Abstract
Baclofen is an oral derivative of gamma-aminobutyric acid (GABA) used to treat muscular spasticity from disorders of the central nervous system. However, it is also being used for a variety of other conditions such as musculoskeletal pain, myoclonus, and alcohol withdrawal. The elimination of baclofen is heavily dependent on intact renal function, and the contraindication for use in patients with insufficient renal function is not well recognized by healthcare providers. Here, the authors report a series of mild to severe cases of baclofen intoxication in patients with end-stage renal disease. In all cases, baclofen was initiated by either inpatient or outpatient healthcare providers and the patients generally presented with altered mentation, somnolence, and/or respiratory depression. All patients were treated with aggressive hemodialysis and made a full recovery. This paper will briefly review the literature regarding baclofen intoxication, safety of baclofen use in renal disease, and efficacy of extra-corporeal therapy in the treatment of baclofen intoxication.
Baclofen toxicity is an under-recognized and treatable cause of encephalopathy and respiratory failure among patients with acute kidney injury or chronic kidney disease. Cases of baclofen neurotoxicity are frequently iatrogenic, poorly recognized, and result in significant mortality, morbidity, and hospital resource utilization. Here, we present five cases involving patients with end-stage renal disease who became symptomatic with low doses of baclofen administered for a variety of conditions. All patients came to the attention of the nephrology hemodialysis service and were treated with daily hemodialysis or continuous renal replacement until resolution of symptoms. We suspect that a number of patients with milder presentations are not recognized in both the inpatient and outpatient settings.
Case Presentations
Case 1
A 48-year-old man with stage 5 chronic kidney disease (eGFR 8 ml/minute), alcoholism, substance abuse, and chronic hepatitis C with liver cirrhosis was involved in a motor vehicle collision. He was cleared of injury and found to have hepatic encephalopathy. At this time, he had a Glasgow Coma Scale (GCS) of 14, serum ammonia of 147 μmol/l, and blood urea nitrogen of 63 mg/dl. He was treated with lactulose and hemodialysis via a tunneled catheter. After 3 days of hemodialysis treatments, the ammonia level fell to 26 μmol/l, the blood urea nitrogen to 12 mg/dl, and his mental status improved. Given his elevated blood pressure, there was concern for alcohol withdrawal and baclofen 10 mg by mouth three times daily was initiated for treatment of withdrawal. Two days later, he was transferred to another hospital, where all medications, including baclofen, were continued. His mental status slowly declined and 2 days after transfer he was found unresponsive, apneic, with a GCS of 3 and a flaccid paralysis. He was intubated and transferred to the Medical Intensive Care Unit. Extensive workup including lumbar puncture revealed no explanation for the worsening encephalopathy. Baclofen toxicity was suspected and daily, high-efficiency hemodialysis was undertaken and mental status improved back to baseline.
Cases 2a-b
A 72-year-old woman with type 2 diabetes mellitus, peripheral arterial disease, and ESRD on thrice weekly hemodialysis was started on baclofen 5 mg by mouth three times a day for leg cramps. Two days after starting baclofen, she became increasingly confused and was unable to participate with activities of daily living. In the emergency department she was awake, but not oriented to person, place, or time exhibiting a fluent aphasia characterized by marked perseveration. Vital signs and laboratory data were within normal limits. Infection was excluded. Because of concern for acute baclofen neurotoxicity, she underwent daily high-efficiency hemodialysis for at least 4 hours. She improved after her second session of dialysis and was discharged to her skilled nursing facility with instructions to discontinue baclofen. However, baclofen was continued and she was readmitted to the hospital shortly after discharge for altered mental status. As before, she improved significantly with daily hemodialysis and discontinuation of the baclofen.
