Abstract
Introduction
Substantial research has been dedicated to understanding the reasons for the dramatic rise in obesity rates in the U.S. in the last two decades. Animal studies and epidemiologic studies in children have suggested that air pollution might contribute to weight gain. This study investigates the association between ambient air pollution and weight gain over 16 years of follow-up (1995–2011) in a large cohort of African American women in the U.S.
Methods
This study assessed associations of fine particulate matter, ozone, and nitrogen dioxide with weight gain using a linear random effects model. All analyses were conducted in 2015.
Results
There was no statistically significant association between weight change and fine particulate matter (average weight change over 16 years per interquartile range [2.9 μg/m3], 0.12 kg; 95% CI= −0.10, 0.35) and ozone (0.16 kg per interquartile range [6.7 ppb]; 95% CI= −0.11, 0.43). There was a small decrease in weight associated with nitrogen dioxide (−0.50 per interquartile range [9.7 ppb]; 95% CI= −0.77, −0.23).
Conclusions
The results do not provide support for an association of air pollution with weight gain in African American adult women.
Introduction
Extensive research has focused on understanding the reasons for the substantial rise in U.S. obesity rates in the last several decades. Changes in energy intake and physical activity1 have been identified as important causes.
Animal studies and epidemiologic studies in children have suggested that air pollution might be related to weight gain and obesity. Mouse models have shown an association of exposure to fine particulate matter (PM2.5)4–6 and benzo[a]pyrene7 with metabolic dysfunction, including insulin resistance, inflammation, and central adiposity. Mice exposed to diesel exhaust prenatally had greater weight gain in adulthood.8
Increased prenatal exposure to polycyclic aromatic hydrocarbons was associated with higher BMI at age 5 years.9 Several other studies in children and adolescents also suggest an effect of air pollution on BMI.10 A cross-sectional study of total urinary polycyclic aromatic hydrocarbon metabolites in National Health and Nutrition Examination Survey participants aged 6–19 years found positive associations between polycyclic aromatic hydrocarbon levels and BMI, waist circumference, and obesity.11 In two longitudinal cohorts of children in southern California, higher traffic density12 and near-roadway pollution13 were associated with higher BMI at age 18 years. In children enrolled in kindergarten or first grade in southern California, traffic pollution was associated with a 0.4-unit increase in annual BMI in those with the highest exposure relative to the lowest exposure.14 The effect decreased as children neared adolescence. A small cross-sectional study of adults in Boston15 found an association between serum leptin levels, a correlate with body fat content, and black carbon levels.
No epidemiologic studies of the relation of air pollution and weight have been conducted among adults.
The present analyses assess the association of levels of PM2.5, nitrous dioxide (NO2), and ozone (O3) with weight gain over 16 years of follow-up in the Black Women’s Health Study (BWHS), a prospective study of African American women from across the U.S. Previous BWHS studies found weight gain to be inversely associated with eating more fruits and vegetables and positively associated with eating more meat and fried foods,16 lower levels of parental education and current education,17 living in less dense versus more dense neighborhoods,18 living in disadvantaged neighborhoods relative to wealthier neighborhoods,19 and reporting more experiences of racism.20
Methods
Study Population
The BWHS was established in 1995, when 59,000 black women aged 21–69 years were recruited mainly from subscribers to Essence magazine, a general readership magazine targeted to black women.21 The baseline questionnaire elicited information on demographic and lifestyle factors, reproductive history, and medical conditions. The cohort is followed biennially with mailed and web-based health questionnaires. Follow-up of the original cohort is complete for 88% of the potential person years through eight questionnaire cycles. The study protocol was approved by the IRB of Boston University School of Medicine. Participants indicate consent by completing and returning the questionnaires.
The present analyses included data from the baseline questionnaire (1995) and eight subsequent follow-up cycles (1997–2011). Women were excluded at baseline from the analytic cohort if they were aged >55 years (n=5,715), did not live in any of 56 metropolitan areas in the U.S. (n=5,302), had a history of cancer at baseline or during follow-up (n=5,249), reported baseline weight <80 pounds (36.32 kg) or >300 pounds (227 kg) (n=539), had a history of gastric bypass surgery (asked in 1999, n=181), had no follow-up (n=456), or had no pollutant data (n=5,458). Women were censored when they reached age 55 years (n=31,184 observations over follow-up), to limit follow-up to the ages at which most weight gain occurs in the BWHS.16 Women were excluded from a cycle if they had reported giving birth within the past 2 years (n=7,612 follow-up observations), were missing information on SES (n=6484), or did not provide information on their weight (n=67,850 follow-up observations). Overall, the 38,374 women contributed 144,580 observations over the 16-year period, with a median of four observations per woman (range, 1–8). Women excluded because they did not live in the 56 metro areas did not differ from the included women in terms of mean age, BMI, or prevalence of diabetes or hypertension at baseline.
