Abstract
Background
Central serous chorioretinopathy (CSC) is characterized by serous detachment of the neural retina with dysfunction of the choroid and retinal pigment epithelium (RPE). The effects on the retina are usually self limited, although some people are left with irreversible vision loss due to progressive and permanent photoreceptor damage or RPE atrophy. There have been a variety of interventions used in CSC, including, but not limited to, laser treatment, photodynamic therapy (PDT), and intravitreal injection of anti‐vascular endothelial growth factor (anti‐VEGF) agents. However, it is not known whether these or other treatments offer significant advantages over observation or other interventions. At present there is no evidence‐based consensus on the management of CSC. Due in large part to the propensity for CSC to resolve spontaneously or to follow a waxing and waning course, the most common initial approach to treatment is observation. It remains unclear whether this is the best approach with regard to safety and efficacy.
Objectives
To compare the relative effectiveness of interventions for central serous chorioretinopathy.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2015, Issue 9), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to February 2014), EMBASE (January 1980 to October 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 5 October 2015.
Selection criteria
Randomized controlled trials (RCTs) that compared any intervention for CSC with any other intervention for CSC or control.
Data collection and analysis
Two review authors independently selected studies and extracted data. We pooled data from all studies using a fixed‐effect model. For interventions applied to the eye (i.e. not systemic interventions), we synthesized direct and indirect evidence in a network meta‐analysis model.
Main results
We included 25 studies with 1098 participants (1098 eyes) and follow‐up from 16 weeks to 12 years. Studies were conducted in Europe, North and South America, Middle East, and Asia. The trials were small (most trials enrolled fewer than 50 participants) and poorly reported; often it was unclear whether key aspects of the trial, such as allocation concealment, had been done. A substantial proportion of the trials were not masked.
The studies considered a variety of treatments: anti‐VEGF (ranibizumab, bevacizumab), PDT (full‐dose, half‐dose, 30%, low‐fluence), laser treatment (argon, krypton and micropulse laser), beta‐blockers, carbonic anhydrase inhibitors, Helicobactor pylori treatment, and nutritional supplements (Icaps, lutein); there were only one or two trials contributing data for each comparison. We downgraded for risk of bias and imprecision for most analyses, reflecting study limitations and imprecise estimates. Network meta‐analysis (as planned in our protocol) did not help to resolve this uncertainty due to a lack of trials, and problems with intransitivity, particularly with respect to acute or chronic CSC.
Low quality evidence from two trials suggested little difference in the effect of anti‐VEGF (ranibizumab or bevacizumab) or observation on change in visual acuity at six months in acute CSC (mean difference (MD) 0.01 LogMAR (logarithm of the minimal angle of resolution), 95% confidence interval (CI) ‐0.02 to 0.03; 64 participants). CSC had resolved in all participants by six months. There were no significant adverse effects noted.
Low quality evidence from one study (58 participants) suggested that half‐dose PDT treatment of acute CSC probably results in a small improvement in vision (MD ‐0.10 logMAR, 95% CI ‐0.18 to ‐0.02), less recurrence (risk ratio (RR) 0.10, 95% CI 0.01 to 0.81) and less persistent CSC (RR 0.12, 95% CI 0.01 to 1.02) at 12 months compared to sham treatment. There were no significant adverse events noted.
Low quality evidence from two trials (56 participants) comparing anti‐VEGF to low‐fluence PDT in chronic CSC found little evidence for any difference in visual acuity at 12 months (MD 0.03 logMAR, 95% CI ‐0.08 to 0.15). There was some evidence that more people in the anti‐VEGF group had recurrent CSC compared to people treated with PDT but, due to inconsistency between trials, it was difficult to estimate an effect. More people in the anti‐VEGF group had persistent CSC at 12 months (RR 6.19, 95% CI 1.61 to 23.81; 34 participants).
Two small trials of micropulse laser, one in people with acute CSC and one in people with chronic CSC, provided low quality evidence that laser treatment may lead to better visual acuity (MD ‐0.20 logMAR, 95% CI ‐0.30 to ‐0.11; 45 participants). There were no significant adverse effects noted.
Other comparisons were largely inconclusive.
We identified 12 ongoing trials covering the following interventions: aflibercept and eplerenone in acute CSC; spironolactone, eplerenone, lutein, PDT, and micropulse laser in chronic CSC; and micropulse laser and oral mifepristone in two trials where type of CSC not clearly specified.
Authors' conclusions
CSC remains an enigmatic condition in large part due to a natural history of spontaneous improvement in a high proportion of people and also because no single treatment has provided overwhelming evidence of efficacy in published RCTs. While a number of interventions have been proposed as potentially efficacious, the quality of study design, execution of the study and the relatively small number of participants enrolled and followed to revealing endpoints limits the utility of existing data. It is not clear whether there is a clinically important benefit to treating acute CSC which often resolves spontaneously as part of its natural history. RCTs comparing individual treatments to the natural history would be valuable in identifying potential treatment groups for head‐to‐head comparison. Of the interventions studied to date, PDT or micropulse laser treatment appear the most promising for study in future trials.
Plain language summary
Interventions for central serous chorioretinopathy
Review question What is the effect of treatments for central serous chorioretinopathy (CSC)? Is any treatment better than any other treatment?
Background CSC is a disorder of the back of the eye. The 'retina' (which captures light and turns it into electric impulses to be sent to the brain) becomes detached. CSC typically affects young and middle‐aged adults, particularly men. It can lead to problems with vision. Most people who develop CSC recover on their own but some people continue to have problems and can lose vision permanently. A variety of treatments have been proposed for CSC including laser treatment and injections of biological agents to reduce the amount of fluid in the back of the eye.
Study characteristics The evidence is current to 5 October 2015. A total of 1098 participants were enrolled from Brazil, China, Germany, India, Iran, Italy, Japan, Mexico, South Korea, Thailand, Turkey, the UK and the US. All enrolled participants were similar with respect to age and most were men. The participants had varying severity of the disease; some displayed symptoms for less than 20 days up to six months. Most studies did not report their source of funding, four studies were industry funded, and six studies were non‐industry funded.
Key results The studies considered a wide range of treatments. As a result, there were not enough studies of any one treatment to provide good evidence of treatment effects. In general, no significant side effects were noted.
Quality of the evidence The overall quality of the presently available published evidence was either low or very low. This finding indicates that future published research is very likely to have an important impact on the conclusions currently provided in this review.
Summary of findings
Summary of findings for the main comparison. Interventions for central serous chorioretinopathy: direct comparisons.
| Interventions for central serous chorioretinopathy: direct comparisons | ||||||
|
Patient or population: people with central serous chorioretinopathy Settings: eye hospital | ||||||
|
Comparison (intervention vs. comparator) |
Anticipated absolute effects (95% CI) | Effect estimate from direct comparison | Comments | |||
| Relative effect (95% CI) | No of participants (studies) | Quality | ||||
| Risk with comparator | Mean difference (95% CI) Negative values are in favor of intervention; positive values in favor of comparator | |||||
| Change in visual acuity at 12 months (logMAR) | ||||||
| Anti‐VEGF vs. observation | ‐ | 0.01 LogMAR (‐0.02 to 0.03) | ‐ | 64 (2) | Low1,2 | Both studies enrolled participants with acute CSC and reported mean change in visual acuity at 6 months |
| Anti‐VEGF vs. low‐fluence PDT | ‐ | 0.03 logMAR (‐0.08 to 0.15) | ‐ | 56 (2) | Low1,2 | Both studies enrolled participants with chronic CSC |
| Anti‐VEGF and 50% PDT vs. 50% PDT | ‐ | 0.30 logMAR (0.09 to 0.51) | ‐ | 15 (1) | Low1,2 | Participants had chronic CSC |
| 6‐dose anti‐VEGF vs. 4‐dose anti‐VEGF | ‐ | ‐0.02 logMAR (‐0.31 to 0.27) | ‐ | 12 (1) | Low1,2 | Participants had chronic CSC and were followed to 6 months |
| 50% PDT vs. observation or sham treatment | ‐ | ‐0.10 logMAR (‐0.18 to ‐0.02) | ‐ | 58 (1) | Low1,2 | Participants had acute CSC |
| 30% PDT vs. PDT | ‐ | ‐0.16 logMAR (‐0.22 to ‐0.10) | ‐ | 60 (1) | Low1,2 | Type of CSC not specified |
| 30% PDT vs. 50% PDT | ‐ | ‐0.12 logMAR (‐0.15 to ‐0.08) | ‐ | 60 (1) | Low1,2 | Type of CSC not specified |
| 50% PDT vs. PDT | ‐ | 0.04 logMAR (‐0.04 to 0.12 | ‐ | 60 (1) | Low1,2 | Type of CSC not specified |
| Selective retina therapy vs. observation | ‐ | ‐0.13 logMAR (‐0.24 to ‐0.01) | ‐ | 30 (1) | Low1,2 | Participants had acute CSC, followed up to 3 months |
| Micropulse diode laser vs. sham laser | ‐ | ‐0.38 logMAR (‐0.56 to ‐0.20) | ‐ | 15 (1) | Low1,2 | Participants had chronic CSC |
| Antioxidant vs. placebo | ‐ | 0.01 logMAR (‐0.04 to 0.06) | ‐ | 14 (1) | Low1,2 | Lutein and acute CSC |
| Propranolol vs. placebo | ‐ | 0.01 logMAR (‐0.07 to 0.09) | ‐ | 60 (1) | Low1,2 | Type of CSC not specified |
| Carbonic anhydrase inhibitors vs. placebo | See comment | ‐ | ‐ | 13 (1) | ‐ | Outcome not reported |
| Helicobacter pylori treatment vs. placebo | ‐ | ‐0.04 logMAR (‐0.07 to ‐0.02) | ‐ | 103 (2) | Low1,2 | Participants had acute CSC, follow‐up 12‐16 weeks |
|
Comparison (intervention vs. comparator) |
Anticipated absolute effects (95% CI) | Effect estimate from direct comparison | Comments | |||
| Risk with comparator* | Risk with intervention | Relative effect (95% CI) | No of participants (studies) | Quality | ||
| Persistent CSC at 12 months | ||||||
| Anti‐VEGF vs. observation | See comment | ‐ | ‐ | 64 (2) | ‐ | Participants had acute CSC. Both trials reported that all participants in treatment and control groups were resolved by 6 months |
| Anti‐VEGF vs. low‐fluence PDT | 111 per 1000 | 688 per 1000 (179 to 1000) | RR 6.19 (1.61 to 23.81) | 34 (1) | Low1,2 | Participants had chronic CSC |
| Anti‐VEGF and 50% PDT vs. 50% PDT | 143 per 1000 | 126 (10 to 1000) | RR 0.88 (0.07 to 11.54) | 15 (1) | Very low1,2,3 | Participants had chronic CSC |
| 6‐dose anti‐VEGF vs. 4‐dose anti‐VEGF | See comment | ‐ | ‐ | 12 (1) | ‐ | Outcome not reported |
| 50% PDT vs. sham treatment | 211 per 1000 | 25 per 1000 (2 to 215) | RR 0.12 (0.01 to 1.02) | 58 (1) | Low1,2 | Participants had acute CSC |
| 30% PDT vs. PDT | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| 30% PDT vs. 50% PDT | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| 50% PDT vs. PDT | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| Selective retina therapy vs. observation | See comment | ‐ | ‐ | 30 (1) | ‐ | Outcome not reported |
| Micropulse diode laser | See comment | ‐ | ‐ | 15 (1) | ‐ | Outcome not reported |
| Antioxidant vs. placebo | See comment | ‐ | ‐ | 51 (1) | ‐ | People in the antioxidant group were less likely to have "complete resolution" at 3 months (RR 0.35, 95% CI 0.13 to 0.95; 51 participants) |
| Propranolol vs. placebo | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| Brinzolamide vs. placebo | 167 per 1000 | 48 (2 to 1000) | RR 0.29 (0.01 to 6.07) | 13 (1) | Very low2,3 | Participants had acute CSC |
| Helicobacter pylori treatment vs. placebo | 314 per 1000 | 210 (113 to 383) | RR 0.67 (0.36 to 1.22) | 103 (2) | Low1,2 | Participants had acute CSC |
|
Comparison (intervention vs. comparator) |
Anticipated absolute effects (95% CI) | Effect estimate from direct comparison | ||||
| Risk with comparator* | Risk with intervention | Relative effect (95% CI) | No of participants (studies) | Quality | Comment | |
| Recurrent CSC at 12 months | ||||||
| Anti‐VEGF vs. observation | See comment | ‐ | ‐ | 64 (2) | ‐ | Outcome not reported |
| Anti‐VEGF vs. low‐fluence PDT | See comment | ‐ | ‐ | 56 (2) | Very low1,2,4 | Participants had chronic CSC. The 2 studies had different results for this outcome (I2 = 71%). In Bae 2011, there was a much higher risk of recurrence in the anti‐VEGF group (ranibizumab) compared with the PDT group (RR 19.83, 95% CI 1.19 to 330.50; 21 eyes); in Semeraro 2012, there was also an increased risk of recurrence in the anti‐VEGF (bevacizumab) group but the size of the effect was much smaller and the CIs include 1 (no effect) (RR 1.46, 95% CI 0.59 to 3.58; 22 eyes) |
| Anti‐VEGF and 50% PDT vs. 50% PDT | See comment | ‐ | ‐ | 15 (1) | ‐ | Outcome not reported |
| 6‐dose anti‐VEGF vs. 4‐dose anti‐VEGF | See comment | ‐ | ‐ | 12 (1) | ‐ | Outcome not reported |
| 50% PDT vs. sham treatment | 267 per 1000 | 27 per 1000 (3 to 216) | RR 0.10 (0.01 to 0.81) | 53 (1) | Low1,2 | Participants had acute CSC |
| 30% PDT vs. PDT | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| 30% PDT vs. 50% PDT | See comment | ‐ | ‐ | 60 (1) | ‐ | Outcome not reported |
| 50% PDT vs. PDT | 270 per 1000 | 338 per 1000 (154 to 737) | RR 1.25 (0.57 to 2.73) | 60 (1) | ‐ | Type of CSC not specified |
| Selective retina therapy vs. observation | See comment | ‐ | ‐ | 30 (1) | ‐ | Outcome not reported |
| Micropulse diode laser | See comment | ‐ | ‐ | 15 (1) | ‐ | Outcome not reported |
| Antioxidant vs. placebo | 143 per 1000 | 46 (4 to 456) | RR 0.32 (0.03 to 3.19) | 36 (1) | Very low2,3 | Participants had acute CSC |
| Propranolol vs. placebo | 167 per 1000 | 100 (27 to 382) | RR 0.60 (0.16 to 2.29) | 60 (1) | Low1,2 | Type of CSC not reported |
| Brinzolamide vs. placebo | 314 per 1000 | 140 (20 to 953) | RR 0.21 (0.03 to 1.43 | 13 (1) | Low1,2 | Participants had acute CSC |
| Helicobacter pylori treatment vs. placebo | See comment | ‐ | ‐ | 103 (2) | ‐ | Outcome not reported |
| Adverse effects | ||||||
| All studies reported no ocular or systematic adverse effects, or did not comment on adverse effects | ||||||
anti‐VEGF: anti‐vascular endothelial growth factor; CI: confidence interval; CSC: central serous chorioretinopathy; logMAR: logarithm of the minimal angle of resolution; PDT: photodynamic therapy; RR: risk ratio.
* Risk was estimated from the comparator group in the included studies
1 Downgraded for imprecision (‐1)
2 Downgraded for risk of bias (‐1)
3 Downgraded for imprecision (‐2)
4 Downgraded for inconsistency (‐1)
Background
Description of the condition
Central serous chorioretinopathy (CSC) has been known by many names including capillarospastic central retinitis, central angiospastic retinopathy and central serous retinopathy (CSR). The hallmark of CSC is the accumulation of subretinal fluid between the neurosensory retina and the retinal pigment epithelium (RPE). The incidence of CSC is greater in men than women; a survey conducted in Minnesota (US) reported that the annual incidence of CSC was higher among men (9.9 per 100,000; 95% confidence interval (CI) 7.4 to 12.4) compared with women (1.7 per 100,000; 95% CI 0.7 to 2.7) (Kitzmann 2008). The odds of CSC is higher among people taking corticosteroids than people not taking corticosteroids (odds ratio (OR) 37.1, 95% CI 6.2 to 221.8) (Haimovici 2004). Type A personality, body type and age are also potential factors correlated with CSC (Nicholson 2013; Yannuzzi 1987). The precise etiology of CSC remains unknown, but the pathogenesis appears to involve dysfunction of the choroid (the major blood vessel network serving the outer portion of the retina) and RPE (Prunte 1996).
The location and amount of subretinal fluid determines what symptoms are experienced. CSC is commonly associated with fluid accumulation under the macula and detachment of the retina. When the detachment occurs in the central macula, symptoms may include reduction of best‐corrected visual acuity (BCVA), distortion of vision, changes in image size, altered color vision, a decrease in contrast sensitivity, the perception of blind spots, or a combination of these symptoms (Cassin 2006; Gass 1967; Wang 2008). Symptoms typically present acutely in one eye without pain. There may be no symptoms when the fluid is located outside the macula. Cases of bilateral involvement are not uncommon, though symptoms may be present in one eye only. People seeking treatment generally have reduced vision, distorted vision, or both. The physician's goal is to improve the visual acuity and other visual symptoms and prevent permanent vision loss related to RPE and outer retinal atrophy by eliminating the fluid between the neurosensory retina and RPE.
CSC is classified as acute or chronic depending on multiple considerations. Various clinical investigators have used different cut‐off time points (e.g. persistent fluid for less than six months or longer than six months) to define acute versus chronic CSC (Nicholson 2013). Others have approached the classification differently: for example acute CSC is defined as the first attempted treatment to improve visual acuity and chronic CSC is defined as being refractory to treatment (Chan 2008; Quin 2013).
The diagnosis of CSC is made by dilated fundus exam combined with imaging of the retina and choroid with optical coherence tomography (OCT), fluorescein angiography (FA), indocyanine green angiography (ICGA), or combinations of these imaging techniques (Nicholson 2013; Quin 2013; Wang 2008). OCT is an imaging technique that allows for the identification of and quantification of subretinal fluid as well as estimation of the thickness of the choroid, which may be abnormally thickened in the setting of CSC. In some cases, OCT may reveal pathologic changes of CSC that are subtle on fundoscopy, such as shallow subretinal fluid, small pigment epithelial detachments, and retinal atrophy that can occur with chronic disease (Montero 2005). FA and ICGA are imaging techniques that allow for the identification of abnormal leakage of fluid from the choroidal and retinal vessels as well as through the RPE layer. Serial exams and strategic choices among these imaging techniques are used to follow the progress of disease and response to treatment.
Many diseases of the choroid, RPE, and retina can produce serous detachment of the neurosensory retina. Considerations for differential diagnosis in CSC include disorders that involve central vision loss associated with central neurosensory retinal detachment. These include choroidal neovascularization (CNV), pattern dystrophy, optic disc pits, polypoidal choroidal vasculopathy, choroidal melanoma, and choroidal metastasis (Yanoff 2013). Choroidal hemangioma, uveitis, Harada disease, optic neuritis, papilledema, vitreous traction, macular holes, and systemic hypertension can also produce neural retinal detachments (Gass 1967). While in its earliest stages retinal detachment due to small tears or holes may present as subretinal fluid, the peripheral location, subsequent course, and discovery of retinal defect usually removes it from the differential diagnosis. Pigment epithelial detachments most often in the setting of macular degeneration are sometimes confused with CSC.
Generally, acute CSC has an excellent prognosis including full visual recovery to premorbid levels (Klein 1974; Loo 2002; Maruko 2010). However, people with chronic CSC with long‐standing subretinal fluid accumulation may develop RPE atrophy and changes in the neurosensory retina that result in a permanent loss of visual function (Baran 2005). While recovery of visual acuity usually occurs within one to four months (Klein 1974; Mudvari 2007; Nicholson 2013), some visual abnormalities, such as alterations in night vision, contrast sensitivity, and color vision, may persist. It has also been observed that the severity of the disease is directly proportional to its duration (Castro‐Correia 1992). One‐third to one‐half of CSC cases will recur in one year (Loo 2002). A waxing and waning course is not unusual and contributes to the difficulty in attributing visual improvement to treatment benefit. A minority of cases become chronic in nature and these may progress to diffuse abnormalities in the RPE and permanently poor vision (Baran 2005). Certain features and coexistent conditions are associated with lower final visual acuity, such as recurrent foveal detachments, chronic foveal detachment, CNV, subretinal fibrosis, subfoveal RPE atrophy, and diffuse involvement.
Management of CSC usually involves careful observation with risk factor modification. Corticosteroid use is the most frequent modifiable risk factor for CSC and physicians first may reduce corticosteroid use to treat CSC (Bouzas 2002). While discontinuation of corticosteroids can benefit some people, some people do not respond and many are not taking corticosteroids at all. Persistent submacular fluid or reduced visual acuity are potential indications for next treatment, as are cases where untreated CSC has previously resulted in a poor visual outcome in the fellow eye (Nicholson 2013). Rare indication may include vocational needs in, for example, airline pilots, professional athletes, or police/military officers.
Description of the intervention
Treatments for CSC generally target the RPE, choroid, or both. The RPE is responsible for maintaining the blood‐retinal barrier between the retina and choroid as well as for removing any subretinal fluid that accumulates. In CSC, the source of the subretinal fluid is the choroidal vasculature. Treatments for CSC aim to improve the ability of the RPE to remove the subretinal fluid, to diminish leakage from the choroidal vessels, or to decrease fluid flux across the RPE barrier. Determining the effectiveness of treatments for CSC is difficult as a waxing and waning of disease activity is typical of the natural history. This natural variation and the tendency for people to present when their symptoms are worse creates uncertainty of whether disease improvement is the result of an intervention or the natural course of the disease. Further confounding the assessment of treatment response is a lack of direct correlation between the person's visual symptoms and the amount of subretinal fluid present (Maalej 2014; Nicholson 2013; Quin 2013; Shuler 2006; Wang 2008).
There have been a variety of interventions used, or proposed for use, in CSC. These interventions include laser treatments, most commonly photodynamic therapy (PDT); intravitreal injections of anti‐vascular endothelial growth factor (anti‐VEGF) agents; medications that alter steroid hormones; and others.
How the intervention might work
The interventions have various potential modes of action directed at either accelerating absorption of subretinal fluid or decreasing the production of fluid that accumulates in the subretinal space. Presently the target cells are in the choroidal vascular network and the RPE.
Laser treatments
Argon laser photocoagulation
Argon laser photocoagulation uses a low‐intensity green or yellow argon laser to coagulate tissue by heat generated from an intense beam of light focused on the RPE (Hofstetter 2000). Lower intensity laser with longer duration and moderate spot size (100 to 200 microns) is preferred to minimize the likelihood of rupture of Bruch's membrane, subsequent development of CNV, and development of progressive atrophy over time in the area of the laser treatment (Robertson 1983). This procedure is commonly used to accelerate the absorption of subretinal fluid in acute and chronic CSC. Typically, laser burns are applied to areas of focal leakage that have been identified on FA as the principal sources of subretinal fluid. The mechanism of subretinal fluid resolution after laser photocoagulation treatment is not well understood. Benefit has been hypothesized to result from the sealing of focal defects in the RPE monolayer, the recruitment of healthy RPE cells after laser injury as a healing response, or the direct stimulation of improved pumping function of RPE cells near the treated areas (Mitsui 1969).
Micropulse diode laser photocoagulation
Micropulse diode laser treatment involves a series of repetitive ultrashort laser pulses that more broadly treat the RPE (Sivaprasad 2010). Improved RPE function is proposed to result from the targeted cells' response to therapy. Direct effects on points of leakage at the level of the RPE also have been postulated. Because of the relatively small amounts of energy delivered, little adverse thermal effect on the underlying neural retina and choroid is anticipated (Chen 2008; Ricci 2004; Roisman 2013). Historically, targeting of focal leaks outside of the macula with thermal laser has been more common than with micropulse laser; however, treatment of diffuse disease with micropulse treatment is increasing in use.
Verteporfin photodynamic therapy
Verteporfin photodynamic therapy (VPDT) has been used to treat acute CSC and to prevent recurrences (Nicholson 2013). The exact mechanism of PDT in treating chronic CSC is not known, but the treatment effects are postulated to result from short‐term choriocapillaris hypoperfusion (decreased blood flow through choroid vessels) and long‐term choroidal vascular remodeling, leading to reduction in choroidal congestion, vascular hyperpermeability, and extravascular leakage (Chan 2003). At present, PDT typically is used in cases of CSC involving the macula that have not responded to other treatments or observation. Despite potential benefits of PDT, there may be dose‐dependent complications such as the development of RPE atrophy, choriocapillaris ischemia, CNV, and RPE tear (Cardillo Piccolino 2003; Kim 2009; Schlötzer‐Schrehardt 2002; Schmidt‐Erfurth 2002). Some studies have reported the treatment of CSC with modified PDT parameters, including reduced dose of verteporfin, reduced time of treatment, or reduced fluence (energy/area/second) of laser. These modifications to treatment parameters have been hypothesized to reduce the risk of complications while maintaining the potential treatment benefit (Chan 2008; Lai 2006; Reibaldi 2010).
Anti‐vascular endothelial growth factor agents
The aim of anti‐VEGF therapy is to stop neovascular vessel growth and leakage. Anti‐VEGF agents bind to, and block the effects of, vascular endothelial growth factor (VEGF) thereby slowing down the growth of new blood vessels in the eye and reducing vascular permeability. Therefore, anti‐VEGFs may have a role in eyes with CSC that are complicated by secondary CNV. Anti‐VEGF therapy is typically not expected to provide benefit in cases of CSC that are not complicated by active CNV. Increased levels of VEGF have not been found in aqueous humor of people with CSC (Lim 2010).
There are several anti‐VEGF agents used by ophthalmologists: aflibercept (Eylea®), bevacizumab (Avastin®), ranibizumab (Lucentis®), and pegaptanib (Macugen®). Currently each of these, with the exception of bevacizumab, has been approved by the US Food and Drug Administration (FDA) for use in the eye (FDA 2015). Anti‐VEGF agents often are used for, and have been shown to be effective for, a number of ocular diseases; however, none of these medications has been approved specifically for use in the treatment of CSC or for the treatment of CNV in the setting of CSC.
Medications that alter steroid hormones
The exact role of steroids in CSC pathogenesis is not well understood. Proposed mechanisms in the choroid include effects on vascular autoregulation, potentiation of vascular reactivity, or prothrombotic steroid effect (Nicholson 2013). The following medications that target steroid hormone pathways have been proposed for treatment of CSC but are not currently licensed for that indication.
Ketoconazole
Ketoconazole is an anti‐fungal agent used to treat candidiasis, chronic mucocutaneous candidiasis, oral thrush, candiduria, blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, or paracoccidioidomycosis (FDA 2015).
Ketoconazole is an anti‐fungal agent that is thought to halt endogenous glucocorticoid production in part by inhibiting the conversion of 11‐deoxycortisol to cortisol. Because of this inhibition, ketoconazole was established as effective in the treatment of Cushing disease (Chou 2000; Winquist 1995). Thus, it is believed that, given the association of corticosteroids in the pathogenesis of CSC, lowering endogenous cortisol production by pharmacologic intervention would be a rational approach to the treatment of CSC (Jampol 2002; Meyerle 2007).
Mifepristone
Mifepristone (RU‐486) is classified as a high‐affinity, glucocorticoid receptor antagonist (Clark 2008). It is used to end an early pregnancy for women who have been pregnant for 49 days (seven weeks) or less since their last menstrual period began. The rationale for its use in CSC is similar to that for ketoconazole.
Rifampin
Rifampin, also known as rifampicin, is an antibacterial drug that is typically used to treat tuberculosis and meningococcal carriers. It is believed to suppress endogenous glucocorticoid production by inducing cytochrome P450 3A4 (Guengerich 1999); and altering reactions in steroid synthesis.
Finasteride
In addition to glucocorticoids, androgens, such as testosterone, have been implicated in the pathophysiology of CSC (Ahad 2006; Grieshaber 2007). Finasteride is a 5‐alfa‐reductase inhibitor that prevents conversion of testosterone to dihydrotestosterone, the latter of which has a higher binding affinity to androgen receptors (Forooghian 2011). Finasteride (5 mg) is used to treat benign prostatic hyperplasia (enlarged prostate gland), while finasteride (1 mg) is used to treat male pattern hair loss (androgenetic alopecia).
Eplerenone and spironolactone
It has been proposed that CSC results from over‐activation of the mineralocorticoid receptor pathway in the choroid. These receptors are bound and activated by mineralocorticoids, such as aldosterone, and by glucocorticoids. Eplerenone and spironolactone are aldosterone receptor antagonists; therefore, they inhibit binding of both aldosterone and glucocorticoids to mineralocorticoid receptors. Eplerenone is used to treat hypertension among people with stable left ventricle systolic dysfunction and congestive heart failure after an acute myocardial infarction (FDA 2015). Spironolactone is used to establish the diagnosis of primary hyperaldosteronism by therapeutic trial. The retinal and choroidal vasculature of the rat expresses glucocorticoid and mineralocorticoid receptors, and aldosterone injection causes choroidal enlargement in this animal model (Zhao 2012). Because of these findings, Bousquet 2013 treated 13 participants with chronic CSC with eplerenone and noted a decrease in mean macular thickness and subretinal fluid.
Carbonic anhydrase inhibitors
Acetazolamide is a carbonic anhydrase inhibitor used to treat chronic simple (open‐angle) glaucoma, secondary glaucoma, preoperatively in acute angle‐closure glaucoma to lower intraocular pressure, and prevention of amelioration. It is being used off‐label to treat CSC. It has been investigated on the basis that inhibition of carbonic anhydrase IV in the RPE seems to promote resorption of subretinal fluid and retinal adhesion (Cox 1988).
Helicobacter pylori treatment
Several studies indicated that people with CSC may have a higher incidence of serum anti‐Helicobacter pylori antibodies and that the treatment for H. pylori could have a positive impact on the outcome of the disease (Cotticelli 2006). It has been posited that an immune response to host proteins of the choroidal vasculature and RPE may be caused by molecular mimicry with antigens of H. pylori (Giusti 2004).
Aspirin
In some cases of CSC, increased levels of plasminogen activator inhibitor have been demonstrated compared with controls (Iijima 1999). Consequently, it has been suggested that hypercoagulability (abnormal blood coagulation that may lead to blood clots) may play a role in CSC pathogenesis (Cotticelli 2006).
Hypothalamic–pituitary–adrenal axis regulation
The hypothalamic‐pituitary‐adrenal (HPA) axis is a complex feedback system among the hypothalamus, pituitary gland, and adrenal glands. The HPA axis responds to stress by modifying hormone levels released by the glands. Increased stress levels can increase the amount of glucocorticoid in the body. Hormone level information feeds back to the HPA axis; high levels of glucocorticoids are believed to suppress HPA axis activity. Medications that may alter the HPA axis regulation include anti‐glucocorticoids and antidepressants.
Why it is important to do this review
Currently there is no consensus on the management of CSC. Due to the recurrent and chronic nature of some cases of CSC and uncertainty relating to best therapy, CSC remains a significant threat to vision and vocational stability. An evidence synthesis is needed to assess the relative effectiveness of interventions in order to determine which are the most promising and to identify any necessary future primary research.
