Abstract
Oxytocin (OT) and vasopressin (AVP) are important hypothalamic neuropeptides that regulate peripheral physiology, and have emerged as important modulators of brain function, particularly in the social realm. OT structure and the genes that ultimately determine structure are highly conserved among diverse eutherian mammals, but recent discoveries have identified surprising variability in OT and peptide structure in New World monkeys (NWM), with five new OT variants identified to date. This review explores these new findings in light of comparative OT/AVP ligand evolution, documents coevolutionary changes in the oxytocin and vasopressin receptors (OTR and V1aR), and highlights the distribution of neuropeptidergic neurons and receptors in the primate brain. Finally, the behavioral consequences of OT and AVP in regulating NWM sociality are summarized, demonstrating important neuromodulatory effects of these compounds and OT ligand-specific influences in certain social domains.
Keywords: oxytocin, vasopressin, New World monkey, social behavior, monogamy, oxytocin receptor, vasopressin receptor
1. Introduction
Oxytocin (OT), a nine amino acid neuropeptide hormone, has been characterized by some (Lee et al., 2009), not completely tongue-in-cheek, as “the Great Facilitator of Life”. For eutherian (placental) mammals, this characterization appears apt. OT is a critical mediator for two of the fundamental defining reproductive characteristics of eutherian mammals: placental birth and lactation. Further, OT has been shown to be critical in the formation and maintenance of mother-infant bonds in mammals (Rilling and Young, 2014), a social process that further enhances the likelihood of offspring survivorship and hence reproductive success. There is also a growing interest in OT and the related neurohypophyseal nonapetide arginine vasopressin (AVP) and their analogues in the regulation of social behavior beyond the maternal context, including social attachments among adults, social cognition, and aggression (Albers, 2015; Caldwell and Young III, 2006; Donaldson and Young, 2008; Goodson, 2008; Insel, 2010; Kelly and Goodson, 2014) and more complex human social traits, including social dysfunction (Feldman et al., 2016; Grinevich et al., 2015; LoParo and Waldman, 2014).
OT and AVP have important regulatory and modulatory roles in a host of processes. These nine amino acid peptides are synthesized primarily in magnocellular neurosecretory neurons in the paraventricular and supraoptic nuclei of the hypothalamus. The peripheral neuroendocrine effects of OT and AVP are mediated by the release of neuropeptides into peripheral circulation from the posterior pituitary (Kiss and Mikkelsen, 2005; Waite et al., 2014). Central effects of these modulatory neuropeptides are produced via projections to a host of forebrain regions that have high expression of OT and AVP receptors (Ludwig and Leng, 2006; Stoop, 2014, 2012). Both neuropeptides provide significant input to nuclei in the Social Brain Network (Newman, 1999). Oxytocinergic signaling impacts nuclei that are important in the regulation of attachment, parental care, reward, emotional intelligence, and social memory, and vasopressinergic signaling affects nodes in the network that regulate aggression, attachment, social memory, and parental care (Albers, 2015; Donaldson and Young, 2008; Kelly and Goodson, 2014) via both direct neuronal signaling and through volume transmission (Fuxe et al., 2012).
Until recently, every review on nonapeptide evolution and structure has indicated, almost axiomatically, that oxytocin structure is absolutely conserved (i.e., identical) in all species of eutherian mammals. This statement has been made in the earlier literature (see, for example: Acher et al., 1994; Donaldson and Young, 2008; Insel 2010; Lee et al., 2009) and even as recently as 2014 (Gruber, 2014). A similar claim has been made for AVP in eutherian mammals (with a few notable and documented exceptions; see Section 3.2). A recent review on the molecular genetics of the nucleotide sequences coding for the mature OT and AVP peptides in eutherian mammals lends credence to this perspective. Wallis (2012) conducted a comparative assessment of the 27 nucleotides in the oxytocin (OXT) and the arginine-vasopressin (AVP) genes that specifically code for the nine OT and AVP amino acids, and quantified the degree of conservation of structure in these molecules via dN/dS ratios (the number of nonsynonymous nucleotide substitutions relative to the number of synonymous substitutions). Ratios of less than 1.0 implies stabilizing selection for protein structure, while ratios of greater than 1.0 imply positive selection for diverse nucleotide sequences (Yang, 2007). dN/dS for eutherian OXT is strikingly low: 0.009. The absence of significant mutations in the OXT gene in eutherians is not surprising, given that structural alterations in the neuropeptide have the potential to fundamentally modify ligand binding properties with its cell membrane-bound receptor, and subsequently alter the modulatory effects of OT on cell signaling. Altering these biochemical processes could thereby disrupt the critical roles of oxytocin in mediating both peripheral and central processes associated with mammalian reproduction. Although eutherian mammals express three variants of AVP-like molecules (Wallis, 2012), dN/dS ratios for the ligand-coding region of AVP are also remarkably low (0.005), again suggesting extreme conservation of AVP ligand structure across eutherian mammals.
The present review will explore emerging discoveries in nonapeptide biology in the New World monkeys (NWM) of South and Central America, nested within the broader comparative biology of nonapeptide structure and function. We will summarize very recent developments characterizing the genomic coding regions for OT and AVP demonstrating that while AVP structure is conserved within this group of primates (as it is in most eutherian mammals), OT structure is highly variable in this group, with six variants identified to date. These findings challenge the common consensus of strict evolutionary conservation of OT within eutherian mammals. Further, social monogamy is a rare mating system among mammals (estimated between 3 – 9% of mammalian species; (Kleiman, 1977; Lukas and Clutton-Brock, 2013). However, the incidence of social monogamy among NWM, in various forms, is exceptionally high – more than 62% of the 117 species in this primate group are classified as socially monogamous (Lukas and Clutton-Brock, 2013). It has not escaped our attention, then, that the one mammalian taxon in which social monogamy is the norm among species also represents the only mammalian taxon where there multiple documented mutations in the OXT gene. This exposition on ligand variation in NWM is followed by a corresponding genomic analysis of receptors for OT (OXTR) and AVP (AVPR1A) within this group, in which a strong coevolutionary relationship between ligand and receptor variation is demonstrated, and we discuss the potential functional consequences of this variation for ligand-receptor binding and subsequent cell signaling. Third, the potential relevance of neuropeptide ligand and receptor variation for behavioral profiles is explored by reviewing the status of OT and AVP signaling systems in the brain, including the source and projections of OT and AVP synthesizing neurons, and the distribution of neuropeptide receptors in the primate forebrain. Finally, we review the important role of neuropeptides in modulating sociality in NWM, based on studies that explore correlations between neuropeptides and social behavior, and those that manipulate neuropeptide function using receptor agonists and antagonists.
2. Structure, evolution, and genetics of nonapetide signaling molecules
The OT/AVP family of neuropeptides shares a number of common structural features associated with the functional signaling molecules. In addition to possessing nine amino acid residues, all known vertebrate and invertebrate OT/AVP-like ligands share a N-terminal six-residue ring structure (ring) formed by a disulfide bridge between cysteine residues at positions 1 and 6, and a flexible C-terminal three-residue flexible tail structure (tail), typically with proline and glycine at positions 7 and 9, respectively. Finally, with few exceptions, the amino acid asparagine is found in position 5 in across phyla in all OT/AVP-like ligands.
A host of invertebrate species express one OT/AVP-like ligand, suggesting a very early evolutionary origin (~600 million years ago; Mya) for these signaling molecules (Beets et al., 2013; Gruber, 2014; Koehbach et al., 2013). Most vertebrates express two forms: an OT- and an AVP-like ligand. The presence of these two forms in primitive jawed fish suggests an evolutionary origin for distinct OT/AVP ligands in vertebrates at least 500 million years ago (Gwee et al., 2009, 2008; Wallis, 2012). Further, the similarity in structure (the two neuropeptide families differ primarily at positions 3 and 8) and the presence of the coding regions of the genes in close proximity on the same chromosome both suggest a gene duplication event, rather than an independent origin of the two nonapeptide lineages (Acher, 1993; Sawyer, 1977). The OT lineage (isotocin-mesotocin-oxytocin) is presumed to have evolutionary origins in the service of reproductive function, while the AVP lineage (vasotocin-vasopressin) is presumed to have origins in the regulation of fluid homeostasis (Acher, 1996).
Both OT and AVP are synthesized by genes characterized by three exons and two introns. Both genes code for a large preprohormone, consisting of a signal peptide, the mature neuropeptide, and a neurophysin. Peptide hormones are cleaved from this macromolecule during axonal transport and in terminal vesicles by a number of peptidases, after which they are released via vesicular fusion at axonal terminals (Caldwell and Young, 2006; Lee et al., 2009). The transcriptional organization of neurohypophseal genes varies across vertebrate phylogeny. In nonmammalian vertebrates and the opossum, genes for the two nonapeptides are arranged tail-to-head, indicating that transcription occurs from the same DNA strand. In contrast, AVP and OT genes are arranged tail-to-tail in all eutherian mammals (Gwee et al., 2009, 2008; Wallis, 2012; Yamashita and Kitano, 2013), indicating transcription of the neuropeptide in opposing directions. A similar tail-to-tail arrangement is also found in the monotreme platypus (Wallis, 2012). Given that monotremes diverged from the mammalian lineage leading to both marsupials and eutherian mammals, this suggests that either the tail-to-tail genetic orientation evolved twice on independent lineages (monotremes and eutherians) or a single reorganization occurred once and was reversed in marsupials (Wallis, 2012; Yamashita and Kitano, 2013). The intergenic region between AVP and OXT genes in eutherian mammals varies from approximately 3 – 10 kilobase pairs, and contains multiple regulatory regions that are important for OT and AVP gene expression in the hypothalamus (Young and Gainer, 2003; Young and Gainer, 2009).
3. Nonapeptide variation in New World monkeys
3.1. Primate taxonomy, evolutionary history, and NWM speciation
For the non-primatologist, we provide a brief review of primate phylogeny and evolution, with particular attention to diversification and taxonomy of NWM. Primates as an Order diverged from other mammalian forms approximately 80-90 million years ago (Mya; (Perelman et al., 2011), and are classified into five major groups based on morphological characteristics and genomically-derived phylogenies. Evolutionary ancient primates include Lemuriformes (Malagazy lemurs, African lorises and allied species), and Tarsiformes (Southeast Asian tarsiers). Among the Simiiformes, or monkey and ape-like primates, there are four broad categories: the New World monkeys distributed in South and Central America (parvorder Platyrrhini; described in more detail below), African and Asian Old World monkeys (superfamily Cercopithecoidea; e.g., macaques and baboons), and two groups of hominoid primates: Asian gibbons and siamangs (family Hylobatidae, the so-called ‘lesser apes’), and the hominid primates (orangutans, gorillas, chimpanzees, bonobos, and humans).
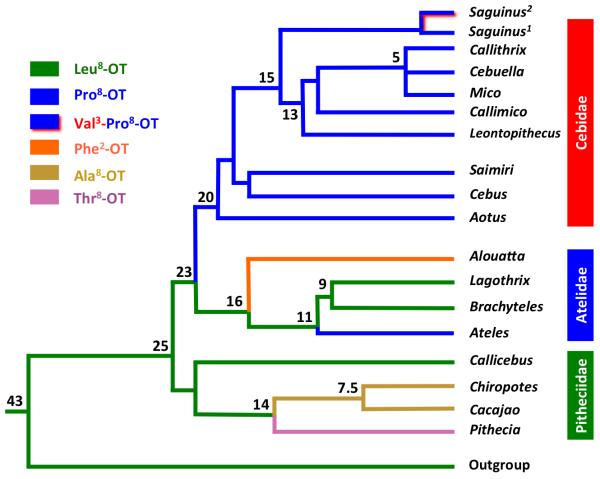
NWM comprise the parvorder Platyrrhini, and estimates based on molecular clocks suggest a divergence of this taxon from Old World primates and hominoids ~43 Mya (Perelman et al., 2011; Schneider and Sampaio, 2015; Wildman et al., 2009). A consensus phylogeny for this group based on nucleotide sequences is shown in Figure 1. Within this taxon there are 17 genera that are classified into three families: Pithecidae (Callicebus, Cacajao, Pithecia, and Chiropotes), Atelidae (Ateles, Brachyteles, Lagothrix, and Alouatta), and Cebidae (Saimiri, Cebus, Aotus, Saguinus, Leontopithecus, Callimico, Cebuella, Mico, and Callithrix). As shown in Figure 1, Pithecidae diverged from Atelidae/Cebidae ~25 Mya, and the divergence between Atelidae and Cebidae occurred ~23 Mya. There are commonalities among NWM (e.g., all species are predominantly arboreal) along with important differences among taxonomic groups (e.g., feeding niches range from primarily folivorous (e.g., Aloutta, Brachyteles) or frugivorous (e.g., Ateles, Pithecia, Chiropotes, Cacajao), to more mixed dietary niches of fruits, leaves, and vertebrate and invertebrate prey and tree exudates (Callicebus and most Cebidae; Rosenberger, 1992). Pitheciidae and Atelidae are generally large-bodied frugivore/folivore species (1.0 – 3.0 kg and 7.0 – 9.0 kg, respectively), while Cebidae are small-bodied species with more varied diets (0.12 – 2.5 kg; Smith and Jungers, 1997).
Figure 1.
Consensus phylogeny of New World monkeys and oxytocin ligand variation. Phylogenetic relationships among families and genera of New World monkeys represent common agreements among recent molecular phylogenies (Perelman et al., 2011; Schneider and Sampaio, 2015; Wildman et al., 2009). Colored lines represent different oxytocin ligand variants and their hypothesized parsimonious origins. Numbers at tree nodes represent estimated time of taxonomic branching (Million years ago; Mya); n.b.: branch lengths are not drawn to scale.
3.2 OT and AVP ligand structure in New World monkeys
Until 2011, no genetic analyses had been conducted on OT/AVP ligands or receptors in NWM. The impetus behind the first sequencing effort for nonapeptide ligands in this cluster of primates arose from the inability to measure plasma oxytocin concentrations in squirrel monkeys (Saimiri), a common primate model for biomedical research, using conventional radioimmunoassay (K. Parker, personal communication). The outcome suggested that OT in Saimiri did not cross-react with an antibody generated against consensus mammalian OT in the same fashion as the consensus mammalian OT standard preparation (Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly-NH2). To explore the remote possibility that the Saimiri OT ligand varied from consensus OT, Lee et al. (2011) sequenced OXT coding regions from genomic DNA in five species of NWM. These analyses revealed an unexpected single in-frame nonsyonymous nucleotide substitution (NS) in the OXT codon for the 8th amino acid (CCG → CTG) in four of the five species (all from the family Cebidae: Saimiri, Aotus, Cebus, and Callithrix; Callicebus maintained the consensus mammalian nucleotides). This single NS leads to an amino acid substitution in position 8 of the oxytocin molecule from leucine to proline, thus yielding a unique variant of OT (Pro8-OT) among eutherian mammals. RT-PCR of mRNA extracts from Saimiri confirmed the accurate transcription of this OT variant. Finally, mass spectroscopy of posterior pituitary extracts from Saimiri, along with a synthetic Pro8-OT standard, revealed that the altered codon for position 8 is translated into a mature Pro8-OT peptide. Bioinformatics analyses on OXT nucleotide sequences conducted at the time of publication revealed only one other eutherian mammal with Pro8-OT (the northern tree shrew, Tupaia). Thus, New World primates represent the first eutherian mammalian taxon in which OT structural variants were documented, and these variants appeared in some, but not all, NWM.
