Abstract
Introduction: Amniotic fluid, once thought to merely provide protection and room for necessary movement and growth for the fetus, is now understood to be a highly complex and dynamic system that is studied as a data point to interpret fetal wellbeing.
Methods: Assessment of amniotic fluid volume is now routine when performing a sonographic evaluation of fetal status and is an important consideration in the assessment and management of perinatal morbidity and mortality. 1 , 2 In this review, we will cover the dynamics that affect amniotic fluid volume, review methods for measurement and quantification of volume, review definitions for normative data as related to neonatal outcomes, and provide evidence based guidance on the workup and management options for oligoydramnios and polyhydramnios in singleton and twin pregnancies. Conclusions: When abnormalities of fluid exist, appropriate workup to uncover the underlying etiology should be initiated as adverse fetal outcomes are sometimes associated with these variations from normalcy.
Keywords: amniotic fluid volume, oligohydramnios, polyhydramnios
Dynamics
In order to understand how variations in amniotic fluid volume (AFV) can affect the fetus, we must first describe amniotic fluid dynamics. In early pregnancy, the factors that affect AFV are not well known. It is known that the osmolality of amniotic fluid (AF) and maternal plasma are the same, suggesting that the fluid is a transudate of maternal plasma either across the placental surface or through the fetal skin. 1 The fetal skin is non‐keratinised until week 22–25, allowing it to act as a membrane through which amniotic fluid can readily pass. 2 Beyond 24 weeks, the surfaces in the mouth and nose can act to exchange fluid, but this is not considered a major source of AFV regulation. 3
The major pathways affecting AF in the latter half of pregnancy are pulmonary excretion, fetal urine production, fetal swallowing, intramembranous movement between fetal blood and the placenta, and transmembranous movement across the amnion and chorion. A fundamental source of AF is the fetal renal system. This is evidenced by the almost complete lack of AF in fetuses with renal agenesis. Evidence of a functioning renal system first starts around 8–11 weeks when urine is initially observed in the fetal bladder. The dilute urine that is produced is thought to cause the observed drop in osmolality and sodium concentration in the AF that persists until delivery. 1 As pregnancy advances, urinary byproducts are observed at two to three times the concentration found in fetal plasma. 4 Estimates of fetal urine output have been made by 3D measurements of bladder volume and timed intervals and have been found to range between 7 and 70 mL/hour from 24 weeks until delivery in two separate studies. 5 , 6
Akin to renal agenesis being associated with low amniotic fluid, disruption in fetal swallowing is associated with excess AFV. The human fetus demonstrates swallowing around the same time that urine production begins. 7 The ovine model has historically been an accurate approximation of human fetal development. 2 Studies evaluating the volume of AF swallowed by the fetus were done primarily in sheep models, and found to range between 8.75 and 43.2 mL/hr. 8 , 9 Ovine studies have also shown that the amount of AF swallowed daily in late gestation is correlated to the AFV, suggesting the fetus can react and attempt to regulate its amniotic fluid surroundings. It is not, however, thought to be a major regulator of AFV, 10 although the fetus is able to modulate swallowing. Primate studies performed by Minei and Suzuki 11 showed initial development of polyhydramnios with esophageal ligation, but the hydramnios had resolved prior to delivery.
Fluid is also excreted from the fetal pulmonary system. It has been demonstrated in fetal sheep by Brace, et al. 12 that half of secreted lung fluid enters the amniotic fluid, and the other half is swallowed upon exit from the trachea. As further evidence of the entrance of secreted lung fluid into amniotic fluid, the phospholipids found in amniotic fluid are produced by pulmonary cells and excreted in fetal urine. The trachea acts as a one‐way valve in most situations preventing amniotic fluid from entering the lungs. Intra‐amniotic injection of contrast media by Liley, et al. 13 resulted in few cases of detectable contrast found in the fetal or neonatal lung. Additionally, meconium staining is not infrequent with a rate in the literature of 7–27% based on the population, whereas meconium aspiration past the trachea is rare, anywhere from less than a percent up to 5% and usually associated with neonatal hypoxia. 14 , 15 Aspiration of uncontaminated amniotic fluid has been reported as a cause of neonatal death confirmed at autopsy. 16 , 17
Intramembranous exchange of fluid describes the reabsorption of fluid and solutes from the amniotic compartment to the fetal blood via the amnion. 18 This is thought to be regulated at least partially by aquaporins found in the human chorioamniotic membranes and the placenta, which have been found to be adaptive to abnormal AF levels and may represent a possible therapeutic target for abnormal AFV regulation. 19 – 21 As the fluid requirements of the fetus increase with increasing gestation, water flow from the amniotic cavity to the fetal circulation via fetal membranes increases up to 400 mL/day. 22 The osmotic gradient between the amniotic fluid and fetal blood also drives fluids and solutes from the amniotic fluid into the fetal blood. 4 The flow of solutes and fluid, although bidirectional, are not necessarily equal. 1 , 23 Transmembranous movement of amniotic fluid describes the transport of water and solutes from the maternal circulation to the amniotic compartment via the placenta. 18 This has been found to be an insignificant contribution to amniotic fluid dynamics.24
Methods of quantifying amniotic fluid
Measurement of amniotic fluid volume can be made directly, indirectly, or estimated sonographically. Direct measurement is done at the time of cesarean or uterine hysterotomy. 25 Indirect measurement is done via amniocentesis by dye‐dilution techniques. Dye‐dilution using para‐amino hippurate has been shown to be representative of actual AFV obtained by direct measurement at the time of cesarean delivery. 26 Because these techniques to measure amniotic fluid volume are time consuming, invasive, and may require laboratory support, amniotic fluid volumes are usually estimated by ultrasound. Magnetic resonance imaging (MRI) has also been evaluated as a means for estimating AFV 27 ; however, this is an impractical approach to everyday screening.
There are four methods of sonographic evaluation of amniotic fluid volume: subjective assessment, 2 × 2 measurement, single deepest pocket (SDP), which is also known as maximum vertical pocket (MVP), and amniotic fluid index (AFI).
Amniotic fluid index, first proposed by Phelan and Rutherford, 28 is the summation of the vertical diameter of the largest pocket in each of the four quadrants with the maternal umbilicus as a central reference point. The transducer should be oriented in the longitudinal plane and there should be a minimum horizontal measurement of one centimeter for each pocket. The single deepest vertical pocket was proposed by Chamberlain 29 and is simply found by identifying the largest pocket of amniotic fluid after a global assessment and selecting the largest vertical measurement with a minimum horizontal measurement of one centimeter. 30 A two‐diameter pocket (cm 2 ) is calculated by multiplying the depth and width of the largest single pocket. 31
The advantages of sonographic estimates are that they are simple to perform, easy to teach to residents, midwives, and nurses, and are reproducible. The disadvantage is that sonograms are two dimensional representations of a complex, three‐dimensional structure with limited impact on clinical outcome. The reproducibility of these measurements was shown by Magann, et al. 32 comparing all four measurements in singleton pregnancies with dye‐dilution technique across different operator experiences. The accuracy of subjective estimates ranged from 65–70% and the accuracy of the three sonographic estimates were similar, ranging from 59–67%. 32 , 33 These were similar across operator experience. Alarmingly, none of the techniques consistently identified abnormal AFVs (oligohydramnios and polyhydramnios). The accuracy in twin pregnancies in a similar study was even more dismal, ranging from 7–29%. 34
Throughout multiple studies and trials, no single sonographic method has emerged superior to the others. A randomised trial comparing the use of SDP versus AFI during modified biophysical profiles in complicated pregnancies showed no difference in delivery mode, NICU admission, umbilical artery pH, or Apgar score. The only significant finding was that AFI identified more pregnancies as oligohydramnios than SDP. 35 This was confirmed in two meta‐analyses 36 , 37 comparing the measurement of AFI to SDP. AFI over‐diagnosed clinically insignificant oligohydramnios leading to superfluous intervention without a difference in outcome, arguing that the use of SDP for sonographic volume assessment 37 may be better.
