2014;77:209–213. doi: 10.5935/0004-2749.20140054. [DOI] [PubMed] [Google Scholar]
- 36.Taira BR, Singer AJ, McClain SA, Lin F, Rooney J, Zimmerman T, Clark RA. Rosiglitazone, a PPAR-gamma ligand, reduces burn progression in rats. J Burn Care Res. 2009;30:499–504. doi: 10.1097/BCR.0b013e3181a28e37. [DOI] [PubMed] [Google Scholar]
- 37.Pershadsingh HA, Moore DM. PPARgamma Agonists: Potential as Therapeutics for Neovascular Retinopathies. PPAR Res. 2008;2008:164273. doi: 10.1155/2008/164273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelman L, Fruchart JC, Auwerx J. An update on the mechanisms of action of the peroxisome proliferator-activated receptors (PPARs) and their roles in inflammation and cancer. Cell Mol Life Sci. 1999;55:932–943. doi: 10.1007/s000180050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and inflammation: From basic science to clinical applications. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S41–S45. doi: 10.1038/sj.ijo.0802499. [DOI] [PubMed] [Google Scholar]
- 40.Kostadinova R, Wahli W, Michalik L. PPARs in diseases: Control mechanisms of inflammation. Curr Med Chem. 2005;12:2995–3009. doi: 10.2174/092986705774462905. [DOI] [PubMed] [Google Scholar]
- 41.Chen M, Matsuda H, Wang L, Watanabe T, Kimura MT, Igarashi J, Wang X, Sakimoto T, Fukuda N, Sawa M, et al. Pretranscriptional regulation of Tgf-β1 by PI polyamide prevents scarring and accelerates wound healing of the cornea after exposure to alkali. Mol Ther. 2010;18:519–527. doi: 10.1038/mt.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchiyama M, Shimizu A, Masuda Y, Nagasaka S, Fukuda Y, Takahashi H. An ophthalmic solution of a peroxisome proliferator-activated receptor gamma agonist prevents corneal inflammation in a rat alkali burn model. Mol Vis. 2013;19:2135–2150. [PMC free article] [PubMed] [Google Scholar]
- 43.Sener G, Sehirli AO, Gedik N, Dülger GA. Rosiglitazone, a PPAR-gamma ligand, protects against burn-induced oxidative injury of remote organs. Burns. 2007;33:587–593. doi: 10.1016/j.burns.2006.10.381. [DOI] [PubMed] [Google Scholar]
- 44.Pershadsingh HA, Benson SC, Marshall, Kurtz TW, Pravenec M, King JC, Stopa EG, Famiglietti EV. Ocular diseases and peroxisome proliferator-activated receptor-γ (PPAR-γ) in mammalian eye. Soc Neurosci Abstr. 1999;25:2193. [Google Scholar]
- 45.Balachandar S, Katyal A. Peroxisome proliferator activating receptor (PPAR) in cerebral malaria (CM): A novel target for an additional therapy. Eur J Clin Microbiol Infect Dis. 2011;30:483–498. doi: 10.1007/s10096-010-1122-9. [DOI] [PubMed] [Google Scholar]
- 46.Pan H, Chen J, Xu J, Chen M, Ma R. Antifibrotic effect by activation of peroxisome proliferator-activated receptor-γ in corneal fibroblasts. Mol Vis. 2009;15:2279–2286. [PMC free article] [PubMed] [Google Scholar]
- 47.Kaul D, Anand PK, Khanna A. Functional genomics of PPAR-gamma in human immunomodulatory cells. Mol Cell Biochem. 2006;290:211–215. doi: 10.1007/s11010-006-9169-8. [DOI] [PubMed] [Google Scholar]
- 48.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, Mannick JA, Lederer JA. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 49.Bashir S, Sharma Y, Elahi A, Khan F. Macrophage polarization: The link between inflammation and related diseases. Inflamm Res. 2016;65:1–11. doi: 10.1007/s00011-015-0874-1. [DOI] [PubMed] [Google Scholar]