Given the remarkable discovery of a novel OT ligand in New World primates, this taxon has been analyzed in detail with regard to the genetics of OT and AVP signaling systems. In 2015, two laboratories independently assessed variation in coding regions of OXT in New World primates (Ren et al., 2015; Vargas-Pinilla et al., 2015). Ren et al. (2015) sampled genomic DNA from at least one species in each genera, with multiple individuals per genus, and the results were contrasted with representatives of all major primate clades, including Hominidae, Old World primates, tarsiers, and Strepsirrhini (lemurs, galagos, and pottos). Supplemental Table 1 provides nucleotide sequences and predicted OT ligand structure for these species, with reference sequences and amino acid residues from mice (Mus) and rats (Rattus). Several important points derive from these comparative genomic data. First, the OXT gene in all species other than NWM codes for consensus mammalian, or Leu8-OT. In all ape and Old World primate species, OXT nucleotide sequences are identical to those in humans. Second, while tarsiers and one genus of strepsirrhine primate (Otolemur) possess one or two synonymous nucleotide differences in codons 8 or 9, these species are also characterized by Leu8-OT. Third, rats and mice possess a terminal synonymous substitution in the last codon, but OXT in these species also codes for Leu8-OT. The feature that stands out most prominently is the prevalence of NSs in OXT in every genus of NWM, which in turn code for five distinct forms of OT in addition to consensus mammalian Leu8-OT, for a total of six OT ligand variants in this taxon. At least one genus in all three NWM families possesses a ligand variant. Pithecidae displays three OT ligands, all varying at position 8 in the OT molecule (Leu8-, Thr8-, and Ala8-OT). Primates in the family Atelidae also display three OT ligands (Leu8-, Pro8-, and Phe2-OT). None of the genera in the family Cebidae express Leu8-OT, but all genera have a NS in the 8th codon (CTG → CCG) leading to a proline residue in the 8th position. Most of these substitutions were also confirmed by Vargas-Pinilla et al. (2015), with the additional finding that some species of Saguinus also possess at second NS at the 3rd position (ATC → GTC), producing a residue substitution of isoleucine → valine, yielding Val3-Pro8-OT.
The distribution of these OT ligand variants is mapped on to NWM phylogeny in Figure 1. Given the phylogeny of the NWM, it is likely that the ancestor of NWM expressed Leu8-OT, since two of the three NWM families, and all of the Old World primates and all other known eutherian mammals share this trait. Within the Pitheciidae, Callicebus maintains Leu8-OT, and two mutations at least ~14 Mya led to Thr and Ala substitutions at position 8 in Pithecia and Chiropotes/Cacajao, respectively. Within the Atelids, at least two independent mutations likely occurred, one at ~16 Mya leading to Phe2-OT in Alouatta, and one at ~11 Mya leading to Pro8-OT in Ateles. The branching of Cebidae from the other NWM families ~23 Mya was likely associated with a mutation that led to the second instance of Pro8-OT in NWM, and a recent nucleotide substitution has led to a Val substitution at position 3 in three species of bare-faced tamarins (Saguinus), in addition to the Cebidae-specific Pro substitution at position 8. Given the presence of multiple OT variants within NWM families, it is clear that mutations in the mature peptide coding regions of OXT continued after separation of the three distinct clades, and some mutations have occurred in recent evolutionary time.
Amino acids in NWM OT ligands are conserved at positions 1, 4-7, and 9. Each of the amino acid substitutions in positions 2, 3, and 8 in NWM represents at least one physicochemical change (polarity, charge, or hydrophobicity) from the corresponding residue in consensus mammalian OT. Hence these changes can be classified as ‘radical’ amino acid substitutions (Zhang, 2000), leading to altered biochemical properties that could have important implications for receptor binding and subsequent cell signaling (Koehbach et al., 2013). For instance, Phe2-OT is the most hydrophobic variant, Leu8-OT is intermediate, while Pro8-OT is the most hydrophilic of the variants (Ren et al., 2015). While the Pro8-OT variant is the most hydrophilic of the OT variants, it is still more hydrophobic than AVP. It is currently unknown whether and to what extent these substitutions and the corresponding changes in hydrophobicity may lead to differences in blood brain barrier permeability across the OT variants. Given the current uncertainty about the possible peripheral and central actions of peripherally administered neuropeptides (see Section 6.4), the potential differences in permeability based on hydrophobicity and protein structure warrant further investigation.
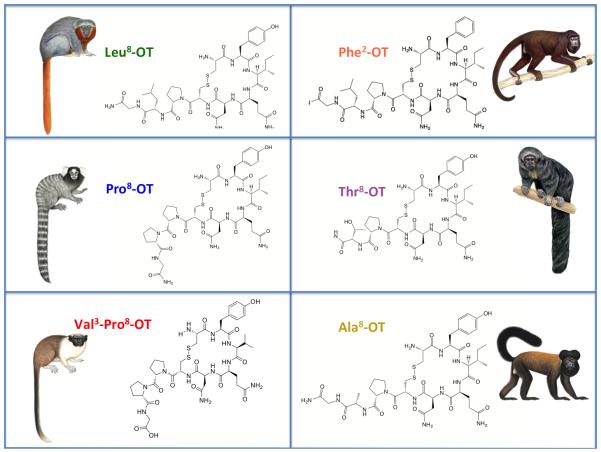
The Pro substitution in the 8th position among all genera in Cebids (and one genus in Atelidae) represents a significant modification to the OT ligand structure, since this amino acid places constraints on rotational structure of the peptide due to restricted side-chain flexibility. Further, the tandem Pro-Pro sequence in positions 7 and 8 produces a polyproline helix, a feature that can significantly alter ligand-cell membrane interaction profiles (Geisler and Chmielewski, 2009). The change from Leu8-OT to Pro8-OT has been identified as a more dramatic change in the molecular architecture of OT than the change from mesotocin to consensus mammalian OT (in which Isoleucine in replaced by Leucine at position 8; Stoop, 2012), with the potential for significant functional changes in the mechanisms of OT binding and signaling. Figure 2 portrays two-dimensional structural models of the six NWM OT variants, and it is clear that the Pro8 substitution in two OT variants produces a dramatic alteration in the structure of the ‘tail’ portion of the OT molecule, whereas the other residue substitutions produce only minor, but still potentially significant, alterations in chemical structure.
Figure 2.
Two-dimension structural models of the six OT variants identified in NWM to date. Exemplar species are Callicebus (Leu8-OT), Callithrix (Pro8-OT), Saguinus bicolor (Val3-Pro8-OT), Alouatta (Phe2-OT), Pithecia (Thr8-OT), and Chiropotes (Ala8-OT). Illustrations © 2015 Stephen D. Nash / IUCN SSC Primate Specialist Group.
In contrast to OT, all primate species, including NWM, have AVP coding sequences that yielded identical amino acid sequences for AVP (Supplemental Table 2). In hominoids and Old World primates, all nucleotides in the coding region for mature peptide are conserved, with no substitutions. In NWM, nucleotides associated with codons for positions 1, 3, 4, 5, and 8 are completely conserved. Several individual nucleotide substitutions among species were noted for in codons for positions 2, 6, 7, and 9 that did not alter amino acid residues at those positions. The most common was the terminal nucleotide (C → T), found in representatives from each of the three NWM families. Thus, in spite of considerable variation in OT ligands among NWM, all species studied to date possess consensus mammalian AVP: Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2.
Table 1 summarizes the current state of knowledge regarding the structure of nonapeptide variants among vertebrates and invertebrates. Regarding OT-like ligands, there are broad swaths of vertebrates that possess relative conserved ligand structure (e.g., OT in most eutherian mammals, mesotocin in birds and cold-blooded vertebrates). However, the amino acid structure of OT ligands presented in Table 1 reveal that there are at least two evolutionary ‘hot-spots’ where diversity in OT ligand structure is apparent in limited taxonomic groups: sharks, skates, and rays (five OT-like ligand variants) and NWM (six OT ligand variants). It is clear from the latter hot-spot (NWM) that the notion of a common, completely-conserved OT ligand among eutherian mammals is no longer a tenable position. The existence of six OT variants among NWM reveals that evolutionary constraints against variation in OT structure does not apply to all taxa. As whole genome sequencing is applied to additional taxa among the eutherian mammals, additional variants may be identified. For instance, a recent search on OT coding nucleotides among eutherian mammals suggests at least two additional variants: Leu3-OT in armadillo, and Thr4-Val8-OT in big brown bats (data accessed from NCBI; 12/15/2015). These variants have yet to be confirmed with more detailed sequencing, but they suggest that further exploration of diverse mammalian lineages may reveal yet additional variants of this “highly conserved” neuropeptide. The selective pressures that led to OT ligand variation in sharks and New World primates have not been identified, but remain an important question for comparative neuroendocrinologists.
Table 1.
Summary of amino acid structure in OT-like and VP-like compounds in vertebrate and invertebrate animals
| Nonapeptide | Amino Acid Position | Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin-like | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Leu8-OT | Cys | Tyr | Ile | Gln | Asn | Cys | Pro | Leu | Gly | Most mammals |
| Pro8-OT | Cys | Tyr | Ile | Gln | Asn | Cys | Pro | Pro | Gly | NWM and Tree shrew |
| Thr8-OT | Cys | Tyr | Ile | Gln | Asn | Cys | Pro | Thr | Gly | NWM |
| Ala8-OT | Cys | Tyr | Ile | Gln | Asn | Cys | Pro | Ala | Gly | NWM |
| Phe2-OT | Cys | Phe | Ile | Gln | Asn | Cys | Pro | Leu | Gly | NWM |
| Val3-Pro8-OT | Cys | Tyr | Val | Gln | Asn | Cys | Pro | Pro | Gly | NWM |
| Leu3-OT | Cys | Tyr | Leu | Gln | Asn | Cys | Pro | Leu | Gly | Armadillo |
| Thr4-Val8-OT | Cys | Tyr | Ile | Thr | Asn | Cys | Pro | Val | Gly | Bat |
| Mesotocin | Cys | Tyr | Ile | Gln | Asn | Cys | Pro | Ile | Gly | Noneutherian tetrapods; lobe-finned fishes |
| Isotocin | Cys | Tyr | Ile | Ser | Asn | Cys | Pro | Leu | Gly | Ray-finned fishes |
| Seritocin | Cys | Tyr | Ile | Gln | Ser | Cys | Pro | Ile | Gly | Egyptian toad |
| Asvatocin | Cys | Tyr | Ile | Asn | Asn | Cys | Pro | Val | Gly | Sharks, rays, skates |
| Phasitocin | Cys | Tyr | Phe | Asn | Asn | Cys | Pro | Ile | Gly | Sharks, rays, skates |
| Phasvatocin | Cys | Tye | Phe | Asn | Asn | Cys | Pro | Val | Gly | Sharks, rays, skates |
| Aspargatocin | Cys | Tyr | Ile | Asn | Asn | Cys | Pro | Leu | Gly | Sharks, rays, skates |
| Valitocin | Cys | Tyr | Ile | Gln | Asn | Cys | Pro | Val | Gly | Sharks, rays, skates |
| Glumitocin | Cys | Tyr | Ile | Ser | Asn | Cys | Pro | Gln | Gly | Sharks, rays, skates |
|
| ||||||||||
| Vasopressin-like | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Arg-vasopressin | Cys | Tyr | Phe | Gln | Asn | Cys | Pro | Arg | Gly | Most mammals |
| Lys8-vasopressin | Cys | Tyr | Phe | Gln | Asn | Cys | Pro | Lys | Gly | Suidae, Hippopotamidae |
| Thr4-vasopressin | Cys | Tyr | Phe | Thr | Asn | Cys | Pro | Arg | Gly | Tenrec |
| Phe2-vasopressin | Cys | Phe | Phe | Gln | Asn | Cys | Pro | Arg | Gly | Wallaby |
| Vasotocin | Cys | Tyr | Ile | Gln | Asn | Cys | Pro | Arg | Gly | Nonmammalian vertebrates |
| Cephalatocin | Cys | Tyr | Phe | Arg | Asn | Cys | Pro | Ile | Gly | Molluscs |
| Octopressin | Cys | Phe | Trp | Thr | Ser | Cys | Pro | Ile | Gly | Molluscs |
| Lys-Conopressin | Cys | Phe | Ile | Arg | Asn | Cys | Pro | Lys | Gly | Molluscs |
| Arg-Conopressin | Cys | Ile | Ile | Arg | Asn | Cys | Pro | Arg | Gly | Molluscs |
| Echinotocin | Cys | Phe | Ile | Ser | Asn | Cys | Pro | Lys | Gly | Echinoderms |
| Inotocin | Cys | Leu | Ile | Thr | Asn | Cys | Pro | Arg | Gly | Arthropods |
| Crustacean VP/OT | Cys | Phe | Ile | Thr | Asn | Cys | Pro | Pro | Gly | Arthropods |
| Annetocin | Cys | Phe | Val | Arg | Asn | Cys | Pro | Thr | Gly | Annelids |
| Hirudotocin | Cys | Phe | Ile | Arg | Asn | Cys | Pro | Leu | Gly | Annelids |
| Nematocin | Cys | Phe | Leu | Asn | Ser | Cys | Pro | Arg | Tyr | Nematodes |
Blue shading = Eutherian mammals, grey shading = invertebrates
Red residues differ from consensus eutherian mammal OT and AVP
Sources: Acher et al. 1995; Beets et al., 2013; Gruber 2014; Gwee et al. 2009; NCBI; Ren et al., 2014, 2015; Vargas-Pinilla, et al., 2015; Wallis 2012
4. OT and AVP Receptor Diversity in NWM
Neuropeptide signaling requires four elements: (1) the synthesis of the peptide modulator by cells of origin, (2) the transport of the peptide to target cells, (3) recognition and binding of the peptide by appropriate cellular receptors, and (4) modification of cell function via intracellular signaling cascades. In the case of OT and AVP signaling, the latter two functions are accomplished by cell membrane bound G protein-coupled receptors (GPCRs). Four receptor types have been identified in mammals: a single receptor that binds OT (OTR), and three receptor subtypes that bind AVP (V1aR, V1bR, and V2R). These receptors are members of the Class I (or A) rhodopsin-like GPCR family. Receptors in this family are characterized by a flexible N-terminus in the extracellular domain, four intracellular and three extracellular loops separated by seven helical transmembrane (TM) domains, and an intracellular C-terminus (Gimpl and Fahrenholz, 2001). OTRs are distributed widely in target tissues throughout the periphery and in the brain (see Section 5). V2R is primarily expressed in the kidney, and mediates the antidiuretic effects of AVP (Bankir, 2001) and will not be discussed further. V1aR and V1bR are likewise expressed widely in peripheral tissue, but are also expressed throughout the central nervous system. Growing evidence suggests an important role for V1bR in behavioral and neuroendocrine modulation (Griebel et al., 2003; Smith et al., 2016; Stevenson and Caldwell, 2012). However, V1a-like receptors are the most common and widely distributed vasopressin receptor in the vertebrate brain (Albers, 2015) and have been studied in the greatest detail from both a phylogenetic and behavioral perspective, and hence will be the focus of our attention in this section.
The binding and subsequent cellular consequences for OT and AVP are well established. The ring portion of OT interacts with the first extracellular loop of OTR, while the 3-residue tail structure interacts with the extracellular N-terminal domain (Gimpl and Fahrenholz, 2001; Zingg and Laporte, 2003). AVP binding, in contrast, involves interactions with amino acid residues in all three extracellular loop elements and residues in the N-terminus proximal to the first TM segment. (Hawtin et al., 2005; Thibonnier et al., 2000). OTR and V1aR are classic neuromodulators, mediating both short- and long-term changes in neural and hence behavioral outcomes (Owen et al., 2013). OT ligand binding to OTR activates multiple G proteins that can exert diverse effects on cell function, including stimulation of cAMP (Gs), inhibition of adenylyl cyclase (Gi/o), stimulation of potassium channel currents (Gi) and activation of phospholipase C (Gq). AVP ligand binding to V1aR activates two potential signaling cascades via Gq and Gi/o proteins, which can open nonspecific cationic channels or close K+ channels (reviewed in Caldwell et al., 2008; Gimpl and Fahrenholz, 2001). In both cases, multiple changes in cell function arise from G protein activation, including mobilization of Ca2+ ions from intracellular stores, alteration of voltage and ligand gated ion channels, and induction of enzymes for neurotransmitter biosynthesis (Stoop, 2014, 2012).
In this section, we discuss phylogenetic differences in the neuropeptide receptor structure from a broad ‘telescope view’ across the full animal kingdom and also a specific ‘microscope view’ on the Order Primates. This latter discussion is framed in the context of genera-level differences in OT ligand structure in NWM, and we discuss how specific amino acid substitutions in the OTR and the V1aR in NWM might alter receptor binding and signaling. This analysis focuses on interspecific differences in OTR and V1aR structure in three important NWM model species that vary in both OT ligand and in social structure: Callithrix: (Pro8-OT; socially monogamous), Callicebus (Leu8-OT; socially monogamous), and the Saimiri (Pro8-OT; nonmonogamous). Amino acid substitutions among these three genera relative to other primates (Homo, Macaca, and Otolemur), highlight potentially important sites for differences in ligand binding, intracellular signaling cascades, and potential downstream effects on neural circuits that regulate social behavior.