As there are several different possible interventions, not all of which will have been compared in head‐to‐head studies, a network meta‐analysis, if possible, will provide quantitative comparisons of interventions and a treatment hierarchy useful for decision makers.
Objectives
To compare the relative effectiveness of interventions for central serous chorioretinopathy.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs) in this review.
Types of participants
We included studies of adults (aged 18 years or over) with CSC diagnosed using either OCT or FA, or as defined by study investigators.
Types of interventions
We included trials that compared any intervention for CSC with any other intervention for CSC or control. The control could be placebo, sham treatment, no treatment, or observation. We excluded trials of traditional Chinese medicine. This was a protocol amendment ‐ see Differences between protocol and review.
We specified the following interventions in our protocol:
argon laser photocoagulation;
micropulse diode laser photocoagulation;
VPDT (full‐dose, half‐dose or half‐fluence);
anti‐VEGF agents;
any other intervention (including the use of the specific medical treatments detailed above).
Figure 1 presents a theoretical treatment network based on interventions in current use and the classes of treatment as listed above. In our protocol, we planned to construct an alternative formulation of the network using specific anti‐VEGF agents (i.e. ranibizumab, bevacizumab, and aflibercept) and possibly different treatment regimens for laser and PDT); however, due to the sparse number of trials identified, this alternative formulation resulted in two disconnected networks and we felt it was unwise to proceed.
1.
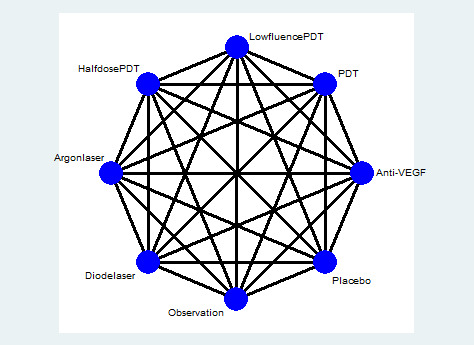
Theoretical treatment network.
A key assumption of the network is that any participant that met the inclusion criteria is, in principle, equally likely to be randomized to any of the interventions included in the network. In our protocol, we planned to exclude trials of treatment for H. pylori from the network as it is likely that these trials have only recruited participants with evidence of H. pylori infection and, therefore, the participants are unlikely to be comparable to participants enrolled in other trials. We identified a few trials of systemic treatments (e.g. antioxidant supplements, beta‐blockers). We felt that it was unlikely that participants would be randomized to a comparison of these interventions and ocular interventions (i.e. interventions applied directly to the eye), so we excluded these systemic interventions from the network. This was a protocol amendment ‐ see Differences between protocol and review.
Types of outcome measures
Primary outcomes
Mean change in BCVA of CSC eyes from baseline (before treatment) to 12 months, measured by a LogMAR (logarithm of the minimal angle of resolution) chart or equivalent.
Secondary outcomes
Proportion of CSC eyes with a recurrence of CSC between baseline and 12 months, as defined by study investigators.
Proportion of CSC eyes with persistent CSC, as defined by study investigators.
Mean change in contrast sensitivity from baseline to 12 months, measured using the Pelli‐Robson chart or equivalent.
Mean change in central retinal subfield thickness (CRST) from baseline and 12 months, measured using OCT.
Proportion of CSC eyes with BCVA 20/40 or better at 12 months.
Proportion of CSC eyes with BCVA 20/200 or worse at 12 months.
Quality of life at 12 months, measured using a validated questionnaire.
Adverse events (e.g. loss of vision due to treatment, retinal atrophy, CNV).
Follow‐up: we analyzed any measurement within the period of six to 18 months' follow‐up as the 12‐month measurement when a measurement at 12 months was not available.
BCVA; when logMAR score was not reported, we used the following formula to convert the number of letters read on an Early Treatment Diabetic Retinopathy Study (ETDRS) chart to logMAR score: logMAR = (total number of letters on the chart‐number of letters read correctly) x 0.02.
The total number of participant at risk of recurrence of CSC between baseline and 12 months was calculated using:
total number of participants at risk for each group = number randomized ‐ number lost to follow‐up ‐ number of participants with persistent CSC.
We planned to estimate the relative ranking of the competing interventions according to the following outcomes:
mean change in BCVA of CSC eyes from baseline and 12 months;
proportion with a recurrence of CSC between baseline and 12 months;
proportion of participants with one or more adverse events.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2015, Issue 9), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to February 2014), EMBASE (January 1980 to October 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 5 October 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), ISRCTN (Appendix 4), ClinicalTrials.gov (Appendix 5), and the ICTRP (Appendix 6).
Searching other resources
We searched the reference lists of all included studies and the Science Citation Index for papers that have cited included studies. We did not handsearch conference proceedings or journals specifically for the purposes of this review.
Data collection and analysis
Selection of studies
Two review authors independently screened titles and abstracts identified from searches using web‐based software (Covidence); and classified each reference as 'relevant', 'possibly relevant', or 'definitely not relevant'. We resolved any discrepancy by discussion. We retrieved full‐text reports of all records classified as 'relevant' or 'possibly relevant', and grouped citations by study. Two review authors independently assessed the eligibility for each study based on the full‐text reports. We resolved any discrepancy during full‐text assessment by discussion. We documented reasons for excluding studies after review of the full‐text reports. We contacted trial investigators for clarification of study eligibility as needed.
For potentially eligible studies identified from trial registers we did the following:
if the study had a completion date more than two years previously, we looked for publications of this trial and contacted the investigators as necessary to obtain published or unpublished data from the trial;
if an eligible study had a completion date less than two years previously, or in the future, we documented the study in the ongoing studies section of the review.
Data extraction and management
We adapted data collection forms developed and piloted by Cochrane Eyes and Vision using web‐based software (Systematic Review Data Repository). Two review authors independently extracted data from each study using all available study reports (protocols, journal publications, conference abstracts, etc.). We resolved any discrepancy by discussion. We abstracted data relevant to study design, methods, participants' characteristics, intervention, table of included studies, and outcomes (Appendix 7). We contacted trial investigators at the email address listed in their publications where we needed clarification. If we received no response after two weeks or we were unable to find current contact information, we used the data as available.
Assessment of risk of bias in included studies
We used the 'Risk of bias' tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors independently assessed each trial for each of the risk of bias domains listed below. We classified each domain as 'low risk', 'high risk', or 'unclear risk' for each trial. We resolved any disagreement by discussion. We discussed the potential impact of trials with high or unclear risks of bias on the treatment effect.
Sequence generation (selection bias)
Low risk of bias: computer‐generated, random number table.
Unclear risk of bias: not clearly described or not reported.
High risk of bias: non‐random process (e.g. alternation) (we excluded these trials).
Allocation concealment (selection bias)
Low risk of bias: data co‐ordination center, opaque sealed envelope.
Unclear risk of bias: low risk (random) sequence generation but not described clearly how this was assigned/stored.
High risk of bias: investigator was involved in sequence generation or assignment, or both.
Masking (blinding) of participants and study personnel (performance bias)
Low risk of bias: masking reported.
Unclear risk of bias: masking not reported or not reported clearly (e.g. 'double blinded' without explicit description of masking) but treatments similar.
High risk of bias: no masking or masking not reported clearly (e.g. 'double blinded' without explicit description of masking) and treatments different (e.g. intervention versus observation).
Masking of outcome assessors (detection bias)
Low risk of bias: masking of outcome assessors reported.
Unclear risk of bias: masking of outcome assessors not reported or not reported clearly (e.g. 'double blinded' without explicit description of masking) but treatments similar.
High risk of bias: no masking of outcome assessors or masking not reported clearly (e.g. 'double blinded' without explicit description of masking) and treatments different (e.g. intervention versus observation).
Incomplete outcome data (attrition bias)
Low risk of bias: missing data less than 20% and no obvious reason why loss to follow‐up should be related to outcome.
Unclear risk of bias: not reported or 20% or greater loss to follow‐up but follow‐up similar in both groups.
High risk of bias: loss to follow‐up different in different groups or follow‐up clearly related to outcome.
Selective outcome reporting (reporting bias)
Low risk of bias: all outcomes reported as per protocol or trial registry entry.
Unclear risk of bias: protocol and trial registry not available for comparison.
High risk of bias: reported primary/secondary outcomes different from protocol/trial registry or outcomes mentioned in methods section not reported in results.
Other biases (e.g. funding source)
Low risk of bias: reported either non‐industry funding or reported no conflict of interest, or both, but did not report either industry funded or conflict of interest.
Unclear risk of bias: source of funding and conflict of interest not reported.
High risk of bias: industry funding or declared conflict of interest, or both.
Measures of treatment effect
Dichotomous data
For dichotomous outcome variables, we used risk ratios (RRs) with corresponding 95% confidence intervals (CIs) to measure the treatment effect. Dichotomous variables were BCVA 20/40 or better, BCVA 20/200 or worse, recurrence/persistence, and adverse events at one year.
Continuous data
For continuous variables, we used mean differences (MDs) and 95% CIs to measure the treatment effect. The continuous variables were change in BCVA, CRST, contrast sensitivity, and quality‐of‐life scores.
None of the included studies reported continuous outcomes using different scales. However, when future studies are included in the review, we will use the standardized mean difference (SMD) whenever a continuous outcomehas been measured on different scales, as may be the case for quality‐of‐life outcomes. The SMD expresses the size of the intervention effect in each study relative to the variability observed in that study. If one scale increases with severity while another decreases, we will ensure that all the scales point in the same direction either by multiplying the mean values of studies using one type of scale by ‐1 or by subtracting the mean from the maximum possible value for the scale.
We presented results from the network meta‐analysis as summary effect sizes (RRs or MDs) for each possible pair of treatments.
Unit of analysis issues
Eyes and people
The unit of analysis was the person as CSC generally involves one symptomatic eye at the time of presentation. As far as we could determine, all included studies only included one eye per participant and none of the included studies enrolled bilateral CSC cases.
Studies with multiple treatment groups
We treated multi‐arm studies as multiple independent two‐arm studies in the network meta‐analysis.
Dealing with missing data
We contacted all study authors, but they were unable to provide more data. Authors were given two weeks to respond to our request. When we received no response, we proceeded using the available data. We did not attempt imputation for missing data. We conducted meta‐analysis only when there was sufficient quantitative information (e.g. measures of variability, number of participants at risk). Otherwise, we described the results narratively.
Assessment of heterogeneity
Assessment of clinical and methodological heterogeneity within treatment comparisons
To evaluate the presence of clinical heterogeneity, we generated descriptive statistics for trial and study population characteristics across all eligible trials that compared each pair of interventions. We assessed the presence of clinical heterogeneity within each pairwise comparison by comparing these characteristics.
Assessment of transitivity across treatment comparisons
We planned to assess the assumption of transitivity epidemiologically by comparing the clinical and methodological characteristics of sets of studies grouped by treatment comparisons. We expected the transitivity assumption will hold as long as treatment comparisons were not related to:
study design (parallel group or within‐person);
acute or chronic CSC;
date the study was conducted;
whether the trial was industry sponsored.
In the event, all trials were parallel group, so we did not consider this factor.
Assessment of reporting biases
None of the meta‐analyses included 10 or more studies, so we did not prepare a funnel plot as planned in our protocol. We assessed selective outcome reporting bias using the 'Risk of bias' tool.
Data synthesis
Methods for direct treatment comparisons
We performed standard pairwise meta‐analyses using a random‐effects model in Review Manager 5 (RevMan 2014). We used a fixed‐effect model when there were fewer than three studies.
Methods for indirect and mixed comparisons
We performed network meta‐analysis using the methodology of multivariate meta‐analysis model where different treatment comparisons are treated as different outcomes (White 2012). For this analysis, we used the 'mvmeta' command in STATA (StataCorp, 2011; Stata Statistical Software: Release 13. College Station, TX) (White 2009; White 2011).
We planned to estimate the ranking probabilities for all treatments of being at each possible rank of intervention effectiveness (e.g. best to worst) and then to calculate the surface under the cumulative ranking curve (SUCRA) and mean ranks, but did not do so due to a lack of data in the network.
Assessment of statistical heterogeneity
Assumptions when estimating the heterogeneity
In standard pairwise meta‐analyses, we estimated heterogeneity variances for each pairwise comparison. In network meta‐analysis, we assumed a common estimate for the heterogeneity variance across the different comparisons.
Measures and tests for heterogeneity
We assessed statistically the presence of heterogeneity within each pairwise comparison using the I2 statistic (Higgins 2003). The I2 statistic measures the percentage of variability that cannot be attributed to random error.
The assessment of statistical heterogeneity in the entire network was based on the magnitude of the heterogeneity variance parameter (τ2) estimated from the network meta‐analysis models.
Assessment of statistical inconsistency
Local approaches for evaluating inconsistency
To evaluate the presence of inconsistency locally, we used the loop‐specific approach (Bucher 1997). This method evaluates the consistency assumption in each closed loop of the network separately as the difference between direct and indirect estimates for a specific comparison in the loop (inconsistency factor). Then, the magnitude of the inconsistency factors and their 95% CIs can be used to infer the presence of inconsistency in each loop. We assumed a common heterogeneity estimate within each loop. We planned to present the results of this approach graphically in a forest plot using the 'ifplot' command in STATA (Chaimani 2013), but in the event, there were not enough loops to make this necessary.
Global approaches for evaluating inconsistency
To check the assumption of consistency in the entire network, we used the 'design‐by‐treatment' model using the 'mvmeta' command in STATA (Higgins 2012). This method accounts for different sources of inconsistency that can occur when studies with different designs (two‐arm trials versus three‐arm trials) give different results as well as disagreement between direct and indirect evidence. Using this approach, we judged the presence of inconsistency from any source in the entire network based on a Chi2 test.
Subgroup analysis and investigation of heterogeneity
There were insufficient data available to perform subgroup analyses by type of CSC (acute versus chronic).
Sensitivity analysis
We planned the following sensitivity analyses but there were not enough studies contributing to each analysis to enable this.
In standard pairwise comparisons or meta‐analyses, we planned to exclude the following studies to determine their impact on effect size for the primary outcome:
studies with high risk of bias on any domain;
studies with unpublished data only; and
industry‐funded studies.
'Summary of findings' table
We prepared a 'Summary of findings' table for all comparisons including relative and absolute effects for the following outcomes: mean change in BCVA, persistent CSC, recurrence of CSC and adverse effects. We used GRADE (Guyatt 2011) to assess the overall quality of the evidence for each outcome in pairwise and network meta‐analyses (Puhan 2014).
Results
Description of studies
Results of the search
The electronic searches yielded 1168 references (Figure 2). The Trials Search Co‐ordinator scanned the search results, removed 299 duplicates and then removed 229 references that were not relevant to the scope of the review. We screened the remaining 640 reports and discarded 554 records as not relevant. We obtained 86 full‐text reports for potential inclusion in the review and we included 25 studies (see Characteristics of included studies table) and excluded 49 studies (see Characteristics of excluded studies table). We also included 12 reports of ongoing studies and will assess the data for these studies when the results become available (see Characteristics of ongoing studies table).
2.
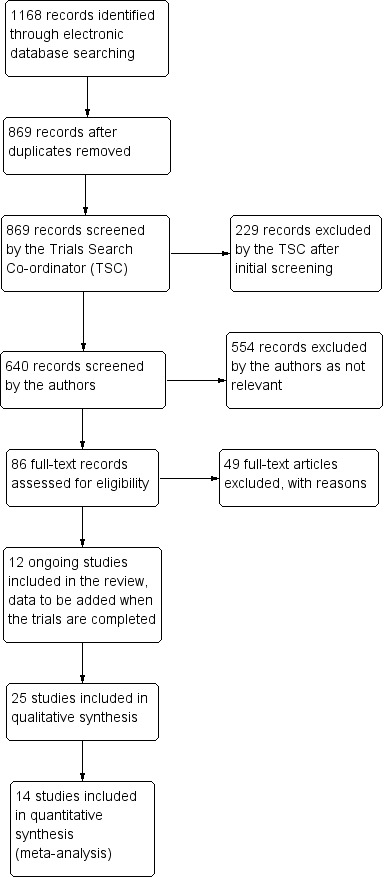
Study flow diagram.
Included studies
We included 25 studies of 30 full‐text articles, of which four studies only had abstracts available (Boscia 2008; Brancato 1994; Chan 2006; Coskun 2014). We contacted the authors of studies with abstracts only; authors of Boscia 2008 and Brancato 1994 did not respond, authors of Coskun 2014 reported not publishing the abstract as a full‐text article, and authors of Chan 2006 were not able to provide the corresponding full‐text. We provided a summary describing each of the included studies in the Characteristics of included studies table. Thirteen of 25 included studies did not report either the number of eyes or number of participants enrolled; we assumed that all included studies were parallel RCTs, where only one eye per participant was enrolled (Bae 2011; Boscia 2008; Brancato 1994; Browning 1993; Chan 2006; Klatt 2011; Leaver 1979; Ontiveros‐Orozco 2004; Rahbani‐Nobar 2011; Sawa 2014; Shang 1999; Verma 2004; Zhang 2012).
Types of participants
A total of 1098 participants from 25 included studies were enrolled from Brazil, China, Germany, India, Iran, Italy, Japan, Mexico, South Korea, Thailand, Turkey, the UK, and the US. The baseline characteristics of participants in all trials were similar with respect to age (mean age ranged from 35.0 to 50.8 years). However, 79% (685 of 872 participants) of participants were men; three studies did not report the number of men and women enrolled (Boscia 2008; Coskun 2014; Shang 1999). One study enrolled men only (Pitcher 2015).
Nine studies enrolled participants with acute CSC alone, six studies enrolled participants with chronic CSC alone, and two studies enrolled participants with both acute and chronic CSC. Seven studies did not specify type of CSC. The definition of acute CSC varied between studies; the duration of onset ranged from less than 20 days to six months. Likewise, the definition of chronic CSC varied; the duration of onset ranged from greater than 12 weeks to six months.
Types of interventions
Five classes of interventions were investigated: laser treatments (argon laser photocoagulation, micropulse diode laser photocoagulation, PDT), anti‐VEGF, medications that alter steroid hormones (carbonic anhydrase inhibitors), H. pylori treatment, and other treatments (antioxidant, calcium antagonist, beta‐blocker). In the studies of PDT, where it was specified, treatment was applied to areas of choroidal hyperpermeability, usually identified using ICGA.
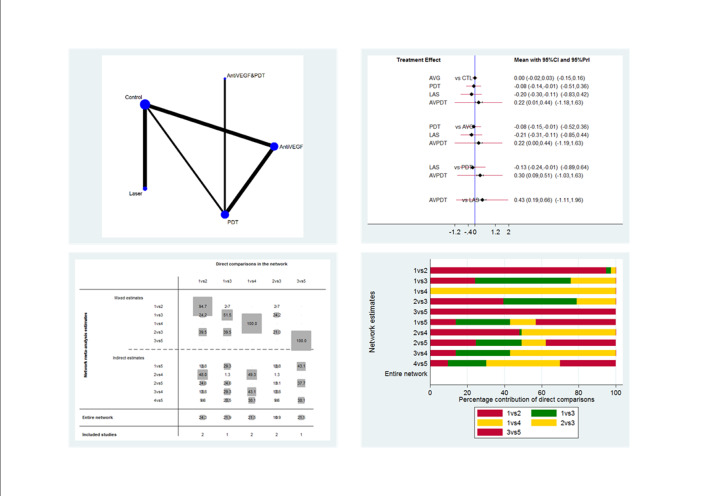
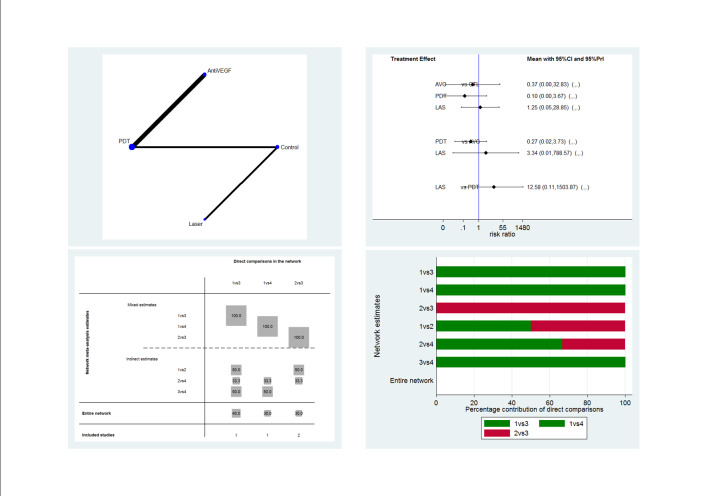
The 25 included studies alluded to 19 pair‐wise comparisons:
anti‐VEGF versus observation;
anti‐VEGF versus PDT;
50% PDT plus anti‐VEGF versus 50% PDT;
different dose regimens of anti‐VEGF (six‐dose versus four‐dose aflibercept);
50% PDT versus observation or sham treatment;
50% PDT versus PDT;
30% PDT versus PDT;
30% PDT versus 50% PDT;
laser versus observation or sham treatment;
micropulse diode laser versus argon laser;
indirect argon laser versus direct argon laser;
antioxidant supplements versus placebo;
beta‐blocker versus placebo;
beta‐blocker versus calcium antagonist;
H. pylori treatment versus placebo or observation;
carbonic anhydrase inhibitors versus placebo;
yellow versus red wavelength laser;
yellow versus green wavelength laser;
red versus green wavelength laser.
Types of outcomes
Fourteen studies were include in the quantitative analysis. Studies reported BCVA was measured using Snellen chart and LogMAR. When authors reported the number of letters read on a logMAR chart, we converted it to a LogMAR score (Types of outcome measures). Many included studies did not clearly describe the adverse events reported, often not reporting which adverse events were collected.
Follow‐up duration ranged from 16 weeks to 12 years. None of the studies reported the proportion of CSC with BCVA 20/40 or better at 12 months, proportion of CSC with BCVA 20/200 or better at 12 months, or quality of life at 12 months.
Excluded studies
We excluded 49 studies of 47 full‐text articles and two trial registries. We documented our reasons for exclusion in the Characteristics of excluded studies table. The reasons for exclusion were: 37 studies were not RCTs, 10 studies were of traditional Chinese medication, one study (a trial registry) was terminated early due to lack of enrollment, and one study (a trial registry) enrolled participants with age‐related macular degeneration.
Ongoing studies
We identified 12 ongoing studies from the trials registers. These studies are evaluating the following interventions and comparators.
In acute CSC:
PDT versus observation (EUCTR2009‐017959‐98‐NL);
aflibercept versus sham injection (NCT01971190);
eplerenone versus placebo (NCT01990677; NCT02215330).
In chronic CSC;
lutein versus placebo (JPRN‐UMIN000005372);
spironolactone versus placebo (NCT01552044);
eplerenone versus placebo (NCT01990677; NCT02153125);
three doses of PDT (50%, 40%, 30%) (NCT01630863);
half‐dose versus half‐fluence PDT (NCT01019668);
half dose PDT versus micropulse diode laser (NCT01797861).
Note: trial NCT01990677 includes both acute and chronic CSC separately.
Type of CSC not clearly specified:
micropulse diode laser versus observation (NCT01982383);
short‐term oral mifepristone versus placebo (NCT02354170).
Risk of bias in included studies
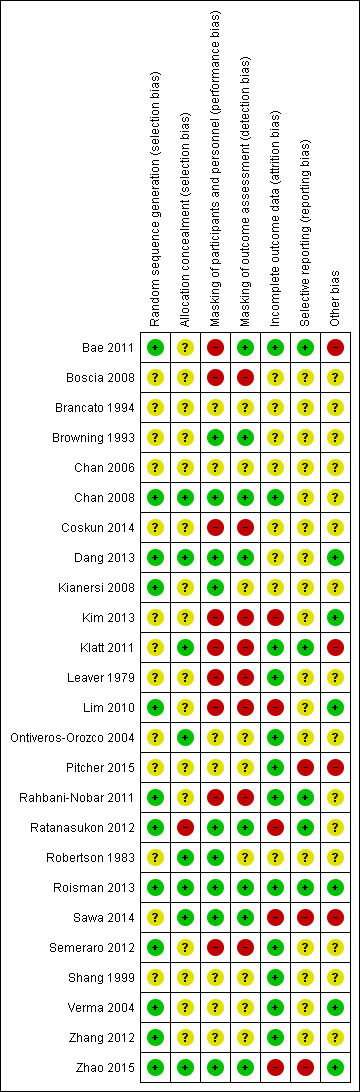
Figure 3 provides a summary of our judgments for each risk of bias domain for the included studies.
3.
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
We judged 12 of 25 included studies at low risk of bias as the authors explicitly reported the method used to generate a random sequence (e.g. computer‐generated, randomization software, random table, random block size design). We judged the remaining 13 studies at unclear risk of bias as the authors did not clearly describe or report the method of randomization. None of the included studies were judged at high risk of bias.
We judged eight of 25 included studies at low risk of bias as the authors explicitly reported appropriate methods to conceal the assignment sequence (e.g. data coordination center, opaque sealed envelope). We judged 16 of 24 included studies at unclear risk of bias as they had an appropriate method of random sequence generation, but did not clearly describe how the random sequence was assigned or stored, or they did not clearly describe how the random sequence was generated or stored. We judged one included study at high risk of bias as one of the investigators was involved in the sequence generation and assignment (Ratanasukon 2012).
Masking of participants and personnel (performance bias)
We judged each study based on the interventions administered to each group; studies had different treatments (e.g. laser versus observation) and studies with similar treatments (e.g. 50% PDT versus 30% PDT). Nine studies compared different treatments (Bae 2011; Boscia 2008; Coskun 2014; Kim 2013; Klatt 2011; Leaver 1979; Lim 2010; Rahbani‐Nobar 2011; Semeraro 2012). None of these studies explicitly reported masking and so we judged them at high risk of performance bias.
The remaining 16 studies had similar treatments. Nine of these 16 studies were judged at low risk of bias as the authors had explicitly stated that participants were masked. We judged seven of these 15 studies at unclear risk of bias as there was no information on masking or reported 'double blinded' without explicit description of how they were masked.
Masking of outcome assessors (detection bias)
Eight studies reported that outcome assessors were masked to treatment group and we judged them at low risk of detection bias (Bae 2011; Browning 1993; Chan 2008; Dang 2013; Ratanasukon 2012; Roisman 2013; Sawa 2014; Zhao 2015). Eight studies did not report any masking and the treatments were different and so we judged them at high risk of detection bias (Boscia 2008; Coskun 2014; Kim 2013; Klatt 2011; Leaver 1979; Lim 2010; Rahbani‐Nobar 2011; Semeraro 2012). In the remainder, it was unclear in general because the groups were similar but masking was not explicitly reported.
Incomplete outcome data
We judged five studies at high risk of attrition bias. Kim 2013 did not report the number of people randomized and loss to follow‐up by group and the final numbers analyzed were identical between treatment and observation group (20/20). Lim 2010 did not follow up 25% of participants and did not report which group they were in. Sawa 2014 excluded 5/39 participants after randomization but did not report which group they were in. Ratanasukon 2012 lost 3/29 participants to follow‐up at three months in intervention group and 4/29 in the control group (4/29) but at 12 months, only assessed seven participants in each group. So there was a low risk at three months and high risk for 12 months outcomes. In Zhao 2015, there were different losses to follow‐up in each group (13% compared with 6%).
We judged 12 studies at low risk of attrition bias because loss to follow‐up was less than 20% and there was no obvious reason why loss to follow‐up should be related to outcome (Bae 2011; Chan 2008; Klatt 2011; Leaver 1979; Ontiveros‐Orozco 2004; Pitcher 2015; Rahbani‐Nobar 2011; Roisman 2013; Semeraro 2012; Shang 1999; Verma 2004; Zhang 2012).
In the remaining studies, it was unclear whether attrition bias was a problem, usually because of a lack of information.
Selective reporting
Only five studies were at low risk of selective reporting, that is, outcomes reported a priori (usually on a trials registry entry) were reported in the published paper (Bae 2011; Klatt 2011; Rahbani‐Nobar 2011; Ratanasukon 2012; Roisman 2013). In most cases it was unclear because we did not have access to the trial protocol and the trial was not registered with a publicly available database. For Pitcher 2015, Sawa 2014, and Zhao 2015, there was some evidence of selective reporting. Sawa 2014 measured, but did not report, BCVA and only reported resolution of CSC for the intervention (lutein) group. Pitcher 2015 included additional outcomes that were not specified in the trial registry entry. Zhao 2015 specified the primary outcome at clinicaltrials.gov (NCT01574430) was "change from baseline in BCVA", but primary outcomes specified in the published report were OCT‐based improvement rate and FA‐based improvement rate at six and 12 months. They did report BCVA, but it was not defined as primary outcome (Zhao 2015).
Other potential sources of bias
We judged four studies at risk of bias either because they were industry funded or there was a declared conflict of interest (Bae 2011; Klatt 2011; Pitcher 2015; Sawa 2014). Six studies were at low risk of bias because they were non‐industry funded or the authors declared they had no conflicts of interest, or both (Dang 2013; Kim 2013; Lim 2010; Roisman 2013; Verma 2004; Zhao 2015). For the remainder of the studies, it was unclear, usually because the studies did not report funding sources and conflicts of interest.
Effects of interventions
See: Table 1
Pairwise meta‐analysis (direct comparisons)
Anti‐vascular endothelial growth factor versus observation
Two trials compared anti‐VEGF therapy to observation; both studies were conducted in South Korea (Kim 2013; Lim 2010). Both studies enrolled participants with acute CSC, which was defined as CSC of less than three months' duration. A total of 82 people (82 eyes) were randomized in these two trials and 64 people were followed up to six months. Both trials were at high risk of performance, detection, and attrition bias.
In Kim 2013, a single dose of ranibizumab (0.5 mg/0.05 mL) was given at baseline and participants followed up for six months. In Lim 2010, a single dose of bevacizumab (1.25 mg/0.05 mL) was given within one week of diagnosis and participants were followed up for six months.
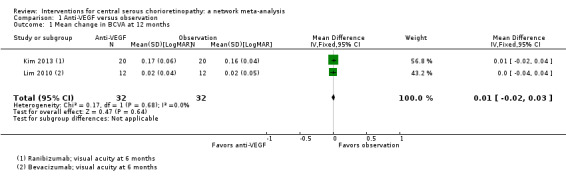
Mean change in best‐corrected visual acuity between baseline and 12 months
Both trials reported visual acuity at six months, which was similar in the anti‐VEGF and observation groups (MD 0.01 LogMAR, 95% CI ‐0.02 to 0.03; 64 eyes; I2 = 0%; Analysis 1.1). We judged this to be low quality evidence downgrading for risk of bias (‐1) and indirectness (‐1) as the outcome was only measured at six and not 12 months.
1.1. Analysis.
Comparison 1 Anti‐VEGF versus observation, Outcome 1 Mean change in BCVA at 12 months.
Recurrence of central serous chorioretinopathy at 12 months
Neither trial reported recurrence of CSC at 12 months.
Persistent central serous chorioretinopathy at 12 months
Both trials reported that all participants in treatment and control groups (total of 64 eyes) were resolved by six months (i.e. did not have persistent CSC).
Mean change in contrast sensitivity between baseline and 12 months
Neither trial reported mean change in contrast sensitivity between baseline and 12 months.
