Abstract
Background
Primary spontaneous pneumothorax (PSP) is treated based on studies that have predominantly consisted of tall male subjects. Here we determined recurrence of PSP in average-statured menstruating women and studied prevalence of catamenial pneumothorax (CP) in this population.
Methods
Males and menstruating females, aged 18-55 years, without underlying lung disease or substance abuse were retrospectively studied between 2009-2015. A chest pathologist reviewed all specimens for thoracic endometriosis. Kaplan-Meier curves were constructed to determine recurrence.
Results
The median age of females (n=33) and males (n=183) was 33.4 and 31.6 years, respectively. In females, nine (27%) had left-sided and 24 (73%) had right-sided PSP, treated with tube thoracostomy. Recurrence occurred in 21 (64%) females with median follow up of 14 months and was treated with thoracoscopic pleurodesis. Right PSP had higher recurrence (70%) compared to left (56%, p=0.02). Four females (12%) presented with recurrent tension pneumothorax within six months. Eight (24%) patients had PSP within 72 hours of menses, meeting clinical criteria of CP. All these were placed on hormonal suppression after initial episode but went on to develop recurrence that was treated with pleurodesis. However, classic endometrial glands were not found in any biopsy specimens obtained during the thoracoscopy. In contrast to female subjects, only 8 (4.4%) average-statured males had recurrence (p<0.001) with a median follow up of 16 months.
Conclusions
PSP in healthy average-statured menstruating women has high recurrence compared to male counterparts. CP is a clinical diagnosis and often recurs despite hormonal suppression therapy.
Keywords: catamenial, pneumothorax, endometriosis
Patients with initial episode of primary spontaneous pneumothorax (PSP) are typically treated with tube thoracostomy, whereas those with an ipsilateral recurrence are offered surgical pleurodesis. This recommendation is based on prior studies demonstrating infrequent recurrence following the initial pneumothorax, but higher recurrence rates with subsequent episodes of PSP [1]. However, these studies have largely consisted of tall males while female patients are highly underrepresented [2, 3]. As such, the natural history of PSP in the young females who are not tall remains undetermined. Whether PSP in such female patients be managed according to the prior studies with male predominance, therefore, needs further investigation.
While several hypotheses have been proposed for the pathogenesis of PSP in males, the mechanisms of PSP in young healthy females remain unknown. It has been suggested that a significant number of pneumothoraces in young females might be related to thoracic endometriosis syndrome [4] or catamenial pneumothorax (CP). CP is defined as pneumothorax occurring within 72 hours of menstruation and has high recurrence rates, despite medical therapy for endometriosis [4]. Gross findings such as diaphragmatic fenestrations might be noticed at the time of surgery in patients with CP. However, the true prevalence of catamenial etiology in female patients with PSP as well as morphological and histological evidence of thoracic endometriosis in patients clinically diagnosed with CP remains undetermined.
We recently observed a series of young healthy females, who were not tall, with high recurrence rates of PSP in our practice. Therefore, we hypothesized that these female patients might have higher recurrence rates compared to male counterparts. Henceforth, we investigated the recurrence rates of PSP in female patients. Additionally, since CP has been proposed as a mechanism for PSP, we further assessed the prevalence of thoracic endometriosis in these patients.
Patients and Methods
Study Cohort
We retrospectively identified all women treated at our center for PSP between May 2009 and June 2015. Inclusion criteria included menstruating females between the ages of 18-55 years, of height less than or equal to one standard deviation from average American female (<169.2 centimeters, www.cdc.gov). Exclusion criteria included a history of trauma as a cause of pneumothorax, pregnancy, known lung disease or presence of parenchymal lung disease on chest imaging, current smokers or smoking exposure > 10 pack years, marijuana or other drug inhalation. During the same study period, we also included male patients less than one standard deviation from average American male height (<183.6 centimeters) with the same inclusion and exclusion criteria as outlined for the female subjects. The study was approved by the Institutional Review Board.