Measures
Height, weight, and weight at age 18 years were reported at baseline and weight was updated on all follow-up questionnaires. A validation study among 115 participants found the Spearman correlation coefficient for self-reported weight (176 pounds) and of technician-measured weight (181 pounds) was 0.97 (p<0.001), and for self-reported height (64.4 inches) and technician-measured height (64.0 inches) was 0.93 (p<0.001).22 Smoking history, alcohol consumption, parity, menopausal status, and hours/week spent in vigorous exercise (ascertained with: On average, during the past year, how many hours each week did you spend in vigorous activity, such as basketball, swimming, running, aerobics?) were obtained at baseline and updated on follow-up questionnaires. In 1995 and 2001, dietary data were obtained with a modification of the short form Block–National Cancer Institute food frequency questionnaire.23 Factor analysis of 35 food groups identified two dietary patterns: high intake of vegetables and fruit and high intake of meat and fried food.16 Information was also obtained on household income (2003), educational attainment (1995, 2003), and perceptions and experiences of racism (1997) adapted from an instrument developed by Williams et al.24 Two summary racism variables were created, an everyday racism score based on responses to five questions (e.g., How often do people act as if they think you are not intelligent?), and a lifetime racism score based on three questions about discrimination on the job, in housing, and by police.20
Residential addresses to which questionnaires were mailed from 1995 to 2009 were geocoded and linked to U.S. Census data at the block group level. A neighborhood SES score was created using factor analysis of seven Census variables (median household income; median housing value; percentage of households receiving interest, dividend, or net rental income; percentage of adults aged ≥25 years that completed college; percentage of families with children headed by a single woman; percentage of population living below the poverty line; and percentage African American), with higher scores indicating higher neighborhood SES.
Levels of PM2.5 at participants’ residential locations for 1999–2008 were estimated using a two-stage modeling strategy that incorporated land use regression (LUR) and Bayesian Maximum Entropy approaches.25 Models with PM2.5 measurements were developed from the Environmental Protection Agency’s Air Quality System of 1,464 monitoring locations. LUR was used to construct a deterministic model that identified various measures of traffic, land use, and population as fixed predictors and then applied Bayesian Maximum Entropy methods to the set of monthly spatiotemporal residuals from the LUR model. Cross-validation based on leave-out samples of about 10% showed strong agreement between observed and predicted PM2.5 levels (R2=0.79). Annual NO2 levels at BWHS participant residential locations at the block group level for 2000–2010 were estimated using a LUR model that incorporated fixed-site ambient NO2 monitoring station data, satellite-derived estimates of ground-level NO2 concentrations, and satellite- and ground-based land use data sets.26 The LUR model was developed using measured annual-average concentrations at 369 monitoring stations and from 81,670 satellite-derived ground-level NO2 estimates. The R2 comparing measured with predicted NO2 levels was 0.80. Ozone levels for the years 2007–2008 were estimated using the Environmental Protection Agency Models-3/Community Multiscale Air Quality model with a resolution of 12 km.27 Estimates are made at the centroid of each Census tract in the coterminous U.S. Daily estimates for 8-hour maximum levels were compiled into annual average of daily peak concentrations. Validation analyses suggested that the model predicted ambient ozone concentrations well.28 For example, correlations with held-out locations for daily predictions ranged from 0.61 to 0.86 at three sites in the eastern U.S.
For each pollutant, any given location was assigned the average of all available values for that location (i.e., 1999–2008 for PM2.5). At each questionnaire cycle, women were assigned the average pollutant value associated with the one residential address where they were living during that cycle. A sensitivity analysis considered an exposure variable incorporating the average pollutant values at all residential locations over follow-up and weighted by time spent at each address.
