Abstract
Nonalcoholic fatty liver disease (NAFLD) is commonly diagnosed in obese or overweight individuals. However, lean individuals with NAFLD are not rare but represent one significant end of the phenotypic spectrum of NAFLD. Although initial observations between obese and lean NAFLD reveal some metabolic parallels, these associations vary widely given differences in study populations and metabolic parameters assessed. The role of body composition in risk assessment is significant and incompletely assessed during most clinical encounters. Recent multinational investigation reveals an increased mortality in lean individuals with NASH. Many aspects of lean NAFLD need further exploration including epidemiology, clinical risk assessment, histologic changes unique to lean NAFLD, genetic and pathophysiologic mechanisms predisposing at risk individuals, natural history and treatment strategies in this underrecognized population.
Keywords: lean, nonobese, normal weight, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH)
Introduction
Nonalcoholic fatty liver disease is now the most common chronic liver disease in the developing and developed world. NAFLD most often presents in states of nutrient excess and obesity with enhanced peripheral adiposity. It is strongly associated with the metabolic syndrome, diabetes and dyslipidemia. However, there is emerging evidence of NAFLD in lean or normal weight individuals. Lean NAFLD may involve similar pathophysiologic pathways given the overlap in phenotypic expression with obese NAFLD – bearing strong associations with insulin resistance and dyslipidemia. The mechanism by which these metabolic effects emerge independent of obesity and increased adiposity are not well known but may hint at genetic risk factors not present in obese NAFLD.
We will explore the spectrum of lean NAFLD and describe its associated metabolic effects, clinical outcomes and areas for future investigation.
BMI and Body composition
The definition of lean BMI (body mass index) is most often regarded as <25 kg/m2. The utility of this measure in some populations, especially Asians, is under scrutiny.
For the purposes of this review, the definitions of lean were in accordance with the World Health Organization (WHO) for international populations. It cannot be overemphasized, however, that BMI-associated risk by race and ethnicity is not to be ignored and may hint at underlying genetic mechanisms that place individuals at risk for NAFLD. With this understanding, we will agree in this article to consider lean as 18.5 < BMI (kg/m2) < 25 and nonobese as 18.5 < BMI <=30,for all racial and ethnic groups. Although the American Dietetic Association (ADA) considers the ideal body mass between 20 and 25, the World Health Organization (WHO), classifies a normal BMI as 18.50 to 24.99 for international populations, while acknowledging increased risk for type 2 diabetes and cardiovascular disease at lower BMIs in Asian populations [1].
BMI is commonly used as a surrogate of body fat content, however the utility of that assessment for true body composition, especially in the lean population, may be insufficient.
Lean NAFLD: One end of a phenotypic spectrum
On a metabolic continuum, there exists a classification of metabolically healthy obese with normal insulin sensitivity without increased mortality risk from cardiometabolic disease, generally thought of as the metabolically healthy obese. On the other end of that spectrum, there exist lean individuals who display insulin resistance, hyperinsulinemia and atherogenic dyslipidemia; these individuals are commonly referred to as metabolically obese normal weight (MONW). MONW individuals are ostensibly distributed along racial and ethnic lines, with Asians developing significant metabolic disease outcomes at lower BMIs than other ethnic groups. The prevalence of lean NAFLD has been described in different ethnic populations, mainly Asian: 20% in India [2], 15.2 % in Japan [3], 15% in China [4], 12% in Greece (5), 12.6% South Korea (6), as well as in Iceland (7) and the US (Dela Cruz abstract and (8)). Well established is that the presence of liver fat, independent of BMI, is strongly associated with increased metabolic risk for insulin resistance.
Despite the association between hepatic steatosis and insulin resistance, there have been differences in other metabolic parameters across ethnic subgroups. In 2004, Kim et al. examined 768 nonobese, nondiabetic individuals over the age of 30 in South Korea. The authors excluded diabetics (defined as anyone with a history of diabetes or fasting glucose >126 mg/dL) and other subjects with risk factors for fatty liver or chronic liver disease; they included subjects with BMI <= 30 and > 18.5 kg/m2) to obtain non-obese subjects for comparison to obese (BMI>30). The nonobese subjects were further subdivided into normal weight (18.5 ≤ BMI< 25) and overweight (25 ≤ BMI <30). In comparison with their overweight counterparts, normal weight subjects in Korea with NAFLD displayed increased prevalence of hypertriglyceridemia, hyperuricemia, insulin resistance and central obesity but a lower prevalence of diabetes, hypertension, hypertriglyceridemia, low –HDL cholesterol, central obesity and metabolic syndrome than in other studies of lean NAFLD (compared lean to non-lean, included obese, distribution unknown) (6).