- 50.Valvis SM, Waithman J, Wood FM, Fear MW, Fear VS. The Immune Response to Skin Trauma Is Dependent on the Etiology of Injury in a Mouse Model of Burn and Excision. J Invest Dermatol. 2015;135:2119–2128. doi: 10.1038/jid.2015.123. [DOI] [PubMed] [Google Scholar]
- 51.Fletcher HA, Keyser A, Bowmaker M, Sayles PC, Kaplan G, Hussey G, Hill AV, Hanekom WA. Transcriptional profiling of mycobacterial antigen-induced responses in infants vaccinated with BCG at birth. BMC Med Genomics. 2009;2:10. doi: 10.1186/1755-8794-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Arpa N, D'Amelio L, Accardo-Palumbo A, Pileri D, Mogavero R, Amato G, Napoli B, Alessandro G, Lombardo C, Conte F. Skin dendritic cells in burn patients. Ann Burns Fire Disasters. 2009;22:175–178. [PMC free article] [PubMed] [Google Scholar]
- 53.Rani M, Zhang Q, Scherer MR, Cap AP, Schwacha MG. Activated skin γδ T-cells regulate T-cell infiltration of the wound site after burn. Innate Immun. 2015;21:140–150. doi: 10.1177/1753425913519350. [DOI] [PubMed] [Google Scholar]
- 54.Schwacha MG, Zhang Q, Rani M, Craig T, Oppeltz RF. Burn enhances toll-like receptor induced responses by circulating leukocytes. Int J Clin Exp Med. 2012;5:136–144. [PMC free article] [PubMed] [Google Scholar]
- 55.Yamada K, Ueta M, Sotozono C, Yokoi N, Inatomi T, Kinoshita S. Upregulation of Toll-like receptor 5 expression in the conjunctival epithelium of various human ocular surface diseases. Br J Ophthalmol. 2014;98:1116–1119. doi: 10.1136/bjophthalmol-2013-304645. [DOI] [PubMed] [Google Scholar]
- 56.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 57.Drage MG, Pecora ND, Hise AG, Febbraio M, Silverstein RL, Golenbock DT, Boom WH, Harding CV. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell Immunol. 2009;258:29–37. doi: 10.1016/j.cellimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cornick SM, Noronha SA, Noronha SM, Cezillo MV, Ferreira LM, Gragnani A. Toll like receptors gene expression of human keratinocytes cultured of severe burn injury. Acta Cir Bras. 2014;29(Suppl 3):33–38. doi: 10.1590/S0102-86502014001700007. [DOI] [PubMed] [Google Scholar]
- 59.Shupp JW, Nasabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res. 2010;31:849–873. doi: 10.1097/BCR.0b013e3181f93571. [DOI] [PubMed] [Google Scholar]
- 60.Kitano A, Okada Y, Yamanka O, Shirai K, Mohan RR, Saika S. Therapeutic potential of trichostatin A to control inflammatory and fibrogenic disorders of the ocular surface. Mol Vis. 2010;16:2964–2973. [PMC free article] [PubMed] [Google Scholar]
- 61.Chistyakov DV, Aleshin SE, Astakhova AA, Sergeeva MG, Reiser G. Regulation of peroxisome proliferator-activated receptors (PPAR) a and -γ of rat brain astrocytes in the course of activation by toll-like receptor agonists. J Neurochem. 2015;134:113–124. doi: 10.1111/jnc.13101. [DOI] [PubMed] [Google Scholar]
- 62.Chistyakov DV, Aleshin S, Sergeeva MG, Reiser G. Regulation of peroxisome proliferator-activated receptor β/δ expression and activity levels by toll-like receptor agonists and MAP kinase inhibitors in rat astrocytes. J Neurochem. 2014;130:563–574. doi: 10.1111/jnc.12757. [DOI] [PubMed] [Google Scholar]
- 63.Ding JL, Zhou ZG, Zhou XY, Zhou B, Wang L, Wang R, Zhan L, Sun XF, Li Y. Attenuation of acute pancreatitis by peroxisome proliferator-activated receptor-α in rats: The effect on Toll-like receptor signaling pathways. Pancreas. 2013;42:114–122. doi: 10.1097/MPA.0b013e3182550cc4. [DOI] [PubMed] [Google Scholar]