Definitions
Catamenial pneumothorax was defined as spontaneous pneumothorax occurring within 72 hours of menstruation, as previously reported [4, 5]. At the time of initial evaluation all patients were queried regarding the timing of their last menstrual cycle and the onset of symptoms to assess potential correlation. Recurrence of pneumothorax was diagnosed by radiographic evidence of pneumothorax at least two weeks following resolution of initial episode. Those patients who never had radiographic resolution of first episode, or developed a pneumothorax within two weeks, were defined as failure of initial management.
Surgical technique
All patients with recurrence of primary pneumothorax underwent video-assisted thoracoscopic (VATS) evaluation of the chest cavity and diaphragm for signs of thoracic endometriosis, including nodules and fenestrations. Visceral and parietal pleura were examined for nodular lesions, and lung for blebs or nodules. All visible foci of implants concerning for endometriosis in lung parenchyma were resected and sent for pathological evaluation. Surgical resection of apical blebs, removal of pleural implants, and repair of diaphragmatic fenestrations was carried out as appropriate. In all patients either a mechanical pleurodesis or subtotal pleurectomy was performed.
Statistical analysis
Clinical and demographic variables were identified using a prospectively maintained database. Statistical analysis was performed using GraphPad Prism software (GraphPad, San Diego, CA). Kaplan Meier curves were constructed using GraphPad Prism software and the recurrence rates between right and left pneumothorax compared using log-rank test. Patients were censored at the time of their last clinical follow up. Development of ipsilateral recurrence during the follow up was considered a positive event on the Kaplan Meier curves. The threshold for statistical significance was defined as α of 0.05.
Results
Study Cohort
During the study period from May 2009 - June 2015, 33 female patients met the inclusion criteria for the study. Their clinical and demographic profiles are presented in Table 1. The median age was 33.4 years (range 19 – 50) with a median follow up time of 14 months. Nine women (27%) had a left-sided spontaneous pneumothorax while the majority, 24 (73%) had right-sided pneumothoraces. All patients were initially managed with tube thoracostomy. There were no treatment failures after tube thoracostomy, as defined by lack of resolution of pneumothorax or development of recurrent pneumothorax within two weeks. Overall twenty-one patients (63.6%) had a recurrence of pneumothorax following management by tube thoracostomy during the study period. Right-sided pneumothorax was associated with ipsilateral recurrence in 16/24 (67%) patients, whereas left sided pneumothorax was associated with ipsilateral recurrence in 5/9 (56%) patients. Two patients refused any surgical treatment after developing ipsilateral recurrence and were treated with tube thoracostomy. The remaining 19 patients (58% of the original cohort) underwent surgical intervention as described in Table 2. In contrast, 183 male patients were treated with PSP during this study period with the same height as well as inclusion/ exclusion criteria. The median age was 31.6 years (range 20 – 57) with a median follow up time of 16 months. The mean height and weight were 176 ± 6.1 cms and 173.5 ± 26.5 lbs, respectively. Seventy-five (41%) had a left-sided spontaneous pneumothorax while 108 (51%) had right-sided pneumothorax. Of these only 8 (4.4%, p<0.001 compared to female patients) recurred with a median follow up of 16 months and were successfully treated with surgical pleurodesis.
Table 1. Clinical and demographic profile of study patients.
| Age (mean, yrs) | 34±2.0 | |
|
| ||
| Laterality | ||
|
| ||
| Right | 24/33 (73%) | |
|
| ||
| Left | 9/33 (27%) | |
|
| ||
| Height (cms) | 164 ± 4.1 cms | |
|
| ||
| Weight (lbs) | 134.7 ± 20.9 | |
|
| ||
| BMI | 21.9 ± 2.9 | |
|
| ||
| Co-morbidites | Gastroesophageal Reflux | 2 (6%) |
| Hypertension | 3 (9%) | |
| Hypothyroidism | 3 (9%) | |
|
| ||
| Pelvic Endometriosis | 3/33 (9%) | |
|
| ||
| Association with Menstrual Cycle | 8/33 (24%) | |
|
| ||
| Overall recurrence (n=33) | 21/33 (63%) | |
|
| ||
| Follow up (median, months) | 14 | |
|
| ||
| Surgical Procedure (n=19) | ||
|
| ||
| Mechanical Pleurodesis | 12/19 (63%) | |
|
| ||
| Subtotal Pleurectomy | 7/19 (37%) | |
|
| ||
| Intraoperative Findings (n=19) | ||
|
| ||
| Apical fibrosis/ blebs | 11/19 (58%) | |
|
| ||
| Diaphragmatic fenestrations | 4/19 (21%) | |
|
| ||
| Nodular deposits | 3/19 (16%) | |
|
| ||
| Histological Findings (n=19) | ||
|
| ||
| Endometriosis | 0 | |
|
| ||
| Chronic changes of fibrosis | 19/19 (100%) | |
|
| ||
| Hemosiderin laiden macrophages | 3/19 (16%) | |
cms=centimeters; lbs=pounds; BMI = Body Mass Index.