Mean change in central retinal thickness between baseline and 12 months
Both studies measured central retinal thickness using an OCT. There was no evidence for a difference between the two groups (MD 8.73 µm, 95% CI ‐18.08 to 35.54; 64 participants; I2 = 20%; Analysis 1.2) We judged this to be low quality evidence downgrading for risk of bias (‐1) and indirectness (‐1).
1.2. Analysis.
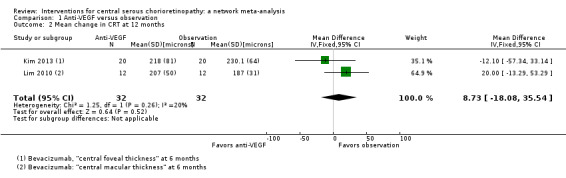
Comparison 1 Anti‐VEGF versus observation, Outcome 2 Mean change in CRT at 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
Neither trial reported BCVA 20/40 or better at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
Neither trial reported BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
Neither trial reported quality of life at 12 months.
Adverse events
Both studies reported that there were no adverse effects of anti‐VEGF treatment. Kim 2013 specified that they looked for systemic and ocular adverse events.
Anti‐vascular endothelial growth factor versus photodynamic therapy
Two studies compared anti‐VEGF to PDT and were conducted in South Korea (Bae 2011) and Italy (Semeraro 2012). Both studies enrolled participants with chronic CSC: in Bae 2011 this was defined as "chronic CSC with visual disturbance persisting for >6 months or recurrent CSC"; in Semeraro 2012 this was defined as "either persistence of subretinal fluid detected on optical coherence topography (OCT) for at least 3 months after diagnosis or more than 3 recurrences in at least 3 months with gravitational RPE atrophy". A total of 54 participants (56 eyes) were randomized in these trials and all were followed up to nine months (Semeraro 2012) and 12 months (Bae 2011). We judged both studies at high risk of performance bias; Semeraro 2012 was also at high risk of detection bias; Bae 2011 was industry funded.
In both studies, PDT was "low fluence", which means that they used a light dose of 25 J/cm2. In Bae 2011, ranibizumab (0.5 mg/0.05 mL) was given at baseline, one month, and two months; in Semeraro 2012, bevacizumab (1.25 mg) was given at baseline and then as needed after four weeks.
Mean change in best‐corrected visual acuity between baseline and 12 months
Visual acuity was similar between the two groups (MD 0.03 logMAR, 95% CI ‐0.08 to 0.15; 56 eyes; I2 = 0%; Analysis 2.1). We judged this to be low quality evidence downgrading for risk of bias (‐1) and imprecision (‐1) as we cannot exclude a clinically important effect.
2.1. Analysis.
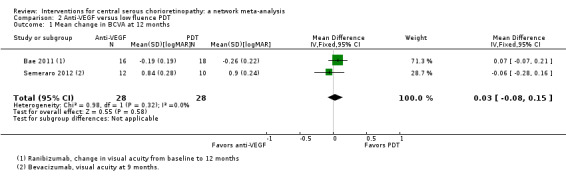
Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 1 Mean change in BCVA at 12 months.
Recurrence of central serous chorioretinopathy at 12 months
The two studies had different results for this outcome (I2 = 71%; Analysis 2.2). In Bae 2011, there was a much higher risk of recurrence in the anti‐VEGF group (ranibizumab) compared with the PDT group (RR 19.83, 95% CI 1.19 to 330.50; 21 eyes); in Semeraro 2012 there was also an increased risk of recurrence in the anti‐VEGF (bevacizumab) group but the size of the effect was much smaller and the CIs included 1 (i.e. no effect) (RR 1.46, 95% CI 0.59 to 3.58; 22 eyes). Note the denominator in these studies is smaller as only the eyes where CSC had resolved were at risk of recurrence. We judged this to be very low quality of evidence downgrading for risk of bias (‐1), imprecision (‐1), and inconsistency (‐1); we are very uncertain as to the size of the effect.
2.2. Analysis.
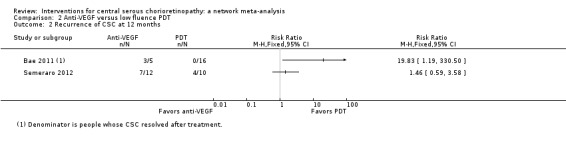
Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 2 Recurrence of CSC at 12 months.
Persistent central serous chorioretinopathy at 12 months
Only Bae 2011 reported persistent CSC at 12 months. People in the anti‐VEGF group (ranibizumab) were more likely to have persistent CSC at 12 months (RR 6.19, 95% CI 1.61 to 23.81; 34 eyes; Analysis 2.3). We judged this to be low quality evidence and downgraded for risk of bias (‐1) and imprecision (‐1).
2.3. Analysis.
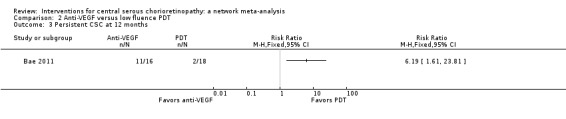
Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 3 Persistent CSC at 12 months.
Mean change in contrast sensitivity between baseline and 12 months
Neither trial reported mean change in contrast sensitivity between baseline and 12 months.
Mean change in central retinal thickness between baseline and 12 months
The two studies found different results for central retinal thickness (I2 = 69%; Analysis 2.4). In Bae 2011, there was a greater reduction in thickness in the PDT group (MD 31.30 µm, 95% CI ‐3.46 to 66.06); in Semeraro 2012, there was a greater reduction in the anti‐VEGF group (bevacizumab) (MD ‐13.00, 95% CI ‐46.05 to 20.05). We judged this to be very low quality of evidence downgrading for risk of bias (‐1), imprecision (‐1), and inconsistency (‐1); we are very uncertain as to the size of the effect.
2.4. Analysis.
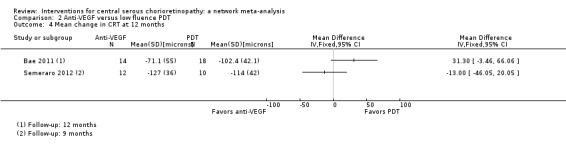
Comparison 2 Anti‐VEGF versus low fluence PDT, Outcome 4 Mean change in CRT at 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
Neither trial reported BCVA 20/40 or better at 12 months.
Central serous chorioretinopathy eyes with best‐corrected visual acuity 20/200 or worse at 12 months
Neither trial reported CSC eyes with BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
Neither trial reported quality of life at 12 months.
Adverse events
Both studies reported that no systemic or ocular adverse events related to the drugs or procedures were observed.
Anti‐vascular endothelial growth factor plus 50% photodynamic therapy versus 50% photodynamic therapy alone
One study conducted in Turkey compared anti‐VEGF (bevacizumab 1.25 mg single dose three days after PDT) plus PDT to PDT alone (Coskun 2014). The PDT was "half dose" (i.e. the dose of verteporfin used was half that usually delivered (3 mg/m2)). The study enrolled 15 participants with chronic CSC (duration six months) and followed them up for a mean of 12 months in the anti‐VEGF plus PDT group and nine months in the PDT alone group. This study was reported in an abstract only and was largely judged at unclear risk of bias apart from performance and detection bias where we judged them at high risk of bias because masking was not mentioned and the treatments were obviously different.
Mean change in best‐corrected visual acuity between baseline and 12 months
At follow‐up, the mean logMAR visual acuity in the anti‐VEGF plus PDT group was 0.36 (standard deviation (SD) 0.25) and in the PDT alone group was 0.06 (SD 0.15). This gives an MD in favor of PDT alone of 0.30 logMAR (95% CI 0.09 to 0.51; Analysis 3.1). We judged this to be low quality evidence downgrading for risk of bias (‐1) and imprecision (‐1) as the CIs include clinically unimportant effects.
3.1. Analysis.
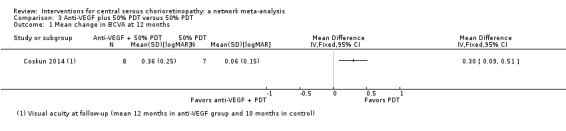
Comparison 3 Anti‐VEGF plus 50% PDT versus 50% PDT, Outcome 1 Mean change in BCVA at 12 months.
Recurrence of central serous chorioretinopathy at 12 months
The trial did not report recurrence of CSC at 12 months.
Persistent central serous chorioretinopathy at 12 months
CSC resolved in 7/8 eyes in the anti‐VEGF plus PDT group and 6/7 eyes in the PDT alone group (RR 0.88, 95% CI 0.07 to 11.54; Analysis 3.2). We judged this to be very low quality evidence and downgraded for risk of bias (‐1) and imprecision (‐2) due to very wide CIs. We are very uncertain as to the size of the effect.
3.2. Analysis.
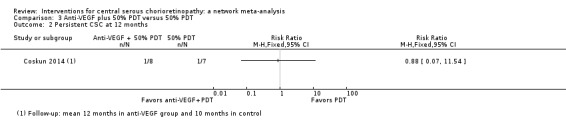
Comparison 3 Anti‐VEGF plus 50% PDT versus 50% PDT, Outcome 2 Persistent CSC at 12 months.
Mean change in contrast sensitivity between baseline and 12 months
The trial did not report mean change in contrast sensitivity between baseline and 12 months.
Mean change in central retinal thickness between baseline and 12 months
In the anti‐VEGF plus PDT group, the central macular thickness was 203 µm (SD 45) and in the PDT alone group it was 187 µm (SD 15) (MD 16.00 µm, 95% CI ‐17.10 to 49.10; Analysis 3.3). We judged this to be low quality evidence; we downgraded for risk of bias (‐1) and imprecision (‐1).
3.3. Analysis.
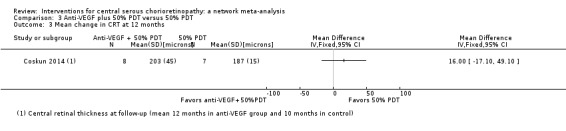
Comparison 3 Anti‐VEGF plus 50% PDT versus 50% PDT, Outcome 3 Mean change in CRT at 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
The trial did not report BCVA 20/40 or better at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
The trial did not report BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
The trial did not report quality of life at 12 months.
Adverse events
The trial did not report adverse events.
Six‐dose anti‐vascular endothelial growth factor versus four‐dose anti‐vascular endothelial growth factor
One study from the US compared two treatment regimens: aflibercept 2.0 mg/0.05 mL administered six times (at baseline, one, two, three, four, and five weeks) versus four times (at baseline, one, two, and four weeks) (Pitcher 2015). The study enrolled 12 participants and followed them for six months. This study was poorly reported with mostly unclear risk of bias and high risk of reporting bias. The study received industry funding.
Mean change in best‐corrected visual acuity between baseline and 12 months
There was a similar change in logMAR acuity over six months in the two groups. In the group given six doses, on average visual acuity improved by 0.1 logMAR (SD 0.32) and in the group given four doses it improved by 0.08 logMAR (SD 0.16). This was an MD of 0.02 logMAR units greater improvement (95% CI ‐0.31 to 0.27) in the six‐doses group but with wide CIs (Analysis 4.1). We judged this to be low quality evidence downgrading for risk of bias (‐1) and imprecision (‐1).
4.1. Analysis.
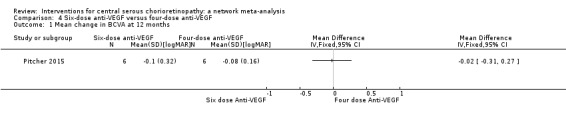
Comparison 4 Six‐dose anti‐VEGF versus four‐dose anti‐VEGF, Outcome 1 Mean change in BCVA at 12 months.
Recurrence of central serous chorioretinopathy at 12 months
The trial did not report recurrence of CSC at 12 months.
Persistent central serous chorioretinopathy at 12 months
The trial did not report persistent CSC at 12 months.
Mean change in contrast sensitivity between baseline and 12 months
The trial did not report mean change in contrast sensitivity between baseline and 12 months.
Mean change in central retinal thickness between baseline and 12 months
The two groups had similar change in central macular thickness over six months with an MD of 26.50 µm (95% CI ‐123.41 to 176.41; 12 participants). Both groups experienced a decrease: in the six‐dose group of ‐80 µm (SD 103.7) and in the four‐dose group of ‐103 µm (SD 156). We judged this to be low quality evidence downgrading for risk of bias (‐1) and imprecision (‐1).
Best‐corrected visual acuity 20/40 or better at 12 months
The trial did not report BCVA 20/40 or better at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
The trial did not report BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
The trial did not report quality of life at 12 months.
Adverse events
The trial reported there were no adverse events.
Photodynamic therapy versus observation or sham treatment
Two studies compared PDT to no PDT and were conducted in Italy (Boscia 2008) and China (Hong Kong) (Chan 2008).
Boscia 2008 was reported in an abstract only and there was no information on our review outcomes. We contacted the investigators (twice) for more information but did not receive a reply. In the abstract report of Boscia 2008 no actual data were reported. In the results section, the following statement was made "No significant changes in all parameters were seen in untreated group. An improvement of far and near BCVA were seen in comparison with both baseline (ANOVA, p=0,008 and 0,000), and control group (t‐TEST, p=0,010 at p=0,000), with the greatest effect at week 24. In all treated eyes a complete resolution of subretinal fluid was observed, with significant reduction of central macular thickness. On week 24, in treated eyes, a significant improvement in mean fixation stability was also observed (ANOVA, p=0,011). No recurrence and/or adverse event occurred in any of the treated patients during the follow‐up".
Therefore, the following review outcomes were only available for Chan 2008.
Chan 2008 enrolled people with acute CSC, which was defined as less than three months' duration. The study enrolled 63 people (63 eyes) and followed up 58 people to 12 months. The study was low risk of bias in most domains and unclear for two domains: selective outcome reporting (where we did not have access to protocol or trials register entry to check this) and other bias (one of the authors declared a conflict of interest but this was only one of five authors and not the first author). Chan 2008 compared 'half dose' PDT (verteporfin 3 mg/m2) to sham PDT treatment (saline infusion and laser application as for PDT).
Mean change in best‐corrected visual acuity between baseline and 12 months
At 12 months, the visual acuity in the PDT group was better than the visual acuity in the sham PDT group (MD ‐0.10, 95% CI ‐0.18 to ‐0.02). We judged this to be low quality evidence, downgrading for imprecision (‐1) (as the CIs included a clinically unimportant effect) and risk of bias (‐1) (Analysis 5.1).
5.1. Analysis.
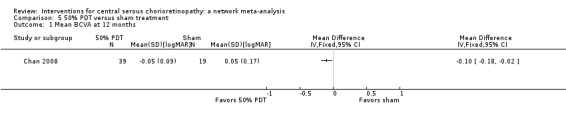
Comparison 5 50% PDT versus sham treatment, Outcome 1 Mean BCVA at 12 months.
Recurrence of central serous chorioretinopathy at 12 months
CSC resolved in 38 eyes in the PDT group and one of these 38 eyes had a recurrence by 12 months. In the control group, 15/19 resolved and four of these 15 had a recurrence (RR 0.10, 95% CI 0.01 to 0.81; Analysis 5.2). We judged this to be low quality evidence downgrading for risk of bias (‐1) and imprecision (‐1), as there were very few events.
5.2. Analysis.
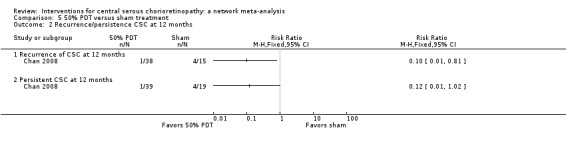
Comparison 5 50% PDT versus sham treatment, Outcome 2 Recurrence/persistence CSC at 12 months.
Persistent central serous chorioretinopathy at 12 months
One of 29 eyes had persistent CSC in the PDT group compared with 4/39 eyes in the control group (RR 0.12, 95% CI 0.01 to 1.02; Analysis 5.2). We judged this to be low quality evidence; downgrading for risk of bias (‐1) and imprecision (‐1).
Mean change in contrast sensitivity between baseline and 12 months
The trial did not report mean change in contrast sensitivity between baseline and 12 months
Mean change in central retinal thickness between baseline and 12 months
The CRT was thinner in the PDT group compared with the sham PDT group at 12 months (MD ‐117.00 μm, 95% CI ‐205.71 to ‐28.29; Analysis 5.3). We judged this to be low quality evidence, downgrading for risk of bias (‐1) and imprecision (‐1), as the CIs included a clinically unimportant effect.
5.3. Analysis.
Comparison 5 50% PDT versus sham treatment, Outcome 3 Mean CRT at 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
The trial did not report BCVA 20/40 or better at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
The trial did not report BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
The trial did not report quality of life at 12 months.
Adverse events
Chan 2008 reported that no systemic or ocular adverse events related to the PDT procedure were observed.
30% photodynamic therapy compared with 50% photodynamic therapy compared with photodynamic therapy
One study compared 30% PDT, 50% PDT, and PDT (Zhang 2012). This study was conducted in China and enrolled 90 eyes of 90 participants with 30 participants in each group and followed them up to 12 months. The type of CSC was not specified. We largely judged this study to be at unclear risk of bias because of problems with reporting.
One additional study, also conducted in China, compared 30% PDT with 50% PDT (Zhao 2015). This study enrolled 129 participants (129 eyes) and followed 117 participants to 12 months. We judged this study to be at low risk of bias in most domains with the exception of attrition bias (6% of the 30% PDT group were lost to follow‐up compared to 13% of the 50% PDT group) and selective outcome reporting (primary and secondary outcomes were designated differently on the trial register entry and the published report).
Both studies applied 50 J/cm2 light energy for 83 seconds but varied the dose of verteporfin: full dose (6 mg/m2), 50% dose (3 mg/m2), and 30% dose (2 mg/m2) (Zhang 2012), and 1.8 mg/m2 (Zhao 2015).
Mean change in best‐corrected visual acuity between baseline and 12 months
30% photodynamic therapy compared with photodynamic therapy
The mean change in visual acuity for the 30% PDT group was 0.03 logMAR (SD 0.02) and the mean change in the PDT group was 0.19 logMAR (SD 0.16). This gives an MD in favor of 30% PDT group (MD ‐0.16 logMAR, 95% CI ‐0.22 to ‐0.10; Analysis 6.1). We judged this to be moderate quality evidence downgrading for risk of bias (‐1).
6.1. Analysis.
Comparison 6 30% PDT versus PDT, Outcome 1 Mean BCVA at 12 months.
50% photodynamic therapy compared with photodynamic therapy
The mean change in visual acuity for the 50% PDT group was 0.23 logMAR (SD 0.15) and the mean change in the PDT group was 0.19 logMAR (SD 0.16). We judged this to be low quality evidence downgrading for risk of bias (‐1) and imprecision (‐1) due to wide CIs. We are uncertain as to the size of the effect (MD 0.04, 95% CI ‐0.04 to 0.12; Analysis 7.1).
7.1. Analysis.
Comparison 7 50% PDT versus PDT, Outcome 1 Mean BCVA at 12 months.
30% photodynamic therapy compared with 50% photodynamic therapy
The MD was favor of 30% PDT group over 50% PDT (MD ‐0.12, 95% CI ‐0.15 to ‐0.08; 177 participants; 2 studies; Analysis 8.1). We judged this to be moderate quality evidence downgrading for risk of bias (‐1).
8.1. Analysis.
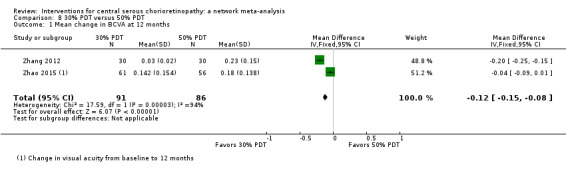
Comparison 8 30% PDT versus 50% PDT, Outcome 1 Mean change in BCVA at 12 months.
Recurrence of central serous chorioretinopathy at 12 months
30% photodynamic therapy compared with photodynamic therapy
CSC resolved in 30 eyes in the 30% PDT group and 22 of these 30 eyes had a recurrence by 12 months. In the control group, 30 eyes resolved and eight of these 30 had a recurrence (RR 2.75, 95% CI 1.46 to 5.17; Analysis 6.2). We judged this to be moderate quality evidence downgrading for risk of bias (‐1).
6.2. Analysis.
Comparison 6 30% PDT versus PDT, Outcome 2 Recurrence of CSC at 12 months.
50% photodynamic therapy compared with photodynamic therapy
CSC resolved in 30 eyes in the 50% PDT group and 10 of these 30 eyes had a recurrence by 12 months. In the PDT group, 30 eyes resolved and eight of these 30 had a recurrence (RR 1.25, 95% CI 0.57 to 2.73; 60 participants; 1 study; Analysis 7.2). We judged this low quality evidence, downgrading for risk of bias (‐1) and imprecision (‐1).
7.2. Analysis.
Comparison 7 50% PDT versus PDT, Outcome 2 Recurrence of CSC at 12 months.
30% photodynamic therapy compared with 50% photodynamic therapy
The risk of recurrence of CSC for 30% PDT at 12 months was 2.5 times higher than that of 50% PDT group (RR 2.50, 95% CI 1.54 to 4.06; 153 participants; Analysis 8.2). We judged this to be moderate quality evidence downgrading for risk of bias (‐1).
8.2. Analysis.
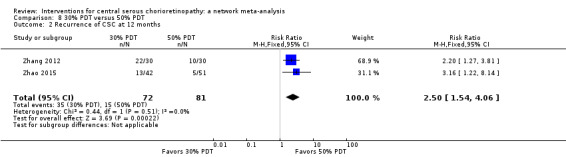
Comparison 8 30% PDT versus 50% PDT, Outcome 2 Recurrence of CSC at 12 months.
Persistent central serous chorioretinopathy at 12 months
30% photodynamic therapy compared with photodynamic therapy
Zhang 2012 did not report 30% PDT compared with PDT.
50% photodynamic therapy compared with photodynamic therapy
Zhang 2012 did not report 50% PDT compared with PDT.
30% photodynamic therapy compared with 50% photodynamic therapy
Data from Zhao 2015 showed that CSC was persistent in 19 of 61 eyes in the 30% PDT group and in four of 56 eyes in the 50% PDT group (RR 4.36, 95% CI 1.58 to 12.04; Analysis 8.3). We judged this to be moderate quality evidence downgrading for risk of bias (‐1).
8.3. Analysis.
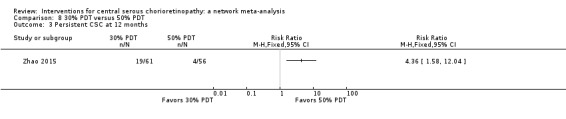
Comparison 8 30% PDT versus 50% PDT, Outcome 3 Persistent CSC at 12 months.
Mean change in contrast sensitivity between baseline and 12 months
Neither study reported mean change in contrast sensitivity between baseline and 12 months.
Mean change in central retinal thickness between baseline and 12 months
30% photodynamic therapy compared with photodynamic therapy
The mean change in central retinal thickness was reduced by 10.8 μm (SD 6.52) in the 30% PDT group and reduced by 52.13 µm (SD 9.06) in the PDT group. This means that on average there was a greater reduction in central retinal thickness in the PDT group (MD 41.33 µm, 95% CI 37.34 to 45.32; 60 participants; Analysis 6.3). We judged this to be moderate quality evidence downgrading for risk of bias (‐1).
6.3. Analysis.
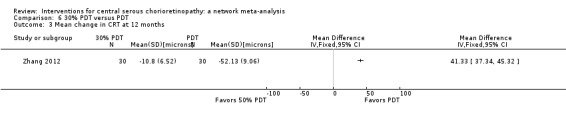
Comparison 6 30% PDT versus PDT, Outcome 3 Mean change in CRT at 12 months.
50% photodynamic therapy compared with photodynamic therapy
The mean change in central retinal thickness was reduced 55.66 μm (SD 0.21) in the 50% PDT group and reduced by 52.13 μm (SD 9.06) in the PDT group. This means that there was a small greater reduction in central retinal thickness in the 50% PDT group (MD ‐3.53, 95% CI ‐6.77 to ‐0.29; Analysis 7.3). We judged this to be low quality evidence downgrading for risk of bias (‐1) and imprecision (‐1).
7.3. Analysis.
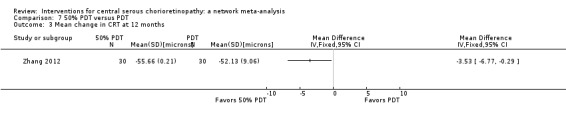
Comparison 7 50% PDT versus PDT, Outcome 3 Mean change in CRT at 12 months.
30% photodynamic therapy compared with 50% photodynamic therapy
There was a greater mean reduction in central retinal thickness in the 50% PDT group (MD 44.90, 95% CI 42.57 to 47.23; 177 participants; Analysis 8.4). We judged this to be moderate quality evidence downgrading for risk of bias (‐1).
8.4. Analysis.
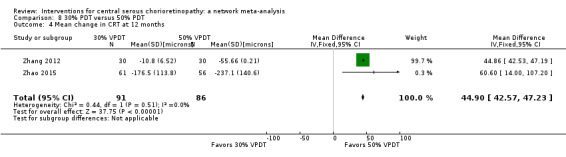
Comparison 8 30% PDT versus 50% PDT, Outcome 4 Mean change in CRT at 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
Neither study reported BCVA 20/40 or better at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
Neither study reported BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
Neither study reported quality of life at 12 months.
Adverse events
Zhang 2012 did not report on adverse events.
Zhao 2015 reported that no ocular adverse event occurred; one participant developed nausea because of an allergic reaction to verteporfin.
Laser versus observation or sham treatment
Four studies compared laser to observation or sham laser treatment.
Klatt 2011 was conducted in Germany and enrolled 30 eyes with acute CSC (30 participants) in a trial of micropulse laser ("selective retina therapy") compared with observation with follow‐up of three months. Selective retina therapy was Q‐switched neodymium‐doped yttrium lithium fluoride (Nd:YLF) laser with a wavelength of 527 nm. They used a spot diameter of 200 µm and pulse repetition rate of 100 Hz. We judged this study at high risk of performance and detection bias and the authors reported conflict of interest.
Leaver 1979 was conducted in the UK and compared direct argon laser photocoagulation to observation in 67 people with acute CSC. Four participants were lost to follow‐up at 12 months. With the exception of two subfoveal leaks, direct treatment was applied using the "Coherent Radiation 800" argon laser to the leaking spot using burns of 50 to 200 µm in diameter. The study was at high risk of performance and detection bias and otherwise was not clearly reported (being quite an old study) and was largely at unclear risk of bias for the other domains.
Robertson 1983 was conducted in the US and enrolled 41 participants (42 eyes) with acute CSC. The study sample was stratified according to the site of leakage. In eyes where the leakage site was in the papillomacular bundle, or within 500 µm of the capillary‐free zone of the macula, eyes were randomly assigned either to indirect argon laser photocoagulation or sham treatment. In eyes where the leakage site was outside the papillomacular bundle, and more than 500 µm from the capillary‐free zone, eyes were randomly allocated to direct or indirect laser photocoagulation ‐ this comparison is discussed below. Participants receiving indirect laser photocoagulation received three argon laser burns directed to the pigment epithelium in an area remote from the fovea, the papillomacular bundle, and the fluorescein leakage site. The laser beam diameter was 200 µm and the burn duration was 0.2 seconds at a power setting of 80 to 120 mW. In the sham technique, the laser beam was switched in the "off" position. We judged the study at low risk of performance and detection bias but unclear risk for other domains (being an older study relevant aspects were not reported).
Roisman 2013 was conducted in Brazil and compared micropulse diode laser to sham photocoagulation in 15 people with chronic CSC. The laser treatment consisted of subthreshold 810‐nm diode micropulse laser. We judged the study to be largely at low risk of bias.
Mean change in best‐corrected visual acuity between baseline and 12 months
This outcome was available for only two of the four studies (Klatt 2011; Roisman 2013). In Leaver 1979, Snellen acuities were converted to a numerical scale from 1 (6/4) to 5 (6/12). The mean acuity at six months was 3.1 in the laser group and 2.9 in the control group. In Robertson 1983, mean visual acuity was not reported.
The two studies that reported mean change in BCVA between baseline and 12 months had high statistical heterogeneity (I2 = 82%) but both were in favor of laser treatment (Analysis 9.1). In Klatt 2011, the difference between treatment and control at three months in improvement in vision was ‐0.13 logMAR (95% CI ‐0.24 to ‐0.01) in favor of selective retina therapy. In Roisman 2013, the difference between final visual acuity at six months was ‐0.38 logMAR (95% CI ‐0.56 to ‐0.20) in favor of micropulse diode laser treatment.
9.1. Analysis.
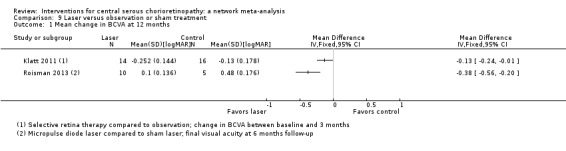
Comparison 9 Laser versus observation or sham treatment, Outcome 1 Mean change in BCVA at 12 months.
Recurrence of central serous chorioretinopathy at 12 months
One study reported on recurrence of CSC at 12 months (Robertson 1983). Similar numbers of the indirect laser group (5/15) compared with the sham laser group had a recurrence during the study (RR 1.25, 95% CI 0.41 to 3.77; Analysis 9.2). We judged this to be very low quality evidence downgrading for risk of bias (‐1) and imprecision (‐2).
9.2. Analysis.
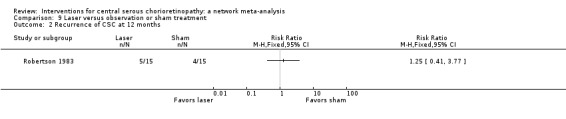
Comparison 9 Laser versus observation or sham treatment, Outcome 2 Recurrence of CSC at 12 months.
Persistent central serous chorioretinopathy at 12 months
None of the studies clearly reported persistent CSC at 12 months.
Mean change in contrast sensitivity between baseline and 12 months
None of the studies reported mean change in contrast sensitivity between baseline and 12 months
Mean change in central retinal thickness between baseline and 12 months
None of the studies reported mean change in central retinal thickness between baseline and 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
None of the studies reported BCVA 20/40 or better at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
None of the studies reported BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
None of the studies reported quality of life at 12 months.
Adverse events
Klatt 2011 reported that no adverse effects related to micropulse laser were observed.
Roisman 2013 did not specifically report on adverse events but did note "no evidence of retinal damage induced by the treatment".
Leaver 1979 and Robertson 1983 did not provide any information on adverse effects.
Micropulse diode laser versus argon laser
One trial conducted in India compared micropulse diode laser to argon laser (Verma 2004). The study enrolled 30 people with CSC (not specified whether acute or chronic) and allocated them to 810‐nm diode laser or 514‐nm argon laser and followed them up for 12 weeks. We judged the trial to be at low/unclear risk of bias.
Mean change in best‐corrected visual acuity between baseline and 12 months
This was reported in Snellen decimal acuity.
| Mean (SD) Snellen decimal acuity | ||
|
Micropulse diode laser (15 participants) |
Argon laser (15 participants) |
|
| Baseline | 0.29 (0.14) | 0.32 (0.16) |
| 12 weeks | 1.06 (0.09) | 0.98 (0.14) |
Recurrence of central serous chorioretinopathy at 12 months
None of the 30 participants had a recurrence before 12 weeks.
Persistent central serous chorioretinopathy at 12 months
None of the 30 participants had persistent CSC at 12 weeks.