Case 3
A 47-year-old woman with HIV on antiretroviral therapy and ESRD on chronic hemodialysis was admitted for hypertensive urgency and altered mental status. One month prior to admission, she was started on baclofen 5 mg by mouth three times daily as needed for the myoclonus. On admission to the hospital, mind altering medications were held, including baclofen and gabapentin 300 mg/day. She improved with supportive care and routine maintenance hemodialysis, but myoclonus persisted. On hospital day 4, baclofen was ordered at 5 mg by mouth three times per day. On hospital day 10, she had a tonic–clonic seizure in the evening and was treated with valproic acid. The following day, she arrived at the dialysis unit poorly responsive with hypoxic–hypercarbic respiratory failure due to hypoventilation. She was intubated and placed on mechanical ventilation in the MICU. A tonic–clonic seizure occurred and the patient was started on continuous veno-venous hemodiafiltration via a non-tunneled catheter. Baclofen was discontinued and mental status subsequently improved.
Case 4
A 65-year-old man with type 2 diabetes mellitus, coronary artery disease, and ESRD on chronic hemodialysis was started on baclofen 10 mg three times daily for postoperative neck pain following a recent cervical laminectomy. Following his second dose of baclofen, he became drowsy, lethargic, and had two separate mechanical falls. After the third dose, he became completely unarousable and was brought to the emergency department. On evaluation, vital signs were within normal limits and there was no localizing evidence of infection. He was able to open his eyes briefly to sternal rub, but was otherwise unresponsive and flaccid on exam. Head and neck imaging were unremarkable. Serum glucose was normal. Urgent overnight hemodialysis was initiated for presumed acute baclofen neurotoxicity. After one HD treatment, he was still lethargic, but more arousable. Following a second consecutive dialysis treatment, his mental status fully returned to baseline.
Case 5
A 66-year-old man with ESRD on chronic hemodialysis, chronic hepatitis C, and type 2 diabetes mellitus, was started on baclofen 5 mg twice daily for muscle spasms. Within 24 hours, he became less responsive, but the drug was continued. He was brought to the hospital by EMS approximately 48 hours after starting baclofen (four total doses of 5 mg), and he was obtunded, hyporeflexive, and flaccid with a GCS of 4. CT scan of his brain was unremarkable. Additional causes of encephalopathy were excluded. Urgent hemodialysis for three consecutive days was performed due to concern for baclofen toxicity. After the second HD treatment, he had notable improvement in mental status, but was not yet at baseline. Following his third consecutive HD treatment, mental status completely normalized and returned to baseline.
Discussion
Baclofen (β-4-chlorophenyl gamma-aminobutyric acid) is a synthetic derivative of the central, inhibitory neurotransmitter gamma-aminobutyric acid (GABA) (1). Baclofen acts as an agonist of GABAB receptors, which are G protein-coupled receptors that open nearby potassium channels, thereby increasing potassium membrane conductance (2). These actions promote cell membrane polarization, which decreases the likelihood of an action potential, and produces an overall inhibitory effect on central neurons.
After oral administration, baclofen is rapidly absorbed by the gastrointestinal tract. Peak concentrations are seen after 2 hours of ingestion (3). Approximately 10–15% of the drug undergoes hepatic metabolism, while the remaining 85–90% is excreted unchanged by the kidneys (4). Drug clearance follows first-order elimination kinetics with a half-life around 3.5 hours (4). About 35% of serum baclofen is protein bound and it has an apparent volume of distribution of 0.8 l/kg in adults and 2.6 l/kg in children (3,5). Baclofen distributes within the intravascular space and highly perfused organs such as the liver and kidney, but it slowly penetrates the CNS by directly crossing the blood–brain barrier (6,7). At usual doses, it primarily exerts its inhibitory effect on spinal motor neurons. Thus, it is most commonly prescribed to control spasticity associated with spinal cord disorders.
Adverse Effects
The adverse effects associated with baclofen are consistent with its inhibitory effect on the central nervous system. Common side effects at normal doses include transient drowsiness, lethargy, nausea, or orthostasis. Larger doses can cause CNS depression, which manifest as sedation, somnolence, and respiratory depression (8). Less common side effects such as hallucinations, delirium, paraphasia, and perseverative behaviors have also been observed (8,9). In patients with a history of seizure disorders, baclofen has been associated with increased seizure activity (8,10). It is hypothesized that there is greater inhibition of key inhibitory interneurons thus lowering the threshold for seizure activity (11).