- 64.Zhao W, Wang L, Zhang M, Wang P, Zhang L, Yuan C, Qi J, Qiao Y, Kuo PC, Gao C. Peroxisome proliferator-activated receptor gamma negatively regulates IFN-beta production in Toll-like receptor (TLR) 3- and TLR4-stimulated macrophages by preventing interferon regulatory factor 3 binding to the IFN-beta promoter. J Biol Chem. 2011;286:5519–5528. doi: 10.1074/jbc.M110.149823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan S, Lei L, Chen S, Li H, Yan F. Rosiglitazone impedes Porphyromonas gingivalis-accelerated atherosclerosis by down-regulating the TLR/NF-κB signaling pathway in atherosclerotic mice. Int Immunopharmacol. 2014;23:701–708. doi: 10.1016/j.intimp.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 66.Lian M, Luo W, Sui Y, Li Z, Hua J. Dietary n-3 PUFA protects mice from Con A induced liver injury by modulating regulatory T cells and PPAR-γ expression. PLoS One. 2015;10:e0132741. doi: 10.1371/journal.pone.0132741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li T, Wang W, Zhao JH, Zhou X, Li YM, Chen H. Pseudolaric acid B inhibits T-cell mediated immune response in vivo via p38MAPK signal cascades and PPARγ activation. Life Sci. 2015;121:88–96. doi: 10.1016/j.lfs.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 68.Kraft CT, Agarwal S, Ranganathan K, Wong VW, Loder S, Li J, Delano MJ, Levi B. Trauma-induced heterotopic bone formation and the role of the immune system: A review. J Trauma Acute Care Surg. 2016;80:156–165. doi: 10.1097/TA.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiu F, Jeschke MG. Perturbed mononuclear phagocyte system in severely burned and septic patients. Shock. 2013;40:81–88. doi: 10.1097/SHK.0b013e318299f774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferrari G, Bignami F, Giacomini C, Franchini S, Rama P. Safety and efficacy of topical infliximab in a mouse model of ocular surface scarring. Invest Ophthalmol Vis Sci. 2013;54:1680–1688. doi: 10.1167/iovs.12-10782. [DOI] [PubMed] [Google Scholar]
- 71.Yamada J, Dana MR, Sotozono C, Kinoshita S. Local suppression of IL-1 by receptor antagonist in the rat model of corneal alkali injury. Exp Eye Res. 2003;76:161–167. doi: 10.1016/S0014-4835(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 72.Sotozono C, He J, Matsumoto Y, Kita M, Imanishi J, Kinoshita S. Cytokine expression in the alkali-burned cornea. Curr Eye Res. 1997;16:670–676. doi: 10.1076/ceyr.16.7.670.5057. [DOI] [PubMed] [Google Scholar]
- 73.Lu P, Li L, Liu G, Zhang X, Mukaida N. Enhanced experimental corneal neovascularization along with aberrant angiogenic factor expression in the absence of IL-1 receptor antagonist. Invest Ophthalmol Vis Sci. 2009;50:4761–4768. doi: 10.1167/iovs.08-2732. [DOI] [PubMed] [Google Scholar]
- 74.Sakimoto T, Yamada A, Kanno H, Sawa M. Upregulation of tumor necrosis factor receptor 1 and TNF-alpha converting enzyme during corneal wound healing. Jpn J Ophthalmol. 2008;52:393–398. doi: 10.1007/s10384-008-0536-8. [DOI] [PubMed] [Google Scholar]
- 75.Pattamatta U, Willcox M, Stapleton F, Garrett Q. Bovine lactoferrin promotes corneal wound healing and suppresses IL-1 expression in alkali wounded mouse cornea. Curr Eye Res. 2013;38:1110–1117. doi: 10.3109/02713683.2013.811259. [DOI] [PubMed] [Google Scholar]