Mean change in contrast sensitivity between baseline and 12 months
|
Mean absolute value of contrast sensitivity (Cambridge low contrast gratings) |
||
|
Micropulse diode laser (15 participants) |
Argon laser (15 participants) |
|
| Baseline | 98.4 (24.77) | 130.66 (31.95) |
| 12 weeks | 306.0 (46.57) | 215.33 (23.25) |
Mean change in central retinal thickness between baseline and 12 months
The study did not report mean change in central retinal thickness between baseline and 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
The study did not report BCVA 20/40 or better at 12 months
Best‐corrected visual acuity 20/200 or worse at 12 months
The study did not report BCVA 20/200 or worse at 12 months
Quality of life at 12 months
The study did not report quality of life at 12 month.
Adverse events
None of the participants developed subretinal neovascularization. No other complications or adverse effects were mentioned.
Indirect argon laser versus direct argon laser
One study compared indirect and direct argon laser (Robertson 1983). This study was conducted in the US and enrolled 41 participants (42 eyes) with acute CSC. In eyes where the leakage site was outside the papillomacular bundle, and more than 500 µm from the capillary‐free zone, eyes were randomly allocated to direct or indirect laser photocoagulation. Participants receiving indirect laser photocoagulation received three argon laser burns directed to the pigment epithelium. The laser beam diameter was 200 µm and the burn duration was 0.2 seconds at a power setting of 80 to 120 mW. We judged the study at low risk of performance and detection bias but unclear risk for other domains (being an older study relevant aspects were not reported).
Mean change in best‐corrected visual acuity between baseline and 12 months
The study did not report mean change in BCVA between baseline and 12 months.
Recurrence of central serous chorioretinopathy at 12 months
Three of five participants in the indirect group experienced a recurrence by 24 weeks compared with 0/7 in the direct laser group (RR 9.33, 95% CI 0.59 to 148.60; Analysis 10.1). We judged this to be very low quality evidence downgrading for risk of bias (‐1) and imprecision (‐2); we are very uncertain as to the size of the effect.
10.1. Analysis.
Comparison 10 Indirect argon laser versus direct argon laser, Outcome 1 Recurrence of CSC at 12 months.
Persistent central serous chorioretinopathy at 12 months
The study did not report persistent CSC at 12 months.
Mean change in contrast sensitivity between baseline and 12 months
The study did not report mean change in contrast sensitivity between baseline and 12 months.
Mean change in central retinal thickness between baseline and 12 months
The study did not report mean change in central retinal thickness between baseline and 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
The study did not report BCVA 20/40 or better at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
The study did not report BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
The study did not report quality of life at 12 months.
Adverse events
The study did not report adverse events.
Yellow versus red versus green wavelength laser
One study conducted in China compared yellow, red, and green wavelength laser in 90 participants with CSC of duration longer than eight weeks (Shang 1999). We largely judged the trial to be at unclear risk of bias.
Mean change in best‐corrected visual acuity between baseline and 12 months
The study did not report mean change in BCVA between baseline and 12 months.
Recurrence of central serous chorioretinopathy at 12 months
The relative effects of these wavelengths on this outcome were uncertain (Analysis 11.1). We graded these estimates as low quality evidence downgrading for risk of bias (‐1) and imprecision (‐1).
11.1. Analysis.
Comparison 11 Comparison of different laser wavelengths, Outcome 1 Recurrence of CSC at 12 months.
Yellow compared with red
RR 0.60, 95% CI 0.16 to 2.29; 60 eyes.
Yellow compared with green
RR 0.72, 95% CI 0.18 to 2.96; 59 eyes.
Red compared with green
RR 1.21, 95% CI 0.36 to 4.06; 59 eyes.
Persistent central serous chorioretinopathy at 12 months
The study did not report persistent CSC at 12 months.
Mean change in contrast sensitivity between baseline and 12 months
The study did not report mean change in contrast sensitivity between baseline and 12 months.
Mean change in central retinal thickness between baseline and 12 months
The study did not report mean change in central retinal thickness between baseline and 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
The study did not report BCVA 20/40 or better at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
The study did not report BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
The study did not report quality of life at 12 months.
Adverse events
The study did not report adverse events.
Antioxidant supplements versus placebo
Two studies compared antioxidant supplements to placebo.
Ratanasukon 2012 was conducted in Thailand and enrolled 58 participants (58 eyes) with acute CSC (onset within six weeks). These participants were randomly allocated to antioxidants or placebo and followed up at three and 12 months. There was high attrition at 12 months with only 14 people seen at that time point. The antioxidants were supplied as ICaps (Alcon Laboratories) in a version that contained vitamin A (6600 IU), vitamin C (400 mg), vitamin E (150 IU), riboflavin (10 mg), zinc (60 mg), copper (4 mg), selenium (40 mg), manganese (4 mg), and lutein/zeaxanthin (4000 μg). We judged the study at high risk of selection bias and attrition bias.
Sawa 2014 was conducted in Japan and investigated the effects of a lutein supplement (20 mg/day) with follow‐up of four months. The type of CSC and the total number of people randomized was not specified. The total number analyzed was 39. We judged the study at high risk of attrition bias and selective outcome reporting bias and it was funded by the manufacturer of the supplement.
The main aim of Sawa 2014 was to examine at changes in macular pigment optical density; none of our review outcomes were reported. The following outcomes are discuss for Ratanasukon 2012.
Mean change in best‐corrected visual acuity between baseline and 12 months
Visual acuity at 12 months was similar in the antioxidant and placebo groups (MD 0.01, 95% CI ‐0.04 to 0.06; 14 participants; Analysis 12.1). We judged this to be low quality evidence downgrading for risk of bias (‐2).
12.1. Analysis.
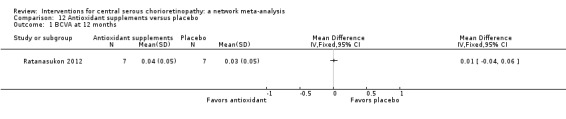
Comparison 12 Antioxidant supplements versus placebo, Outcome 1 BCVA at 12 months.
Recurrence of central serous chorioretinopathy at 12 months
Only three participants had a recurrence, one in the antioxidant group and two in the control group (RR 0.32, 95% CI 0.03 to 3.19; 36 participants; Analysis 12.2). With so few events, we are very uncertain as to the effect and judged this to be very low quality evidence downgrading for risk of bias (‐1) and imprecision (‐2).
12.2. Analysis.
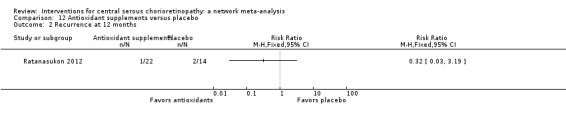
Comparison 12 Antioxidant supplements versus placebo, Outcome 2 Recurrence at 12 months.
Persistent central serous chorioretinopathy at 12 months
The paper did not report persistent CSC at 12 months clearly but the number of people with complete resolution was reported. People in the antioxidant group were less likely to have complete resolution (RR 0.35, 95% CI 0.13 to 0.95; 51 participants; Analysis 12.3). We judged this to be low quality evidence downgrading for risk of bias (‐1) and indirectness (‐1), as it was not precisely the outcome required.
12.3. Analysis.
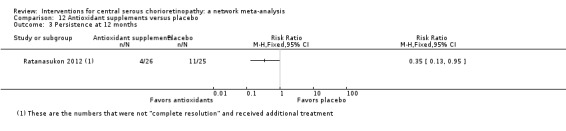
Comparison 12 Antioxidant supplements versus placebo, Outcome 3 Persistence at 12 months.
Mean change in contrast sensitivity between baseline and 12 months
The study did not report mean change in contrast sensitivity between baseline and 12 months.
Mean change in central retinal thickness between baseline and 12 months
There was a mean reduction of 180 µm (SD 15.05) in the antioxidant group compared to 186.22 µm reduction in the placebo group (MD ‐6.22, 95% CI ‐25.13 to 12.69; 14 participants; Analysis 12.4).
12.4. Analysis.
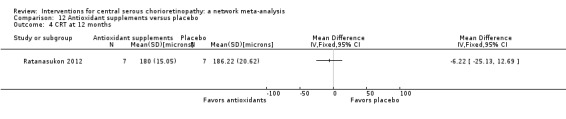
Comparison 12 Antioxidant supplements versus placebo, Outcome 4 CRT at 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
The study did not report BCVA 20/40 or better at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
The study did not report BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
The study did not report quality of life at 12 months.
Adverse events
The study reported "no significant side effects" at three months.
Beta‐blocker versus placebo
Two studies compared beta‐blocker to placebo. They were conducted in US (Browning 1993) and Iran (Kianersi 2008). The studies enrolled a total of 76 eyes (possibly 76 people) and followed them up to four months (Browning 1993) and 12 months (Kianersi 2008).
Browning 1993 looked at the effect of nadolol 40 mg/day in 16 participants and Kianersi 2008 investigated propranolol 40 mg/day delivered as 20 mg twice a day in 60 participants.
We judged both studies at low risk of detection bias because they were placebo controlled, but were largely judged at unclear risk of bias for the other domains because of poor reporting.
Browning 1993 was a short report and did not report any of the review outcomes. The following outcomes are discussed for Kianersi 2008 only (60 eyes).
Mean change in best‐corrected visual acuity between baseline and 12 months
Mean visual acuity in the propranolol group at 12 months was 0.98 logMAR (SD 0.13) and in the placebo group was 0.97 logMAR (SD 0.18) (MD 0.01 logMAR, 95% CI ‐0.07 to 0.09; 60 eyes; Analysis 13.1). We judged this to be moderate quality evidence downgrading for risk of bias (‐1).
13.1. Analysis.
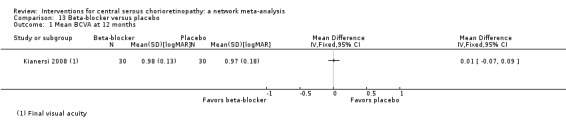
Comparison 13 Beta‐blocker versus placebo, Outcome 1 Mean BCVA at 12 months.
Recurrence of central serous chorioretinopathy at 12 months
The effect of beta‐blocker on recurrence was uncertain (RR 0.60, 95% CI 0.16 to 2.29; 60 eyes; Analysis 13.2). We judged this to be low quality evidence, downgrading for risk of bias (‐1) and imprecision (‐1).
13.2. Analysis.
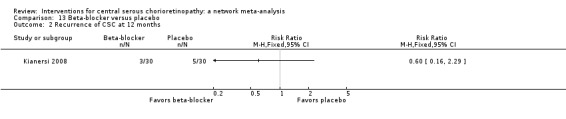
Comparison 13 Beta‐blocker versus placebo, Outcome 2 Recurrence of CSC at 12 months.
Persistent central serous chorioretinopathy at 12 months
The study did not report persistent CSC at 12 months.
Mean change in contrast sensitivity between baseline and 12 months
The study did not report mean change in contrast sensitivity between baseline and 12 months.
Mean change in central retinal thickness between baseline and 12 months
The study did not report mean change in central retinal thickness between baseline and 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
Similar numbers of people in the beta‐blocker and placebo groups had visual acuity 20/40 or better at 12 months (RR 1.10, 95% CI 0.81 to 1.49; 60 participants; Analysis 13.3). We judged this to be low quality evidence, downgrading for risk of bias (‐1) and imprecision (‐1).
13.3. Analysis.
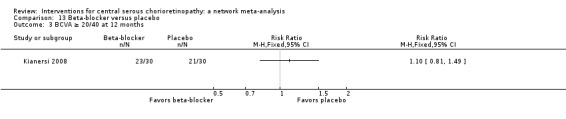
Comparison 13 Beta‐blocker versus placebo, Outcome 3 BCVA ≥ 20/40 at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
The study did not report BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
The study did not report quality of life at 12 months.
Adverse events
The study reported that no adverse events occurred.
Beta‐blocker versus calcium antagonist
One study compared beta‐blocker (propranolol) to a calcium antagonist in 25 participants affected with acute and chronic CSC (Brancato 1994). The study was reported in abstract form only and the abstract did not include any results. We contacted the trialists twice but received no reply.
Topical carbonic anhydrase inhibitors versus placebo
One study conducted in Mexico enrolled 13 participants with acute CSC (less than 20 days' duration) and randomly allocated them to brinzolamide (2%, twice a day) or placebo (polyvinyl alcohol) with follow‐up of six months (Ontiveros‐Orozco 2004). We largely judged the trial to be at unclear risk of bias.
Mean change in best‐corrected visual acuity between baseline and 12 months
The study did not report mean change in BCVA between baseline and 12 months.
Recurrence of central serous chorioretinopathy at 12 months
One of seven participants in the brinzolamide group had a recurrence compared with 4/6 participants in the placebo group (RR 0.21, 95% CI 0.03 to 1.43; Analysis 14.1). We judged this to be low quality evidence downgrading for risk of bias (‐1) and imprecision (‐1).
14.1. Analysis.
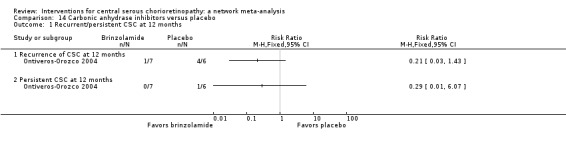
Comparison 14 Carbonic anhydrase inhibitors versus placebo, Outcome 1 Recurrent/persistent CSC at 12 months.
Persistent central serous chorioretinopathy at 12 months
None of seven participants in the brinzolamide group had persistent CSC compared with 1/6 participants in the placebo group (RR 0.29, 95% CI 0.01 to 6.07; Analysis 14.1). We judged this to be very low quality evidence downgrading for risk of bias (‐1) and imprecision (‐2).
Mean change in contrast sensitivity between baseline and 12 months
The study did not report mean change in contrast sensitivity between baseline and 12 months.
Mean change in central retinal thickness between baseline and 12 months
The study did not report mean change in central retinal thickness between baseline and 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
The study did not report BCVA 20/40 or better at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
The study did not report BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
The study did not report quality of life at 12 months.
Adverse events
The study noted no adverse effects of brinzolamide.
Helicobacter pylori treatment versus placebo or observation
Two studies compared H. pylori treatment to no treatment in people with acute CSC who also had H. pylori infection. Dang 2013 was conducted in China and compared H. pylori therapy to placebo in 53 participants with follow‐up of 12 weeks. Rahbani‐Nobar 2011 was conducted in Iran and compared H. pylori treatment with no treatment in 50 participants with follow‐up of 16 weeks. We judged Dang 2013 at low risk of bias in most domains; and Rahbani‐Nobar 2011 at high risk of performance and detection bias.
Mean change in best‐corrected visual acuity between baseline and 12 months
Visual acuity was slightly better in the H. pylori‐treated group (MD ‐0.04 logMAR, 95% CI ‐0.07 to ‐0.02; 103 participants; Analysis 15.1) Heterogeneity was high (I2 = 82%) but the two studies showed similar direction and order of effect. We judged this to be low quality evidence downgrading for risk of bias (‐1) and imprecision (‐1) as the CIs included a clinically unimportant effect.
15.1. Analysis.
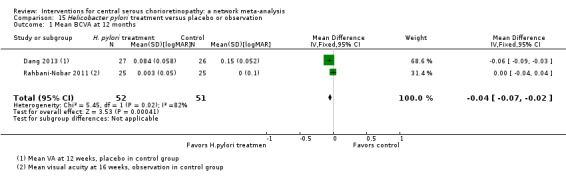
Comparison 15 Helicobacter pylori treatment versus placebo or observation, Outcome 1 Mean BCVA at 12 months.
Recurrence of central serous chorioretinopathy at 12 months
The studies did not report recurrence of CSC at 12 months.
Persistent central serous chorioretinopathy at 12 months
Fewer people in the H. pylori‐treated group had persistent CSC at 12 weeks (Dang 2013) or 16 weeks (Rahbani‐Nobar 2011) of follow‐up (RR 0.67, 95% CI 0.36 to 1.22; 103 participants; Analysis 15.2). We judged this to be low quality evidence, downgrading for risk of bias (‐1) and imprecision (‐1).
15.2. Analysis.
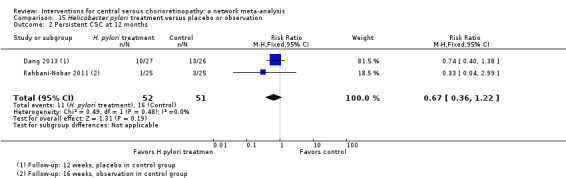
Comparison 15 Helicobacter pylori treatment versus placebo or observation, Outcome 2 Persistent CSC at 12 months.
Mean change in contrast sensitivity between baseline and 12 months
The studies did not report mean change in contrast sensitivity between baseline and 12 months.
Mean change in central retinal thickness between baseline and 12 months
The studies did not report mean change in central retinal thickness between baseline and 12 months.
Best‐corrected visual acuity 20/40 or better at 12 months
The studies did not report BCVA 20/40 or better at 12 months.
Best‐corrected visual acuity 20/200 or worse at 12 months
The studies did not report BCVA 20/200 or worse at 12 months.
Quality of life at 12 months
The studies did not report quality of life at 12 months.
Adverse events
Dang 2013 noted that no systemic or ocular adverse events occurred during follow‐up.
Rahbani‐Nobar 2011 noted that no systemic adverse effects of medication were observed.
Network meta‐analyses (direct and indirect comparisons)
As specified in Types of interventions section, we restricted our network to interventions applied directly to the eye.
We planned to look at three outcomes: visual acuity, recurrence, and adverse events. Almost all studies reported no adverse events, so we are unable to do this analysis.
Visual acuity
Description of network
Figure 4 shows the network plots. With so few trials included in the network it was difficult to assess transitivity (Table 2). It is possible that the inclusion of people with acute or chronic CSC may be different in the different comparisons. This would affect the validity of the network. Overall, there was no evidence for statistical inconsistency (P value = 0.3208). There was only one closed loop (anti‐VEGF, PDT, control) and again no evidence for inconsistency (inconsistency factor = 0.073, P value = 0.321). However, the power of these tests will be low and we cannot exclude the possibility of important inconsistency in the network.
4.
Visual acuity network: network plot, interval plot, contribution matrix and risk of bias.
AVG: Anti‐VEGF PDT: photodynamic therapy LAS: laser AVPDT: anti‐VEGF plus PDT.
1 = control; 2 = anti‐VEGF; 3 = PDT; 4 = laser; 5 = anti‐VEGF plus PDT.
1. Assessment of transitivity across treatment comparisons: visual acuity.
| Treatment comparison | Study | Type of CSC | Date study conducted | Industry sponsored |
| Anti‐VEGF vs. PDT |
Bae 2011 Semeraro 2012 |
Chronic Chronic |
2009‐2012 2009‐2010 |
Yes NR |
| PDT vs. no treatment | Chan 2008 | Acute | 2004‐2005 | NR |
| Laser vs. no treatment | Robertson 1983 | Acute | 1977‐1981 | No |
One additional study for the comparison PDT vs. no treatment was reported in abstract form only and no data on outcome so was not included in the network meta‐analysis (Boscia 2008).
anti‐VEGF: anti‐vascular endothelial growth factor; CSC: central serous chorioretinopathy; NR: not reported; PDT: photodynamic therapy.
Comparative effects
Table 3 summarizes the comparative effects. These are plotted in Figure 4.
2. Comparative effects of ocular interventions for central serous chorioretinopathy: visual acuity.
| Anti‐VEGF | ‐0.08 (‐0.14 to ‐0.01) | ‐0.20 (‐0.30 to ‐0.11) | 0.00 (‐0.02 to 0.03) | 0.22 (0.01 to 0.44) |
| 0.08 (0.01 to 0.14) | PDT | ‐0.13 (‐0.24 to ‐0.01) | 0.08 (0.01 to 0.15) | 0.30 (0.09 to 0.51) |
| 0.20 (0.11 to 0.30) | 0.13 (0.01 to 0.24) | Laser | 0.21 (0.11 to 0.31) | 0.43 (0.19 to 0.66) |
| ‐0.00 (‐0.03 to 0.02) | ‐0.08 (‐0.15 to ‐0.01) | ‐0.21 (‐0.31 to ‐0.11) | Anti‐VEGF and PDT | 0.22 (0.00 to 0.44) |
| ‐0.22 (‐0.44 to ‐0.01) | ‐0.30 (‐0.51 to ‐0.09) | ‐0.43 (‐0.66 to ‐0.19) | ‐0.22 (‐0.44 to ‐0.00) | Control (no treatment or sham treatment) |
Effect estimate is the mean difference (95% confidence interval). Negative values favor the first intervention. In the lower left hand triangle, the first intervention is anti‐VEGF, PDT, laser etc. In the upper right hand triangle, the first intervention is control, anti‐VEGF and PDT, laser etc. So, for example, visual acuity with anti‐VEGF was 0.22 logMAR units better than control 95% CI 0.44 better to 0.01 better.
anti‐VEGF: anti‐vascular endothelial growth factor; logMAR: logarithm of the minimal angle of resolution; PDT: photodynamic therapy.
An alternative formulation of the network based on the specific type of intervention resulted in two disconnected networks and we considered it unwise to proceed further.
Recurrence of central serous chorioretinopathy
Description of network
Figure 5 shows the network plot. There were no closed loops and, therefore, it was not possible to assess consistency. Table 4 shows the assessment of transitivity. Again with few trials it was difficult to assess but type of CSC was a concern.
5.
Recurrence CSC network: network plot, interval plot, contribution matrix and risk of bias.
AVG: Anti‐VEGF; PDT: photodynamic therapy; LAS: laser: CTL: control.
1 = control; 2 = anti‐VEGF; 3 = PDT; 4 = laser.
3. Assessment of transitivity across treatment comparisons: recurrence.
| Treatment comparison | Study | Type of CSC | Date study conducted | Industry sponsored |
| Anti‐VEGF vs. no treatment |
Kim 2013 Lim 2010 |
Acute Acute |
2010‐2011 2008 |
No No |
| Anti‐VEGF vs. PDT |
Bae 2011 Semeraro 2012 |
Chronic Chronic |
2009‐2012 2009‐2010 |
Yes NR |
| Anti‐VEGF + PDT vs. PDT alone | Coskun 2014 | Chronic | NR (published 2014) | NR |
| PDT vs. no treatment | Chan 2008 | Acute | 2004‐2005 | NR |
| Laser vs. no treatment |
Klatt 2011 Robertson 1983 Roisman 2013 |
Acute Acute Chronic |
2007‐2008 1977‐1981 NR (published 2013) |
NR No NR |
One additional study for the comparison PDT vs no treatment was reported in abstract form only and no data on outcome so was not included in the network meta‐analysis (Boscia 2008).
anti‐VEGF: anti‐vascular endothelial growth factor; CSC: central serous chorioretinopathy; NR: not reported; PDT: photodynamic therapy.
Comparative effects
Table 5 summarizes the comparative effects. These are plotted in Figure 5. All estimates were very uncertain. The contribution matrix shows that all the evidence either came from direct or indirect estimates, reflecting the lack of closed loops in the network (Figure 5).
4. Comparative effects of ocular interventions for CSC: recurrence.
| Anti‐VEGF | 0.27 (0.02 to 3.73) | 3.34 (0.01 to 788.57) | 2.67 (0.03 to 234.08) |
| 3.77 (0.27 to 52.94) | PDT | 12.58 (0.11 to 1503.87) | 10.07 (0.27 to 371.91) |
| 0.30 (0.00 to 70.79) | 0.08 (0.00 to 9.50) | Laser | 0.80 (0.03 to 18.46) |
| 0.37 (0.00 to 32.83) | 0.10 (0.00 to 3.67) | 1.25 (0.05 to 28.85) | Control |
Effect estimate is the risk ratio (95% CI).
anti‐VEGF: anti‐vascular endothelial growth factor; logMAR: logarithm of the minimal angle of resolution; PDT: photodynamic therapy.
Discussion
Summary of main results
Given that there were fewer than three studies per treatment comparison, that the available studies generally contained small numbers of participants, and given the methodological limits identified by this method of review, there are at the present time, insufficient data to make robust treatment recommendations. Of note, the present review did not identify a contraindication to observation as an initial management strategy in acute CSC.
Reporting of systemic or ocular adverse events were vague for most included studies. Studies would report "no systemic or ocular adverse events occurred" without reporting which systemic or ocular adverse events were being monitored. Thus, we were unable to make robust conclusions on potential adverse events for each intervention.
A brief summary of the mains results can be found in the Table 1.
We also identified 12 ongoing trials. In separate trials of acute CSC, PDT, aflibercept, and eplerenone are being compared to control (observation versus PDT, sham injection versus aflibercept, or placebo versus eplerenone). In separate trials of chronic CSC, spironolactone, eplerenone, and lutein are being compared to placebo, PDT is being compared to micropulse diode laser, and there are several head‐to‐head comparisons of PDT dose. In two trials, the type of CSC is not clearly specified on the trials register ‐ in these trials, micropulse diode laser is compared to observation and oral mifepristone to placebo.
Overall completeness and applicability of evidence
The present review did not identify a contraindication to current practice, which is observation as an initial management strategy in acute CSC. However, the identified studies do not sufficiently address all of the review objectives as there were too few included studies for each comparison to make robust conclusions. Furthermore, each study identified in this review had varying definitions of acute and chronic CSC, therefore, the review conclusions cannot be isolated to people with either acute or chronic CSC.
One further difficulty with the applicability of the evidence is that different treatments are indicated for different types of CSC: conventional laser may be directed to extrafoveal well‐defined leaks, micropulse laser to juxtafoveal leaks, PDT to subfoveal leaks (but may be less applicable to very large and ill‐defined leaking areas). Oral drugs are often used in any long‐standing fluid.
Quality of the evidence
Overall, with one or two exceptions, we judged the quality of the evidence to be low or very low. This means that further research is very likely to have an important impact on our confidence in the estimates of effect and is likely to change the estimates.
We included 25 studies that enrolled 1098 participants. In general, these trials were small, with fewer than 50 participants. These studies considered a variety of treatments and, in general, there was only one or two trials contributing data for each comparison. The studies were poorly reported and often it was unclear whether key aspects of the trial, such as allocation concealment, had been done. A substantial proportion of the trials were not masked (nine trials), which may put them at risk of performance and detection bias. Problems with follow‐up that may lead to attrition bias was also a potential problem in five trials.
We downgraded for risk of bias and imprecision for most analyses, reflecting the study limitations and imprecise estimates because of the small size of the included studies and few number of trials contributing to the analyses. Network meta‐analysis did not help to resolve this uncertainty due to a lack of trials to construct a reliable network and potential problems with intransivity with respect to acute or chronic CSC.
Potential biases in the review process
This review followed standard Cochrane methods and limited any potential biases in the review process.
Agreements and disagreements with other studies or reviews
To our knowledge, there is no systematic review that has included all interventions of CSC. We identified two reviews evaluating isolated comparisons. Specifically, authors of Chung 2013 evaluated the effects of intravitreal bevacizumab injection and reported that their results were inconclusive. The authors of Ma 2014 compared PDT with other therapy or compared different parameters of PDT. Overall, they concluded that "PDT is a promising therapy for CSC patients". They included case series and observational studies, which are at higher risk of bias and uncontrolled confounding, and they did not distinguish trials and citations, leading to some double counting of results.
For outcomes such as adverse effects that may occur infrequently, trial evidence may not be the best source of information. One review of over 200 participants with chronic CSC treated with PDT concluded that adverse effects were rare. RPE atrophy occurred in 4% of participants and acute severe visual decrease occurred in 1.5% (Lim 2014).
Authors' conclusions
Implications for practice.
Given that there were fewer than three studies per treatment comparison, that the available studies generally contained small numbers of participants, and given the methodological limits identified by this method of review, there are, at the present time, insufficient data to inform treatment decisions. Of note, the present study did not identify a contraindication to observation as an initial management strategy in acute central serous chorioretinopathy (CSC).
Implications for research.
Overall, when considering all of the studies reviewed, and the methods applied, the quality of the presently available data is low or very low. This means that further research is very likely to have an important impact on confidence in the estimates of effect, and is also very likely to change the estimates. This finding identifies well‐designed, prospectively randomized controlled trials with larger numbers of participants, examining the safety and efficacy of treatments for CSC, as potentially high impact areas of study. Due to the recurrent and chronic nature of CSC, the appropriate treatment is one that can result in visual improvement, shorten the duration of symptoms, and reduce the recurrent rate. The benefit will likely extend to patients, care providers, and funders. The somewhat stronger evidence in the areas of thermal laser for focal leakage and the various photodynamic therapy (PDT) regimens suggests that further work to define their risks and benefits, as well as to identify the pertinent patients and entry points for such interventions, is a logical point of emphasis for future trials. Given the complexity of CSC, the following features could be considered in future trials: duration of symptoms and fluid, extent of retinal pigment epithelium derangement, and number and delimitation of leaks. For laser/PDT whether treatment is applied to leaks or RPE abnormalities or choroidal hyperpermeability should be considered. Future research on CSC would benefit from development of a 'core outcome set' including standardized measures of visual acuity and definition of persistence and recurrence, potentially including these as one outcome.
Acknowledgements
We thank Iris Gordon, Trials Search Co‐ordinator for Cochrane Eyes and Vision, for creating and executing the electronic search strategies. We gratefully acknowledge Barbara Hawkins, Kristina Lindsley, Adriani Nikolakopoulou, and Gianni Virgili for their comments to this protocol.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Central Serous Chorioretinopathy] explode all trees #2 central serous near/2 (retinopath* or chorioretinopath*) #3 retinitis near/3 (angiospastic* or serosa or capillarospastic or capillarospastic or angiosplastic) #4 retinopath* near/3 (angiospastic* or serosa or capillarospastic or capillarospastic or angiosplastic) #5 central* near/3 (retinitis or serosa) #6 #1 or #2 or #3 or #4 or #5
Appendix 2. MEDLINE (Ovid) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. Central Serous Chorioretinopathy/ 14. (central serous adj2 (retinopath$ or chorioretinopath$)).tw. 15. (retinitis adj3 (angiospastic$ or serosa or capillarospastic or capillarospastic or angiosplastic)).tw. 16. (retinopath$ adj3 (angiospastic$ or serosa or capillarospastic or capillarospastic or angiosplastic)).tw. 17. (central$ adj3 (retinitis or serosa)).tw. 18. or/13‐17 19. 12 and 18
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (Ovid) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. central serous retinopathy/ 34. (central serous adj2 (retinopath$ or chorioretinopath$)).tw. 35. (retinitis adj3 (angiospastic$ or serosa or capillarospastic or capillarospastic or angiosplastic)).tw. 36. (retinopath$ adj3 (angiospastic$ or serosa or capillarospastic or capillarospastic or angiosplastic)).tw. 37. (central$ adj3 (retinitis or serosa)).tw. 38. or/33‐37 39. 32 and 38
Appendix 4. ISRCTN search strategy
(central serous retinopathy) OR (central serous chorioretinopathy)
Appendix 5. ClinicalTrials.gov search strategy
central serous retinopathy OR central serous chorioretinopathy
Appendix 6. ICTRP search strategy
"central serous retinopathy"
"central serous chorioretinopathy"
Appendix 7. Data on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design | · Parallel group RCTi.e. people randomized to treatment · Within‐person RCTi.e. eyes randomized to treatment · Cluster RCTi.e. communities randomized to treatment · Cross‐over RCTi.e. people randomized to sequence of treatment · Other, specify |
Exclusions after randomization Losses to follow‐up Number randomized/analyzed How were missing data handled? e.g., available case analysis, imputation methods Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Unit of randomization/unit of analysis | · One eye included in study, specify how eye selected · Two eyes included in study, both eyes received same treatment, briefly specify how analyzed(best/worst/average/both and adjusted for within person correlation/both and not adjusted for within person correlation) and specify if mixture one eye and two eye · Two eyes included in study, eyes received different treatments,specify if correct pair‐matched analysis done |
|
| Participants | ||
| Country | Setting Ethnic group Equivalence of baseline characteristics (Y/N) |
|
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are only reported for the people who were followed up only, please indicate. | |
| Number (%) of men and women | ||
| Average age and age range | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | ||
| Intervention (n= ) Comparator (n= ) |
· Number of people randomized to this group · Drug (or intervention) name · Dose · Frequency · Route of administration |
|
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports | List outcomes Adverse events reported (Y/N) Length of follow‐up and intervals at which outcomes assessed |
Planned/actual length of follow‐up |
Data and analyses
Comparison 1. Anti‐VEGF versus observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in BCVA at 12 months | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.02, 0.03] |
| 2 Mean change in CRT at 12 months | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 8.73 [‐18.08, 35.54] |
Comparison 2. Anti‐VEGF versus low fluence PDT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in BCVA at 12 months | 2 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.08, 0.15] |
| 2 Recurrence of CSC at 12 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Persistent CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Mean change in CRT at 12 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Anti‐VEGF plus 50% PDT versus 50% PDT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in BCVA at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Persistent CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in CRT at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Six‐dose anti‐VEGF versus four‐dose anti‐VEGF.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in BCVA at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Mean change in CRT at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
4.2. Analysis.
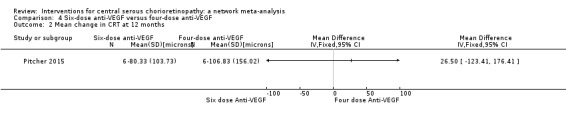
Comparison 4 Six‐dose anti‐VEGF versus four‐dose anti‐VEGF, Outcome 2 Mean change in CRT at 12 months.