Table 2. Gross and pathologic findings of patients undergoing surgical treatment for PSP recurrence.
AWR= Apical Wedge Resection, MP= Mechanical Pleurodesis, SP= Subtotal Pleurectomy
| Patient | Surgical Procedure | Intraoperative findings | Pathology |
|---|---|---|---|
| 1 | AWR, MP | apical fibrosis | mesothelial hyperplasia and reactive epithelial cell changes |
| 2 | AWR, MP | apical bleb, fibrosis | mesothelial hyperplasia and reactive epithelial cell changes |
| 3 | AWR, MP | right middle lobe bleb | mesothelial hyperplasia and reactive epithelial cell changes |
| 4 | AWR, MP | apical bleb | mesothelial hyperplasia and reactive epithelial cell changes; hemosiderin laden macrophages |
| 5 | AWR, MP | diffuse parietal pleural thickening | mesothelial hyperplasia and reactive epithelial cell changes |
| 6 | AWR, SP | apical bleb | mesothelial hyperplasia and reactive epithelial cell changes |
| 7 | AWR, MP | apical bleb | mesothelial hyperplasia and reactive epithelial cell changes |
| 8 | AWR, SP | apical bleb | mesothelial hyperplasia and reactive epithelial cell changes |
| 9 | AWR, SP | apical scarring | mesothelial hyperplasia and reactive epithelial cell changes, hemosiderin laden macrophages |
| 10 | AWR, MP | subpleural nodule hemorrhagic, apical fibrosis | mesothelial hyperplasia and reactive epithelial cell changes |
| 11 | AWR, MP | diaphragmatic fenestrations | mesothelial hyperplasia and reactive epithelial cell changes |
| 12 | AWR, SP | None | mesothelial hyperplasia and reactive epithelial cell changes |
| 13 | AWR, MP | diaphragmatic fenestrations | mesothelial hyperplasia and reactive epithelial cell changes |
| 14 | AWR, SP | diaphragmatic fenestrations | mesothelial hyperplasia and reactive epithelial cell changes; hemosiderin laden macrophages |
| 15 | AWR, MP | apical bleb | mesothelial hyperplasia and reactive epithelial cell changes |
| 16 | AWR, MP | single nodular deposit on parietal pleural | mesothelial hyperplasia and reactive epithelial cell changes |
| 17 | AWR, SP | multiple nodular deposits on parietal pleural, diaphragmatic fenestrations | mesothelial hyperplasia and reactive epithelial cell changes |
| 18 | AWR, SP | None | mesothelial hyperplasia and reactive epithelial cell changes |
| 19 | AWR, MP | Apical bleb | Mesothelial hyperplasia and reactive epithelial cell changes |
The surgical procedures included apical wedge resection in all patients and mechanical pleurodiesis (12/19, 63%) or subtotal pleurectomy (7/19, 37%). During thoracoscopy, nodular deposits were noted over the diaphragm in three (16%) patients, and four (21%) patients had diaphragmatic fenestrations. When diaphragmatic fenestrations were noted, plication of the diaphragm was performed to cover the defects. Histology of the apical wedge resections confirmed blebs/subpleural fibrosis in 11 (58%) of operated patients while analysis of the diaphragmatic nodules as well as the fenestrations revealed mesothelial hyperplasia and reactive changes but no endometrial glands or stroma.