4.1. Phylogenetic Analyses of OT and AVP Receptor Diversity
Studying the OT/AVP nonapeptide family and the corresponding structure of their cognate receptors serves as an interesting test case in ligand-receptor coevolution (Markov et al., 2008), given the long evolutionary history of these peptide systems (~600 million years) and evolutionary changes in ligand structure. There are conserved elements of the peptide ligands (positions 1, 6, and 9) but also considerable variability in other positions (2-5 and 8; see Table 1). The variable amino acids at positions 2-5 and 8 are likely responsible for species-specific recognition at the receptor level and may underlie the multitude of functions subserved by nonapeptides. Consequently, we should be expect changes in ligand structure to be associated with changes in receptor structure.
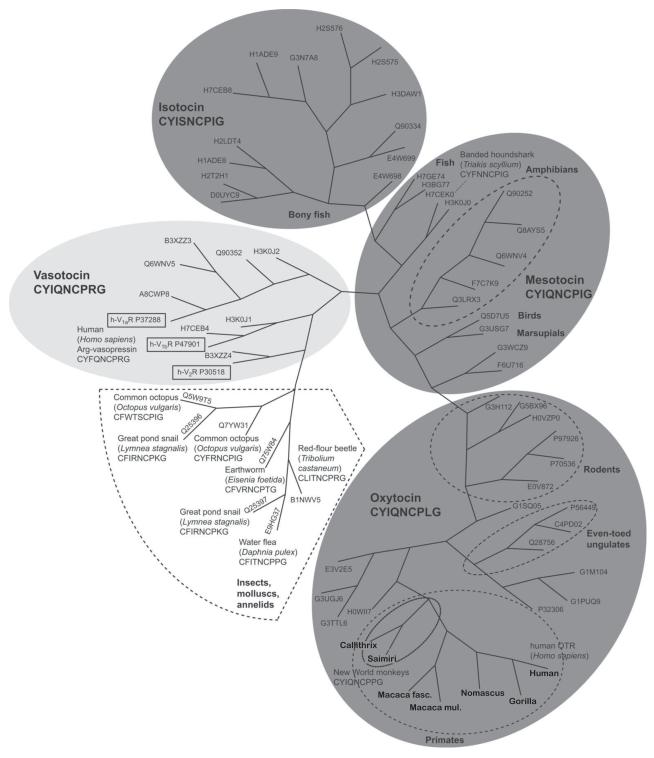
A survey of nucleotide sequences for OT/AVP-like GPRCs among 69 representative invertebrate and vertebrate species revealed considerable ligand-receptor coevolution (Fig. 3) (Koehbach et al., 2013; Liutkeviciute and Gruber, 2015). Several points can be taken from this analysis. First, receptors for invertebrate species that only possess vasopressin-like ligands clearly cluster together and are distinct from all nonapeptide receptors for vertebrates. Second, receptor clusters for AVP-like ligands in vertebrates are clearly differentiated from the clusters that characterize receptors for OT-like ligands. Third, and perhaps most importantly, receptor structure for different species expressing the three classic OT-like ligand variants (isotocin, mesotocin, and oxytocin) show high receptor sequence similarity among common ligands, but are clearly differentiated across OT-like ligands. Variation in receptor structure within a common OT ligand closely matches the consensus mammalian phylogeny by the clustering of OTR structure for primates, ungulates, and rodents, all species that exhibit OT (with the important exception of NWM, where exceptional variation in OT-like ligands is present). This analysis clearly highlights the important and pervasive coevolutionary relationship between variation in ligand structure and parallel changes in receptor structure.
Figure 3.
Diversity in nonapeptide receptor structure and ligand variation. Trees represent phylogenies of OT-like and AVP-like receptors in 69 invertebrate and vertebrate species. Published protein sequences were analyzed with ClustalW and the resulting phylogenies were generated. Nonapeptide receptors in invertebrates are in shown in white, receptors for AVP-like ligands in light gray, and OT-like compounds (isotocin, mesotocin, and oxytocin) in dark gray. For primates, genus names for OTR are indicated; for other species, the six-digit identifiers indicate UniProt KB entry numbers (www.uniprot.org). For mesotocin and oxytocin, species from related taxa (Class or Order) are indicated by dashed lines. Modified from a figure originally published in Koehbach et al. (2013): doi:10.1042/BST20120256
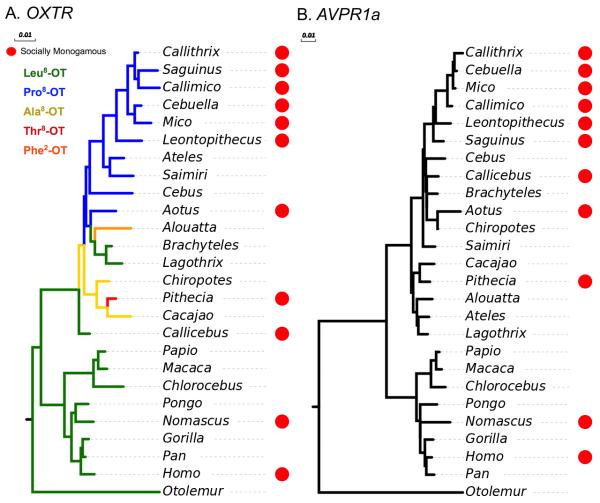
When a similar phylogenetic analysis is conducted on nonapeptide receptor variability among primates, taxa can be differentiated on the basis of receptor nucleotide sequences (Fig. 4a), in most cases with significant bootstrap support (Ren et al., 2014, 2015). While OTR sequence phylogenies across broad animal phyla parallel consensus phylogeny, examining OTR sequence phylogeny within the NWMs demonstrates OTR sequence phylogenies also significantly group by OT variants, social monogamy (Ren et al., 2015), and paternal care (Vargas-Pinilla et al., 2015). Importantly, some noteworthy deviations from the consensus NWM phylogenies (see Fig. 1) can be identified (Ren et al., 2015). Specifically, Ateles (family Atelidae), has an OTR structure that is more similar to the genera in Cebidae than to its congeners Brachyteles and Lagothrix. As shown in Section 3.2, Ateles is the only genera of NWM outside the Cebidae to express Pro8-OT, and all Cebids express this OT variant. In this case, then, OT ligand variant is a better predictor of OTR structure than conventional phylogenetic relationships. In support of this notion, (Ren et al., 2015) also systematically contrasted OTR amino acids in Callithrix to those of Otolemur. Humans and Callitrix are more closely related to each other than to Otolemur, but Otolemur and human share the Leu8-OT. Differences in OTR structure based solely on evolutionary distance would result in more amino acid differences between Homo and Otolemur than between Homo and Callithrix. However, the Callithrix OTR has more residue differences than the Otolemur OTR, particularly in the N-terminus that has been shown to be critical for ligand recognition and binding. These two cases strongly suggest strong coevolutionary relationships between OT ligands and receptors among the primates, especially in the case of Pro8-OT species. OTR variability among primates and especially NWM has been significantly associated with the occurrence of two important social phenotypes in NWM: social monogamy (Ren et al., 2015) and paternal care in (Vargas-Pinilla et al., 2015). Another independent survey of the OTR variability in primates demonstrated important differences in amino acid motifs in OT coding regions across socially monogamous primates (e.g., humans, gibbons, owl monkeys, titi monkeys, saki monkeys; Babb et al., 2015). This finding suggests that monogamy has evolved independently in Great Apes and NWMs by potentially different molecular mechanisms (Babb et al., 2015). Overall, the association between OTR variability and sociality suggest that, at least in part, these systems are involved in the evolution and maintenance of these social phenotypes in NWM (Ren et al., 2015; Vargas-Pinilla et al., 2015).
Figure 4.
NWM phylogenies derived from neuropeptide receptor nucleotide sequences. (A) phylogenetic reconstruction of NWM derived from OXTR nucleotide sequences. Branch colors represent different OT variants as shown in the legend. (B) phylogenetic reconstruction of NWM derived from AVPR1a nucleotide sequences. For both phylogenies, red circles represent genera generally considered to be socially monogamous. Scale bars indicate the branch length in nucleotide substitutions per site. The phylogenies are reconstructed from neuropeptide receptor sequences published by Ren et al. (2015, 2014) using ClustalW.
With regard to the AVP system, while there are no known variants in the AVP ligand in NWM, V1aR structure in NWM differs significantly from receptors in other primate genera especially in the N- and C-termini. Among NWM there have been many physico-chemically radical and conservative amino acid substitutions in the N-terminus, third intracellular loop, and C-terminus when compared to the human V1aR (Ren et al., 2014). Moreover, a phylogeny generated using AVPR1a nucleotide sequences groups socially monogamous species more closely than grouping from canonical phylogenies. There are several substitutions unique to the callitrichines, a clade characterized by social monogamy (Fig. 4b; Ren et al., 2014). One noteworthy variation from the consensus phylogeny is Callicebus, a socially monogamous NWM in the family Pitheciidae. Rather than sharing V1aR similarity with the other members of the pithiceds, Callicebus V1aR is more similar to the Pro8-OT Cebidae including the socially monogamous callitrichines. Moreover, while Callicebus and callitrichines are separated by the greatest evolutionary distance among NWM, the socially monogamous Callicebus and callitrichine AVPR1a sequences are more similar than the callitrichines and other non-socially monogamous Cebidae V1aR sequences. Specifically, the major ligand recognition and binding regions in V1aR and the major signal transduction regions both showed multiple radical physico-chemical substitutions, four of which (Gly172, Cys241, Asn319, Val399) were significantly associated with NWM classified as socially monogamous (Ren et al., 2014).
4.2. Neuropeptide receptor variation in NWM
4.2.1. Substitutions in OTR ligand binding region
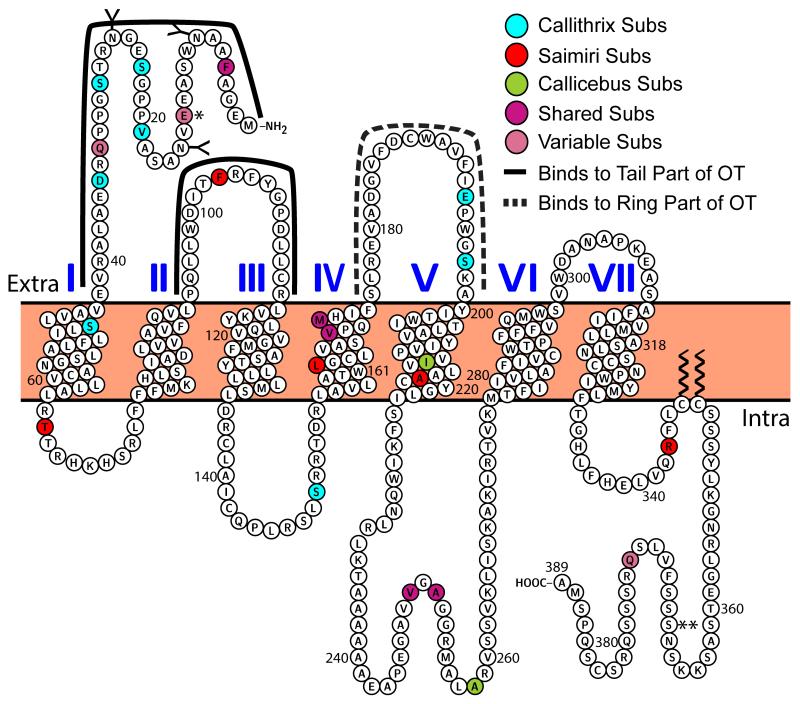
Many of the amino acid substitutions in the OT receptors of NWM are found in the N-terminus the putative binding region for the C-tail of the OT ligand (Gimpl and Fahrenholz, 2001). It is reasonable that structural changes to the C-terminus tail of the Pro8-OT ligand might result in compensatory amino acid substitutions in the N-terminus of the receptor (Ren et al., 2015). Figure 5 shows the results of an analysis comparing the OTR structure of three NWM genera, Callithrix, Callicebus, and Saimiri, to those of other representative primate groups (Homo, Macaca and Otolemur), highlighting substitutions present in at least one of these three NWM genera but not in any of the comparison primates (see Supplemental Table S3 for an OTR protein alignment representing all NWM genera). Most of the residues that have been shown or are predicted to be critical for receptor binding or function (reviewed in Gimpl and Fahrenholz, 2001; Koehbach et al., 2013) are conserved in the OTR of NWM species (Ren et al., 2015; Vargas-Pinilla et al., 2015). This is not unexpected, because experimental substitutions of these residues typically result in a receptor that does not bind or signal (Gimpl and Fahrenholz, 2001). One important exception is in Saimiri. This Pro8-OT species has a substitution at F103Y, which is a key residue of the putative binding pocket of OTR (Gimpl and Fahrenholz, 2001; Vargas-Pinilla et al., 2015). Vargas-Pinilla et al. (2015) identified this residue as homologous to Tyr115 in V1aR, which is critical for AVP binding and may confer increased binding affinity for AVP to Saimiri OTR (Chini et al., 1995). A Phe at this position is important for binding to ligands with a Leu or Iso at position 8 (Koehbach et al. 2013). This residue may also exhibit intraspecific polymorphism because one of the receptor sequences available for S. sciureus shows Phe115, and one shows Tyr115 (Lee et al., 2011; Ren et al., 2015; Vargas-Pinilla et al., 2015). These insights make this residue an attractive target for future ligand-receptor binding studies.
Figure 5.
Schematic of the Callithrix oxytocin receptor. Different colored residues indicate amino acid substitutions (subs) that are common among Callithrix, Saimiri, and Callicebus, shared by two of these genera, or unique to one genus, relative to representative primates from other major taxonomic groups (Homo, Macaca and Otolemur). The solid and dashed lines showing putative binding regions for the ring vs. tail portion of OT are adapted from (Gimpl and Fahrenholz, 2001). * Indicates a unique substitution in Callithrix in addition to a variable shared NWM substitution. ** indicates S368L substitution in Saguinus reported by (Vargas-Pinilla et al., 2015). Generated using Protter (Omasits et al., 2014).
There are several amino acid substitutions in the OTR N-terminus of Callithrix compared to Homo, Macaca, and Otolemur. Included in these substitutions are R33Q (a substitution also present in rats, voles, sheep, and cows; Wesley et al., 2002) and N35D. These residues flank Arg34, which is a residue critical for OT binding (Wesley et al., 2002), and though replacement of Arg33 and Asn35 with Ala does not affect OT binding, the callitrichine N35D substitution is homologous to the D33 in the vasopressin V2 receptor, which is adjacent to its critical R32 (Wesley et al., 2002). Like the F103Y substitution in Saimiri, this vasopressin receptor-like substitution may be a possible site for altered crosstalk between Pro8-OT and the V2 receptor and represents a residue that warrants further study. In contrast to Saimiri and Callithrix, there are no amino acid substitutions in the N-terminus or extracellular loops that are unique to Callicebus, a socially monogamous Leu8-OT species. There are, however, substitutions in the intracellular and TM domains (discussed in section 4.2.3), suggesting that potential differences between human and Callicebus OTR function will likely occur through differences in intracellular signaling rather than receptor binding.