Comparison 5. 50% PDT versus sham treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean BCVA at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence/persistence CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Persistent CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean CRT at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. 30% PDT versus PDT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean BCVA at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in CRT at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. 50% PDT versus PDT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean BCVA at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in CRT at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 8. 30% PDT versus 50% PDT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in BCVA at 12 months | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.15, ‐0.08] |
| 2 Recurrence of CSC at 12 months | 2 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.54, 4.06] |
| 3 Persistent CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Mean change in CRT at 12 months | 2 | 177 | Mean Difference (IV, Fixed, 95% CI) | 44.90 [42.57, 47.23] |
Comparison 9. Laser versus observation or sham treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in BCVA at 12 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mean change in CRT at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
9.3. Analysis.
Comparison 9 Laser versus observation or sham treatment, Outcome 3 Mean change in CRT at 12 months.
Comparison 10. Indirect argon laser versus direct argon laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 11. Comparison of different laser wavelengths.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Yellow compared with red | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Yellow compared with green | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Red compared with green | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Antioxidant supplements versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BCVA at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Persistence at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 CRT at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 13. Beta‐blocker versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean BCVA at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 BCVA ≥ 20/40 at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 14. Carbonic anhydrase inhibitors versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent/persistent CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Recurrence of CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Persistent CSC at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Helicobacter pylori treatment versus placebo or observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean BCVA at 12 months | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.07, ‐0.02] |
| 2 Persistent CSC at 12 months | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.36, 1.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bae 2011.
| Methods |
Study design: parallel randomized controlled trial Number randomized: 16 eyes of NR participants in anti‐VEGF group 18 eyes of NR participants in PDT group Exclusions after randomization: 0 in anti‐VEGF group 0 in PDT group Number analyzed: 16 eyes of NR participants in anti‐VEGF group 18 eyes of NR participants in PDT group Unit of analysis: mixed, some participants had 1 eye included, some participants had both eyes included Losses to follow‐up: 2 eyes of participants in anti‐VEGF group 0 in PDT group How were missing data handled?: NA Power calculation: power = 80% and sample size = 34 eyes total (17 eyes in each group) |
|
| Participants |
Country: South Korea
Mean age (SD) (years): 50.8 (7.7) overall 48.9 (7.5) in anti‐VEGF group 51.4 (8.2) in PDT group Gender (%): 28 men (82%) and 6 women (18%) in Total group 13 men (81%) and 3 women (19%) in anti‐VEGF group 15 men (83%) and 3 women (17%) in PDT group Participants with chronic CSC were included as defined as: "chronic CSC with visual disturbance persisting for >6 months or recurrent CSC. Recurrent CSC was defined as the recurrence of serous retinal detachment on optical coherence tomography (OCT) associated with visual symptoms after complete recovery of ocular manifestations; the first episode occurred >6 months before screening and the current episode persisted >3 months with sustained SRF on OCT" Inclusion criteria: BCVA 0‐1.0 logMAR; presence of SFF persisting for > 3 months on OCT; presence of multifocal/diffuse RPE decompensation with leakage on the FA; and choroidal vascular hyperpermeability and abnormal dilation of the choroidal vasculature on ICGA Exclusion criteria: history of treatment including PDT, focal laser photocoagulation, intravitreal injection of steroid or anti‐VEGF agent in the study eye; evidence of CNV or polypoidal choroidal vasculopathy; any other ocular diseases that can affect visual acuity, including diabetic retinopathy, retinal vascular occlusion, or ocular inflammatory diseases; media opacity that interferes with adequate image acquisition; history of any intraocular surgery except uncomplicated cataract surgery > 3 months before enrollment; history of systemic steroid or anti‐VEGF treatment in the preceding 12 months; uncontrolled glaucoma with intraocular pressure > 21 mm Hg despite treatment; uncontrolled hypertension, diabetes, or history of cerebrovascular accident or myocardial infarction; and pregnancy Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: anti‐VEGF (ranibizumab) Dose: 0.5 mg/0.05 mL Frequency: baseline, 1 and 2 months Control: low‐fluence PDT Light dose: 25 J/cm2 Verteporfin concentration: 6 mg/m2 |
|
| Outcomes | Length of follow‐up: 12 months Primary outcome, as defined in study reports: primary efficacy outcome was proportion of eyes that maintained the complete absorption of SRF until 12 months without any rescue treatment Secondary outcomes, as defined in study reports: serial changes from baseline of mean change in logMAR BCVA; mean change in CRT obtained by OCT; proportion of eyes with resolved leakage on FA; proportion of eyes with resolved choroidal hyperpermeability on ICGA; and fluid‐free interval, which was defined as the interval between the time when the SRF was completely absorbed without any rescue treatment and when re‐accumulation of the fluid occurred Adverse events reported: yes Intervals at which outcomes assessed: 1, 3, 6, 9, and 12 months | |
| Notes |
Full study name: Low‐fluence photodynamic therapy versus ranibizumab for chronic central serous chorioretinopathy: one‐year results of a randomized trial
Type of study: published full‐text
Funding sources: Novartis Korea, Seoul, Korea
Disclosures of interest: reported explicitly none of the authors had any financial relationship Trial registry: NCT01325181 (clinicaltrials.gov) Study period: July 2009 to September 2012 (as reported in the trial registry) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized to receive low‐fluence PDT or the intravitreal injections of ranibizumab with an equal allocation ratio by means of permuted block randomization" p. 559 |
| Allocation concealment (selection bias) | Unclear risk | "Subjects and the treating ophthalmologist (S.H.B.) were not masked to the treatment modalities" p. 559 Unclear if the allocation was masked before enrollment |
| Masking of participants and personnel (performance bias) | High risk | "Subjects and the treating ophthalmologist (S.H.B.) were not masked to the treatment modalities" p. 559 |
| Masking of outcome assessment (detection bias) | Low risk | "The investigator (J.H.) and the other examiners for BCVA measurement, OCT, FA, and ICGA were masked to treatment allocation" p. 559 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/34 participants (< 10%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes on trial registry entry reported in paper |
| Other bias | High risk | Received industry funding (Novartis Korea, Seoul, Korea). Stated that "the sponsor or funding organization had no role in the design or conduct of this research. No conflicting relationship exists for any author" |
Boscia 2008.
| Methods |
Study design: unclear ‐ refers only to eyes
Number randomized: 8 eyes of NR participants in PDT group 8 eyes of NR participants in observation group Exclusions after randomization: not reported Number analyzed: NR Unit of analysis: unclear Losses to follow‐up: NR How were missing data handled?: NA Power calculation: NR |
|
| Participants |
Country: Italy (probably)
Mean age (SD) (years): NR for overall and by group Gender (%): did not report number of men and women overall or by group Participants with chronic CSC were included as defined as: not defined Inclusion criteria: BCVA 0.2‐1 logMAR; presence of SRF or serous pigment epithelial detachment on OCT (or both) without regression for ≥ 3 months, RPE leakage on FA and choroidal vascular hyperpermeability on confocal SLO‐ICGA Exclusion criteria: any previous treatment for CSC; evidence of other chorioretinal disorders; media opacities; and treatment with systemic steroids Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: low‐fluence PDT light dose: 25 J/cm2 Verteporfin concentration: NR Control: observation |
|
| Outcomes | Length of follow‐up: 24 weeks Primary outcome, as defined in study reports: far BCVA (logMAR, using ETDRS charts) and near BCVA (logMAR, using MNRead Acuity Charts); CMT (OCT3, Zeiss‐Humphrey) Secondary outcomes, as defined in study reports: macular sensitivity and stability of fixation determined using microperimetry (Nidek MP1) Adverse events reported: yes Intervals at which outcomes assessed: 1, 4, 12, and 24 weeks | |
| Notes |
Full study name: Low fluence photodynamic therapy in chronic central serous chorioretinopathy: blind randomized clinical trial of efficacy and safety
Type of study: published abstract
Funding sources: NR
Disclosures of interest: NR Trial registry: not registered Study period: NR Contacted study authors and received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | High risk | Different groups and the study described as being "blind" but no information on masking |
| Masking of outcome assessment (detection bias) | High risk | Different groups and the study described as being "blind" but no information on masking |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Brancato 1994.
| Methods |
Study design: cross‐over randomized controlled trial
Number randomized: NR eyes of 25 participants in total, By group NR Exclusions after randomization: NR Number analyzed: NR Unit of analysis: unclear Losses to follow‐up: NR How were missing data handled?: NA Power calculation: NR Unusual study design: "two weeks of treatment with propranolol, then one week of wash‐out; two weeks of treatment with nimodizin and again one of wash‐out; two weeks of placebo treatment" |
|
| Participants | Participants with both acute or chronic CSC were enrolled Country: NR Inclusion criteria: NR Equivalence of baseline characteristics: unclear |
|
| Interventions |
Intervention: beta‐blocker (propranolol) Dose: NR Frequency: NR Duration: NR Control: calcium antagonist (nimodizin) Dose: NR Frequency: NR Duration: NR |
|
| Outcomes |
Length of follow‐up: NR
Primary outcome: no outcomes defined Secondary outcomes: no outcomes defined Adverse events reported: no Intervals at which outcomes assessed: NR |
|
| Notes |
Full study name: Treatment of central serous chorioretinopathy with beta‐blockers and calcium antagonists
Type of study: published abstract
Funding sources: NR
Disclosures of interest: NR Trial registry: not registered Study period: NR Contacted study authors and received no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Both interventions were pills but no information on how similar the pills were |
| Masking of outcome assessment (detection bias) | Unclear risk | Both interventions were pills but no information on how similar the pills were |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Browning 1993.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: NR eyes of 8 participants in beta‐blocker group NR eyes of 8 participants in placebo group Exclusions after randomization: NR Number analyzed: NR Unit of analysis: individual Losses to follow‐up: NR How were missing data handled?: NR Power calculation: NR |
|
| Participants |
Country: US Mean age (SD) (years): 41.5 (NR) in total 41 (NR) in beta‐blocker group 42 (NR) in placebo group Gender (%): 9 men (56%) and 7 women (44%) in total 4 men (50%) and 4 women (50%) in beta‐blocker group 5 men (63%) and 3 women (38%) in placebo group Type of CSC not specified Inclusion criteria: "all patients had serous retinal detachments and consistent fluorescein angiograms. no patient had vitreous cells, uncontrolled hypertension, recent pregnancy, or other causes of serous retinal detachment" Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: beta‐blocker (nadolol) Dose: 40 mg Frequency: daily Duration: NR Control: placebo |
|
| Outcomes | Length of follow‐up: 4 months Primary and secondary outcomes not differentiated Adverse events reported: no Intervals at which outcomes assessed: 4 months | |
| Notes |
Full study name: Nadolol in the treatment of central serous retinopathy
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: NR Trial registry: not registered Study period: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "I evaluated the effect of the nonselective beta‐blocker nadolol in a prospective, randomized, double‐masked trial" p. 770 No further information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Low risk | Placebo‐controlled trial |
| Masking of outcome assessment (detection bias) | Low risk | Placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Chan 2006.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 15 eyes of 15 participants in total By group NR Exclusions after randomization: NR Number analyzed: NR Unit of analysis: individual Losses to follow‐up: NR How were missing data handled?: NR Power calculation: NR |
|
| Participants | Type of CSC not specified Country: US Mean age (SD) (years): Total and by group NR Gender (%): total and by group NR Inclusion criteria: NR Equivalence of baseline characteristics: unclear |
|
| Interventions |
Intervention: low‐dose transpupillary thermotherapy Control: sham laser |
|
| Outcomes |
Length of follow‐up: 3 months
Primary and secondary outcomes not differentiated Outcomes: proportion of eyes with resolved CSC Adverse events reported: no Intervals at which outcomes assessed: 3 months |
|
| Notes |
Full study name: Low‐dose transpupillary thermotherapy for the treatment of central serous chorioretinopathy
Type of study: published abstract
Funding sources: NR
Disclosures of interest: NR Trial registry: not registered Study period: NR Contacted study authors who were unable to provide a reference to the full‐text |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Fifteen eyes of 15 patients with CSR [CSC] were randomly assigned" No further information on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Control group had sham therapy but no details on how this was done |
| Masking of outcome assessment (detection bias) | Unclear risk | Control group had sham therapy but no details on how this was done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Chan 2008.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 42 eyes of 42 participants in PDT group 21 eyes of 21 participants in placebo group Exclusions after randomization: 0 in PDT group 0 in placebo group Number analyzed: 39 eyes of 39 participants in PDT group 19 eyes of 19 participants in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 3 eyes of 3 participants in PDT group 2 eyes of 2 participants in placebo group How were missing data handled?: NR Power calculation: power = 85% and sample size = yes, power = 85% and sample size = 63 participants total (42 participants in PDT group and 21 participants in placebo group) |
|
| Participants |
Participants with acute CSC were included and defined as: acute symptomatic CSC of ≤ 3 months' duration Country: China Mean age (SD) (years): 41.0 (6.7) in total 40.3 (5.8) in PDT group 42.4 (8.4) in placebo group Gender (%): 54 men (86%) and 9 women (14%) in total 38 men (90%) and 4 women (10%) in PDT group 16 men (76%) and 5 women (24%) in placebo group Inclusion criteria: people with BCVA ≥ 20/200; presence of SRF involving the fovea on OCT; presence of active angiographic leakage on FA caused by CSC but not CNV or other diseases; and abnormal dilated choroidal vasculature and other features on ICGA consistent with the diagnosis of CSC Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: 50% PDT Light dose: 50 J/cm2 Verteporfin concentration: 3 mg/m2 Control: placebo (30 mL normal saline infused instead of verteporfin, and laser applied in the same manner as in the verteporfin group) |
|
| Outcomes | Length of follow‐up: 12 months Primary outcome, as defined in study reports: proportion of eyes with complete absorption of SRF at 12 months Secondary outcomes, as defined in study reports: serial changes in logMAR BCVA, OCT CFT, FA, and ICGA, and systemic and ocular complications during the study Adverse events reported: yes Intervals at which outcomes assessed: 1, 3, 6, 9, and 12 months | |
| Notes |
Full study name: Half‐dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one‐year results of a randomized controlled trial
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: "Dr Lai has served as a consultant to an advisory board of Novartis, Inc" Trial registry: not registered Study period: December 2004 to December 2005, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was generated using a computerized randomization table kept centrally by a research nurse, and the group allocation was performed before drug preparation and infusion" p. 1757 |
| Allocation concealment (selection bias) | Low risk | "The randomization sequence was generated using a computerized randomization table kept centrally by a research nurse, and the group allocation was performed before drug preparation and infusion. All patients and investigators were masked to the treatment allocation group by wrapping the infusion syringes externally with aluminum foil" p. 1757 |
| Masking of participants and personnel (performance bias) | Low risk | "All patients and investigators were masked to the treatment allocation group by wrapping the infusion syringes externally with aluminum foil" p. 1757 |
| Masking of outcome assessment (detection bias) | Low risk | "All patients and investigators were masked to the treatment allocation group by wrapping the infusion syringes externally with aluminum foil" p. 1757 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall 5/58 participants (< 10%) were lost to follow‐up; 3/39 PDT group lost to follow‐up; 2/19 of placebo group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Source of funding not reported. A conflict of interest was declared but only for 1 of the 5 authors and not the first author: "Dr Lai has served as a consultant to an advisory board of Novartis, Inc. All other authors have no financial interest to declare" |
Coskun 2014.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 8 eyes of 8 participants in anti‐VEGF group 7 eyes of 7 participants in PDT group Exclusions after randomization: NR Number analyzed: 8 eyes of 8 participants in anti‐VEGF group 7 eyes of 7 participants in PDT group Unit of analysis: participant, 1 eye per person Losses to follow‐up: NR How were missing data handled?: NR Power calculation: NR |
|
| Participants |
Country: Turkey
Mean age (SD) (years): NR in total 46.5 (11.5) in anti‐VEGF group 56.1 (7.5) in PDT group Gender (%): NR Participants with chronic CSC were included and defined as: eyes with symptomatic chronic CSC (duration 6 months) Inclusion criteria: eyes with symptomatic chronic CSC Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: 50% PDT + anti‐VEGF (bevacizumab) Light dose: 50 J/cm2 Verteporfin concentration: 3 mg/m2 Bevacizumab dose: 1.25 mg Bevacizumab duration: single dose, 3 days after PDT Control: 50% PDT Light dose: 50 J/cm2 Verteporfin concentration: 3 mg/m2 |
|
| Outcomes |
Length of follow‐up: 12 months
Primary and secondary outcomes not differentiated Outcomes: time to CSC resolution; BCVA; CMT Adverse events reported: no Intervals at which outcomes assessed: 1, 3, 6, and 12 months |
|
| Notes |
Full study name: Combined half dose photodynamic therapy with verteporfin and intravitreal bevacizumab for chronic central serous chorioretinopathy
Type of study: published abstract
Funding sources: NR
Disclosures of interest: NR Trial registry: not registered Study period: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | Groups different and no mention of masking |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Dang 2013.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 32 eyes of 32 participants in Helicobacter pylori treatment group 32 eyes of 32 participants in placebo group Exclusions after randomization: NR Number analyzed: 27 eyes of 27 participants in H. pylori group 26 eyes of 26 participants in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 5 eyes of 5 participants in H. pylori group 6 eyes of 6 participants in placebo group How were missing data handled?: NR Power calculation: NR |
|
| Participants |
Country: China
Mean age (SD) (years): NR in total 35.66 (5.47) in H. pylori group 34.85 (5.53) in placebo group Gender (%): 43 men (81%) and 10 women (19%) in total 22 men (81%) and 5 women (19%) in H. pylori group 21 men (81%) and 5 women (19%) in placebo group Participants with chronic CSC were included and defined as: duration > 12 weeks Inclusion criteria: single idiopathic leakage detected by FA excluding any other diseases; SRF confirmed by OCT (3D OCT‐2000; TOPCON Corporation, Tokyo, Japan); H. pylori infection diagnosed according to a specific protocol 1 pupillary diameter); diffused retinal pigment epitheliopathy; aged < 20 years old and > 70 years old; and pregnancy, steroid use, and any other systemic diseases Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention:Helicobacter pylori treatment Drug (dose): omeprazole 20 mg, clarithromycin 500 mg, and amoxicillin 1000 mg Frequency: twice a day Duration: 14 days Control: placebo |
|
| Outcomes | Length of follow‐up: 12 weeks Primary and secondary outcomes not differentiated Outcomes: disappearance rate of SRF; BCVA; and central retinal sensitivity Adverse events reported: yes, but not descriptive; "during the follow‐up visit, no systemic or ocular adverse events occurred" p. 358 Intervals at which outcomes assessed: 2, 4, 8, and 12 weeks | |
| Notes |
Full study name: The effect of eradicating Helicobacter pylori on idiopathic central serous chorioretinopathy patients
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: reported explicitly none of the authors has any financial relationship Trial registry: not registered Study period: September 2010 to December 2012, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned through a web‐based data entry system maintained at the Data Coordinating Center (The MEDABC Corporation, Zhengzhou, Henan, People's Republic of China), with equal probability of receiving either H. pylori eradication (referred to as the active treatment group) or placebo drugs (referred to as the control group) using a permuted‐block design with random block sizes" p. 356 |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned through a web‐based data entry system maintained at the Data Coordinating Center (The MEDABC Corporation, Zhengzhou, Henan, People's Republic of China), with equal probability of receiving either H. pylori eradication (referred to as the active treatment group) or placebo drugs (referred to as the control group) using a permuted‐block design with random block sizes" p. 356 |
| Masking of participants and personnel (performance bias) | Low risk | "The control group received an identical placebo that was the same color, size, and had the same identification name as the treatment. The placebos were taken in the same manner as the study drugs. Both drugs were also in identical opaque bottles and prepared by one nonclinician research assistant" p. 356 |
| Masking of outcome assessment (detection bias) | Low risk | "The control group received an identical placebo that was the same color, size, and had the same identification name as the treatment. The placebos were taken in the same manner as the study drugs. Both drugs were also in identical opaque bottles and prepared by one nonclinician research assistant" p. 356 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "A total of 64 eyes in 64 patients were enrolled and randomized equally into two groups. Eleven eyes (17.18%) were lost to follow‐up or did not yield enough data (five eyes in the active treatment group and six eyes in the control group). A total of 53 eyes (82.81%) were included in the study." p. 357 Although similar drop‐outs loss to follow‐up was approaching 20% and no information on reasons for loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry not available for comparison |
| Other bias | Low risk | Source of monetary support not reported and conflict of interest was declared: "the authors report no conflicts of interest in this work" |
Kianersi 2008.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 30 eyes of 30 participants in beta‐blocker group 30 eyes of 30 participants in placebo group Exclusions after randomization: 0 in beta‐blocker group 0 in placebo group Number analyzed: 30 eyes of 30 participants in beta‐blocker group 30 eyes of 30 participants in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 0 in beta‐blocker group 0 in placebo group How were missing data handled?: NR Power calculation: NR |
|
| Participants |
Country: Iran
Mean age (SD) (years): 35 (8) in total 34 (7) in beta‐blocker group 36 (8) in placebo group Gender (%): 44 men (73%) and 16 women (27%) in total 23 men (77%) and 7 women (23%) in beta‐blocker group 21 men (70%) and 9 women (30%) in placebo group The type of CSC was not specified Inclusion criteria: no contraindication for propranolol use; no other eye disease such as cataract, retinal disorders, etc., which causes diminish visual activity; no indication for laser therapy Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: beta‐blocker (propranolol) Dose: 20 mg Frequency: twice daily Duration: NR Control: placebo |
|
| Outcomes | Length of follow‐up: 12 months Primary and secondary outcomes not differentiated Outcomes: psychological tension; subretinal fleck; recurrence of CSC; visual acuity Adverse events reported: yes, laser therapy; kidney transplantation; multiple sclerosis; kidney disease caused by steroid use Intervals at which outcomes assessed: weekly for 1 year | |
| Notes |
Full study name: Effects of propranolol in patients with central serous chorioretinopathy
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: NR Trial registry: not registered Study period: 2003 to 2004, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients met the inclusion criteria were referred to the second author (FF), and according to the table of random numbers, they were assigned randomly in two groups; half of patients received propranolol (treatment group) and the other half received placebo with the shape and color similar to propranolol" p. 104 |
| Allocation concealment (selection bias) | Unclear risk | "Patients met the inclusion criteria were referred to the second author (FF), and according to the table of random numbers, they were assigned randomly in two groups; half of patients received propranolol (treatment group) and the other half received placebo with the shape and color similar to propranolol" p. 104 |
| Masking of participants and personnel (performance bias) | Low risk | "Patients met the inclusion criteria were referred to the second author (FF), and according to the table of random numbers, they were assigned randomly in two groups; half of patients received propranolol (treatment group) and the other half received placebo with the shape and color similar to propranolol" p. 104 |
| Masking of outcome assessment (detection bias) | Unclear risk | "Patients met the inclusion criteria were referred to the second author (FF), and according to the table of random numbers, they were assigned randomly in two groups; half of patients received propranolol (treatment group) and the other half received placebo with the shape and color similar to propranolol" p. 104 This suggests that the research staff may have been unmasked to the treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Kim 2013.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 25 eyes of 25 participants in anti‐VEGF group 25 eyes of 25 participants in placebo group Exclusions after randomization: NR Number analyzed: 20 eyes of 20 participants in anti‐VEGF group 20 eyes of 20 participants in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 5 eyes of 5 participants in anti‐VEGF group 5 eyes of 5 participants in placebo group How were missing data handled?: NR Power calculation: NR |
|
| Participants |
Country: South Korea
Mean age (SD) (years): 43.05 (7.46) in anti‐VEGF group 41.40 (7.80) in placebo group Gender (%): 22 men (55%) and 18 women (45%) in total 12 men (60%) and 8 women (40%) in anti‐VEGF group 10 men (50%) and 10 women (50%) in placebo group Participants with acute CSC were included and defined as: < 3 months' duration Inclusion criteria: NR Exclusion criteria: people who had received any previous treatment, including PDT or focal thermal laser photocoagulation for CSC, or who had evidence of CNV, polypoidal choroidal vasculopathy, or other maculopathy on fundus examination, FA, or ICGA Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: anti‐VEGF (ranibizumab) Dose: 0.5 mg/mL Frequency: single dose at baseline Control: observation |
|
| Outcomes | Length of follow‐up: 6 months Primary outcome, as defined in study reports: time from baseline to complete resolution of neurosensory retinal detachment during follow‐up Secondary outcomes, as defined in study reports: serial changes in logMAR BCVA, OCT CFT, FA, and ICGA and the systemic and ocular complications during the study Adverse events reported: yes Intervals at which outcomes assessed: 1, 2, 3, 4, 5, 6 months | |
| Notes |
Full study name: Intravitreal ranibizumab for acute central serous chorioretinopathy
Type of study: published full‐text
Funding sources: 2012 Research Grant from Kangwon National University
Disclosures of interest: NR Trial registry: not registered Study period: January 2010 to December 2011, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized into the IVRI [anti‐VEGF] group or the observation group at a ratio of 1: 1" p. 153 No details on how the random allocation generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | High risk | Masking not reported and treatments different (injection vs. no treatment) |
| Masking of outcome assessment (detection bias) | High risk | Masking not reported and treatments different (injection vs. no treatment) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported how many were randomized by group and loss to follow‐up by group. Final numbers analyzed identical between treatment and observation group (20/20) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry not available for comparison |
| Other bias | Low risk | Funding from non‐profit and conflict of interest was not reported. "This study was supported by 2012 Research Grant from Kangwon" |
Klatt 2011.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 14 eyes of NR participants in micropulse laser group ("selective retina therapy") 16 eyes of NR participants in placebo group Exclusions after randomization: 0 in micropulse laser group ("selective retina therapy") 0 in placebo group Number analyzed: 14 eyes of number of participants NR in micropulse laser group ("selective retina therapy") 16 eyes of number of participants NR in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 0 in micropulse laser group ("selective retina therapy") 0 in placebo group How were missing data handled?: NR Power calculation: power = 80% and sample size = 62 eyes |
|
| Participants |
Country: Germany
Mean age (SD) (years): 43.8 (5.6) in total 42.8 (5.5) in micropulse laser group ("selective retina therapy") 44.7 (5.7) in placebo group Gender (%): 26 men (87%) and 4 women (13%) in total 14 men (100%) and 0 women (0%) in micropulse laser group ("selective retina therapy") 12 men (75%) and 4 women (25%) in placebo group Participants with acute CSC were included and defined as: acute symptomatic CSC and a documented disease progression of at least 3 months' duration Inclusion criteria: minimum age 18 years; minimum history 3 months of reduced visual acuity; minimum BCVA of 20 ETDRS letters (20/200); presence of SRF on OCT; and presence of active angiographic leakage in FA Exclusion criteria: other retinal diseases; glaucoma; cataract or other media opacities, which preclude color fundus photography and FA; previous PDT or continuous wave laser photocoagulation for CSC; systemic corticosteroid treatment, Cushing disease, renal diseases, pregnancy, and breastfeeding Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: micropulse laser ("selective retina therapy") Q‐switched neodymium‐doped yttrium lithium fluoride (Nd:YLF) laser wavelength: 527 nm spot diameter; 200 µm pulse repetition rate: 100 Hz Control: observation |
|
| Outcomes | Length of follow‐up: 3 months Primary outcome, as defined in study reports: change of best corrected Early Treatment Diabetic Retinopathy Study visual acuity (BCVA); change in SRF as measured by OCT Secondary outcomes, as defined in study reports: rate of complete absorption of SRF; presence of leakage in FA; systemic or ocular adverse effects Adverse events reported: yes Intervals at which outcomes assessed: 1 and 3 months | |
| Notes |
Full study name: Selective retina therapy for acute central serous chorioretinopathy
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: "Johann Roider, Ralf Brinkmann and Reginald Birngruber hold patents on selective retina therapy. Carsten Klatt, Mark Saeger, Till Oppermann, Erk Porksen, Felix Treumer, Jost Hillenkamp and Elfriede Fritzer have no competing interests" Trial registry: NCT00987077 (clinicaltrials.gov) Study period: April 2007 to June 2008, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered, opaque, sealed envelopes were prepared on the basis of the accomplished randomisation" p. 84 |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | "At all visits, BCVA was assessed using ETDRS charts at 4 m distance. The investigator was blinded" p. 84 Masking for other outcomes not reported and visual acuity assessment may be affected by the fact that the patient knew which group they were in |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All patients kept their follow‐up appointment and were included in the analysis" p. 84 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as stated in the trials registry entry (NCT00987077; clinicaltrials.gov) |
| Other bias | High risk | Source of monetary support was not reported and conflict of interest was declared: "Johann Roider, Ralf Brinkmann and Reginald Birngruber hold patents on selective retina therapy. Carsten Klatt, Mark Saeger, Till Oppermann, Erk Porksen, Felix Treumer, Jost Hillenkamp and Elfriede Fritzer have no competing interests" |
Leaver 1979.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: NR eyes of 35 participants in argon laser group NR eyes of 35 participants in placebo group Exclusions after randomization: NR Number analyzed: NR eyes of 32 participants in argon laser group NR eyes of 31 participants in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: NR eyes of 3 participants in argon laser group NR eyes of 4 participants in placebo group How were missing data handled?: NR Power calculation: NR |
|
| Participants |
Country: UK
Mean age (SD) (years): 40.1 (NR) in total NR in argon laser group NR in placebo group Gender (%): 53 men (84%) and 10 women (16%) in total NR in argon laser group NR in placebo group Participants with both acute or chronic CSC were enrolled Inclusion criteria: corrected visual acuity ≥ 6/12; retina detached at macula; RPE defects < 1 disc diameter; no symptomatic improvement since onset; no subretinal exudates present; no cystic retinal edema present; no associated ocular disease (e.g. drusen, congenital pit of the disc, generalized RPE dystrophy, etc.); consent to participate in the study after explanation of aims and methods Equivalence of baseline characteristics: NR |
|
| Interventions | Intervention: argon laser (direct) Control: observation | |
| Outcomes | Length of follow‐up: 12.1 years Primary and secondary outcomes not differentiated Outcomes: time to recovery; visual acuities; hue discrimination Adverse events reported: no Intervals at which outcomes assessed: "weekly intervals for 4 weeks and then at monthly intervals until symptoms improved, the retina flattened, and there was no identifiable leakage on fluoresce in fundus angiography (FFA). Thereafter examinations were carried out at 3‐ and 6‐monthly intervals. Longest follow‐up 12 years" | |
| Notes |
Full study name: Argon laser photocoagulation in the treatment of central serous retinopathy
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: NR Trial registry: not registered Study period: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on how the random allocation generated: "randomised cards from sealed envelopes" p. 675 |
| Allocation concealment (selection bias) | Unclear risk | "...randomised cards from sealed envelopes" (p. 675) but not enough information on how sealed envelopes were prepared e.g. sequentially number? opaque? |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | Groups different and no mention of masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/70 (10%) missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Lim 2010.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 32 eyes of number of participants NR in total By group NR Exclusions after randomization: NR Number analyzed: 12 eyes of 12 participants in anti‐VEGF group 12 eyes of 12 participants in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 8 eyes of number of participants NR in total; by group NR How were missing data handled?: NR Power calculation: NR |
|
| Participants |
Country: South Korea
