Abstract
Experience with clinical liver xenotransplantation has largely involved the transplantation of livers from nonhuman primates. Experience with pig livers has been scarce. This brief review will be restricted to assessing the potential therapeutic impact of pig liver xenotransplantation in acute liver failure and the remaining barriers that currently do not justify clinical trials. A relatively new surgical technique of heterotopic pig liver xenotransplantation is described that might play a role in bridging a patient with acute liver failure until either the native liver recovers or a suitable liver allograft is obtained. Other topics discussed include the possible mechanisms for the development of the thrombocytopenis that rapidly occurs after pig liver xenotransplantation in a primate, the impact of pig complement on graft injury, the potential infectious risks, and potential physiologic incompatibilities between pig and human. There is cautious optimism that all of these problems can be overcome by judicious genetic manipulation of the pig. If liver graft survival could be achieved in the absence of thrombocytopenia or rejection for a period of even a few days, there may be a role for pig liver transplantation as a bridge to allotransplantation in carefully selected patients.
Introduction
Liver transplantation offers several advantages for the treatment of patients with acute or fulminant liver failure or end-stage chronic liver disease, but is limited by the shortage of deceased human donor organs. In patients with acute liver failure, usually induced by chemical or viral hepatitis, the onset of disease is sudden and identification of a suitable donor organ is frequently not possible before permanent neurologic injury and/or death occurs.
In the USA, data from the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network indicate that in 2014 the number of patients on the waiting list for a human donor liver was close to 16,000. Of these, only 6,729 received a transplant.1 Of close to 10,000 who did not receive a transplant, 3,178 died or were removed from the waiting list because they were too sick to undergo a major operative procedure, which is approximately 20% of those on the waiting list and 30% of those who did not receive a transplant.
Support during the critical period when the patient is in acute liver failure may be aimed at (i) ‘bridging’ the patient to liver allotransplantation in order to prevent irreversible cerebral injury, or (ii) gaining time for regeneration of a damaged native liver, if this is considered likely.
Potential solutions to the problem include (i) an artificial liver device, (ii) the transplantation of hepatocytes or (iii) hepatocyte-like expanded human stem cells, (iv) ex vivo pig or nonhuman primate (NHP) liver perfusion, or (v) the transplantation of a genetically-engineered pig liver. Regenerative medicine techniques whereby a human or pig liver is decellularized and recelluarized with cells from the potential recipient would not be applicable to patients with acute liver failure. This brief review will be restricted to assessing the potential therapeutic impact of pig liver xenotransplantation in acute liver failure and the remaining barriers that currently do not yet justify clinical trials.
Clinical experience with ex vivo pig liver perfusion
Early experience has been reviewed by Hara et al.2 In the late 1960s and early 1970s, at least 141 ex vivo pig liver perfusions were performed to treat 87 patients with liver failure, but then this therapeutic option was largely superseded for several years by orthotopic liver allotransplantation. Neurologic improvement to at least hepatic coma grade III or II has been documented in most patients. These clinical trials have provided valuable immunologic information. The data suggested that unmodified (wild-type) pig livers may be rejected less vigorously than other pig organs, possibly because hepatic failure is accompanied by diminished complement levels, although there may be additional reasons.
In a small clinical trial by Levy et al3 livers from pigs transgenic for the human complement-regulatory proteins (regulators of complement activation, RCA), CD55 (human decay-accelarating factor [hDAF]) and CD59, were extracorporeally perfused in 2 patients with acute hepatic failure for 6.5h and 10h, respectively, as bridging to successful allotransplantation. The histopathological findings in these cases were similar to those described with nontransgenic pig livers. Of interest, the authors made no mention of whether thrombocytopenia developed.
Clinical experience with pig liver xenotransplantation
Following the development of techniques of vascular anastomosis at the beginning of the 20th century, organ xenotransplantation became possible. Most of the early attempts at clinical organ xenotransplantation used NHP species as sources of the organ (reviewed in 2), although there were a few attempts using the pig and other nonprimate mammals, but without significant success.4–6
Only 1 of these experiences related to the transplantation of a pig liver. In 1993, Makowka and his colleagues performed the only heterotopic pig liver xenotransplant, with the aim of performing orthotopic allotransplantation when a human donor became available, at which time the auxiliary pig liver would have been removed.7,8 The patient was a 26-year-old woman with a 14-year history of autoimmune hepatitis who was admitted to hospital with grade III encephalopathy. Hepatitis C was detected serologically. She was listed with the UNOS at the highest priority. Despite aggressive medical therapy, the patient continued to deteriorate, with increasing encephalopathy and coagulopathy.
A wild-type (genetically-unmodified) pig liver was transplanted heterotopically. Before transplantation, circulating natural anti-pig antibodies were removed by plasmapheresis and ex vivo perfusion of the donor pig kidneys. After transplantation, the liver xenograft clearly functioned, as documented by bile production, stabilization of prothrombin levels, and reduction in the levels of lactic acid and the enzymes aspartate aminotransferase and alanine aminotransferase. Unfortunately, this did not result in any improvement in the neurologic status of the patient, who died after 34 hours from irreversible brain damage.
Despite the removal of >90% of the recipient’s natural xenoantibodies prior to transplantation, antibody rapidly returned and was associated with complement-mediated injury of the graft. A liver biopsy obtained 3 hours posttransplantation showed deposition of antibody and complement components, and endothelial swelling, suggesting early graft rejection. At the time of death, the pig liver showed thrombosis and ischemic necrosis. Importantly, however, no mention was made as to whether thrombocytopenia was documented, as seen in pig-to-NHP models (see below). Nevertheless, this experience demonstrated the ability of a pig liver to function, at least temporarily, in a human recipient and to provide some metabolic support during acute liver failure.
Experimental pig liver xenotransplantation in nonhuman primates
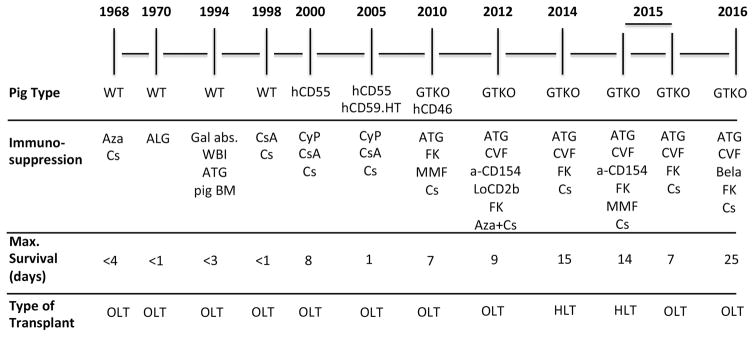
There have been several reports of liver xenotransplantation between species of NHPs [reviewed in 2–42], but, for a number of reasons,9 it is very unlikely that NHPs will be acceptable as sources of livers for clinical transplantation in the future. The pig-to-NHP model is increasingly being investigated to assess strategies aimed at advancing towards clinical xenotransplantation (Figure 1).
Figure 1.
Time-line in experimental pig liver xenotransplantation in NHPs.
Abbreviations: a-CD154 = anti-CD154 monoclonal antibodies; ALG = antilymphocyte globulin; ATG = antithymocyte globulin; Aza = azathioprine; Bela = belatacept; BM = bone marrow; Cs = corticosteroids; CsA = cyclosporine; CVF = cobra venom factor; CyP = cyclophosphamide; FK = tacrolimus; Gal abs = extracorporeal anti-Gal antibody adsorption; GTKO = 1,3-galactosyltransferase gene-knockout; hCD46 = expression of the human regulator of complement, hCD46; hCD55 = expression of the human regulator of complement, CD55; HLT = heterotopic liver transplantation; HT = H-transferase; LoCD2b = anti-CD2 monoclonal antibody; MMF = mycophenolate mofetil; OLT = orthotopic liver transplantation; WBI = whole body irradiation; WT = wild-type.
