Abstract
Objective
Mindfulness-based cognitive therapy (MBCT) is a programme developed to prevent depression relapse, but has been applied for other disorders. Our objective was to systematically review and meta-analyse the evidence on the effectiveness and safety of MBCT for the treatment of mental disorders.
Methods
Searches were completed in CENTRAL, MEDLINE, EMBASE, LILACS, PsychINFO, and PsycEXTRA in March 2011 using a search strategy with the terms ‘mindfulness-based cognitive therapy’, ‘mindfulness’, and ‘randomised controlled trials’ without time restrictions. Selection criteria of having a randomised controlled trial design, including patients diagnosed with mental disorders, using MBCT according to the authors who developed MBCT and providing outcomes that included changes in mental health were used to assess 608 reports. Two reviewers applied the pre-determined selection criteria and extracted the data into structured tables. Meta-analyses and sensitivity analyses were completed.
Results
Eleven studies were included. Most of them evaluated depression and compared additive MBCT against usual treatment. After 1 year of follow-up MBCT reduced the rate of relapse in patients with three or more previous episodes of depression by 40% (5 studies, relative risk [95% confidence interval]: 0.61 [0.48, 0.79]). Other meta-analysed outcomes were depression and anxiety, both with significant results but unstable in sensitivity analyses. Methodological quality of the reports was moderate.
Conclusion
Based on this review and meta-analyses, MBCT is an effective intervention for patients with three or more previous episodes of major depression.
Keywords: depression, mental disorders, meta-analysis, mindfulness-based cognitive therapy, randomised controlled trials, systematic review
Introduction
Mental disorders account for 13% of the global burden of disease, represent a significant burden of disability, and are projected to continue to rise (World Health Organization, 2004). Mindfulness-based cognitive therapy (MBCT) (Segal et al., 2002), initially developed to prevent relapse or recurrence of major depressive disorder (MDD), is now being studied to treat a variety of mental health disorders. The purpose of this research was to conduct a systematic review and meta-analysis of randomised controlled trials (RCTs) assessing the efficacy and safety of MBCT for the treatment of mental disorders.
MBCT is a programme in which contemplative practices and cognitive therapy techniques are combined and delivered by MBCT-trained therapists in standard 8-week units (Segal et al., 2002). The patient visits the therapist, participates in cognitive therapy sessions, and learns mindfulness techniques, breathing and physical exercises of relaxation. Patients are coached to continue these exercises at home through recordings and notes.
The number of practitioners who use the technique is growing – nurses, nurse practitioners, physicians, psychologists, counselors, etc. MBCT has been described in the nursing literature as an innovative approach to relieve distress for individuals suffering from medical and psychiatric illnesses (O’Haver Day and Horton-Deutsch, 2004). Priority recommendations for the implementation of the National Institute for Health and Clinical Excellence new depression guidelines include managing depression in people with physical and chronic illnesses and include using group-based cognitive-behaviour therapy (Kendrick and Peveler, 2010). Nurses and advanced practice nurses interested in mental healthcare are often eager to trial novel or alternative therapeutic approaches. Evidence of efficacy, indications and specific patient populations is necessary to support implementation of therapeutic interventions.
The number of studies assessing the efficacy of MBCT is growing as well; therefore, a systematic description is needed. Systematic reviews on MBCT have been published (Chiesa and Serretti, 2011; Coelho et al., 2007; Fjorback et al., 2011; Hofmann et al., 2010; Piet and Hougaard, 2011). However, this review differs by including solely RCTs, by conducting sensitivity analyses on drop-out rates, and by having a different analytic strategy.
Methods
A systematic protocol was developed and implemented for this research (Galante, 2009).
Literature search and study selection
In March 2011 the following databases were searched: CENTRAL, MEDLINE, EMBASE, LILACS, PsychINFO, and PsycEXTRA. The terms ‘mindfulness-based cognitive therapy’, ‘meditation’ and ‘mindfulness’, and ‘randomised controlled trials’ were used with language limit of English and Spanish. Two reviewers independently excluded reports that did not meet inclusion criteria based on title and abstract. Full published reports were obtained for the remainder, and inclusion criteria were applied. References were scanned for further RCTs.
Included studies had to: (1) be RCTs; (2) include patients with mental disorders diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2012), the International Statistical Classification of Diseases (World Health Organization, 2012) or a validated diagnostic scale; (3) deliver MBCT according to recommendations of Segal and colleagues (2002), or with minimal adaptations made but still called MBCT by the study authors; and (4) include a change in mental health as an outcome variable.
Data abstraction
The data were extracted independently by two reviewers and entered into data extraction forms designed for the review. Studies were assessed for methodological quality according to the Cochrane Reviewers’ Handbook assessment tool (The Cochrane Collaboration, 2008). Disagreements between reviewers were satisfactorily resolved by discussion.
Analysis
Studies were grouped according to the type of outcome investigated and the follow-up period. In studies in which authors divided their patient populations into groups, the divisions for analysis were retained. Data obtained using the same measure and which were reported as continuous variables (or scales with a sufficient number of points to treat variables as continuous) were pooled using the weighted mean difference (WMD) with a 95% confidence interval (95% CI). When different measures were used to evaluate the same result in a comparison, data were grouped by calculating the standardised mean difference (SMD) with 95% CI. Final values were used. Dichotomous outcomes were analysed by calculating relative risk (RR) grouped in each comparison.
In order to determine whether combining the results was appropriate, χ2 and I2 tests of heterogeneity were performed. The p-value for χ2 was set conservatively at 0.1. I2 band values were interpreted according to the Cochrane Reviewers’ Handbook (The Cochrane Collaboration, 2008), which recommends interpreting I2 values below 40% as non-significant heterogeneity. Subgroup analyses were done according to type of disorder, stage, co-morbidities and multifactorial interventions. The potential effect of publication bias was assessed by analysing funnel plot asymmetries when meta-analysis of at least five studies could be carried out and when no significant heterogeneity was found. To obtain more conservative estimates a random effects model for the meta-analyses was used. Finally, sensitivity analyses were conducted to explore the influence of studies with significant dropout rates (>20%) on effect size. Results are reported according to QUOROM guidelines (Moher et al., 1999).
Results
Eleven studies meeting selection criteria were identified (Figure 1). The characteristics of included studies are provided in Table 1. Some of the studies were reported in multiple publications. Data could not be obtained for three conference papers either because efforts to contact the authors were unsuccessful, or because authors did not supply the requested information after contacting them (Fearson and Chadwick, 2007; Katzman et al., 2003; Welch, 2005). All but one study compared MBCT to treatment as usual (TAU) (Segal et al., 2010). As a conservative approach the study reported by Kuyken et al. (2008) was included in the MBCT + TAU versus TAU because 25% of the MBCT patients continued their medication.
Figure 1.
