Abstract
A tandem olefin metathesis/oxidative cyclization has been developed to synthesize 2,5-disubstituted tetrahydrofuran (THF) diols in a stereocontrolled fashion from simple olefin precursors. The ruthenium metathesis catalyst is converted into an oxidation catalyst in the second step and is thus responsible for both catalytic steps. The stereochemistry of the 1,5-diene intermediate can be controlled through the choice of catalyst and the type of metathesis conducted. This olefin stereochemistry then controls the THF diol stereochemistry through a highly stereospecific oxidative cyclization.
Assisted tandem catalysis is an attractive synthetic strategy where a single catalyst is added at the outset of the reaction and is intentionally converted into a new species via a change in reaction conditions for a second catalytic step.1 Ruthenium alkylidene catalysts have been frequently used in assisted tandem catalysis in order to couple a metathesis step with other transformations, including but not limited to dihydroxylation, hydrogenation, and isomerization, showcasing the versatility of these complexes.2 We sought to further explore the dual nature of these alkylidene complexes in tandem catalysis.
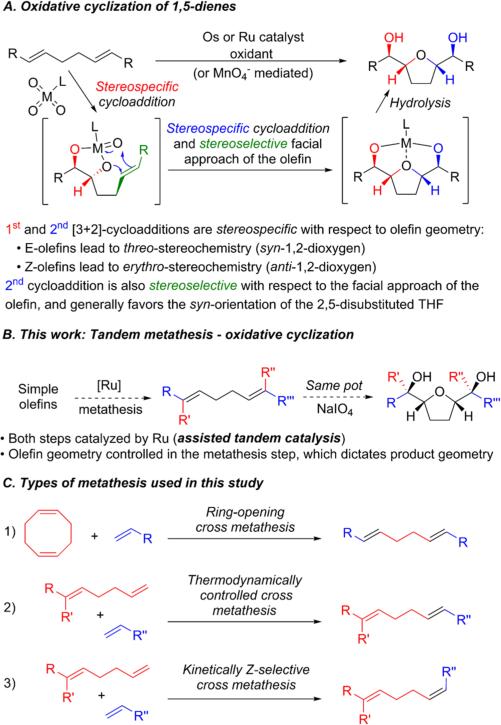
The Ru- or Os-catalyzed oxidative cyclization of 1,5-dienes is a convenient method to prepare 2,5-disubstituted tetrahydrofuran (THF) diols in a stereocontrolled manner.6 Furthermore, these THF diols are present in a wide variety of complex natural products (Figure 1). While olefin metathesis could be envisioned as a practical way to assemble the 1,5-diene substrate, there have been no reports of utilizing alkylidene catalysts for oxidative cyclization. Herein we report the development of a tandem metathesis/oxidative cyclization capable of assembling a diverse set of THF diols, where the diol stereochemistry is determined from the intermediate olefin geometry set in the metathesis step.
Figure 1.
Natural products3 containing the THF diol motif from the Annonaceous acetogenin4 and macrolide families.5
The oxidative cyclization of 1,5-dienes mediated by permanganate was discovered in 1965 by Klein and Rojahn.7 Later, it was found that RuO48 or OsO49 can catalyze this transformation in the presence of a suitable oxidant, such as NaIO4 (Figure 2A). In 1979, Baldwin and Walba independently demonstrated the stereospecificity of the oxidative cyclization with respect to olefin geometry.10 The currently accepted mechanism involves initial [3 + 2]-cycloaddition with one of the olefins.6 Subsequently, the metallo ester intermediate undergoes a second [3 + 2]-cycloaddition with the pendant olefin, followed by hydrolysis, furnishing a THF diol. Each olefin dioxygenation step is stereospecific with respect to olefin geometry, analogous to olefin dihydroxylation. The cyclization step is also stereoselective with respect to the facial approach of the olefin and generally favors the cis-2,5-disubstituted THF.
Figure 2.