4.2.2. Substitutions in V1aR ligand binding region
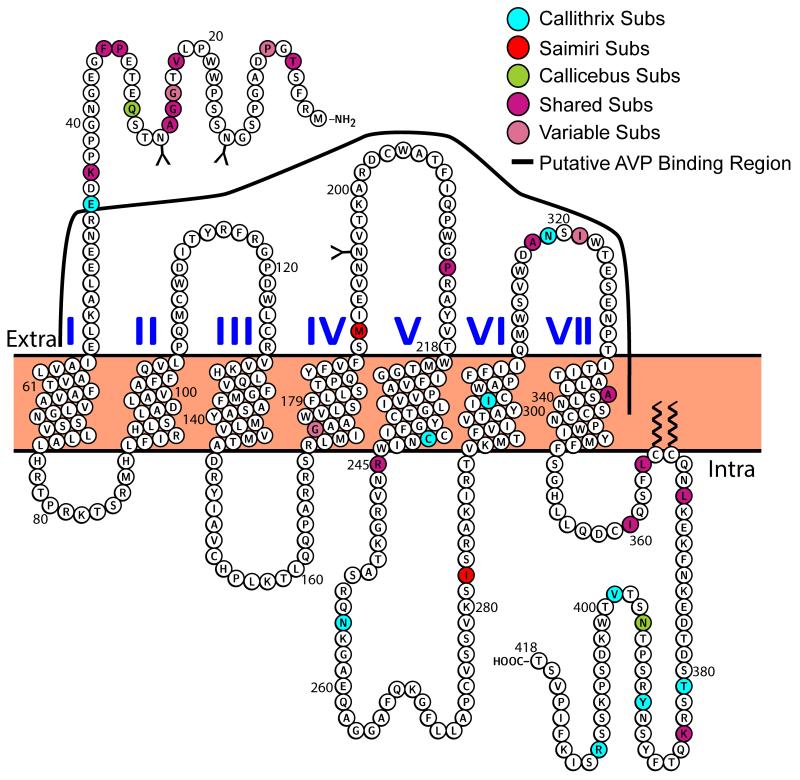
The V1aR has undergone significant modification among NWM (Ren et al., 2014). Because there is considerable crosstalk between OT and V1aR (Manning et al., 2012), one might expect that variation in the V1aR of NWM will reflect OT ligand structure. Figure 6 shows the analysis comparing the V1aR structure of three NWM genera, Callithrix, Callicebus, and Saimiri, to those of other representative primate groups (Homo, Macaca and Otolemur), highlighting substitutions present in at least one of these three NWM genera but not in any of the comparison primates (see Supplemental Table S4 for a V1aR protein alignment among all NWM genera). Like the OTR, most of the critical sites for AVPV1aR binding are conserved (Barberis et al., 1998; Chini et al., 1995; Mouillac et al., 1995; Thibonnier et al., 2000), with one notable exception. Saimiri, Callithrix, and Callicebus all have a S213P substitution, which is predicted using computer simulations to interact with the AVP ligand (Thibonnier et al., 2000). Additionally, the substitution in marmosets at V45E is adjacent to the highly conserved Arg46 (Hawtin et al., 2005). It is worth noting that many of the changes in the V1aR N-terminus are not in the AVP binding region, which is proximal to the first TM segment (Hawtin et al., 2005; Thibonnier et al., 2000). The distal N-terminus might, however, be important for OT-V1aR binding, particularly given that Pro8-OT is expected to be more hydrophilic than Leu8-OT (Ren et al., 2015), and thus may not penetrate as deeply into the hydrophobic cell membrane as AVP or Leu8-OT.
Figure 6.
Schematic of the Callithrix vasopressin 1a receptor. See Figure 5 legend for details. The solid line showing putative binding region is adapted from Hawtin et al. (2005) and Thibonnier et al. (2000).
These residue differences present in NWM make the V1aR a target for both pharmacological and behavioral experiments, especially in conjunction with the OTR and research questions involving ligand-receptor crosstalk between OT-V1aR and AVP-OTR. A host of evidence reveals significant OT and V1aR crosstalk in other model species, including pharmacological studies (Gimpl and Fahrenholz, 2001; Gruber et al., 2012), physiological studies (Chini et al., 1996; Meyer-Lindenberg et al., 2011), and neuropeptide binding and behavioral changes (Loyens et al., 2012; Schorscher-Petcu et al., 2010; Song et al., 2014). Pharmacological assessment of whether NWM amino acid substitutions in OTR and V1aR alter affinities for alternative nonapeptide ligands represents an important unanswered question.
4.2.3. Substitutions in OTR and V1aR signal transduction regions
As described above in Section 4, OTR and V1aR exert their modulatory effect via changes in G protein signaling cascades. There are several amino acid substitutions in the intracellular domains of NWM OTR and V1aR that warrant highlighting in this context. Many of these substitutions involve Ser or Thr, which may indicate changes in phosphorylation sites. For example, the V1aR substitutions M381T in Callithrix and N396S in Callicebus each create tripeptide Ser/Thr clusters. Additionally, (Vargas-Pinilla et al., 2015) identified a new phosphorylation site in the OTR of Callithrix at R149S, and the shortening of a Ser cluster in the OTR of Saguinus that is important for β-arrestin mediated desensitization and receptor recycling (Innamorati et al., 1998; Oakley et al., 2001; Vargas-Pinilla et al., 2015). There are also several substitutions in the V1aR and the OTR of Callithrix, Callicebus, and Saimiri near the palmitoylation site in the C-terminus. Experimental truncation of the C-terminus before the palmitoylation site results in reductions in receptor expression, binding affinity, and Ca2+ response, and an overall shift from Gq to Gi coupling (Hoare et al., 1999). Whether any of the intracellular substitutions highlighted here have any significant effects on receptor binding and intracellular signaling remains to be determined, but the published surveys of NWM neuropeptide diversity have opened the door for experimental assays of receptor function with respect to ligand and receptor variation.
5. Central distribution of neuropeptidergic neurons and receptors in primates
To exert significant effects on social and other behavioral processes, OT and AVP neurons in the PVN/SON must communicate with relevant brain regions, which in turn must contain cells that express receptors for these neuropeptides. Knowledge of the sites of origin for neuropeptides, and particularly the location of targets for these signaling molecules, can provide initial insights into the role(s) of these signaling systems in modulating social behavior. A recent comparative review highlighted commonalities and differences in a broad taxonomic context (Grinevich et al., 2015). We focus here on contrasts between the two mammalian taxa for which substantial information is available (OT/AVP signaling in the primate versus rodent brain), and then characterize common and unique pathways NWM vs. Old World monkeys and humans.
The distribution of OT-ergic and AVP-ergic neurons, and cellular receptors for OT (OTR) and AVP (V1aR, V1bR, V2R) have been well characterized in rodents (Albers, 2015; Johnson and Young, 2015) and other vertebrates (Goodson and Kabelik, 2009; O’Connell and Hofmann, 2012, 2011), and this distribution includes substantial overlap with the social behavior network (SBN; Newman, 1999). The SBN includes forebrain and midbrain nuclei and has extensive connectivity with the mesolimbic reward system, which is also rich in neuropeptidergic neurons and receptors. O’Connell and Hofmann (2011) proposed that the SBN can be characterized as an integrated social decision-making network that regulates both motivational components of sociality along with adaptive processing of salient social stimuli. Thus, this section will provide a summary and synthesis of what is known about the distribution of OT and AVP neurons and their cognate receptors in the SBN of the primate brain, with a particular focus on NWM. A summary of OT-ergic and AVP-ergic neurons, their central projections, and OTR and V1aR binding is presented for brain regions in the neurohypophyseal tract, the SBN, the mesolimbic reward system, as well as select brain regions involved in social/cognitive behavior, and select sensory processing centers, and an overall summary of these data can be found in Table 2.
Table 2.
Central distribution of neuropeptidergic neurons and their receptors in human and non-human primates
| Measure | Genus | Neurohypophyseal tract |
Social Behavior Network |
Mesolimbic Reward System |
Social/cognitive Processing |
Sensory Processing |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PVN | SON | POA | AH | VMH | PAG | LS | BNST | MeA | Str | NAcc | VP | blA | HIP | VTA | CeA | CC | IC | PFC | NTS | SC | NBM | V1 | OB | ||
| OT-ir neurons | Callithrix 12 | + + | + + | + | |||||||||||||||||||||
| Macaca 3 | + | + | |||||||||||||||||||||||
| Homo 8 | + | + | − | ||||||||||||||||||||||
|
| |||||||||||||||||||||||||
| OT-ir fibers | Callithrix 12 | + + | + + | + | + | ||||||||||||||||||||
| Macaca 3 | + + | + + | + | ||||||||||||||||||||||
|
| |||||||||||||||||||||||||
| OTR binding | Callithrix 11 | − | − | + + | − | + | +* | ||||||||||||||||||
| Callicebus 7 | + | − | + + | − | + | + | + | + | |||||||||||||||||
| Macaca1,6 | +f | +m | +f | − m | +m | +m | − m | ||||||||||||||||||
| Homo2,9,10 | + | + | + | + | − | + | + | − | − | + | + | + | + | + | |||||||||||
|
| |||||||||||||||||||||||||
| AVP-ir neurons | Callithrix12,13 | + + | + + | + | + | − | − | − | |||||||||||||||||
| Macaca 3 | + | + | + | + | + | + | |||||||||||||||||||
| Homo4,5,8 | + | + | + | ||||||||||||||||||||||
|
| |||||||||||||||||||||||||
| AVP-ir fibers | Callithrix12,13 | + + | + + | + | + | + | − | ||||||||||||||||||
| Macaca 3 | + + | − | + + | + + | + + | + + | + + | + | |||||||||||||||||
|
| |||||||||||||||||||||||||
| V1aR binding | Callithrix11,13 | + − | − | + | + | + + | + + | + | + | + | − | + | + + | + | + + | ||||||||||
| Callicebus 7 | + + | + + | + + | + | + | + | + | + | |||||||||||||||||
| Macaca 14 | + | + | + | + | + + | + | + | + | + | + | − | + | |||||||||||||
| Homo 9 | + | + | + | − | − | − | |||||||||||||||||||
Note. OT and AVP immunoreactivity and OTR and V1aR binding are represented by + + (dense immunoreactivity, receptor binding), + (present immunoreactivity, receptor binding), and − (no immunoreactivity, receptor binding detected) in specified brain regions. Blank cells represent a lack of information on the presence or absence of immunoreactivity or receptor binding in that region. Data for all measures are not available for all genera.
Macaca fascicularis.
Macaca mulatta.
Unpublished observation in Freeman et al., 2014a.
5.1. OT and AVP synthesis and their projections in the primate brain
The distribution of OT- and AVP-immunoreactive (OT-ir; AVP-ir) neurons appear to be relatively conserved across mammals, with OT-containing neurons present in the neurohypophyseal tract (PVN and SON) and AVP-containing neurons present in the PVN, SON, and SCN (Landgraf and Neumann, 2004; Ludwig and Leng, 2006; Sofroniew, 1983). Surprisingly little is known about the distribution of OT and AVP neurons and their projections in humans and other primates. To date, OT-ir and AVP-ir pathways in the brain have only been identified in three primate genera (Callithrix, Macaca, and Homo). OT- and AVP-ir in primates predominantly resemble the pattern of immunostaining in rodents. As expected, OT- and AVP-containing neurons have been identified in the PVN and SON in Callithrix (Wang et al., 1997a, 1997b), Macaca (Caffé et al., 1989), and Homo (Ishunina and Swaab, 1999). AVP-ir neurons are found in the BNST in these genera (Caffé et al., 1989; Fliers et al., 1986; Ishunina and Swaab, 1999; Wang et al., 1997a). However, no extrahypothalamic OT neurons were found in either Macaca or Homo (Caffé et al., 1989; Ishunina and Swaab, 1999), but OT-ir neurons were found in the BNST and meA of Callithrix (Wang et al., 1997a, 1997b). Thus, OT- and AVP-containing neurons are mainly localized to the neurohypophyseal tract in primates, but one species of NWM expressed OT-ir in extrahypothalamic regions of the SBN. This pattern of OT-ir and AVP-ir expression is consistent with patterns reported in rodents.
OT- and AVP-producing neurons in primates project to multiple regions of the social decision-making network. In Callithrix, localization studies based on antibodies generated to Leu8-OT revealed OT-ir fibers in the neurohypophyseal tract, BNST, meA, and VMH. AVP-ir fibers were also revealed within the neurohypophyseal tract and BNST, as well as the NAcc. In Macaca, dense concentrations of OT fibers were found in the amygdala and NTS, and AVP-ir was shown to project to the amygdala, BNST, PAG, VTA, hippocampus, and NTS, but not the LS (Caffé et al., 1989). The distribution of central OT- and AVP-producing neurons and their projections in these primate genera is quite dissimilar than rodent central OT and AVP systems. One apparent difference between rodent and primate distribution of central neuropeptide systems is the presence of AVP-ir fibers in the LS. Among rodents, the LS is a key regulator of social recognition (Everts and Koolhaas, 1997; Lukas et al., 2013) and aggression (Veenema et al., 2010) and expresses high levels of AVP-ir (n.b.: there is some variation in AVP system parameters in the LS across rodents). In Callithrix marmosets, there was low, but detectable, AVP immunoreactivity in the LS (confirmed by in situ hybridization; Wang et al., 1997b), but the fibers did not form a dense plexus as found in several rodent species (Caffé et al., 1987; de Vries and Ruijs, 1983). Despite this potentially important difference in the LS, projections from oxytocinergic and vasopressinergic neurons appear to be relatively conserved, with significant overlap in the neurohypophyseal tract as well as key areas within the social decision-making network (e.g., BNST, VTA, amygdala), suggesting that the activity of the OT and AVP systems within these regions could serve, in part, as a final pathway for regulating species-appropriate social behavior.
Knowledge of the distribution of neuropeptidergic tracts should be followed up with studies of the functional release of these signaling molecules in social contexts to confirm their role in modulating social function. The use of microdialysis to measure regionally-specific neuronal release of neuropeptides has provided important mechanistic insights into neuropeptide modulation in a variety of species and a host of social contexts, including maternal behavior, social recognition, social preference, social reward, social memory, and social buffering (Bosch and Neumann, 2012; Dölen et al., 2013; Dumais et al., 2016; Kendrick, 2013; Lukas et al., 2013; Smith and Wang, 2014; Zoicas et al., 2014). To our knowledge no studies have utilized microdialysis in behaving primates to evaluate the role of real-time release of neuropeptides in stimulating social behavior, or in response to salient social stimuli. Thus, future studies that utilize this and related approaches to monitor moment-to-moment neuronal release of OT and AVP will provide significant translational information regarding the specific social modules and neuropeptide circuits that are activated in a social context.
5.2. Central distribution of OT and AVP receptors in the primate brain
5.2.1. OTR and V1aR in the social decision-making network
Among rodents, there is pronounced interspecific variation in OTR and V1aR binding in the central nervous system that reflects differential social organization and mating strategies (Barrett et al., 2013; Beery et al., 2008; Insel and Shapiro, 1992; Mooney et al., 2015; Ophir et al., 2012; Young et al., 2011). Interest in identifying the density and distribution neuropeptide receptors in primates has been apparent for decades. Despite the apparent interest in mapping OT and AVP receptors in the brains of primates (Ichimiya et al., 1988; Kawata and Sano, 1982; Sukhov et al., 1993; Ueda et al., 1983), progress lags significantly behind rodent research in this area. The limited information on the distributions of OTR and V1aR in primate neural tissue is due in part to the lower selectivity for radioligands targeting OTR (125I-OVTA) and V1aR (125I-LVA) autoradiography, relative to high selectivity of radioligands for these receptors in rodents, as well as the promiscuous binding profile of both radioligands for OTR and V1aR (Freeman et al., 2014a, 2014b; Manning et al., 2012; Toloczko et al., 1997). Two studies (Freeman et al., 2014a, 2014b) have used a pharmacologically-advised design to account for the problematic binding affinity profiles. The procedure involves co-incubating neural tissue with the radioligand along with an unlabeled and competitive selective receptor antagonist. This allows for competition between the unlabeled antagonist and the radioligand, revealing the localization of the receptor of interest. Thus, much of what is known about OTR and V1aR density and distribution in the primate brain (Loup et al., 1991, 1989; Schorscher-Petcu et al., 2009; Wang et al., 1997b; Young et al., 1999) should be cautiously evaluated in light of the limitations of radioligands utilized for receptor autoradiography in primates.
OTR and V1aR distributions have been evaluated in four primate species: Callithrix, Callicebus, Macaca, and Homo. There are strikingly different profiles for these receptors across species, potentially reflecting both species-level differences in social structure and mating strategies as well as the secular effects of phylogeny. The distribution of V1aR is much more widespread across the primate brain compared to the distribution of OTR. V1aRs are found in nuclei within the social decision-making network, including in the hypothalamus, LS, NAcc, VP, BNST, hippocampus and the extended amygdala. OTRs are less widely and densely distributed, but include expression in hypothalamic nuclei, NAcc, VP, LS, and hippocampus.
Despite the widespread distribution of OTR and V1aR across the social decision-making network, there are pronounced taxonomic differences in the localization of these neuropeptide receptors in primates. In particular, Callithrix has dense OTR expression in the NAcc (Schorscher-Petcu et al., 2009). Since OT activity within the NAcc is critical for pair-bond formation in socially monogamous rodents (Insel and Shapiro, 1992; Lim et al., 2004) and normative social processes in nonmonogamous rodents (Dölen et al., 2013), we might expect that OTR expression in the NAcc to be conserved within primates as well. However, Callicebus does not exhibit OTR binding in the NAcc, and instead densely expresses V1aR (confirmed by in situ hybridization; Freeman et al., 2014b). Callithrix and Callicebus have relatively similar social structures, characterized by high levels of sociality, selective breeding, maintenance of long-term male-female relationships, and biparental care (Digby, 1995; Fernandez-Duque et al., 2009; Spence-Aizenberg et al., 2015). That Callithrix and Callicebus exhibit drastically different expression patterns OTR and V1aR raises the intriguing possibility that OT and AVP systems produce similar social phenotypes via different neural mechanisms.