Mean age (SD) (years): 43.2 (9.0) in total 45.6 (10.4) in anti‐VEGF group 40.7 (7.0) in placebo group Gender (%): 20 men (83%) and 4 women (17%) in total 9 men (75%) and 3 women (25%) in anti‐VEGF group 11 men (92%) and 1 women (8%) in placebo group Participants with acute CSC were included and defined as: "patients with symptomatic CSC of less than a 3‐month duration" Inclusion criteria: diagnosis of CSC was established by the presence of serous macular detachment on fundus examination and dilated choroidal vasculature and hyperpermeability on ICGA Exclusion criteria: participants who had received any previous treatment, including PDT or focal thermal laser photocoagulation for CSC, or who had evidence of CNV, polypoidal choriovasculopathy, or other maculopathy on clinical examination, FA, or ICGA Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: anti‐VEGF (bevacizumab) Dose: 1.25 mg/0.05 mL Frequency: single dose injected < 1 week after diagnosis Control: observation |
|
| Outcomes | Length of follow‐up: 6 months Primary outcome, as defined in study reports: time measured from baseline to complete absorption of SRF during follow‐up Secondary outcomes, as defined in study reports: serial changes in the logMAR visual acuity and OCT central 1‐mm macular thickness Adverse events reported: yes Intervals at which outcomes assessed: 1, 2, 3, 4, 5, and 6 months | |
| Notes |
Full study name: The effect of intravitreal bevacizumab in patients with acute central serous chorioretinopathy
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: reported explicitly none of the authors has any financial relationship Trial registry: not registered Study period: March 2008 to August 2008, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was generated using a computerized randomization table" p. 156 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | Groups different and no mention of masking |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/32 (25%) of participants not followed up and not reported which group they were in |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Low risk | Source of monetary support not reported and conflict of interest declared: "no potential conflict of interest relevant to this article was reported" |
Ontiveros‐Orozco 2004.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 16 participants were included in the study, but allocation not reported NR eyes of NR participants in carbonic anhydrase inhibitor group NR eyes of NR participants in placebo group Exclusions after randomization: 3 Number analyzed: Number of eyes NR of 7 participants in carbonic anhydrase inhibitor group Number of eyes NR of 6 participants in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: NR How were missing data handled?: NA Power calculation: NR |
|
| Participants |
Participants with acute CSC were included and define as: participants with "symptoms for less than 20 days, no previous treatment" Country: Mexico Mean age (SD) (years): NR in total NR in carbonic anhydrase inhibitors group NR in placebo group Gender (%): 9 men (69%) and 4 women (31%) in total 4 men (57%) and 3 women (43%) in carbonic anhydrase inhibitors group 5 men (83%) and 1 women (17%) in placebo group Inclusion criteria: CSC idiopathic; fluorangiography showing hyperfluorescence associated with serous retinal pigment epithelial detachment; with 3 months of follow‐up after recovery and provide informed consent Exclusion criteria: "any patients older than 50 years, patients that had any additional pre‐existing ocular pathology or systematic pathology. Any patients were eliminated that did not comply with their appointments, studies, or that had received any medications during their development" Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: carbonic anhydrase inhibitors (brinzolamide) Dose: 2% Duration: twice daily Control: placebo (polyvinyl alcohol) |
|
| Outcomes | Length of follow‐up: 6 months Primary outcome, as defined in study reports: time to clinical recovery Secondary outcomes, as defined in study reports: "complications: RPE atrophy, recurrence, persistence of subretinal fluid and appearance of neovascular membranes. Each complication received a value according to the severity (from 0 for the absence of complications to 4 for the presence of neovascular membranes)" Adverse events reported: reported no adverse effects Intervals at which outcomes assessed: weekly for the first month, then monthly for 3 months, and 6 months | |
| Notes |
Full study name: Brinzolamide for topical treatment coroidorretinopatía idiopathic central serous
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: NR Trial registry: not registered Study period: June 2002 to October 2003, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | The labeling and drug control was done by third person not related with the study p. 134 |
| Masking of participants and personnel (performance bias) | Unclear risk | Similar groups (both groups' treatment were eye drops), but no information on masking |
| Masking of outcome assessment (detection bias) | Unclear risk | Similar groups (both groups' treatment were eye drops), but no information on masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Pitcher 2015.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 6 eyes of 6 participants in 6‐dose group 6 eyes of 6 participants in 4‐dose group Exclusions after randomization: 0 in 6‐dose group 0 in 4‐dose group Number analyzed: 6 eyes of 6 participants in 6‐dose group 6 eyes of 6 participants in 4‐dose group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 0 in 6‐dose group 0 in 4‐dose group How were missing data handled?: NA Power calculation: NR |
|
| Participants |
Country: USA
Mean age (SD) (years): NR in total 53.50 (9.61) in 6‐dose group 53.83 (12.80) in 4‐dose group Gender (%): all participants were men Participants with chronic CSC were included as defined as: "persistent CSCR [CSC] demonstrated by subfoveal fluid (SFF) on optical coherence tomography (OCT) for greater than 3 months" Inclusion criteria: age ≥ 18 years; ability to provide informed consent; persistent CSC demonstrated by SFF on OCT for > 3 months; active leakage on FA at the time of enrolment; evidence of hyperpermeability and abnormal dilation of choroidal vasculature on ICGA; BCVA between 20/25 and 20/320. Only 1 eye for each participant was included in participants with bilateral CSC Exclusion criteria: concurrent progressive retinal or substantial ocular disease in the study eye; prior treatment for CSC in the study eye (anti‐VEGF, PDT, or laser) within 3 months prior to enrolment; presence of CNV or polypoidal choroidal vasculopathy on enrolment imaging; history of intraocular surgery except uncomplicated cataract surgery > 3 months prior to enrolment; prior treatment with systemic anti‐VEGF agents or steroid agents within the preceding 12 months; uncontrolled glaucoma; history of cerebrovascular accident or myocardial infarction; pregnancy Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: 6‐dose group (aflibercept) Dose: 2.0 mg/0.05 mL Duration: single dose at baseline, 1, 2, 3, 4, and 5 weeks Control: 4‐dose group (aflibercept) Dose: 2.0 mg/0.05 mL Duration: single dose at baseline, 1, 2, and 4 weeks |
|
| Outcomes |
Length of follow‐up: 6 months
Primary and secondary outcomes not differentiated Outcomes listed as: occurrence of ocular or systemic adverse events; mean change from baseline in ETDRS letter score; percentage of eyes with 20/40 or better vision; percentage of eyes with ≥ 15‐letter gain from baseline; percentage of eyes with < 15‐letter loss from baseline; mean change in CMT; mean change in SFF manually measured by a masked observer; percentage of eyes with complete resolution of macular fluid on OCT; mean change in subfoveal choroidal thickness as measured via enhanced depth imaging; and percentage of eyes with absence of leakage on FA Adverse events reported: yes Intervals at which outcomes assessed: 6 months |
|
| Notes |
Full study name: A prospective pilot study of intravitreal aflibercept for the treatment of chronic central serous chorioretinopathy: the CONTAIN study
Type of study: published full‐text
Funding sources: Regeneron Pharmaceuticals
Disclosures of interest: "none" Trial registry: NCT01710332 (clinicaltrials.gov) Study period: November 2012 to May 2013, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised to two groups" p. 849 Did not clear describe method of randomization |
| Allocation concealment (selection bias) | Unclear risk | Did not describe clearly how the sequence generation was assigned/stored |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking not reported |
| Masking of outcome assessment (detection bias) | Unclear risk | SFF height was determined by a masked observer using the digital caliper function to measure distance from the hyper‐reflective RPE to the photoreceptor outer segments on a b‐scan through the foveal center point. Choroidal thickness was determined by a masked observer using the digital caliper to measure distance from the inner border of the choroido‐scleral interface to the hyper‐reflective RPE |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | High risk | Did not clearly define the primary and secondary outcomes in the published full‐text, while the trial registry had primary and secondary outcomes were clearly defined. Reported 5 additional outcomes not defined in the trial registry: "mean change in CMT; mean change in SFF manually measured by a masked observer; percentage of eyes with complete resolution of macular fluid on OCT; mean change in subfoveal choroidal thickness as measured via enhanced depth imaging; and percentage of eyes with absence of leakage on FA |
| Other bias | High risk | Industry funding |
Rahbani‐Nobar 2011.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: NR eyes of 25 participants in Helicobacter pylori group NR eyes of 25 participants in placebo group Exclusions after randomization: 0 in H. pylori group 0 in placebo group Number analyzed: NR eyes of 25 participants in H. pylori group NR eyes of 25 participants in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 0 in H. pylori group 0 in placebo group How were missing data handled?: NA Power calculation: NR |
|
| Participants |
Participants with acute CSC were included but definition not given Country: Iran Mean age (SD) (years): NR in total 32.54 (4.57) in H. pylori group 34.24 (4.78) in placebo group Gender (%): 41 men (82%) and 9 women (18%) in total 21 men (84%) and 4 women (16%) in H. pylori group 20 men (80%) and 5 women (20%) in placebo group Inclusion criteria: to accept participation in the study by signing an informed consent; not having been treated with antibiotics, a proton pomp inhibitor, corticosteroids, or sympathomimetic drugs for 3 months before the study; no history of previous ocular surgery Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention:H. pylori Drugs: metronidazole and amoxicillin Dose: 500 mg Frequency: 3 times daily Duration: 2 weeks Drug: omeprazole Dose: NR Frequency: once daily Duration: 6 weeks Control: observation |
|
| Outcomes |
Length of follow‐up: 16 weeks
Primary and secondary outcomes not differentiated Outcomes: average neuroretinal and/or pigment epithelial detachment; number of cases that reached zero subretinal fluid value; subretinal fluid level; subretinal fluid reabsorption time; mean visual acuity Adverse events reported: yes Intervals at which outcomes assessed: 2, 4, 6, 8, 12, and 16 weeks |
|
| Notes |
Full study name: The effect of Helicobacter pylori treatment on remission of idiopathic central serous chorioretinopathy
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: NR Trial registry: NCT00817245 (clinicaltrials.gov) Study period: February 2008 to January 2010, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were divided in two groups using random allocation software" p. 100 |
| Allocation concealment (selection bias) | Unclear risk | "The patients were divided in two groups using random allocation software" p. 100, but no information specifically on allocation concealment |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | Groups different and no mention of masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Outcomes on clinical trials registry entry (NCT00817245) were reported in the full text |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Ratanasukon 2012.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 29 eyes of 29 participants in antioxidant group 29 eyes of 29 participants in placebo group Exclusions after randomization: NR Number analyzed: 26 eyes of 26 participants in antioxidant group 25 eyes of 25 participants in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 3 eyes of 3 participants in antioxidant group 4 eyes of 4 participants in placebo group How were missing data handled?: NR Power calculation: power = 80% and sample size = 58 participants in total (29 participant in each group) |
|
| Participants |
Country: Thailand
Mean age (SD) (years): 40.4 (NR) in total 41.28 (5.07) in antioxidant group 39.48 (6.95) in placebo group Gender (%): 48 men (83%) and 10 women (17%) in total 23 men (79%) and 6 women (21%) in antioxidant group 25 men (86%) and 4 women (14%) in placebo group Participants with acute CSC were included and defined as: "onset within 6 weeks" Inclusion criteria: people with acute CSC (within 6 weeks of onset), aged 30‐50 years, new or recurrent attack (symptom‐free ≥ 6 months), FA‐confirmed diagnosis with inkblot or smoke‐stack leakage, OCT by Status OCT showing definite SRF and the people's ability for proper follow‐up Exclusion criteria: chronic CSC (> 6 weeks), multiple attacks (> 2 times), large pigment epithelial detachment (> 1 disc diameter), multiple pigment epithelial detachment or diffuse retinal pigment epitheliopathy, younger or older ages, follow‐up time < 3 months, complicated CSC such as secondary CNV detected from FA, pregnancy, steroid use and people with contraindication from high‐dose antioxidant therapy such as heavy smokers, and people with lung cancer, thyrotoxicosis, renal stones and anemia (hematocrit < 30%) Equivalence of baseline characteristics: NR |
|
| Interventions | Intervention: antioxidant (Icaps: vitamin A (6600 IU); vitamin C (400 mg), vitamin E (150 IU); riboflavin (10 mg); zinc (60 mg); copper (4 mg); selenium (40 mg); manganese (4 mg) and lutein/zeaxanthin (4000 μg)) Control: placebo | |
| Outcomes | Length of follow‐up: 3 months planned but 12 months reported (with high attrition) Primary outcome, as defined in study reports: change in visual acuity; change in CMT recorded by OCT during every visit Secondary outcomes, as defined in study reports: number of participants with SRF at each follow‐up time, the number of participants who showed FA leakage at the end of the 3rd month and participants who received additional treatments in each group Adverse events reported: yes Intervals at which outcomes assessed: 1, 2, 3, 6, and 12 months | |
| Notes |
Full study name: High‐dose antioxidants for central serous chorioretinopathy; the randomized placebo‐controlled study
Type of study: published full‐text
Funding sources: "study and placebo drugs were contributed by Alcon Laboratories (Thailand)"
Disclosures of interest: reported explicitly none of the authors has any financial relationship Trial registry: NCT00963131 (clinicaltrials.gov) Study period: December 2004 to December 2008, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomly assigned to high‐dose antioxidant or placebo drugs. The randomization was computer generated with a 1:1 ratio, block lengths of 4 and random numbers were coded to all bottles. Moreover, the codes were in envelops until the end of the study" p. 2 |
| Allocation concealment (selection bias) | High risk | "The corresponding author generated the allocation sequence, enrolled and assigned the patients to any additional treatments when needed" p. 2 |
| Masking of participants and personnel (performance bias) | Low risk | "The patients were randomly assigned to high‐dose antioxidant or placebo drugs. The randomization was computer generated with a 1:1 ratio, block lengths of 4 and random numbers were coded to all bottles. Moreover, the codes were in envelops until the end of the study" p. 2 |
| Masking of outcome assessment (detection bias) | Low risk | "The patients were randomly assigned to high‐dose antioxidant or placebo drugs. The randomization was computer generated with a 1:1 ratio, block lengths of 4 and random numbers were coded to all bottles. Moreover, the codes were in envelops until the end of the study" p. 2 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/29 lost to follow‐up at 3 months in intervention group and 4/29 lost to follow‐up in control group but at 12 months only 7 participants seen in each group. So low risk of bias for 3 months and high risk of bias for 12 months outcomes |
| Selective reporting (reporting bias) | Low risk | Trial registry entry was available for comparison, and all outcomes were reported as per the trial registry |
| Other bias | Unclear risk | "Study and placebo drugs were contributed by Alcon Laboratories (Thailand)" but authors reported that none of the authors had any financial relationship |
Robertson 1983.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 15 eyes of 15 participants in argon (direct) laser group 15 eyes of 15 participants in sham laser group 7 eyes of 7 participants in argon (direct) laser group 5 eyes of 5 participants in argon (indirect) laser group Exclusions after randomization: NR Number analyzed: 15 eyes of 15 participants in argon (direct) laser group 15 eyes of 15 participants in sham laser group 7 eyes of 7 participants in argon (direct) laser group 5 eyes of 5 participants in argon (indirect) laser group Unit of analysis: participant, 1 eye per person Losses to follow‐up: NR How were missing data handled?: NR Power calculation: NR Unusual study design: eyes were divided into 2 groups depending on the site of leakage (determined by FA) "We assigned eyes in which the leakage site was in the papillomacular bundle or within 500 μm of the capillary‐free zone of the macula to Group A" "We assigned eyes in which the leakage site was outside the papillomacular bundle and more than 500 μm from the capillary‐free zone to Group B" |
|
| Participants |
Country: US
Mean age (SD) (years): NR in total 39.7 (NR) in argon (direct) laser group NR in sham laser group 41.3 (NR) in argon (direct) laser group 44.8 (NR) in argon (indirect) laser group Gender (%): 29 men (69%) and 13 women (31%) in total 10 men (67%) and 5 women (33%) in (direct) laser group 9 men (60%) and 6 women (40%) in sham laser group 5 men (71%) and 2 women (29%) in (direct) laser group 5 men (100%) and 0 women (0%) in (indirect) laser group Participants with acute CSC were included and defined as: "central serous chorioretinopathy of recent onset" p. 458 Inclusion criteria: no evidence of previous ocular surgery, trauma, photocoagulation, cloudy media, vitreous inflammation, retinal vessel occlusion, hypertensive or diabetic retinopathy, vitreoretinal traction, congenital optic pits, mass lesion, or any other unusual retinal or choroidal abnormality Equivalence of baseline characteristics: NR |
|
| Interventions | Group A intervention: argon laser (indirect laser photocoagulation) Group A control: sham laser Group B intervention: argon laser (direct laser photocoagulation) Group B control: argon laser (indirect laser photocoagulation | |
| Outcomes | Length of follow‐up: examined at 6 months recurrences reported to 18 months Primary and secondary outcomes not differentiated Outcomes: resolution of CSC as determined by 2 observers by following criteria: subretinal fluid absent on biomicroscopic exam and stereoscopic FA showed no active leakage; visual acuity measured by Sloan chart; visual fields Adverse events reported: no Intervals at which outcomes assessed: 2 weeks, 6 months, 18 months | |
| Notes |
Full study name: Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy
Type of study: published full‐text
Funding sources: Grant EY0 1709 from the National Eye Institute and by the Mayo Foundation
Disclosures of interest: NR Trial registry: not registered Study period: May 1977 to January 1981, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on how the random allocation generated: "After the patient was positioned at the laser, a sealed envelope containing the randomized treatment assignment was opened. The assignments, worked out and kept by the statistician, directed the physician (D.M.R. in all cases) to administer direct, indirect, or sham laser photocoagulation" p. 459 |
| Allocation concealment (selection bias) | Low risk | "After the patient was positioned at the laser, a sealed envelope containing the randomized treatment assignment was opened. The assignments, worked out and kept by the statistician, directed the physician (D.M.R. in all cases) to administer direct, indirect, or sham laser photocoagulation." p. 459 |
| Masking of participants and personnel (performance bias) | Low risk | "The subject did not learn which of the three forms of treatment he or she had received until the study ended six months later" pp. 459‐460 |
| Masking of outcome assessment (detection bias) | Unclear risk | Similar groups (both groups' treatment were laser), but no information on masking of outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Funded by government sources but conflicts of interest were not reported |
Roisman 2013.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 10 eyes of 10 participants in micropulse diode laser group 5 eyes of 5 participants in placebo group Exclusions after randomization: 0 in micropulse diode laser group 0 in placebo group Number analyzed: 10 eyes of 10 participants in micropulse diode laser group 5 eyes of 5 participants in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 0 in micropulse diode laser group 0 in placebo group How were missing data handled?: NR Power calculation: NR |
|
| Participants |
Country: Brazil
Mean age (SD) (years): NR in total 39.5 (7.7) in micropulse diode laser group 44.2 (5.8) in placebo group Gender (%): 10 men (67%) and 5 women (33%) in total 7 men (70%) and 3 women (30%) in micropulse diode laser group 3 men (60%) and 2 women (40%) in placebo group Participants with chronic CSC were included and defined as: "CSC lasting more than 6 months were enrolled" Inclusion criteria: NR Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: micropulse diode laser Wavelength: 810 nm FastPulse laser; Opto, Brazil Control: sham laser |
|
| Outcomes | Length of follow‐up: 12 months Primary outcome, as defined in study reports: change in visual acuity after 3 months Secondary outcomes, as defined in study reports: change in CMT after 3 months Adverse events reported: no Intervals at which outcomes assessed: 1, 3, 6, and 12 months | |
| Notes |
Full study name: Micropulse diode laser treatment for chronic central serous chorioretinopathy: a randomized pilot trial
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: reported explicitly none of the authors has any financial relationship Trial registry: NCT01327170 (clinicaltrials.gov) Study period: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The 15 patients were randomized 2:1 through double‐masked random draw into two groups" p. 466 |
| Allocation concealment (selection bias) | Low risk | "The 15 patients were randomized 2:1 through double‐masked random draw into two groups" p. 466 |
| Masking of participants and personnel (performance bias) | Low risk | Double‐masked with placebo group |
| Masking of outcome assessment (detection bias) | Low risk | Double‐masked with placebo group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the trial registry were reported |
| Other bias | Low risk | Source of monetary funding not reported but the conflict of interest declared: "the authors have no financial or proprietary interest in the materials presented herein" |
Sawa 2014.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: Total number randomized was 44 Unclear number in each group Exclusions after randomization: 5 people excluded after randomization but not reported to which groups they belonged Number analyzed: NR eyes of 20 participants in antioxidant group NR eyes of 19 participants in placebo group Unit of analysis: participant, 1 eye per person Losses to follow‐up: NR How were missing data handled?: NR Power calculation: NR |
|
| Participants | The type of CSC was not specified Country: Japan Mean age (SD) (years): 49 (10) in total 51.2 (9) in antioxidant group 46.6 (8.3) in placebo group Gender (%): 35 men (90%) and 4 women (10%) in total 19 men (95%) and 1 women (5%) in antioxidant group 16 men (84%) and 3 women (16%) in placebo group Inclusion criteria: previous regular intake of lutein or zeaxanthin, or both; corticosteroid treatment; disturbance of ocular media; other retinal disorders such as age‐related macular degeneration, polypoidal choroidal vasculopathy, retinal vein occlusion, or diabetic retinopathy Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: antioxidant (lutein) Dose: 20 mg/day Control: placebo |
|
| Outcomes | Length of follow‐up: 4 months Primary outcome, as defined in study reports: macular pigment optical density and plasma lutein concentration Secondary outcomes, as defined in study reports: NR Adverse events reported: no Intervals at which outcomes assessed: 1 and 4 months | |
| Notes |
Full study name: Effects of a lutein supplement on the plasma lutein concentration and macular pigment in patients with central serous chorioretinopathy
Type of study: published full‐text
Funding sources: Santen Pharmaceutical Co., Ltd
Disclosures of interest: reported explicitly none of the authors has any financial relationship Trial registry: not registered Study period: March 2011 to June 2012, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "The study was a randomized, double‐masked, placebo‐controlled trial" p. 5239 |
| Masking of participants and personnel (performance bias) | Low risk | "The study was a randomized, double‐masked, placebo‐controlled trial" p. 5239 |
| Masking of outcome assessment (detection bias) | Low risk | "The study was a randomized, double‐masked, placebo‐controlled trial" p. 5239 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 people excluded after randomization but not reported to which groups they belonged |
| Selective reporting (reporting bias) | High risk | BCVA measured but not reported. Only reported resolution of CSC for the intervention (lutein) group |
| Other bias | High risk | Funded by manufacturer of the supplement. Authors reported not having any conflict of interest |
Semeraro 2012.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 12 eyes of 12 participants in anti‐VEGF group 10 eyes of 10 participants in PDT group Exclusions after randomization: 12 eyes of 12 participants in anti‐VEGF group 10 eyes of 10 participants in PDT group Number analyzed: 12 eyes of 12 participants in anti‐VEGF group 10 eyes of 10 participants in PDT group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 0 in anti‐VEGF group 0 in PDT group How were missing data handled?: NR Power calculation: NR |
|
| Participants |
Participants with chronic CSC were included and defined as: "...either persistence of subretinal fluid detected on optical coherence tomography (OCT) for at least 3 months after diagnosis or more than 3 recurrences in at least 3 months with gravitational RPE atrophy" and "The inclusion criteria consisted of presence of CSC with chronic foveal detachment of the neuroepithelium (C3 months) and no previous treatment for CSC" Country: Italy Mean age (SD) (years): NR in total 35.2 (6) in anti‐VEGF group 36 (8) in PDT group Gender (%): 13 men (59%) and 9 women (41%) in total 7 men (58%) and 5 women (42%) in anti‐VEGF group 6 men (60%) and 4 women (40%) in PDT group Inclusion criteria: presence of CSC with chronic foveal detachment of the neuroepithelium (≥ 3 months) and no previous treatment of CSC Exclusion criteria: ages > 50 years; chronic systemic disease; pregnancy; any uncontrolled ocular disease; and presence of occult or minimally classic choroidal neovascular lesions, scarring, or atrophy within the lesion Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: anti‐VEGF (bevacizumab) Dose: 1.25 mg Duration: single dose at baseline and then as needed after 4 weeks Control: low fluence PDT Light dose: 25 J/cm2 Verteporfin concentration: NR |
|
| Outcomes | Length of follow‐up: 9 months Primary and secondary outcomes not differentiated Outcomes: change in macular thickness; the number of eyes with recurrence; stabilization of the lesions; the number of retreatments Adverse events reported: no Intervals at which outcomes assessed: 9 months | |
| Notes |
Full study name: Intravitreal bevacizumab versus low‐fluence photodynamic therapy for treatment of chronic central serous chorioretinopathy
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: NR Trial registry: not registered Study period: February 2009 to April 2010, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Enrolled patients were randomly assigned to group 1 or group 2 by random block permutation in accordance with a computer‐generated randomization list" p. 609 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | High risk | Groups different and no mention of masking |
| Masking of outcome assessment (detection bias) | High risk | Groups different and no mention of masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Shang 1999.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: NR eyes of 30 participants in yellow laser group NR eyes of 30 participants in red laser group NR eyes of 30 participants in green laser group Exclusions after randomization: 0 in yellow group 0 in red group 0 in green group Number analyzed: NR eyes of 30 participants in yellow group NR eyes of 30 participants in red group NR eyes of 30 participants in green group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 0 in yellow group 0 in red group 0 in green group How were missing data handled?: NR Power calculation: NR |
|
| Participants | The type of CSC was not specified Country: China Mean age (SD) (years): 40.0 (6.3) in total 43.7 (7.6) in yellow group 39.9 (7.0) in red group 39.5 (5.0) in green group Gender (%): 79 men (89%) and 10 women (11%) in total NR in yellow group NR in red group NR in green group Inclusion criteria: people with CSC validated by eye exam, Amsler chart examine and FFA; duration of the disease > 8 weeks; corrected visual acuity ≤ 0.8; distance between fundus fluorescein leakage point showed by FFA and central fovea of macula > 250 µm Exclusion criteria: NR Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention 1: yellow Intervention 2: red Control: green |
|
| Outcomes | Length of follow‐up: 12 months Primary and secondary outcomes not differentiated Outcomes: visual acuity; light sensitivity; recurrent rate; disease course; photocoagulation energy; photocoagulation spot expansion Adverse events reported: yes, made mention of protrusion, proliferation/diffusion, RPE complications Intervals at which outcomes assessed: 12 months | |
| Notes |
Full study name: Wavelength selection in management of central serous chorioretinopathy
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: NR Trial registry: not registered Study period: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | No mention of masking |
| Masking of outcome assessment (detection bias) | Unclear risk | No mention of masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Verma 2004.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: NR eyes of 15 participants in micropulse diode laser group NR eyes of 15 participants in argon laser group Exclusions after randomization: 0 in micropulse diode laser group 0 in argon laser group Number analyzed: NR eyes of 15 participants in micropulse diode laser group NR eyes of 15 participants in argon laser group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 0 in micropulse diode laser group 0 in argon laser group How were missing data handled?: NR Power calculation: NR |
|
| Participants |
Country: India
Mean age (SD) (years): NR in total 34.06 (2.54) in micropulse diode laser group 34.66 (3.23) in argon laser group Gender (%): 25 men (83%) and 5 women (17%) in total 12 men (80%) and 3 women (20%) in micropulse diode laser group 13 men (87%) and 2 women (13%) in argon laser group The type of CSC was not specified Inclusion criteria: ages < 50 years; type I central serous retinopathy with a single leak on FA that was at least 300 μm away from fovea; presence of an indication for laser treatment (recurrence, occupational, history of poor visual outcome in fellow eye); no history of any treatment in the past Exclusion Criteria: participants with multiple leak central serous retinopathy; type 2 or type 3 central serous retinopathy or leak at papillomacular bundle or leak within 300 μm from the foveal center; people with ocular pathology such as CNV, choroidal inflammatory, or neoplastic disorder or a congenital optic nerve pit Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: micropulse diode laser Wavelength: 810 nm Control: argon laser (NR) Wavelength: 514 nm |
|
| Outcomes | Length of follow‐up: 12 weeks Primary and secondary outcomes not differentiated Outcomes: mean BCVA; mean contrast sensitivity Adverse events reported: no Intervals at which outcomes assessed: 4, 8, and 12 weeks | |
| Notes |
Full study name: Comparative evaluation of diode laser versus argon laser photocoagulation in patients with central serous retinopathy: a pilot, randomized controlled trial ISRCTN84128484
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: reported explicitly none of the authors has any financial relationship Trial registry: ISRCTN84128484 (ICTRP) Study period: January 1998 to June 2000, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "They were randomly assigned into 2 groups according to the statistical random table using sequence generation" p. 2 |
| Allocation concealment (selection bias) | Unclear risk | "The allocation of patients into 2 groups was done by a person who was not involved in the study and the sequence was concealed until interventions were assigned to prevent bias" p. 2 |
| Masking of participants and personnel (performance bias) | Unclear risk | Similar groups but no information on masking |
| Masking of outcome assessment (detection bias) | Unclear risk | Similar groups but no information on masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Low risk | Source of monetary support not reported and conflict of interest declared: "the author(s) declare that they have no competing interests" |
Zhang 2012.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 30 eyes of 30 participants in 30% PDT group 30 eyes of 30 participants in 50% PDT group 30 eyes of 30 participants in PDT group Exclusions after randomization: 0 in 30% PDT group 0 in 50% PDT group 0 in PDT group Number analyzed: 30 eyes of 30 participants in 30% PDT group 30 eyes of 30 participants in 50% PDT group 30 eyes of 30 participants in PDT group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 0 in 30% PDT group 0 in 50% PDT group 0 in PDT group How were missing data handled?: NR Power calculation: NR |
|
| Participants | The type of CSC was not specified Country: China Mean age (SD) (years): NR in total 33.8 (5.5) in 30% PDT group 34.2 (5.2) in 50% PDT group 32.0 (4.1) in PDT group Gender (%): 25 men (83%) and 5 women (17%) in total 8 men (27%) and 22 women (73%) in 30% PDT group 11 men (37%) and 19 women (63%) in 50% PDT group 9 men (30%) and 21 women (70%) in PDT group Inclusion criteria: aged < 45 years with conscious visual distortion of imagery, darkened vision or changes of smaller imagery vision; anterior segments do not have anything in particular or abnormal. Fundus exam should show macula regions or macula peripheral region gray spots with irregular formation or circular type infection, arching or ring‐shaped hemorrhage, including various degrees of retinal edema; through FFA exam, low fluorescent shows in the early exudative lesion focus, hemorrhage spots always cover the fluorescein, but exudative lesion focus and mild bleeding spot could show CNV with typical petal shaped or trochoid shaped high fluorescent. As the time of radiography gets longer, fluorescein leakage could be observed. It is splinter high fluorescent, the scale and scope is similar to the gray exudative lesion focus; excluding external injury related, myopia related, or other established causes of non‐age‐related macular degeneration CNV; people who have not received laser photocoagulation, intraocular injections, or invasive surgical ocular treatments Exclusion criteria: history of penicillin allergy or systemic illnesses or issues with fluorescence or other systems of sexually transmitted disease that could cause intolerance of FFA treatment and PDT treatment; serious corneal illness, cataracts, blood volume, light opacity interfering mediums that can disturb the treatment plan and examination plan of the study; corneal endothelial cell scarring, shows FA has not created leakage; poor participant compliance with those who cannot complete follow‐up times Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: 30% PDT Light dose: 50 J/cm2 Verteporfin concentration: 2 mg/m2 Intervention: 50% PDT Light dose: 50 J/cm2 Verteporfin concentration: 3 mg/m2 Control: PDT Light dose: 50 J/cm2 Verteporfin concentration: 6 mg/m2 |
|
| Outcomes |
Length of follow‐up: 12 months
Primary and secondary outcomes not differentiated
Outcomes: BCVA value change; number of eyes show CNV change; CFT value change; number of treatments; recurrence of CSC Adverse events reported: no Intervals at which outcomes assessed: 3, 6, and 12 months, but not all participants had 12 month |
|
| Notes |
Full study name: Different doses of verteporfin photodynamic therapy for central exudative chorioretinopathy
Type of study: published full‐text
Funding sources: NR
Disclosures of interest: NR Trial registry: not registered Study period: January 2006 to December 2009, as reported in the full‐text article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence specified and outlined in methods. Low risk of random sequence generation. The study used a number sequence table to assign proper randomization for groups. They used a verified and structured numbering sequence of 1‐90 that was aligned for row and column and considered from smallest value to largest. They then randomized and recorded each randomly generated assignment figure |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Similar groups but no information on masking |
| Masking of outcome assessment (detection bias) | Unclear risk | Similar groups but no information on masking |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol and trial registry entry not available for comparison |
| Other bias | Unclear risk | Conflict of interest and source of funding not reported |
Zhao 2015.