(A) Oxidative cyclization of 1,5-dienes catalyzed by Ru or Os or mediated by Mn. (B) Proposed tandem metathesis/oxidative cyclization. (C) Types of olefin metathesis used in this study to generate the intermediate 1,5-dienes with control of olefin geometry.
Ruthenium alkylidene complexes have been shown to be converted into dihydroxylation catalysts upon treatment with NaIO4, and this has been used in the development of tandem metathesis/dihydroxylation.11 While the precise nature of the oxidized ruthenium species has not been firmly established, if this species is also capable of catalyzing oxidative cyclization, then 1,5-dienes accessible via olefin metathesis could be transformed into 2,5-disubstituted THF diols in a stereocontrolled fashion (Figure 2B).
We envisioned that olefin metathesis could be a powerful approach to synthesizing the requisite 1,5-dienes necessary for oxidative cyclization with control of olefin geometry (Figure 2C). Cross metathesis is well studied and can achieve high E-selectivity through thermodynamic control for many substrate classes, including acrylates and bulky terminal olefins.12 Additionally, considerable effort in recent years has focused on metathesis catalysts capable of achieving high kinetic Z-selectivity.13 More recently, stereoretentive cross metathesis has also been developed.14
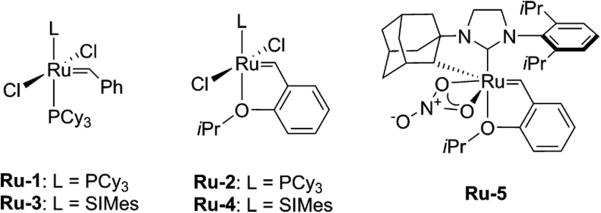
In order to test whether ruthenium alkylidene complexes are competent in oxidative cyclization, we chose to study the oxidation of bisacrylate 1. Using conditions optimized for ruthenium catalyzed dihydroxylation (NaIO4, catalytic CeCl3, 3:3:1 EtOAc/MeCN/H2O),15 first generation, second generation, and cyclometalated alkylidene complexes Ru-1–5 (Figure 3) all catalyzed the desired oxidative cyclization to give THF diol 2a (Table 1). While full conversion of 1 was observed in all cases, first generation complexes Ru-1,2 produced significantly more byproducts, leading to lower yields of 2a. Second generation catalysts Ru-3,4 gave comparable yields (64% and 63%, entries 3 and 4). CeCl3 has been proposed to facilitate hydrolysis of ruthenate ester intermediates via acid catalysis as well as increase the redox potential.14c,16 In the absence of CeCl3, THF diol 2a was still obtained, albeit in lower yield (42%, entry 6). A control experiment in the absence of ruthenium led to complete recovery of bisacrylate 1, indicating that ruthenium is necessary for oxidation to occur (entry 7).
Figure 3.
Ruthenium alkylidene catalysts examined in this study.
Table 1.
Comparison of Various Ru Alkylidene Catalysts in the Oxidative Cyclization of Bisacrylate 1

| ||||
|---|---|---|---|---|
| entry | catalyst | deviation from above | 1 (%)a | 2a (%)a |
| 1 | Ru-1 | – | 0 | 36 |
| 2 | Ru-2 | – | 0 | 37 |
| 3 | Ru-3 | – | 0 | 64 |
| 4 | Ru-4 | – | 0 | 63 |
| 5 | Ru-5 | – | 0 | 36 |
| 6 | Ru-4 | no CeCl3 | 0 | 42 |
| 7 | none | – | 100 | 0 |
Determined by 1H NMR using mesitylene as an internal standard.
E,E-Bisacrylates such as 1 can be conveniently synthesized by ring-opening cross metathesis of 1,5-cyclooctadiene (3) with an α,β-unsaturated ester in the presence of a second generation catalyst such as Ru-3 or Ru-4.17 A tandem metathesis/oxidative cyclization was thus examined using 1,5-cyclooctadiene (3) as a 1,5-diene precursor. Ring-opening cross metathesis of 3 with a series of acrylates, followed by sequential treatment of the crude solution with NaIO4 and catalytic CeCl3, produced the desired meso-THF diols 2 (Table 2). Both aryl and alkyl acrylates were effective. Upon scale-up to 2.5 mmol of 3, phenyl acrylate derived THF diol 2b was obtained in 52% yield (964 mg, entry 2). While the yields are moderate, we feel that the high degree of molecular complexity that can be achieved with a single catalyst renders the method highly attractive.18,19
Table 2.