Modification of ultrasound quantification
The use of color Doppler has been added as a means to identify the umbilical cord in amniotic fluid in an effort to better diagnose oligohyramnios. It has, however, not been shown to aid in identification of pregnancies with adverse outcomes. Using dye‐determined AFV as the benchmark, Magann, et al. 38 compared gray scale ultrasonography to Doppler color ultrasonography and found that Doppler not only over‐diagnosed oligohydramnios, but labeled 37% of women with normal AFV as having oligohydramnios. The use of color Doppler is not recommended when evaluating amniotic fluid volume.
Sahin, et al. 39 attempted to apply the Cavalieri method, a mathematical estimate of volumes, to estimate AFV by ultrasound images. Their results correlated with concurrent AFI measurements, and although this is a promising theory, it is complicated to perform and has not been correlated with pregnancy outcome or directly measured AFV.
3D ultrasound technology has found relevance in the evaluation of the fetus; however, it has not yet found relevance in the assessment of amniotic fluid. There has only been one attempt to assess third trimester AFV with 3D ultrasonography. 40 The authors of that study concluded that 3D volume data sets are reliable for determining AFV, however, AFV was determined subjectively by the sonographer based on five volume acquisitions and compared to the value obtained during the 2D ultrasound; there was no attempt at calculating a volume based on a gold standard. Interestingly, in the first trimester, growth charts have been developed for gestational sac volume and embryonic volume, the ratio of which correlates positively with gestational age. 41 Clinical applications for this data have not yet been developed, but normal versus abnormal early pregnancies are being investigated to determine if these parameters can help predict early adverse outcomes such as miscarriage.
Figure 1.
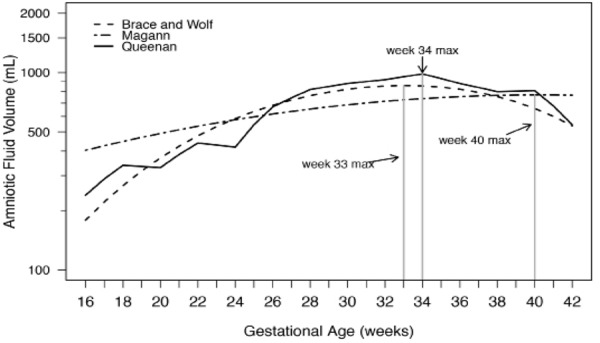
From Magann, et al. 2 . Comparison of normal amniotic fluid volumes (dye determined or directly measured) across gestation: Brace and Wolf 41 Magann, et al 42 and Queenan et al 26 .
Normal volume
Normality can be defined in two ways. Mathematically, it can represent the majority of the population as expressed by area under the curve in a Gaussian distributed population. Typically, the lower and upper 5% are excluded as ‘abnormal’. Normality can also be defined in terms of outcome. Undesired outcomes are tracked and measurements associated with these outcomes are defined as ‘abnormal’. This second definition is more clinically useful, but much more difficult to define.
Amniotic fluid volume has been defined in both of these manners as well. The 5th and 95th percentiles have been defined for AFI, SDP, the 2‐diameter pocket, and dye‐directed techniques across gestational ages, as fluid levels have been found to vary significantly throughout pregnancy. Nomograms for amniotic fluid in normal pregnancies have been developed by Queenan, et al. by dye‐determined methods 42 ; Moore and Cayle by AFI 43 ; Brace and Wolfe by dye‐dilution and direct measurement 44 ; and Magann, et al. by dye‐dilution, direct measurement, and sonographic estimate. 45 , 46 It is worth noting that although for diagnostic purposes a single set of cutoffs for abnormal amniotic fluid volumes are used throughout a pregnancy, the actual values vary by each week. In the figure below, two studies showed amniotic fluid volume increasing steadily in early gestation and remain relatively stable between 22 and 38 weeks, thereafter declining until delivery. The third nomogram found that amniotic fluid volume continues to increase throughout gestation with a mean volume of approximately 800 mL at term. Table 1 shows typical references for normal ranges of amniotic fluid volume based on sonographic estimates.
Table 1.
Normal values of amniotic fluid estimates.
| Ultrasound estimate | Olighydramnios | Normal Values | Polyhydramnios |
|---|---|---|---|
| AFI | < 5 cm | 5–25 cm | > 25 cm |
| SDP | < 2 cm | 2–8 cm | > 8 cm |
| 2 diameter pocket | < 4 cm 2 | 4–50 cm 2 | > 50 cm 2 |
Modified from Magann EF. Olighydramnios: Amniotic Fluid and the Clinical Relevance of the Sonographically Estimated Amniotic Fluid Volume, J Ultrasound Med 2011; 30 (11): 1573–85, and The amniotic fluid index, single deepest pocket, and 2‐diameter pocket in normal human pregnancy, Am J Obstet Gynecol, 2000; 182 (6): 1581–8.
The outer boundaries of AFI in normal term and postdate pregnancies (5th and 95th percentiles) was found to be 6.8–19.6 cm and 6.7–17.4 cm respectively by Moore and Cayle 44 in a study of 791 normal pregnancies. Magann, et al. 46 , 47 established 5th–95th percentiles respectively as 4.2–14.9 cm for AFI at gestational age 37–41 weeks. The 5th–95th percentile was 2.3–6 cm for SDP, and 4.6–38.3cm 2 for 2‐dimensional pocket in the same study.
A study of over 15,000 patients done by Shanks, et al. 47 showed that AFI less than the 5th percentile versus a cutoff of 5 cm better predicts fetuses at risk for newborn intensive care (NICU) admission, although this study has been criticised for its retrospective design and the variable length of time between the time the measurement was obtained and the time of delivery. Additionally, an investigation of 291 pregnancies with dye‐determined AFVs in which the 3rd and 5th percentiles of the AFI were assessed to determine if either of these percentiles was superior to the fixed cut off of < 5 for the AFI to detect the 75 pregnancies with oligohydramnios discovered that both the percentiles (3rd and 5th) and the fixed cut off (< 5) poorly identified oligohydramnios, but neither was superior to the other. 48
Oligohydramnios
Oligohydramnios has been defined as an AFV that is less than 200 mL 25 or 500 mL. 49 By ultrasound techniques, it has been estimated as an SDP less than 2 cm, 50 AFI less than 5 cm 28 , 51 or an AFI that is below the 5th percentile for gestational age, 35 , 43 or a subjectively low AFV. 32 Borderline fluid has been defined as an AFI between 5–8 cm or 5–10 cm and has been associated with fetal malformations if diagnosed at age 24–34 weeks. 52 , 53
Low amniotic fluid can be due to underproduction, loss, or can be idiopathic. Underproduction can be the result of absent or dysfunctional kidneys, urinary tract obstruction, abnormal placental function, or maternal dehydration. Loss is due to rupture of membranes. Appropriate workup for this abnormal vital sign is the review of maternal history, assessment for evidence of membrane rupture, anatomic evaluation of the renal system and bladder, and assessment of placental function and fetal growth.
