Abstract
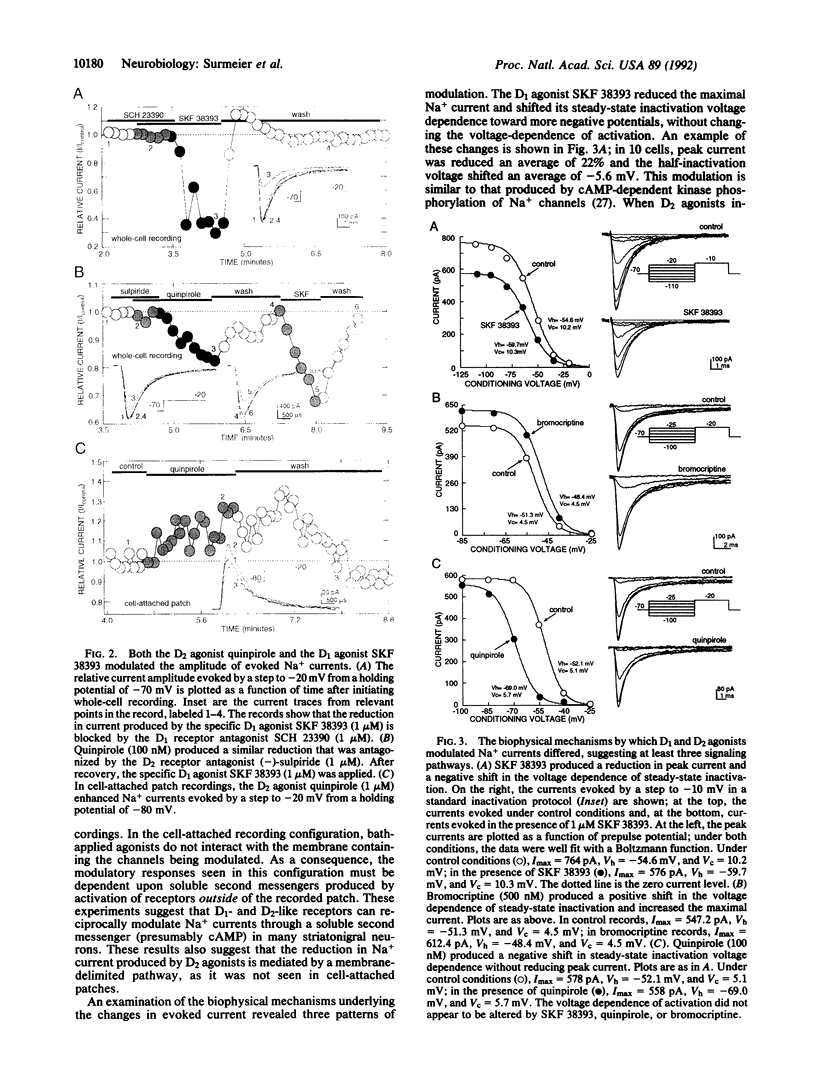
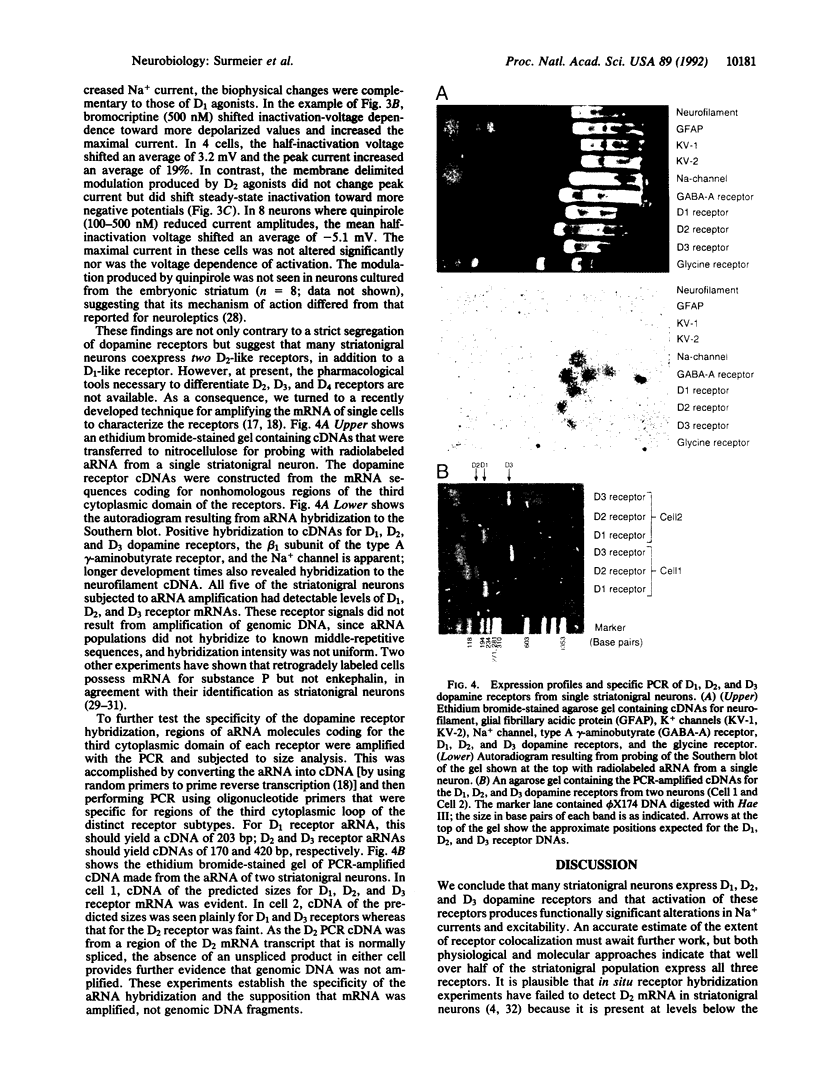
Dopaminergic neurons of the substantia nigra provide one of the major neuromodulatory inputs to the neostriatum. Recent in situ hybridization experiments have suggested that postsynaptic dopamine receptors are segregated in striatonigral and striatopallidal neurons. We have tested this hypothesis in acutely isolated, retrogradely labeled striatonigral neurons by examining the neuromodulatory effects of selective dopaminergic agonists on Na currents and by probing single-cell antisense RNA populations with dopamine receptor cDNAs. In most of the neurons examined (20/31), the application of the D1 dopamine receptor agonist SKF 38393 reduced evoked whole-cell Na+ current. The D2 agonists quinpirole and bromocriptine had mixed effects; in most neurons (23/42), whole-cell Na+ currents were reduced, but in others (8/42), currents were increased. In cell-attached patch recordings, bath application of SKF 38393 decreased currents as in whole-cell recordings, whereas quinpirole consistently (6/10) enhanced currents--suggesting that D2-like receptors could act through membrane delimited and non-delimited pathways. Changes in evoked current were produced by modulation of peak conductance and modest shifts in the voltage dependence of steady-state inactivation. Antisense RNA probes of dopamine receptor cDNA Southern blots consistently (5/5) revealed the presence of D1, D2, and D3 receptor mRNA in single striatonigral neurons. These findings argue that, contrary to a strict receptor segregation hypothesis, many striatonigral neurons colocalize functional D1, D2, and D3 receptors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike A., Ohno Y., Sasa M., Takaori S. Excitatory and inhibitory effects of dopamine on neuronal activity of the caudate nucleus neurons in vitro. Brain Res. 1987 Aug 25;418(2):262–272. doi: 10.1016/0006-8993(87)90094-1. [DOI] [PubMed] [Google Scholar]
- Akins P. T., Surmeier D. J., Kitai S. T. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature. 1990 Mar 15;344(6263):240–242. doi: 10.1038/344240a0. [DOI] [PubMed] [Google Scholar]
- Bertorello A. M., Hopfield J. F., Aperia A., Greengard P. Inhibition by dopamine of (Na(+)+K+)ATPase activity in neostriatal neurons through D1 and D2 dopamine receptor synergism. Nature. 1990 Sep 27;347(6291):386–388. doi: 10.1038/347386a0. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Mroz E. A., Tappaz M. L., Leeman S. E. On the origin of substance P and glutamic acid decarboxylase (GAD) in the substantia nigra. Brain Res. 1977 Oct 28;135(2):315–323. doi: 10.1016/0006-8993(77)91034-4. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Mercuri N., Stanzione P., Stefani A., Bernardi G. Intracellular studies on the dopamine-induced firing inhibition of neostriatal neurons in vitro: evidence for D1 receptor involvement. Neuroscience. 1987 Mar;20(3):757–771. doi: 10.1016/0306-4522(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Misgeld U., Dodt H. U. Intrinsic membrane properties of neostriatal neurons can account for their low level of spontaneous activity. Neuroscience. 