- 76.Shin YJ, Hyon JY, Choi WS, Yi K, Chung ES, Chung TY, Wee WR. Chemical injury-induced corneal opacity and neovascularization reduced by rapamycin via TGF-β1/ERK pathways regulation. Invest Ophthalmol Vis Sci. 2013;54:4452–4458. doi: 10.1167/iovs.13-11684. [DOI] [PubMed] [Google Scholar]
- 77.Ling S, Li W, Liu L, Zhou H, Wang T, Ye H, Liang L, Yuan J. Allograft survival enhancement using doxycycline in alkali-burned mouse corneas. Acta Ophthalmol. 2013;91:e369–e378. doi: 10.1111/aos.12070. [DOI] [PubMed] [Google Scholar]
- 78.Xiao O, Xie ZL, Lin BW, Yin XF, Pi RB, Zhou SY. Minocycline inhibits alkali burn-induced corneal neovascularization in mice. PLoS One. 2012;7:e41858. doi: 10.1371/journal.pone.0041858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeon HS, Yi K, Chung TY, Hyon JY, Wee WR, Shin YJ. Chemically injured keratocytes induce cytokine release by human peripheral mononuclear cells. Cytokine. 2012;59:280–285. doi: 10.1016/j.cyto.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 80.Cairns B, Maile R, Barnes CM, Frelinger JA, Meyer AA. Increased Toll-like receptor 4 expression on T cells may be a mechanism for enhanced T cell response late after burn injury. J Trauma. 2006;61:293–298. doi: 10.1097/01.ta.0000228969.46633.bb. discussion 298–299. [DOI] [PubMed] [Google Scholar]
- 81.Planck SR, Rich LF, Ansel JC, Huang XN, Rosenbaum JT. Trauma and alkali burns induce distinct patterns of cytokine gene expression in the rat cornea. Ocul Immunol Inflamm. 1997;5:95–100. doi: 10.3109/09273949709085057. [DOI] [PubMed] [Google Scholar]
- 82.De Nuccio C, Bernardo A, Cruciani C, De Simone R, Visentin S, Minghetti L. Peroxisome proliferator activated receptor-γ agonists protect oligodendrocyte progenitors against tumor necrosis factor-alpha-induced damage: Effects on mitochondrial functions and differentiation. Exp Neurol. 2015;271:506–514. doi: 10.1016/j.expneurol.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 83.Shimada K, Furukawa H, Wada K, Korai M, Wei Y, Tada Y, Kuwabara A, Shikata F, Kitazato KT, Nagahiro S, et al. Protective Role of Peroxisome Proliferator-Activated Receptor-γ in the Development of Intracranial Aneurysm Rupture. Stroke. 2015;46:1664–1672. doi: 10.1161/STROKEAHA.114.007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mirza RE, Fang MM, Novak ML, Urao N, Sui A, Ennis WJ, Koh TJ. Macrophage PPARγ and impaired wound healing in type 2 diabetes. J Pathol. 2015;236:433–444. doi: 10.1002/path.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lan LF, Zheng L, Yang X, Ji XT, Fan YH, Zeng JS. Peroxisome proliferator-activated receptor-γ agonist pioglitazone ameliorates white matter lesion and cognitive impairment in hypertensive rats. CNS Neurosci Ther. 2015;21:410–416. doi: 10.1111/cns.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang RC, Jiang DM. PPAR-γ agonist pioglitazone affects rat gouty arthritis by regulating cytokines. Genet Mol Res. 2014;13:6577–6581. doi: 10.4238/2014.August.28.2. [DOI] [PubMed] [Google Scholar]
- 87.Cheng Y, Dong Z, Liu S. β-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 mice through CB2 receptor activation and the PPARγ pathway. Pharmacology. 2014;94:1–12. doi: 10.1159/000362689. [DOI] [PubMed] [Google Scholar]
- 88.Bhattarai G, Lee YH, Yi HK. Peroxisome proliferator activated receptor gamma loaded dental implant improves osteogenesis of rat mandible. J Biomed Mater Res B Appl Biomater. 2015;103:587–595. doi: 10.1002/jbm.b.33207. [DOI] [PubMed] [Google Scholar]