| Methods |
Study design: parallel randomized controlled trial
Number randomized: 65 eyes of 65 participants in 30% PDT group 64 eyes of 64 participants in 50% PDT group Exclusions after randomization: 0 in 30% PDT group 0 in 50% PDT group Number analyzed: 61 eyes of 61 participants in 30% PDT group 56 eyes of 56 participants in 50% PDT group Unit of analysis: participant, 1 eye per person Losses to follow‐up: 4 eyes of 4 participants in 30% PDT group 9 eyes of 9 participants in 50% PDT group How were missing data handled?: NR Power calculation: power = 80% and sample size = 112 participants (56 participants in each group) |
|
| Participants |
Country: China
Mean age (SD) (years): NR in total 42.5 (5.6) in 30% PDT group 43.1 (5.3) in 50% PDT group Gender (%): 87 men (74%) and 30 women (26%) in total 47 men (77%) and 14 women (23%) in 30% PDT group 40 men (71%) and 16 women (29%) in 50% PDT group Participants with acute CSC were included and defined as: "symptoms occurred for the first time, as an episode duration of less than 6 months, or there was a medical record that could prove the presence of subretinal fluid (SRF) for less than 6 months if the patient was asymptomatic" Inclusion criteria: symptoms occurred for the first time, as an episode duration of < 6 months, or there was a medical record that could prove the presence of SRF for < 6 months if the participant was asymptomatic; ages 18‐50 years; presence of SRF involving the macula and detected using OCT; active fluorescein leakage during FA and abnormal dilated choroidal vasculature detected using ICGA Exclusion criteria: previous PDT, focal photocoagulation, intravitreal injections of anti‐VEGF, or ocular surgery; other macular abnormalities such as CNV or polypoidal choroidal vasculopathy; choroidopathy that may affect choroidal thickness; any retinal vascular disease that may have fluorescein leakage during FA; history of porphyria or photosensitivity; severe impaired kidney or liver function or unstable heart condition (or a combination of these); pregnancy; inability to obtain photographs or to perform FA or ICGA; use of steroid systemically or topically in the last 6 months Equivalence of baseline characteristics: NR |
|
| Interventions |
Intervention: 30% PDT Light dose: 50 J/cm2 Verteporfin concentration: 1.8 mg/m2 Control: 50% PDT Light dose: 50 J/cm2 Verteporfin concentration: 3 mg/m2 |
|
| Outcomes | Length of follow‐up: 12 months Primary outcome, as defined in study reports: proportion of eyes with complete absorption of SRF; proportion of eyes with complete disappearance of fluorescein leakage at 6 and 12 months Secondary outcomes, as defined in study reports: SRF recurrent rate; the fluorescein leakage recurrent rate at 12 months; mean BCVA; the retinal thickness of the foveal center; the maximum retinal thickness at each scheduled visit Adverse events reported: yes Intervals at which outcomes assessed: 2 weeks, 1, 3, 6, and 12 months | |
| Notes |
Full study name: A 50% vs 30% dose of verteporfin (photodynamic therapy) for acute central serous chorioretinopathy: one‐year results of a randomized clinical trial
Type of study: published full‐text
Funding sources: Capital Health Research and Development of Special Funding grant D101100050010026 and National Science and Technology Major Project grant 2011ZX09302‐007‐02
Disclosures of interest: reported explicitly none of the authors has any financial relationship Trial registry: NCT01574430 (clinicaltrials.gov) Study period: March 2011 to February 2012, as reported in the full‐text |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was generated using a computerized randomization table" p. 334 |
| Allocation concealment (selection bias) | Low risk | "All patients, examiners, investigators, and research assistants at the reading centers were masked to the treatment allocation group" p. 334 |
| Masking of participants and personnel (performance bias) | Low risk | "All patients, examiners, investigators, and research assistants at the reading centers were masked to the treatment allocation group" p. 334 |
| Masking of outcome assessment (detection bias) | Low risk | "All patients, examiners, investigators, and research assistants at the reading centers were masked to the treatment allocation group" p. 334 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Level of lost to follow‐up was not the same in each group: 4/65 (6%) participants in 30% PDT group and 8/64 (13%) participants 50% PDT group |
| Selective reporting (reporting bias) | High risk | Primary outcome reported at clinicaltrials.gov (NCT01574430) was change from baseline in BCVA, but study primary outcome were OCT‐based improvement rate and FA‐based improvement rate at 6 and 12 months. BCVA was reported, but not defined as primary outcome |
| Other bias | Low risk | Reported no conflicts of interest and non‐industry funded |
anti‐VEGF: anti‐vascular endothelial growth factor; BCVA: best‐corrected visual acuity; CFT: central foveal thickness; CMT: central macular thickness; CNV: choroidal neovascularization; CRT: central retinal thickness; CSC: central serous chorioretinopathy; FA: fluorescein angiography; FFA: fundus fluorescein angiography; ICGA: indocyanine green angiography; logMAR: logarithm of the minimal angle of resolution; NA: not applicable as no missing data or unclear if there is missing data; NR: not reported; OCT: optical coherence tomography; p: page; PDT: photodynamic therapy; RPE: retinal pigment epithelium; SD: standard deviation; SFF: subfoveal fluid; SRF: subretinal fluid; SLO‐ICGA: scanning laser indocyanine green angiography.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ainiwaer 2014 | Wrong treatment arms and included traditional Chinese medicine; compared Argon laser to xueshuantong |
| Arevalo 2013 | Not RCT |
| Aydin 2013 | Not RCT |
| Beger 2012 | Not RCT |
| Behnia 2013 | Not RCT |
| Bi 2000 | Not RCT |
| Boscia 2007 | Not RCT |
| Bruha 1972 | Not RCT |
| Cervera 2008 | Not RCT |
| Chrapek 2015 | Not RCT; randomization not clearly described |
| Demirel 2014 | Not RCT |
| Di 2013 | Wrong treatment arms and included traditional Chinese medicine; compared argon laser to traditional medication (vitamin C, E, inosine, rutin, xueshuantong, difrarel) |
| Earl 2014 | Not RCT |
| Fang 2013 | Wrong treatment arms and included traditional Chinese medicine; compared jolethin combined with argon laser (argon laser and oral jolethin, vitamin B1, inosine and venoruton tablets) to traditional medication (oral lecithin complex iodine, vitamin B1, inosine, venoruton tablets) |
| Feily 2009 | Not RCT |
| Haas 2004 | Not RCT |
| Heinrich 1974 | Not RCT |
| Huang 2006 | Wrong treatment arms and included traditional Chinese medicine; compared argon laser and iodine treatment to argon laser |
| Khosla 1997 | Not RCT |
| Koss 2012 | Not RCT |
| Kurimoto 1969 | Not RCT |
| Lee 2011 | Not RCT |
| Li 2010 | Not RCT |
| Lim 2011 | Not RCT |
| Liu 2009 | Not RCT |
| Long 2011 | Wrong treatment arms and included traditional Chinese medicine; compared krypton laser combined with danshen and inosine to krypton laser combined with anisodine |
| Lyons 1977 | Not RCT |
| Mackowiakowa 1987 | Not RCT |
| Miyashita 1971 | Not RCT |
| NCT01256580 | Wrong participants. RCT enrolled participants with age‐related macular degeneration |
| NCT01585441 | This study was terminated early due to lack of enrollment |
| Novak 1987 | Not RCT |
| Okamoto 2015 | Not RCT |
| Ozdemir 2014 | Not RCT |
| Peng 2010 | Not RCT |
| Radian 1984 | Not RCT |
| Sanchez‐Pacheco 2010 | Not RCT |
| Takagi 1965 | Not RCT |
| Tewari 1986 | Not RCT; randomization not clearly described |
| Wang 2009a | Not RCT |
| Wang 2009b | Wrong treatment arms and included traditional Chinese medicine; compared therapeutic alliance group (injected subcutaneously compound anisodine injection 2 mL combined with joletion tablets taking) to joletion tablets group |
| Watzke 1974 | Wrong participants |
| Watzke 1979 | Wrong participants |
| Wu 2010 | Wrong treatment arms and included traditional Chinese medicine; compared jolethin combined with argon laser to argon laser |
| Xu 2013 | Not RCT |
| Xu 2014 | Wrong treatment arms and included traditional Chinese medicine; compared anisodine injection to traditional medication (oral medication such as adenosine triphosphate, inosine, vitamin) |
| Ye 2013 | Wrong treatment arms and included traditional Chinese medicine; compared argon laser combined with xueshuantong (laser combined with compound xueshuantong capsule) to argon laser |
| Zhang 2014 | Not RCT |
| Zheng 2013 | Wrong treatment arms and included traditional Chinese medicine; compared hyperbaric oxygen and iodized lecithin to hyperbaric oxygen |
RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
EUCTR2009‐017959‐98‐NL.
| Trial name or title | Early Treatment of Patients with Central Serous Retinopathy: a Randomized Controlled Trial ‐ CSR & PDT |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: adults, elderly Gender: men and women "Poor prognostic acute CSR [CSC]" |
| Interventions | NR PDT versus observation |
| Outcomes |
Primary outcomes: visual acuity (BCVA ETDRS) at 1 year of follow‐up Secondary outcomes: NR |
| Starting date | 25 March 2010 |
| Contact information | NR |
| Notes | Sponsor name: Rotterdam Eye Hospital |
JPRN‐UMIN000005372.
| Trial name or title | Study on the Effects of Supplements Containing Lutein on Chronic Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: ≤ 40 years Gender: male and female |
| Interventions |
Intervention 1: multivitamins, minerals, and lutein Control: placebo |
| Outcomes |
Primary outcomes: rate of spontaneous resolution of CSC, changes in macular volume measured by OCT Secondary outcomes: NR BCVA |
| Starting date | NR |
| Contact information | Tsutomu Yasukawa Nagoya City University Graduate School of Medical Sciences Department of Ophthalmology and Visual Science |
| Notes |
Sponsor name: Nagoya City University Graduate School of Medical Sciences Source of funding: Santen Pharmaceutical Co., Ltd |
NCT01019668.
| Trial name or title | Central Serous Chorioretinopathy Treated by Modified Photodynamic Therapy |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: 18‐75 years Gender: men and women |
| Interventions |
Intervention 1: verteporfin PDT, half‐dose Intervention 2: verteporfin PDT, half‐fluence |
| Outcomes |
Primary outcomes: effectiveness of both modification for the treatment of chronic CSC, fluorescent leakage as regards to BCVA OCT changes Secondary outcomes: detrimental influence on choroidal perfusion, represented by the decrease of fluorescent intensity in ICGA |
| Starting date | November 2008 |
| Contact information | Cheng‐Kuo Cheng, MD Assistant Professor and Attending Physician of Ophthalmology Shin‐Kong Wu Ho‐Su Memorial Hospital, School of Medicine, Fu‐Jen Catholic University |
| Notes |
Sponsor name: Shin Kong Wu Ho‐Su Memorial Hospital Source of funding: NR |
NCT01552044.
| Trial name or title | Effect of Spironolactone in Treating Chronic Non‐Resolutive Central Serous Chorioretinitis |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: 18‐60 years Gender: male and female |
| Interventions |
Intervention 1: spironolactone 25 mg/day Intervention 2: placebo |
| Outcomes |
Primary outcomes: change in central macular thickness at 1 and 3 months, subretinal fluid decrease of 40 microns or more Secondary outcomes: NR |
| Starting date | January 2012 |
| Contact information | Francine Behar‐Cohen, MD, PhD Hotel‐Dieu of Paris, France |
| Notes |
Sponsor name: Institut National de la Santé Et de la Recherche Médicale, France Source of funding: NR |
NCT01630863.
| Trial name or title | The Safe Effective Light Dose of Photodynamic Therapy for Chronic Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: 20‐70 years Gender: men and women |
| Interventions |
Intervention 1: 50% group (power of PDT is applied to the participants at 50% of the full energy based on TAP study) Intervention 2: 40% group (power of PDT is applied to the participants at 40% of the full energy based on TAP study) Intervention 3: 30% group (power of PDT is applied to the participants at 30% of the full energy based on TAP study) |
| Outcomes |
Primary outcomes: change in BCVA at 1, 3, 6 and months Secondary outcomes: change in central retinal thickness, success rate, recurrence rate, and complications at 1, 3, and 6 months |
| Starting date | June 2012 |
| Contact information | Min Sagong Yeungnam University College of Medicine Daegu, Republic of Korea |
| Notes |
Sponsor name: Yeungnam University College of Medicine Source of funding: NR |
NCT01797861.
| Trial name or title | Prospective Randomized Controlled Treatment Trial for Chronic Central Serous Chorioretinopathy (PLACE) |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: ≥ 18 years Gender: men and women |
| Interventions |
Intervention 1: half‐dose PDT "In the PDT treatment arm, all patients will receive an intravenous drip through which half‐dose (3 mg/m2) verteporfin (Visudyne ®) is administered, with an infusion time of 10 minutes. At 15 minutes after the start of the infusion, PDT laser treatment is performed with standard 50 J/cm2 fluency, a wavelength of 689 nm, and a treatment duration of 83 seconds. If there still is subretinal fluid on OCT scan at Evaluation Visit 1 (6‐8 weeks after Treatment Visit 1 / the first treatment with half‐dose PDT), a second treatment with half‐dose PDT will be performed (Treatment Visit 2)" Intervention 2: micropulse laser (ML) treatment ML treatment with an 810 nm diode laser will be performed of the areas identified on mid‐phase ICG angiography. Multiple laser spots will be applied, covering the leakage area on mid‐phase ICG angiography. The area(s) that has to be treated is determined based on those hyperfluorescent area(s) on mid‐phase (approximately 10 minutes) ICG‐angiography that correspond to subretinal fluid accumulation in the macula on the OCT scan and hyperfluorescent "hot spots" on the mid‐phase (3 minutes) fluorescein angiogram. If there still is subretinal fluid on OCT scan at Evaluation Visit 1 (6‐8 weeks after Treatment Visit 1 / the first ML treatment), a second ML treatment will be performed (Treatment Visit 2) |
| Outcomes |
Primary outcomes: absence of subretinal fluid on OCT scan Secondary outcomes: BCVA |
| Starting date | December 2013 |
| Contact information | Camiel JF Boon, MD PhD FEBO Leiden University Medical Center, Netherlands Myrte Breukink, MD Radboud University Nijmegen Medical Centre, Institute of Ophthalmology, Netherlands |
| Notes |
Sponsor name: Radboud University Source of funding: NR |
NCT01971190.
| Trial name or title | Efficacy and Safety of Intravitreal Aflibercept Injection for Subacute Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: 18‐60 years Gender: men and women |
| Interventions |
Intervention 1: aflibercept (Eylea) 2 mg intravitreal injection at baseline, 1 and 2 months Control: sham injection at baseline, 1 and 2 months |
| Outcomes |
Primary outcomes: change in central subfield thickness from baseline to 1, 2, 3, 4, 5, and 6 months Secondary outcomes: percentage of eyes achieving complete resolution of subretinal fluid at 6 months, percentage of eyes achieving 20/20 vision at 6 months, number of aflibercept injections needed to achieve a complete resolution at 6 months, change in subfoveal choroidal thickness from baseline using EDI‐OCT at 1, 2, 3, 4, 5, and 6 months, adverse effects of intravitreal aflibercept (Eylea) injection up to 6 months |
| Starting date | October 2013 |
| Contact information | Young Hee Yoon, MD Asan Medical Center, Republic of Korea |
| Notes |
Sponsor name: Asan Medical Center Source of funding: NR |
NCT01982383.
| Trial name or title | Study on the Use of Micropulse Laser to Treat Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: 30‐60 years Gender: men and women "New diagnosis of CSC" |
| Interventions |
Intervention 1: micropulse laser treatment "Patient's randomized to ML treatment would be treated with the following settings: 200 micron spot size, 0.2 second duration, 15% duty cycle, and 300 milliWatt power. Their eyes would be dilated prior to treatment with standard mydriatic medications, including Tropicamide and Phenylephrine" Control: no treatment "Patients randomized to this treatment arm, will not receive treatment for CSC. They will continue to be observed at month 1 and month 3. If any worsening of pathology is found during the follow‐up visits, the patient will be removed from the study and given appropriate standard of care by the attending" |
| Outcomes |
Primary outcomes: resolution of fluid build‐up within 1 week to 3 months after the laser procedure is completed Secondary outcomes: NR |
| Starting date | November 2012 |
| Contact information | Khadijah Abdallah, MPH George Washington University, District of Columbia, United States |
| Notes |
Sponsor name: George Washington University Source of funding: NR |
NCT01990677.
| Trial name or title | Eplerenone for the Treatment of Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: ≥ 18 years Gender: men and women |
| Interventions |
Intervention 1: eplerenone 25 mg ‐ chronic CSC diagnosis "Dosing will begin at 25mg Eplerenone taken orally , one time, each day for 58 days. Throughout the 58 day treatment period dosage will be adjusted. The adjustment will be based on serum potassium and creatine levels from blood draws done at Day 12 and Day 33. From the 25 mg starting dosage, the dosage will either be increased to 50 mg a day or reduced to placebo, one time, each day" Intervention 2: placebo ‐ chronic CSC diagnosis "Dosing will begin with placebo and will stay as placebo throughout the study. The placebo pills will be taken orally, once daily, for 58 days. The placebo pills will be compounded to be of similar composition to the eplerenone tablets, without the active ingredient" Intervention 3: eplerenone 25 mg ‐ acute CSC diagnosis "Dosing will begin at 25mg Eplerenone taken orally , one time, each day for 28 days. Throughout the 28 day treatment period, dosage will be adjusted based on serum potassium and creatine levels from blood draws done on Day 12. From the 25 mg starting dosage, the dosage will either be increased to 50 mg a day or reduced to placebo, one time, each day" Intervention 4: placebo ‐ acute CSC diagnosis "Dosing will begin with placebo and will stay as placebo throughout the study. The placebo pills will be taken orally, once daily, for 28 days. The placebo pills will be compounded to be of similar composition to the eplerenone tablets, without the active ingredient" |
| Outcomes |
Primary outcomes: absence of subfoveal (retinal) fluid based on spectral domain OCT measurement at 1 month in acute CSC and 2 months in chronic CSC participants Secondary outcomes: mean change in subfoveal fluid height based on OCT measurement at 1 month in acute CSC and 2 months in chronic CSC participants |
| Starting date | October 2013 |
| Contact information | Brian Burke, MPH Wills Eye Hospital |
| Notes |
Sponsor name: Wills Eye Hospital, Philadelphia, United States Source of funding: NR |
NCT02153125.
| Trial name or title | Eplerenone for the Treatment of Chronic Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: 18‐65 years Gender: men and women |
| Interventions |
Intervention 1: eplerenone 25 mg given daily for 1 week, followed by 50 mg given for a total of 3 months since commencement of treatment Intervention 2: placebo |
| Outcomes |
Primary outcomes: decrease of at least 10% in subretinal fluid thickness as measured by OCT at 6 months Secondary outcomes: NR |
| Starting date | April 2014 |
| Contact information | Michaella Goldstein, MD Tel Aviv Souraski Medical Center, Israel |
| Notes |
Sponsor name: Tel‐Aviv Sourasky Medical Center Source of funding: NR |
NCT02215330.
| Trial name or title | A Study of the Beneficial Effects of Eplerenone on Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: ≥ 21 years Gender: men and women |
| Interventions |
Intervention: eplerenone 25 mg pills triturated and filled into capsules Control: sugar pill (maltodextrin filled into capsules) |
| Outcomes |
Primary outcomes: difference in the number of successful treatments after 16 weeks, defined as complete absence of subretinal fluid on SD‐OCT Secondary outcomes: change in visual acuity between eplerenone and placebo at 16 weeks, change in retinal thickness between eplerenone and placebo at 16 weeks, change in retinal volume between eplerenone and placebo at 16 weeks |
| Starting date | October 2014 |
| Contact information | Oliver Findl, MD, Prof, MBA Vienna Institute for Research in Ocular Surgery, Department of Ophthalmology Hanusch Hospital Vienna, Vienna, Austria |
| Notes |
Sponsor name: Oliver Findl, MD, Prof, MBA Source of funding: NR |
NCT02354170.
| Trial name or title | Short‐Term Oral Mifepristone for Central Serous Chorioretinopathy |
| Methods | Study design: parallel group RCT |
| Participants |
Population age: ≥ 18 years Gender: men and woman |
| Interventions |
Intervention 1: 1 x 300 mg mifepristone tablet, taken once daily for 4 weeks Intervention 2: 3 x 300 mg mifepristone tablets (900 mg dose), taken once daily for 4 weeks Control: placebo taken once daily for 4 weeks |
| Outcomes |
Primary outcomes: resolution of sub‐retinal fluid at 4 weeks after treatment, presence or absence of subretinal fluid on spectral‐domain OCT after 4 weeks of treatment with mifepristone 300 or 900 mg daily, compared with placebo Secondary outcomes: change in subretinal fluid or intraretinal fluid (or both) at weeks 1, 2, 4, and 8, BCVA at weeks 1, 2, 4, and 8, change in ETDRS BCVA compared with baseline at weeks 1, 2, 4, and 8, change in macular thickness at weeks 1, 2, 4, and 8, change in foveal thickness at weeks 1, 2, 4, and 8, change compared with baseline in thickness of subretinal fluid under the fovea on OCTat weeks 1, 2, 4, and 8, change in choroidal thickness at weeks 1, 2, 4, and 8, dye leakage in vasculature at week 4 and 8, change in OCT characteristics in the fellow eye at week 8, proportion of acute versus chronic CSC participants at week 8, proportion of acute versus chronic CSC participants as determined at baseline, with the above outcomes analyzed for each subgroup; safety and tolerability characteristics at week 8 |
| Starting date | January 2015 |
| Contact information | Roger A Goldberg, MD, MBA Bay Area Retina Associates Walnut Creek, California, United States Jeffery S Heier, MD Ophthalmic Consultants of Boston Boston, Massachusetts, United States |
| Notes |
Sponsor Name: Roger Goldberg, MD, MBA Source of funding: Bay Area Retina Associates, Ophthalmic Consultants of Boston |
BCVA: best‐corrected visual acuity; CSC: central serous chorioretinopathy (also known as CSR: central serous retinopathy); ETDRS: Early Treatment Diabetic Retinopathy Study; ICGA: indocyanine green angiography; NR: not reported; OCT: optical coherence tomography; PDT: photodynamic therapy; RCT: randomized controlled trial.
Differences between protocol and review
We made the following amendments to our protocol (Salehi 2015).
We excluded trials of traditional Chinese medicine. This is because we did not have a clear rationale for these treatments and these interventions may not be applicable to the settings covered by this review.
We restricted the network to interventions applied directly to the eye (ocular interventions) because we felt that a key assumption of the network ‐ participants should be equally likely to be randomized to any of the interventions ‐ would be unlikely to hold otherwise.
We did not consistently contact trial investigators for clarification of risk of bias as 'unclear' based on unreported or poorly reported information: some of the studies were completed many years ago and we took the judgment that the information was unlikely to be forthcoming.
We omitted to describe the GRADE assessment and 'Summary of findings' table in our protocol and have included that in the review methods.
The following methods set out in our protocol were not done due to lack of data. They may be applicable in future editions of the review.
Unit of analysis
If any studies enroll bilateral CSC cases and randomize eyes in participants to intervention versus comparator (within‐person study), we will refer to Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions as a guide for analysis of matched data (Higgins 2011b).
Assessing reporting bias
When future versions of this review's meta‐analysis include 10 or more studies, we will investigate small‐study effects using a funnel plot. The funnel plot will have the effect estimate on the horizontal axis and the standard error on the vertical axis for each trial. We will conduct a qualitative interpretation of funnel plot asymmetry using guidance from Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011).
Contributions of authors
Each author has undertaken all of the following tasks listed:
conceived or designed the study, or both;
drafted the review or commented on it critically for intellectual content;
provided final approval of the document to be published.
MS, AL, and JE performed study screening and data extraction.
Sources of support
Internal sources
-
National Institute of Health Research (NIHR), UK.
This review was funded through the NIHR Cochrane Incentive Scheme and partly funded Jennifer Evan's salary.
External sources
Cochrane Eyes and Vision US Project, supported by cooperative agreement 1 U01 EY020522, National Eye Institute, National Institutes of Health, Department of Health and Human Services, USA.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
None known.
New
References
References to studies included in this review
Bae 2011 {published data only}
- Bae SH, Heo J, Kim C, Kim TW, Shin JY, Lee JY, et al. Low‐fluence photodynamic therapy versus ranibizumab for chronic central serous chorioretinopathy: one‐year results of a randomized trial. Ophthalmology 2014;121(2):558‐65. [DOI] [PubMed] [Google Scholar]
- Bae SH, Heo JW, Kim C, Kim TW, Lee JY, Song SJ, et al. A randomized pilot study of low‐fluence photodynamic therapy versus intravitreal ranibizumab for chronic central serous chorioretinopathy. American Journal of Ophthalmology 2011;152(5):784‐92. [DOI] [PubMed] [Google Scholar]
Boscia 2008 {published data only}
- Boscia F, Michele R, Antonio L, Nicola C, Claudio F, Salvatore F, et al. Low fluence photodynamic therapy in chronic central serous chorioretinopathy: blind randomized clinical trial of efficacy and safety. The Macula Society 2008:118.
- Boscia F, Reibaldi M, Longo A, Faro S, Uva M, Cardascia N, et al. Low fluence photodynamic therapy in chronic central serous chorioretinopathy: blind randomised clinical trial of efficacy and safety. Investigative Ophthalmology and Visual Science 2008:ARVO E‐abstract 3278.
Brancato 1994 {published data only}
- Brancato R, Bandello F. Treatment of central serous chorioretinopathy with beta‐blockers and calcium antagonists. The Macula Society 1994:114.
Browning 1993 {published data only}
Chan 2006 {published data only}
- Chan PS, Uy HS, Salvosa F, Silva PA. Low dose transpupillary thermotherapy for the treatment of central serous chorioretinopathy. American Academy of Ophthalmology 2006:290.
Chan 2008 {published data only}
- Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half‐dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one‐year results of a randomized controlled trial. Ophthalmology 2008;115(10):1756‐65. [DOI] [PubMed] [Google Scholar]
- Wu ZH, Lai RY, Yip YW, Chan WM, Lam DS, Lai TY. Improvement in multifocal electroretinography after half‐dose verteporfin photodynamic therapy for central serous chorioretinopathy: a randomized placebo‐controlled trial. Retina 2011;31(7):1378‐86. [DOI] [PubMed] [Google Scholar]
Coskun 2014 {published data only}
- Coskun E, Gurler B, Erbagci I. Combined half dose photodynamic therapy with verteporfin and intravitreal bevacizumab for chronic central serous chorioretinopathy. Ophthalmologica 2014;232:56. [Google Scholar]
Dang 2013 {published data only}
- Dang Y, Mu Y, Zhao M, Li L, Guo Y, Zhu Y. The effect of eradicating Helicobacter pylori on idiopathic central serous chorioretinopathy patients. Therapeutics and Clinical Risk Management 2013; Vol. 9, issue 1:355‐60. [DOI] [PMC free article] [PubMed]
Kianersi 2008 {published data only}
- Kianersi F, Fesharaki F. Effects of propranolol in patients with central serous chorioretinopathy. Journal of Research in Medical Sciences 2008;13(3):103‐7. [Google Scholar]
Kim 2013 {published data only}
- Kim M, Lee SC, Lee SJ. Intravitreal ranibizumab for acute central serous chorioretinopathy. Ophthalmologica 2013;229(3):152‐7. [DOI] [PubMed] [Google Scholar]
Klatt 2011 {published data only}
Leaver 1979 {published data only}
- Ficker L, Vafidis G, While A, Leaver P. Long term results of treatment of central serous retinopathy ‐ a preliminary report. Transactions of the Ophthalmological Societies of the United Kingdom 1986;105(Pt 4):473‐5. [PubMed] [Google Scholar]
- Ficker L, Vafidis G, While A, Leaver P. Long‐term follow‐up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. British Journal of Ophthalmology 1988;72(11):829‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. British Journal of Ophthalmology 1979; Vol. 63, issue 10:674‐7. [DOI] [PMC free article] [PubMed]
Lim 2010 {published data only}
- Lim JW, Ryu SJ, Shin MC. The effect of intravitreal bevacizumab in patients with acute central serous chorioretinopathy. Korean Journal of Ophthalmology 2010;24(3):155‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ontiveros‐Orozco 2004 {published data only}
- Ontiveros‐Orozco I, Garcia‐Franco R, Levine‐Berebichez A, Celis‐Suazo B, Rojas‐Juarez S. Topical brinzolamide for the treatment of idiopathic central serous chorioretinopathy. Investigative Ophthalmology and Visual Science 2004; Vol. 45:ARVO E‐abstract 529.
