Abstract
Background
Time to intrinsicoid deflection (ID), the time from onset of the QRS complex to the peak of the R wave on the electrocardiogram, represents delayed ventricular activation and suggests that impaired myocardial function is present. It is unknown whether delayed time to ID is predictive of future heart failure (HF) events.
Hypothesis
Delayed time to ID is predictive of future HF events.
Methods
A total of 6394 participants (mean age, 62 ± 10 years; 54% women; 38% whites, 28% blacks, 22% Hispanics, 12% Chinese Americans) without clinically apparent cardiovascular disease or major ventricular conduction delay (QRS ≥120 ms) from the Multi‐Ethnic Study of Atherosclerosis were included. Time to ID was automatically measured from baseline electrocardiograms (2000–2002) as the maximum value in leads V5 and V6. Cox regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between time to ID and HF.
Results
Over a median follow‐up of 11.2 years, a total of 217 (3.4%) participants developed HF (incidence rate per 1000 person‐years: 3.33, 95% CI: 2.91‐3.80). In a multivariable Cox regression analysis adjusted for demographics, cardiovascular risk factors, and potential confounders, each 10‐ms increase in maximum time to ID was associated with an increased risk for HF (HR: 1.42, 95% CI: 1.15‐1.74). The results remained similar when stratified by age, sex, and race/ethnicity.
Conclusions
Delayed time to ID is able to identify individuals at risk for developing HF before major ventricular conduction delays (eg, bundle branch block) are evident.
Keywords: Heart failure/cardiac transplantation/cardiomyopathy/myocarditis, Electrocardiography ambulatory ECG, Epidemiology
1. INTRODUCTION
Intrinsicoid deflection (ID) corresponds to the peak of the R wave.1 The time from beginning of the QRS complex to peak R wave is described as the time to ID. This measure represents the time for excitation to spread from the endocardial to the epicardial surface of the left ventricle. Time to ID has been proposed to represent a more accurate measure of delayed left ventricular (LV) activation due to underlying structural abnormalities than QRS duration, which measures global ventricular activation.2
Several epidemiological studies have shown that the risk of incident heart failure (HF) increases with QRS prolongation, including the presence of left bundle branch block.3, 4, 5, 6, 7 In this framework, it is clear that delayed global LV activation increases one's risk for developing HF. However, the risk afforded by a more accurate measure of delayed LV activation, such as time to ID, has not been examined.
We hypothesized that time to ID will be predictive of future HF events, as it is more likely to detect delayed LV activation due to underlying abnormalities of LV function. We tested this hypothesis in individuals free of apparent cardiovascular disease and major ventricular conduction delays in the population‐based Multi‐Ethnic Study of Atherosclerosis (MESA).
2. METHODS
2.1. Study Population
Details of MESA have been reported previously.8 Briefly, between July 2000 and September 2002, a total of 6814 persons were recruited at 6 field centers (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota). Participants were required to be between 45 and 84 years of age and to have no clinical cardiovascular disease. All participants provided informed consent and the study protocol was approved by the institutional review boards at each participating institution. For the purpose of this analysis, participants were excluded if they were missing the following: baseline time to ID measurements (n = 104), HF follow‐up data (n = 5), or baseline characteristics (n = 28). We also excluded those with major ventricular conduction delays (QRS duration ≥120 ms, including complete left and right bundle branch blocks; n = 283).
2.2. Baseline Characteristics
Participant characteristics were collected during the initial MESA visit. Age, sex, race/ethnicity, income, and education were self‐reported. Annual income was categorized as < $20 000 or ≥ $20 000, and education was categorized as “high school or less,” or “some college or more.” Smoking was defined as ever (eg, current or former) vs never smoker. Blood samples were obtained after a 12‐hour fast, and measurements of total cholesterol, high‐density lipoprotein cholesterol, and plasma glucose were used. Diabetes mellitus (DM) was defined as fasting glucose ≥126 mg/dL or a history of DM medication use. Blood pressure was measured for each participant after 5 minutes in the seated position; systolic measurements were recorded 3 separate times, and the mean of the last 2 values was used. The use of aspirin, statins, and antihypertensive medications was self‐reported. Body mass index (BMI) was computed as the weight in kilograms divided by the square of the height in meters. Left ventricular hypertrophy was defined by the Cornell criteria (R‐wave amplitude in lead aVL + S‐wave amplitude in lead V3 ≥2.8 mV in males and ≥2.0 mV in females) using baseline electrocardiogram (ECG) data.9 Resting heart rate also was obtained from baseline ECGs.