Similar to Callithrix and Callicebus, V1aR is widely distributed throughout the brain of Macaca, with notable expression in the social-decision making network as well in regions conventionally identified within social/cognitive circuits, including the CeA, PFC, CC, and IC (confirmed by in situ hybridization; Young et al., 1999). In Homo, V1aR was less widely distributed across the social-decision making network (Loup et al., 1991) relative to Callithrix, Callicebus, and Macaca, although fewer brain regions were sampled. Macaca OTR was less widely distributed in the social-decision making network compared to V1aR (Boccia et al., 2001; Freeman et al., 2014a). Interestingly, binding of labeled OT in Homo was shown across the social-decision making network, but was notably absent from the NAcc and hippocampus (Boccia et al., 2013; Loup et al., 1991, 1989), similar to Callithrix. In sum, across a sampling of New World, Old World, and hominid primates, cellular receptors for OT and AVP are widely distributed across the social-decision making network, but there is pronounced variation in the presence and density of OTR and V1aR, potentially guiding important differences in social phenotype.
5.2.2. OTR and V1aR in select sensory processing centers
In human and non-human primates, processing of social information is largely guided by the visual and auditory systems (and to a lesser extent, olfactory systems). In particular, initiating and maintaining visual contact with another individual is a critical prerequisite for navigating the social environment, establishing and maintaining dominance hierarchies, as well developing long-term social bonds and cooperative relationships. As expected, Callithrix, Callicebus, Macaca, and Homo have high levels of OTR expression in the SC and NBM (Boccia et al., 2013, 2001; Freeman et al., 2014a, 2014b; Loup et al., 1991, 1989; Schorscher-Petcu et al., 2009), important regulators of selective attention and visual streaming. The NTS and V1 also show positive binding for OTR in primates, but OTR expression in these regions is not consistently reported across these studies. In Callithrix and Callicebus, V1aR expression is less widespread, but is apparent across several critical sensory processing centers. Callithrix show dense binding for V1aR in the NTS and OB, with moderate binding in the NBM; V1aR binding for the SC or V1 was not reported (Schorscher-Petcu et al., 2009; Wang et al., 1997b). The dense binding for V1aR in the OB of Callithrix is intriguing since marmosets utilize olfactory signals as a primary means of social communication (Epple, 1972; Smith et al., 2001; Ziegler, 2013). Callicebus show binding for V1aR in the SC and V1; V1aR binding for the NTS, NBM, or OB was not reported (Freeman et al., 2014b). V1aR in sensory areas in Macaca and Homo are much less widespread, and was only found in V1 in Macaca. Macaca showed no V1aR binding in the SC, and Homo showed no V1aR binding in either the SC or NBM (Freeman et al., 2014a; Loup et al., 1991). These results suggest an important modulatory role of neuropeptides (particularly OT) in processing visual (e.g., facial recognition, directing eye gaze) and multimodal social stimuli among all primates, and an important role for AVP in these processes in Callithrix. The overall patterns of receptor distribution in the visual processing areas in primates is consistent with the importance and predominance of visual stimuli in primate social relationships, akin to the central role of olfaction, and the rich expression of OTR in odor-processing networks, in rodents (Freeman et al., 2014b).
5.3. Sex differences in central OT and AVP pathways
OT and AVP are known to regulate social behavior in sex-specific ways, which is likely regulated in part by sex differences in OT and AVP synthesis and in the density and distribution of OTR and V1aR in the brain. The sexually dimorphic nature of AVP, and to a lesser extent OT, in the rodent brain is fairly well characterized, (De Vries and Panzica, 2006; Dumais and Veenema, 2015). Generally, OT and AVP system parameters are not different between males and females across rodents, with no detectable differences in the neurohypophyseal tract and hypothalamic nuclei (see Dumais and Veenema, 2015). However, in several species the expression of central AVP-ir neurons/fibers and V1aR is higher in males than females, , notably in the LS and BSNT. OT system parameters also differ between males and females in some species of rodents, with males having more OT-ir neurons/fibers and greater OTR density and distribution in some species and females having higher OT system parameters in other species (see Dumais and Veenema, 2015). Thus, sex differences in central OT and AVP system parameters appear to be species- and brain region-specific, and these differences likely contribute in part to differences in social behavior across and within species.
In contrast to the well-characterized central OT and AVP pathways in rodents, information about the sexually dimorphic nature of these pathways in primates is remarkably limited. Of the studies that included both males and females and included an analysis of sex, no sex differences were found in OT-ir or AVP-ir of Macaca or Homo (Caffé et al., 1989; Fliers et al., 1986). Furthermore, male and female Callithrix do not have dimorphic central distributions of OT-ir neurons (Wang et al., 1997a). However, AVP-ir in the BSNT was dimorphic in Callithrix, with males expressing more AVP-ir neurons than females (Wang et al., 1997a), suggesting that AVP projections from the BNST may be particularly important for the expression of sex-specific behavior in Callithrix.
No data are available regarding sex differences in the central distribution of OTR in non-human primates. The limited information on sex differences in V1aR shows that male and female Macaca (Young et al., 1999) and Homo (Loup et al., 1991) display similar density and distribution of V1aR. In Homo, males and females do no differ in the number or size of OT-ir neurons in the neurohypophyseal tract (Fliers et al., 1985; Ishunina and Swaab, 1999; Wierda et al., 1991). Interestingly, there is a sex difference in the size of AVP-ir neurons in Homo under 50 years of age, with men having significantly larger AVP neurons than women (Ishunina and Swaab, 1999). Given the widespread sexual dimorphism in neuropeptide cells and receptors in rodents, additional studies in primates are critical for identifying the contribution of OT and AVP to sexually-dimorphic behavior patterns in primates.
5.4. Limitations and caveats regarding nonapeptide localization in the brain of NWM
This section makes it abundantly clear that there remain large gaps in the neuroanatomical map of OT-ergic and AVP-ergic neurons, their central projections, and the density and distribution of cellular receptors in human and non-human primates. The pharmacological limitations of radioligands used for receptor autoradiography in primates argues strongly for adopting pharmacologically-advised designs to account for interspecific variation in receptor binding properties. Of particular relevance for NWM is the need for data on the binding affinities of the newly-identified NWM OT variants described in Section 2, and knowledge of their competitive binding interactions with known labeled neuropeptide compounds. Further, given the significant structural changes in OT ligands, especially Pro8-OT, and the NWM-specific changes in OTR and V1aR, information on ‘cross-talk’ between ligands and receptors is critical. The ability of OT agonists to displace labeled AVP receptor antagonists in Callithrix (Schorscher-Petcu et al., 2009) provides a hint that cross-talk is a likely possibility in NWM.
6. Neuropeptide Diversity and Social Behavior in New World monkeys
The important role of neuropeptide signaling in regulating a host of behavioral processes in invertebrate and especially vertebrate animals, especially in the social realm, has been firmly established (Albers, 2015; Caldwell and Young III, 2006; Donaldson and Young, 2008; Taghert and Nitabach, 2012; Wircer et al., 2015). In this review, the data summarized in Section 5 clearly demonstrate that neuropeptide-synthesizing neurons and target cells that express appropriate receptors in primates, including NWM, are located in key nodes of the SBN. While it is generally recognized that the conserved function of nonapeptides across species is to maximize reproductive success through the modulation of reproductive physiology and behavior (Knobloch and Grinevich, 2014), comparative analyses have revealed that the effects of OT and AVP on social behavior are as complex and diverse as the differences in reproductive and social strategies found across the animal kingdom (Goodson, 2008; Kelly and Ophir, 2015).
The notion that the conserved function of nonapeptides can be made manifest via alternative phenotypic routes raises the interesting question of whether the multiple OT ligand variants identified in NWM (Section 3.2) have functional consequences for species differences in social behavior. As described in Section 1, NWM are important models for social behavior because many species show intraspecific and idiosyncratic variability in behaviors that are translatable to humans including pair-bonding, biparental care, and high levels of cooperation and aggression. The majority of studies performed to date have focused on marmosets (Callithrix) and tamarins (Saguinus), although a handful of studies have examined squirrel monkeys (Saimiri), capuchin monkeys (Cebus), and titi monkeys (Callicebus). While OT ligand variation has been statistically associated with the presence of social monogamy (Ren et al., 2015) and paternal care (Vargas-Pinilla et al., 2015) across NWM genera, there are some important comparative tests to further elucidate how OT variants influence species-specific social behavior. For instance, titi monkeys are socially monogamous and exhibit biparental cooperation, but express the Leu8-OT ligand. Conversely, squirrel monkeys and capuchins are non-monogamous and uniparental, yet both possess the Pro8-OT ligand.
In this section we review the available literature on neuropeptides and social behavior in NWMs. These studies expand and complement what has been learned from other vertebrate taxa (Hofmann et al., 2014), and demonstrate the utility of NWMs to advance our understanding of the human social brain and behavior. The overarching theme, as summarized by the meta-analysis of results in Table 3, is that neuropeptides modulate social behavior in NWM by maintaining and enhancing established social relationships in a species-, and to a lesser degree, ligand-specific fashion.
Table 3.
Summary of social/behavioral effects of experimental OT/AVP treatments and correlations between peripheral measures of neuropeptides in New World monkeys
| Social Behavior | Species | Pro8- OT |
Leu8- OT |
OTa | OT- peri |
AVP | V1aRa | Specific Effects |
|---|---|---|---|---|---|---|---|---|
| Social Proximity | Callithrix | ↑ | ↓ | OTa reduced proximity and approach behavior, and OT increased overall huddling and proximity initiation. 1 |
||||
| Callithrix | ↑/↓ | 0 | 0 | OT reduced proximity toward opposite-sex strangers and OT increased proximity with pairmates in females. Effects were specific to the Pro8-OT ligand. 2 |
||||
| Callithrix | ↑ | ↑ | 0 | OT treated partners received increased proximity from pairmates. 3 | ||||
| Callithrix | ↑ | Within-pair OT correlations are higher for affiliative dyads. 4 | ||||||
| Saguinus | ↑ | OT concentrations were higher within pairs that showed increased huddling. 5 |
||||||
| Cebus | ↓ | OT increased social distance between partners in a food-sharing task. 6 | ||||||
| Titi | ↑ | AVP increased greater social contact with pairmates in males. 7 | ||||||
| Grooming | Callithrix | ↑ | ↑ | 0 | OT treated partners received increased grooming from pairmates. 3 | |||
| Saguinus | ↑ | OT concentrations were higher within pairs that showed increased grooming. 5 |
||||||
| Sexual Behavior | Callithrix | ↓ | 0 | 0 | OT increased latency to open-mouth displays toward strangers. No effects of OT toward pairmates. 2 |
|||
| Saguinus | ↑ | OT concentrations were higher within pairs that showed increased sexual behavior. 5 |
||||||
| Social Buffering | Callithrix | 0 | 0 | ↓ | OTa reduced proximity from their pairmate during a social separation stressor. 8 |
|||
| Parental Care | Callithrix | ↑ | 0 | ↑ | 0 | OT decreased latency to respond to infant stimuli in males. AVP decreased latency to respond to infant stimuli in females. 9 |
||
| Callithrix | ↑ | 0 | OT increased paternal tolerance of food stealing from infants. 10 | |||||
| Food Sharing | Callithrix | 0 | ↓ | OTa reduced food sharing toward pairmates. 1 | ||||
| Callithrix | ↓ | 0 | 0 | OT reduced 'altruistic' food sharing toward opposite-sex strangers. This effect was specific to the Pro8-OT. 11 |
||||
| Callithrix | 0 | 0 | 0 | OT did not alter food inequity aversion toward pairmates or opposite-sex strangers. 12 |
||||
| Cebus | ↓ | OT reduced passive food sharing between partners and strangers. 8 |
OT = oxytocin, OTa = OT antagonist, OT – peri = peripherally measured OT concentrations, AVP = vasopressin, V1aRa = vasopressin antagonist. ↑ = increased social behavior. ↓ = decreased social behavior. 0 = no significant effect on social behavior. Blank = no data available.
All Smith et al., 2011;
Snowdon et al., 2011;
Mustoe et al., in press. Data for all measures are not available for all genera. Administered of neuropeptide agonists for all studies used intranasal treatment except 10, which utilized intracerebroventricular administration.
6.1. Pair-bond Formation and Maintenance
Social monogamy is uncommon among mammals, but the incidence is higher among primates and is especially high in NWM. The high level of social monogamy found among NWM is a topic that has received recent attention and debate. Researchers have argued for the use of more nuanced definitions and classification of social systems in primates (Díaz-Muñoz and Bales, 2016), and this is especially important given that there is considerable variability in sociosexual and parental behavior both between and within species (Díaz-Muñoz, 2016).
NWM show a diverse range of monogamous and non-monogamous social systems. In marmosets and other callitrichines, social monogamy is characterized by high levels of sociality with a pair-mate (Ågmo et al., 2012; Digby, 1995; Schaffner et al., 1995), biparental care and alloparental infant care (Mota et al., 2006), pronounced stress responses to social disruption or separation from the partner (Rukstalis and French, 2005), and high levels of intrasexual aggression toward adult strangers (French and Inglett, 1989; Ross et al., 2004; Ross and French, 2011). Furthermore, and unlike prairie voles that display a consistently strong preference for their partner over an opposite-sex stranger (reviewed in: Johnson and Young, 2015; Young et al., 2011; Young and Wang, 2004; c.f. Ophir et al., 2008), marmosets display a more flexible pattern of sociosexual preferences (Cavanaugh et al., 2014; Smith et al., 2010). Furthermore, in some cases callitrichines are also polyandrous or polygynous (Dietz and Baker, 1993; Goldizen, 1988; Nievergelt, 2000; Saltzman et al., 2009). Other NWM such as titi and owl monkeys show similar patterns of behavior, but exhibit less extra-pair interactions and hence are ‘more’ socially monogamous than most callitrichines (Díaz-Muñoz and Bales, 2016; Fernandez-Duque, 2007; Spence-Aizenberg et al., 2015). Squirrel monkeys and capuchins, conversely, exhibit non-monogamous social systems. Squirrel monkeys live in large mixed-sex groups and exhibit seasonal sexual dimorphism and capuchins exhibit multi-male/multi-female hierarchical social organization (Boinski, 1987; Fedigan, 1993; Janson, 1986). While marmosets show flexible social relationships among adults, it is important to note that the marmoset mating system more closely resembles the more strict social monogamy in titi and owl monkeys than in nonmonogamous NWM. Ultimately, callitrichine social monogamy can be viewed as similar to the flexible mating strategies found among humans (Chapais, 2013).
6.1.1. Peripheral Measures of Neuropeptides and Affiliation
Studies of NWM examining the relationship between OT and pair bond formation/maintenance come in two main forms. The first strategy employs measuring peripheral concentrations of neuropeptides and correlating these neuropeptide concentrations with behaviors of interest. For example, basal concentrations of OT in tamarins (Saguinus) are correlated between pair-bonded male and females suggesting that these shared OT levels result from complementary levels of affiliative behavior within the pair. Similarly, the synchrony between basal OT concentrations and affiliation was strongest in marmoset dyads that had stronger social bonds (Finkenwirth et al., 2015). In both studies, it is important to note that OT synchrony was measured by the correlation in basal OT concentrations between partners in more vs. less affiliative dyads, and absolute OT concentrations were not correlated with affiliative behavior directly. Importantly, these neuropeptide-behavior relationships are not limited to any specific behavior, but rather neuropeptides are positively associated with a variety of affiliative behaviors involved in pair-bonds including sociosexual behavior, grooming, proximity, and food sharing. In general, the association between OT and affiliative behavior appears to be strongest when the quality of the social bond is strongest, and these studies complement findings of increased urinary excretion of OT metabolites and stronger social bonding in chimpanzees (Pan; Crockford et al., 2013; Wittig et al., 2014).