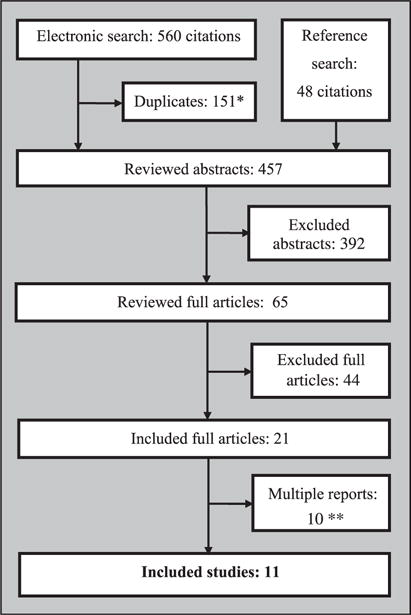
Study selection flow chart.
*Citations that were present in more than one database.
**For some studies more than one report was published.
Table 1.
Characteristics of included studies.
| Study no. | Publication | Type | No. | MBCT duration | Follow-up | Location | Agesa | Patients | Experimental group | Control groups | Primary outcomes | Secondary outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Teasdale et al., 2000, 2002; Williams et al., 2000, 2008 | RCT multi-center | 145 | 8 weeks | 1 yr | Great Britain, Canada | 18–65 | Patients with MD (≥2 episodes) in remission | MBCT + TAU | TAU | Relapse that meets DSM-III criteria for MD | BDI II, MACAM, use of AD, type of memory |
| 2 | Ma and Teasdale, 2004; Williams et al., 2008 | RCT | 75 | 8 weeks | 1 yr | Great Britain | 18–65 | Patients with (≥MD 2 episodes) in remission | MBCT + TAU | TAU | Relapse that meets DSM-III –R criteria for MD | BDI II |
| 3 | Kuyken et al., 2008 | RCT | 123 | 8 weeks | 1 yr | Great Britain | ≥18 | Patients with MD (≥3 episodes) in remission, taking ADs | MBCT +support to discontinue or reduce ADs | Maintenance ADs | Relapse that meets DSM-III criteria for MD | Characteristics of relapses, BDI II, HAM-D, psychiatric co-morbidities, WHOQOL-BREF, cost |
| 4 | Barnhofer et al., 2007; Crane et al., 2008; Hepburn et al., 2009; Williams et al., 2008 | RCT | 68 | 8 weeks | 1 yr | Great Britain | 18–65 | Bipolar and unipolar patients with MD (≥1 episode) in remission with suicidal ideation | MBCT + TAU | TAU | Prefrontal alpha-asymmetry during sleep EEG, BDI II, BAI, White Bear Suppression Inventory | |
| 5 | Godfrin and van Heeringen, 2010 | RCT | 106 | 8 weeks | 1 yr | Belgium | ≥18 | Patients with MD (≥3 episodes) in remission | MBCT + TAU | TAU | Relapse that meets DSM-IV-TR criteria for MD | BDI II, HAM-D, POMS, QLDS |
| 6 | Bondolfi et al., 2010 | RCT | 60 | 8 weeks | 1 yr | Switzerland | 18–65 | Patients with MD (≥3 episodes) in remission | MBCT + TAU | TAU | Relapse that meets DSM-IV criteria for MD | BDI II, frequency of practice of full attention during the study |
| 7 | Barnhofer et al., 2009; Hargus et al., 2010 | RCT | 31 | 8 weeks | absent | Great Britain | 18–65 | Patients with MD (≥3 episodes) with suicidal ideation | MBCT + TAU | TAU | Relapse that meets DSM-IV criteria for MD | BDI II and Beck scale for sucicidal ideation |
| 8 | Foley et al., 2010 | RCT | 115 | 8 weeks | 12 weeks | Australia | ≥18 | Oncology patients with depression | MBCT + TAU | TAU | HAM-D, HAM-A, DASS, (FACT–G) | Frieburg Mindfulness Inventory |
| 9 | Piet et al., 2010 | RCT crossover | 26 | 8 weeks | 1 yr | Denmark | 18–25 | Patients with social phobia | MBCT | GCBT | LSAS, SPS and SIAS | SPC,SCL-90-R, Symptom Checklist- 90-Revised, IPP, FNE, SDS, BDI II, BAI |
| 10 | Britton et al., 2010; Shahar et al., 2010 | RCT | 26 | 8 weeks | absent | USA | 24–64 | Antidepressant-free individuals with partial or totally remission of DM (≥3 episodes), with residual sleep complaints | MBCT + TAU | TAU | Polysomnographic sleep profiles | BDI II, sleep diaries |
| 11 | Segal et al., 2010 | RCT | 84 | 8 weeks | 18 months | Canada | 18–65 | Patients with MD (≥2 episodes) in remission | MBCT + support to discontinue ADs | 2 groups: Placebo and maintenance ADs | Time to relapse/recurrence of DM | – |
Male and female patients.
Yr: year; RCT: randomised controlled trial; MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; DM: major depression; BDI: Beck depression inventory; MACAM: measure of awareness and coping in autobiographical memory; AD: antidepressants; HAM-D: Hamilton rating scale for depression; WHOQOL-BREF: World Health Organization Quality of Life instrument; EEG: electroencephalogram; BAI: Beck anxiety inventory; POMS: profile of moods scale; QLDS: quality of life in depression scale; HAM-A: Hamilton anxiety rating scale; DASS: depression, anxiety stress scale; FACT-G: functional assessment of cancer therapy-general; GCBT: group cognitive-behavioural therapy; SPC: social phobia composite; SPS: social phobia scale; SIAS: social interaction scale; LSAS: Liebowitz social anxiety scale; SCL-90-R: symptom checklist-90-revised; IPP: inventory of interpersonal problems; FNE: fear of negative evaluation; SDS: Shehan disability scale.
Table 2 outlines the methodological quality of included reports. Due to the nature of the intervention, double blinding cannot be implemented with interventionists. However, most studies blinded evaluators and interviewers to intervention. Allocation concealment was adequate in all of the studies. Some reports did not include all outcomes listed in methods. Therefore, there is the potential for publication bias. In the majority of the reports randomisation method was not fully described.
Table 2.
Risk of bias table.