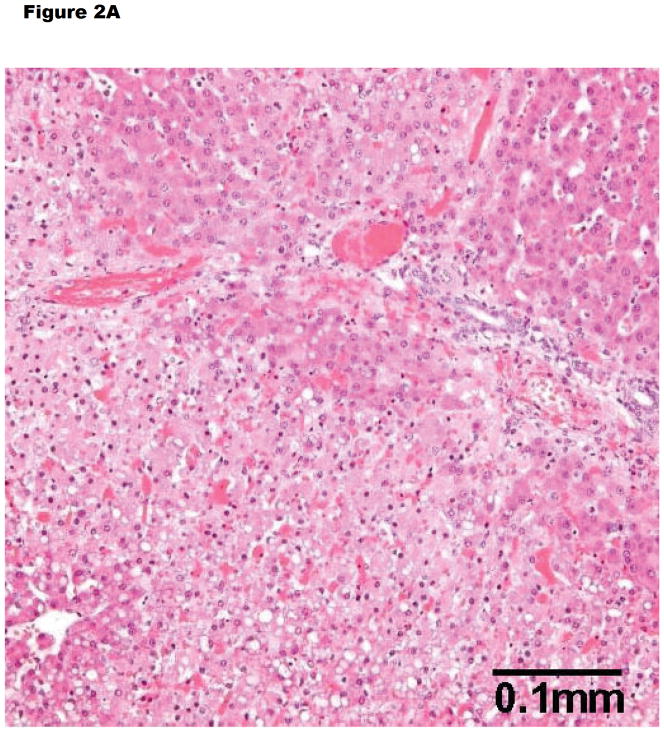
In untreated NHPs, wild-type pig livers have generally undergone early antibody-mediated rejection (intravascular thrombosis, hemorrhagic necrosis, endothelial cell injury, deposition of IgM, IgG, C3), though no intravascular fibrin aggregation is seen (Figure 2A). For example, Ramirez et al reported 3 baboons that survived for <12 hours and showed features of hyperacute rejection (Table 1).10,11 Subsequently, Ekser et al provided sequential data on the development of hyperacute rejection in this model.12
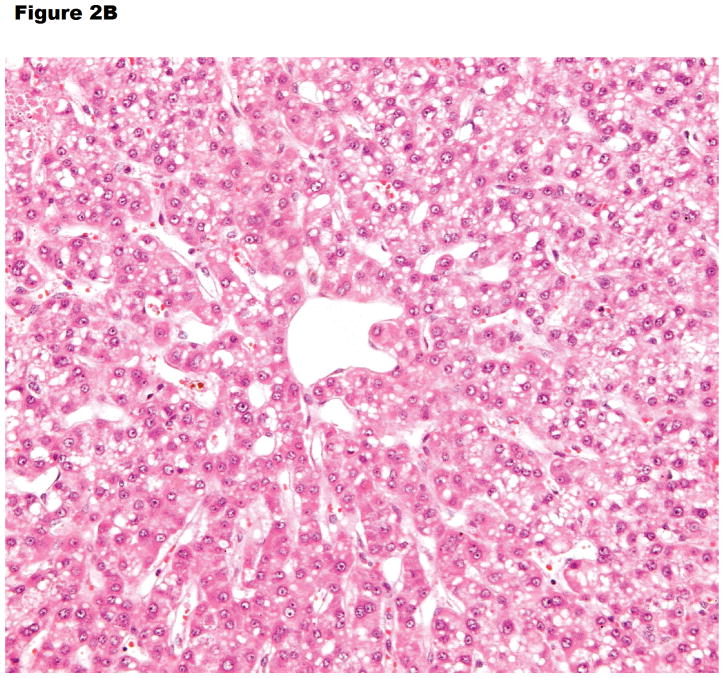
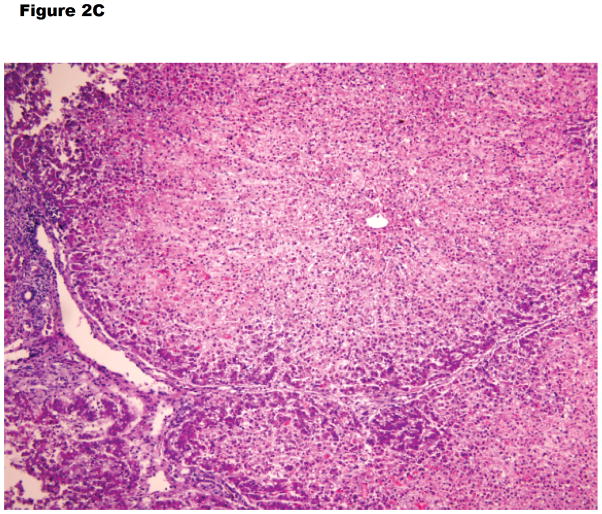
Figure 2.
Histopathology of (A) hyperacute rejection (<24 hours) in a wild-type pig liver transplanted orthotopically into a baboon, (B) a GTKO/hCD46 orthotopic pig liver graft in a baboon that survived for 6 days, and (C) a pig left liver lobe graft in a Tibetan monkey that survived for 14 days
(A) WT pig-to-baboon liver xenotransplantation at 1 h (x200). Severe hepatocellular vacuolar change, focal hepatocyte necrosis, and few thrombi.
(B) Vacuolar hepatocellular cytoplasmic change with minimal hepatocellular necrosis on postoperative day 6 (x200).
(C) The graft shows some lymphocyte infiltration in the portal area, but no major features of antibody-mediated or cellular rejection (x100).
Table 1.
Experience in experimental liver xenotransplantation between pigs and nonhuman primates
| Donor pig | Recipient | Type of transplant | n | Survival (days) | (Ref) | |
|---|---|---|---|---|---|---|
| WT | Baboon | OLT | 7 | <1–3 | } | 96–98 |
| WT | Rhesus monkey | OLT | 3 | <1 | } | |
| WT | Chimpanzee | OLT | 1 | <1 | } | |
| WT | Cynomolgus monkey | OLT | 4 | <1–3 | } | 99 |
| WT | Baboon | OLT | 2 | |||
| WT | Rhesus monkey | OLT | 6 | <1 | } | 38,100 |
| WT | Baboon | HLT | 2 | <1 | } | |
| WT | Baboon | OLT | 4 | <1 | } | 10,11 |
| hCD55 | Baboon | OLT | 2 | 4, 8 | } | |
| WT | Baboon | OLT | 4 | <1 | } | 16 |
| hCD55, hCD59, HT | Baboon | OLT | 5 | <1 | ||
| WT | Baboon | OLT | 1 | <1 | 12,17,18 | |
| GTKO, CD46 | Baboon | OLT | 10 | <1–7 | ||
| MGH MS, GTKO | Baboon | OLT | 3 | 6 8,9 | 19 | |
| MGH MS, GTKO | Baboon | HLT | 3 | 6,9,15 | 25 | |
| WZMS, GTKO | Tibetan monkey | HLT | 3 | 2,5,14 | 28 | |
| MGH MS, GTKO | Baboon | OLT | 6 | 1,3,5,5,6,7 | 26 | |
| MGH MS GTKO | Baboon | OLT | 1 | 25 | 27 | |
CD46, CD55, CD59 = human complement-regulatory proteins
GTKO = α1,3-galactosyltransferase gene-knockout
HLT = heterotopic (auxiliary) liver transplantation
HT= H-transferase
MGH MS = Massachusetts General Hospital miniature swine
OLT = orthotopic liver transplantation
WZ MS = Wu Zhanshen miniature swine
WT = wild-type (genetically unmodified)
When genetically-engineered pigs have been the source of the liver, the results have significantly improved. In the same series of experiments by Ramirez et al10,11 2 baboons transplanted with livers from pigs transgenic for the human RCA, CD55 (human decay-accelerating factor, hDAF) survived for 4 and 8 days, respectively (Table 1). Neither liver xenograft demonstrated histopathologic features of hyperacute rejection. This study indicated that, when hyperacute rejection is abrogated by the expression of hCD55, the porcine liver can maintain reasonable levels of coagulation factors and protein in the baboon for up to 8 days, although factors II, VII, and X decreased significantly. The authors confirmed studies in rodents that established that the recipient progressively acquires the protein profile of the donor species.13–15 In a subsequent brief report by this group, livers from pigs transgenic for 2 hRCAs (CD55, CD59) and H-transferase survived only 13–24 hours in baboons.16
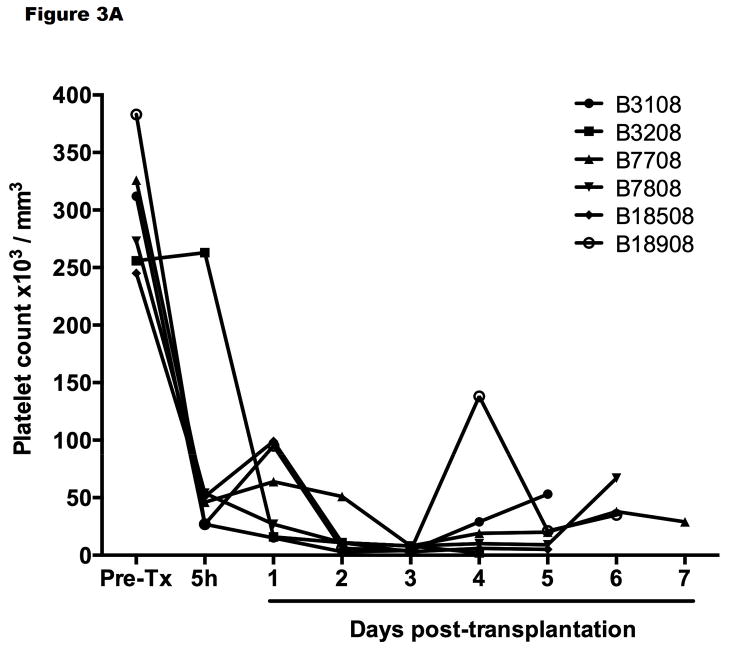
Ekser et al reported their experience of orthotopic pig liver xenotransplantation in baboons with grafts from α1,3-galactosyltransferase gene-knockout (GTKO) pigs or GTKO pigs transgenic for the human RCA, CD46 (GTKO/hCD46) and a clinically applicable immunosuppressive regimen (Table 1).12,17 Profound thrombocytopenia developed within one hour posttransplantation (Figure 3A), which resulted in spontaneous hemorrhages in various native organs and in the liver graft, limiting recipient survival to a maximum of 7 days. However, throughout much of this time, hepatic function was documented near-normal to normal (as assessed by liver enzymes, coagulation factors, coagulation assays, and production of porcine-specific proteins).18 There was no definitive humoral and cellular rejection (as documented on biopsies taken at 2 hours or at the time of euthanasia or death (at 4–7 days posttransplant) (Figure 2B).