6.1.2. Intranasal Neuropeptides and Pair-bond Maintenance
A second strategy to evaluate the influence of neuropeptides on pair-bond formation and maintenance involves direct treatment with neuropeptide agonists and antagonists. Many studies on humans have used intranasal (IN) treatments of OT and AVP to study changes in behavior and have gone as far as to suggest that these neuropeptides may have therapeutic benefits with regard to some social deficits (Meyer-Lindenberg et al., 2011; Young and Barrett, 2015). Recently, researchers have adopted this methodology in NWM and have revealed important modulatory influences of neuropeptides on social behavior. Male and female marmosets treated with an IN Leu8-OT agonist showed enhanced affiliation toward pairmates during the first 3 weeks of cohabitation (Smith et al., 2010). Additionally marmosets treated with a nonpeptide OTa (L-368,899) reduced huddling behavior and food sharing with their partner over the same time period (Smith et al. 2010). Overall, the suppression of OT activity through OT antagonist treatments had a stronger impact on affiliation than IN OT agonists. These modest effects of the IN OT agonist may have been due, at least in part, to the use of Leu8-OT compound instead of Pro8-OT, which had yet to be identified in marmosets (Lee et al., 2011; Wallis, 2012).
Recent studies using the Pro8-OT variant have shown more robust effects on sociosexual behavior in marmosets. Treatment with IN Pro8-OT facilitated fidelity with a long-term partner by decreasing the amount of time marmosets spent in close proximity with an opposite-sex stranger, and increasing the latency to exhibit sociosexual behavior toward a stranger in a partner-preference paradigm (Cavanaugh et al., 2014). This effect was specific to the Pro8-OT variant, as treatment with Leu8-OT did not alter partner/stranger preferences. The effect of OT treatment produced differential effects in males and females. Females treated with Pro8-OT drastically reduced their time spent with a stranger in favor of interacting with their partner, while males treated with Pro8-OT spent less time with both the stranger and their partner (Cavanaugh et al., 2014). IN OT also modified social behavior by altering the behavior of untreated partners toward their treated partner. Female marmosets initiated and maintained proximity more often when their mate was treated with IN OT compared to when their mate was treated with a saline control, suggesting that OT makes male marmosets more ‘socially attractive’ (Cavanaugh et al., 2015). Unlike the measure of partner preference in marmosets, however, this effect was ligand-specific: females modified behavior toward male partners similarly when males were treated with either Pro8-OT or Leu8-OT. Overall, IN OT treatments in marmoset have different effects in affiliative behavior toward mates and strangers. IN OT enhances social contact and affiliation with mates and inhibits sociality toward strangers. Enhanced affiliation with a current breeding partner and decreased affiliation toward unfamiliar opposite-sex partners are two social responses that serve to enhance and maintain long-term pair bonds in marmosets.
To date, only one study has directly tested the role of AVP on pair-bond behavior in NWM (Jarcho et al., 2011). Untreated male titi monkeys showed greater interest in and contact with unfamiliar females than their long-term pairmates. However, when males were treated with a high dose of AVP, they increased affiliation with their long-term pairmate compared to opposite-sex strangers (Jarcho et al., 2011). This effect was absent in the low dose of AVP. Overall, high doses of intranasal AVP reduced male interactions with unfamiliar females and increased interactions with pair-bonded partners. This study only tested AVP-treated males, so it is currently unknown whether there are any sex differences in AVP-induced facilitation of social contact. In both cases, then, neuropeptide treatment (OT in the case of marmosets, AVP in the case of titi monkeys) both reduced interest in extrapair partners and enhanced sociality toward pairmates.
Many studies in socially monogamous mammals have found that neuropeptides modify partner preferences and affiliative contact behavior between partners, but pair bonds can be maintained through a variety of other behavioral mechanisms and the presence of a mate can even alter how individuals respond to stressors. Marmosets and titi monkeys, for example, show distress and activated HPA activity in response to isolation from their family or their pairmate, but the presence of a familiar conspecific can buffer an individual’s stress response (Hennessy et al., 1995; Rukstalis and French, 2005; Smith et al., 1998). OT is known to have ‘salubrious’ effects on social stress (Smith and Wang, 2012) and OT may underlie the suppression of HPA activity via social interactions (DeVries et al., 2003). Specifically, OT has an anti-stress effect in squirrel monkeys, with IN OT treated monkeys exhibiting lower ACTH concentrations compared to saline-treated monkeys 90 minutes following a social isolation stressors (Parker et al., 2005). Importantly, this effect was specific to ACTH, but not cortisol, suggesting the anti-stress effect of OT in squirrel monkeys is at the level of the hypothalamus and/or pituitary, and not via effects on adrenal responsivity to ACTH stimulation.
OT treatment also modulates how pair-bonded marmosets respond to stressors (Cavanaugh et al., 2016). The presence of a mate during a stressor significantly attenuated HPA-axis activity in females, but not males, relative to when marmosets experienced a stressor in complete isolation. However, blocking OT signaling in marmosets with an OTa led to significantly higher cortisol levels in males during the stressor, suggesting that OT may reduce the stress-induced elevations in cortisol levels. Additionally, male and female marmosets treated with an OTa spent significantly less time in close proximity to their mate during the stressor. These results suggest that the OT system may modulate HPA-axis activity by promoting the expression of proximity behavior with a close social partner (Cavanaugh et al., 2016). Whether this effect is socially-specific to a pair-bonded partner in marmosets and other NWM warrants further investigation, but this finding suggests that blocking, but not augmenting, the OT system modulates the social buffering effects deriving from the presence of a mate during a stressor in marmosets.
6.2. Parental Care and Motivation
OT mediates mother-infant attachments via both physiological mechanisms (e.g., uterine contraction, milk-let down) and also behavioral mechanisms (e.g., mother-infant bonding; (Rilling and Young, 2014). In cooperatively breeding primates like callitrichines and humans, fathers also participate in the rearing of offspring and form attachments with offspring. Studies on humans have shown increased concentrations of OT following interaction with their infants in both mothers and fathers (Feldman et al., 2010), suggesting that OT reinforces father-infant attachments despite the lack of direct exposure to the neuroendocrine consequences of infant suckling and lactation. In callitrichines, cascades of other neuroendocrine changes occur in both mothers and fathers following the birth of offspring. These effects include changes in estrogen, testosterone, and prolactin based on a variety of social contexts including parental experience and sex (Nunes et al., 2001, 2000; Storey and Ziegler, 2015)
To date, there have been only two studies to assess the effects of neuropeptide treatment on parental care and motivation in NWM (Saito and Nakamura, 2011; Taylor and French, 2015). Marmosets fathers that received intracerebroventricular infusion of both high and low doses of Leu8-OT increased the frequency of food transfer toward older offspring (via reduced refusal to share) and low doses of OT reduced their food refusals toward younger offspring (Saito and Nakamura, 2011). Treatment with Pro8-OT, AVP, and OT and V1aR antagonists also influenced parental responsiveness to infant stimuli in marmosets (Taylor and French, 2015). Both OT and AVP increased parental responsiveness, but in a sex-specific way. Pro8-OT enhanced responsiveness to infant stimuli in males, and AVP enhanced responsiveness to infant stimuli in females. Acute treatment with OTR and V1aR antagonists did not alter parental responsiveness in these studies. It may be that chronic administration of neuropeptide antagonists is required to alter behavioral responses in social contexts (Smith et al., 2010), or that the behavioral consequences of blocking OTR or V1aR are limited to specific behaviors or relationships.
Not surprisingly, there is evidence that parental experience itself can modify neuropeptide signaling. Compared to non-fathers, both first-time and experienced marmoset fathers have increased vasopressin V1aR density in neurons in the prefrontal cortex, as well as increases in the proportion of dendritic spines with V1aR (Kozorovitskiy et al., 2006). Marmoset fathers show high rates of offspring care for newborn and young infants, and rates decrease gradually throughout infant development (French, 2008), and V1aR density in fathers is negatively associated with offspring age. Together these findings suggest that receptor expression is plastic and highly dependent upon day-to-day changes in social interactions between fathers and infants. Experience-dependent changes have also been noted in the OT system in marmosets. Hypothalamic extracts from experienced marmoset fathers contained higher OT levels than those from paternally-inexperienced males (Woller et al., 2012). Thus, parental care is affected by experimentally modifying neuropeptide concentrations, and engaging in parental care (at least paternal care) serves as a potent stimulus to alter both neuropeptide synthesis and release, and receptor expression. This bidirectional relationship between neuropeptides and parental care in NWM therefore constitutes an important avenue to explore the complexity of neuroendocrine-behavior relationships in this primate group.
6.3. Prosocial Behavior and Cooperation
Cooperation is a hallmark trait of most human social relationships. However, the likelihood to engage in prosocial and cooperative behavior is highly flexible and varies considerably across different social contexts and experiences. Recent work has demonstrated that many nonhuman primates behave cooperatively or prosocially with others, even in the face of unequal or unfair outcomes (Brosnan, 2011; Brosnan and de Waal, 2014), yet very little is known about how these preferences vary based on neuroendocrine and social contexts across the diverse OT ligands and social systems found among NWM. Untreated marmosets (Burkart et al., 2007), tamarins (Cronin et al., 2010), and capuchins (Lakshminarayanan and Santos, 2008) have each demonstrated some form of spontaneous other-regarding preferences toward familiar partners (i.e., behavior motivated by benefitsto, and the welfare of, others). However, prosocial propensity in NWM is not always demonstrated (Cronin, 2012), and examining the contextual and neuroendocrine states in which prosocial behavior is expressed is an active research question.
Evidence suggests both OT and specific social contexts are critically important for the expression of prosocial preferences among primates (Brosnan et al., 2015; Chang et al., 2012; Mustoe et al., 2015). While many presume that the effect of OT generally enhances prosociality toward others, marmosets treated with IN OT actually reduced prosocial preferences but in a contextually-specific way (Mustoe et al., 2015). Marmosets exhibit high social tolerance and exhibit other-regarding preferences toward both familiar (Burkart et al., 2007) and unfamiliar partners (Mustoe et al., 2015). However, marmosets treated with IN Pro8-OT reduced ‘altruistic’ food sharing with opposite-sex strangers, but ‘altruistic’ food sharing with their long-term pairmates was unaffected. Reduced prosociality toward strangers was specific to the Pro8-OT variant, as treatment with Leu8-OT and OTa did not alter prosocial behavior. OT treatment may interact with the HPA axis to regulate prosociality, since the OT-mediated reduction in food-sharing with strangers was strongest when donor cortisol levels were lowest (Mustoe et al., 2015). Capuchins treated with a small IN dose of Leu8-OT showed reduced prosocial food sharing (Brosnan et al., 2015). The authors argue this reduction of prosociality following OT treatment was because the OT treatment reduced the typical congregating behavior of capuchins via reducing social proximity between partners (Brosnan et al., 2015). These findings in NWM are similar to social context-dependent effects of OT on prosocial responses in macaques (Chang et al., 2012), highlighting the important point that neuropeptide modulation of sociality does not occur in a vacuum and that context matters (Ebitz and Platt, 2013; Kelly and Ophir, 2015; Platt et al., 2016).
6.4 Limitations and Caveats Regarding Intranasal Neuropeptides
While the experimental manipulation of OT via agonists and antagonists strengthens causal interpretations of findings, there are recent reviews which have called into question the efficacy of IN OT treatments (Leng and Ludwig, 2015) and have cautioned against hasty interpretations of these studies (Walum et al., 2015). Among the concerns are the following:
1) Most studies using IN neuropeptides administer large doses that represent supraphysiological concentrations of neuropeptides, at least in the periphery, and doses are not always consistent across studies.
2) OT and AVP do not abundantly or easily penetrate the blood brain barrier, and it is estimated based on previous studies that, at best, only 0.005% of the IN administered neuropeptides accumulate in CSF within an hour (Leng and Ludwig, 2015). However, it could be the case that small concentrations of neuropeptides penetrating socially relevant brain regions may be sufficient to induce behavioral changes.
3) IN neuropeptides may be directly activating peripheral pathways, which in turn may account for many of the behavioral and motivational changes found following IN OT treatments (i.e., social anxiety, feeding and metabolism, etc.). Additionally it is also possible that that IN OT alters peripheral neural activity alone, and that peripheral transduction pathways may alter central OT release and/or activity via (Ferris et al., 2015; Neumann et al., 2013).
4) Peripheral measures of OT do not directly reflect central OT release, especially measures limited to only basal concentrations or measures in the absence of within-individual changes. Additionally the use of unextracted OT samples for immunoassays has been called into question (McCullough et al., 2013), and potential non-specific OT binding might be further complicated by variation in OT ligands in NWM.
The studies examining the effects of neuropeptides on social behavior in NWM are increasingly important because they repeatedly demonstrate that neuropeptides do not universally enhancing sociality and, in some cases, neuropeptides like OT have no significant influence on socially-relevant behavior (Mustoe et al., 2016). Rather these studies support the more nuanced view that neuropeptides alter sociality in ways that reflect species-specific social relationships and social contexts. Compared to the oversimplified viewed that OT is a “moral molecule” (Zak, 2012), this nuanced view more accurately reflects the ways in which neuropeptides can regulate complex and diverse social phenotypes found across birds, rodents, and primates (Beery, 2015; Bethlehem et al., 2014; Goodson, 2013; van Anders et al., 2013). From forming new social bonds to maintaining old social bonds, these differences in how OT modifies social behavior reflect differences in the salience and motivation underlying specific social relationships, and as a result, it is of the utmost importance to interpret the effects of OT on social behavior in the context of the social relationship (i.e., partner familiarity, social rank, sex; see excellent recent review on this point; Kelly and Ophir, 2015).
7. Conclusions
Without a doubt, the notion that eutherian mammals possess a common structure of the OT ligand can clearly be dismissed. Recent work on the molecular genetics of OXT in NWM indicates multiple nucleotide substitutions in the exonic region that codes for the mature nonapeptide, resulting in the expression of six OT variants. The discovery that different species in the same genus (Saguinus; Vargas-Pinilla et al., 2015) express unique OT variants hints at the possibility that yet additional forms may be present in this taxon. The phylogenetic analysis reveals nucleotide changes that predict distinct amino acids in three positions (2, 3, and 8), suggesting multiple and independent changes across species. It is equally clear that, in spite of the spatial proximity of OXT and AVP on the same chromosome and the common ancestry of these two genes, genomic and hence structural variation in nonapeptides is limited to OT: there are no NSs in AVP among NWM, and all express the common mammalian form of the mature neuropeptide. The reasons for the differential selective modification of OT and not AVP within NWM have yet to be identified.
This review has also identified important variation in the cognate receptors for OT and AVP in NWM, in ways that are linked to both ligand variation (in the case of OTR) and with social structure (in the case of V1aR). The most notable amino acid substitutions in the receptors are in those regions that involve ligand recognition and binding, and include additional substitutions in GPCR elements that mediate intracellular G protein signaling cascades. There are a host of unanswered questions regarding the importance of receptor variation for both neuropeptide systems, including differential affinity and efficacy of OT and AVP ligands when interacting with receptors, the formation of oligomeric forms of OTR-OTR and OTR-V1aR receptors (Devost and Zingg, 2004), and the potential for modified cell signaling cascades, including biased agonism (Kenakin, 2007). This latter potential is particularly intriguing, since both OTR and V1aR alter cell function through multiple G protein signaling routes.
The likely functional consequences of OT and AVP signaling in NWM for modulating behavioral features, especially social processes, is supported both by the widespread distribution of OTR and V1aR in important nodes in the Social Behavior Network, and by the handful of behavioral studies using nonapeptide agonists and antagonists. It is important to note that studies reported herein that map the distribution of oxytocinergic neurons and localize the OTR in Callithrix were conducted prior to the discovery of Pro8-OT in this species, and hence used antibodies and labeled agonists or antagonists optimized for species with Leu8-OT. Definitive knowledge regarding the distribution of neurons of origin for OT and AVP will require reagents that are selective for Pro8-OT and labeled ligands that are selectively bound by species-specific OTRs. Work is just now beginning in our laboratory to characterize the affinity and efficacy of OT ligand variants, AVP, and non-peptide agonists and antagonists against OTR and V1aR from multiple primate species. These studies will provide crucial information that can advise both future pharmacological and behavioral studies.
In the search for agonists and antagonists for altering the peripheral effects of OT and AVP with clinical applications (e.g., premature labor or hypertension), no stone has been left unturned in assessing the impact of ligand variation to assess structure-function relationships, with 100’s if not 1,000’s of compounds evaluated (see, for example, the list of compounds in Manning et al., 2012). However, this approach has not been widely applied in the study of neuropeptide modulation of neural function. In the fields of behavioral science and psychiatry, experimental and therapeutic approaches to normative and pathological states involving oxytocin manipulation have exclusively utilized Leu8-OT (reviews in Shamay-Tsoory and Young, 2016; Zik and Roberts, 2015). We suggest that the NWM represent an important ‘natural experiment’ in assessing the impact of ligand and receptor variation in nonapeptide signaling systems, and the consequent impact on social and behavioral outcomes. The tantalizing links we have identified in this review among OT ligand variability, OTR and V1aR structure, and the unique social phenotype among a majority of NWM all suggest that this group represent an important model that can be exploited to further our knowledge of the role of neurohypophyseal nonapeptides and sociality.