Regardless of the etiology, fetuses in pregnancies complicated by oligohydramnios are at increased risk of adverse outcomes in the form of cord accidents. Oligohydramnios/anhydramnios can also result in fetal lung hypoplasia, malformations, and contracture if it is severe enough and persistent. 54 Oligohydramnios was retrospectively evaluated in 7582 fetuses in high risk pregnancies with normal anatomy. Perinatal mortality was 11% in these pregnancies versus 0.2% in pregnancies with normal fluid based on SDP. Borderline fluid of SDP 1–2 cm was associated with an increased mortality at 3.7%. 50 Morbidity such as cesarean delivery, non‐reassuring fetal heart rate patterns, NICU admission, and meconium aspiration are also increased in pregnancies complicated by oligohydramnios. 55
There is conflicting data that argues that the most significant risk to the normal fetus with incidental oligohydramnios is iatrogenic prematurity resulting from the subsequent rush to delivery. 56 Low AF as determined by dye‐dilution along with SGA has been associated with NICU admission (OR 11.1) but no other measures of infant morbidity 57 in another study. A Cochrane review reminds us that SDP identified fewer pregnancies with oligohydramnios without a difference in clinical outcome, suggesting this is the ideal test for diagnosis of low amniotic fluid if normative data is to be based on outcome. 37
It is worth remembering that oligohydramnios may appear idiopathic at diagnosis, but may be a sign of an anomaly not detected until after birth. Chromosomal anomalies have been found in 13% of pregnancies with olighydramnios, 58 and late diagnosis of oligohydramnios in pregnancies with normal anatomy has also been found to be associated with undiagnosed renal anomalies up to 9.8% of the time. 59
Rupture of membranes (ROM) at any gestational age can be associated with oligohydramnios and should be excluded by patient history or clinical exam. In a single study looking at the diagnosis of ROM, ultrasound alone was shown to have a sensitivity of 19%, specificity of 100% and positive and negative predictive values of 100% and 61% respectively. 60 Most facilities combine ultrasound evaluation with other modalities for diagnosis, such as the evaluation of vaginal fluid with nitrazine, for evidence of ferning, or with monoclonal antibodies to detect placental α‐microglobulin‐1 (PAMG‐1) (Amni Sure International LLC, Cambridge, MA).
Amniotic fluid volume in pregnancies with preterm rupture, although inherently low, can still be a vital sign indicative of eventual fetal outcome. A group of 31 women with rupture before 24 weeks gestation were followed for outcomes based on SDP greater than or less than 1 cm (severe oligohyramnios). The group with SDP > 1 cm was associated with a longer latency in live birth in 60% of pregnancies compared to 8.3% in the group with SDP < 1 cm. The remainder of outcomes, such as sepsis, chorioamnionitis, and abruption, were not statistically different between groups. 61 A separate group found that AFI was a poor predictor of survival in pregnancies with PPROM between 16–24 weeks. 62 In 438 pregnancies with PPROM between 30 and 36 weeks, oligohydramnios as defined by AFI < 5 cm was associated with only decreased latency. 63
Idiopathic oligohydramnios has been found to be responsive to maternal hydration with no difference in IV versus oral hydration acutely or long term. 64 – 67 The effect, however, is short lived without continued efforts at hydration. 68 Oral hydration has been shown to change AFI in pregnancies with isolated gastroschisis. 69 It has also shown to increase AFI in women diagnosed with hypertensive disorders, although to a lesser extent, 70 possibly reflecting the aberrant placental interface in hypertensive pregnancies. Women also placed in the left‐lateral position at rest have been found to have an increase in AFI. 71 It is notable that none of the above studies evaluated clinically important outcomes. Maternal hydration, therefore, has a limited clinical application and may help in cases where adequate amniotic fluid assists in outcome, such as external cephalic versions or amniocentesis.
Management of oligohydramnios is dependent on the gestational age when discovered. If discovered after 37 weeks gestational age, and rupture of membranes ruled out, induction of labor would not be unreasonable. A retrospective cohort study evaluating the consequences of induction in 206 pregnancies with olioghydramnios as compared to 206 spontaneous labors with normal AFV showed a significant increase in operative deliveries (forceps, vacuum and cesarean) citing non‐reassuring fetal status as the driving factor. There was no difference found in neonatal acidosis, Apgar score, NICU admission, or perinatal morbidity and mortality. 72 A prospective study looked at outcomes in both oligohydramnios and polyhydramnios as compared to pregnancies with normal AFV. They reported a 56% induction rate with oligohydramnios and 57% cesarean rate, as well as higher perinatal mortality when all abnormal fluid pregnancies were compared to normal. 73 Unfortunately, there is no direct comparison in either study of outcomes of inductions with low and normal amniotic fluid.
There is a single randomised trial evaluating induction versus expectant management in 54 patients with oligohydramnios beyond 40 weeks. No difference was found in mode of delivery or neonatal Apgar score or cord blood pH. 74 However, in over 500 United States Maternal‐Fetal Medicine members surveyed, 92% would recommend induction without documented lung maturity before 39 weeks, and 35% before 37 weeks, even though only one‐third of respondents felt induction would decrease adverse outcomes. 75 , 76 If expectant management is chosen, there is no consensus on what fetal monitoring is best, or even if fetal monitoring is necessary; however, the use of Doppler monitoring in pregnancies thought to be at risk of placental insufficiency has shown benefit. 77 Consistent maternal oral hydration can be encouraged.
Prior to term, the discovery of oligohydramnios should initiate a sonographic evaluation if not already undertaken, focusing on the renal system and bladder as well as fetal growth and placental function. Chromosomal analysis can be offered to patients, and premature rupture should be excluded.
The Royal Australian and New Zealand College of Obstetricians and Gynaecologists recommend a pregnancy with oligohydramnios be referred to a specialist Obstetrician. 78 The American College of Obstetrics and Gynecology indicates that oligohydramnios may be an indication for induction of labour. 79 The Society of Obstetricians and Gynaecologists of Canada states that antepartum testing may be beneficial. 80 , 81
Polyhyramnios
Polyhydramnios has been defined as an AFV of greater than 2000 mL. 82 By ultrasound techniques, it has been estimated as an SDP greater than 8 cm, 50 AFI greater than 24 cm 51 or 25 cm 83 or above the 95th percentile for gestational age, 35 , 43 or a subjectively high AFV. 32
Elevated amniotic fluid can be due to decreased absorption, overproduction, or be idiopathic. Decreased absorption typically results from a failure of fetal swallowing from etiologies such as tracheal atresia, tracheal or bowel obstruction, or neurologic abnormalities such as anencephaly. Chromosomal abnormalities, 84 non‐immune hydrops, and diabetes are also recognised reasons for polyhydramnios. Twin‐twin transfusion syndrome (TTTS), another origin of hydramnios, will be covered elsewhere. Appropriate workup for this abnormal vital sign is review of the maternal history, possible repeat of 75 g glucose test, anatomic evaluation of the fetus, and assessment of fetal growth.
Older studies of pregnancies with polyhydramnios show an association with macrosomia, premature birth, cesarean delivery, non‐reactive non‐stress tests, perinatal morbidity, and congenital anomalies. 85 , 86 More recent studies are conflicting. Increased rates of congenital malformations are consistently reported; however, perinatal morbidity and mortality vary, 87 , 88 possibly due to the retrospective nature of some studies and possibly due to improvements in management of underlying conditions for polyhydramnios.