2.3. Time to Intrinsicoid Deflection
In MESA, 12‐lead digital ECGs were obtained by trained technicians on GE MAC 1200 ECG system (GE Healthcare, Milwaukee, WI) using standardized procedures. Electrocardiograms were transmitted electronically to the MESA ECG Reading Center located at the Epidemiology Cardiology Research Center (Wake Forest School of Medicine, Winston‐Salem, NC). According to MESA protocol, all filters in the ECG machines were disabled to provide unfiltered measurements. All ECGs were automatically processed, after visual inspection for technical errors and inadequate quality, using the 2001 version of the GE Marquette 12SL program (GE Healthcare). As part of routine quality‐control measures regarding ECG data processing, trained staff perform visual inspection of main ECG waveforms and confirm computer‐detected ECG abnormalities. Time to ID was automatically measured in leads V5 and V6 (the LV chest leads), and the maximum of both values (in milliseconds) was used in the main analysis. QRS duration also was used and computed as the average duration in all leads.
2.4. Heart Failure
The ascertainment of incident HF events has been previously described.10 Participants were followed for incident cardiovascular events from baseline through December 31, 2012. At intervals of 9 to 12 months, a telephone interviewer contacted each participant to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses, procedures, and deaths. Additionally, MESA occasionally identified medical encounters through cohort clinic visits, participant call‐ins, medical‐record abstractions, or obituaries. Next‐of‐kin interviews for out‐of‐hospital cardiovascular deaths also were used.
The outcome of interest for this analysis was the composite of probable and definite HF events. Definite or probable HF required symptoms, such as shortness of breath or edema, as asymptomatic disease is not a MESA endpoint. In addition to symptoms, probable HF required a previous physician diagnosis and also for the patient to be receiving medical treatment for HF. Definite HF required ≥1 other criteria, such as pulmonary edema/congestion by chest x‐ray; dilated ventricle or poor LV function by echocardiography or ventriculography; or evidence of LV diastolic dysfunction. For this analysis, systolic and diastolic HF events were grouped together.
2.5. Statistical Analysis
Baseline characteristics were compared by HF status. Categorical variables were reported as frequency and percentage; continuous variables were recorded as mean ± SD. Statistical significance for categorical variables was tested using the χ2 method and the t test for continuous variables.
Follow‐up time was defined as the time between the baseline time to ID measurement until a diagnosis of HF, death, loss to follow‐up, or end of follow‐up (December 31, 2012). To explore the potential nonlinear association between time to ID and HF, we used a restricted cubic spline model with incorporated knots at the 5th, 50th, and 95th percentiles.11 Kaplan‐Meier estimates were used to compute the cumulative incidence of HF stratified by the maximum time to ID value of 45 ms (delayed time to ID) based on the results of the restricted cubic spline model, and the difference in estimates was compared using the log‐rank procedure. Cox regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between time to ID and HF. P values were computed using the likelihood ratio test. Multivariable models were constructed as follows: Model 1 adjusted for age, sex, race/ethnicity, income, and education; Model 2 adjusted for Model 1 covariates plus systolic blood pressure, heart rate, smoking, DM, BMI, cholesterol, high‐density lipoprotein cholesterol, aspirin, statins, antihypertensive medications, and left ventricular hypertrophy. We tested for interactions between our main effect variable and age (dichotomized at the median age of the study population: 61 years), sex, and race/ethnicity (whites vs nonwhites). A sensitivity analysis was performed in which the association between time to ID and HF was examined by HF subtype (eg, systolic vs diastolic).