Tandem Ring-Opening Cross Metathesis/Oxidative Cyclization of 1,5-Cyclooctadiene with Terminal Olefins
Isolated as a single diastereomer.
Using 2.5 mmol of 3 (964 mg 2b isolated).
Isolated yield after benzoylation (for ease of purification).
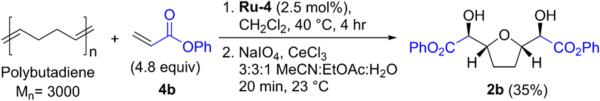
We next wondered whether polybutadiene could be used in place of 1,5-cyclooctadiene (3) in the metathesis step, since the 1,5-olefin units in this polymer are expected to undergo cross metathesis to lead to the same E,E-bisacrylate intermediate as is formed with 3. The tandem sequence was performed using phenyl acrylate and polybutadiene (Mn = 3000, cis-1,4 content = 75%, trans-1,4 content = 24%, vinyl content = 1%), and THF diol 2b was obtained in 35% yield (Scheme 1). This result is valuable since polybutadiene is an inexpensive and widely available 1,5-diene building block. The molecular structure of 2b was determined by X-ray crystallography, as illustrated in Figure 4. The vicinal dioxygen stereocenters are threo, as expected for a syn dioxgenation of an E-olefin, and the THF ring substituents are oriented in a syn arrangement. This observed stereochemistry is consistent with previous reports on oxidative cyclization with RuO4.6
Scheme 1.
Cross Metathesis/Oxidative Cyclization Using Polybutadiene As a Source of 1,5-Diene
Figure 4.
Molecular structure of 2b determined by X-ray crystallography.
Next we examined cross metathesis/oxidative cyclization with unsymmetrical 1,5-dienes containing one hindered olefin (expected to be a spectator in the metathesis step) and one terminal olefin (capable of cross metathesis).12 E-5a underwent cross metathesis with benzyl acrylate followed by oxidative cyclization in sequential fashion to give THF diol 6a in 65% yield (Table 3, entry 1).20 Phenyl acrylate 4b and butyl acrylate 4c reacted in a similar fashion to produce 6b and 6c in 66% and 60% yields, respectively. Z-1,4-Diacetoxy-2-butene 4d also underwent cross metathesis with 5a followed by oxidative cyclization to give monoacetate 6d in 59% yield.
Table 3.
Tandem Cross Metathesis/Oxidative Cyclization of 1,5-Dienes with Terminal Olefins

| |||||
|---|---|---|---|---|---|
| Entry | 5 | 4 | Product | 6 | Yield (%)a |
| l | 5a | 4a |
|
6a | 65 |
| 2 | 5a | 4b |
|
6b | 66 |
| 3 | 5a | 4c |
|
6c | 60 |
| 4 | 5a | 4d |
|
6d | 59b |
| 5 | 5b | 4a |
|
6e | 69 |
| 6 | 5b | 4b |
|
6f | 73 |
| 7 | 5c | 4b |
|
6g | 73 |
| 8 | 5d | 4b |
|
6h | 60 |
| 9 | 5e | 4b |
|
6i | 42 |
| 10 | 5f | 4a |

|
6j | 76 |
| 11 | 5f | 4b |

|
6k | 63 |
Isolated as a single diastereomer.
1.25 of equiv of 4d, 5 mol % Ru-4; see Supporting Information for details.