With an acute overdose, profound CNS depression occurs. Case reports of severe baclofen overdoses (range 80–2500 mg) in the general population describe patients presenting with muscular hypotonia, areflexia, myoclonus, respiratory depression, bradycardia, and seizures (7,8). Severe baclofen intoxications can produce a profound comatose state with absent brain stem reflexes, mimicking brain death (12). In one case, a patient with an acute baclofen intoxication and normal renal function was initially thought to have anoxic brain death and the treating physicians arranged for organ procurement. By hospital day 5, the patient spontaneously regained purposeful movements, eventually leading to a full recovery (12). In the setting of large overdose like this, clearance appears to follow first-order elimination kinetics, but the half-life is significantly extended to 8 hours or even longer (4). Case reports have noted that CNS depression persists despite plasma baclofen levels falling to the therapeutic range, suggesting that CNS clearance is significantly delayed compared to other body compartments (4). Thus, caution is warranted when interpreting serum baclofen levels.
Toxicity in Renal Failure
Because baclofen elimination is heavily dependent on renal clearance, patients with reduced kidney function are at high risk of baclofen intoxication. The earliest case reports noted that patients with ESRD developed altered mental status after taking small doses of baclofen for very short periods of time (13). Case reports of baclofen toxicity in patients with renal insufficiency note that toxicity occurred primarily in patients with ESRD (27 cases, 68%) (14). Of the remainder, 10 patients had chronic kidney disease stage 3–5 (24%), and four had acute kidney injury (9%) (14). Among those with ESRD, 68% were on hemodialysis and 32% were on continuous ambulatory peritoneal dialysis (CAPD). Symptoms often began 2–4 days after starting therapy. The mean daily dose was 20 mg/day with a wide range of doses reported (5–60 mg/day) (14). This is consistent with the findings in our series (see Table 1), where ESRD patient often developed symptoms within the first 24–48 hours on initial divided doses of 10–30 mg/day.
Table 1. Confirmed baclofen toxicity cases from 2011 to 2013.
| Case | Kidney function | Baclofen indication | Dose | Site of drug initiation | Presentation | Length of stay (days) | ICU admission | Mechanical ventilation | No. HD treatments required |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ESRD | ETOH withdrawal | 10 mg TID | Inpatient | AMS, coma, resp. failure | 30 | Yes | Yes | 5 |
| 2a | ESRD | Leg pain | 5 mg TID | Outpatient SNF | AMS, perseveration | 2 | No | No | 2 |
| 2b | ESRD | Leg pain | 5 mg TID | Outpatient SNF | AMS, perseveration | 4 | No | No | 2 |
| 3 | ESRD | Movement disorder | 5 mg TID | Inpatient | AMS, seizure, resp. failure | 27 | Yes | Yes | CVVHDF |
| 4 | ESRD | Neck Pain | 10 mg TID | Outpatient clinic | AMS, somnolence | 4 | No | No | 2 |
| 5 | ESRD | Muscle Spasm | 5 mg BID | Outpatient clinic | AMS, somnolence | 4 | Yes | No | 3 |
AMS, Altered mental status; CVVHDF, Continuous veno-venous hemodiafiltration; SNF, Skilled nursing facility.
The package insert for baclofen tablets recommends a starting dose of 5 mg three times per day, titrated to effect, but not exceeding a total daily dose of 80 mg (15). Despite being primarily renally eliminated, the manufacturer's label and available drug databases have no specific dosage adjustments for patients with renal insufficiency (15,16). The package insert suggests that baclofen should be “given with caution, and it may be necessary to reduce the dosage,” with no mention of dosing adjustments for dialysis patients (15). Based on the pharmacokinetics of the drug, it is possible that appropriate dose reductions could allow the safe use of baclofen in patients with renal insufficiency. However, studies to determine these doses have not been performed, and baclofen is typically absent from comprehensive guidelines about drugs that require adjustment in renal failure (17,18). For these reasons, many practitioners are unaware of the need for dose adjustment as well as the increased risk of toxicity in patients with renal insufficiency.