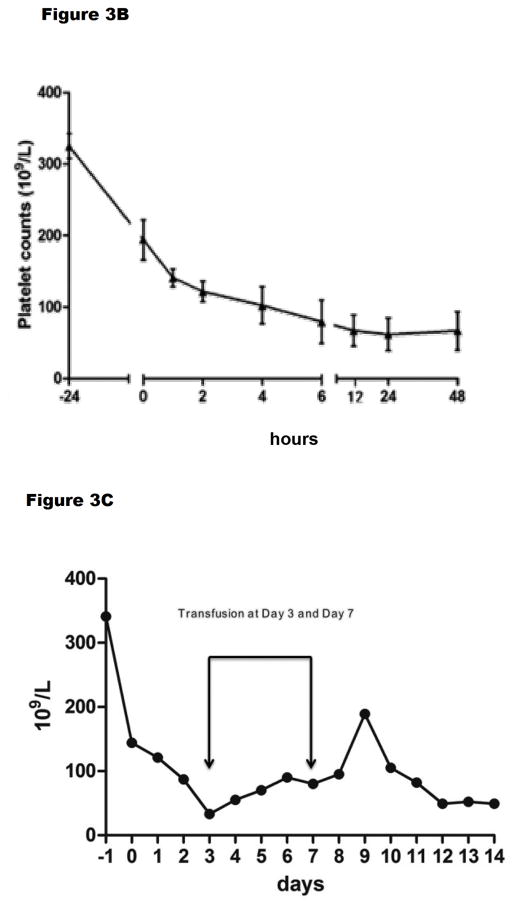
Figure 3.
Platelet counts (A) after GTKO/hCD46 orthotopic pig liver transplantation in baboons (n=6) that survived from 4–7 days, and (B) after a GTKO Wu Zhishan miniature swine heterotopic left liver lobe transplant in Tibetan monkeys (n=3, mean ±SD) within the first 48 hours, and (C) in the Tibetan monkey that survived for 14 days. (A is reproduced with permission from Ekser et al. Transplantation 2010;90:483–493; B is reproduced with permission from Ji H, et al.28
Kim et al reported GTKO Massachusetts General Hospital (MGH) miniature swine orthotopic liver xenotransplantation in baboons with an intensive immunosuppressive regimen (Table 1).19 Their initial 3 cases survived 6, 8, and 9 days, respectively. The 6-day survivor died of spontaneous hemorrhage associated with profound thrombocytopenia, as observed by Ekser et al.12 In the 8-and 9-day survivors, loss of platelets was ameliorated to some extent by blocking fibrinolysis by the administration of aminocaproic acid. Although thrombocytopenia was marginal (platelet counts remained between 40–50,000/mm3), the baboons suffered severe blood loss and sepsis.
The mechanisms by which platelet aggregation and phagocytosis occurred were investigated in vitro.20 Pig hepatocytes, liver sinusoidal endothelial cells, and aortic endothelial cells all induced moderate aggregation of baboon platelets (but not of pig platelets), and sinusoidal endothelial cells were shown to phagocytose baboon platelets (as demonstrated previously with human platelets in an ex vivo perfusion model by Tector and his colleagues.21–24 Phagocytosis could be significantly reduced by certain agents, e.g., aurintricarboxylic acid (which blocks von Willebrand Factor [vWF]).
Yeh et al went on to assess the effect of maintaining the recipient liver in situ and transplanting the pig liver heterotopically.25 Although thrombocytopenia developed as before, the presence of baboon coagulation factors prevented severe spontaneous hemorrhage from occurring. However, features of thrombotic microangiopathy and ischemia developed in the graft (as seen in pig hearts and kidneys), resulting in graft failure in 2 cases. Survival of the 3 grafts or recipients was limited to 6, 9, and 15 days, respectively, with sepsis being a major cause of death.
Recently, Navarro-Alvarez et al reported 7 further cases (6 new, 1 historical) of orthotopic GTKO pig-to-baboon liver xenotransplantation,26 in which they sought to determine the effects of the administration of human coagulation factors (Table 1). Graft and recipient survival was 1 and 3 days (with bolus administration) and 5–7 days (with continuous administration), which was not different from survival of a historical control baboon (6 days). Platelet counts were maintained, but the baboons quickly developed large vessel thrombosis and thrombotic microangiopathy. Several deaths were from infection.
The most recent report 27 documented 25-day survival of a GTKO pig liver graft in a baboon treated with a human prothrombinase concentrate complex. Immunosuppressive therapy included induction with antithymocyte globulin and cobra venom factor, with maintenance with belatacept, tacrolimus, and methylprednisolone. Abnormalities of liver function tests on day 7 were presumed to be associated with rejection, but were reversed by a course of steroid pulses. Early thrombocytopenia began to recover by day 11 and was maximal on day 21 (614,000/mm3) without the need for platelet transfusions. Euthanasia was necessary on day 25 from the development of plantar ulcerations (associated with peripheral edema), progressive cholestasis, hemolysis, and a rising direct bilirubin. The liver showed no macroscopic features of necrosis with all vessels free of thrombus. This report provides encouragement that a pig liver graft might maintain life in a patient with fulminant hepatic failure until either recovery of the native liver occurs (if the pig liver had been transplanted heterotopically) or until an allograft becomes available.