If the geometry of the trisubstituted olefin is inverted, the resulting tertiary alcohol stereochemistry is also expected to invert. When Z-diene 5b was used with benzyl or phenyl acrylate, THF diols 6e and 6f were obtained in 69% and 73% yield, respectively. Hydrogenolysis of benzyl ester 6e yielded the monocarboxylic acid 7a, which provided crystals suitable for X-ray diffraction (Figure 5). The THF 2- and 5-substituents were found to be in a syn configuration, and the vicinal dioxygenation geometries are consistent with a syn-dioxygenation of the olefins. This is in agreement with expectations from previous reports on oxidative cyclization of 1,5-dienes with ruthenium catalysts6 (see Figure 2A).
Figure 5.
Molecular structure of 7a determined by X-ray crystallography.
E- and Z-ethyl substituted olefins 5c and 5d, as well as α,β-unsaturated-1,3-diester 5e, also underwent tandem cross metathesis/oxidative cyclization to provide THF diols 6g–i in 42–73% yield (entries 7–9). Geminal dimethyl substitution on the diene linker was tolerated, as 5f underwent reaction with 4a and 4b to give 6j and 6k in 76% and 63% yield, respectively. The allylic quaternary center presumably provided enough steric hindrance to prevent reversible metathesis of the ethyl acrylate moiety.
Having demonstrated that the hydroxyl stereochemistry of the THF diol depends on the olefin geometry of the 1,5-diene intermediate, we sought to use Z-selective metathesis in order to generate Z-diene intermediates in a catalytic fashion. Given the activity of cyclometalated alkylidene Ru-5 in the oxidative cyclization of 1 (Table 1), and the use of Ru-5 in tandem Z-selective cross metathesis–dihydroxylation,11d we anticipated that this complex would also be able to catalyze the oxidative cyclization of Z-diene intermediates in a stereospecific fashion. Using Ru-5 as catalyst, diene 5a and 5g underwent cross metathesis with an excess of allyl benzoate (5 equiv), to give an E,Z-diene intermediate, which then underwent oxidative cyclization upon exposure to the oxidative conditions to provide diol 6l and 6m in 45% and 30% yield,21 respectively (Scheme 2).
Scheme 2.
Z-Selective Cross Metathesis/Oxidative Cyclization
In conclusion, a tandem olefin metathesis/oxidative cyclization has been developed to generate 2,5-disubstituted THF diols in a stereocontrolled fashion. The stereochemistry of the intermediate 1,5-dienes can be controlled by the choice of catalyst in the metathesis step, and this stereochemistry is translated to the product in the oxidative cyclization. We are currently exploring mechanistic aspects of this process. It is envisioned that this methodology will provide a concise route to biologically important THF diol motifs as well as contribute to a greater understanding of tandem catalysis.
Supplementary Material
ACKNOWLEDGMENTS
This work was financially supported by the ONR (N000141410650), the NIH (5R01M031332-27), and the NSF (CHE-1212767). P.K.D. is grateful to the CIHR for a postodoctoral fellowship, and D.L. thanks the Amgen Scholar Program for a fellowship. L. M. Henling and M. Takase are thanked for X-ray crystallography. NMR spectra were obtained using instruments supported by the NIH (RR027690). Dr. Noah Fine Nathel, Dr. Patrick Montgomery, Dr. Daniel Ziegler, and Dr. Zachary Wickens are thanked for helpful discussions. Materia, Inc. is thanked for the donation of metathesis catalysts.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.6b02653.
Experimental details, characterization data (PDF)
X-ray crystallography data for 2b; CIF file also available from the CCDC (No. 1465061) (CIF)
X-ray crystallography data for 7a; CIF file also available from the CCDC (No. 1465062) (CIF)
The authors declare no competing financial interest.