Statistical Analysis
Linear mixed models were used to estimate weight change during each 2-year cycle. The mixed model accounted for correlation between 2-year weight change within each individual and between individuals nested within the 56 metro areas. Separate models were fit for each pollutant. Regression coefficients, representing 2-year weight change, were multiplied by eight to estimate the total weight change over the 16 years of follow-up and reported per interquartile range (IQR). All models included baseline age, baseline height, baseline BMI (<25, 25–30, ≥30) and a variable for time in 2-year intervals to control for (linear) temporal trends. Potential confounders considered in this study were vegetable/fruit diet score, meat/fried foods diet score in quintiles, neighborhood SES, daily and lifetime racism scores, weight at age 18 years, vigorous exercise (<1 hour/week, 1–2 hours/week, 3–4 hours/week, 5–6 hours/week, ≥7 hours/week), alcohol consumption (never, past, current), parity (nulliparous, one birth, two births, three or more births), menopausal status (pre, post), years since last birth (nulliparous, <5 years, 5–14 years, ≥15 years), income (≤$25,000, $25,001–$50,000, $50,001–$100,000, >$100,000), years of education (≤12 years, 13–15 years, 16 years, ≥17 years), hypertension, diabetes, and smoking status (never, past, <15 cigarettes/day, ≥15 cigarettes/day). Variables that changed the effect estimate of any pollutant value by at least 10%, and thus were in the models, were menopausal status, education, smoking status, neighborhood SES, daily racism, vigorous exercise, and weight at age 18 years. Because similar confounders were detected for each of the three air pollutant exposures using the 10% criteria, the final models for each pollutant included the same covariates. Nonlinearities between each pollutant and 2-year weight change were not found.
There were no violations of the normality assumption for 2-year weight change. Missing data were modeled as a separate category for categorical variables. Women with missing pollutant values were not included in the analysis. Women were censored if they moved out of the study area and when they reached age 55 years.16 Likelihood ratio tests were used to determine if there was a significant interaction between each air pollutant and baseline BMI in 1995 (<25, 25–30, and ≥30) or neighborhood SES (standardized continuous variable). A sensitivity analysis was performed among women who did not move. Pollutant levels were inversely correlated with neighborhood SES; models were run separately for the women in cities where the Spearman correlation between SES and the pollutant was low (≤0.3), moderate (0.3–0.5), and high (≥0.5). All analyses were conducted in 2015 using SAS, version 9.3.
Results
The average levels in 1995 of PM2.5, O3, and NO2 were, respectively, 13.9 μg/m3, 37.5 ppb, and 18.5 ppb. At baseline, PM2.5 levels were positively associated with NO2 and O3, whereas NO2 levels were inversely associated with O3 (Table 1). There was little variation in weight at age 18 years and baseline, BMI, nonsmoking, nondrinking, low levels of exercise, menopausal status, and perceived racism across levels of the pollutants. Nulliparity was positively associated with PM2.5 and NO2 and inversely with O3 levels. Those in the highest-SES areas tended to have lower BMI, exercise more, be nulliparous, and have more education.
Table 1.
Baseline Characteristics of 28,877 Participants With Complete Baseline Data. Results Shown by First and Fifth Quintiles of Baseline Pollutant Values and the Highest Tertile of SES