Recurrence Patterns of Pneumothorax
Overall there was a recurrence rate of 64% in the study cohort at a median follow up of 14 months from the initial episode of pneumothorax (Figure 1A). Right-sided pneumothorax had a higher recurrence (70%) compared to left (56%, p=0.02, Figure 1B). Of the study cohort, four patients (12%) presented with large recurrent pneumothoraces with signs of tension pneumothorax within the first six months of the initial episode. One patient developed a second ipsilateral recurrence following apical resection with subtotal pleurectomy and she was successfully treated with talc poudrage. None of the male patients treated with surgical pleurodesis developed recurrence.
Figure 1. PSP Recurrence rate in the study cohort.
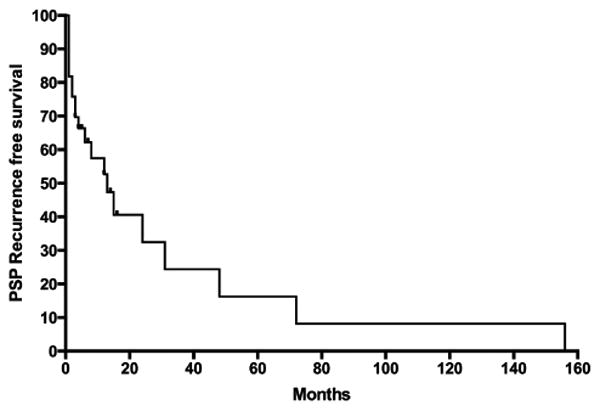
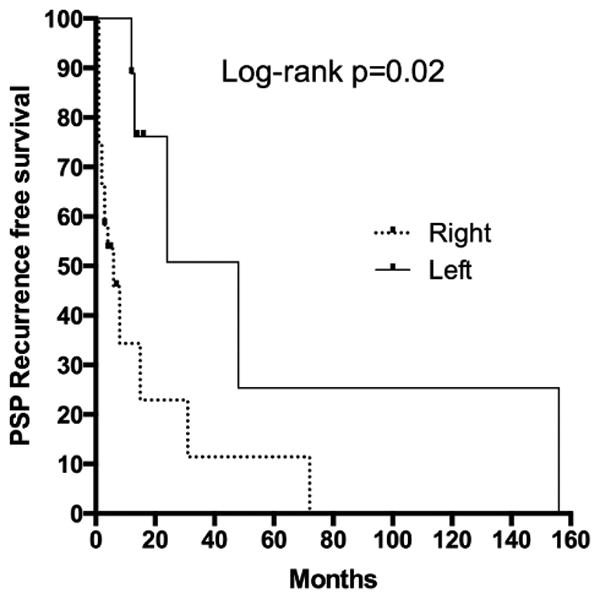
A) Overall recurrence in entire study cohort. B) Recurrence in right and left sided PSP.
Catamenial Pneumothorax
Of the study cohort, 8 (24%) patients reported that their symptoms began within 72 hours of the onset of menstruation. Of these, three patients had a documented history of pelvic endometriosis. All eight patients were either already receiving, or were placed on oral contraception after consultation with a gynecologist. Despite this, all patients developed ipsilateral recurrence of pneumothorax within the first year of the initial episode (Figure 2). Of these, six (75%) were right and two (25%) left sided.
Figure 2. Recurrence in patients clinically diagnosed with Catamenial Pneumothorax (CP, broken line) compared to those with no association with menstrual cycle (Non CP, solid line).