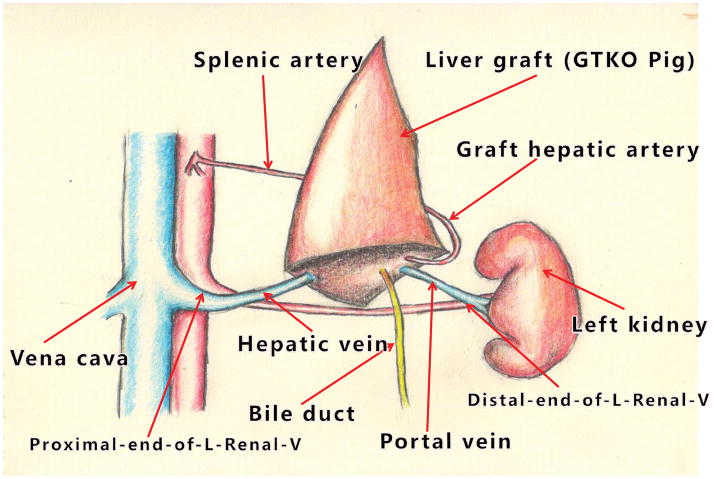
Another recent experience has been gained by Dou’s group from Xi’an, China, who inserted a left liver lobe from GTKO Wu Zhishan miniature swine as auxiliary grafts into Tibetan monkeys (Table 1).28 Their innovative surgical technique is illustrated in Figure 4. Although it requires native splenectomy, the Xi’an technique has the advantage that none of the native liver needs to be excised as the graft fits comfortably into the splenic fossa. (Ekser et al17, Navarro-Alvarez et al26, Yeh et al25, and Shah et al27 all performed splenectomy in order to reduce thrombocytopenia. However, whether splenectomy is immunologically beneficial to the outcome is uncertain, but there was no definitive evidence for this in the early studies of pig heart or kidney xenotransplantation [reviewed in 29.)
Figure 4.
Surgical technique of pig left liver lobe transplantation in Tibetan monkeys. After native splenectomy, the pig liver graft was placed in the splenic recess. The recipient’s left renal vein was divided, the distal end being anastomosed to the graft portal vein, and the proximal end to the graft hepatic vein. The graft hepatic artery was anastomosed end-to-end to the recipient splenic artery (using a microvascular technique with an operating microscope). After reperfusion, the bile duct was drained through the abdominal wall to allow measurement of bile drainage. (Using the same technique in clinical cases of allotransplantation, the bile duct is drained into a Roux-en-Y jejunal loop.)
Of interest, the surgical technique introduced by the Xi’an group was first used successfully in clinical liver allotransplantation in 2007 in a patient with Wilson’s disease, splenomegaly, and hypersplenism, and has since been employed in a further 14 patients, using both living donors (excising segments 2 and 3) and split livers from deceased (donation after cardiac death) donors (Dou K-F, et al, unpublished). Following the work of Olausson et al,30 in 1 patient with >90% panel-reactive antibodies (PRA) in need of kidney transplantation, the donor liver graft was inserted heterotopically before the donor kidney with the intention of preventing antibody-mediated hyperacute rejection of the kidney, which it did successfully.
In their xenotransplantation studies, graft survival, as defined by various parameters of hepatic function, extended between 2 and almost 14 days, although the presence of the native recipient liver would have been beneficial. In none of the 3 monkeys was the fall in platelet count during the first 24 hours as dramatic or as profound (Figure 3B) as in the experience of others (Figure 3A).17,19,25 In the longest-surviving monkey, although the platelet count remained at approximately 50,000/mm3 by the end of the experiment (day 14) (Figure 3C), whether this difference can be accounted for by the fact that the recipient was a Tibetan monkey rather than a baboon, or that the organ-source pig was a Wu Zhishan miniature swine remains uncertain, but needs to be explored.
The study provided further valuable data on the coagulation disorders that develop following pig liver transplantation in NHPs. The authors demonstrated that early activation of recipient tissue factor is largely responsible for coagulation dysregulation (supporting the studies by Lin et al,31,32 in part related to incompatibility between pig tissue factor pathway inhibitor (TFPI) and primate tissue factor, which results in the inefficient inhibition of recipient tissue factor.33 Accompanying in vitro studies indicated that the overexpression of human TFPI by pig bone marrow-derived mesenchymal stromal cells inhibited clotting, which is an observation that may be of potential therapeutic importance and warrants further investigation.34,35
From the above combined studies, we can tentatively conclude that early after transplantation of a liver from a genetically-engineered pig, microscopic and immunohistochemical examination of a liver biopsy has been almost normal, with minimal (patchy) or no deposition of IgM, IgG, C3, C4d, and C5b-9. At necropsy some days later, some livers showed macroscopic changes consistent with cholestasis, except for some patchy dark areas which microscopically showed hemorrhagic necrosis, platelet-fibrin thrombi, monocyte/macrophage margination, and vascular endothelial cell hypertrophy (Figure 2C).12,28,36–38 No cell infiltration was seen. Confocal microscopy has confirmed tissue factor expression on platelets, and platelet and platelet/leukocyte aggregates in liver sinusoids. The minimal or absent deposition of immunoglobulin or complement fractions and absence of a cellular infiltrate suggest that neither antibody- nor cell-mediated rejection plays a major role in injury to genetically-engineered pig liver grafts. Platelet loss and platelet/leukocyte aggregation would appear to be a major problem.21,39 However, if the native liver remains in situ, the production of primate coagulation factors leads to the development of thrombotic microangiopathy in the graft, resulting in ischemic injury and graft destruction (just as in pig heart and kidney xenografts).
Considerable progress in pig organ xenotransplantation into NHPs has been made in the past few years, largely through the availability of pigs with an increasing number of genetic manipulations.40 However, these advances have largely been confined to heart and kidney xenotransplantation,41–48 as the barriers associated with pig lung 41 and liver 2,42,43 xenotransplantation are rather more complex.
Investigation of the mechanisms of platelet loss
The studies summarized above have identified certain barriers specific to the liver that need to be overcome. The major outstanding problem preventing successful long-term pig liver xenotransplantation is the rapid loss of platelets from the recipient within minutes or hours of the transplant. Evidence has been presented to indicate that (i) primate platelets are phagocytosed by pig liver macrophages (Kupffer cells) and sinusoidal endothelial cells,20–23,44 or (ii) may be lost in platelet-leukocyte aggregates in the graft and in certain recipient organs.20,39
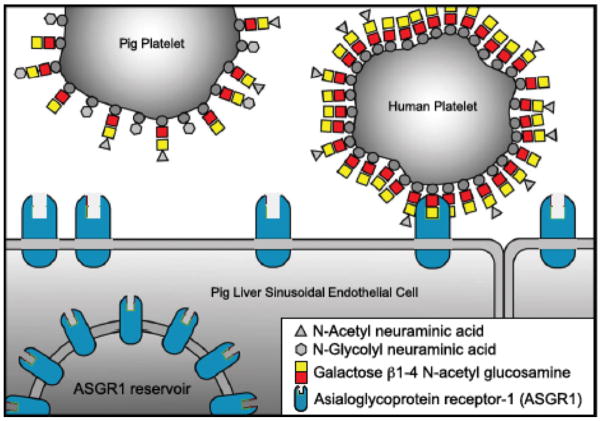
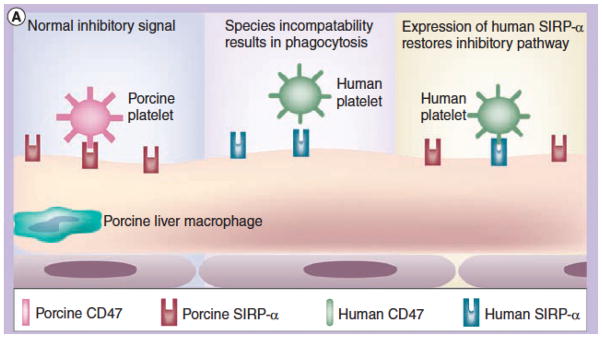
Potential factors that may contribute towards the phagocytosis include (i) the activity of the asialoglycoprotein receptor-1 (ASGR1, present on sinusoidal endothelial cells) (Figure 5) and the macrophage antigen complex-1 (CD11b/CD18; Mac-1, a surface integrin receptor on Kupffer cells),22,44 (ii) interspecies incompatibility and phagocytic dysregulation in the porcine signal regulatory protein-alpha (SIRPα)/human CD47 pathway (Figure 6),41,45–47 and (iii) upregulation of tissue factor expression on activated donor liver sinusoidal endothelial cells activated by the immune response, as well as on recipient platelets and mononuclear cells. Furthermore, ASGR1 is expressed on aortic and systemic arterial vascular endothelium in pigs and this may play a role in binding and phagocytosis of human platelets.48 There may therefore be multiple factors involved, but specific genetic engineering of the organ source pig may negate this problem.
Figure 5.