Supplementary Material
Highlights.
Multiple OT variants have been identified in New World monkeys.
OTR and V1aR phylogenies are associated with ligand variants and social phenotypes.
OT/AVP receptors overlap extensively with the social-decision making network.
OT/AVP modulate social behavior in a context- and ligand-specific fashion.
Acknowledgements
We thank members of the Callitrichid Research Center at the University of Nebraska at Omaha for support and comments on the materials and ideas covered in this paper. Elizabeth Genne-Bacon provided important impetus for pursuing the oxytocin system in New World monkeys. The research from the CRC described in this review was supported in part by funds from the National Institutes of Health (HD042882), the Nebraska Foundation, and from the Office of Research and Creative Activity at UNO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acher R. Molecular evolution of fish neurohypophysial hormones: Neutral and selective evolutionary mechanisms. Gen. Comp. Endocrinol. 1996;102:157–172. doi: 10.1006/gcen.1996.0057. [DOI] [PubMed] [Google Scholar]
- Acher R. Neurohypophysial peptide systems: processing machinery, hydroosmotic regulation, adaptation and evolution. Regul. Pept. 1993;45:1–13. doi: 10.1016/0167-0115(93)90174-7. [DOI] [PubMed] [Google Scholar]
- Acher R, Chauvet J, Chauvet MT. Man and the chimaera. Selective versus neutral oxytocin evolution. Adv. Exp. Med. Biol. 1994;395:615–627. [PubMed] [Google Scholar]
- Albers HE. Species, sex and individual differences in the vasotocin/vasopressin system: Relationship to neurochemical signaling in the social behavior neural network. Front. Neuroendocrinol. 2015;36:49–71. doi: 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb PL, Fernandez-Duque E, Schurr TG. Oxytocin receptor gene sequences in owl monkeys and other primates show remarkable interspecific regulatory and protein coding variation. Mol. Phylogenet. Evol. 2015 doi: 10.1016/j.ympev.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Bankir L. Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc. Res. 2001;51:372–390. doi: 10.1016/s0008-6363(01)00328-5. [DOI] [PubMed] [Google Scholar]
- Barberis C, Mouillac B, Durroux T. Structural bases of vasopressin/oxytocin receptor function. J. Endocrinol. 1998;156:223–229. doi: 10.1677/joe.0.1560223. [DOI] [PubMed] [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm. Behav. 2013;63:518–526. doi: 10.1016/j.yhbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK. Antisocial oxytocin: complex effects on social behavior. Curr. Opin. Behav. Sci. 2015;6:174–182. [Google Scholar]
- Beery AK, Lacey EA, Francis DD. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis) J. Comp. Neurol. 2008;507:1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- Beets I, Temmerman L, Janssen T, Schoofs L. Worm. Taylor & Francis; 2013. Ancient neuromodulation by vasopressin/oxytocin-related peptides; p. e24246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem RA, Baron-Cohen S, van Honk J, Auyeung B, Bos PA. The oxytocin paradox. Front. Behav. Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML, Panicker AK, Pedersen C, Petrusz P. Oxytocin receptors in non-human primate brain visualized with monoclonal antibody. Neuroreport. 2001;12:1723–1726. doi: 10.1097/00001756-200106130-00041. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–164. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- Boinski S. Mating patterns in squirrel monkeys (Saimiri oerstedi) Behav. Ecol. Sociobiol. 1987;21:13–21. [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Horm. Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Brosnan SF. A hypothesis of the co-evolution of cooperation and responses to inequity. Front. Neurosci. 2011;5 doi: 10.3389/fnins.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan SF, de Waal FBM. Evolution of responses to (un)fairness. Science. 2014;346:1251776–1251776. doi: 10.1126/science.1251776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan SF, Talbot CF, Essler JL, Leverett K, Flemming T, Dougall P, Heyler C, Zak PJ. Oxytocin reduces food sharing in capuchin monkeys by modulating social distance. Behaviour. 2015 doi: 10.1163/1568539X-00003268. [DOI] [Google Scholar]
- Burkart JM, Fehr E, Efferson C, van Schaik CP. Other-regarding preferences in a non-human primate: Common marmosets provision food altruistically. Proc. Natl. Acad. Sci. 2007;104:19762–19766. doi: 10.1073/pnas.0710310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffé AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J. Comp. Neurol. 1987;261:237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- Caffé AR, van Ryen PC, van der Woude TP, van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. J. Comp. Neurol. 1989;287:302–325. doi: 10.1002/cne.902870304. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee H-J, Macbeth AH, Young WS. Vasopressin: behavioral roles of an “original” neuropeptide. Prog. Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Young WS., III . Handbook of Neurochemistry and Molecular Neurobiology. Springer; 2006. Oxytocin and vasopressin: genetics and behavioral implications; pp. 573–607. [Google Scholar]
- Cavanaugh J, Carp SB, Rock CM, French JA. Oxytocin modulates behavioral and physiological responses to a stressor in marmoset monkeys. Psychoneuroendocrinology. 2016;66:22–30. doi: 10.1016/j.psyneuen.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Huffman MC, Harnisch AM, French JA. Marmosets treated with oxytocin are more socially attractive to their long-term mate. Front. Behav. Neurosci. 2015;9 doi: 10.3389/fnbeh.2015.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe AC, Taylor JH, French JA. Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology. 2014;49:1–10. doi: 10.1016/j.psyneuen.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc. Natl. Acad. Sci. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapais B. Monogamy, strongly bonded groups, and the evolution of human social structure. Evol. Anthropol. Issues News Rev. 2013;22:52–65. doi: 10.1002/evan.21345. [DOI] [PubMed] [Google Scholar]
- Chini B, Mouillac B, Ala Y, Balestre MN, Trumpp-Kallmeyer S, Hoflack J, Elands J, Hibert M, Manning M, Jard S. Tyr115 is the key residue for determining agonist selectivity in the V1a vasopressin receptor. EMBO J. 1995;14:2176. doi: 10.1002/j.1460-2075.1995.tb07211.x. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Mouillac B, Balestre M-N, Trumpp-Kallmeyer S, Hoflack J, Hibert M, Andriolo M, Pupier S, Jard S, Barberis C. Two aromatic residues regulate the response of the human oxytocin receptor to the partial agonist arginine vasopressin. FEBS Lett. 1996;397:201–206. doi: 10.1016/S0014-5793(96)01135-0. [DOI] [PubMed] [Google Scholar]
- Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbühler K, Deschner T. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B Biol. Sci. 2013;280:20122765. doi: 10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin KA. Prosocial behaviour in animals: the influence of social relationships, communication and rewards. Anim. Behav. 2012;84:1085–1093. [Google Scholar]
- Cronin KA, Schroeder KK, Snowdon CT. Prosocial behaviour emerges independent of reciprocity in cottontop tamarins. Proc. R. Soc. B Biol. Sci. 2010;277:3845–3851. doi: 10.1098/rspb.2010.0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devost D, Zingg HH. Homo-and Hetero-Dimeric Complex Formations of the Human Oxytocin Receptor. J. Neuroendocrinol. 2004;16:372–377. doi: 10.1111/j.0953-8194.2004.01188.x. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiol. Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ, Ruijs RM. The origin of vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- Díaz-Muñoz SL. Complex cooperative breeders: Using infant care costs to explain variability in callitrichine social and reproductive behavior. Am. J. Primatol. 2016;78:372–387. doi: 10.1002/ajp.22431. [DOI] [PubMed] [Google Scholar]
- Díaz-Muñoz SL, Bales KL. “Monogamy” in primates: Variability, trends, and synthesis. Am. J. Primatol. 2016;78:283–287. doi: 10.1002/ajp.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz JM, Baker AJ. Polygyny and female reproductive success in golden lion tamarins, Leontopithecus rosalia. Anim. Behav. 1993;46:1067–1078. [Google Scholar]
- Digby LJ. Social organization in a wild population of Callithrix jacchus: II. Intragroup social behavior. Primates. 1995;36:361–375. [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–4. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Immormino MA, Bredewold R, Veenema AH. Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition. Psychoneuroendocrinology. 2016;64:79–88. doi: 10.1016/j.psyneuen.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol. 2015 doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitz RB, Platt ML. An evolutionary perspective on the behavioral consequences of exogenous oxytocin application. Front. Behav. Neurosci. 2013;7 doi: 10.3389/fnbeh.2013.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple G. Social communication by olfactory signals in marmosets. Int. Zoo Yearb. 1972;12:36–42. [Google Scholar]
- Everts HGJ, Koolhaas JM. Lateral septum vasopressin in rats: role in social and object recognition? Brain Res. 1997;760:1–7. doi: 10.1016/s0006-8993(97)00269-2. [DOI] [PubMed] [Google Scholar]
- Fedigan L. Sex differences and intersexual relations in adult white---faced capuchins (Cebus capucinus) Int. J. Primatol. 1993;14 [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010;35:1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin pathway genes: Evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol. Psychiatry. 2016;79:174–184. doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E. Aotinae: Social monogamy in the only nocturnal haplorhines. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder S, editors. Primates in Perspective. Oxford University Press; New York: 2007. pp. 139–154. [Google Scholar]
- Fernandez-Duque E, Valeggia CR, Mendoza SP. The biology of paternal care in human and nonhuman primates. Annu. Rev. Anthropol. 2009;38:115–130. doi: 10.1146/annurev-anthro-091908-164334. [DOI] [Google Scholar]
- Ferris CF, Yee JR, Kenkel WM, Dumais KM, Moore K, Veenema AH, Kulkarni P, Perkybile AM, Carter CS. Distinct BOLD activation profiles following central and peripheral oxytocin administration in awake rats. Front. Behav. Neurosci. 2015;9 doi: 10.3389/fnbeh.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkenwirth C, van Schaik C, Ziegler TE, Burkart JM. Strongly bonded family members in common marmosets show synchronized fluctuations in oxytocin. Physiol. Behav. 2015;151:246–251. doi: 10.1016/j.physbeh.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliers E, Guldenaar SEF, van den Wal N, Swaab DB. Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Res. 1986;375:363–367. doi: 10.1016/0006-8993(86)90759-6. [DOI] [PubMed] [Google Scholar]
- Fliers E, Swaab DF, Pool CW, Verwer RWH. The vasopressin and oxytocin neurons in the human supraoptic and paraventricular nucleus; changes with aging and in senile dementia. Brain Res. 1985;342:45–53. doi: 10.1016/0006-8993(85)91351-4. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014a;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014b;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Fite JA, Ross CN. Family life in marmosets: causes and consequences of variation in offspring care. In: Bridges RS, editor. The Parental Brain. Elsevier Press; New York: 2008. pp. 461–478. [Google Scholar]
- French JA, Inglett BJ. Female-female aggression and male indifference in response to unfamiliar intruders in lion tamarins. Anim. Behav. 1989;37:487–497. [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Ciruela F, Manger P, Leo G, Díaz-Cabiale Z, Agnati LF. On the role of volume transmission and receptor–receptor interactions in social behaviour: focus on central catecholamine and oxytocin neurons. Brain Res. 2012;1476:119–131. doi: 10.1016/j.brainres.2012.01.062. [DOI] [PubMed] [Google Scholar]
- Geisler I, Chmielewski J. Cationic amphiphilic polyproline helices: Side-chain variations and cell-specific internalization. Chem. Biol. Drug Des. 2009;73:39–45. doi: 10.1111/j.1747-0285.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Goldizen AW. Tamarin and marmoset mating systems: unusual flexibility. Trends Ecol. Evol. 1988;3:36–40. doi: 10.1016/0169-5347(88)90045-6. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Deconstructing sociality, social evolution and relevant nonapeptide functions. Psychoneuroendocrinology. 2013;38:465–478. doi: 10.1016/j.psyneuen.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front Neuroendocr. 2009;30:429–41. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Stemmelin J, Gal CS, Steinberg R. The vasopressin V1b receptor as a therapeutic target in stress-related disorders. Curr Drug Targets CNS Neurol Disord. 2003;2:191–200. doi: 10.2174/1568007033482850. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Sophie Knobloch-Bollmann H, Eliava M, Busnelli M, Chini B. Assembling the puzzle: Pathways of oxytocin signaling in the brain. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Gruber CW. Physiology of invertebrate oxytocin and vasopressin neuropeptides. Exp. Physiol. 2014;99:55–61. doi: 10.1113/expphysiol.2013.072561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber CW, Koehbach J, Muttenthaler M. Exploring bioactive peptides from natural sources for oxytocin and vasopressin drug discovery. Future Med. Chem. 2012;4:1791–1798. doi: 10.4155/fmc.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwee P-C, Amemiya CT, Brenner S, Venkatesh B. Sequence and organization of coelacanth neurohypophysial hormone genes: evolutionary history of the vertebrate neurohypophysial hormone gene locus. BMC Evol. Biol. 2008;8:93. doi: 10.1186/1471-2148-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwee P-C, Tay B-H, Brenner S, Venkatesh B. Characterization of the neurohypophysial hormone gene loci in elephant shark and the Japanese lamprey: origin of the vertebrate neurohypophysial hormone genes. BMC Evol. Biol. 2009;9:47. doi: 10.1186/1471-2148-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawtin SR, Wesley VJ, Simms J, Argent CCH, Latif K, Wheatley M. The N-terminal juxtamembrane segment of the V1a vasopressin receptor provides two independent epitopes required for high-affinity agonist binding and signaling. Mol. Endocrinol. 2005;19:2871–2881. doi: 10.1210/me.2005-0148. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Mendoza SP, Mason WA, Moberg GP. Endocrine sensitivity to novelty in squirrel monkeys and titi monkeys: species differences in characteristic modes of responding to the environment. Physiol. Behav. 1995;57:331–338. doi: 10.1016/0031-9384(94)00250-9. [DOI] [PubMed] [Google Scholar]
- Hoare S, Copland JA, Strakova Z, Ives K, Jeng Y-J, Hellmich MR, Soloff MS. The proximal portion of the COOH terminus of the oxytocin receptor is required for coupling to gq, but not gi independent mechanisms for elevating intracellular calcium concentrations from intracellular stores. J. Biol. Chem. 1999;274:28682–28689. doi: 10.1074/jbc.274.40.28682. [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Beery AK, Blumstein DT, Couzin ID, Earley RL, Hayes LD, Hurd PL, Lacey EA, Phelps SM, Solomon NG. An evolutionary framework for studying mechanisms of social behavior. Trends Ecol. Evol. 2014;29:581–589. doi: 10.1016/j.tree.2014.07.008. others. [DOI] [PubMed] [Google Scholar]
- Ichimiya Y, Emson PC, Shaw FD. Localization of vasopressin mRNA-containing neurons in the hypothalamus of the monkey. Mol. Brain Res. 1988;4:81–85. doi: 10.1016/0169-328x(88)90022-8. [DOI] [PubMed] [Google Scholar]
- Innamorati G, Sadeghi HM, Tran NT, Birnbaumer M. A serine cluster prevents recycling of the V2 vasopressin receptor. Proc. Natl. Acad. Sci. 1998;95:2222–2226. doi: 10.1073/pnas.95.5.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. 1992;89:5981. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus; size changes in relation to age and sex. J. Clin. Endocrinol. Metab. 1999;84:4637–4644. doi: 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- Janson CH. The mating system as a determinant of social evolution in capuchin monkeys (Cebus) Primate Ecol. Conserv. 1986;2:169–179. [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain Behav. 2011;10:375–383. doi: 10.1111/j.1601-183X.2010.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair bonding. Curr. Opin. Behav. Sci. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata M, Sano Y. Immunological identification of the oxytocin and vasopressin neurons in the hypothalamus of the monkey (Macaca fuscata) Anat. Embryol. (Berl.) 1982;165:151–167. doi: 10.1007/BF00305474. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL. Social functions of individual vasopressin–oxytocin cell groups in vertebrates: What do we really know? Front. Neuroendocrinol. 2014;35:512–529. doi: 10.1016/j.yfrne.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Ophir AG. Compared to what: what can we say about nonapeptide function and social behavior without a frame of reference? Curr. Opin. Behav. Sci. 2015;6:97–103. doi: 10.1016/j.cobeha.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Functional selectivity through protean and biased agonism: who steers the ship? Mol. Pharmacol. 2007;72:1393–1401. doi: 10.1124/mol.107.040352. [DOI] [PubMed] [Google Scholar]