1987 Jan;20(1):293–303. doi: 10.1016/0306-4522(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Eberwine J., Yeh H., Miyashiro K., Cao Y., Nair S., Finnell R., Zettel M., Coleman P. Analysis of gene expression in single live neurons. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3010–3014. doi: 10.1073/pnas.89.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley J. C., Maderdrut J. L., Petrusz P. The immunocytochemical localization of enkephalin in the central nervous system of the rat. J Comp Neurol. 1981 Jun 1;198(4):541–565. doi: 10.1002/cne.901980402. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Engber T. M., Mahan L. C., Susel Z., Chase T. N., Monsma F. J., Jr, Sibley D. R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990 Dec 7;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Llano I. Sodium and potassium conductances in somatic membranes of rat Purkinje cells from organotypic cerebellar cultures. J Physiol. 1989 Oct;417:105–122. doi: 10.1113/jphysiol.1989.sp017793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Herrling P. L., Hull C. D. Iontophoretically applied dopamine depolarizes and hyperpolarizes the membrane of cat caudate neurons. Brain Res. 1980 Jun 23;192(2):441–462. doi: 10.1016/0006-8993(80)90896-3. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Yang H. Y., Racagni G., Costa E. Projections of substance P containing neurons from neostriatum to substantia nigra. Brain Res. 1977 Feb 25;122(3):541–544. doi: 10.1016/0006-8993(77)90464-4. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine in the basal ganglia. Its role and therapeutic implications (including the clinical use of L-DOPA). Br Med Bull. 1973 May;29(2):172–178. doi: 10.1093/oxfordjournals.bmb.a070990. [DOI] [PubMed] [Google Scholar]
- Hu X. T., Wachtel S. R., Galloway M. P., White F. J. Lesions of the nigrostriatal dopamine projection increase the inhibitory effects of D1 and D2 dopamine agonists on caudate-putamen neurons and relieve D2 receptors from the necessity of D1 receptor stimulation. J Neurosci. 1990 Jul;10(7):2318–2329. doi: 10.1523/JNEUROSCI.10-07-02318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. T., Wang R. Y. Comparison of effects of D-1 and D-2 dopamine receptor agonists on neurons in the rat caudate putamen: an electrophysiological study. J Neurosci. 1988 Nov;8(11):4340–4348. doi: 10.1523/JNEUROSCI.08-11-04340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. R., Hamill O. P., Prince D. A. Developmental changes in Na+ conductances in rat neocortical neurons: appearance of a slowly inactivating component. J Neurophysiol. 1988 Mar;59(3):778–795. doi: 10.1152/jn.1988.59.3.778. [DOI] [PubMed] [Google Scholar]
- Katz L. C., Burkhalter A., Dreyer W. J. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984 Aug 9;310(5977):498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- Kita H., Kita T., Kitai S. T. Active membrane properties of rat neostriatal neurons in an in vitro slice preparation. Exp Brain Res. 1985;60(1):54–62. doi: 10.1007/BF00237018. [DOI] [PubMed] [Google Scholar]