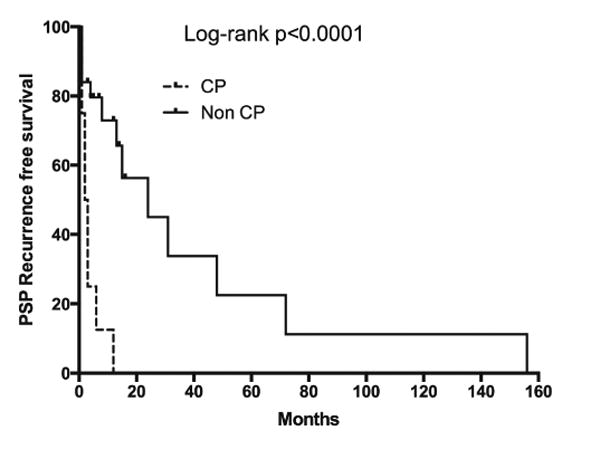
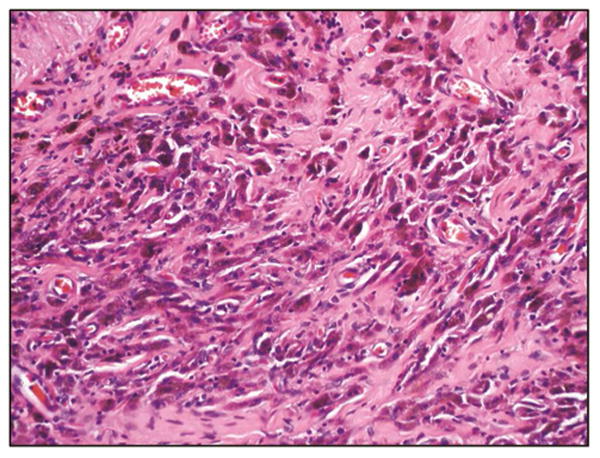
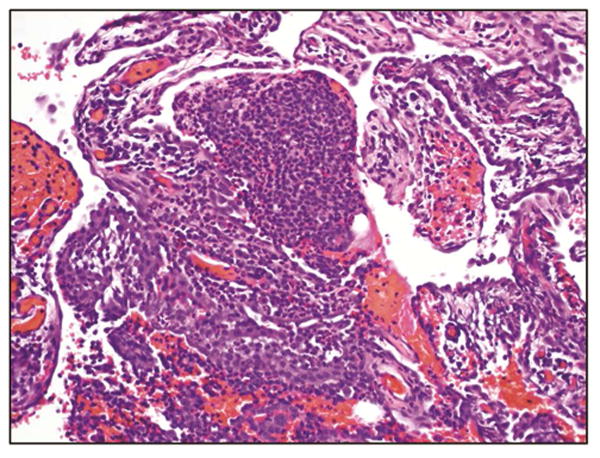
All eight patients with CP underwent surgical therapy at the time of the second episode. Thoracoscopy revealed diaphragmatic fenestrations in four (50%) and nodular deposits in three patients (38%). However, upon careful evaluation, neither endometrial glands nor stroma were found in the tissues resected from any of the patients. We evaluated whether the presence of hemosiderin would be associated with thoracic endometriosis, as previously reported [6]. Biopsy materials from the apical resection, pleural and diaphragm biopsies were evaluated and revealed presence of hemosiderin-laden macrophages in three (38%) patients. These macrophages were found at the site of diaphragmatic fenestrations. Tissues biopsies from all of these patients revealed mesenchymal fibrosis and chronic epithelial cell changes similar to those patients without association of PSP with their menstrual cycles (Figure 3).
Figure 3. Hemosiderin laden macrophages (A) and chronic fibrosis (B) suggestive of catamenial pneumothorax.
Comment
In this study, we investigated the recurrence rates of PSP in menstruating females with no underlying lung disease and those who were not tall. Tall males or patients with marfanoid features can have apical blebs that lead to PSP [3], but this does not apply to normal-statured females who likely have a different pathogenesis of PSP. Tobacco [7, 8] and cannabis [9] smoking are associated with PSP even in the absence of parenchymal lung disease. Deeper inhalation observed frequently in cannabis smokers and valsalva-like maneuvers can lead to PSP [9]. Hence, we excluded active tobacco or cannabis smokers from our study. Therefore, our patients consisted of a homogenous group of menstruating females with no underlying lung disease or obvious cause for PSP. We found a much higher recurrence of pneumothorax in our patient population compared to the published literature [1]. This might suggest that PSP in female patients should be treated more aggressively. This is further emphasized by the fact that about 12% of patients presented with a potentially life-threatening recurrence within 6 months. During the study period, the male patients with same height and inclusion/exclusion had a significantly lower recurrence rate. The overall male recurrence rate was lower in our series compared to published literature likely due to the exclusion of tall males [2, 3].