- Kendrick K. Oxytocin regulation of sheep social. In: Choleris E, Pfaff DW, Kavaliers M, editors. Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior. 2013. pp. 183–192. [Google Scholar]
- Kiss A, Mikkelsen JD. Oxytocin–anatomy and functional assignments: a minireview. Endocr. Regul. 2005;39:97–105. [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q. Rev. Biol. 1977:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Front. Behav. Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehbach J, Stockner T, Bergmayr C, Muttenthaler M, Gruber CW. Insights into the molecular evolution of oxytocin receptor ligand binding. Biochem Soc Trans. 2013;41:197–204. doi: 10.1042/BST20120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat. Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan VR, Santos LR. Capuchin monkeys are sensitive to others’ welfare. Curr. Biol. 2008;18:R999–R1000. doi: 10.1016/j.cub.2008.08.057. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee AG, Cool DR, Grunwald WC, Neal DE, Buckmaster CL, Cheng MY, Hyde SA, Lyons DM, Parker KJ. A novel form of oxytocin in New World monkeys. Biol. Lett. 2011;7:584–587. doi: 10.1098/rsbl.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Macbeth AH, Pagani JH, Young WS., III Oxytocin: The great facilitator of life. Prog. Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Intranasal oxytocin: myths and delusions. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J. Comp. Neurol. 2004;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Liutkeviciute Z, Gruber CW. Evolutionary aspects of physiological function and molecular diversity of the oxytocin/vasopressin signaling system. Molecular Neuroendocrinololgy: From Genome to Physiology. 2015 [Google Scholar]
- LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol. Psychiatry. 2014;20:640–646. doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss JJ. Localization of oxytocin binding sites in the human brainstem and upper spinal cord: an autoradiographic study. Brain Res. 1989;500:223–230. doi: 10.1016/0006-8993(89)90317-x. [DOI] [PubMed] [Google Scholar]
- Loyens E, Vermoesen K, Schallier A, Michotte Y, Smolders I. Proconvulsive effects of oxytocin in the generalized pentylenetetrazol mouse model are mediated by vasopressin 1a receptors. Brain Res. 2012;1436:43–50. doi: 10.1016/j.brainres.2011.11.059. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lukas D, Clutton-Brock TH. The evolution of social monogamy in mammals. Science. 2013;341:526–530. doi: 10.1126/science.1238677. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Veenema AH, Neumann ID. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: Male juvenile versus female adult conspecifics. Psychoneuroendocrinology. 2013;38:916–926. doi: 10.1016/j.psyneuen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol. 2012;24:609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov GV, Paris M, Bertrand S, Laudet V. The evolution of the ligand/receptor couple: A long road from comparative endocrinology to comparative genomics. Mol. Cell. Endocrinol. 2008;293:5–16. doi: 10.1016/j.mce.2008.06.011. [DOI] [PubMed] [Google Scholar]
- McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 2013;37:1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mooney SJ, Coen CW, Holmes MM, Beery AK. Region-specific associations between sex, social status, and oxytocin receptor density in the brains of eusocial rodents. Neuroscience. 2015;303:261–269. doi: 10.1016/j.neuroscience.2015.06.043. [DOI] [PubMed] [Google Scholar]
- Mouillac B, Chini B, Balestre M-N, Elands J, Trumpp-Kallmeyer S, Hoflack J, Hibert M, Jard S, Barberis C. The binding site of neuropeptide vasopressin V1a receptor: Evidence for a major localization within transmembrane regions. J. Biol. Chem. 1995;270:25771–25777. doi: 10.1074/jbc.270.43.25771. [DOI] [PubMed] [Google Scholar]
- Mustoe AC, Cavanaugh J, Harnisch AM, Thompson BE, French JA. Do marmosets care to share? Oxytocin treatment reduces prosocial behavior toward strangers. Horm. Behav. 2015;71:83–90. doi: 10.1016/j.yhbeh.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe AC, Harnisch AM, Hochfelder B, Cavanaugh J, French JA. Inequity aversion strategies between marmosets are influenced by partner familiarity and sex but not by oxytocin. Anim. Behav. 2016;114:69–79. doi: 10.1016/j.anbehav.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior a node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM. Genetic analysis of group composition and breeding system in a wild common marmoset (Callithrix jacchus) population. Int. J. Primatol. 2000;21:1–20. [Google Scholar]
- Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii) Anim. Behav. 2000;60:857–865. doi: 10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii) Horm. Behav. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-β-arrestin complexes after receptor endocytosis. J. Biol. Chem. 2001;276:19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336:1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Omasits U, Ahrens CH, Muller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30:884–886. doi: 10.1093/bioinformatics/btt607. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng D-J, Phelps SM. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm. Behav. 2012;61:445–453. doi: 10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, Wolff JO. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Anim. Behav. 2008;75:1143–1154. [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30:924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Perelman P, Johnson WE, Roos C, Seuánez HN, Horvath JE, Moreira MA, Kessing B, Pontius J, Roelke M, Rumpler Y. A molecular phylogeny of living primates. PLoS Genet. 2011;7:e1001342. doi: 10.1371/journal.pgen.1001342. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Seyfarth RM, Cheney DL. Adaptations for social cognition in the primate brain. Phil Trans R Soc B. 2016;371:20150096. doi: 10.1098/rstb.2015.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Chin KR, French JA. Molecular Variation in AVP and AVPR1a in New World Monkeys (Primates, Platyrrhini): Evolution and Implications for Social Monogamy. PLoS ONE. 2014;9:e111638. doi: 10.1371/journal.pone.0111638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Lu G, Moriyama H, Mustoe AC, Harrison EB, French JA. Genetic Diversity in Oxytocin Ligands and Receptors in New World Monkeys. PLoS ONE. 2015;10:e0125775. doi: 10.1371/journal.pone.0125775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger AL. Evolution of feeding niches in New World monkeys. Am. J. Phys. Anthropol. 1992;88:525–562. doi: 10.1002/ajpa.1330880408. [DOI] [PubMed] [Google Scholar]
- Ross CN, French JA. Female marmosets’ behavioral and hormonal responses to unfamiliar intruders. Am. J. Primatol. 2011;73:1072–1081. doi: 10.1002/ajp.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CN, French JA, Patera KJ. Intensity of aggressive interactions modulates testosterone in male marmosets. Physiol. Behav. 2004;83:437–445. doi: 10.1016/j.physbeh.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, French JA. Vocal buffering of the stress response: exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Horm. Behav. 2005;47:1–7. doi: 10.1016/j.yhbeh.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Nakamura K. Oxytocin changes primate paternal tolerance to offspring in food transfer. J. Comp. Physiol. A. 2011;197:329–337. doi: 10.1007/s00359-010-0617-2. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Digby LJ, Abbott DH. Reproductive skew in female common marmosets: what can proximate mechanisms tell us about ultimate causes? Proc. R. Soc. B Biol. Sci. 2009;276:389. doi: 10.1098/rspb.2008.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer WH. Evolution of active neurohypophysial principles among the vertebrates. Am. Zool. 1977;17:727–737. [Google Scholar]
- Schneider H, Sampaio I. The systematics and evolution of New World primates–A review. Mol. Phylogenet. Evol. 2015;82:348–357. doi: 10.1016/j.ympev.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupré A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci. Lett. 2009;461:217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupré A, Ritchie J, Sorge RE, Crawley JN, Hu S-B, Nishimori K, Young LJ. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J. Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S, Young LJ. Understanding the Oxytocin System and Its Relevance to Psychiatry. Biol. Psychiatry. 2016;79:150. doi: 10.1016/j.biopsych.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm. Behav. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Avram SW, Cymerblit-Sabba A, Song J, Young WS. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol. Psychiatry. 2016 doi: 10.1038/mp.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Horm. Behav. 2012;61:320–330. doi: 10.1016/j.yhbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Jungers WL. Body mass in comparative primatology. J. Hum. Evol. 1997;32:523–559. doi: 10.1006/jhev.1996.0122. [DOI] [PubMed] [Google Scholar]
- Smith TE, McGreer-Whitworth B, French JA. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (Callithrix kuhli) Horm. Behav. 1998;34:211–222. doi: 10.1006/hbeh.1998.1469. [DOI] [PubMed] [Google Scholar]
- Smith TE, Tomlinson AJ, Mlotkiewicz JA, Abbott DH. Female marmoset monkeys (Callithrix jacchus) can be identified from the chemical composition of their scent marks. Chem. Senses. 2001;26:449–458. doi: 10.1093/chemse/26.5.449. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Morphology of vasopressin and oxytocin neurons and their central and vascular projections. Prog. Brain Res. 1983;60:101–114. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill JK, Larkin TE, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence-Aizenberg A, Di Fiore A, Fernandez-Duque E. Social monogamy, male-female relationships, and biparental care in wild titi monkeys (Callicebus discolor) Primates. 2015 doi: 10.1007/s10329-015-0489-8. [DOI] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK. The vasopressin 1b receptor and the neural regulation of social behavior. Horm. Behav. 2012;61:277–282. doi: 10.1016/j.yhbeh.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin in the central nervous system as a basis for their rapid behavioral effects. Curr. Opin. Neurobiol. 2014;29:187–193. doi: 10.1016/j.conb.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Storey AE, Ziegler TE. Primate paternal care: Interactions between biology and social experience. Horm. Behav. 2015 doi: 10.1016/j.yhbeh.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhov RR, Walker LC, Rance NE, Price DL, Young WS., III Vasopressin and oxytocin gene expression in the human hypothalamus. J. Comp. Neurol. 1993;337:295–306. doi: 10.1002/cne.903370210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, French JA. Oxytocin and vasopressin enhance responsiveness to infant stimuli in adult marmosets. Horm. Behav. 2015;75:154–159. doi: 10.1016/j.yhbeh.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibonnier M, Coles P, Conarty DM, Plesnicher CL, Shoham M. A Molecular Model of Agonist and Nonpeptide Antagonist Binding to the Human V1 Vascular Vasopressin Receptor. J. Pharmacol. Exp. Ther. 2000;294:195–203. [PubMed] [Google Scholar]
- Toloczko DM, Young LJ, Insel TR. Are there oxytocin receptors in the primate brain? Ann. N. Y. Acad. Sci. 1997;807:506–509. doi: 10.1111/j.1749-6632.1997.tb51953.x. [DOI] [PubMed] [Google Scholar]
- Ueda S, Kawata M, Sano Y. Identification of serotonin- and vasopressin immunoreactivities in the suprachiasmatic nucleus of four mammalian species. Cell Tissue Res. 1983;234:237–248. doi: 10.1007/BF00213766. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Goodson JL, Kingsbury MA. Beyond“ oxytocin= good”: neural complexities and the flipside of social bonds. Arch. Sex. Behav. 2013;42:1115. doi: 10.1007/s10508-013-0134-9. [DOI] [PubMed] [Google Scholar]
- Vargas-Pinilla P, Paixão-Côrtes VR, Paré P, Tovo-Rodrigues L, Vieira CM, de AG, Xavier A, Comas D, Pissinatti A, Sinigaglia M, Rigo MM. Evolutionary pattern in the OXT-OXTR system in primates: Coevolution and positive selection footprints. Proc. Natl. Acad. Sci. 2015;112:88–93. doi: 10.1073/pnas.1419399112. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm. Behav. 2010;58:273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Waite GN, Geib RW, King MW. Neuropeptides as biologial system integrators: mini review. Biomed. Sci. Instrum. 2014;50:1–11. [PubMed] [Google Scholar]
- Wallis M. Molecular evolution of the neurohypophysial hormone precursors in mammals: Comparative genomics reveals novel mammalian oxytocin and vasopressin analogues. Gen. Comp. Endocrinol. 2012;179:313–318. doi: 10.1016/j.ygcen.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Walum H, Waldman ID, Young LJ. Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Moody K, Newman JD, Insel TR. Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus) Synapse. 1997a;27:14–25. doi: 10.1002/(SICI)1098-2396(199709)27:1<14::AID-SYN2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wang Z, Toloczko D, Young LJ, Moody K, Newman JD, Insel TR. Vasopressin in the forebrain of common marmosets (Callithrix jacchus): studies with in situ hybridization, immunocytochemistry and receptor autoradiography. Brain Res. 1997b;768:147–156. doi: 10.1016/s0006-8993(97)00636-7. [DOI] [PubMed] [Google Scholar]
- Wesley VJ, Hawtin SR, Howard HC, Wheatley M. Agonist-specific, high-affinity binding epitopes are contributed by an arginine in the N-terminus of the human oxytocin receptor. Biochemistry (Mosc.) 2002;41:5086–5092. doi: 10.1021/bi015990v. [DOI] [PubMed] [Google Scholar]
- Wierda M, Goudsmit E, Van der Woude PF, Purba JS, Hofman MA, Bogte H, Swaab DF. Oxytocin cell number in the human paraventricular nucleus remains constant with aging and in Alzheimer’s disease. Neurobiol. Aging. 1991;12:511–516. doi: 10.1016/0197-4580(91)90081-t. [DOI] [PubMed] [Google Scholar]
- Wildman DE, Jameson NM, Opazo JC, Soojin VY. A fully resolved genus level phylogeny of neotropical primates (Platyrrhini) Mol. Phylogenet. Evol. 2009;53:694–702. doi: 10.1016/j.ympev.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Wircer E, Ben-Dor S, Levkowitz G. Non-Mammalian Models for Neurohypophysial Peptides. Mol. Neuroendocr. Genome Physiol. 2015;301 [Google Scholar]
- Wittig RM, Crockford C, Deschner T, Langergraber KE, Ziegler TE, Zuberbühler K. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B Biol. Sci. 2014;281:20133096. doi: 10.1098/rspb.2013.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woller MJ, Sosa ME, Chiang Y, Prudom SL, Keelty P, Moore JE, Ziegler TE. Differential hypothalamic secretion of neurocrines in male common marmosets: parental experience effects? J. Neuroendocrinol. 2012;24:413–421. doi: 10.1111/j.1365-2826.2011.02252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Kitano T. Molecular evolution of the oxytocin–oxytocin receptor system in eutherians. Mol. Phylogenet. Evol. 2013;67:520–528. doi: 10.1016/j.ympev.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Gainer H. Encyclopedia of Neuroscience. Academic Press; Oxford: 2009. Vasopressin/oxytocin and receptors; pp. 51–59. [Google Scholar]
- Young WS, 3rd, Gainer H. Transgenesis and the study of expression, cellular targeting and function of oxytocin, vasopressin and their receptors. Neuroendocrinology. 2003;78:185–203. doi: 10.1159/000073702. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: Insights from a socially monogamous rodent. Front. Neuroendocrinol. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Barrett CE. Can oxytocin treat autism?: We are still at an early stage of assessing oxytocin-based therapy for autism spectrum disorders. Sci. N. Y. NY. 2015;347:825. doi: 10.1126/science.aaa8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J. Neuroendocrinol. 1999;11:291–297. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zak PJ. The moral molecule: The source of love and prosperity. Random House; 2012. [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Rates of conservative and radical nonsynonymous nucleotide substitutions in mammalian nuclear genes. J. Mol. Evol. 2000;50:56–68. doi: 10.1007/s002399910007. [DOI] [PubMed] [Google Scholar]
- Ziegler TE. Social Effects via Olfactory Sensory Stimuli on Reproductive Function and Dysfunction in Cooperative Breeding Marmosets and Tamarins: Role of Scent in Reproductive Success. Am. J. Primatol. 2013;75:202–211. doi: 10.1002/ajp.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zik JB, Roberts DL. The many faces of oxytocin: Implications for psychiatry. Psychiatry Res. 2015;226:31–37. doi: 10.1016/j.psychres.2014.11.048. [DOI] [PubMed] [Google Scholar]
- Zingg HH, Laporte SA. The oxytocin receptor. Trends Endocrinol. Metab. 2003;14:222–227. doi: 10.1016/s1043-2760(03)00080-8. [DOI] [PubMed] [Google Scholar]
- Zoicas I, Slattery DA, Neumann ID. Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology. 2014;39:3027–3035. doi: 10.1038/npp.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.