Potential mechanism of human platelet phagocytosis by pig livers - 1. Human platelets may be selectively phagocytosed by ASGR1-expressing cells, e.g., liver sinusoidal endothelial cells, as human platelets express higher levels of Galβ1,4N-acetylglucosamine (Galβ1,4GlcNAc).
Figure 6.
Potential mechanism of human platelet phagocytosis by pig livers - 2. Schematic representation of CD47-SIRPα (signal regulatory protein-alpha) interaction in relation to natural expression of SIRPα on pig Kupffer cells; (Left) In the organ-source pig, there is a normal inhibitory signal of pig CD47 (in this example expressed on platelets) that is recognized by porcine SIRPα; (Center) After pig liver xenotransplantation, there could be a lack of recognition of human CD47 by pig SIRPα, resulting in phagocytosis of human platelets; (Right) Transgenic expression of human SIRPα on pig Kupffer cells would result in recognition of human CD47 on human platelets, thus inhibiting phagocytosis. (Reproduced with permission from Ekser et al. Expert Rev. Clin. Immunol. 2012;8:621–634)
Although pigs naturally deficient in vWF have been available for some time,49–53 they are extremely difficult to work with in surgical research and, furthermore, do not resolve the problems faced in xenotransplantation. As pig vWF activates recipient primate platelets,54,55 an alternative approach might be to generate pigs in which pig vWF has been knocked out and replaced with human vWF,41 but such pigs are not yet available.
In addition to the reported loss of platelets in NHPs, the ex vivo perfusion of a pig liver with human blood demonstrated that pig macrophages continuously phagocytosed human erythrocytes;56,57 this was unrelated to antibody binding and complement activation, but was associated with direct recognition of erythrocytes by pig Kupffer cells.58,59 A pig liver removed approximately 1 unit of human erythrocytes from the circulation every 24 hours. Phagocytosis of recipient erythrocytes has not been documented in most studies in NHPs, suggesting that the expression of N-glycolylneuraminic acid (NeuGc) on pig erythrocytes and the production of anti-NeuGc antibodies in humans (but not in NHPs which, like pigs, express NeuGc) may be playing a role 60 (reviewed in 61). Pigs in which the gene for the enzyme that adds NeuGc to underlying carbohydrate chains has been deleted have recently become available,62 and Butler et al63 showed that a GTKO/NeuGc-knockout pig liver significantly reduced xenogeneic consumption of human platelets in an ex vivo perfusion study.
The impact of pig complement on the liver graft
Despite these negative aspects of pig liver xenotransplantation, there may be some advantages in comparison with xenotransplantation of the pig heart or kidney. As the liver is the major site of synthesis of complement proteins, except for C1q, factor D, properdin, and C7, impaired hepatic synthetic function contributes to complement deficiency in patients with hepatic disease.64–69 The level of membrane attack complex activity may be much lower than that seen in healthy subjects, reducing the possibility of early graft injury.
Furthermore, after pig liver transplantation in humans (or NHPs), the graft will produce pig complement. In vitro studies have indicated that (i) in the presence of human antibodies, pig complement is associated with less lysis of pig cells than human complement (and thus may be associated with less graft injury), (ii) lysis by pig complement of GTKO pig cells is significantly less than of wild-type pig cells, and (iii) the expression of a human RCA on the pig cell can reduce pig complement-induced lysis of the cells.43 Rees and his colleagues also provided evidence suggesting that human RCAs will successfully inhibit porcine complement.59 In ex vivo liver perfusion with human blood, survival of hCD55 (hDAF) transgenic pig livers for up to 72 hours indicated that a pig liver expressing both pig and human RCAs may provide some protection from injury by pig complement.56,57 Although human complement components will continue to be produced by various recipient cells, the transgenic expression of a hRCA in the graft should be protective.
Independent of the low complement levels seen in patients with hepatic failure, numerous studies have shown that the liver is more resistant than other solid organs to injury from preformed graft antibodies.70–73 In some studies, human serum caused significantly less cytotoxicity when incubated with porcine hepatic sinusoidal endothelial cells than with porcine aortic endothelial cells (25% vs 72%).69 This relative resistance to injury could possibly be related to the liver’s ability to clear soluble immune complexes,69 or to less binding of human IgM and IgG to porcine hepatic sinusoidal endothelial cells when compared to aortic endothelial cells (on flow cytometry), suggesting lower expression of antigens.74
The liver complement levels in patients with liver failure and the possible resistance of the sinusoidal endothelium to injury combine to contribute to protection of the liver from antibody-mediated rejection. Serum from patients with hepatic failure caused significantly less lysis of both porcine aortic (38% versus 72%) and hepatic sinusoidal (9% versus 25%) endothelial cells.69
The potential of sensitization to pig antigens
If a pig liver is employed to bridge the patient to allotransplantation, will exposure to pig tissues result in the production of anti-pig antibodies that might cross-react with alloantigens, thus preventing or being detrimental to allotransplantation? Studies of cross-reactivity between anti-species antibodies and/or sensitized T cells are few (reviewed in 75), but there has been little evidence for the development of cross-reactive cytotoxic antibodies or activated T cells that might mediate humoral or accelerated cellular rejection of a subsequent allograft.76–78
In this respect, the 2 patients who underwent successful liver allotransplantation following relatively transient bridging by ex vivo hemoperfusion of porcine livers (see above) did not develop anti-HLA antibodies.3 Bridging with a bioartificial liver, which incorporated porcine hepatocytes, has also been followed by successful liver allotransplantation.79 A liver allograft transplanted after a bridging pig xenograft would therefore not appear to be at increased risk of either humoral or cellular rejection.
Potential infectious risks
The risks of the transfer of microorganisms with the pig organ to the human recipient and, more importantly, of the transfer of a porcine infectious agent to the community are now considered to be small.80–84 Indeed, the use of animal organs might have some advantages with regard to recurrence of disease, such as viral hepatitis, as some viruses are species-specific. For example, following pig-to-baboon organ transplantation, porcine cytomegalovirus has not been documented to infect the host baboon, and baboon cytomegalovirus has not been seen to infect the transplanted pig organ.85,86 Nevertheless, porcine endogenous retroviruses (PERVs) are present in the genome of every pig cell, and would therefore be transplanted with the organ. Although the risk of PERVs causing any disease process in the recipient or in the community is considered low,83,84,87 using the new CRISPR/Cas9 technology, it is now possible to render pig cells PERV-negative 88 and so it is likely that PERV-negative pigs will become available in the future, if this is believed to be essential.
Most aspects of physiologic compatibility between pig and human have been sparsely investigated or not investigated at all, in part because it is difficult to accurately assess true hepatic function in the presence of an ongoing immune response.89–92 Nevertheless, significant incompatibilities between pig and human hepatic function are almost certain to be present, though some of these may be relative rather than absolute hurdles. If a function, e.g., the synthesis of a key protein, is found to be essential, and that function cannot be achieved by the pig liver, genetic modification of the source pig can be carried out to produce its human protein counterpart.
Conclusions
Attempts at liver xenotransplantation can best be justified if the patient will die rapidly without transplantation. Although prolonged function (i.e., weeks or months) of a transplanted pig liver in a primate cannot yet be guaranteed, in rapidly-deteriorating patients with acute liver failure, liver xenotransplantation (particularly if a GTKO/hRCA pig liver is implanted), could probably be considered as a short-term (days) bridge to allotransplantation as long as thrombocytopenia and coagulopathy can be prevented.93,94 Auxiliary liver xenotransplantation might allow recovery of the native organ. Orthotopic pig liver xenotransplantation would be preferable if the state of the native liver was increasing the risk of mortality to the patient, or if no recovery of the native liver is anticipated. 95
Acknowledgments
Funding
Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute at the University of Pittsburgh has been supported in part by National Institutes of Health grants U01 AI068642, R21 AI074844, and U19 AI090959, and by sponsored research agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA. Work on xenotransplantation in Xijing Hospital and the Fourth Military Medical University has been supported by the National Basic Research Program of China (973 Program; 2015CB554100), the National Natural Science Foundation (81270549 and 81300361), the Xijing Hospital disciplines boosting projects (XJZT12M09 and XJZT13Z01), the Science and Technology Research and Development Program of Shaanxi province (2013K12-18-02), and the National High Technology Research and Development Program (863 Program; 2012AA021005).