In looking further at the relationship of liver fat and ethnic differences for disease susceptibility for type 2 diabetes in lean individuals, Petersen looked at 482 young, lean, healthy, sedentary nonsmoking Eastern Asians, Asian-Indians, Blacks, Caucasians and Hispanics. There was a two-fold increase in hepatic triglyceride content as assessed by proton magnetic resonance spectroscopy in Asian Indian males with insulin resistance (assessed by OGTT by ISI) as opposed to Caucasians. Asian Indian males displayed an increased prevalence of insulin resistance (HOMA-IR) compared to all other ethnic groups. Additionally, markers of inflammatory activation, particularly IL-6, were elevated in Asian Indian males. Complementary studies to look at intracellular lipid content were also undertaken via proton magnetic resonance spectroscopy (MRS) (9) and revealed larger adipocyte size in Asian Indians versus their Caucasian counterparts. This observation, coupled with higher levels of nonesterified fatty acids (NEFAs) and lower levels of adiponectin bear resemblance to traditional obese NAFLD.
The relationship between body composition and insulin resistance in South Asians was then the subject of further study. Chandalia et al. examined the clinical characteristics of body fat content, distribution and function in South Asian men (n=29) and the relationship of those characteristics to insulin resistance compared to Caucasians (n=18). Insulin sensitivity was assessed by euglycemic-hyperinsulinemic clamp (10). Body fat content was calculated by underwater weighing for total body fat, MRI of entire abdomen for intraperitoneal (IP) and subcutaneous abdominal (SA) fat and biopsy of SA fat for adipocyte size. The authors related the insulin resistance seen in young South Asian men with large subcutaneous adipocyte size rather than intraperitoneal fat mass (no difference between groups), implicating truncal fat and dysfunctional adipose tissue than the visceral fat excess.
Looking at the phenotype of lean NASH within the greater context of hepatic steatosis, Das et al found the prevalence of NAFLD within a cohort of active, rural, predominantly poor community in West Bengal, India to be 8.7%. Although not entirely lean (overweight/central obesity present in 7 and 11%), the average BMI was 19.6 +/− 6.6kg/m2. Of particular importance is that 31% of the subjects with NAFLD and elevated ALT had NASH histologically and 2.4% had cirrhosis. The non-obese and lean individuals (average BMI 19.6 +/− 6.6kg/m2) with NAFLD were phenotypically distinct: more subcutaneous fat, higher fasting blood glucose, and higher levels of triglycerides (11). Lean NASH behaves much like obese NASH from a biological standpoint; however the absence of significant adiposity suggests a phenotypic uniqueness with shared biology but raises the possibility of genetic risk.
Studies of lean NAFLD that include histology are limited (see Table 1). Histologic grading systems for non-obese or lean NAFLD and NASH characterize the disease process using the same parameters as obese NAFLD; a more specialized approach to a unique phenotype is an area of unmet need.
Table 1.
Summary of recent studies of lean NAFLD in different ethnic populations.
| Reference | Country | Population | Definition of NAFLD | % NAFLD cases with BMI <25 | Risk factors for NAFLD in lean individuals |
|---|---|---|---|---|---|
| Das (11) | India | General, rural N = 1911 | US or CT *subset with biopsy and elastography | 75 % | Higher BMI (OR 1.2; 95% CI 1.1-1.4; p <0.01) Higher biceps skinfold thickness (OR 1.2; 95% CI 1.1-1.3; p <0.01) |
| Nishioji (3) | Japan | General N = 3271 | US | 15.2% | Waist circumference (male OR 1.11; 95% CI 1.07-1.16; p <0.001) Body fat percent (male 1.13 95% CI 1.07-1.19; p <0.001) |
| Feng (4) | China | General n = 1779 | US | 15% | Met-S (OR = 2.17, 95%CI: 1.17-4.05) |
| Margariti (5) | Greece | NAFLD patients in hepatology clinics n=162 | US | 12% | - |
| Kim (6) | Korea | General, clinic N = 768 | US | 16.1% | Male gender (B 1.12; p=0.046) Waist circumference (B 0.13; p = .001) TG (B 0.004; p = 0.01) HOMA-IR (B 1.74; p 0.03) |
| Younossi (8) | US | National Health and Nutrition Examination Survey III N = 11,613 | US | 7.39% | Age [younger] (OR 0.98, 95% CI 0.97-0.99; p =0.0008) Female (*OR given for male sex 0.60, 95% CI 0.41-0.87, p = 0.008) |
| Dela Cruz (abstract) | Multinational | Multicenter, N =1090 | Liver biopsy | 11.5% | - |
NAFLD nonalcoholic fatty liver disease, BMI body mass index, US ultrasound, CT computed tomography, Met-S Metabolic Syndrome, TG triglycerides, HOMA-IR Homeostatic model assessment – insulin resistance
Lean NAFLD: Outcomes and Natural History
Little is known about the prognosis of lean individuals with NAFLD. In a large, multicenter, biopsy-proven cohort, De la Cruz et al reported increased overall mortality in lean patients than overweight or obese patients with NAFLD [12]. Other studies have shown more metabolic derangements in lean individuals with NAFLD without long term survival data.