| Characteristic | Overall | PM2.5 | NO2 | O3 | SES | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | Third tertile |
|
| PM2.5 (μg/m3) | 13.9 (2.3) |
10.8 (0.99) |
17.3 (1.4) |
12.9 (1.9) |
14.7 (2.6) |
13.0 (1.9) |
14.9 (3.0) |
13.4 (2.5) |
| O3 (ppb) | 37.5 (4.5) |
36.9 (5.2) |
39.4 (5.1) |
40.7 (3.3) |
34.7 (4.2) |
31.9 (1.4) |
44.2 (2.5) |
38.0 (4.7) |
| NO2 (ppb) | 18.5 (6.5) |
15.0 (4.8) |
21.4 (5.7) |
10.0 (2.3) |
28.1 (2.1) |
24.5 (5.7) |
14.7 (6.1) |
16.7 (6.4) |
| Age, years | 37.3 (8.6) |
38.0 (8.5) |
37.3 (8.4) |
37.7 (8.3) |
36.2 (8.7) |
36.6 (8.7) |
37.4 (8.2) |
37.8 (8.4) |
| Height (cm) | 165.0 (7.0) |
165.0 (7.1) |
165.0 (6.9) |
164.9 (6.9) |
165.1 (7.1) |
165.1 (7.1) |
164.8 (6.9) |
165.2 (6.9) |
| Weight at age 18 (kg) | 58.2 (11.3) |
58.0 (11.4) |
58.0 (10.9) |
57.3 (11.0) |
58.7 (11.5) |
59.1 (11.8) |
57.1 (10.6) |
57.4 (10.7) |
| Weight in 1995 (kg) | 74.7 (17.2) |
74.9 (17.5) |
74.0 (17.1) |
74.1 (16.6) |
74.2 (17.4) |
75.0 (17.8) |
73.4 (16.6) |
72.5 (16.3) |
| Daily racism score | 2.5 (1.2) | 2.6 (1.2) |
2.4 (1.2) |
2.5 (1.2) |
2.4 (1.2) |
2.5 (1.2) |
2.5 (1.2) | 2.5 (1.2) |
| N (%) of Participants with characteristic | ||||||||
| BMI | ||||||||
| <25 | 11,744 (40.8) |
2,326 (40.5) |
2,459 (42.7) |
2,410 (41.9) |
2,514 (43.7) |
2,363 (41.1) |
2,511 (43.7) |
4,468 (47.1) |
| 25-30 | 8,991 (31.3) |
1,810 (31.5) |
1,815 (31.6) |
1,813 (31.5) |
1,667 (28.9) |
1,721 (30.0) |
1,796 (31.3) |
2,926 (30.8) |
| > 30 | 8,031 (27.9) |
1,604 (27.9) |
1,479 (25.7) |
1,531 (26.6) |
1,579 (27.4) |
1,661 (28.9) |
1,438 (25.0) |
2,095 (22.1) |
| Prevalent diabetes | 1,058 (3.7) |
219 (3.8) |
204 (3.5) |
202 (3.5) |
204 (3.5) |
200 (3.5) |
204 (3.5) |
249 (2.6) |
| Prevalent hypertension | 5,768 (20.0) |
1,186 (20.6) |
1,128 (19.5) |
1,175 (20.3) |
1,057 (18.3) |
1,095 (19.0) |
1,127 (19.5) |
1,726 (18.1) |
| Never smoker | 18,903 (65.7) |
3,696 (64.5) |
3,935 (68.3) |
4,006 (69.5) |
3,700 (64.3) |
3,620 (63.1) |
4,081 (70.9) |
6,519 (68.6) |
| Never drinker | 16,513 (57.5) |
3,203 (55.9) |
3,300 (57.4) |
3,465 (60.3) |
3,333 (58.1) |
3,163 (55.3) |
3,415 (59.5) |
5,568 (58.8) |
| None or <1 hr/week vigorous exercise |
12,913 (46.3) |
2,466 (44.3) |
2,535 (45.2) |
2,603 (46.4) |
3,019 (54.3) |
2,497 (45.0) |
2,559 (45.5) |
3,807 (41.1) |
| Nulliparous | 11,667 (40.4) |
2,105 (36.6) |
2,420 (41.9) |
2,100 (36.4) |
2,592 (45.0) |
2,572 (44.7) |
2,253 (39.1) |
4,208 (44.2) |
| Premenopausal | 24,056 (89.2) |
4,686 (88.2) |
4,854 (89.7) |
4,700 (87.9) |
5,074 (91.3) |
4,985 (90.9) |
4,755 (88.9) |
7,919 (89.3) |
| Education at least 17 years |
6,305 (21.9) |
1,340 (23.3) |
1,201 (20.8) |
1,443 (25.0) |
1,151 (20.0) |
1,240 (21.6) |
1,233 (21.4) |
3,015 (31.7) |
| Lifetime racism, yes to all three questions |
3,085 (11.2) |
731 (13.2) |
584 (10.6) |
600 (10.8) |
611 (11.1) |
650 (11.8) |
549 (10.0) |
1,156 (12.6) |
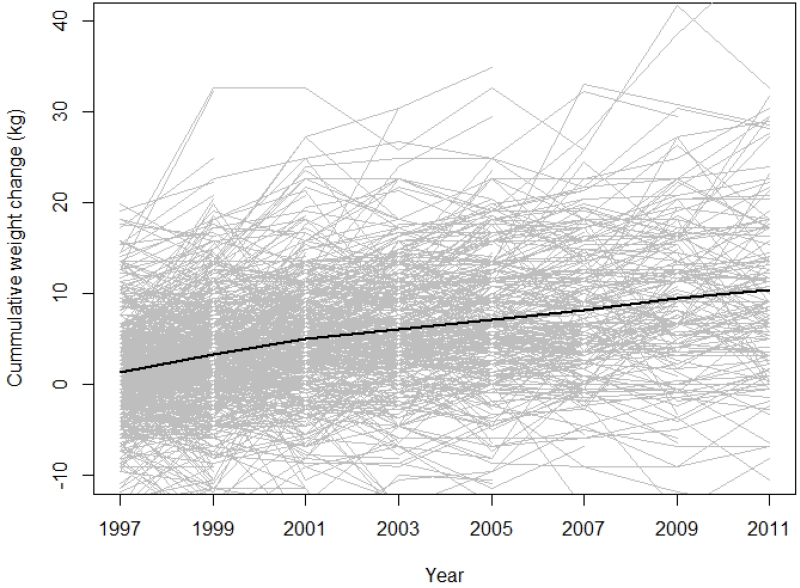
The average weight change for women over the 16-year follow-up period was a gain of 8.8 kg (range, −67.7 to 113.5 kg) (Figure 1). As shown in Table 2, the increase in weight was 0.12 kg (95% CI= −0.10 kg, 0.35 kg), per IQR of PM2.5 (2.9 μg/m3) and 0.16 kg (95% CI= −0.11 kg, 0.43 kg) per IQR of O3 (6.7 ppb). There was a small, statistically significant decrease in weight per IQR of NO2 (9.7 ppb) (−0.50 kg, 95% CI= −0.77 kg, −0.23 kg). Similar results were found in sensitivity analyses that used an exposure metric weighting pollutants by the time spent at each address and that included all pollutants together.