In a recent study of 788 infants with polyhydramnios, the rate of congenital malformations was 2.3% compared to 0.13% in those with normal AF. Those with no major congenital malformation diagnosed were at risk for the development of respiratory distress and hypoglycemia. 89 One hundred eighteen pregnancies with polyhydramnios as defined by SDP > 8 cm with a normal fetal anatomical scan, 75 g glucose test, and TORCH serology, were studied retrospectively by Abele, et al. 90 Eleven of those pregnancies had postnatal abnormalities identified with the majority being gastrointestinal atresia. Another prospective study looked at 7111 pregnancies over a two‐year period starting in 2007. Only 50 patients were found to have polyhydramnios, but out of those 50, perinatal mortality was reported at 42%, with 31% of those being due to congenital anomalies. 73
Polyhydramnios has been linked to maternal pre‐gestational and gestational diabetes and fetal macrosomia. A study of several ultrasound criteria (fetal adipose tissue, asymmetrical macrosomy, cardiac circumference and width, interventricular septum thickness, immature appearance of the placenta, and polyhydramnios) was performed by Perovic, et al. 91 and found to have a sensitivity and specificity of 90.9% and 89.6% respectively. Isolated polyhydramnios has not been found to be a strong independent predictor of gestational diabetes. Known diabetics with polyhydramnios have been found to have an increased hemoglobin A1C indicative of poor diabetic control, however, there was no significant increase in adverse perinatal outcomes in those pregnancies apart from iatrogenic preterm deliveries and elective Cesarean sections not related to fetal macrosomia. 92
Management of pregnancies with polyhydramnios, if identified early, should include sonographic evaluation for anatomical causes, evaluation for maternal diabetes, TORCH serology testing, and consideration of isoimmunisation as a cause. If polyhydramnios is present with a growth restricted fetus, evaluation for chromosomal abnormalities should be undertaken. 93 If polyhydramnios is a late presentation, all the above should be considered, excluding an anatomical evaluation as this should have already been completed. Antenatal testing should be initiated at 24 weeks gestation, although there is no consensus on what type of testing should be begun, or at what interval. 77 This can be left to the discretion of the physician. There are no recommendations for induction of labor if discovered at term.
The Royal Australian and New Zealand College of Obstetricians and Gynaecologists recommend a pregnancy with polyhydramnios be referred to a specialist Obstetrician. 78 Surprisingly, there are currently no practice management recommendations from the American College of Obstetrics (ACOG), Royal College of Obstetrics and Gynaecology (RCOG) or the Society of Obstetricians and Gynaecologists of Canada (SOGC) beyond the statement that antepartum testing may be beneficial by ACOG and SOGC. 80 , 81
Twins and amniotic fluid
When evaluating the health of the amniotic fluid in twin pregnancies, singleton growth curves currently provide the best predictors of adverse outcomes, and the evaluation of fluid with singleton nomograms is frequently used. This approach appears reasonable since in the only evaluation of AFV in third trimester diamniotic twin pregnancies the AFV of each sac was shown to be similar to that of normal singleton pregnancies. 94 Morin and Lim 95 performed a literature review with the Diagnostic Imaging Committee of the Society of Obstetricians and Gynaecologists of Canada and suggest that the SDP be used in each sac with standard definitions for singleton pregnancies, although they state that there is not enough evidence to suggest that one method is more predictive than the others on adverse pregnancy outcome. The AFI has been used to estimate amniotic fluid volume in twins; however, when using the summated AFI (measurement of all four quadrants as is done in singletons but without taking membrane placement into consideration) the measurement poorly predicts oligohydramnios and polyhydramnios. 88
Table 2.
SDP percentiles in twins across gestation.
| EGA (weeks) | 2.5th | 5th | 10th | 50th | 90th | 95th | 97.5th | Twin sacs assessed |
|---|---|---|---|---|---|---|---|---|
| < 17 | 2.02 | 2.11 | 2.54 | 3.96 | 6.48 | 6.86 | 6.88 | 22 |
| 17–19 | 2.13 | 2.14 | 2.64 | 4.07 | 6.56 | 6.97 | 7.02 | 143 |
| 20–22 | 2.22 | 2.23 | 2.79 | 4.22 | 6.60 | 7.04 | 7.21 | 276 |
| 23–25 | 2.23 | 2.28 | 2.93 | 4.38 | 6.67 | 7.19 | 7.45 | 322 |
| 26–28 | 2.29 | 2.37 | 3.06 | 4.53 | 6.80 | 7.44 | 7.79 | 390 |
| 29–31 | 2.43 | 2.46 | 3.20 | 4.67 | 6.91 | 7.64 | 8.09 | 323 |
| 32–34 | 2.44 | 2.51 | 3.32 | 4.80 | 6.98 | 7.76 | 8.27 | 283 |
| 35–37 | 2.41 | 2.54 | 3.39 | 4.90 | 7.01 | 7.81 | 8.39 | 129 |
Reprinted from: Magann EF, Doherty DA, Ennen CS, Chauhan SP, Shields D, Gjesdal SM, Morrison JC. The ultrasound estimation of amniotic fluid volume in diamniotic twin pregnancies and prediction of peripartum outcomes. Am J Obstet Gynecol 2007; 196 (6): 570. e1–6. With permission from Elsevier.
In a single prospective observational study of 299 diamniotic twin pregnancies, SDP was found to be constant between 17 and 37 weeks with an increase in fetal labor intolerance and neonatal morbidity in twin pregnancies with hydramnios. Alternate fixed cutoffs of < 2.2 cm for a low amniotic fluid volume and > 7.5 cm for a high amniotic fluid volume were suggested in diamniotic twin pregnancies to identify pregnancies at risk for adverse intrapartum and neonatal outcomes. 96
Hernandez, et al. 97 performed a retrospective review of 1951 twin pregnancies, both dichorionic and monochorionic, with twin‐twin transfusion syndrome excluded. Hydramnios was identified in 18% of pregnancies, defined as mild (SDP 8–9.9 cm), moderate (SDP 10–11.9 cm), and severe (SPD ≥ 12). Pregnancy outcomes were reviewed and hydramnios was not associated with preterm delivery, fetal growth restriction, NICU admission, or neonatal death in either twin pregnancy. The incidence of major anomalies became more common in increasing hydramnios in both monochorionic and dichorionic pregnancies with a prevalence of almost 20% in severe hydramnios. Severe hydramnios was significantly associated with stillbirth in monochorionic pregnancies (27% P < .001).