In 2014, Dela Cruz et al observed 1090 patients with biopsy proven NAFLD, comparing lean NAFLD (BMI <25 kg/m2) and non-lean (BM>= 25) individuals in order to assess the clinical presentation and long-term prognosis of lean patients with NAFLD. 125 patients with lean NAFLD (BMI 23.1 +/− 1.7) and 965 non-lean (BMI 33.3 +/−6 6.6) patients were enrolled from multiple centers on multiple continents.
Standard scoring systems were used to assess steatosis, inflammation, ballooning and fibrosis. Patients with lean NAFLD were more commonly men, of non-Caucasian race, and had a lower prevalence of diabetes, hypertension, hypertriglyceridemia, low-HDL cholesterol, central obesity and metabolic syndrome as compared to non-lean NAFLD (p <0.004 for all). The lean NAFLD group had significantly lower levels of ALT and less insulin resistance (HOMA-IR), a lower degree of steatosis and less advanced fibrosis, but more severe lobular inflammation than the non-lean NAFLD group (p <0.03 for all). There was no significant difference between groups in age, hepatocyte ballooning or with definitive NASH.
Overall mortality was calculated in a subset of patients (n=483) who underwent biopsy prior to 2005. With 133 +/− 81.3 months of follow-up in this subset, 71 died (14.7%). Cumulative survival was shorter in this group (lean NAFLD) as compared to those with non-lean NAFLD.
Prior studies looked at lean/overweight-NAFLD (BMI <30 kg/m2) group as compared to the obese-NAFLD (BMI > 30 kg/m2) group, and found that patients were younger, male, more insulin resistant and had significant NASH and fibrosis present (61% and 55%)(13). In individuals with advanced liver disease, Vos et al. also looked at 1777 patients undergoing liver biopsy for chronic liver disease. Non obese and non diabetic patients with NAFLD was found in 50 (2.8%) and was the most frequent cause of cryptogenic liver disease (38%)(13).
Pathogenesis
The presence of intrahepatic fat required for NAFLD usually occurs in the setting of decreased physical activity, increased caloric intake relative to expenditure, and for the most part, excess adipose tissue or fat mass. However, in individuals without absolute excesses of adipose tissue (lean), or with fat fractions in excess of skeletal muscle and osseous tissue (i.e. a relative excess), the relationship between intrahepatic fat, skeletal muscle and subcutaneous adipose tissue is of intense interest, especially given the development of metabolic changes independent of peripheral adiposity.