The etiology of the high recurrence rates in the female patients remains unknown. Catamenial pneumothorax has been proposed as the etiology of PSP in these patients. Nevertheless, it is generally considered a rare phenomenon with a previously reported prevalence of 0.9 – 5.6% of all cases of PSP in women [10, 11]. However, others have shown a higher prevalence up to 18-29% [12-14]. We evaluated clinical, morphological, and histological evidence of CP in female patients with PSP after eliminating obvious causes of PSP. Roughly 25% of our patients were clinically diagnosed with CP, defined by association with menses within 72 hours. The higher prevalence of CP in our study and others is likely related to the selection of study cohorts. Furthermore, 50% of our patients with PSP associated with menses revealed morphological features of CP such as nodular deposits and diaphragmatic fenestrations. Thoracoscopy provides improved exposure and magnification during chest exploration that may otherwise be missed at thoracotomy [13]. Therefore, use of video-assisted thoracoscopy (VATS) might also explain the increased detection of morphological features of CP observed in our series as well as others. Nonetheless, classical histological features of endometriosis such as endometrial glands and stroma were lacking in all patients. Instead, all our patients revealed non-specific histological features including mesothelial hyperplasia and reactive epithelial cell changes. Three patients had evidence of hemosiderin-laden macrophages that are suggestive of, but not definitive evidence for, CP. Hence, we postulate that standard histology has poor utility in defining the diagnosis of CP. To improve the histologic evidence of endometriosis, estrogen and progesterone receptor staining has been performed and shown to increase sensitivity [15]. Additionally, immunostaining with CD10 has been reported to be useful. However, the published experience with these markers has also been highly variable 16. We conclude that the diagnosis of catamenial pneumothorax should be made clinically based on the temporal relation of PSP to menses and the identification of gross pathologic findings (nodular brown lesions, diaphragmatic defects, and implants) at the time of surgery.
Approximately 95% of catamenial pneumothoraces occur on the right side and are associated with a high recurrence rate. In our cohort, there was right side predominance of PSP as well in patients clinically diagnosed with CP. Several hypotheses have been postulated to explain the pathogenesis of CP. The coelomic metaplasia theory suggests that a metaplastic transformation of the cells lining the pleural cavity accounts for development of endometrial tissue. This, however, does not account for the observed right-sided predominance. Other theories involving ectopic spread of endometrium via lymphatic or vascular embolization or retrograde menstruation may better explain the increased prevalence of right-sided PSP. Diaphragmatic defects found at thoracoscopy corroborate the transperitoneal-transdiaphragmatic theory of endometrial implant migration. These defects should be repaired by suture plication that eliminates a potential mechanism for endometrial implant migration.
In our series the recurrence rate for primary spontaneous pneumothorax in females was high, particularly for right-sided pneumothoraces. This high early recurrence and potential for life-threatening recurrence may warrant management with surgery at the initial presentation of spontaneous pneumothorax in this patient population. Further prospective studies are necessary to validate our findings. The limitations of our study include its retrospective nature. Additionally, we excluded patients with smoking history or evidence of lung disease. CP can certainly occur in those patients. However, by eliminating those patients, we obtained a more homogenous group to study CP. Data from our study cannot be applied to tall females or those with traumatic and secondary causes of pneumothorax.
We conclude that primary spontaneous pneumothorax in menstruating women with no underlying lung disease has a high recurrence rate. The high recurrence of PSP in this patient population may warrant earlier follow up and/or primary surgical intervention. Furthermore, the diagnosis of CP should be made clinically as pathologic findings of endometrial glands or stroma are rarely identified histologically.
Acknowledgments
Dr Ankit Bharat is supported by NHLBI K08 HL-125940 and an American Lung Association Biomedical Grant.