Twin‐twin transfusion syndrome carries with it a 3–5 fold increase in mortality and morbidity. 98 It is often uncovered with ultrasound evidence of discordant fetal weights and amniotic fluid in monochorionic/diamniotic twins, although it has been documented in monochorionic/monoamniotic pregnancies. 99 When diagnosing TTTS by ultrasound, two criteria are required: monochorionic/diamniotic pregnancy and the presence of oligohydramnios in one sac and polyhydramnios in the other. 100 It has been shown that if the difference of amniotic fluid in the two sacs fail to meet that criteria, but a subjective difference is noted, TTTS will develop in 15% of those pregnancies. 101 The criteria for oligohydramnios and polyhyramnios follow singleton values. Growth discordance and selective growth restriction is sometimes associated with TTTS, but it is not a diagnostic criteria. 100 , 102 Evaluation for TTTS in these pregnancies should begin in the second trimester with weekly surveillance if discordance is detected and every two weeks evaluation for concordant pregnancies. 103 Selective intrauterine growth restricted (sIUGR) twins should be monitored with umbilical artery dopplers, keeping in mind that results will vary based on severity of sIUGR. 102
Management of abnormal amniotic fluid volume in twin pregnancies does not vary much from singleton pregnancies with the single exception of twin‐twin transfusion syndrome. Pregnancies with polyhydramnios‐oligohydramnios sequence or those with diagnosed twin‐twin transfusion syndrome should be referred to Maternal‐Fetal Medicine for evaluation and potential treatment as indicated. The diagnosis of abnormal amniotic fluid in monoamniotic twins does not alter management of the pregnancy, as antenatal testing should begin at viability and delivery planned early. 104 The discovery of abnormal fluid volumes may prompt earlier evaluation of causes such as twin‐twin transfusion syndrome, IUGR, and congenital anomalies. Dichorionic/diamniotic twin pregnancies should be treated as described above for singleton pregnancies. In pregnancies where polyhydramnios is found in both sacs, or in a single shared amnion, fluid reduction may be necessary for maternal comfort, and preterm labor may be encountered more frequently. 105
In summary, amniotic fluid is a highly complex and dynamic system that should be utilised in the interpretation of fetal wellbeing. Routine assessment of amniotic fluid volume has become commonplace with sonography and many options exist for its estimation. Practitioners should be familiar with all of the methods above, but choose one method for which to evaluate their patients, knowing the strengths and limitations of each. When abnormalities of fluid exist, appropriate workup to uncover the underlying etiology should be initiated as adverse fetal outcomes are sometimes associated with these variations from normalcy. Management options should be related to cause, if discovered. The ultimate goal of appropriate timing of delivery while reducing concurrent morbidity and mortality can be challenging, but continued research in this field will no doubt continue to elevate clinical practice to this goal.
Acknowledgements
Donna G Eastham, BA, Department of Obstetrics and Gynecology, University of Arkansas for the Medical Sciences for her editing of the manuscript.
References
- 1. Beall MH, van den Wijngaard JP, van Gemert MJ, Ross MG. Amniotic fluid water dynamics. Placenta 2007; 28: 816–23. [DOI] [PubMed] [Google Scholar]
- 2. Magann EF, Sandlin AT, Ounpraseuth ST. Amniotic fluid and the clinical relevance of the sonographically estimated amniotic fluid volume: oligohydramnios. J Ultrasound Med 2011; 30: 1573–85. [DOI] [PubMed] [Google Scholar]
- 3. Brace RA. Amniotic fluid volume and its relationship to fetal fluid balance: review of experimental data. Semin Perinatol 1986; 10: 103–12. [PubMed] [Google Scholar]
- 4. Gillibrand PN. Changes in the electrolytes, urea and osmolality of the amniotic fluid with advancing pregnancy. J Obstet Gynaecol Br Commonw 1969; 76: 898–905. [DOI] [PubMed] [Google Scholar]
- 5. Lee SM, Park SK, Shim SS, Jun JK, Park JS, Syn HC. Measurement of fetal urine production by three‐dimensional ultrasonography in normal pregnancy. Ultrasound Obstet Gynecol 2007; 30: 281–86. [DOI] [PubMed] [Google Scholar]
- 6. Peixoto‐Filho FM, de Sa RA, Velarde LG, Lopes LM, Ville Y. Normal range for fetal urine production rate by 3–D ultrasound in Brazilian population. Arch Gynecol Obstet 2011; 283: 497–500. [DOI] [PubMed] [Google Scholar]
- 7. Modena AB, Fieni S. Amniotic fluid dynamics. Acta Biomed 2004; 75 Supplement 1: 11–13. [PubMed] [Google Scholar]
- 8. Pritchard JA. Deglutition by Normal and Anencephalic Fetuses. Obstet Gynecol 1965; 25: 289–97. [PubMed] [Google Scholar]
- 9. Tomoda S, Brace RA, Longo LD. Amniotic fluid volume and fetal swallowing rate in sheep. Am J Physiol 1985; 249: R133–38. [DOI] [PubMed] [Google Scholar]
- 10. Brace RA, Anderson DF, Cheung CY. Fetal swallowing as a protective mechanism against oligohydramnios and polyhydramnios in late gestation sheep. Reprod Sci 2013; 20 (3): 326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Minei LJ, Suzuki K. Role of fetal deglutition and micturition in the production and turnover of amniotic fluid in the monkey. Obstet Gynecol 1976; 48: 177–81. [PubMed] [Google Scholar]
- 12. Brace RA, Wlodek ME, Cock ML, Harding R. Swallowing of lung liquid and amniotic fluid by the ovine fetus under normoxic and hypoxic conditions. Am J Obstet Gynecol 1994; 171: 764–70. [DOI] [PubMed] [Google Scholar]
- 13. Assali NS. Pathophysiology of gestation. New York: Academic Press; 1972. [Google Scholar]
- 14. Fischer C, Rybakowski C, Ferdynus C, Sagot P, Gouyon JB. A Population‐Based Study of Meconium Aspiration Syndrome in Neonates Born between 37 and 43 Weeks of Gestation. Int J Pediatr 2012; 2012: 321545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balchin I, Whittaker JC, Lamont RF, Steer PJ. Maternal and fetal characteristics associated with meconium‐stained amniotic fluid. Obstet Gynecol 2011; 117: 828–35. [DOI] [PubMed] [Google Scholar]
- 16. Bolisetty S, Patole SK, McBride GA, Whitehall JS. Neonatal amniotic fluid aspiration syndrome underdiagnosed? Int J Clin Pract 2001; 55: 727–28. [PubMed] [Google Scholar]
- 17. Ikeda N, Yamakawa M, Imai Y, Suzuki T. Sudden infant death from atelectasis due to amniotic fluid aspiration. Am J Forensic Med Pathol 1989; 10: 340–43. [DOI] [PubMed] [Google Scholar]