- 89.Guri AJ, Mohapatra SK, Horne WT, II, Hontecillas R, Bassaganya-Riera J. The role of T cell PPAR γ in mice with experimental inflammatory bowel disease. BMC Gastroenterol. 2010;10:60. doi: 10.1186/1471-230X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amparo F, Sadrai Z, Jin Y, Alfonso-Bartolozzi B, Wang H, Shikari H, Ciolino JB, Chodosh J, Jurkunas U, Schaumberg DA, et al. Safety and efficacy of the multitargeted receptor kinase inhibitor pazopanib in the treatment of corneal neovascularization. Invest Ophthalmol Vis Sci. 2013;54:537–544. doi: 10.1167/iovs.12-11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang X, Han Y, Shao Y, Yi JL. Efficacy of the nucleotide-binding oligomerzation domain 1 inhibitor Nodinhibit-1 on corneal alkali burns in rats. Int J Ophthalmol. 2015;8:860–865. doi: 10.3980/j.issn.2222-3959.2015.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee CM, Jung WK, Na G, Lee DS, Park SG, Seo SK, Yang JW, Yea SS, Lee YM, Park WS, et al. Inhibitory effects of the platelet-activating factor receptor antagonists, CV-3988 and Ginkgolide B, on alkali burn-induced corneal neovascularization. Cutan Ocul Toxicol. 2015;34:53–60. doi: 10.3109/15569527.2014.903573. [DOI] [PubMed] [Google Scholar]
- 93.Giacomini C, Ferrari G, Bignami F, Rama P. Alkali burn versus suture-induced corneal neovascularization in C57BL/6 mice: An overview of two common animal models of corneal neovascularization. Exp Eye Res. 2014;121:1–4. doi: 10.1016/j.exer.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 94.Bignami F, Giacomini C, Lorusso A, Aramini A, Rama P, Ferrari G. NK1 receptor antagonists as a new treatment for corneal neovascularization. Invest Ophthalmol Vis Sci. 2014;55:6783–6794. doi: 10.1167/iovs.14-14553. [DOI] [PubMed] [Google Scholar]
- 95.Koenig Y, Bock F, Kruse FE, Stock K, Cursiefen C. Angioregressive pretreatment of mature corneal blood vessels before keratoplasty: Fine-needle vessel coagulation combined with anti-VEGFs. Cornea. 2012;31:887–892. doi: 10.1097/ICO.0b013e31823f8f7a. [DOI] [PubMed] [Google Scholar]
- 96.Zhou AY, Bai YJ, Zhao M, Yu WZ, Li XX. KH902, a recombinant human VEGF receptor fusion protein, reduced the level of placental growth factor in alkali burn induced-corneal neovascularization. Ophthalmic Res. 2013;50:180–186. doi: 10.1159/000353437. [DOI] [PubMed] [Google Scholar]
- 97.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999;274:9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 98.Vucic E, Dickson SD, Calcagno C, Rudd JH, Moshier E, Hayashi K, Mounessa JS, Roytman M, Moon MJ, Lin J, et al. Pioglitazone modulates vascular inflammation in atherosclerotic rabbits noninvasive assessment with FDG-PET-CT and dynamic contrast-enhanced MR imaging. JACC Cardiovasc Imaging. 2011;4:1100–1109. doi: 10.1016/j.jcmg.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Usui T, Sugisaki K, Iriyama A, Yokoo S, Yamagami S, Nagai N, Ishida S, Amano S. Inhibition of corneal neovascularization by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci. 2008;49:4370–4376. doi: 10.1167/iovs.07-0964. [DOI] [PubMed] [Google Scholar]
- 100.Panigrahy D, Kaipainen A, Huang S, Butterfield CE, Barnés CM, Fannon M, Laforme AM, Chaponis DM, Folkman J, Kieran MW. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc Natl Acad Sci USA. 2008;105:985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hao F, Mu JW, Zhang HJ, Kuang HY, Yu QX, Bai MM, Meng P. Damage to vascular endothelial cells by high insulin levels is associated with increased expression of ChemR23, and attenuated by PPAR-gamma agonist, rosiglitazone. Neuro Endocrinol Lett. 2015;36:59–66. [PubMed] [Google Scholar]