Figure 1.
Weight change over time. Cumulative weight change for a randomly selected group of 1,000 women over the study period.
Note: A smoothing spline is fit to all of the data and shown.
Table 2.
Average Weight Change Over 16 Years (1997-2011) per Interquartile Range (IQR) Increase in Pollutantsa
| Average weight change (SD)b |
PM2.5, IQR=2.9 μg/m3 |
O3, IQR=6.7 ppb | NO2, IQR=9.7 ppb | |
|---|---|---|---|---|
| Overall | 8.6 (11.4) | 0.12 (−0.10, 0.35) | 0.16 (−0.11, 0.43) | −0.50 (−0.77, −0.23) |
| BMI in 1995c | ||||
| BMI <25 (n=11,744) |
10.0 (8.7) | 0.04 (0.01, 0.08) | 0.45 (0.43, 0.47) | −0.77 (−0.85, −0.69) |
| 25< BMI <30 (n=8,991) |
9.6 (10.9) | 0.24 (−0.21, 0.68) | −0.14 (−0.67, 0.38) | −0.06 (−0.58, 0.46) |
| BMI >30 (n=8,031) |
5.5 (14.6) | 0.13 (−0.31, 0.57) | 0.01 (−0.51, 0.53) | −0.55 (−1.06, −0.03) |
| Neighborhood SESd | ||||
| At Q1 SES | 10.4 (0.05) | 0.07 (−0.23, 0.37) | 0.34 (−0.03, 0.70) | −0.67 (−1.04, −0.29) |
| Median SES | 10.2 (0.09) | 0.11 (−0.19, 0.41) | 0.19 (−0.19, 0.56) | −0.52 (−0.90, −0.14) |
| At Q3 SES | 10.0 (0.11) | 0.16 (−0.14, 0.46) | 0.03 (−0.33, 0.40) | −0.36 (−0.74, 0.01) |
Note: Boldface indicates statistical significance (p<0.05).
All models adjusted for diet (western and prudent), menopausal status, education level, smoking status, vigorous activity, everyday racism, height, age, neighborhood SES, weight at age 18 and time.
Weight change values for SES are calculated using a random effects model with SES as the only covariate. Model-based SEs for the estimates are reported, rather than SDs.
Estimates in strata of BMI also include an interaction term for BMI and the pollutant.
Estimates in strata of neighborhood SES also include an interaction term for neighborhood SES and the pollutants.
There was no consistent pattern between weight change and pollutant exposure across BMI or SES categories (Table 2). For example, the highest weight changes for PM2.5 were in the 25< BMI <30 category and the highest-SES areas, whereas the greatest weight changes for O3 and NO2 were in the BMI <25 category and the lowest-SES areas.
Weight change estimates by categories of the magnitude of the correlation coefficient of neighborhood SES and the pollutants are shown in Table 3. When there was low correlation between SES and the pollutant, there was no significant association with weight change. There were inverse associations between weight change and all pollutants where there was substantial correlation between SES and the pollutant, although the association was only significant for NO2 in the middle correlation category.
Table 3.
Average Weight Change Over 16 Years (1997-2011) per Interquartile Range (IQR) Change in Pollutants. Results Shown Separately for Cities With Low (<0.3), Moderate (0.3-0.5), and High (>0.5) Correlation Between Neighborhood SES and the Pollutantsa
| SES and pollutant correlation at the city level | |||
|---|---|---|---|
| Low: |r|<0.3 | Moderate: 0.3 < |r| < 0.5 |
High: |r| > 0.5 |
|
| PM2.5 IQR=2.9 μg/m3 |
0.07 (−0.19, 0.34) m=32 cities |
0.48 (−0.05, 1.01) m=17 cities |
−1.04 (−2.59, 0.51) m=7 cities |
| O3 IQR=6.7 ppm |
0.38 (−0.01, 0.78) m=23 cities |
0.01 (−0.43, 0.45) m=21 cities |
−0.37 (−1.34, 0.60) m=12 cities |
| NO2 IQR=9.7 μg/m3 |
−0.28 (−0.75, 0.19) m=14 cities |
−0.78 (−1.41, −0.16) m=23 cities |
−1.39 (−2.88, 0.10) m=18 cities |
Note: Boldface indicates statistical significance (p<0.05).