An additional analysis was performed using covariates (age, heart rate, systolic blood pressure, BMI, DM, coronary heart disease, valve disease) from the Framingham HF risk score to determine whether delayed time to ID was able to improve prediction of future HF events.12 We computed the Harrell concordance index (C‐index) for the model with and without delayed time to ID using methodology developed for survival analyses.13 The added predictive ability of delayed time to ID was investigated using integrated discrimination improvement (IDI) and relative IDI.14 The IDI quantifies the increase in the difference between mean predicted risks for participants who do and do not develop HF after adding delayed time to ID to the model. Additionally, net reclassification improvement (NRI), which quantifies any desirable change in predicted risk, was computed using the following risk categories: <2.5%, 2.5% to 5%, and >5%. Confidence intervals for both IDI and NRI were computed using bootstrapping with 1000 replicates.15
To compare the predictive ability of delayed time to ID with QRS duration, several sensitivity analyses were performed. We compared the predictive ability of time to ID with the peak R wave to QRS end duration. We also examined the HF risk associated with intraventricular conduction delay (QRS >100 ms) and delayed time to ID (>45 ms) in separate models. Additionally, we examined the HF risk in participants with both intraventricular conduction delay and delayed time to ID to determine if delayed time to ID was able to provide additional prognostic information beyond QRS duration.
The proportional hazards assumption was not violated in our analyses. Statistical significance was defined as P < 0.05. SAS version 9.4 (SAS Institute, Inc., Cary, NC) was used for all analyses.
3. RESULTS
A total of 6394 participants (mean age, 62 ± 10 years; 54% women; 38% whites, 28% blacks, 22% Hispanics, 12% Chinese Americans) were included in the final analysis. Baseline characteristics stratified by incident HF are shown in Table 1.
Table 1.
Baseline Characteristics (N = 6394)
| Characteristic | HF, n = 217 | No HF, n = 6177 | P Valuea |
|---|---|---|---|
| Age, y | 68 ± 8.8 | 62 ± 10 | <0.0001 |
| Male sex | 127 (59) | 2831 (46) | 0.0002 |
| Race/ethnicity | |||
| White | 87 (40) | 2326 (38) | |
| Chinese American | 16 (7.0) | 765 (12) | |
| Black | 63 (29) | 1709 (28) | |
| Hispanic | 51 (24) | 1377 (22) | 0.18 |
| Education, high school or less | 97 (45) | 2239 (36) | 0.011 |
| Income < $20 000 | 81 (37) | 1635 (26) | 0.0004 |
| BMI, kg/m2 | 30 ± 6.3 | 28 ± 5.4 | <0.0001 |
| Ever smoker | 123 (57) | 3028 (49) | 0.027 |
| DM | 77 (35) | 811 (13) | <0.0001 |
| SBP, mm Hg | 139 ± 23 | 126 ± 21 | <0.0001 |
| Total cholesterol, mg/dL | 190 ± 34 | 195 ± 36 | 0.059 |
| HDL‐C, mg/dL | 48 ± 13 | 51 ± 15 | 0.0049 |
| Antihypertensive medications | 126 (58) | 2211 (36) | <0.0001 |
| Aspirin | 76 (35) | 1420 (23) | <0.0001 |
| Statin | 40 (18) | 894 (14) | 0.10 |
| LVH | 19 (8.8) | 204 (3.3) | <0.0001 |
| QRS duration, ms | 94 ± 9.8 | 91 ± 9.7 | 0.0003 |
| Lead V5 time to ID, ms | 35 ± 7.1 | 34 ± 6.8 | 0.13 |
| Lead V6 time to ID, ms | 36 ± 7.8 | 35 ± 7.6 | 0.031 |
| Maximum time to ID, ms | 38 ± 6.9 | 37 ± 6.4 | 0.049 |
| Heart rate, bpm | 66 ± 10 | 63 ± 9.6 | <0.0001 |
Abbreviations: BMI, body mass index; bpm, beats per minute; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; ID, intrinsicoid deflection; LVH, left ventricular hypertrophy; ms, millisecond; SBP, systolic blood pressure; SD, standard deviation; y, years
Data are presented as n (%) or mean ± SD.
Statistical significance for continuous data was tested using the t test; categorical data were tested using the χ2 test.
Over a median follow‐up of 11.2 years, a total of 217 (3.4%) participants developed HF (incidence rate per 1000 person‐years: 3.33, 95% CI: 2.91‐3.80). A dose–response relationship was observed between maximum time to ID and the risk of HF. The Figure 1 shows the association with HF across maximum time to ID values. The risk of future HF events increased considerably with time to ID values >45 ms. See Supporting Information, Figure, in the online version of this article for the cumulative incidence of HF events stratified by time to ID value of 45 ms.