- 18. Beall MH, van den Wijngaard JP, van Gemert MJ, Ross MG. Regulation of amniotic fluid volume. Placenta 2007; 28: 824–32. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Ding S, Shen Q, Wu J, Zhu X. The expression and regulation of aquaporins in placenta and fetal membranes. Front Biosci 2012; 17: 2371–82. [DOI] [PubMed] [Google Scholar]
- 20. Jiang SS, Zhu XJ, Ding SD, Jiang LL, Jiang WX, Zhu XQ. Expression and localization of Aquaporins 8 and 9 in term placenta with oligohydramnios. Reprod Sci 2012; 19(12):1276–84. [DOI] [PubMed] [Google Scholar]
- 21. Damiano AE. Review: Water channel proteins in the human placenta and fetal membranes. Placenta 2011; 32 Supplement 2: S207–11. [DOI] [PubMed] [Google Scholar]
- 22. Mann SE, Nijland MJ, Ross MG. Mathematic modeling of human amniotic fluid dynamics. Am J Obstet Gynecol 1996; 175: 937–44. [DOI] [PubMed] [Google Scholar]
- 23. Faber JJ, Anderson DF. Absorption of amniotic fluid by amniochorion in sheep. Am J Physiol Heart Circ Physiol 2002; 282: H850–54. [DOI] [PubMed] [Google Scholar]
- 24. Gilbert WM, Brace RA. The missing link in amniotic fluid volume regulation: intramembranous absorption. Obstet Gynecol 1989; 74: 748–54. [PubMed] [Google Scholar]
- 25. Horsager R, Nathan L, Leveno KJ. Correlation of measured amniotic fluid volume and sonographic predictions of oligohydramnios. Obstet Gynecol 1994; 83: 955–58. [DOI] [PubMed] [Google Scholar]
- 26. Magann EF, Whitworth NS, Files JC, Terrone DA, Chauhan SP, Morrison JC. Dye‐dilution techniques using aminohippurate sodium: do they accurately reflect amniotic fluid volume? J Matern Fetal Neonatal Med 2002; 11: 167–70. [DOI] [PubMed] [Google Scholar]
- 27. Zaretsky MV, McIntire DD, Reichel TF, Twickler DM. Correlation of measured amnionic fluid volume to sonographic and magnetic resonance predictions. Am J Obstet Gynecol 2004; 191: 2148–53. [DOI] [PubMed] [Google Scholar]
- 28. Phelan JP, Smith CV, Broussard P, Small M. Amniotic fluid volume assessment with the four‐quadrant technique at 36–42 weeks' gestation. J Reprod Med 1987; 32: 540–42. [PubMed] [Google Scholar]
- 29. Chamberlain PF, Manning FA, Morrison I, Harman CR, Lange IR. Ultrasound evaluation of amniotic fluid volume. II. The relationship of increased amniotic fluid volume to perinatal outcome. Am J Obstet Gynecol 1984; 150: 250–54. [DOI] [PubMed] [Google Scholar]
- 30. Johnson JM, Harman CR, Lange IR, Manning FA. Biophysical profile scoring in the management of the postterm pregnancy: an analysis of 307 patients. Am J Obstet Gynecol 1986; 154: 269–73. [DOI] [PubMed] [Google Scholar]
- 31. Magann EF, Nolan TE, Hess LW, Martin RW, Whitworth NS, Morrison JC. Measurement of amniotic fluid volume: accuracy of ultrasonography techniques. Am J Obstet Gynecol 1992; 167: 1533–37. [DOI] [PubMed] [Google Scholar]
- 32. Magann EF, Perry KG Jr, Chauhan SP, Anfanger PJ, Whitworth NS, Morrison JC. The accuracy of ultrasound evaluation of amniotic fluid volume in singleton pregnancies: the effect of operator experience and ultrasound interpretative technique. J Clin Ultrasound 1997; 25: 249–53. [DOI] [PubMed] [Google Scholar]
- 33. Magann EF, Chauhan SP, Barrilleaux PS, Whitworth NS, Martin JN. Amniotic fluid index and single deepest pocket: weak indicators of abnormal amniotic volumes. Obstet Gynecol 2000; 96: 737–40. [DOI] [PubMed] [Google Scholar]
- 34. Magann EF, Chauhan SP, Whitworth NS, Anfanger P, Rinehart BK, Morrison JC. Determination of amniotic fluid volume in twin pregnancies: ultrasonographic evaluation versus operator estimation. Am J Obstet Gynecol 2000; 182: 1606–09. [DOI] [PubMed] [Google Scholar]
- 35. Chauhan SP, Doherty DD, Magann EF, Cahanding F, Moreno F, Klausen JH. Amniotic fluid index vs single deepest pocket technique during modified biophysical profile: a randomized clinical trial. Am J Obstet Gynecol 2004; 191: 661–67. Discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 36. Magann EF, Chauhan SP, Doherty DA, Magann MI, Morrison JC. The evidence for abandoning the amniotic fluid index in favor of the single deepest pocket. Am J Perinatol 2007; 24: 549–55. [DOI] [PubMed] [Google Scholar]
- 37. Nabhan AF, Abdelmoula YA. Amniotic fluid index versus single deepest vertical pocket: a meta‐analysis of randomized controlled trials. Int J Gynaecol Obstet 2009; 104: 184–88. [DOI] [PubMed] [Google Scholar]
- 38. Magann EF, Chauhan SP, Barrilleaux PS, Whitworth NS, McCurley S, Martin JN. Ultrasound estimate of amniotic fluid volume: color Doppler overdiagnosis of oligohydramnios. Obstet Gynecol 2001; 98: 71–74. [DOI] [PubMed] [Google Scholar]
- 39. Sahin B, Alper T, Kokcu A, Malatyalioglu E, Kosif R. Estimation of the amniotic fluid volume using the Cavalieri method on ultrasound images. Int J Gynaecol Obstet 2003; 82: 25–30. [DOI] [PubMed] [Google Scholar]
- 40. Bromley B, Shipp TD, Benacerraf B. Assessment of the third‐trimester fetus using 3–dimensional volumes: a pilot study. J Clin Ultrasound 2007; 35: 231–37. [DOI] [PubMed] [Google Scholar]
- 41. Rousian M, Koning AH, Hop WC, van der Spek PJ, Exalto N, Steegers EA. Gestational sac fluid volume measurements in virtual reality. Ultrasound Obstet Gynecol 2011; 38: 524–29. [DOI] [PubMed] [Google Scholar]
- 42. Queenan JT, Thompson W, Whitfield CR, Shah SI. Amniotic fluid volumes in normal pregnancies. Am J Obstet Gynecol 1972; 114: 34–38. [DOI] [PubMed] [Google Scholar]
- 43. Moore TR, Cayle JE. The amniotic fluid index in normal human pregnancy. Am J Obstet Gynecol 1990; 162: 1168–73. [DOI] [PubMed] [Google Scholar]
- 44. Brace RA, Wolf EJ. Normal amniotic fluid volume changes throughout pregnancy. Am J Obstet Gynecol 1989; 161: 382–88. [DOI] [PubMed] [Google Scholar]
- 45. Magann EF, Bass JD, Chauhan SP, Young RA, Whitworth NS, Morrison JC. Amniotic fluid volume in normal singleton pregnancies. Obstet Gynecol 1997; 90: 524–28. [DOI] [PubMed] [Google Scholar]
- 46. Magann EF, Sanderson M, Martin JN, Chauhan S. The amniotic fluid index, single deepest pocket, and two‐diameter pocket in normal human pregnancy. Am J Obstet Gynecol 2000; 182: 1581–88. [DOI] [PubMed] [Google Scholar]
- 47. Shanks A, Tuuli M, Schaecher C, Odibo AO, Rampersad R. Assessing the optimal definition of oligohydramnios associated with adverse neonatal outcomes. J Ultrasound Med 2011; 30: 303–07. [DOI] [PubMed] [Google Scholar]
- 48. Magann EF, Doherty DA, Chauhan SP, Busch FW, Mecacci F, Morrison JC. How well do the amniotic fluid index and single deepest pocket indices (below the 3rd and 5th and above the 95th and 97th percentiles) predict oligohydramnios and hydramnios? Am J Obstet Gynecol 2004; 190: 164–69. [DOI] [PubMed] [Google Scholar]