| Study no. | Appropriate randomisation method? | Concealment of allocation? | Blinding? | Free selective reporting of outcomes? | Loss to follow-up treated correctly? | Free of other biases? |
|---|---|---|---|---|---|---|
| 1 | Unclear | Yes | No Evaluation of results was blinded |
No Some of the data collected were not available (BDI II: not reported in Teasdale et al., 2000 and partially reported in Williams et al., 2008) |
Unclear Loss to follow-up was low and balanced between groups (3% in TAU and 5% in MBCT + TAU), but did not provide the reasons for the losses |
Unclear Contamination bias evaluation due to group intervention was not significant. Co-morbidities were taken into consideration, ITT analysis was conducted |
| 2 | Unclear | Yes | No Evaluation of results was blinded |
Yes | Unclear Loss to follow-up was low and balanced between groups (2.6% in TAU and 2.7% in MBCT + TAU), reasons for to loss follow-up not described |
Unclear Contamination bias evaluation due to group intervention was not significant. Co-morbidities were taken into consideration, ITT analysis was conducted |
| 3 | Yes | Yes | No Reviewers were blinded to group allocation |
Yes | Unclear Loss to follow-up was low (9.7% in TAU and 3.3% in MBCT + TAU), but groups were not balanced and the reasons for the losses were only partially explained |
Unclear Contamination bias evaluation due to group intervention was not significant. Co-morbidities were taken into consideration, ITT analysis was conducted |
| 4 | Unclear | Yes | No Reviewers were blinded to group allocation |
No Some results of interest were incompletely reported and could not be included in the meta-analysis (ITT analysis in Crane et al., 2008) |
Unclear Loss to follow-up was high (34% in TAU and 9% in MBCT + TAU for some results, 15% in TAU and 23% in MBCT + TAU for Williams et al., 2008 results), and reasons for the losses were not described |
No ITT analysis unspecified (e.g. Hepburn et al., 2009) or partially reported (e.g. Crane et al., 2008) |
| 5 | Yes | Yes | No No blinding |
Yes | Unclear Loss to follow-up was high (22.2% TAU and 34.6% MBCT + TAU), and reasons for the losses were only partially explained |
No ITT analysis was conducted but loss to follow-up was high and authors did not specify how missing data were dealt with. Co-morbidities were taken into consideration |
| 6 | Unclear | Yes | No Raters were blinded to group allocation |
Yes Not all outcomes pre-specified in the methods section were reported. Authors did provide missing data after being contacted |
Unclear Loss to follow-up was low (3.4% TAU and 12.9% in MBCT + TAU), but reasons for the losses were not described |
Unclear Co-morbidities were taken into consideration, ITT analysis was conducted |
| 7 | Yes | Yes | No Reviewers were blinded to group allocation |
Yes | Unclear Loss to follow-up was low (6.7% TAU and 12.5% in MBCT + TAU), but reasons for the losses were not described. Final data from lost participants were imputed by carrying forward baseline data |
Unclear Co-morbidities were taken into consideration, ITT analysis was conducted |
| 8 | Yes | Yes | No Reviewers were blinded to group allocation |
Yes | Unclear Loss to follow-up was moderate (16.7% TAU and 10.9% in MBCT + TAU), but reasons were only partially explained. Final data from lost participants were imputed by carrying forward baseline data |
Unclear Co-morbidities were taken into consideration, ITT analysis was conducted |
| 9 | Unclear | Yes | No | Yes All outcomes pre-specified in the methods section were reported |
Yes Loss to follow-up losses was high (21.4% in MBCT and 8.3% in GCBT) and reasons for the losses were provided |
Unclear Co-morbidities were taken into consideration, ITT analysis was conducted |
| 10 | Unclear | Yes | No Researchers who collected Post- baseline data were blinded |
Yes All outcomes pre-specified in the methods section were reported |
Unclear Loss to follow-up was moderate (17.4% in TAU and 10.3% in MBCT) and reasons for losses were partially reported |
No No ITT analysis |
| 11 | Yes | Yes | No Evaluators blinded to treatment allocation |
No Some of the secondary outcomes stated in the methods section are not reported |
No Loss to follow-up was high (25% in AD, 20% in placebo and 19.2% in MBCT), reasons for the losses were provided |
Unclear Co-morbidities were taken into consideration, ITT analysis was conducted |
MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; BDI: Beck depression inventory; ITT: intention to treat; GCBT: group cognitive-behavioural therapy; AD: maintenance antidepressants group.
Effects of the intervention
Meta-analyses were conducted for results including relapse rates, depression (scales were not mixed because same studies reported different scales) and anxiety (mixed scales). Stress and quality of life were reported in more than one study, but the scales for each were too distinct to standardise and combine for a meta-analysis. Other outcomes were reported with insufficient data to conduct meta-analyses. Adverse effects were not assessed in any of the studies.
Relapse rate at 1 year post-intervention for patients with three or more previous episodes of depression
As shown in Figure 2, 430 participants contributed to this outcome. Of participants in the MBCT + TAU group 38% relapsed, compared to 62% in the TAU group. The difference between the two groups was significant in favour of MBCT + TAU (RR [95% CI]: 0.61 [0.48, 0.79]). No statistical heterogeneity was identified (p = 0.22, I2 = 31%). The number needed to treat to avoid a relapse was 4 (95% CI 2.6–9.1).
Figure 2.
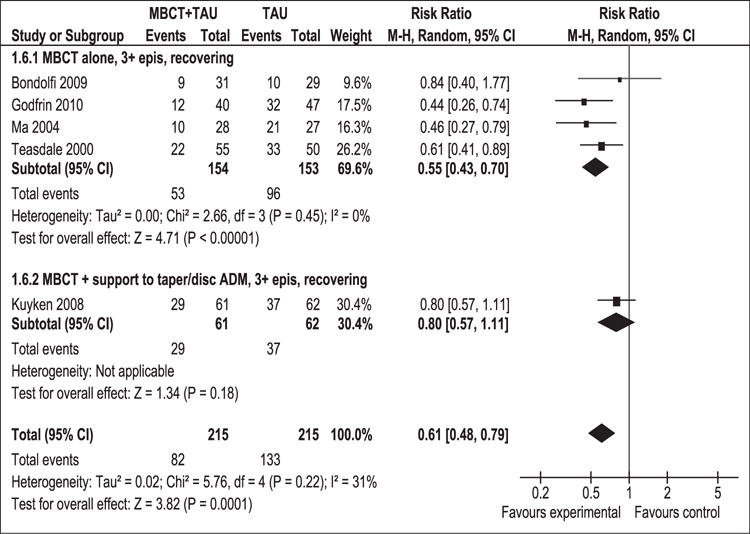
Meta-analysis (risk ratio). Relapse rate at 1 year post-intervention for patients with 3 or more previous episodes of depression.
MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; 95% CI: 95% confidence interval; M-H: Mantel–Haenszel; random: random effects model; epis: episodes.
Subgroup analysis examined patients with MDD (≥3 episodes) in remission receiving MBCT alone or in combination with support (MBCT+) to discontinue or reduce the amount of anti-depressant medications (ADs) taken. The overall result in the MBCT alone subgroup was significant (RR [95% CI]: 0.55 [0.43–0.70]) with absence of heterogeneity (p = 0.45) (I2 = 0%). Results remained unchanged with sensitivity analysis (RR [95% CI]: 0.66 [0.52, 0.85]) (Figure 3).
Figure 3.
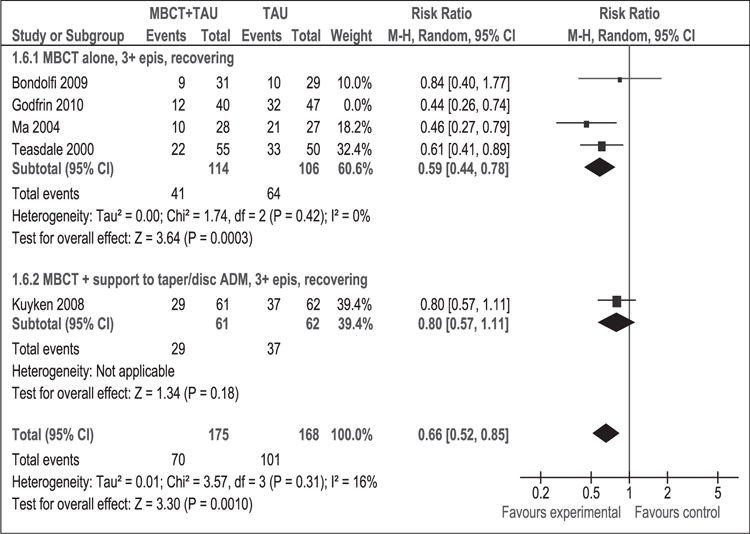
Sensitivity analysis (risk ratio). Relapse rate at 1 year post-intervention for patients with 3 or more previous episodes of depression.
MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; 95% CI: 95% confidence interval; M-H: Mantel–Haenszel; random: random effects model; epis: episodes.
Depression measured with HAM-D at 1 year post-intervention
The Hamilton Rating Scale for Depression (HAM-D) scale ranges from 0 (minimal depression) to 53 (severe depression) (Hamilton, 1967). There is no consensus on the clinically significant difference. However, a difference of 3–3.1 points has been considered valid (National Collaborating Centre for Mental Health, 2004).
As shown in Figure 4, 242 participants contributed to this outcome. The difference in depression mean scores between the MBCT + TAU group and the TAU group was significant in favour of MBCT + TAU (WMD [95% CI]: −2.46 [−4.36 to −0.56]). This result suggests that MBCT + TAU decreased the average degree of depression at 1-year post-intervention compared to TAU. The result of the overall χ2 test for heterogeneity was not significant (p = 0.99, I2 = 0%), indicating that combining these studies was appropriate.
Figure 4.
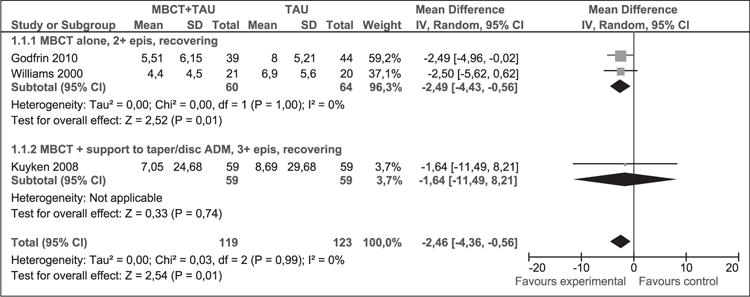
Meta-analysis (mean difference). Depression measured with HAM-D at 1 year post-intervention. MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; SD: standard deviation; 95% CI: 95% confidence interval; IV: inverse variance; epis: episodes; disc: discontinue; HAM-D: Hamilton rating scale for depression; ADM: antidepressant medication.
In sensitivity analysis results did not remain stable (Figure 5). While results continue to favour the MBCT + TAU group, they were no longer significant (WMD [95% CI]: −2.42 [−5.40, 0.55]). There was no statistical heterogeneity (p = 0.87, I2 = 0%).
Figure 5.
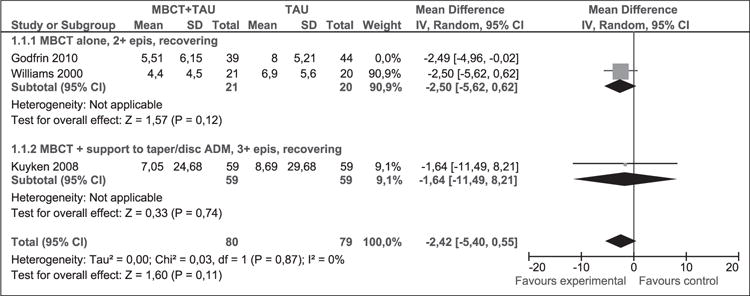
Sensitivity analysis (mean difference). Depression measured with HAM-D at 1 year post-intervention.
MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; SD: standard deviation; 95% CI: 95% confidence interval; IV: inverse variance; epis: episodes; disc: discontinue; HAM-D: Hamilton rating scale for depression; ADM: antidepressant medication.
Depression measured with BDI-II at 1 year post-intervention
The Beck Depression Inventory – second edition (BDI-II) ranges from 0 (minimal depression) to 63 (severe depression) (Beck et al., 1996), and a difference of 5 points is considered clinically relevant (Hiroe et al., 2005).
As shown in Figure 6, 190 participants contributed to this outcome. The difference in depression mean scores between the MBCT + TAU group and the TAU group was significant in favour of MBCT + TAU (WMD [95% CI]: −10.39 [−15.66 to 5.12]). There was no statistical heterogeneity (p = 0.50, I2 = 0%) and findings were clinically significant. Sensitivity analysis could not be conducted due to only two studies contributing to this outcome.
Figure 6.
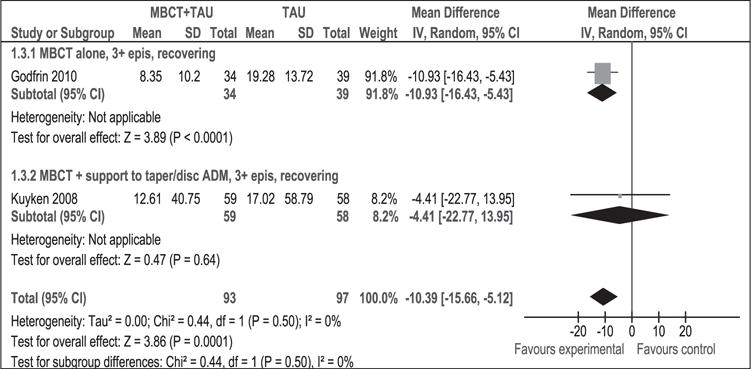
Meta-analysis (mean difference). Depression measured with BDI-II at 1 year post-intervention. MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; SD: standard deviation; 95% CI: 95% confidence interval; IV: inverse variance; epis: episodes; disc: discontinue; BDI: Beck depression inventory; ADM: antidepressant medication.
Depression measured with HAM-D at post-intervention
As shown in Figure 7, 316 participants contributed to this outcome. The difference in depression mean scores between the MBCT + TAU group and the TAU group was significant in favour of MBCT + TAU (WMD [95% CI]: −4.31 [−5.79 to −2.83]). The result of the χ2 test for heterogeneity was not significant (p = 0.79, I2 = 0%). This result suggests that MBCT + TAU decreased the average degree of depression at post-intervention compared to TAU using HAM-D. Results remained stable in sensitivity analysis (WMD [95% CI]: −3.88 [−6.07, −1.69]) (Figure 8).
Figure 7.
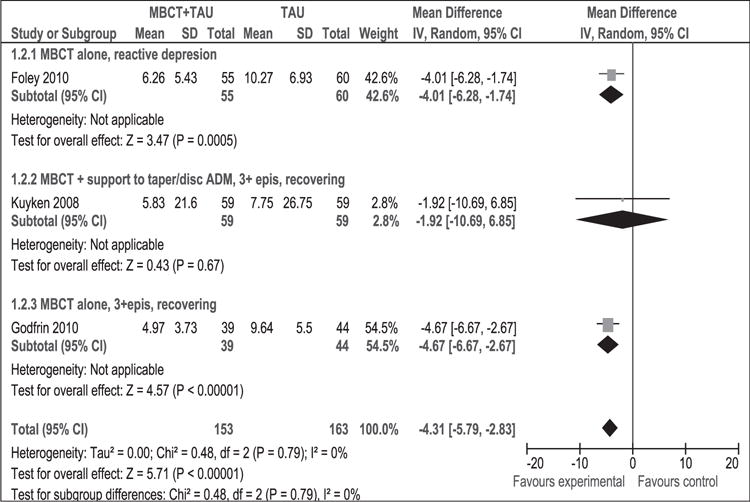
Meta-analysis (mean difference). Depression measured with HAM-D at post-intervention. MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; SD: standard deviation; 95% CI: 95% confidence interval; IV: inverse variance; epis: episodes; disc: discontinue; HAM-D: Hamilton rating scale for depression; ADM: antidepressant medication.