Footnotes
Notes: Article type = Original Article
IC by Berry to come.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl 2):ii18–31. doi: 10.1136/thx.2010.136986. [DOI] [PubMed] [Google Scholar]
- 2.Guo Y, Xie C, Rodriguez RM, Light RW. Factors related to recurrence of spontaneous pneumothorax. Respirology. 2005;10(3):378–84. doi: 10.1111/j.1440-1843.2005.00715.x. [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ, 3rd, Hepper NG, Offord KP. Influence of height on the risk of spontaneous pneumothorax. Mayo Clin Proc. 1981;56(11):678–82. [PubMed] [Google Scholar]
- 4.Blanco S, Hernando F, Gomez A, Gonzalez MJ, Torres AJ, Balibrea JL. Catamenial pneumothorax caused by diaphragmatic endometriosis. J Thorac Cardiovasc Surg. 1998;116(1):179–80. doi: 10.1016/S0022-5223(98)70264-8. [DOI] [PubMed] [Google Scholar]
- 5.Visouli AN, Zarogoulidis K, Kougioumtzi I, et al. Catamenial pneumothorax. J Thorac Dis. 2014;6(Suppl 4):S448–60. doi: 10.3978/j.issn.2072-1439.2014.08.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legras A, Mansuet-Lupo A, Rousset-Jablonski C, et al. Pneumothorax in women of child-bearing age: an update classification based on clinical and pathologic findings. Chest. 2014;145(2):354–60. doi: 10.1378/chest.13-1284. [DOI] [PubMed] [Google Scholar]
- 7.Bense L, Eklund G, Wiman LG. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest. 1987;92(6):1009–12. doi: 10.1378/chest.92.6.1009. [DOI] [PubMed] [Google Scholar]
- 8.Sadikot RT, Greene T, Meadows K, Arnold AG. Recurrence of primary spontaneous pneumothorax. Thorax. 1997;52(9):805–9. doi: 10.1136/thx.52.9.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman AL, Sullivan JT, Passero MA, Lewis DC. Pneumothorax in polysubstance-abusing marijuana and tobacco smokers: three cases. J Subst Abuse. 1993;5(2):183–6. doi: 10.1016/0899-3289(93)90061-f. [DOI] [PubMed] [Google Scholar]
- 10.Shearin RP, Hepper NG, Payne WS. Recurrent spontaneous pneumothorax concurrent with menses. Mayo Clin Proc. 1974;49(2):98–101. [PubMed] [Google Scholar]
- 11.Nakamura H, Konishiike J, Sugamura A, Takeno Y. Epidemiology of spontaneous pneumothorax in women. Chest. 1986;89(3):378–82. doi: 10.1378/chest.89.3.378. [DOI] [PubMed] [Google Scholar]
- 12.Bagan P, Le Pimpec Barthes F, Assouad J, Souilamas R, Riquet M. Catamenial pneumothorax: retrospective study of surgical treatment. Ann Thorac Surg. 2003;75(2):378–81. doi: 10.1016/s0003-4975(02)04320-5. discusssion 381. [DOI] [PubMed] [Google Scholar]
- 13.Alifano M, Roth T, Broet SC, Schussler O, Magdeleinat P, Regnard JF. Catamenial pneumothorax: a prospective study. Chest. 2003;124(3):1004–8. doi: 10.1378/chest.124.3.1004. [DOI] [PubMed] [Google Scholar]
- 14.Ciriaco P, Negri G, Libretti L, et al. Surgical treatment of catamenial pneumothorax: a single centre experience. Interact Cardiovasc Thorac Surg. 2009;8(3):349–52. doi: 10.1510/icvts.2008.190975. [DOI] [PubMed] [Google Scholar]
- 15.Flieder DB, Moran CA, Travis WD, Koss MN, Mark EJ. Pleuro-pulmonary endometriosis and pulmonary ectopic deciduosis: a clinicopathologic and immunohistochemical study of 10 cases with emphasis on diagnostic pitfalls. Hum Pathol. 1998;29(12):1495–503. doi: 10.1016/s0046-8177(98)90021-1. [DOI] [PubMed] [Google Scholar]