Abbreviations
- GTKO
α1,3-galactosyltransferase gene-knockout
- NeuGc
N-glycolylneuraminic acid
- NHP
nonhuman primate
- PERV
porcine endogenous retrovirus
- RCA
regulator of complement
- TFPI
tissue factor pathway inhibitor
- UNOS
United Network for Organ Sharing
- vWF
von Willebrand Factor
Footnotes
Disclosures: The authors declare no conflicts of interest.
Authors’ Contributions
DKCC - prepared the initial draft with K-FD and BE, coordinated the contributions from the other authors, and prepared the final manuscript.
K-FD – contributed significantly to the initial draft, and approved the final manuscript.
K-ST – contributed revisions to the initial draft and approved the final manuscript.
Z-XY - contributed revisions to the initial draft and approved the final manuscript.
AJT - contributed revisions to the initial draft and approved the final manuscript.
BE – helped prepare the initial draft, helped coordinate the contributions from the other authors, and approved the final manuscript.
References
- 1.UNOS. [Accessed October 20, 2015];United Network for Organ Sharing. Http://www.unos.org. Updated 2016.
- 2.Hara H, Gridelli B, Lin YJ, Marcos A, Cooper DK. Liver xenografts for the treatment of acute liver failure: clinical and experimental experience and remaining immunologic barriers. Liver Transpl. 2008;14:425–434. doi: 10.1002/lt.21476. [DOI] [PubMed] [Google Scholar]
- 3.Levy MF, Crippin J, Sutton S, et al. Liver allotransplantation after extracorporeal hepatic support with transgenic (hCD55/hCD59) porcine livers: clinical results and lack of pig-to-human transmission of the porcine endogenous retrovirus. Transplantation. 2000;69:272–280. doi: 10.1097/00007890-200001270-00013. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DK. A brief history of cross-species organ transplantation. Proc (Bayl Univ Med Cent) 2012;25:49–57. doi: 10.1080/08998280.2012.11928783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deschamps JY, Roux FA, Sai P, Gouin E. History of xenotransplantation. Xenotransplantation. 2005;12:91–109. doi: 10.1111/j.1399-3089.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi S, Cooper DK. Clinical xenotransplantation: past, present and future. Ann R Coll Surg Engl. 1997;79:13–19. [PMC free article] [PubMed] [Google Scholar]
- 7.Makowa L, Cramer DV, Hoffman A, et al. The use of a pig liver xenograft for temporary support of a patient with fulminant hepatic failure. Transplantation. 1995;59:1654–1659. doi: 10.1097/00007890-199506270-00002. [DOI] [PubMed] [Google Scholar]
- 8.Makowka L, Wu GD, Hoffman A, et al. Immunohistopathologic lesions associated with the rejection of a pig-to-human liver xenograft. Transplant Proc. 1994;26:1074–1075. [PubMed] [Google Scholar]
- 9.Cooper DK, Bottino R. Recent advances in understanding xenotransplantation: implications for the clinic. Expert Rev Clin Immunol. 2015;11:1379–1390. doi: 10.1586/1744666X.2015.1083861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez P, Chavez R, Majado M, et al. Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days. Transplantation. 2000;70:989–998. doi: 10.1097/00007890-200010150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez P, Yelamos J, Parrilla P, Chavez R. Hepatic xenotransplantation will benefit from strategies aimed to reduce complement activation. Liver Transpl. 2001;7:562–563. doi: 10.1002/lt.500070618. [DOI] [PubMed] [Google Scholar]
- 12.Ekser B, Klein E, He J, et al. Genetically-engineered pig-to-baboon liver xenotransplantation: histopathology of xenografts and native organs. PLoS One. 2012;7:e29720. doi: 10.1371/journal.pone.0029720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valdivia LA, Fung JJ, Demetris AJ, et al. Donor species complement after liver xenotransplantation. The mechanism of protection from hyperacute rejection. Transplantation. 1994;57:918–922. doi: 10.1097/00007890-199403270-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celli S, Valdivia LA, Fung JJ, Kelly RH. Early recipient-donor switch of the complement type after liver xenotransplantation. Immunol Invest. 1997;26:589–600. doi: 10.3109/08820139709088543. [DOI] [PubMed] [Google Scholar]
- 15.Valdivia LA, Lewis JH, Celli S, et al. Hamster coagulation and serum proteins in rat recipients of hamster xenografts. Transplantation. 1993;56:489–490. doi: 10.1097/00007890-199308000-00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez P, Montoya MJ, Rios A, et al. Prevention of hyperacute rejection in a model of orthotopic liver xenotransplantation from pig to baboon using polytransgenic pig livers (CD55, CD59, and H-transferase) Transplant Proc. 2005;37:4103–4106. doi: 10.1016/j.transproceed.2005.09.186. [DOI] [PubMed] [Google Scholar]
- 17.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 18.Ekser B, Echeverri GJ, Hassett AC, et al. Hepatic function after genetically engineered pig liver transplantation in baboons. Transplantation. 2010;90:483–493. doi: 10.1097/TP.0b013e3181e98d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K, Schuetz C, Elias N, et al. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation. 2012;19:256–264. doi: 10.1111/j.1399-3089.2012.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng Q, Yeh H, Wei L, et al. Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells. PLoS One. 2012;7:e47273. doi: 10.1371/journal.pone.0047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burlak C, Paris LL, Chihara RK, et al. The fate of human platelets perfused through the pig liver: implications for xenotransplantation. Xenotransplantation. 2010;17:350–361. doi: 10.1111/j.1399-3089.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- 22.Paris LL, Chihara RK, Reyes LM, et al. ASGR1 expressed by porcine enriched liver sinusoidal endothelial cells mediates human platelet phagocytosis in vitro. Xenotransplantation. 2011;18:245–251. doi: 10.1111/j.1399-3089.2011.00639.x. [DOI] [PubMed] [Google Scholar]
- 23.Paris LL, Chihara RK, Sidner RA, Tector AJ, Burlak C. Differences in human and porcine platelet oligosaccharides may influence phagocytosis by liver sinusoidal cells in vitro. Xenotransplantation. 2012;19:31–39. doi: 10.1111/j.1399-3089.2011.00685.x. [DOI] [PubMed] [Google Scholar]
- 24.Paris LL, Estrada JL, Li P, et al. Reduced human platelet uptake by pig livers deficient in the asialoglycoprotein receptor 1 protein. Xenotransplantation. 2015;22:203–210. doi: 10.1111/xen.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh H, Machaidze Z, Wamala I, et al. Increased transfusion-free survival following auxiliary pig liver xenotransplantation. Xenotransplantation. 2014;21:454–464. doi: 10.1111/xen.12111. [DOI] [PubMed] [Google Scholar]
- 26.Navarro-Alvarez N, Shah JA, Zhu A, et al. The effects of exogenous administration of human coagulation factors following pig-to-baboon liver xenotransplantation. Am J Transplant. doi: 10.1111/ajt.13647. [published online ahead of print November 27th, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah JA, Navarro-Alvarez N, DeFazio M, et al. A bridge to somewhere: 25-day survival after pig-to-baboon liver xenotransplantation. Ann Surg. doi: 10.1097/SLA.0000000000001659. [published online ahead of print January 28th, 2016] [DOI] [PubMed] [Google Scholar]
- 28.Ji H, Li X, Yue S, et al. Pig BMSCs transfected with human TFPI combat species incompatibility and regulate the human TF pathway in vitro and in a rodent model. Cell Physiol Biochem. 2015;36:233–249. doi: 10.1159/000374067. [DOI] [PubMed] [Google Scholar]