- 49. Dildy GA 3rd, Lira N, Moise KJ Jr, Riddle GD, Deter RL. Amniotic fluid volume assessment: comparison of ultrasonographic estimates versus direct measurements with a dye‐dilution technique in human pregnancy. Am J Obstet Gynecol 1992; 167: 986–94. [DOI] [PubMed] [Google Scholar]
- 50. Chamberlain PF, Manning FA, Morrison I, Harman CR, Lange IR. Ultrasound evaluation of amniotic fluid volume. I. The relationship of marginal and decreased amniotic fluid volumes to perinatal outcome. Am J Obstet Gynecol 1984; 150: 245–49. [DOI] [PubMed] [Google Scholar]
- 51. Baron C, Morgan MA, Garite TJ. The impact of amniotic fluid volume assessed intrapartum on perinatal outcome. Am J Obstet Gynecol 1995; 173: 167–74. [DOI] [PubMed] [Google Scholar]
- 52. Petrozella LN, Dashe JS, McIntire DD, Leveno KJ. Clinical significance of borderline amniotic fluid index and oligohydramnios in preterm pregnancy. Obstet Gynecol 2011; 117: 338–42. [DOI] [PubMed] [Google Scholar]
- 53. Magann EF, Chauhan SP, Hitt WC, Dubil EA, Morrison JC. Borderline or marginal amniotic fluid index and peripartum outcomes: a review of the literature. J Ultrasound Med 2011; 30: 523–28. [DOI] [PubMed] [Google Scholar]
- 54. Oyelese Y. Placenta, umbilical cord and amniotic fluid: the not‐less‐important accessories. Clin Obstet Gynecol 2012; 55: 307–23. [DOI] [PubMed] [Google Scholar]
- 55. Casey BM, McIntire DD, Bloom SL, et al. Pregnancy outcomes after antepartum diagnosis of oligohydramnios at or beyond 34 weeks' gestation. Am J Obstet Gynecol 2000; 182: 909–12. [DOI] [PubMed] [Google Scholar]
- 56. Melamed N, Pardo J, Milstein R, Chen R, Hod M, Yogev Y. Perinatal outcome in pregnancies complicated by isolated oligohydramnios diagnosed before 37 weeks of gestation. Am J Obstet Gynecol 2011; 205: 241 e1–6. [DOI] [PubMed] [Google Scholar]
- 57. Magann EF, Haas DM, Hill JB, Chauhan SP, Watson EM, Learman LA. Oligohydramnios, small for gestational age and pregnancy outcomes: an analysis using precise measures. Gynecol Obstet Invest 2011; 72: 239–44. [DOI] [PubMed] [Google Scholar]
- 58. Stoll C, Alembik Y, Roth MP, Dott B. Study of 224 cases of oligohydramnios and congenital malformations in a series of 225,669 consecutive births. Community Genet 1998; 1: 71–77. [DOI] [PubMed] [Google Scholar]
- 59. Leibovitch L, Kuint J, Rosenfeld E, Schushan‐Eisen I, Weissmann‐Brenner A, Maayan‐Metzger A. Short‐term outcome among term singleton infants with intrapartum oligohydramnios. Acta Paediatr 2012; 101: 727–30. [DOI] [PubMed] [Google Scholar]
- 60. Martinez de Tejada B, Boulvain M, Dumps P, Bischof P, Meisser A, Irion O. Can we improve the diagnosis of rupture of membranes? The value of insulin‐like growth factor binding protein–1. BJOG 2006; 113: 1096–99. [DOI] [PubMed] [Google Scholar]
- 61. Storness‐Bliss C, Metcalfe A, Simrose R, Wilson RD, Cooper SL. Correlation of residual amniotic fluid and perinatal outcomes in periviable preterm premature rupture of membranes. J Obstet Gynaecol Can 2012; 34: 154–58. [DOI] [PubMed] [Google Scholar]
- 62. Hunter TJ, Byrnes MJ, Nathan E, Gill A, Pennell CE. Factors influencing survival in pre‐viable preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2012; 25: 1755–61. [DOI] [PubMed] [Google Scholar]
- 63. Coolen J, Kabayashi K, Wong K, Mayes DC, Bott N, Demianczuk N. Influence of oligohydramnios on preterm premature rupture of the membranes at 30 to 36 weeks' gestation. J Obstet Gynaecol Can 2010; 32: 1030–34. [DOI] [PubMed] [Google Scholar]
- 64. Cosmi E, Patrelli TS, Gizzo S, Cosmi E, Carpano MG, Di Gangi S, et al. Maternal hydration therapy improves the quantity of amniotic fluid and the pregnancy outcome in third‐trimester isolated oligohydramnios: a controlled randomized institutional trial. J Ultrasound Med 2012; 31: 239–44. [DOI] [PubMed] [Google Scholar]
- 65. Ghafarnejad M, Tehrani MB, Anaraki FB, Mood NI, Nasehi L. Oral hydration therapy in oligohydramnios. J Obstet Gynaecol Res 2009; 35: 895–900. [DOI] [PubMed] [Google Scholar]
- 66. Hofmeyr GJ, Gulmezoglu AM. Maternal hydration for increasing amniotic fluid volume in oligohydramnios and normal amniotic fluid volume. Cochrane Database Syst Rev 2002;CD000134. [DOI] [PubMed]
- 67. Magann EF, Doherty DA, Chauhan SP, Barrilleaux SP, Verity LA, Martin JN Jr. Effect of maternal hydration on amniotic fluid volume. Obstet Gynecol 2003; 101: 1261–65. [DOI] [PubMed] [Google Scholar]
- 68. Malhotra B, Deka D. Duration of the increase in amniotic fluid index (AFI) after acute maternal hydration. Arch Gynecol Obstet 2004; 269: 173–75. [DOI] [PubMed] [Google Scholar]
- 69. Brizot ML, Liao AW, Nomura RM, Francisco RP, Zugaib M. Changes in amniotic fluid index after maternal oral hydration in pregnancies with fetal gastroschisis: initial observations. Fetal Diagn Ther 2010; 28: 87–91. [DOI] [PubMed] [Google Scholar]
- 70. Malhotra B, Deka D. Effect of maternal oral hydration on amniotic fluid index in women with pregnancy‐induced hypertension. J Obstet Gynaecol Res 2002; 28: 194–98. [DOI] [PubMed] [Google Scholar]
- 71. Ulker K, Temur I, Karaca M, Ersoz M, Volkan I, Gul A. Effects of maternal left lateral position and rest on amniotic fluid index: a prospective clinical study. J Reprod Med 2012; 57: 270–76. [PubMed] [Google Scholar]
- 72. Manzanares S, Carrillo MP, Gonzalez‐Peran E, Puertas A, Montoya F. Isolated oligohydramnios in term pregnancy as an indication for induction of labor. J Matern Fetal Neonatal Med 2007; 20: 221–24. [DOI] [PubMed] [Google Scholar]
- 73. Guin G, Punekar S, Lele A, Khare S. A prospective clinical study of feto‐maternal outcome in pregnancies with abnormal liquor volume. J Obstet Gynaecol India 2011; 61: 652–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ek S, Andersson A, Johansson A, Kublicas M. Oligohydramnios in uncomplicated pregnancies beyond 40 completed weeks. A prospective, randomised, pilot study on maternal and neonatal outcomes. Fetal Diagn Ther 2005; 20: 182–85. [DOI] [PubMed] [Google Scholar]
- 75. Schwartz N, Sweeting R, Young BK. Practice patterns in the management of isolated oligohydramnios: a survey of perinatologists. J Matern Fetal Neonatal Med 2009; 22: 357–61. [DOI] [PubMed] [Google Scholar]
- 76. Munn MB. Management of oligohydramnios in pregnancy. Obstet Gynecol Clin North Am 2011; 38: 387–95. [DOI] [PubMed] [Google Scholar]
- 77. O'Neill E, Thorp J. Antepartum evaluation of the fetus and fetal well being. Clin Obstet Gynecol 2012; 55: 722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. RANZCOG. Maternal suitability for models of care, and indications for referral within and between models of care (C‐Obs 30). 2012.
