Abstract
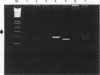
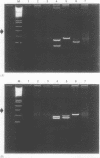
AIMS--To evaluate polymerase chain reaction (PCR) amplification of T cell receptor (TCR) beta and gamma chain genes as a means of demonstrating monoclonality in T cell lymphomas using histological samples; to compare the performance of PCR with Southern blot analysis. METHODS--TCR-beta, TCR-gamma and immunoglobulin heavy chain (IGH) genes were analysed using PCR in 55 cases of T cell lymphoma (28 frozen tissue and 27 paraffin wax embedded samples), diagnosed using morphological and immunohistochemical criteria. The 28 frozen samples were subjected to Southern blot analysis using TCR-beta, TCR-gamma and IGH gene probes. Twenty five B cell lymphomas and 21 non-neoplastic lymphoid tissue samples were used as controls. RESULTS--Using TCR-beta PCR, monoclonality was detected in 24 (44%) of 55 T cell lymphomas compared with 43 (78%) of 55 using TCR-gamma PCR and in 82% with both techniques. Five (9%) of 55 T cell lymphomas were IGH PCR positive. None of the non-neoplastic lymphoid control samples were PCR positive. All B cell lymphomas showed a polyclonal pattern with TCR-beta PCR while a single B cell lymphoma was positive using TCR-gamma primers. With TCR-beta PCR, a monoclonal result was seen in 12 (43%) of 28 frozen samples of T cell lymphoma, compared with 23 (82%) of 28 using Southern blot analysis. With TCR-gamma PCR, 19 (68%) of 28 frozen tissue samples were positive, compared with 26 (93%) of 28 using Southern blot analysis. A single case showed IGH rearrangement by Southern blot analysis. CONCLUSION--TCR-gamma PCR should be the method of choice for analysis of clonality in paraffin wax embedded sections of lymphoproliferative lesions, as TCR-beta PCR has a high false negative rate. Southern blot analysis remains the most successful technique when sufficient fresh tissue samples and resources are available.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertness V., Kirsch I., Hollis G., Johnson B., Bunn P. A., Jr T-cell receptor gene rearrangements as clinical markers of human T-cell lymphomas. N Engl J Med. 1985 Aug 29;313(9):534–538. doi: 10.1056/NEJM198508293130902. [DOI] [PubMed] [Google Scholar]
- Bourguin A., Tung R., Galili N., Sklar J. Rapid, nonradioactive detection of clonal T-cell receptor gene rearrangements in lymphoid neoplasms. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8536–8540. doi: 10.1073/pnas.87.21.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossman J., Uppenkamp M., Sundeen J., Coupland R., Raffeld M. Molecular genetics and the diagnosis of lymphoma. Arch Pathol Lab Med. 1988 Feb;112(2):117–127. [PubMed] [Google Scholar]
- Diss T. C., Pan L., Peng H., Wotherspoon A. C., Isaacson P. G. Sources of DNA for detecting B cell monoclonality using PCR. J Clin Pathol. 1994 Jun;47(6):493–496. doi: 10.1136/jcp.47.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller A. C., Griesser H., Schilling C. V., Wacker H. H., Dallenbach F., Bartels H., Kuse R., Mak T. W., Lennert K. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988 Dec;133(3):549–556. [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. G., Rabbitts T. H. The sequence of a human immunoglobulin epsilon heavy chain constant region gene, and evidence for three non-allelic genes. EMBO J. 1982;1(5):655–660. doi: 10.1002/j.1460-2075.1982.tb01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flug F., Pelicci P. G., Bonetti F., Knowles D. M., 2nd, Dalla-Favera R. T-cell receptor gene rearrangements as markers of lineage and clonality in T-cell neoplasms. Proc Natl Acad Sci U S A. 1985 May;82(10):3460–3464. doi: 10.1073/pnas.82.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudie R. B., Karim S. N., Mills K., Alcorn M., Lee F. D. A sensitive method of screening for dominant T cell clones by amplification of T cell gamma gene rearrangements with the polymerase chain reaction. J Pathol. 1990 Nov;162(3):191–196. doi: 10.1002/path.1711620304. [DOI] [PubMed] [Google Scholar]
- Greenberg J. M., Quertermous T., Seidman J. G., Kersey J. H. Human T cell gamma-chain gene rearrangements in acute lymphoid and nonlymphoid leukemia: comparison with the T cell receptor beta-chain gene. J Immunol. 1986 Sep 15;137(6):2043–2049. [PubMed] [Google Scholar]
- Greiner T. C., Raffeld M., Lutz C., Dick F., Jaffe E. S. Analysis of T cell receptor-gamma gene rearrangements by denaturing gradient gel electrophoresis of GC-clamped polymerase chain reaction products. Correlation with tumor-specific sequences. Am J Pathol. 1995 Jan;146(1):46–55. [PMC free article] [PubMed] [Google Scholar]
- Gross G. G., Schwartz V. L., Stevens C., Ebert E. C., Blumberg R. S., Balk S. P. Distribution of dominant T cell receptor beta chains in human intestinal mucosa. J Exp Med. 1994 Oct 1;180(4):1337–1344. doi: 10.1084/jem.180.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Stein H., Banks P. M., Chan J. K., Cleary M. L., Delsol G., De Wolf-Peeters C., Falini B., Gatter K. C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994 Sep 1;84(5):1361–1392. [PubMed] [Google Scholar]