REFERENCES
- 1.a Fogg DE, dos Santos EN. Coord. Chem. Rev. 2004;248:2365–2379. [Google Scholar]; b Ajamian A, Gleason JL. Angew. Chem., Int. Ed. 2004;43:3754–3760. doi: 10.1002/anie.200301727. [DOI] [PubMed] [Google Scholar]; c Wasilke J-C, Obrey SJ, Baker RT, Bazan GC. Chem. Rev. 2005;105:1001–1020. doi: 10.1021/cr020018n. [DOI] [PubMed] [Google Scholar]; d Chapman C, Frost C. Synthesis. 2007;2007:1–21. [Google Scholar]; e Shindoh N, Takemoto Y, Takasu K. Chem. -Eur. J. 2009;15:12168–12179. doi: 10.1002/chem.200901486. [DOI] [PubMed] [Google Scholar]
- 2.a Alcaide B, Almendros P, Luna A. Chem. Rev. 2009;109:3817–3858. doi: 10.1021/cr9001512. [DOI] [PubMed] [Google Scholar]; b Alcaide B, Almendros P. Chem. - Eur. J. 2003;9:1258–1262. doi: 10.1002/chem.200390142. [DOI] [PubMed] [Google Scholar]
- 3.a McLaughlin JL, Shi G, Zeng L, Gu Z, MacDougal JM. Heterocycles. 1995;41:1785–1796. (+)-cis-Sylvaticin. [Google Scholar]; b Abdel Ghani SB, Chapman JM, Figadère B, Herniman JM, Langley GJ, Niemann S, Brown RCD. J. Org. Chem. 2009;74:6924–6928. doi: 10.1021/jo9012578. cis-Uvariamicin I A. [DOI] [PubMed] [Google Scholar]; c Pettit GR, Cragg GM, Polonsky J, Herald DL, Goswami A, Smith CR, Moretti C, Schmidt JM, Weisleder D. Can. J. Chem. 1987;65:1433–1435. (+)-Rolliniastatin 1. [Google Scholar]; d Řezanka T, Hanuš L, Dembitsky VM. Eur. J. Org. Chem. 2003;2003:4073–4079. (−)-Chagosensine. [Google Scholar]
- 4.a Alali FQ, Liu X-X, McLaughlin JL. J. Nat. Prod. 1999;62:504–540. doi: 10.1021/np980406d. [DOI] [PubMed] [Google Scholar]; b Bermejo A, Figadère B, Zafra-Polo M-C, Barrachina I, Estornell E, Cortes D. Nat. Prod. Rep. 2005;22:269–303. doi: 10.1039/b500186m. [DOI] [PubMed] [Google Scholar]
- 5.Lorente A, Lamariano-Merketegi J, Albericio F, Alvarez M. Chem. Rev. 2013;113:4567–4610. doi: 10.1021/cr3004778. [DOI] [PubMed] [Google Scholar]
- 6.Piccialli V. Synthesis. 2007;2007:2585–2607. [Google Scholar]
- 7.Klein E, Rojahn W. Tetrahedron. 1965;21:2353–2358. [Google Scholar]
- 8.Carlsen PHJ, Katsuki T, Martin VS, Sharpless KB. J. Org. Chem. 1981;46:3936–3938. [Google Scholar]
- 9.de Champdoré M, Lasalvia M, Piccialli V. Tetrahedron Lett. 1998;39:9781–9784. [Google Scholar]
- 10.a Baldwin JE, Crossley MJ, Lehtonen E-MM. J. Chem. Soc., Chem. Commun. 1979:918–920. [Google Scholar]; b Walba DM, Wand MD, Wilkes MC. J. Am. Chem. Soc. 1979;101:4396–4397. [Google Scholar]
- 11.a Beligny S, Eibauer S, Maechling S, Blechert S. Angew. Chem., Int. Ed. 2006;45:1900–1903. doi: 10.1002/anie.200503552. [DOI] [PubMed] [Google Scholar]; b Scholte AA, An MH, Snapper ML. Org. Lett. 2006;8:4759–4762. doi: 10.1021/ol061837n. [DOI] [PubMed] [Google Scholar]; c Neisius NM, Plietker B. J. Org. Chem. 2008;73:3218–3227. doi: 10.1021/jo800145x. [DOI] [PubMed] [Google Scholar]; d Dornan PK, Wickens ZK, Grubbs RH. Angew. Chem., Int. Ed. 2015;54:7134–7138. doi: 10.1002/anie.201501505. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Kato H, Ishigame T, Oshima N, Hoshiya N, Shimawaki K, Arisawa M, Shuto S. Adv. Synth. Catal. 2011;353:2676–2680. [Google Scholar]; f Schmidt B, Krehl S. Chem. Commun. 