Role of Extracorporeal Therapy
Only a handful of published case reports address the efficacy of extracorporeal therapy in treating baclofen intoxication. Because baclofen has a low molecular weight (213 Daltons), low volume of distribution, and a low degree of plasma protein binding, it is readily removed by hemodialysis (19,20). Case reports of ESRD patients with baclofen toxicity consistently describe rapid resolution of symptoms following hemodialysis (13,14). One case report calculated the clearance of baclofen with hemodialysis in a patient with acute kidney injury and acute baclofen intoxication (19). Using a high-flux dialysis membrane (Gambro Polyflux 140H), a single dialysis session of 4 hours (Qb 230 ml/minute, Qd 500 ml/minute) resulted in a clearance of 2.14 ml/second or 120 ml/minute, essentially the same as a normal glomerular filtration rate (19). A clearance study in another baclofen intoxicated ESRD patient found that a 4-hour dialysis session eliminated 79% of the circulating drug and reduced the half-life from 15 hours to 2 hours (21). In our series, patients with milder symptoms recovered after 2–3 consecutive hemodialysis treatments (see Table 1). The two patients with more severe presentations (cases 1 and 3 in Table 1) required more days of renal replacement therapy until resolution of symptoms.
Continuous renal replacement therapy (CRRT) can also be used to treat severe baclofen intoxication. One case report describes a clearance study using continuous veno-venous hemofiltration (CVVH). In a severe intoxication involving a patient with normal renal function, CVVH was initiated with an Ultraflux AV 600 S hemofilter (1.4 m2), Qb of 200 ml/minute, and prefilter replacement fluid at a rate of 4.8 l/hour (22). Total drug clearance was estimated to be 6.6 l/h, the patient's endogenous clearance was estimated to be 4.2 l/h, and the CVVH clearance was calculated to be 2.4 l/h (22). Therefore, the addition of CVVH was equal to about 57% of the patient's total renal and hepatic clearance.
There are no data available regarding the clearance of baclofen with peritoneal dialysis. One small, uncontrolled case series observed no significant differences in time to recovery between 3 patients treated with CAPD and 12 patients treated with hemodialysis (23). A compilation of heterogeneous baclofen intoxication cases in ESRD patients found that the range of time to recovery appears longer in patients treated with CAPD (range 48–120 hours) compared to those treated with hemodialysis (range 2–120 hours) (14).
Management
The initial step in managing known or suspected baclofen intoxication relies on supportive care. The patient should be closely monitored for respiratory compromise, as endotracheal intubation and mechanical ventilation may be necessary (24). If the overdose is confirmed and acute, then activated charcoal is indicated (24). Efforts should also be focused on supporting hemodynamics and optimizing renal blood flow and glomerular filtration to maximize renal clearance in patients with reasonable kidney function.
If baclofen intoxication is suspected, serum baclofen levels can be measured using high-performance liquid chromatography–mass spectrometry (HPLC-MS). In many cases, this test is performed in a reference lab and the result will not be immediately available. The therapeutic range for serum baclofen is between 100 and 400 ng/ml, with symptoms usually developing above 400 ng/ml (22). Supratherapeutic concentrations of baclofen in the setting of new symptoms are supportive of the diagnosis of baclofen intoxication. However, in many cases, symptoms are present despite baclofen concentrations within the therapeutic range (4,13,21).