- 102.Sarayba MA, Li L, Tungsiripat T, Liu NH, Sweet PM, Patel AJ, Osann KE, Chittiboyina A, Benson SC, Pershadsingh HA, Chuck RS. Inhibition of corneal neovascularization by a peroxisome proliferator-activated receptor-gamma ligand. Exp Eye Res. 2005;80:435–442. doi: 10.1016/j.exer.2004.10.009. [DOI] [PubMed] [Google Scholar]; Exp Eye Res. 2005;80:435–442. doi: 10.1016/j.exer.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 103.Zhang H, Wei T, Jiang X, Li Z, Cui H, Pan J, Zhuang W, Sun T, Liu Z, Zhang Z, Dong H. PEDF and 34-mer inhibit angiogenesis in the heart by inducing tip cells apoptosis via up-regulating PPAR-γ to increase surface FasL. Apoptosis. 2016;21:60–68. doi: 10.1007/s10495-015-1186-1. [DOI] [PubMed] [Google Scholar]
- 104.Gronkiewicz KM, Giuliano EA, Kuroki K, Bunyak F, Sharma A, Teixeira LB, Hamm CW, Mohan RR. Development of a novel in vivo corneal fibrosis model in the dog. Exp Eye Res. 2016;143:75–88. doi: 10.1016/j.exer.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Donnelly KS, Giuliano EA, Sharm A, Mohan RR. Suberoylanilide hydroxamic acid (vorinostat): Its role on equine corneal fibrosis and matrix metalloproteinase activity. Vet Ophthalmol. 2014;17(Suppl 1):61–68. doi: 10.1111/vop.12129. [DOI] [PubMed] [Google Scholar]
- 106.Zhou Q, Yang L, Qu M, Wang Y, Chen P, Wang Y, Shi W. Role of senescent fibroblasts on alkali-induced corneal neovascularization. J Cell Physiol. 2012;227:1148–1156. doi: 10.1002/jcp.22835. [DOI] [PubMed] [Google Scholar]
- 107.Jeon KI, Phipps RP, Sime PJ, Huxlin KR. Inhibitory effects of PPARγ ligands on TGF-β1-induced CTGF expression in cat corneal fibroblasts. Exp Eye Res. 2015;138:52–58. doi: 10.1016/j.exer.2015.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoon YS, Kim SY, Kim MJ, Lim JH, Cho MS, Kang JL. PPARγ activation following apoptotic cell instillation promotes resolution of lung inflammation and fibrosis via regulation of efferocytosis and proresolving cytokines. Mucosal Immunol. 2015;8:1031–1046. doi: 10.1038/mi.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luo H, Zhu H, Zhou B, Xiao X, Zuo X. MicroRNA-130b regulates scleroderma fibrosis by targeting peroxisome proliferator-activated receptor γ. Mod Rheumatol. 2015;25:595–602. doi: 10.3109/14397595.2014.1001311. [DOI] [PubMed] [Google Scholar]
- 110.Zoccal KF, Paula-Silva FW, Bitencourt CS, Sorgi CA, Bordon KC, Arantes EC, Faccioli LH. PPAR-γ activation by Tityus serrulatus venom regulates lipid body formation and lipid mediator production. Toxicon. 2015;93:90–97. doi: 10.1016/j.toxicon.2014.11.226. [DOI] [PubMed] [Google Scholar]
- 111.Wang C, Zeng L, Zhang T, Liu J, Wang W. Tenuigenin prevents IL-1β-induced inflammation in human osteoarthritis chondrocytes by suppressing pi3k/akt/nf-κb signaling pathway. Inflammation. 2016;39:807–812. doi: 10.1007/s10753-016-0309-3. [DOI] [PubMed] [Google Scholar]
- 112.Poon MW, Yan L, Jiang D, Qin P, Tse HF, Wong IY, Wong DS, Tergaonkar V, Lian Q. Inhibition of RAP1 enhances corneal recovery following alkali injury. Invest Ophthalmol Vis Sci. 2015;56:711–721. doi: 10.1167/iovs.14-15268. [DOI] [PubMed] [Google Scholar]