2011;47:5879–5881. doi: 10.1039/c1cc11347j. [DOI] [PubMed] [Google Scholar]; g Schmidt B, Krehl S, Hauke S. J. Org. Chem. 2013;78:5427–5435. doi: 10.1021/jo4005684. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee AK, Choi T-L, Sanders DP, Grubbs RH. J. Am. Chem. Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 13.a Endo K, Grubbs RH. J. Am. Chem. Soc. 2011;133:8525–8527. doi: 10.1021/ja202818v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rosebrugh LE, Herbert MB, Marx VM, Keitz BK, Grubbs RH. J. Am. Chem. Soc. 2013;135:1276–1279. doi: 10.1021/ja311916m. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Malcolmson SJ, Meek SJ, Sattely ES, Schrock RR, Hoveyda AH. Nature. 2008;456:933–937. doi: 10.1038/nature07594. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Herbert MB, Grubbs RH. Angew. Chem., Int. Ed. 2015;54:5018–5024. doi: 10.1002/anie.201411588. [DOI] [PubMed] [Google Scholar]; e Shahane S, Bruneau C, Fischmeister C. ChemCatChem. 2013;5:3436–3459. [Google Scholar]; f Meek SJ, O'Brien RV, Llaveria J, Schrock RR, Hoveyda AH. Nature. 2011;471:461–466. doi: 10.1038/nature09957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johns AM, Ahmed TS, Jackson BW, Grubbs RH, Pederson RL. Org. Lett. 2016;18:772–775. doi: 10.1021/acs.orglett.6b00031. [DOI] [PubMed] [Google Scholar]
- 15.a Shing TKM, Tai VW-F, Tam EKW. Angew. Chem., Int. Ed. Engl. 1994;33:2312–2313. [Google Scholar]; b Shing TKM, Tam EKW, Tai VW-F, Chung IHF, Jiang Q. Chem. - Eur. J. 1996;2:50–57. [Google Scholar]; c Plietker B, Niggemann M. J. Org. Chem. 2005;70:2402–2405. doi: 10.1021/jo048020x. [DOI] [PubMed] [Google Scholar]
- 16.Plietker B, Niggemann M. Org. Lett. 2003;5:3353–3356. doi: 10.1021/ol035335a. [DOI] [PubMed] [Google Scholar]
- 17.a Roe SJ, Legeay J-C, Robbins D, Aggarwal P, Stockman RA. Chem. Commun. 2009:4399–4401. doi: 10.1039/b906301c. [DOI] [PubMed] [Google Scholar]; b Boufroura H, Mauduit M, Drège E, Joseph D. J. Org. Chem. 2013;78:2346–2354. doi: 10.1021/jo302435a. [DOI] [PubMed] [Google Scholar]
- 18.The ring-opening cross metathesis was optimized independently (see Supporting Information for details) and generally results in >95% conversion under optimized conditions. The oxidation step is primarily responsible for the moderate yields, due to byproduct formation (the intermediate dienoate is fully consumed). Ongoing studies are directed at identifying byproducts.
- 19.The minor diastereomer with respect to the 2- and 5-substituents on the THF ring was not identified, and thus a diastereoselectivity for the oxidative cyclization cannot be reported. In the literature, diastereoselectivity is generally high when the 1,5-diene contains a H substituent at the 2- and 5-positions (see ref 6).
- 20.As was the case for Table 2, yields in Table 3 are primarily limited by the oxidation step. Conversion after the cross metathesis step was generally >95% when investigated independently.
- 21.The moderate yields are due in part to the statistical nature of the Z-selective cross metathesis, byproduct formation in the oxidation step, and difficulty in removing the 1,2-diol byproduct derived from the allyl benzoate homodimerization intermediate.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.