In one case series of adolescents who acutely ingested baclofen, 14-hour postingestion baclofen concentrations correlated strongly with the number of days on mechanical ventilation, but later reduced concentrations did not correlate well with persistent neurologic symptoms (25). It has also been observed that ESRD patients with baclofen neurotoxicity can have delayed resolution of neurologic symptoms days after the serum level normalizes with hemodialysis (13,21). Animal studies have shown that the rate of baclofen elimination from the CNS is significantly slower compared to that of the serum (26). Several case reports of patients treated conservatively even observed a ‘rebound’ effect in serum baclofen levels 3–4 days after admission (27,28). This slow equilibration between the two compartments likely explains the disassociation between serum levels and symptoms in many cases. Therefore, there is little utility in measuring serial serum baclofen levels as the response to treatment can be reasonably determined by clinical examination.
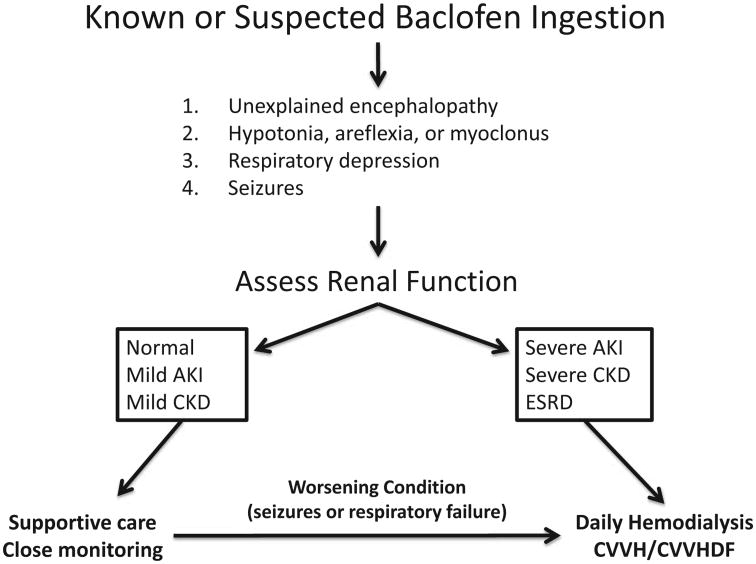
Figure 1 outlines an approach to the patient with known or suspected baclofen toxicity. If the symptoms are mild and renal function is adequate, then supportive care and close monitoring is sufficient (24). In patients with diminished renal function or ESRD who present with a change in mental status or other symptoms shortly after taking baclofen, dialysis remains the only rapid and effective treatment available to remove the drug. Baclofen is effectively cleared through intermittent hemodialysis or continuous renal replacement therapy (19–22). High-efficiency hemodialysis is the preferred treatment strategy unless hemodynamic instability necessitates the use of continuous renal replacement therapies. For patients managed with intermittent hemodialysis, we recommend daily, extended length (4–5 hours) hemodialysis with a high-efficiency dialysis membrane until the resolution of symptoms. Among patients receiving supportive care, should renal failure develop or should they clinically decline (new seizure activity or hypoventilatory respiratory failure), immediate treatment with hemodialysis would then be indicated.
Fig. 1.
Approach to the patient with baclofen toxicity.
Conclusion
In summary, baclofen toxicity is an under-recognized and treatable cause of encephalopathy and respiratory failure among patients with absent or diminished kidney function. Cases of baclofen neurotoxicity are frequently iatrogenic and result in significant mortality, morbidity, and hospital resource utilization. Unfortunately, there are currently no published dosing guidelines for the safe use of baclofen in patients with chronic kidney disease, and many providers are unaware of the signs and symptoms of baclofen toxicity. Based on our case series and review of the available literature, we recommend as “expert opinion” the following guidelines for the safe use of baclofen. In patients with mild chronic kidney disease (eGFR > 60 ml/minute), we recommend using the lowest, effective dose of baclofen according to the manufacturer's labeling. For patients with moderate or severe chronic kidney disease (eGFR < 60 ml/minute), there is insufficient evidence to provide safe dosing recommendations, so we would recommend avoiding the use of baclofen and seek out a safer, alternative option if one exists. For patients with ESRD or acute kidney injury, we strongly recommend against the use of baclofen, as the risk of severe toxicity is unacceptably high in these two groups.