- 113.Saika S, Miyamoto T, Yamanaka O, Kato T, Ohnishi Y, Flanders KC, Ikeda K, Nakajima Y, Kao WW, Sato M, et al. Therapeutic effect of topical administration of SN50, an inhibitor of nuclear factor-κB, in treatment of corneal alkali burns in mice. Am J Pathol. 2005;166:1393–1403. doi: 10.1016/S0002-9440(10)62357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ma Z, Piao T, Wang Y, Liu J. Astragalin inhibits IL-1β-induced inflammatory mediators production in human osteoarthritis chondrocyte by inhibiting nf-κb and MAPK activation. Int Immunopharmacol. 2015;25:83–87. doi: 10.1016/j.intimp.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 115.Shen M, Yuan F, Jin J, Yuan Y. The effect of TC14012 on alkali burn-induced corneal neovascularization in mice. Ophthalmic Res. 2014;52:17–24. doi: 10.1159/000358201. [DOI] [PubMed] [Google Scholar]
- 116.Gardner JC, Noel JG, Nikolaidis NM, Karns R, Aronow BJ, Ogle CK, McCormack FX. G-CSF drives a posttraumatic immune program that protects the host from infection. J Immunol. 2014;192:2405–2417. doi: 10.4049/jimmunol.1302752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Choo J, Lee Y, Yan XJ, Noh TH, Kim SJ, Son S, Pothoulakis C, Moon HR, Jung JH, Im E. A Novel Peroxisome Proliferator-activated Receptor (PPAR)γ Agonist 2-Hydroxyethyl 5-chloro-4,5-didehydrojasmonate Exerts Anti-Inflammatory Effects in Colitis. J Biol Chem. 2015;290:25609–25619. doi: 10.1074/jbc.M115.673046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pires AS, Souza VC, Paula RS, Toledo JO, Lins TC, Moraes CF, Córdova C, Pereira RW, Nóbrega OT. Pro-inflammatory cytokines correlate with classical risk factors for atherosclerosis in the admixed Brazilian older women. Arch Gerontol Geriatr. 2015;60:142–146. doi: 10.1016/j.archger.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 119.Zhang F, Sun D, Chen J, Guan N, Huo X, Xi H. Simvastatin attenuates angiotensin II-induced inflammation and oxidative stress in human mesangial cells. Mol Med Rep. 2015;11:1246–1251. doi: 10.3892/mmr.2014.2871. [DOI] [PubMed] [Google Scholar]
- 120.Xu S, Song H, Huang M, Wang K, Xu C, Xie L. Telmisartan inhibits the proinflammatory effects of homocysteine on human endothelial cells through activation of the peroxisome proliferator-activated receptor-δ pathway. Int J Mol Med. 2014;34:828–834. doi: 10.3892/ijmm.2014.1834. [DOI] [PubMed] [Google Scholar]
- 121.Qin L, Gong C, Chen AM, Guo FJ, Xu F, Ren Y, Liao H. Peroxisome proliferator-activated receptor γ agonist rosiglitazone inhibits migration and invasion of prostate cancer cells through inhibition of the CXCR4/CXCL12 axis. Mol Med Rep. 2014;10:695–700. doi: 10.3892/mmr.2014.2232. [DOI] [PubMed] [Google Scholar]
- 122.Dong W, Wang X, Bi S, Pan Z, Liu S, Yu H, Lu H, Lin X, Wang X, Ma T, Zhang W. Inhibitory effects of resveratrol on foam cell formation are mediated through monocyte chemotactic protein-1 and lipid metabolism-related proteins. Int J Mol Med. 2014;33:1161–1168. doi: 10.3892/ijmm.2014.1680. [DOI] [PubMed] [Google Scholar]
- 123.Higashihara H, Kokura S, Imamoto E, Ueda M, Naito Y, Yoshida N, Yoshikawa T. Hypoxia-reoxygenation enhances interleukin-8 production from U937 human monocytic cells. Redox Rep. 2004;9:365–369. doi: 10.1179/135100004225006894. [DOI] [PubMed] [Google Scholar]