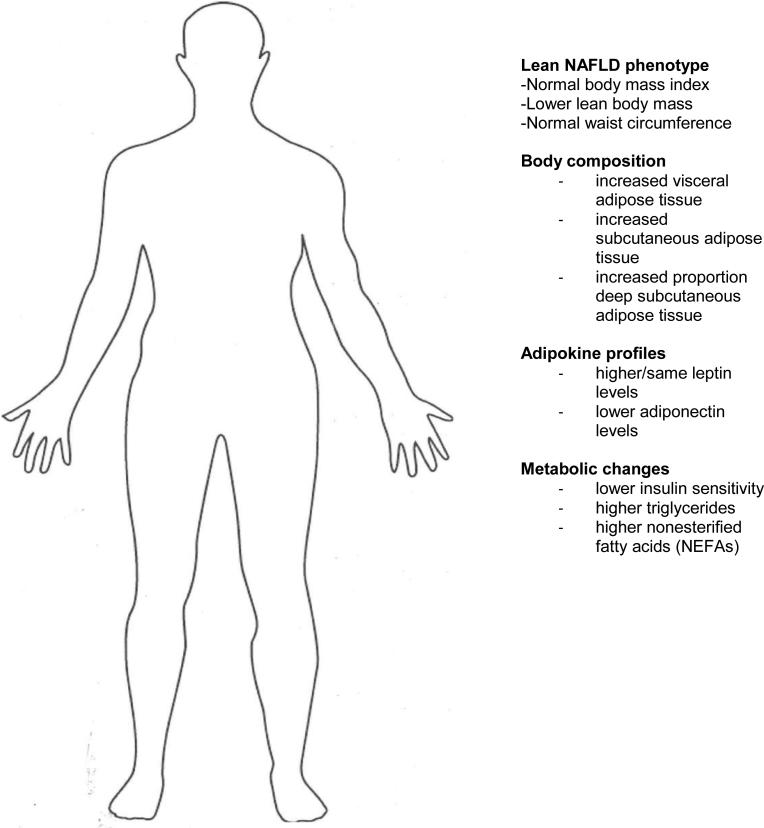
Excess adipose tissue is generally regarded as metabolically harmful; however there is evidence that it is protective against overnutrition by serving as a buffer against metabolic risk factors (14). Using lipodystrophy as an example, the deficiency of adipose tissue leads to the redistribution of fat to skeletal muscle and liver with the resultant metabolic syndrome including severe insulin resistance (15). In these individuals, leptin deficiency induces overnutrition and leads to severe ectopic fat accumulation and resultant severe metabolic syndrome. However, in non-obese NASH, leptin levels have been found to be higher or the same compared with controls, rather than lower (as seen in lipodystrophy); adiponectin levels are notably lower in most studies (16) (See Figure 1).
Figure 1. Phenotypic and Pathophysiologic Changes Associated with Lean NAFLD.
Phenotypic characteristics, body composition, chemokine and metabolic changes observed in individuals with lean NAFLD as compared with lean individuals without NAFLD. Adapted from Das and Chowdhury (23), with permission from Springer.
Metabolically obese individuals with normal weight have a body composition that favors visceral adiposity as opposed to peripheral adiposity. The fatty acid theory may help to explain how this happens. The fatty acid theory identifies elevation of plasma nonesterified fatty acids (NEFA) as the mediating factor between ectopic fat and metabolic risk. Therefore adipose tissue stores determine plasma NEFA levels, but may be affected by the distribution of these stores with upper body obesity generally having higher levels of NEFAs (15). Furthermore, the impact of visceral adipose is expanded, increased portal NEFA are derived from visceral depots in addition to subcutaneous adipose (17-19). This portal NEFAs may help explain hepatic steatosis and dyslipidemia associated with NAFLD (20, 21). Although functional differences between upper body and lower body fat are apparent, the determinants for the distribution of those fat stores are unclear. The role of site specific developmental genes are suspected(22). Independent of the fatty acid theory, dietary carbohydrate is also a notable source of plasma NEFA.
Therapeutics
Apart from cases of lipodystrophy misclassified as lean NAFLD, there are no specific therapeutic options for this particular phenotype. Classical recommendations for managing obese or overweight NAFLD such as weight loss are not available for these individuals; however exercise independent of weight loss, particularly resistance exercise may play an unknown role given fatty acid utilization by muscle. The effectiveness of various treatment modalities (including pharmacotherapeutic options) on lean NAFLD and its associated comorbidities is an area for future investigation.
Conclusions
Lean NAFLD represents a significant portion of individuals with NAFLD. Given the absence of traditional risk factors, especially with regards to ethnic differences with BMI, the development of hepatic steatosis is often underrecognized. Although lean NAFLD represents one end of a metabolic spectrum with similar physiologic consequences as obese NAFLD vis-à-vis the development of insulin resistance, the underlying pathogenesis may be quite different. The lean NAFLD phenotype is an ideal population for genetic studies given the unknown contributions of genetics to body composition and the downstream consequences of variable fat distribution and the expression of genes within adipocytes. Differences in adipocyte expansion capabilities may translate into ectopic fat storage in the liver.