- 29.Lambrigts D, Sachs DH, Cooper DK. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation. 1998;66:547–561. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- 30.Olausson M, Mjornstedt L, Norden G, et al. Successful combined partial auxiliary liver and kidney transplantation in highly sensitized cross-match positive recipients. Am J Transplant. 2007;7:130–136. doi: 10.1111/j.1600-6143.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 31.Lin CC, Chen D, McVey JH, Cooper DK, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702–709. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CC, Cooper DK, Dorling A. Coagulation dysregulation as a barrier to xenotransplantation in the primate. Transpl Immunol. 2009;21:75–80. doi: 10.1016/j.trim.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowan PJ, Robson SC, d’Apice AJ. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16:214–221. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ezzelarab M, Ezzelarab C, Wilhite T, et al. Genetically-modified pig mesenchymal stromal cells: xenoantigenicity and effect on human T-cell xenoresponses. Xenotransplantation. 2011;18:183–195. doi: 10.1111/j.1399-3089.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Ezzelarab MB, Ayares D, Cooper DK. The potential role of genetically-modified pig mesenchymal stromal cells in xenotransplantation. Stem Cell Rev. 2014;10:79–85. doi: 10.1007/s12015-013-9478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekser B, Burlak C, Waldman JP, et al. Immunobiology of liver xenotransplantation. Expert Rev Clin Immunol. 2012;8:621–634. doi: 10.1586/eci.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekser B, Lin CC, Long C, et al. Potential factors influencing the development of thrombocytopenia and consumptive coagulopathy after genetically modified pig liver xenotransplantation. Transpl Int. 2012;25:882–896. doi: 10.1111/j.1432-2277.2012.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo Y, Kosanke S, Mieles L, et al. Comparative histopathology of hepatic allografts and xenografts in the nonhuman primate. Xenotransplantation. 1998;5:197–206. doi: 10.1111/j.1399-3089.1998.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 39.Ezzelarab M, Ekser B, Gridelli B, Iwase H, Ayares D, Cooper DK. Thrombocytopenia after pig-to-baboon liver xenotransplantation: where do platelets go? Xenotransplantation. 2011;18:320–327. doi: 10.1111/j.1399-3089.2011.00679.x. [DOI] [PubMed] [Google Scholar]
- 40.Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D. The role of genetically-engineered pigs in xenotransplantation research. J Pathol. 2016;238:288–299. doi: 10.1002/path.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper DK, Ekser B, Burlak C, et al. Clinical lung xenotransplantation--what donor genetic modifications may be necessary? Xenotransplantation. 2012;19:144–158. doi: 10.1111/j.1399-3089.2012.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ekser B, Gridelli B, Veroux M, Cooper DK. Clinical pig liver xenotransplantation: how far do we have to go? Xenotransplantation. 2011;18:158–167. doi: 10.1111/j.1399-3089.2011.00642.x. [DOI] [PubMed] [Google Scholar]
- 43.Hara H, Campanile N, Tai HC, et al. An in vitro model of pig liver xenotransplantation--pig complement is associated with reduced lysis of wild-type and genetically modified pig cells. Xenotransplantation. 2010;17:370–378. doi: 10.1111/j.1399-3089.2010.00602.x. [DOI] [PubMed] [Google Scholar]
- 44.Chihara RK, Paris LL, Reyes LM, et al. Primary porcine Kupffer cell phagocytosis of human platelets involves the CD18 receptor. Transplantation. 2011;92:739–744. doi: 10.1097/TP.0b013e31822bc986. [DOI] [PubMed] [Google Scholar]
- 45.Ide K, Wang H, Tahara H, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104:5062–5066. doi: 10.1073/pnas.0609661104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navarro-Alvarez N, Yang YG. CD47: a new player in phagocytosis and xenograft rejection. Cell Mol Immunol. 2011;8:285–288. doi: 10.1038/cmi.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang YG. CD47 in xenograft rejection and tolerance induction. Xenotransplantation. 2010;17:267–273. doi: 10.1111/j.1399-3089.2010.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bongoni AK, Kiermeir D, Denoyelle J, et al. Porcine extrahepatic vascular endothelial asialoglycoprotein receptor 1 mediates xenogeneic platelet phagocytosis in vitro and in human-to-pig ex vivo xenoperfusion. Transplantation. 2015;99:693–701. doi: 10.1097/TP.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 49.Badimon L, Badimon JJ, Rand J, Turitto VT, Fuster V. Platelet deposition on von Willebrand factor-deficient vessels. Extracorporeal perfusion studies in swine with von Willebrand’s disease using native and heparinized blood. J Lab Clin Med. 1987;110:634–647. [PubMed] [Google Scholar]
- 50.Brouland JP, Egan T, Roussi J, et al. In vivo regulation of von Willebrand factor synthesis: von Willebrand factor production in endothelial cells after lung transplantation between normal pigs and von Willebrand factor-deficient pigs. Arterioscler Thromb Vasc Biol. 1999;19:3055–3062. doi: 10.1161/01.atv.19.12.3055. [DOI] [PubMed] [Google Scholar]
- 51.Cantu E, Balsara KR, Li B, et al. Prolonged function of macrophage, von Willebrand factor-deficient porcine pulmonary xenografts. Am J Transplant. 2007;7:66–75. doi: 10.1111/j.1600-6143.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- 52.Lau CL, Cantu E, 3rd, Gonzalez-Stawinski GV, et al. The role of antibodies and von Willebrand factor in discordant pulmonary xenotransplantation. Am J Transplant. 2003;3:1065–1075. doi: 10.1034/j.1600-6143.2003.00190.x. [DOI] [PubMed] [Google Scholar]
- 53.Meyer C, Wolf P, Romain N, et al. Use of von Willebrand diseased kidney as donor in a pig-to-primate model of xenotransplantation. Transplantation. 1999;67:38–45. doi: 10.1097/00007890-199901150-00006. [DOI] [PubMed] [Google Scholar]
- 54.Schulte am Esch J, 2nd, Cruz MA, Siegel JB, Anrather J, Robson SC. Activation of human platelets by the membrane-expressed A1 domain of von Willebrand factor. Blood. 1997;90:4425–4437. [PubMed] [Google Scholar]
- 55.Schulte Am Esch J, 2nd, Robson SC, Knoefel WT, Hosch SB, Rogiers X. O-linked glycosylation and functional incompatibility of porcine von Willebrand factor for human platelet GPIb receptors. Xenotransplantation. 2005;12:30–37. doi: 10.1111/j.1399-3089.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 56.Luo Y, Levy G, Ding J, et al. HDAF transgenic pig livers are protected from hyperacute rejection during ex vivo perfusion with human blood. Xenotransplantation. 2002;9:36–44. doi: 10.1034/j.1399-3089.2002.0o140.x. [DOI] [PubMed] [Google Scholar]
- 57.Rees MA, Butler AJ, Chavez-Cartaya G, et al. Prolonged function of extracorporeal hDAF transgenic pig livers perfused with human blood. Transplantation. 2002;73:1194–1202. doi: 10.1097/00007890-200204270-00003. [DOI] [PubMed] [Google Scholar]
- 58.Ide K, Ohdan H, Kobayashi T, Hara H, Ishiyama K, Asahara T. Antibody- and complement-independent phagocytotic and cytolytic activities of human macrophages toward porcine cells. Xenotransplantation. 2005;12:181–188. doi: 10.1111/j.1399-3089.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 59.Rees MA, Butler AJ, Negus MC, Davies HF, Friend PJ. Classical pathway complement destruction is not responsible for the loss of human erythrocytes during porcine liver perfusion. Transplantation. 2004;77:1416–1423. doi: 10.1097/01.tp.0000121135.24688.a3. [DOI] [PubMed] [Google Scholar]
- 60.Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha 1–3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J. 1996;13:947–953. doi: 10.1007/BF01053190. [DOI] [PubMed] [Google Scholar]
- 61.Padler-Karavani V, Varki A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18:1–5. doi: 10.1111/j.1399-3089.2011.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lutz AJ, Li P, Estrada JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 63.Butler JR, Paris LL, Blankenship RL, et al. Silencing porcine CMAH and GGTA1 genes significantly reduces xenogeneic consumption of human platelets by porcine livers. Transplantation. 2016;100:571–576. doi: 10.1097/TP.0000000000001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tector AJ, Fridell JA, Ruiz P, et al. Experimental discordant hepatic xenotransplantation in the recipient with liver failure: implications for clinical bridging trials. J Am Coll Surg. 2000;191:54–64. doi: 10.1016/s1072-7515(00)00293-3. [DOI] [PubMed] [Google Scholar]