- Haynes B. F., Hensley L. L., Jegasothy B. V. Phenotypic characterization of skin-infiltrating T cells in cutaneous T-cell lymphoma: comparison with benign cutaneous T-cell infiltrates. Blood. 1982 Aug;60(2):463–473. [PubMed] [Google Scholar]
- Kanavaros P., Farcet J. P., Gaulard P., Haioun C., Divine M., Le Couedic J. P., Lefranc M. P., Reyes F. Recombinative events of the T cell antigen receptor delta gene in peripheral T cell lymphomas. J Clin Invest. 1991 Feb;87(2):666–672. doi: 10.1172/JCI115044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchingman G. R., Rovigatti U., Mauer A. M., Melvin S., Murphy S. B., Stass S. Rearrangement of immunoglobulin heavy chain genes in T cell acute lymphoblastic leukemia. Blood. 1985 Mar;65(3):725–729. [PubMed] [Google Scholar]
- LeFranc M. P., Forster A., Baer R., Stinson M. A., Rabbitts T. H. Diversity and rearrangement of the human T cell rearranging gamma genes: nine germ-line variable genes belonging to two subgroups. Cell. 1986 Apr 25;45(2):237–246. doi: 10.1016/0092-8674(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Lefranc M. P., Chuchana P., Dariavach P., Nguyen C., Huck S., Brockly F., Jordan B., Lefranc G. Molecular mapping of the human T cell receptor gamma (TRG) genes and linkage of the variable and constant regions. Eur J Immunol. 1989 Jun;19(6):989–994. doi: 10.1002/eji.1830190606. [DOI] [PubMed] [Google Scholar]
- Lefranc M. P., Forster A., Rabbitts T. H. Rearrangement of two distinct T-cell gamma-chain variable-region genes in human DNA. 1986 Jan 30-Feb 5Nature. 319(6052):420–422. doi: 10.1038/319420a0. [DOI] [PubMed] [Google Scholar]
- Lorenzen J., Jux G., Zhao-Höhn M., Klöckner A., Fischer R., Hansmann M. L. Detection of T-cell clonality in paraffin-embedded tissues. Diagn Mol Pathol. 1994 Jun;3(2):93–99. doi: 10.1097/00019606-199406000-00005. [DOI] [PubMed] [Google Scholar]
- Macintyre E. A., d'Auriol L., Duparc N., Leverger G., Galibert F., Sigaux F. Use of oligonucleotide probes directed against T cell antigen receptor gamma delta variable-(diversity)-joining junctional sequences as a general method for detecting minimal residual disease in acute lymphoblastic leukemias. J Clin Invest. 1990 Dec;86(6):2125–2135. doi: 10.1172/JCI114951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. P., Sloane J. P., Kabarowski J. H., Matutes E., Wiedemann L. M. A simplified method of detection of clonal rearrangements of the T-cell receptor-gamma chain gene. Diagn Mol Pathol. 1992 Sep;1(3):173–179. [PubMed] [Google Scholar]
- McCarthy K. P., Sloane J. P., Kabarowski J. H., Matutes E., Wiedemann L. M. The rapid detection of clonal T-cell proliferations in patients with lymphoid disorders. Am J Pathol. 1991 Apr;138(4):821–828. [PMC free article] [PubMed] [Google Scholar]
- Pelicci P. G., Knowles D. M., 2nd, Dalla Favera R. Lymphoid tumors displaying rearrangements of both immunoglobulin and T cell receptor genes. J Exp Med. 1985 Sep 1;162(3):1015–1024. doi: 10.1084/jem.162.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnett D. N., Sinha R., Kabak S., Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to "benign monoclonal gammapathy". J Exp Med. 1994 Feb 1;179(2):609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. E., Tunnacliffe A., Smith W. J., Rabbitts T. H. Complexity of human T-cell antigen receptor beta-chain constant- and variable-region genes. Nature. 1984 Dec 6;312(5994):541–545. doi: 10.1038/312541a0. [DOI] [PubMed] [Google Scholar]
- Slack D. N., McCarthy K. P., Wiedemann L. M., Sloane J. P. Evaluation of sensitivity, specificity, and reproducibility of an optimized method for detecting clonal rearrangements of immunoglobulin and T-cell receptor genes in formalin-fixed, paraffin-embedded sections. Diagn Mol Pathol. 1993 Dec;2(4):223–232. [PubMed] [Google Scholar]
- Theodorou I., Raphaël M., Bigorgne C., Fourcade C., Lahet C., Cochet G., Lefranc M. P., Gaulard P., Farcet J. P. Recombination pattern of the TCR gamma locus in human peripheral T-cell lymphomas. J Pathol. 1994 Dec;174(4):233–242. doi: 10.1002/path.1711740402. [DOI] [PubMed] [Google Scholar]
- Trainor K. J., Brisco M. J., Wan J. H., Neoh S., Grist S., Morley A. A. Gene rearrangement in B- and T-lymphoproliferative disease detected by the polymerase chain reaction. Blood. 1991 Jul 1;78(1):192–196. [PubMed] [Google Scholar]
- Uppenkamp M., Andrade R., Sundeen J., Raffeld M., Coupland R., Cossman J. Diagnostic interpretation of T gamma gene rearrangement: effect of polyclonal T cells. Hematol Pathol. 1988;2(1):15–24. [PubMed] [Google Scholar]
- Wood G. S., Tung R. M., Haeffner A. C., Crooks C. F., Liao S., Orozco R., Veelken H., Kadin M. E., Koh H., Heald P. Detection of clonal T-cell receptor gamma gene rearrangements in early mycosis fungoides/Sezary syndrome by polymerase chain reaction and denaturing gradient gel electrophoresis (PCR/DGGE). J Invest Dermatol. 1994 Jul;103(1):34–41. doi: 10.1111/1523-1747.ep12389114. [DOI] [PubMed] [Google Scholar]