- Le Moine C., Normand E., Bloch B. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., West J. W., Lai Y., Scheuer T., Catterall W. A. Functional modulation of brain sodium channels by cAMP-dependent phosphorylation. Neuron. 1992 Jun;8(6):1151–1159. doi: 10.1016/0896-6273(92)90135-z. [DOI] [PubMed] [Google Scholar]
- Marchetti C., Carbone E., Lux H. D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986 Feb;406(2):104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Ogata N., Tatebayashi H. Modulation of sodium current kinetics by chlorpromazine in freshly-isolated striatal neurones of the adult guinea-pig. Br J Pharmacol. 1989 Dec;98(4):1173–1184. doi: 10.1111/j.1476-5381.1989.tb12662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Tatebayashi H. Sodium current kinetics in freshly isolated neostriatal neurones of the adult guinea pig. Pflugers Arch. 1990 Jul;416(5):594–603. doi: 10.1007/BF00382695. [DOI] [PubMed] [Google Scholar]
- Sah P., Gibb A. J., Gage P. W. The sodium current underlying action potentials in guinea pig hippocampal CA1 neurons. J Gen Physiol. 1988 Mar;91(3):373–398. doi: 10.1085/jgp.91.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert B., VanDongen A. M., Kirsch G. E., Brown A. M. Beta-adrenergic inhibition of cardiac sodium channels by dual G-protein pathways. Science. 1989 Aug 4;245(4917):516–519. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Monsma F. J., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992 Feb;13(2):61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Giros B., Martres M. P., Bouthenet M. L., Schwartz J. C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990 Sep 13;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Stoof J. C., Verheijden P. F. D-2 receptor stimulation inhibits cyclic AMP formation brought about by D-1 receptor stimulation in rat neostriatum but not nucleus accumbens. Eur J Pharmacol. 1986 Sep 23;129(1-2):205–206. doi: 10.1016/0014-2999(86)90358-4. [DOI] [PubMed] [Google Scholar]
- Surmeier D. J., Stefani A., Foehring R. C., Kitai S. T. Developmental regulation of a slowly-inactivating potassium conductance in rat neostriatal neurons. Neurosci Lett. 1991 Jan 14;122(1):41–46. doi: 10.1016/0304-3940(91)90188-y. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Armstrong C. M. Fast-deactivating calcium channels in chick sensory neurons. J Gen Physiol. 1988 Aug;92(2):197–218. doi: 10.1085/jgp.92.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura N., Higashi H., Nishi S. Hyperpolarizing and depolarizing actions of dopamine via D-1 and D-2 receptors on nucleus accumbens neurons. Brain Res. 1986 Jun 11;375(2):368–372. doi: 10.1016/0006-8993(86)90760-2. [DOI] [PubMed] [Google Scholar]
- Van Gelder R. N., von Zastrow M. E., Yool A., Dement W. C., Barchas J. D., Eberwine J. H. Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1663–1667. doi: 10.1073/pnas.87.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. J., Wang R. Y. Electrophysiological evidence for the existence of both D-1 and D-2 dopamine receptors in the rat nucleus accumbens. J Neurosci. 1986 Jan;6(1):274–280. doi: 10.1523/JNEUROSCI.06-01-00274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]