- 79.Practice Bulletin No AC. 107: Induction of labor. Obstet Gynecol 2009; 114: 386–97. [DOI] [PubMed] [Google Scholar]
- 80. ACOG practice bulletin. Antepartum fetal surveillance. Number 9, October 1999 (replaces Technical Bulletin Number 188, January 1994). Clinical management guidelines for obstetrician‐gynecologists. Int J Gynaecol Obstet 2000; 68: 175–85. [PubMed] [Google Scholar]
- 81. Liston R, Sawchuck D, Young D. Fetal health surveillance: antepartum and intrapartum consensus guideline. J Obstet Gynaecol Can 2007; 29: S3–56. [PubMed] [Google Scholar]
- 82. Magann EF, Morton ML, Nolan TE, Martin JN Jr, Whitworth NS, Morrison JC. Comparative efficacy of two sonographic measurements for the detection of aberrations in the amniotic fluid volume and the effect of amniotic fluid volume on pregnancy outcome. Obstet Gynecol 1994; 83: 959–62. [DOI] [PubMed] [Google Scholar]
- 83. Phelan JP, Ahn MO, Smith CV, Rutherford SE, Anderson E. Amniotic fluid index measurements during pregnancy. J Reprod Med 1987; 32: 601–04. [PubMed] [Google Scholar]
- 84. Brady K, Polzin WJ, Kopelman JN, Read JA. Risk of chromosomal abnormalities in patients with idiopathic polyhydramnios. Obstet Gynecol 1992; 79: 234–38. [PubMed] [Google Scholar]
- 85. Phelan JP, Park YW, Ahn MO, Rutherford SE. Polyhydramnios and perinatal outcome. J Perinatol 1990; 10: 347–50. [PubMed] [Google Scholar]
- 86. Magann EF, Doherty DA, Lutgendorf MA, Magann MI, Chauhan SP, Morrison JC. Peripartum outcomes of high‐risk pregnancies complicated by oligo‐ and polyhydramnios: a prospective longitudinal study. J Obstet Gynaecol Res 2010; 36: 268–77. [DOI] [PubMed] [Google Scholar]
- 87. Ott WJ. Reevaluation of the relationship between amniotic fluid volume and perinatal outcome. Am J Obstet Gynecol 2005; 192: 1803–9; discussion 9. [DOI] [PubMed] [Google Scholar]
- 88. Rochelson B, Wagner J, Shmoys S. The clinical significance of resolving polyhydramnios. Ultrasound Obstet Gynecol 1992; 2: 321–24. [DOI] [PubMed] [Google Scholar]
- 89. Leibovitch L, Schushan‐Eisen I, Kuint J, Weissmann‐Brenner A, Maayan‐Metzger A. Short‐term outcome for term and near‐term singleton infants with intrapartum polyhydramnios. Neonatology 2012; 101: 61–67. [DOI] [PubMed] [Google Scholar]
- 90. Abele H, Starz S, Hoopmann M, Yazdi B, Rall K, Kagan KO. Idiopathic Polyhydramnios and Postnatal Abnormalities. Fetal Diagn Ther 2012; 32 (4): 251–5. [DOI] [PubMed] [Google Scholar]
- 91. Perović M, Garalejić E, Gojnić M, Arsić B, Pantić I, Bojović DJ, et al. Sensitivity and specificity of ultrasonography as a screening tool for gestational diabetes mellitus. J Matern Fetal Neonatal Med 2012; 25: 1348–53. [DOI] [PubMed] [Google Scholar]
- 92. Idris N, Wong SF, Thomae M, Gardener G, McIntyre DH. Influence of polyhydramnios on perinatal outcome in pregestational diabetic pregnancies. Ultrasound Obstet Gynecol 2010; 36: 338–43. [DOI] [PubMed] [Google Scholar]
- 93. Barnhard Y, Bar‐Hava I, Divon MY. Is polyhydramnios in an ultrasonographically normal fetus an indication for genetic evaluation? Am J Obstet Gynecol 1995; 173: 1523–27. [DOI] [PubMed] [Google Scholar]
- 94. Magann EF, Whitworth NS, Bass JD, Chauhan SP, Martin JN Jr, Morrison JC. Amniotic fluid volume of third‐trimester diamniotic twin pregnancies. Obstet Gynecol 1995; 85: 957–60. [DOI] [PubMed] [Google Scholar]
- 95. Morin L, Lim K. Ultrasound in twin pregnancies. J Obstet Gynaecol Can 2011; 33: 643–56. [DOI] [PubMed] [Google Scholar]
- 96. Magann EF, Doherty DA, Ennen CS, Chauhan SP, Shields D, Gjesdal SM, Morrison JC. The ultrasound estimation of amniotic fluid volume in diamniotic twin pregnancies and prediction of peripartum outcomes. Am J Obstet Gynecol 2007; 196: 570 e1–6; discussion e6–8. [DOI] [PubMed] [Google Scholar]
- 97. Hernandez JS, Twickler DM, McIntire DD, Dashe JS. Hydramnios in twin gestations. Obstet Gynecol 2012; 120: 759–65. [DOI] [PubMed] [Google Scholar]
- 98. Stein RG, Diessner J, Frieauff E, Zollner U, Rehn M, Dietl J, Hönig A. Acute foeto‐foetal transfusion syndrome–case report and review of the literature. Z Geburtshilfe Neonatol 2012; 216: 147–49. [DOI] [PubMed] [Google Scholar]
- 99. Shveiky D, Ezra Y, Schenker JG, Rojansky N. Monoamniotic twins: an update on antenatal diagnosis and treatment. J Matern Fetal Neonatal Med 2004; 16: 180–86. [DOI] [PubMed] [Google Scholar]
- 100. Simpson LL. Twin‐twin transfusion syndrome. Am J Obstet Gynecol 2013; 208: 3–18. [DOI] [PubMed] [Google Scholar]
- 101. Huber A, Diehl W, Zikulnig L, Bregenzer T, Hackeloer BJ, Hecher K. Perinatal outcome in monochorionic twin pregnancies complicated by amniotic fluid discordance without severe twin‐twin transfusion syndrome. Ultrasound Obstet Gynecol 2006; 27: 48–52. [DOI] [PubMed] [Google Scholar]
- 102. Moise KY, Kugler L, Jones T. Contemporary management of complicated monochorionic twins. J Obstet Gynecol Neonatal Nurs 2012; 41: 434–44, quiz 45–46. [DOI] [PubMed] [Google Scholar]
- 103. Carver A, Haeri S, Moldenhauer J, Wolfe HM, Goodnight W. Monochorionic diamniotic twin pregnancy: timing and duration of sonographic surveillance for detection of twin‐twin transfusion syndrome. J Ultrasound Med 2011; 30: 297–301. [DOI] [PubMed] [Google Scholar]
- 104. Su LL. Monoamniotic twins: diagnosis and management. Acta Obstet Gynecol Scand 2002; 81: 995–1000. [DOI] [PubMed] [Google Scholar]
- 105. Inde Y, Miyake H, Takaya A, Ono S, Igarashi M, Suzuki S. A case of monochorionic‐diamniotic twin pregnancy with polyhydramnios‐polyhydramnios sequence. J Nippon Med Sch 2009; 76: 93–95. [DOI] [PubMed] [Google Scholar]