Figure 1 outlines a general approach to the patient with symptomatic baclofen intoxication. When kidney function is impaired or supportive measures fail, hemodialysis is the most effective treatment to remove this drug. In our case series, all patients were treated with aggressive hemodialysis and eventually made a full recovery. Nephrologists and pharmacists should make efforts to educate other healthcare providers about this important and poorly recognized contraindication in our patient population.
Acknowledgments
Funding: J.K.R. is supported by the Duke Training Grant in Nephrology (5T32DK007731). M.A.S. is funded by Career Development Award IK2BX002240 from the Department of Veterans Affairs, Office of Research and Development, Biomedical Laboratory Research and Development Service.
References
- 1.May CR. Baclofen overdose. Ann Emerg Med. 1983;12:171–173. doi: 10.1016/s0196-0644(83)80562-9. [DOI] [PubMed] [Google Scholar]
- 2.Reis GM, Duarte ID. Baclofen, an agonist at peripheral GABAB receptors, induces antinociception via activation of TEA-sensitive potassium channels. Br J Pharmacol. 2006;149:733–739. doi: 10.1038/sj.bjp.0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wuis EW, Dirks MJ, Vree TB, Van der Kleijn E. Pharmacokinetics of baclofen in spastic patients receiving multiple oral doses. Pharm Weekbl Sci. 1990;12:71–74. doi: 10.1007/BF01970149. [DOI] [PubMed] [Google Scholar]
- 4.Gerkin R, Curry SC, Vance MV, Sankowski PW, Meinhart RD. First-order elimination kinetics following baclofen overdose. Ann Emerg Med. 1986;15:843–846. doi: 10.1016/s0196-0644(86)80388-2. [DOI] [PubMed] [Google Scholar]
- 5.Wiersma HE, van Boxtel CJ, Butter JJ, van Aalderen WM, Omari T, Benninga MA. Pharmacokinetics of a single oral dose of baclofen in pediatric patients with gastroesophageal reflux disease. Ther Drug Monit. 2003;25:93–98. doi: 10.1097/00007691-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Kochak GM, Rakhit A, Wagner WE, Honc F, Waldes L, Kershaw RA. The pharmacokinetics of baclofen derived from intestinal infusion. Clin Pharmacol Ther. 1985;38:251–257. doi: 10.1038/clpt.1985.167. [DOI] [PubMed] [Google Scholar]
- 7.Leung NY, Whyte IM, Isbister GK. Baclofen overdose: defining the spectrum of toxicity. Emerg Med Australas. 2006;18:77–82. doi: 10.1111/j.1742-6723.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- 8.Young RR, Delwaide PJ. Drug therapy: spasticity (second of two parts) N Engl J Med. 1981;304:96–99. doi: 10.1056/NEJM198101083040207. [DOI] [PubMed] [Google Scholar]
- 9.Liu HC, Tsai SC, Liu TY, Chi CW. Baclofen-induced frontal lobe syndrome: case report. Paraplegia. 1991;29:554–556. doi: 10.1038/sc.1991.80. [DOI] [PubMed] [Google Scholar]
- 10.Sauneuf B, Totouom HK, Savary B, Varin L, Dupeyrat J, Ramakers S, Hanouz JL. Clinical and EEG features of acute intrathecal baclofen overdose. Clin Neurol Neurosurg. 2012;114:84–86. doi: 10.1016/j.clineuro.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Mott DD, Bragdon AC, Lewis DV, Wilson WA. Baclofen has a proepileptic effect in the rat dentate gyrus. J Pharmacol Exp Ther. 1989;249:721–725. [PubMed] [Google Scholar]