Figure 8.
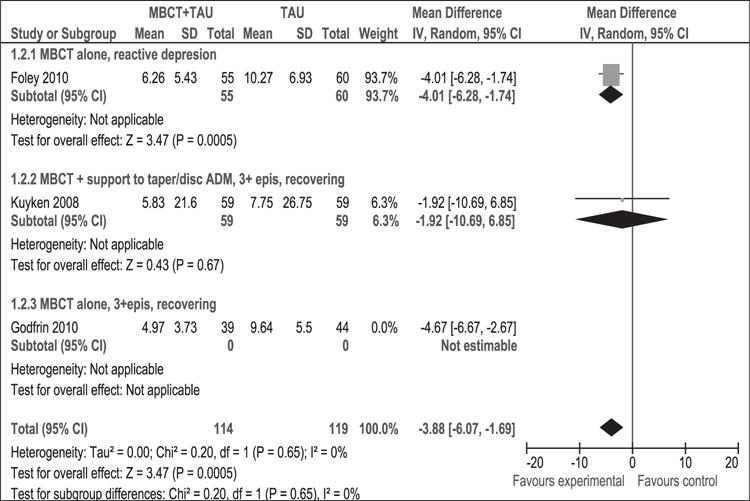
Sensitivity analysis (mean difference). Depression measured with HAM-D at post-intervention. MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; SD: standard deviation; 95% CI: 95% confidence interval; IV: inverse variance; epis: episodes; disc: discontinue; HAM-D: Hamilton rating scale for depression; ADM: antidepressant medication.
Depression measured with BDI-II at post-intervention
As shown in Figure 9, 291 participants contributed to this outcome. The meta-analysis of the BDI-II scale favoured MBCT + TAU intervention: the average degree of depression decreased (WMD [95% CI]: −7.33 [−12.12, −2.54]) compared to TAU. This difference is clinically significant; however, there was statistical heterogeneity (p = 0.002, I2 = 73%), which cautions the appropriateness of combining the studies.
Figure 9.
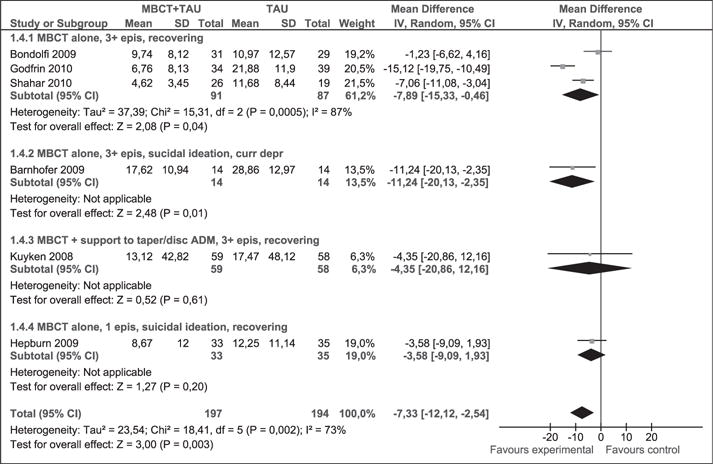
Meta-analysis (mean difference). Depression measured with BDI-II at post-intervention. MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; SD: standard deviation; 95% CI: 95% confidence interval; IV: inverse variance; epis: episodes; disc: discontinue; BDI: Beck depression inventory; ADM: antidepressant medication.
Subgroup analysis examined the effect of MBCT alone or in combination with support to discontinue or reduce the amount of ADs taken by patients with MDD (≥3 episodes) in remission or with current depression and a history of suicidal ideation. The heterogeneity of the overall effect of the meta-analysis may have been influenced by data from the first group. The likely cause of these results can be tracked by comparing the studies. Although patient profiles and interventions were similar between arms in the study conducted by Godfrin and van Heeringen (2010) there was a significant loss of patient follow-up (22.2% in one group and 34.6% in the other), likely contributing to the gap between subgroups. The remainder of the subgroups analysed included only one study in each.
Sensitivity analysis (Figure 10) excluded the study by Godfrin and van Heeringen and statistical heterogeneity disappeared (p = 0.20, I2 = 35%). Results remained stable (WMD [95% CI]: −5.68 [−9.88, −1.49]).
Figure 10.
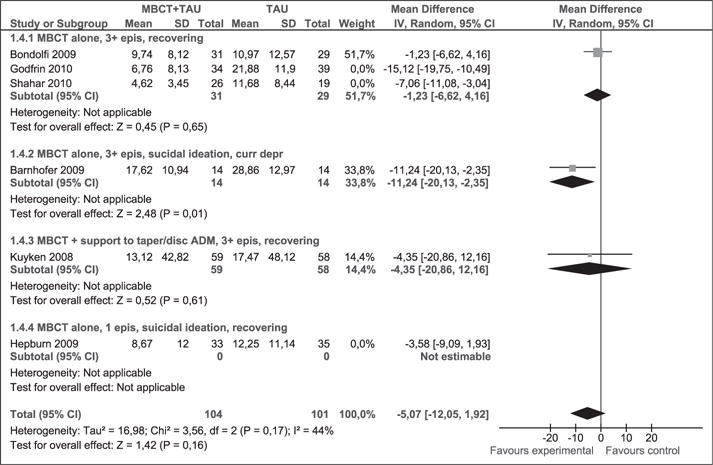
Sensitivity analysis (mean difference). Depression measured with BDI-II at post-intervention. MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; SD: standard deviation; 95% CI: 95% confidence interval; IV: Inverse variance; epis: episodes; disc: discontinue; BDI: Beck depression inventory; ADM: antidepressant medication.
Anxiety at post-intervention
As Figure 11 shows, 149 participants contributed to this outcome, which favoured MBCT + TAU intervention: the average degree of anxiety decreased compared to TAU (SMD [95% CI]: −0.42 [−0.74, −0.09]). The result of the tests for heterogeneity were not significant (p = 0.55, I2 = 0%). Anxiety was measured with different scales in each of the studies (Foley et al., 2010; Williams et al., 2008). Because patients are heterogeneous, differences may be due to subgroup differences rather than to the use of different scales.
Figure 11.
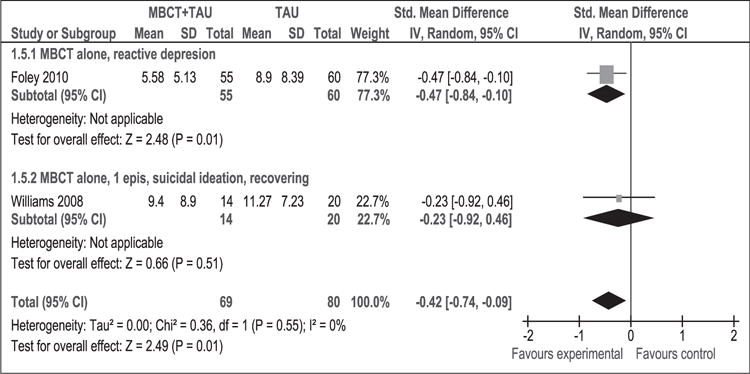
Meta-analysis (standardised mean difference). Anxiety at post-intervention.
MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; SD: standard deviation; 95% CI: 95% confidence interval; IV: inverse variance; epis: episodes.
Evaluation of publication bias
Funnel plots (Figures 12 and 13) do not show major asymmetry, indicating that there is no clear evidence of publication bias. However, due to the low number of studies this bias could not be assessed for all outcomes.
Figure 12.
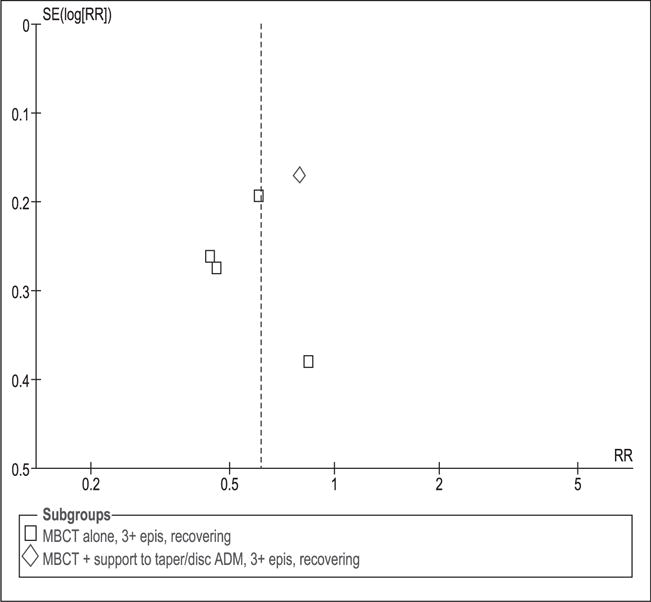
Funnel plot to evaluate publication bias. Relapse rate at 1 year post-intervention for patients with 3 or more previous episodes of depression.
MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; SD: standard deviation; 95% CI: 95% confidence interval; IV: inverse variance; epis: episodes; disc: discontinue; ADM: antidepressant medication; SE: standard error; RR: relative risk.
Figure 13.
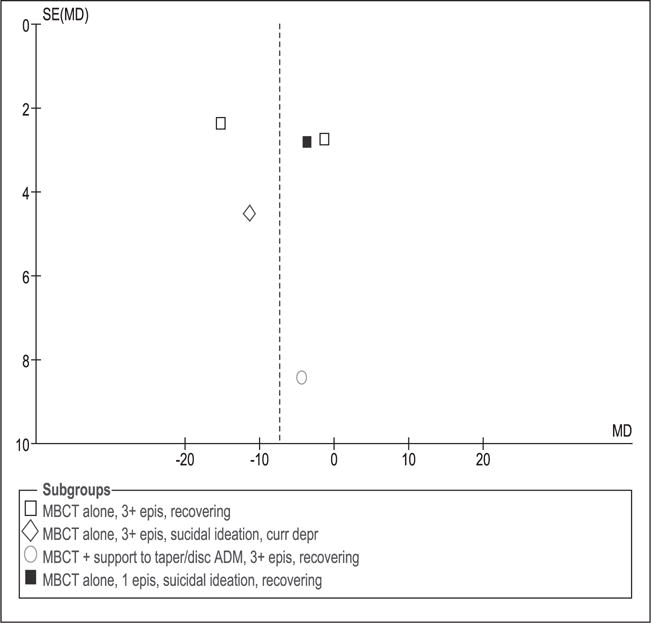
Funnel plot to evaluate publication bias. Depression measured with BDI-II at post-intervention. MBCT: mindfulness-based cognitive therapy; TAU: treatment as usual; SD: standard deviation; 95% CI: 95% confidence interval; IV: inverse variance; epis: episodes; disc: discontinue; ADM: antidepressant medication; SE: standard error; MD: mean difference; curr depr: current depression.
Discussion
The main results indicate that MBCT + TAU are more effective at preventing episodes or prolonging time between episodes of depression than TAU alone. Patients with recurrent depression (≥3 episodes) treated with MBCT + TAU have on average 40% fewer relapses compared to patients undergoing TAU alone. One relapse is avoided for every four patients treated with MBCT in comparison to those receiving TAU. This effect is statistically significant and remained stable under sensitivity analysis. Given that MBCT teaches techniques that should be practiced on a daily basis to maintain its effectiveness over time, long-term studies are particularly important. Rate of relapse was the primary outcome of the majority of the studies identified and included in this systematic review and meta-analysis; therefore sample sizes were calculated for relapse rates giving methodological robustness to our findings. Furthermore, a relapse rate is a more robust and objective measure compared to self-reported measures. Depressive symptoms at 1 year post-intervention measured by HAM-D and BDI-II scales were statistically significant (and clinically significant at least in the case of HAM-D), but did not remain stable. New trials with adequate methodological quality are needed to further evaluate this outcome.
Unsurprisingly, the results of the meta-analysis of individuals who had two previous episodes of depression at one year of follow-up (data not demonstrated) showed no significant differences. The particular difference in the number of previous episodes of depression is supported by the hypothesis of differential activation (Teasdale, 1988). This hypothesis states that with each new relapse in depression the strength of the association between negative mood and dysfunctional patterns of thought and rumination increases in such a way that every time it is less necessary for a stressful stimulus to reproduce the relapse.
In a recent study that could not be meta-analysed because of the unique comparison groups, recurrently (>1 episode) depressed patients in remission were randomised to receive ADs, MBCT plus a discontinuation of ADs, or placebo instead of ADs (Segal et al., 2010). After 18 months of follow-up results showed that among unstable remitters, patients in both MBCT and ADs showed a 73% decrease in hazard of relapse compared with placebo (p = 0.03). In contrast, stable remitters showed no differences. These results suggest that MBCT offers protection against relapse on par with that of maintenance AD pharmacotherapy. This was the most important result with intent-to-treat or available case analysis among the reported studies that could not be meta-analysed.
Other significant single RCT results with intent-to-treat or available case analysis at one year of follow-up comparing MBCT to TAU include a reduction in the number of diagnosed psychiatric co-morbidities (Kuyken et al., 2008), a reduction of depressive symptoms and anger, an increase of strength and an improvement in the quality of life (Foley et al., 2010; Godfrin and van Heeringen, 2010). MBCT was also shown to do as well as group cognitive therapy in decreasing social phobia symptoms (Piet et al., 2010). However, there were no significant differences for the use of ADs (Teasdale et al., 2000), the amount, duration, severity and degree of distress of relapses, quality of life measured by the OMS scale, the total cost of treatment per year during the year of follow-up (Kuyken et al., 2008), and fatigue and tension (Godfrin and van Heeringen, 2010). The remainder of the results included in this systematic review had shorter or no follow-up periods so it is uncertain whether the results are maintained over time.