- Ontiveros‐Orozco I, García‐Franco R, Levine‐Berevichez A, López‐Star EM, Rojas‐Juárez S, Celis‐Suazo B, et al. Brinzolamide for topical treatment coroidorretinopatía idiopathic central serous [Brinzolamida tópica para el tratamiento de lacoroidorretinopatía serosa central idiopática]. Revista Mexicana de Oftalmología 2006;80(3):132‐7. [Google Scholar]
Pitcher 2015 {published data only}
- Pitcher JD 3rd, Witkin AJ, DeCroos FC, Ho AC. A prospective pilot study of intravitreal aflibercept for the treatment of chronic central serous chorioretinopathy: the CONTAIN study. British Journal of Ophthalmology 2015;99(6):848‐52. [DOI] [PubMed] [Google Scholar]
Rahbani‐Nobar 2011 {published data only}
- Rahbani‐Nobar MB, Javadzadeh A, Ghojazadeh L, Rafeey M, Ghorbanihaghjo A. The effect of Helicobacter pylori treatment on remission of idiopathic central serous chorioretinopathy. Molecular Vision 2011;17:99‐103. [PMC free article] [PubMed] [Google Scholar]
Ratanasukon 2012 {published data only}
- Ratanasukon M, Bhurayanontachai P, Jirarattanasopa P. High‐dose antioxidants for central serous chorioretinopathy; the randomized placebo‐controlled study. BMC Ophthalmology 2012;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 1983 {published data only}
- Robertson DM, Ilstrup D. Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy. American Journal of Ophthalmology 1983;95(4):457‐66. [DOI] [PubMed] [Google Scholar]
Roisman 2013 {published data only}
- Roisman L, Magalhaes FP, Lavinsky D, Moraes N, Hirai FE, Cardillo JA, et al. Micropulse diode laser treatment for chronic central serous chorioretinopathy: a randomized pilot trial. Ophthalmic Surgery Lasers and Imaging 2013;44(5):465‐70. [DOI] [PubMed] [Google Scholar]
Sawa 2014 {published data only}
Semeraro 2012 {published data only}
- Semeraro F, Romano MR, Danzi P, Morescalchi F, Costagliola C. Intravitreal bevacizumab versus low‐fluence photodynamic therapy for treatment of chronic central serous chorioretinopathy. Japanese Journal of Ophthalmology 2012;56(6):608‐12. [DOI] [PubMed] [Google Scholar]
Shang 1999 {published data only}
- Shang Q, Liu C, Wei S, Shi F, Yang A. Wavelength selection in management of central serous chorioretinopathy. Chinese Journal of Ophthalmology 1999;35(6):413‐5. [PubMed] [Google Scholar]
Verma 2004 {published data only}
- Verma L, Sinha R, Venkatesh P, Tewari HK. Comparative evaluation of diode laser versus argon laser photocoagulation in patients with central serous retinopathy: a pilot, randomized controlled trial. ISRCTN84128484. BMC Ophthalmology 2004;4(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang YL, You ZP, Wang CY. Different doses of verteporfin photodynamic therapy for central exudative chorioretinopathy. Chinese Journal of Experimental Ophthalmology 2012;30(11):1030‐5. [Google Scholar]
Zhao 2015 {published data only}
- Zhao M, Zhang F, Chen Y, Dai H, Qu J, Dong C, et al. A 50% vs 30% dose of verteporfin (photodynamic therapy) for acute central serous chorioretinopathy: one‐year results of a randomized clinical trial. JAMA Ophthalmology 2015;133(3):333‐40. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ainiwaer 2014 {published data only}
- Ainiwaer K, Xiong LJ. Efficacy of compound Xueshuantong combined laser therapy on central serous chorioretinopathy. International Eye Science 2014;14(10):1841‐3. [Google Scholar]
Arevalo 2013 {published data only}
- Arevalo JF, Espinoza JV. Combined photodynamic therapy with verteporfin and intravitreal anti‐vascular endothelial growth factor therapy for chronic central serous chorioretinopathy. Graefes Archive for Clinical and Experimental Ophthalmology 2013;251(1):403‐4. [DOI] [PubMed] [Google Scholar]
Aydin 2013 {published data only}
- Aydin E. The efficacy of intravitreal bevacizumab for acute central serous chorioretinopathy. Journal of Ocular Pharmacology and Therapeutics 2013;29(1):10‐3. [DOI] [PubMed] [Google Scholar]
Beger 2012 {published data only}
- Beger I, Koss M J, Koch F. Treatment of central serous chorioretinopathy: micropulse photocoagulation versus bevacizumab. Der Ophthalmologe 2012;109(12):1224‐32. [DOI] [PubMed] [Google Scholar]
Behnia 2013 {published data only}
- Behnia M, Khabazkhoob M, Aliakbari S, Abadi A E, Hashemi H, Pourvahidi P. Improvement in visual acuity and contrast sensitivity in patients with central serous chorioretinopathy after macular subthreshold laser therapy. Retina 2013;33(2):324‐8. [DOI] [PubMed] [Google Scholar]
Bi 2000 {published data only}
- Bi HS, Liu L, Wang XR, Yang MY. Treatment of central serous chorioretinopathy with krypton yellow and green laser photocoagulation. Ophthalmology 2000;9(3):166‐8. [Google Scholar]
Boscia 2007 {published data only}
- Boscia F, Caradscia N, Furino C, Dammacco R, Sborgia G, Reibaldi M. Photodynamic therapy with low fluence PDT for chronic central serous chorioretinopathy: a short term pilot study. Investigative Ophthalmology and Visual Science 2007; Vol. 48:ARVO E‐Abstract 4151.
Bruha 1972 {published data only}
- Bruha H. Therapy of the so‐called chorioretinitis centralis serosa with the laser‐coagulator. Klinische Monatsblatter fur Augenheilkunde 1972;160(3):340‐6. [PubMed] [Google Scholar]
Cervera 2008 {published data only}
- Cervera E, Montero J, Torralba C, Palomares P, Hernandez M. Low dose photodynamic therapy for chronic central serous chorioretinopathy. Archivos de la Sociedad Espanola de Oftalmologia 2008;83(9):525‐6. [DOI] [PubMed] [Google Scholar]
Chrapek 2015 {published data only}
- Chrapek O, Jirkova B, Kandrnal V, Rehak J, Sin M. Treatment of central serous chorioretinopathy with beta‐blocker metipranolol. Biomedical Papers of the Medical Faculty of Palacky University in Olomouc, Czech Republic 2015;159(1):120‐3. [DOI] [PubMed] [Google Scholar]
Demirel 2014 {published data only}
- Demirel S, Ozmert E, Batioglu F. The comparison of reduced‐fluence photodynamic therapy with 577 nm yellow‐wavelength pattern micropulse laser therapy in cases with chronic central serous chorioretinopathy: short term results. Ophthalmologica 2014;232(Suppl 2):60. [Google Scholar]
Di 2013 {published data only}
- Di Y, Gui DM, Yang HW, Chen XL. Changes in visual function before and after laser photocoagulation of central serous chorioretinopathy. International Eye Science 2013;13(2):336‐8. [Google Scholar]
Earl 2014 {published data only}
- Earl JB, Lee CS, Yom V, Stavern GP, Abuattieh M, Chin‐Yee D, et al. Visual cycle suppression via patching in central serous chorioretinopathy. Ophthalmology 2014;121(12):2502‐4. [DOI] [PubMed] [Google Scholar]
Fang 2013 {published data only}
- Fang TB, Yan H, Xu ZR. Argon laser treatment of central serous chorioretinopathy. International Eye Science 2013;13(4):740‐2. [Google Scholar]
Feily 2009 {published data only}
- Feily A, Namazi MR. The potential utility of coumarin and horse chestnut extract for treatment of central serous chorioretinopathy. Nigerian Journal of Medicine 2009;18(4):434‐5. [PubMed] [Google Scholar]
Haas 2004 {published data only}
- Haas A, Weger M, Furschuss‐Wolff P, Bachernegg M. Photodynamic therapy in patients with central serous chorioretinopathy. Spektrum der Augenheilkunde 2004;18(2):104. [Google Scholar]
Heinrich 1974 {published data only}
- Heinrich MR. Central serous retinopathy and alpha‐blockaders. Bulletin des Sociétés d'Ophtalmologie de France 1974;74(5‐6):681‐3. [PubMed] [Google Scholar]
Huang 2006 {published data only}
- Huang Y, Wang Z, Zhang M. Clinical effectiveness of lecithin‐bound iodine on central serous chorioretinopathy following laser photocoagulation. Chinese Ophthalmic Research 2006;24(5):546‐8. [Google Scholar]
Khosla 1997 {published data only}
- Khosla PK, Rana SS, Tewari HK, Azad RU, Talwar D. Evaluation of visual function following argon laser photocoagulation in central serous retinopathy. Ophthalmic Surgery and Lasers 1997;28(8):693‐7. [PubMed] [Google Scholar]
Koss 2012 {published data only}
- Koss MJ, Beger I, Koch FH. Subthreshold diode laser micropulse photocoagulation versus intravitreal injections of bevacizumab in the treatment of central serous chorioretinopathy. Eye 2012;26(2):307‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurimoto 1969 {published data only}
- Kurimoto S, Fukunaga K, Momomura K. Effect of adenosine monophosphate on retinitis centralis. Nihon Ganka Kiyo 1969;20(8):841‐50. [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee JY, Chae JB, Yang SJ, Kim JG, Yoon YH. Intravitreal bevacizumab versus the conventional protocol of photodynamic therapy for treatment of chronic central serous chorioretinopathy. Acta Opthalmologica 2011;89(3):e293‐4. [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li XJ, Zhang JS. Intravitreal bevacizumab injection for chronic central serous chorioretinopathy. Chinese Medical Journal 2010;123(15):2145‐7. [PubMed] [Google Scholar]
Lim 2011 {published data only}
- Lim JW, Kang SW, Kim YT, Chung SE, Lee SW. Comparative study of patients with central serous chorioretinopathy undergoing focal laser photocoagulation or photodynamic therapy. British Journal of Ophthalmology 2011;95(4):514‐7. [DOI] [PubMed] [Google Scholar]
Liu 2009 {published data only}
- Liu ZC, Li R. 532 Laser photocoagulation for the treatment of central serous chorioretinopathy. International Journal of Ophthalmology 2009;9(12):2451‐2. [Google Scholar]
Long 2011 {published data only}
- Long F, Cao SJ, Wang LP, Qi F, Xiu WW. Study in krypton laser combined with compound anisodine for central serous chorioretinopathy. International Journal of Ophthalmology 2011;11(2):330‐1. [Google Scholar]
Lyons 1977 {published data only}
- Lyons DE. Conservative management of central serous retinopathy. Transactions of the Ophthalmological Societies of the United Kingdom 1977;97(1):214‐6. [PubMed] [Google Scholar]
Mackowiakowa 1987 {published data only}
- Mackowiakowa A, Pecoldowa K, Szwarcowa C. Comparison of late results of conservative treatment and laser coagulation of central serous chorioretinopathy [Porownanie wynikow odleglych po leczeniu zachowawczym i laserokoagulacji choroidoretinopatii surowiczej srodkowej]. Klinika Oczna 1987;89(4):162‐4. [PubMed] [Google Scholar]
Miyashita 1971 {published data only}
- Miyashita S, Sakai M, Kanai A, Hayashi E. Successful treatment of central serous retinopathy by tranexamic acid injection. Nippon Ganka Gakkai Zasshi 1971;75:603‐8. [PubMed] [Google Scholar]
NCT01256580 {unpublished data only}
- NCT01256580. Pilot study of intravitreal bevacizumab vs. combination therapy for choroidal neovascularization secondary to causes other than age‐related macular degeneration. clinicaltrials.gov/ct2/show/NCT01256580 (accessed 16 October 2015).
NCT01585441 {unpublished data only}
- NCT01585441. Phase II, randomized, placebo‐controlled study for the evaluation of finasteride in the treatment of chronic central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT01585441 (accessed 16 October 2015).
Novak 1987 {published data only}
- Novak MA, Singerman LJ, Rice TA. Krypton and argon laser photocoagulation for central serous chorioretinopathy. Retina 1987;7(3):162‐9. [DOI] [PubMed] [Google Scholar]
Okamoto 2015 {published data only}
Ozdemir 2014 {published data only}
- Ozdemir O, Erol MK. Morphologic changes and visual outcomes in resolved central serous chorioretinopathy treated with ranibizumab. Cutaneous and Ocular Toxicology 2014;33(2):122‐6. [DOI] [PubMed] [Google Scholar]
Peng 2010 {published data only}
- Peng QH, Peng J, Wu QL, Tan HY. Clinical study of method of activating blood and diuresis on central serous. International Journal of Ophthalmology 2010;10(7):1284‐6. [Google Scholar]
Radian 1984 {published data only}
- Radian AB, Mocanu C. Enzymatic treatment of central serous retinopathy. Revista de Chirurgie, Oncologie, Radiologie, ORL, Oftalmologie, Stomatologie. Oftalmologie 1984;28(4):299‐300. [PubMed] [Google Scholar]
Sanchez‐Pacheco 2010 {published data only}
- Sanchez‐Pacheco Tardon L, Pardo Saiz A, Nebot Martinez J, Jornet Montana S. Mifepristone as a treatment alternative to chronic serous chorioretinopathy. Farmacia Hospitalaria 2010;34(1):44‐5. [DOI] [PubMed] [Google Scholar]
Takagi 1965 {published data only}
- Takagi M. Statistic studies of retinitis centralis. Nippon Ganka Kiyo 1965;16(7):420‐3. [PubMed] [Google Scholar]
Tewari 1986 {published data only}
- Tewari HK, Azad R, Khosla PK, Kumar A. Argon laser photocoagulation in treatment of central serous retinopathy ‐ a prospective randomized study. Indian Journal of Ophthalmology 1986;34:251‐3. [PubMed] [Google Scholar]
Wang 2009a {published data only}
- Wang XX. Clinical analysis of argon laser in treating central serous chorioretinopathy. International Journal of Ophthalmology 2009;9(2):384‐5. [Google Scholar]
Wang 2009b {published data only}
- Wang TT, Xu GX. Clinical observation of alliance application of compound anisodine and joletion in the treatment of central serous chorioretinopathy. International Journal of Ophthalmology 2009;9(6):1169‐71. [Google Scholar]
Watzke 1974 {published data only}
- Watzke RC, Burton TC, Leaverton P. Ruby laser photocoagulation therapy of central serous retinopathy. A preliminary report. Modern Problems in Ophthalmology 1974;12(0):242‐6. [PubMed] [Google Scholar]
Watzke 1979 {published data only}
- Watzke RC, Burton TC, Woolson RF. Direct and indirect laser photocoagulation of central serous choroidopathy. American Journal of Ophthalmology 1979;88(5):914‐8. [DOI] [PubMed] [Google Scholar]
Wu 2010 {published data only}
- Wu SJ, Zeng ZC. Clinical observation of Jolethin combined with argon laser for central serous chorioretinopathy. International Journal of Ophthalmology 2010;10(12):2295‐7. [Google Scholar]
Xu 2013 {published data only}
- Xu B. Efficacy comparison of 577nm micro‐pulse laser and drug in the treatment of central serous chorioretinopathy. International Eye Science 2013;13(8):1688‐90. [Google Scholar]
Xu 2014 {published data only}
- Xu JF, Chen KC. Treatment of juxtafoveal central serous chorioretinopathy by compound anisodine injection. International Eye Science 2014;14(4):701‐3. [Google Scholar]
Ye 2013 {published data only}
- Ye J, Yang XY, Zeng S. Compound Xueshuantong capsule joint laser treating central serous chorioretinopathy. International Eye Science 2013;13(6):1160‐2. [Google Scholar]
Zhang 2014 {published data only}
- Zhang J, Xue GM, Zhang QY, Cao Y. Clinical observation of laser treatment for central serous chorioretinopathy choroidal diseases and study on relationship between visual acuity with leakage location. International Eye Science 2014;14(5):872‐4. [Google Scholar]
Zheng 2013 {published data only}
- Zheng TF, Qin YY. Hyperbaric oxygen therapy combined with jolethin in treating central serous chorioretinopathy. International Eye Science 2013;13(10):2124‐6. [Google Scholar]
References to ongoing studies
EUCTR2009‐017959‐98‐NL {unpublished data only}
- EUCTR2009‐017959‐98‐NL. Early treatment of patients with central serous retinopathy: a randomized controlled trial ‐ CSR & PDT. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009‐017959‐98‐NL (accessed 16 October 2015).
JPRN‐UMIN000005372 {unpublished data only}
- JPRN‐UMIN000005372. Study on the effects of supplements containing lutein on spontaneous resolution in eyes with chronic central serous chorioretinopathy. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000005372 (accessed 16 October 2015).
NCT01019668 {unpublished data only}
- NCT01019668. Central serous chorioretinopathy treated by modified photodynamic therapy. clinicaltrials.gov/ct2/show/NCT01019668 (accessed 16 October 2015).
NCT01552044 {unpublished data only}
- NCT01552044. Effect of spironolactone in treating chronic non‐resolutive central serous chorioretinitis [Evaluation de la spironolactone dans le traitement des choriorétinites séreuses centrales non résolutives à trois mois]. clinicaltrials.gov/ct2/show/NCT01552044 (accessed 16 October 2015).
NCT01630863 {unpublished data only}
- NCT01630863. The safe effective light dose of photodynamic therapy for chronic central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT01630863 (accessed 16 October 2015).
NCT01797861 {unpublished data only}
- Breukink MB, Downes SM, Querques G, Dijk EH, Hollander AI, Blanco‐Garavito R, et al. Comparing half‐dose photodynamic therapy with high‐density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy (the PLACE trial): study protocol for a randomized controlled trial. TRIALS 2015;16:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01797861. A prospective randomized controlled multicentre trial comparing half‐dose photodynamic therapy (PDT) with high‐density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy (CSC). clinicaltrials.gov/ct2/show/NCT01797861 (accessed 16 October 2015).
NCT01971190 {unpublished data only}
- NCT01971190. Efficacy and safety of intravitreal aflibercept injection for subacute central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT01971190 (accessed 16 October 2015).
NCT01982383 {unpublished data only}
- NCT01982383. A prospective study of the use of micropulse 577nm laser treatment in central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT01982383 (accessed 16 October 2015).
NCT01990677 {unpublished data only}
- NCT01990677. Eplerenone for the treatment of central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT01990677 (accessed 16 October 2015).
NCT02153125 {unpublished data only}
- NCT02153125. Eplerenone for the treatment of chronic central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT02153125 (accessed 16 October 2015).
NCT02215330 {unpublished data only}
- NCT02215330. A randomized, double‐masked, placebo controlled study of the beneficial effects of eplerenone on central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT02215330 (accessed 16 October 2015).
NCT02354170 {unpublished data only}
- NCT02354170. Short‐term oral mifepristone for central serous chorioretinopathy. A placebo‐controlled dose ranging study of mifepristone in the treatment of CSC (STOMP‐CSC). clinicaltrials.gov/ct2/show/NCT02354170 (accessed 16 October 2015).
Additional references
Ahad 2006
- Ahad MA, Chua CN, Evans NM. Central serous chorioretinopathy associated with testosterone therapy. Eye 2006;20(4):503‐5. [DOI] [PubMed] [Google Scholar]
Baran 2005
- Baran NV, Gürlü VP, Esgin H. Long‐term macular function in eyes with central serous chorioretinopathy. Clinical and Experimental Ophthalmology 2005;33(4):369‐72. [DOI] [PubMed] [Google Scholar]
Bousquet 2013
- Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar‐Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina 2013;33(10):2096‐102. [DOI] [PubMed] [Google Scholar]
Bouzas 2002
- Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Survey of Ophthalmology 2002;47(5):431‐48. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50:683‐91. [DOI] [PubMed] [Google Scholar]
Cardillo Piccolino 2003
- Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003;23(6):752‐63. [DOI] [PubMed] [Google Scholar]
Cassin 2006
- Cassin B. Dictionary of Eye Terminology. 5th Edition. Gainesville, Florida: Triad Publishing Company, 2006. [Google Scholar]
Castro‐Correia 1992
- Castro‐Correia J, Countinho MF, Rosas V, Maia J. Long‐term follow‐up of central serous retinopathy in 150 patients. Documenta Ophthalmologica 1992;81(4):379‐86. [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2003
- Chan WM, Lam DS, Lai TY, Yuen KS, Liu DT, Chan CK, et al. Treatment of choroidal neovascularization in central serous chorioretinopathy by photodynamic therapy with verteporfin. American Journal of Opthalmology 2003;136(5):836‐45. [DOI] [PubMed] [Google Scholar]
Chen 2008
- Chen SN, Hwang JF, Tseng LF, Lin CJ. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology 2008;115(12):2229‐34. [DOI] [PubMed] [Google Scholar]
Chou 2000
- Chou SC, Lin JD. Long‐term effects of ketoconazole in the treatment of residual or recurrent Cushing's disease. Endocrine Journal 2000;47(4):401‐6. [DOI] [PubMed] [Google Scholar]
Chung 2013
- Chung YR, Seo EJ, Lew HM, Lee KH. Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta‐analysis and review. Eye 2013;27:1339‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2008
- Clark JA, Flick RB, Pai LY, Szalayova I, Key S, Conley RK, et al. Glucocorticoid modulation of tryptophan hydroxylase‐2 protein in raphe nuclei and 5‐hydroxytryptophan concentrations in frontal cortex of C57/Bl6 mice. Molecular Psychiatry 2008;13(5):498‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotticelli 2006
- Cotticelli L, Borrelli M, D'Alessio AC, Menzione M, Villani A, Piccolo G, et al. Central serous chorioretinopathy and Helicobacter pylori. European Journal of Ophthalmology 2006;16(2):274‐8. [DOI] [PubMed] [Google Scholar]
Covidence
- Covidence. www.covidence.org (accessed 20 April 2015).
Cox 1988
- Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. JAMA Ophthalmology 1988;106(9):1190‐5. [DOI] [PubMed] [Google Scholar]
FDA 2015
- Drugs@FDA. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/drugsatfda/ (accessed 25 August 2015).
Forooghian 2011
- Forooghian F, Meleth AD, Cukras C, Chew EY, Wong WT, Meyerle CB. Finasteride for chronic central serous chorioretinopathy. Retina 2011;31(4):766‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gass 1967
- Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. American Journal of Ophthalmology 1967;63(3):S1‐139. [PubMed] [Google Scholar]
Giusti 2004
- Giusti C. Association of Helicobacter pylori with central serous chorioretinopathy: hypotheses regarding pathogenesis. Medical Hypotheses 2004;63(3):524‐7. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Grieshaber 2007
- Grieshaber MC, Staub JJ, Flammer J. The potential role of testosterone in central serous chorioretinopathy. British Journal of Ophthalmology 2007;91(1):118‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guengerich 1999
- Guengerich FP. Cytochrome P‐450 3A4: regulation and role in drug metabolism. Annual Review of Pharmacology and Toxicology 1999;39:1‐17. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64:380‐2. [DOI] [PubMed] [Google Scholar]
Haimovici 2004
- Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S, Central Serous Chorioretinopathy Case‐Control Study Group. Risk factors for central serous chorioretinopathy: a case‐control study. Ophthalmology 2004;111(2):244‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2012
- Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3:98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofstetter 2000
- Hofstetter WH, Griffin JR, Berman MS, Everson RW. Visual Science and Related Clinical Terms. 5th Edition. Woburn: Butterworth‐Heinemann, 2000. [Google Scholar]
Iijima 1999
- Iijima H, Iida T, Murayama K, Imai M, Gohdo T. Plasminogen activator inhibitor 1 in central serous chorioretinopathy. American Journal of Ophthalmology 1999;127(4):477‐8. [DOI] [PubMed] [Google Scholar]
Jampol 2002
- Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology 2002;109(10):1765‐6. [DOI] [PubMed] [Google Scholar]
Kim 2009
- Kim SW, Oh J, Oh IK, Huh K. Retinal pigment epithelial tear after half fluence PDT for serous pigment epithelial detachment in central serous chorioretinopathy. Ophthalmic Surgery, Lasers and Imaging Retina 2009;40(3):300‐3. [DOI] [PubMed] [Google Scholar]
Kitzmann 2008
- Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980‐2002. Ophthalmology 2008;115(1):169‐73. [DOI] [PubMed] [Google Scholar]
Klein 1974
- Klein ML, Buskirk EM, Friedman E, Gragoudas E, Chandra S. Experience with nontreatment of central serous choroidopathy. Archives of Ophthalmology 1974;91(4):247‐50. [DOI] [PubMed] [Google Scholar]
Lai 2006
- Lai TY, Chan WM, Li H, Lai RY, Liu DT, Lam DS. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. British Journal of Ophthalmology 2006;90(7):869‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2014
- Lim JI, Glassman AR, Aiello LP, Chakravarthy U, Flaxel CJ, Spaide RF. Collaborative retrospective macula society study of photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 2014;121:1073‐8. [DOI] [PubMed] [Google Scholar]
Loo 2002
- Loo RH. Factors associated with reduced visual acuity during long‐term follow‐up of patients with idiopathic central serous chorioretinopathy. Retina 2002;22(1):19‐24. [DOI] [PubMed] [Google Scholar]
Ma 2014
- Ma J, Meng N, Xu X, Zhou F, Qu Y. System review and meta‐analysis on photodynamic therapy in central serous chorioretinopathy. Acta Ophthalmologica 2014;92:e594‐601. [DOI] [PubMed] [Google Scholar]
Maalej 2014
- Maalej A, Khallouli A, Wathek C, Rannen R, Gabsi S. Central serous chorioretinopathy: clinical‐anatomic correlations. Journal Français d'Ophtalmologie 2014;37(10):787‐95. [DOI] [PubMed] [Google Scholar]
Maruko 2010
- Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology 2010;117(9):1792‐9. [DOI] [PubMed] [Google Scholar]
Meyerle 2007
- Meyerle CB, Freund KB, Bhatnagar P, Shah V, Yannuzzi LA. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy. Retina 2007;27(7):943‐6. [DOI] [PubMed] [Google Scholar]
Mitsui 1969
- Mitsui Y, Matsubara M, Kanagawa M. Xenon light‐exposure as a treatment of central serous retinopathy (a preliminary report). Nippon Ganka Kiyo 1969;20(3):291‐4. [PubMed] [Google Scholar]
Montero 2005
- Montero JA, Ruiz‐Moreno JM. Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. British Journal of Ophthalmology 2005;89(5):562‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mudvari 2007
- Mudvari SS, Goff MJ, Fu AD, McDonald HR, Johnson RN, Ai E. The natural history of pigment epithelial detachment associated with central serous chorioretinopathy. Retina 2007;27(9):1168‐73. [DOI] [PubMed] [Google Scholar]
Nicholson 2013
- Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Survey of Ophthalmology 2013;58(2):103‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prunte 1996
- Prunte C, Flammer J. Choriodal capillary and venous congestion in central serous chorioretinopathy. American Journal of Ophthalmology 1996;121(1):26‐34. [DOI] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
Quin 2013
- Quin G, Liew G, Ho I, Gillies M, Fraser‐Bell S. Diagnosis and interventions for central serous chorioretinopathy: review and update. Clinical and Experimental Ophthalmology 2013;41(2):187‐200. [DOI] [PubMed] [Google Scholar]
Reibaldi 2010
- Reibaldi M, Cardascia N, Longo A, Furino C, Avitabile T, Faro S, et al. Standard‐fluence versus low‐fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. American Journal of Ophthalmology 2010;149(2):307‐15. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ricci 2004
- Ricci F, Missiroli F, Cerulli L. Indocyanine green dye‐enhanced micropulsed diode laser: a novel approach to subthreshold RPE treatment in a case of central serous chorioretinopathy. European Journal of Ophthalmology 2004;14(1):74‐82. [DOI] [PubMed] [Google Scholar]
Schlötzer‐Schrehardt 2002
- Schlötzer‐Schrehardt U, Viestenz A, Naumann GO, Laqua H, Michels S, Schmidt‐Erfurth U. Dose‐related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefe's Archive for Clinical and Experimental Ophthalmology 2002;240(9):748‐57. [DOI] [PubMed] [Google Scholar]
Schmidt‐Erfurth 2002
- Schmidt‐Erfurth U, Michels S, Barbazetto I, Laqua H. Photodynamic effects on choroidal neovascularization and physiological choroid. Investigative Ophthalmology and Visual Science 2002;43(3):830‐41. [PubMed] [Google Scholar]
Shuler 2006
- Shuler RK, Mruthyunjaya P. Diagnosing and managing central serous chorioretinopathy. EyeNet Magazine, February 2006. Available at www.aao.org/publications/eyenet/200602/pearls.cfm (accessed 13 March 2015).
Sivaprasad 2010
- Sivaprasad S, Elagouz M, McHugh D, Shona O, Dorin G. Micropulsed diode laser therapy: evolution and clinical applications. Survey of Ophthalmology 2010;55(6):516‐30. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Systematic Review Data Repository
- Systematic Review Data Repository (SRDR). srdr.ahrq.gov/ (accessed 20 April 2015).
Wang 2008
- Wang M, Munch IC, Hasler PW, Prunte C, Larsen M. Central serous chorioretinopathy. Acta Opthalmologica 2008;86(2):126‐45. [DOI] [PubMed] [Google Scholar]
White 2009
- White IR. Multivariate random‐effects meta‐analysis. Stata Journal 2009;9:40‐56. [Google Scholar]
White 2011
- White IR. Multivariate random‐effects meta‐regression: updates to mvmeta. Stata Journal 2011;11:255‐70. [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Research Synthesis Methods 2012;3:111‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winquist 1995
- Winquist EW, Laskey J, Crump M, Khamsi F, Shepherd FA. Ketoconazole in the management of paraneoplastic Cushing's syndrome secondary to ectopic adrenocorticotropin production. Journal of Clinical Oncology 1995;13(1):157‐64. [DOI] [PubMed] [Google Scholar]
Yannuzzi 1987
- Yannuzzi LA. Type‐A behavior and central serous chorioretinopathy. Retina 1987;7(2):111‐31. [DOI] [PubMed] [Google Scholar]
Yanoff 2013
- Yanoff M, Duker J. Ophthalmology. 4th Edition. Saunders, 2013. [Google Scholar]
Zhao 2012
- Zhao M, Célérier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli M, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. Journal of Clinical Investigation 2012;122(7):2672‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Salehi 2015
- Salehi M, Wenick A, Law HA, Evans J, Gehlbach P. Interventions for central serous chorioretinopathy: a network meta‐analysis. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011841] [DOI] [PMC free article] [PubMed] [Google Scholar]