Footnotes
Compliance with Ethics Guidelines:
Conflicts of Interest:
JW and AJS declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent:
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Consultation WHOE Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 2.Bhat G, Baba CS, Pandey A, Kumari N, Choudhuri G. Insulin resistance and metabolic syndrome in nonobese Indian patients with non-alcoholic fatty liver disease. Trop Gastroenterol. 2013;34(1):18–24. doi: 10.7869/tg.2012.86. [DOI] [PubMed] [Google Scholar]
- 3.Nishioji K, Sumida Y, Kamaguchi M, Mochizuki N, Kobayashi M, Nishimura T, et al. Prevalence of and risk factors for non-alcoholic fatty liver disease in a non-obese Japanese population, 2011-2012. J Gastroenterol. 2015;50(1):95–108. doi: 10.1007/s00535-014-0948-9. [DOI] [PubMed] [Google Scholar]
- 4.Feng RN, Du SS, Wang C, Li YC, Liu LY, Guo FC, et al. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J Gastroenterol. 2014;20(47):17932–40. doi: 10.3748/wjg.v20.i47.17932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margariti E, Deutsch M, Manolakopoulos S, Papatheodoridis GV. Non-alcoholic fatty liver disease may develop in individuals with normal body mass index. Ann Gastroenterol. 2012;25(1):45–51. [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164(19):2169–75. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 7.Kim LJ, Nalls MA, Eiriksdottir G, Sigurdsson S, Launer LJ, Koster A, et al. Associations of visceral and liver fat with the metabolic syndrome across the spectrum of obesity: the AGES-Reykjavik study. Obesity (Silver Spring) 2011;19(6):1265–71. doi: 10.1038/oby.2010.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91(6):319–27. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 9.Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A. 2006;103(48):18273–7. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandalia M, Lin P, Seenivasan T, Livingston EH, Snell PG, Grundy SM, et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS One. 2007;2(8):e812. doi: 10.1371/journal.pone.0000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Das K, Das K, Mukherjee PS, Ghosh A, Ghosh S, Mridha AR, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51(5):1593–602. doi: 10.1002/hep.23567. [This study is one of the early prospective, biopsy-proven studies that described lean NAFLD in a nonclassical phenotype. The implications for the developed and developing world are not insignificant in terms of disease burden.] [DOI] [PubMed] [Google Scholar]
- 12**.Dela Cruz AC, et al. Characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease. DDW. 2014 Abstract 379. [This large, multinational study with histologically proven NAFLD in lean individuals demonstrated increased mortality in this population compared with non-lean NAFLD. Although limited to an abstract, these outcomes highlight significant areas for future investigation.] [Google Scholar]
- 13.Vos B, Moreno C, Nagy N, Fery F, Cnop M, Vereerstraeten P, et al. Lean non-alcoholic fatty liver disease (Lean-NAFLD): a major cause of cryptogenic liver disease. Acta Gastroenterol Belg. 2011;74(3):389–94. [PubMed] [Google Scholar]
- 14.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117(9):2621–37. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy SM. Adipose tissue and metabolic syndrome: too much, too little or neither. Eur J Clin Invest. 2015;45(11):1209–17. doi: 10.1111/eci.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musso G, Gambino R, Durazzo M, Biroli G, Carello M, Faga E, et al. Adipokines in NASH: postprandial lipid metabolism as a link between adiponectin and liver disease. Hepatology. 2005;42(5):1175–83. doi: 10.1002/hep.20896. [DOI] [PubMed] [Google Scholar]
- 17.Roust LR, Jensen MD. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes. 1993;42(11):1567–73. doi: 10.2337/diab.42.11.1567. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113(11):1582–8. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Z, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes. 1999;48(8):1586–92. doi: 10.2337/diabetes.48.8.1586. [DOI] [PubMed] [Google Scholar]
- 20.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49(3):791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vega GL, Chandalia M, Szczepaniak LS, Grundy SM. Metabolic correlates of nonalcoholic fatty liver in women and men. Hepatology. 2007;46(3):716–22. doi: 10.1002/hep.21727. [DOI] [PubMed] [Google Scholar]
- 22*.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11(2):90–100. doi: 10.1038/nrendo.2014.185. [The role of adiposity and body composition has clear implications in the development of lean NAFLD. This review elaborates on the connections between adipose tisue and the metabolic syndrome that are important for hepatologists to know.] [DOI] [PubMed] [Google Scholar]
- 23.Das K, Chowdhury A. Lean NASH: distinctiveness and clinical implication. Hepatol Int. 2013;7(Suppl 2):806–13. doi: 10.1007/s12072-013-9477-5. [DOI] [PubMed] [Google Scholar]