- 65.Colten HR. Biosynthesis of complement. Adv Immunol. 1976;22:67–118. doi: 10.1016/s0065-2776(08)60548-9. [DOI] [PubMed] [Google Scholar]
- 66.Ellison RT, 3rd, Horsburgh CR, Jr, Curd J. Complement levels in patients with hepatic dysfunction. Dig Dis Sci. 1990;35:231–235. doi: 10.1007/BF01536768. [DOI] [PubMed] [Google Scholar]
- 67.Morgan BP, Gasque P. Extrahepatic complement biosynthesis: where, when and why? Clin Exp Immunol. 1997;107:1–7. doi: 10.1046/j.1365-2249.1997.d01-890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tector AJ, Berho M, Fridell JA, et al. Rejection of pig liver xenografts in patients with liver failure: implications for xenotransplantation. Liver Transpl. 2001;7:82–89. doi: 10.1053/jlts.2001.21281. [DOI] [PubMed] [Google Scholar]
- 69.Tector AJ, Elias N, Rosenberg L, et al. Mechanisms of resistance to injury in pig livers perfused with blood from patients in liver failure. Transplant Proc. 1997;29:966–969. doi: 10.1016/s0041-1345(96)00331-4. [DOI] [PubMed] [Google Scholar]
- 70.Tector AJ, Chen X, Soderland C, Tchervenkov JI. Complement activation in discordant hepatic xenotransplantation. Xenotransplantation. 1998;5:257–261. doi: 10.1111/j.1399-3089.1998.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 71.Platt JL. Xenotransplantation of the liver: is more complement control needed? Liver Transpl. 2001;7:933–934. doi: 10.1002/lt.500071017. [DOI] [PubMed] [Google Scholar]
- 72.Nakamura K, Murase N, Becich MJ, et al. Liver allograft rejection in sensitized recipients. Observations in a clinically relevant small animal model. Am J Pathol. 1993;142:1383–1391. [PMC free article] [PubMed] [Google Scholar]
- 73.Manez R, Kelly RH, Kobayashi M, et al. Immunoglobulin G lymphocytotoxic antibodies in clinical liver transplantation: studies toward further defining their significance. Hepatology. 1995;21:1345–1352. doi: 10.1002/hep.1840210519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cattan P, Zhang B, Braet F, et al. Comparison between aortic and sinusoidal liver endothelial cells as targets of hyperacute xenogeneic rejection in the pig to human combination. Transplantation. 1996;62:803–810. doi: 10.1097/00007890-199609270-00018. [DOI] [PubMed] [Google Scholar]
- 75.Cooper DK, Tseng YL, Saidman SL. Alloantibody and xenoantibody cross-reactivity in transplantation. Transplantation. 2004;77:1–5. doi: 10.1097/01.TP.0000105116.74032.63. [DOI] [PubMed] [Google Scholar]
- 76.Baertschiger RM, Dor FJ, Prabharasuth D, Kuwaki K, Cooper DK. Absence of humoral and cellular alloreactivity in baboons sensitized to pig antigens. Xenotransplantation. 2004;11:27–32. doi: 10.1111/j.1399-3089.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 77.Key T, Schuurman HJ, Taylor CJ. Does exposure to swine leukocyte antigens after pig-to-nonhuman primate xenotransplantation provoke antibodies that cross-react with human leukocyte antigens? Xenotransplantation. 2004;11:452–456. doi: 10.1111/j.1399-3089.2004.00167.x. [DOI] [PubMed] [Google Scholar]
- 78.Ye Y, Luo Y, Kobayashi T, et al. Secondary organ allografting after a primary “bridging” xenotransplant. Transplantation. 1995;60:19–22. doi: 10.1097/00007890-199507150-00004. [DOI] [PubMed] [Google Scholar]
- 79.Baquerizo A, Mhoyan A, Kearns-Jonker M, et al. Characterization of human xenoreactive antibodies in liver failure patients exposed to pig hepatocytes after bioartificial liver treatment: an ex vivo model of pig to human xenotransplantation. Transplantation. 1999;67:5–18. doi: 10.1097/00007890-199901150-00003. [DOI] [PubMed] [Google Scholar]
- 80.Paradis K, Langford G, Long Z, et al. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 81.Onions D, Cooper DK, Alexander TJ, et al. An approach to the control of disease transmission in pig-to-human xenotransplantation. Xenotransplantation. 2000;7:143–155. doi: 10.1034/j.1399-3089.2000.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fishman JA. Screening of source animals and clinical monitoring for xenotransplantation. Xenotransplantation. 2007;14:349–352. [Google Scholar]
- 83.Wynyard S, Nathu D, Garkavenko O, Denner J, Elliott R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation. 2014;21:309–323. doi: 10.1111/xen.12102. [DOI] [PubMed] [Google Scholar]
- 84.Denner J, Mueller NJ. Preventing transfer of infectious agents. Int J Surg. 2015;23:306–311. doi: 10.1016/j.ijsu.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mueller NJ, Kuwaki K, Dor FJ, et al. Reduction of consumptive coagulopathy using porcine cytomegalovirus-free cardiac porcine grafts in pig-to-primate xenotransplantation. Transplantation. 2004;78:1449–1453. doi: 10.1097/01.tp.0000141361.68446.1f. [DOI] [PubMed] [Google Scholar]
- 86.Mueller NJ, Livingston C, Knosalla C, et al. Activation of porcine cytomegalovirus, but not porcine lymphotropic herpesvirus, in pig-to-baboon xenotransplantation. J Infect Dis. 2004;189:1628–1633. doi: 10.1086/383351. [DOI] [PubMed] [Google Scholar]
- 87.Denner J, Tonjes RR. Infection barriers to successful xenotransplantation focusing on porcine endogenous retroviruses. Clin Microbiol Rev. 2012;25:318–343. doi: 10.1128/CMR.05011-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang L, Guell M, Niu D, et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs) Science. 2015;350:1101–1104. doi: 10.1126/science.aad1191. [DOI] [PubMed] [Google Scholar]
- 89.Hammer C. Evolutionary obstacles to xenotransplantation. In: Cooper DKCKE, Platt JL, White DJG, editors. Xenotransplantation. 2. Heidelberg: Springer; 1997. pp. 716–735. [Google Scholar]
- 90.Hammer C. Physiological obstacles after xenotransplantation. Ann N Y Acad Sci. 1998;862:19–27. doi: 10.1111/j.1749-6632.1998.tb09113.x. [DOI] [PubMed] [Google Scholar]
- 91.Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13:488–499. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 92.Kanazawa A, Platt JL. Prospects for xenotransplantation of the liver. Semin Liver Dis. 2000;20:511–522. doi: 10.1055/s-2000-13159. [DOI] [PubMed] [Google Scholar]
- 93.Ekser B, Gridelli B, Tector AJ, Cooper DK. Pig liver xenotransplantation as a bridge to allotransplantation: which patients might benefit? Transplantation. 2009;88:1041–1049. doi: 10.1097/TP.0b013e3181ba0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Horton PJ, Chaudhury P, Rochon C, Metrakos P, Tchervenkov J. Should trials of liver xenotransplantation proceed in toxic fulminant hepatic failure? Xenotransplantation. 2006;13:483. doi: 10.1111/j.1399-3089.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 95.Tector J. New Hope for Liver Xenotransplantation. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001680. [DOI] [PubMed] [Google Scholar]
- 96.Calne RY. Organ transplantation between widely disparate species. Transplant Proc. 1970;2:550–556. [PubMed] [Google Scholar]
- 97.Calne RY, Davis DR, Pena JR, et al. Hepatic Allografts and xenografts in primates. Lancet. 1970;7638:103–106. doi: 10.1016/s0140-6736(70)90462-9. [DOI] [PubMed] [Google Scholar]
- 98.Calne RY, White HJ, Herbertson BM, et al. Pig to baboon liver xenografts. Lancet. 1968;7553:1176–1178. doi: 10.1016/s0140-6736(68)91869-2. [DOI] [PubMed] [Google Scholar]
- 99.Powelson J, Cosimi AB, Austen W, Jr, et al. Porcine-to-primate orthotopic liver transplantation. Transplant Proc. 1994;26:1353–1354. [PubMed] [Google Scholar]
- 100.Mieles L, Ye Y, Luo Y, et al. Auxiliary liver allografting and xenografting in the nonhuman primate. Transplantation. 1995;59:1670–1676. doi: 10.1097/00007890-199506270-00005. [DOI] [PubMed] [Google Scholar]