MBCT has been predominantly implemented for depressive patients. However, as seen in medical, nursing, and other arenas involved in mental healthcare, depression is a symptom that is present in many psychiatric and psychological conditions; therefore, the theoretical foundations of MBCT are relevant to the whole spectrum of mental health pathologies. Moreover, depression is highly prevalent in patients with physical illness and in aging populations. The populations analysed in most studies included in this review suffered from serious and recurrent depression. More RCTs to evaluate the intervention in populations with less severity are needed.
Comparing results with other reviews
Two systematic reviews on MBCT RCT’s without meta-analyses were published before (Coelho et al., 2007; Fjorback et al., 2011). Findings in the current study agree with Coelho et al. (2007) and Fjorback et al. (2011) in highlighting that because of the nature of the control groups results cannot be attributed to specific effects of MBCT. More clinical studies with long-term follow-up are needed to better understand and confirm specific effects of MBCT. Problems which can surface when traditional statistical analyses are applied to interventions in which groups are used were also pointed out previously (Williams et al., 2008). Groups of patients are able to influence each other’s outcomes and thus variables are no longer necessarily independent.
A systematic review and meta-analysis on MBCT was recently published (Chiesa and Serretti, 2011) in which non-randomised trials were included and more conservative analyses were conducted by presenting diagnostic subgroup analyses only. Nonetheless, in spite of the differences between Chiesa’s work and the current review, the main conclusions are similar.
Piet and Hougaard (2011) published another systematic review on MBCT that included patients with MDD only. Their findings on relapse prevention were similar to those of this review, but they added a meta-analysis including MDD patients with any number of episodes and the results were significant (RR = 0.66 for MBCT compared to treatment as usual or placebo controls). Other differences with this review are that Piet and Hougaard were less conservative when including Kuyken et al.’s study in a meta-analysis comparing MBCT against ADs (yet getting not significant results), that they did not have a previous formal protocol, that they did not use the Cochrane tool to assess the methodological quality of the studies, and that they did not explore drop-out rates in sensitivity analyses. Piet and Hougaard (2011) made a final remark we found interesting: that it may be premature to exclude patients with 2 MDD relapses from future studies since not enough data have been collected.
A meta-analytic review was published (Hofmann et al., 2010) on the effect of mindfulness-based therapies on depression and anxiety, obtaining moderate effect sizes. However, as this analysis is pre-post and uncontrolled, the validity of the results is much lower than that of meta-analyses of RCTs, such as those presented in this review.
Limitations
Important limitations of the current review are the low number of studies in the meta-analyses and the fact that only dichotomous variables were used to measure relapse rates. In addition, mental health problems are chronic or long-term conditions but outcomes were not reported to assess long-term effects beyond the first year of follow-up.
Although participants in all the reported studies were depressed or had been depressed in the past, the heterogeneity among studies was high. To counter this limitation subgroup analyses were conducted. Although this study was performed as per the version of the Cochrane Handbook available at the time, a new version is now in place (The Cochrane Collaboration, 2011). The most relevant update concerning this review is that the risk of bias table was slightly expanded. Finally, the search strategy used to support this review was thorough. However, grey literature data could have been further assessed through contacting key informants.
Despite these limitations it is concluded that MBCT is an effective tool at least for patients with three or more previous episodes of major depression.
Implications for practice.
With increasing use of MBCT across a range of practitioners, the clinical relevance of MBCT can be considered. Findings from this systematic meta-analysis can be used to inform nurses and other mental health practitioners on the efficacy, patient population and type of mental illnesses which best respond to MBCT technique based on findings from randomised control trials.
Key points for policy, practice and research.
Patients with recurrent depression (three episodes or more) treated with additive MBCT have on average 40% fewer relapses at one year of follow-up compared to patients undergoing treatment as usual.
Improvements in depression and anxiety with additive MBCT were significant at one year of follow-up but unstable in sensitivity analyses.
More studies with active control groups and long-term follow-ups are needed to better understand the specific effects of MBCT.
Depression is a symptom that is present in many conditions. More high quality RCTs are needed to evaluate MBCT in populations with varying depression severity as well as diagnosis with multiple co-morbidities.
Acknowledgments
We would like to thank Daniel Comandé for assisting with the search strategy and obtaining the studies, to Lucila Rey Ares for her help with article selection, to Sabrina Grosman for her help with Spanish to English language translation, to Agustin Ciapponi for his methodological support, and to John Gallacher and Marie-Jet Bekkers without whom this work could not be completed.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Biographies
Patricia F. Pearce (PhD, FNP-BC, FAANP) has over 30 years of experience as a nurse, nurse practitioner, nurse researcher, and educator. She is experienced in qualitative, quantitative, and mixed methods research, and her specific interest area is in making technology work effectively and efficiently, in research and clinical arenas. Her research has been funded through intramural awards, as well as awards from the American Nurses Foundation and NIH. She has presented locally, regionally, nationally and internationally and published on a wide array of topics. Dr Pearce holds a PhD in Nursing with minor in Informatics (University of North Carolina Chapel Hill), a Master’s in Public Health (Tulane University School of Public Health), and Master’s in Nursing (Mississippi University for Women). She is currently Associate Professor, Loyola University, New Orleans, LA.
Sarah J. Iribarren (RN) is a Nursing PhD Candidate at the University of Utah, College of Nursing (anticipated completion Spring 2013) and has completed a certificate in Global Health from the Department of Family and Preventative Medicine. She received a B.S. degree in Biology and Spanish and B.S. in Nursing from Washington State University. Her current PhD programme was developed to encompass training from multiple disciplines, including: nursing, public/international health, anthropology, epidemiology, statistics and informatics. She received an NIH-NRSA grant for her dissertation research which was conducted at a public pulmonary reference hospital in the Province of Buenos Aires, Argentina and recently completed an NIH/Fogarty International Clinical Research Scholar Program in Buenos Aires.
Julieta Galante (MD, MSc) is a physician and PhD candidate at Cardiff University School of Medicine. She completed her medical studies and MSc in clinical effectiveness at the University of Buenos Aires, Argentina. She has been working in research in the UK and Argentina for the last 10 years in the areas of epidemiology, health economics and cognitive science. Her research interests include the processes and effects of mind-body techniques, prevention and promotion in mental health, and cutting edge methodologies in clinical research.
Footnotes
Conflict of interest statement
None declared.
Contributor Information
Julieta Galante, PhD candidate, Institute of Primary Care and Public Health, Cardiff University, UK.