- 124.Akahori T, Sho M, Hamada K, Suzaki Y, Kuzumoto Y, Nomi T, Nakamura S, Enomoto K, Kanehiro H, Nakajima Y. Importance of peroxisome proliferator-activated receptor-gamma in hepatic ischemia/reperfusion injury in mice. J Hepatol. 2007;47:784–792. doi: 10.1016/j.jhep.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 125.Sakimoto T, Ishimori A. Anti-inflammatory effect of topical administration of tofacitinib on corneal inflammation. Exp Eye Res. 2016;145:110–117. doi: 10.1016/j.exer.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 126.Ma J, Zhou D, Fan M, Wang H, Huang C, Zhang Z, Wu Y, Li W, Chen Y, Liu Z. Keratocytes create stromal spaces to promote corneal neovascularization via MMP13 expression. Invest Ophthalmol Vis Sci. 2014;55:6691–6703. doi: 10.1167/iovs.14-14746. [DOI] [PubMed] [Google Scholar]
- 127.Zhang H, Li C, Baciu PC. Expression of integrins and MMPs during alkaline-burn-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2002;43:955–962. [PubMed] [Google Scholar]
- 128.Yang JW, Lee SM, Oh KH, Park SG, Choi IW, Seo SK. Effects of topical chondrocyte-derived extracellular matrix treatment on corneal wound healing, following an alkali burn injury. Mol Med Rep. 2015;11:461–467. doi: 10.3892/mmr.2014.2722. [DOI] [PubMed] [Google Scholar]
- 129.Iwanami H, Ishizaki M, Fukuda Y, Takahashi H. Expression of matrix metalloproteinases (MMP)-12 by myofibroblasts during alkali-burned corneal wound healing. Curr Eye Res. 2009;34:207–214. doi: 10.1080/02713680802687809. [DOI] [PubMed] [Google Scholar]
- 130.Bian F, Pelegrino FS, Tukler Henriksson JT, Pflugfelder SC, Volpe EA, Li DQ, de Paiva CS. Differential Effects of Dexamethasone and Doxycycline on Inflammation and MMP Production in Murine Alkali-Burned Corneas Associated with Dry Eye. Ocul Surf. 2016;14:242–254. doi: 10.1016/j.jtos.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang SJ, Jo H, Kim KA, Ahn HR, Kang SW, Jung SH. Diospyros kaki Extract Inhibits Alkali Burn-Induced Corneal Neovascularization. J Med Food. 2016;19:106–109. doi: 10.1089/jmf.2014.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ke Y, Wu Y, Cui X, Liu X, Yu M, Yang C, Li X. Polysaccharide hydrogel combined with mesenchymal stem cells promotes the healing of corneal alkali burn in rats. PLoS One. 2015;10:e0119725. doi: 10.1371/journal.pone.0119725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu J, Lu H, Huang R, Lin D, Wu X, Lin Q, Wu X, Zheng J, Pan X, Peng J, et al. Peroxisome proliferator activated receptor-gamma ligands induced cell growth inhibition and its influence on matrix metalloproteinase activity in human myeloid leukemia cells. Cancer Chemother Pharmacol. 2005;56:400–408. doi: 10.1007/s00280-005-1029-9. [DOI] [PubMed] [Google Scholar]
- 134.Motoki T, Kurobe H, Hirata Y, Nakayama T, Kinoshita H, Rocco KA, Sogabe H, Hori T, Sata M, Kitagawa T. PPAR-γ agonist attenuates inflammation in aortic aneurysm patients. Gen Thorac Cardiovasc Surg. 2015;63:565–571. doi: 10.1007/s11748-015-0576-1. [DOI] [PubMed] [Google Scholar]
- 135.Kato T, Saika S, Ohnishi Y. Effects of the matrix metalloproteinase inhibitor GM6001 on the destruction and alteration of epithelial basement membrane during the healing of post-alkali burn in rabbit cornea. Jpn J Ophthalmol. 2006;50:90–95. doi: 10.1007/s10384-005-0287-8. [DOI] [PubMed] [Google Scholar]
- 136.Fini ME, Cui TY, Mouldovan A, Grobelny D, Galardy RE, Fisher SJ. An inhibitor of the matrix metalloproteinase synthesized by rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1991;32:2997–3001. [PubMed] [Google Scholar]