- 12.Sullivan R, Hodgman MJ, Kao L, Tormoehlen LM. Baclofen Overdose Mimicking Brain Death. Clin Toxicol. 2010;48:609–610. doi: 10.3109/15563650.2011.654209. [DOI] [PubMed] [Google Scholar]
- 13.Chen KS, Bullard MJ, Chien YY, Lee SY. Baclofen toxicity in patients with severely impaired renal function. Ann Pharmacother. 1997;31:1315–1320. doi: 10.1177/106002809703101108. [DOI] [PubMed] [Google Scholar]
- 14.El-Husseini A, Sabucedo A, Lamarche J, Courville C, Peguero A. Baclofen Toxicity in Patients with Advanced Nephropathy: proposal for New Labeling. Am J Nephrol. 2011;34:491–495. doi: 10.1159/000333247. [DOI] [PubMed] [Google Scholar]
- 15.Baclofen (tablet) [package insert] Sellersville, PA: Teva Pharmaceuticals; 2013. [Google Scholar]
- 16.Baclofen . Lexi-Drugs, Lexicomp Online. Hudson, OH: Lexi-Comp Inc; [Accessed May 7, 2015]. [Google Scholar]
- 17.Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75:1487–1496. [PubMed] [Google Scholar]
- 18.Aronoff GR. Drug Prescribing in Renal Failure Dosing Guidelines for Adults. 5th. Philadelphia, PA: American College of Physicians; 2007. [DOI] [PubMed] [Google Scholar]
- 19.Brvar M, Vrtovec M, Kovac D, Kozelj G, Pezdir T, Bunc M. Haemodialysis clearance of baclofen. Eur J Clin Pharmacol. 2007;63:1143–1146. doi: 10.1007/s00228-007-0371-8. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh MJ, Chen SC, Weng TI, Fang CC, Tsai TJ. Treating baclofen overdose by hemodialysis. Am J Emerg Med. 2012;30:1654, e5–7. doi: 10.1016/j.ajem.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Wu VC, Lin SL, Lin SM, Fang CC. Treatment of baclofen overdose by haemodialysis: a pharmacokinetic study. Nephrol Dial Transplant. 2005;20:441–443. doi: 10.1093/ndt/gfh297. [DOI] [PubMed] [Google Scholar]
- 22.Meulendijks D, Khan S, Koks CH, Huitema AD, Schellens JH, Beijnen JH. Baclofen overdose treated with continuous venovenous hemo-filtration. Eur J Clin Pharmacol. 2015;71:357–361. doi: 10.1007/s00228-014-1802-y. [DOI] [PubMed] [Google Scholar]
- 23.Chen YC, Chang CT, Fang JT, Huang CC. Baclofen neurotoxicity in uremic patients: is continuous ambulatory peritoneal dialysis less effective than intermittent hemodialysis? Ren Fail. 2003;25:297–305. doi: 10.1081/jdi-120018730. [DOI] [PubMed] [Google Scholar]
- 24.Dart R. Medical Toxicology. 3rd. Philadelphia, PA: Lippincott Williams and Wilkins; 2003. [Google Scholar]
- 25.Perry HE, Wright RO, Shannon MW, Woolf AD. Baclofen overdose: drug experimentation in a group of adolescents. Pediatrics. 1998;101:1045–1048. doi: 10.1542/peds.101.6.1045. [DOI] [PubMed] [Google Scholar]
- 26.Faigle JW, Keberle H. The chemistry and kinetics of Lioresal. Postgrad Med J. 1972;48(Suppl. 5):9–13. [PubMed] [Google Scholar]
- 27.Weiβhaar GF, Hoemberg M, Bender K, Bangen U, Herkenrath P, Eifinger F, Rothschild M, Roth B, Oberthuer A. Baclofen intoxication: a “fun drug” causing deep coma and nonconvulsive status epilepticus—a case report and review of the literature. Eur J Pediatr. 2012;171:1541–1547. doi: 10.1007/s00431-012-1780-y. [DOI] [PubMed] [Google Scholar]
- 28.Ghose K, Holmes KM, Matthewson K. Complications of baclofen overdosage. Postgrad Med J. 1980;56:865–886. doi: 10.1136/pgmj.56.662.865. [DOI] [PMC free article] [PubMed] [Google Scholar]