Model adjusts for diet (western and prudent), menopausal status, education level, smoking status, vigorous activity, everyday racism, lifetime racism, height, age, neighborhood SES, weight at age 18 and time.
Among non-movers (n=10,307), the baseline characteristics were similar to the whole cohort, except they were slightly older (mean age, 41 years), heavier (32% were obese), and less likely to be nulliparous (30%) (data not shown). Weight changes were slightly greater than in the overall cohort (Appendix Table 1). Results by BMI categories and across values of neighborhood SES were similar to those in the overall cohort (Appendix Table 2).
Discussion
In this large prospective cohort study of African American women, exposure to PM2.5 and O3 was not associated with 16-year weight gain. There was no consistent trend in weight gain across categories of BMI. Observed magnitudes of 16-year weight change associated with levels of air pollution were small: The largest gain in weight was the nonsignificant 0.45 kg (1.0 pound) per IQR of O3, and the greatest weight loss was 0.8 kg (1.7 pounds) per IQR of NO2, both observed in the leanest women. The weight change associated with other exposures in BWHS was more substantial. For example, different diet patterns led to changes in weight from 1 kg (2.2 pounds) to 2 kg (4.4 pounds) over 10 years. Over just 4 years, having one child compared with no children was associated with an increase in weight of 2.3 kg (5.1 pounds) among women who were obese at baseline.29 The magnitudes of weight change observed in the present study were most similar to those observed for neighborhood SES, where weight gain over 6 years among women living in neighborhoods of lowest SES was 0.6 kg (1.32 pounds) greater than for women living in neighborhoods of highest SES.19 Compared with the overall average weight change of 8.84 kg (19.4 pounds) over the 16-year follow-up period, the contribution of any pollutant, if real, was very small. However, owing to the ubiquity of air pollution exposure, even small contributions may have public health import, especially among certain subgroups (e.g., lean women).
There was a statistically significant loss of 0.50 kg over 16 years associated with each IQR increase in NO2. This result may have occurred by chance or reveal underlying challenges in analyses of air pollution where multiple, highly correlated attributes of an individual’s environment can potentially play a role in the association. In cities where there is a very small correlation between air pollution and SES, the effects of the pollutants on weight change were small and not significant, contrasting with the significant weight loss for all pollutants when there was a strong correlation between air pollution and SES, possibly reflecting the complex relationships of air pollution, neighborhood SES, and associated factors.
In the analyses, the overall mean of air pollution estimates at a particular address was used for that address over the entire follow-up period, accounting for spatial but not temporal variation. Most of the variance in the pollutants levels over follow-up was spatial, not temporal. In addition, in other analyses of PM2.5 and diabetes incidence,30 and NO2 and diabetes incidence (P Coogan, Boston University, unpublished observations, 2016), results were similar regardless of adjustment for temporal variation in pollutant levels. Simulation results indicate that the choice of metric for temporal adjustment is relatively robust, as long as time-varying values are averaged.31
Ambient pollutant levels were estimated only at each woman’s residential address, where, on average, time–activity studies show that Americans spend on average 67% of their time.32 There were no exposure measures based on personal monitoring devices or information on indoor air quality. The models for PM2.5 and NO2 relied on government monitoring sites, which tend to be located away from major roadways. It is therefore likely that the models were unable to capture completely the fine-scale variation that occurs around major roadways, resulting in underestimation on NO2 and PM2.5 in areas of high pollution, biasing results toward the null.
The study population is not representative of all black women in the U.S. Women with less than a high school education, about 15% of U.S. black women,33 are under-represented in BWHS, so results may not be generalizable to the least-educated women. The analysis was limited to women who were aged <55 years living in metropolitan areas. Thus, the results may not apply to women who are older, of low educational levels, and living in rural areas.
Currently, the studies showing potential biological mechanisms by which air pollution may affect metabolism have been conducted in mice.4,5 Epidemiologic evidence comes from several studies in children. In one of the childhood studies, when the authors controlled for many factors related to neighborhood and community structure (i.e., connectivity of the street network, number of fast food outlets within 500 m, greenness around home, number of active recreational programs for children and traffic density), the effect of traffic density on attained BMI was reduced by 20% and no longer significant whereas the impact of NOx remained positive and significant.14 It is possible that the impact of air pollution on weight gain is most prominent in children and teenagers, and has no appreciable effect in adults. In a cross-sectional study of serum leptin levels in adults, there was a small association between black carbon exposure and serum leptin levels, but only when one of multiple modeling strategies was used.15
Conclusions
In this large cohort of African American women, there was no material association between air pollution and 16-year weight change, although there was a statistically significant, small inverse association of NO2 levels and weight change. Although associations varied over levels of baseline BMI, neighborhood SES, and the magnitude of the correlation of neighborhood SES and air, there was no consistent pattern. These data do not provide strong support for an association of air pollution and weight gain in adult African American women.