Sarah J. Iribarren, Nursing PhD candidate, College of Nursing, University of Utah, USA
Patricia F. Pearce, Associate Professor, School of Nursing, Loyola University, New Orleans, USA
References
- American Psychiatric Association. DSM. 2012 Available at: http://www.psych.org/practice/dsm (accessed 21 August 2012)
- Barnhofer T, Crane C, Hargus E, et al. Mindfulness-based cognitive therapy as a treatment for chronic depression: a preliminary study. Behaviour Research and Therapy. 2009;47(5):366–373. doi: 10.1016/j.brat.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhofer T, Duggan D, Crane C, et al. Effects of meditation on frontal alpha-asymmetry in previously suicidal individuals. Neuroreport. 2007;18(7):709–712. doi: 10.1097/WNR.0b013e3280d943cd. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, et al. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. Journal of Personlality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bondolfi G, Jermann F, der Linden MV, et al. Depression relapse prophylaxis with mindfulness-based cognitive therapy: Replication and extension in the Swiss health care system. Journal of Affective Disorders. 2010;122(3):224–231. doi: 10.1016/j.jad.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton WB, Bootzin RR, Cousins JC, et al. The contribution of mindfulness practice to a multicomponent behavioral sleep intervention following substance abuse treatment in adolescents: A treatment-development study. Substance Abuse. 2010;31(2):86–97. doi: 10.1080/08897071003641297. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Mindfulness based cognitive therapy for psychiatric disorders: A systematic review and meta-analysis. Psychiatry Research. 2011;187(3):441–453. doi: 10.1016/j.psychres.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Coelho HF, Canter PH, Ernst E. Mindfulness-based cognitive therapy: Evaluating current evidence and informing future research. Journal of Consulting and Clinical Psychology. 2007;75(6):1000–1005. doi: 10.1037/0022-006X.75.6.1000. [DOI] [PubMed] [Google Scholar]
- Crane C, Barnhofer T, Duggan D, et al. Mindfulness-based cognitive therapy and self-discrepancy in recovered depressed patients with a history of depression and suicidality. Cognitive Therapy and Research. 2008;32(6):775–787. [Google Scholar]
- Fearson S, Chadwick PM. Is mindfulness-based cognitive therapy helpful for people with diabetes?. The Fifth World Congress of Behavioural and Cognitive Therapies; 11–14 July; Barcelona, Spain. 2007. [Google Scholar]
- Fjorback LO, Arendt M, Ornbol E, et al. Mindfulness-based stress reduction and mindfulness-based cognitive therapy: A systematic review of randomized controlled trials. Acta Psychiatrica Scandinavica. 2011;124(2):102–119. doi: 10.1111/j.1600-0447.2011.01704.x. [DOI] [PubMed] [Google Scholar]
- Foley E, Baillie A, Huxter M, et al. Mindfulness-based cognitive therapy for individuals whose lives have been affected by cancer: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2010;78(1):72–79. doi: 10.1037/a0017566. [DOI] [PubMed] [Google Scholar]
- Galante J. Thesis protocol: Revisión Sistemática y metanalisis: terapia cognitiva basada en la atención plena para transtornos mentales (Spanish) University of Buenos Aires; Argentina: 2009. Available at: http://www.iecs.org.ar/iecs-visor-publicacion.php?cod_publicacion=1076&origen_publicacion=publicaciones. [Google Scholar]
- Godfrin KA, van Heeringen C. The effects of mindfulness-based cognitive therapy on recurrence of depressive episodes, mental health and quality of life: A randomized controlled study. Behaviour Research and Therapy. 2010;48(8):738–746. doi: 10.1016/j.brat.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hargus E, Crane C, Barnhofer T, et al. Effects of mindfulness on meta-awareness and specificity of describing prodromal symptoms in suicidal depression. Emotion. 2010;10(1):34–42. doi: 10.1037/a0016825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn SR, Crane C, Barnhofer T, et al. Mindfulness-based cognitive therapy may reduce thought suppression in previously suicidal participants: Findings from a preliminary study. British Journal of Clinical Psychology. 2009;48(2):209–215. doi: 10.1348/014466509X414970. [DOI] [PubMed] [Google Scholar]
- Hiroe T, Kojima M, Yamamoto I, et al. Gradations of clinical severity and sensitivity to change assessed with the Beck Depression Inventory-II in Japanese patients with depression. Psychiatry Research. 2005;135(3):229–235. doi: 10.1016/j.psychres.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, et al. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman MA, Welch A, Kitchen K, et al. Mindfulness-based cognitive therapy and life-role functioning in comorbid mood, anxiety, and substance-related disorders: A pilot study. The 156th Annual Meeting of the American Psychiatric Association; San Francisco, CA. 17–22 May 2003.2003. [Google Scholar]
- Kendrick T, Peveler R. Guidelines for the management of depression: NICE work? British Journal of Psychiatry. 2010;197(5):345–347. doi: 10.1192/bjp.bp.109.074575. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76(6):966–978. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: Replication and exploration of differential relapse prevention effects. Journal of Consulting and Clinical Psychology. 2004;72(1):31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. Lancet. 1999;354(9193):1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health. Depression: Management of Depression in Primary and Secondary Care (Clinical Guideline 23) London, UK: National Institute for Clinical Excellence; 2004. [Google Scholar]
- O’Haver Day P, Horton-Deutsch S. Using mindfulness-based therapeutic interventions in psychiatric nursing practice-part I: Description and empirical support for mindfulness-based interventions. Archives of Psychiatric Nursing. 2004;18(5):164–169. doi: 10.1016/j.apnu.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: A systematic review and meta-analysis. Clinical Psychology Review. 2011;31(6):1032–1040. doi: 10.1016/j.cpr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Piet J, Hougaard E, Hecksher MS, et al. A randomized pilot study of mindfulness-based cognitive therapy and group cognitive-behavioral therapy for young adults with social phobia. Scandinavian Journal of Psychology. 2010;51(5):403–410. doi: 10.1111/j.1467-9450.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Archives of General Psychiatry. 2010;67(12):1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York, NY: Guilford Press; 2002. [Google Scholar]
- Shahar B, Britton WB, Sbarra DA, et al. Mechanisms of change in mindfulness-based cognitive therapy for depression: Preliminary evidence from a randomized controlled trial. International Journal of Cognitive Therapy. 2010;3(4):402–418. [Google Scholar]
- Teasdale JD. Cognitive vulnerability to persistent depression. Cognition & Emotion. 1988;2(3):247–274. [Google Scholar]
- Teasdale JD, Moore RG, Hayhurst H, et al. Metacognitive awareness and prevention of relapse in depression: Empirical evidence. Journal of Consulting and Clinical Psychology. 2002;70(2):275–287. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JMG, et al. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. 2008 Available at: www.cochrane-handbook.org (accessed 1 February 2008)
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. 2011 Available at: www.cochrane-handbook.org (accessed 20 August 2012)
- Welch A. Mindfulness-based cognitive therapy and life-role functioning in co-morbid mood, anxiety and substance-related disorders: A pilot study. The 25TH Annual Conference of the Anxiety Disorders Association of America; Seattle, WA. 17–20 March.2005. [Google Scholar]
- Williams JMG, Russell I, Russell D. Mindfulness-based cognitive therapy: Further issues in current evidence and future research. Journal of Consulting and Clinical Psychology. 2008;76(3):524–529. doi: 10.1037/0022-006X.76.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG, Segal ZV, Teasdale JD, et al. Mindfulness-based cognitive therapy reduces overgeneral autobiographical memory in formerly depressed patients. Journal of Abnormal Psychology. 2000;109(1):150–155. doi: 10.1037//0021-843x.109.1.150. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The world health report 2004: Changing history. 2004 Available at: http://www.who.int/whr/2004/en/ (accessed 21 August 2004)
- World Health Organization. International Classification of Diseases (ICD) 2012 Available at: http://www.who.int/classifications/icd/en/ (accessed 21 August 2012)