Supplementary Material
Acknowledgments
This research was funded by grants from the National Institute of Environmental Health Sciences (ES019573) and the National Cancer Institute (R01 CA 058420, UM1CA164974). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mattes R. Energy intake and obesity: ingestive frequency outweighs portion size. Physiol Behav. 2014;134:110–118. doi: 10.1016/j.physbeh.2013.11.012. http://dx.doi.org/10.1016/j.physbeh.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Stijnen P, Tuand K, Varga T, Franks P, Aertgeerts B, Creemers J. The association of common variants in PCSK1 with obesity: a HiGE review and meta-analysis. Am J Epidemiol. 2014;180(11):1051–1065. doi: 10.1093/aje/kwu237. http://dx.doi.org/10.1093/aje/kwu237. [DOI] [PubMed] [Google Scholar]
- 3.Waalen J. The genetics of human obesity. Transl Res. 2014;164(4):293–301. doi: 10.1016/j.trsl.2014.05.010. http://dx.doi.org/10.1016/j.trsl.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Liu C, Xu Z, et al. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci. 2011;124(1):88–98. doi: 10.1093/toxsci/kfr211. http://dx.doi.org/10.1093/toxsci/kfr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X, Yavar Z, Verdin M, et al. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. 2010;30(12):2518–2527. doi: 10.1161/ATVBAHA.110.215350. http://dx.doi.org/10.1161/ATVBAHA.110.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Q, Yue P, Deiuliis J, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119(4):538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irigaray P, Ogier V, Jacquenet S, et al. Benzo[a]pyrene impairs beta-adrenergic stimulation of adipose tissue lipolysis and causes weight gain in mice. A novel molecular mechanism of toxicity for a common food pollutant. FEBS J. 2006;273(7):1362–1372. doi: 10.1111/j.1742-4658.2006.05159.x. http://dx.doi.org/10.1111/j.1742-4658.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- 8.Bolton JL, Smith SH, Huff NC, et al. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012;26(11):4743–4754. doi: 10.1096/fj.12-210989. http://dx.doi.org/10.1096/fj.12-210989. [DOI] [PubMed] [Google Scholar]
- 9.Rundle A, Hoepner L, Hassoun A, et al. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol. 2012;175(11):1163–1172. doi: 10.1093/aje/kwr455. http://dx.doi.org/10.1093/aje/kwr455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConnell R, Gillilan FD, Goran M, Allayee H, Hricko A, Mittelman S. Does near-roadway air pollution contribute to childhood obesity? Pediatr Obes. 2016;11(1):1–3. doi: 10.1111/ijpo.12016. http://dx.doi.org/10.1111/ijpo.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scinicariello F, Buser MC. Urinary Polycyclic Aromatic Hydrocarbons and Childhood Obesity : NHANES (2001-2006) Environ Health Perspect. 2014;122(3):299–303. doi: 10.1289/ehp.1307234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerrett M, McConnell R, Chang CCR, et al. Automobile traffic around the home and attained body mass index: a longitudinal cohort study of children aged 10-18 years. Prev Med (Baltim) 2010;50(Suppl 1):S50–S58. doi: 10.1016/j.ypmed.2009.09.026. http://dx.doi.org/10.1016/j.ypmed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mcconnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang C. Research | Children ’ s Health A Longitudinal Cohort Study of Body Mass Index and Childhood Exposure to Secondhand Tobacco Smoke and Air Pollution: The Southern California Children’s Health Study. Environ Health Perspect. 2015;123(4):360–366. doi: 10.1289/ehp.1307031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerrett M, McConnell R, Wolch J, et al. Traffic-related air pollution and obesity formation in children: a longitudinal, multilevel analysis. Environ Health. 2014;13(1):49. doi: 10.1186/1476-069X-13-49. http://dx.doi.org/10.1186/1476-069X-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Eliot MN, Kuchel G, et al. Long-term exposure to ambient air pollution and serum leptin in older adults: results from the MOBILIZE Boston study. J Occup Environ Med. 2014;56(9):e73–e77. doi: 10.1097/JOM.0000000000000253. http://dx.doi.org/10.1097/JOM.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boggs DA, Palmer JR, Spiegelman D, Stampfer MJ, Adams-campbell LL, Rosenberg L. Dietary patterns and 14-y weight gain in African American women. Am J Clin Nutr. 2011;6:86–94. doi: 10.3945/ajcn.111.013482. http://dx.doi.org/10.3945/ajcn.111.013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coogan PF, Wise LA, Cozier YC, Palmer JR, Rosenberg L. Lifecourse Educational Status in Relation to Weight Gain in African American Women. Ethn Dis. 2012;22 [PMC free article] [PubMed] [Google Scholar]
- 18.Coogan PF, White LF, Evans SR, et al. Longitudinal assessment of urban form and weight gain in African-American women. Am J Prev Med. 2011;40(4):411–418. doi: 10.1016/j.amepre.2010.12.013. http://dx.doi.org/10.1016/j.amepre.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coogan PF, Cozier YC, Krishnan S, et al. Neighborhood socioeconomic status in relation to 10-year weight gain in the Black Women’s Health Study. Obesity (Silver Spring) 2010;18(10):2064–2065. doi: 10.1038/oby.2010.69. http://dx.doi.org/10.1038/oby.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cozier YC, Yu J, Coogan PF, Bethea TN, Rosenberg L, Palmer JR. Racism, segregation, and risk of obesity in the black women’s health study. Am J Epidemiol. 2014;179(7):875–883. doi: 10.1093/aje/kwu004. http://dx.doi.org/10.1093/aje/kwu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg L, Adams-Campbell L, Palmer J. The Black Women’s Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc. 1995;50(2):56–58. [PubMed] [Google Scholar]
- 22.Carter-Nolan P, Adams-Campbell L, Makambi K, Lewis S, Palmer J, Rosenberg L. Validation of physical activity instruments: Black Women’s Health Study. Ethn Dis. 2006;16(4):943–947. [PubMed] [Google Scholar]
- 23.Block G, Hartman A, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1(1):58–64. doi: 10.1097/00001648-199001000-00013. http://dx.doi.org/10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Williams DR, Yu Y, Jackson JS, Anderson NB. Racial Differences in Physical and Mental Health. J Heal Psychol. 1997;2(3):335–351. doi: 10.1177/135910539700200305. http://dx.doi.org/10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 25.Beckerman B, Jerrett M, Serre M, et al. A Hybrid Approach to Estimating National Scale Spatiotemporal Variability of PM2.5 in the Contiguous United States. Env Sci Technol. 2013;47(13):7233–7241. doi: 10.1021/es400039u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novotny E, Bechle M, Millet D, Marshall J. National satellite-based land-use regression: NO2 in the United States. Env Sci Technol. 2011;45(10):4407–4414. doi: 10.1021/es103578x. http://dx.doi.org/10.1021/es103578x. [DOI] [PubMed] [Google Scholar]
- 27.U.S. E.P.A Office of Research and Development . Science Algorithms of the EPA Models-3 Community Multiscale Air Quality (CMAQ) Modeling System. Washington, DC: 1999. [Google Scholar]
- 28.Berrocal V, Gelfand A, Holland D. Space-time data fusion under error in computer model output: An application to modeling air quality. Biometrics. 2012;68:837–848. doi: 10.1111/j.1541-0420.2011.01725.x. http://dx.doi.org/10.1111/j.1541-0420.2011.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg L, Palmer J, Wise L, Horton N, Kumanyika S, Adams-Campbell L. A prospective study of the effect of childbearing on weight gain in AFrican-American women. Obes Res. 2003;11:1526–1535. doi: 10.1038/oby.2003.204. http://dx.doi.org/10.1038/oby.2003.204. [DOI] [PubMed] [Google Scholar]
- 30.Coogan PF, White L, Yu J, et al. PM2.5 and Diabetes and Hypertension Incidence in the Black Women’s Health Study. Epidemiology. 2016;27(2):202–210. doi: 10.1097/EDE.0000000000000418. http://dx.doi.org/10.1097/EDE.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White LF, Yu J, Jerrett M, Coogan PF. Temporal aspects of air pollutant measures in epidemiologic analysis: a simulation study. Sci Rep. 2016;6:19691. doi: 10.1038/srep19691. http://dx.doi.org/10.1038/srep19691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leech JA, Nelson WC, Burnett RT, et al. It’s about time: a comparison of Canadian and American time-activity patterns. J Expo Anal Environ Epidemiol. 2002;12(6):427–432. doi: 10.1038/sj.jea.7500244. http://dx.doi.org/10.1038/sj.jea.7500244. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Census Bureau [August 23, 2009];Educational Attainment in the US, March 1999, Table 1. Education attainment of the population 15 years and over by age, sex, race, and Hispanic origin. www.census.gov/population/www/socdemo/education/p20-528.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.