Figure 1.
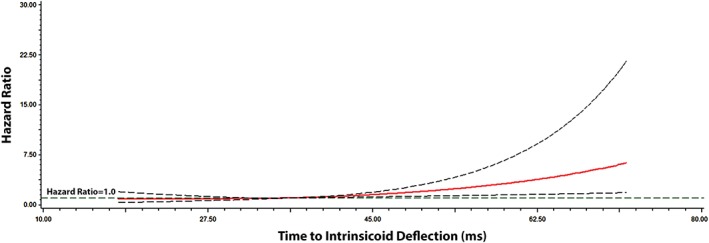
Multivariable risk of HF by time to ID. Each HR was computed with the median time to ID value of 36 ms as the reference and was adjusted for age, sex, race/ethnicity, education, income, heart rate, SBP, smoking, DM, BMI, cholesterol, HDL‐C, aspirin, statins, antihypertensive medications, and LVH. Dotted lines represent the 95% CI. Abbreviations: BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; HR, hazard ratio; ID, intrinsicoid deflection; LVH, left ventricular hypertrophy; ms, millisecond; SBP, systolic blood pressure.
In a Cox regression analysis adjusted for sociodemographics, cardiovascular risk factors, and potential confounders, each 10‐ms increase in maximum time to ID was associated with an increased risk for HF (Table 2). Similar results were obtained when individual time to ID measurements in V5 and V6 were used (Table 2). The results did not vary when the analysis was stratified by age, sex, or race/ethnicity (Table 3).
Table 2.
Risk of HFa
| Model 1, HR (95% CI)b | P Value | Model 2, HR (95% CI)c | P Value | |
|---|---|---|---|---|
| Lead V5 time to ID | 1.22 (1.01‐1.48) | 0.039 | 1.28 (1.05‐1.56) | 0.013 |
| Lead V6 time to ID | 1.29 (1.08‐1.54) | 0.0052 | 1.34 (1.12‐1.61) | 0.0014 |
| Maximum time to ID | 1.35 (1.10‐1.65) | 0.0040 | 1.42 (1.15‐1.74) | 0.0010 |
| Peak R wave to QRS end | 1.07 (0.93‐1.22) | 0.38 | 0.98 (0.85‐1.13) | 0.80 |
Abbreviations: BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; HR, hazard ratio; ID, intrinsicoid deflection; LVH, left ventricular hypertrophy; ms, millisecond; SBP, systolic blood pressure.
HR presented for time to ID per 10‐ms increase.
Adjusted for age, sex, race/ethnicity, education, and income.
Adjusted for Model 1 covariates plus heart rate, SBP, smoking, DM, BMI, cholesterol, HDL‐C, aspirin, statins, antihypertensive medications, and LVH.
Table 3.
Risk of HF by Age, Sex, and Race/Ethnicitya
| Events/No. at Risk | Model 1, HR (95% CI)b | P Value | Model 2, HR (95% CI)c | P Value | Interaction P Valued | |
|---|---|---|---|---|---|---|
| Age, ye | 0.56 | |||||
| <61 | 45/2979 | 1.19 (0.75‐1.89) | 0.47 | 1.30 (0.82‐2.06) | 0.27 | |
| ≥61 | 172/3415 | 1.32 (1.05‐1.67) | 0.017 | 1.37 (1.08‐1.73) | 0.0087 | |
| Sex | 0.14 | |||||
| M | 127/2958 | 1.50 (1.15‐1.96) | 0.0028 | 1.61 (1.23‐2.12) | 0.0006 | |
| F | 90/3436 | 1.16 (0.84‐1.60) | 0.37 | 1.20 (0.86‐1.67) | 0.27 | |
| Race/ethnicity | 0.69 | |||||
| White | 87/2413 | 1.27 (0.92‐1.75) | 0.14 | 1.32 (0.95‐1.81) | 0.095 | |
| Nonwhite | 130/3981 | 1.41 (1.08‐1.84) | 0.011 | 1.45 (1.10‐1.90) | 0.0079 |
Abbreviations: BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; F, female; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; HR, hazard ratio; ID, intrinsicoid deflection; LVH, left ventricular hypertrophy; M, male; ms, millisecond; SBP, systolic blood pressure.
HR presented for maximum time to ID per 10‐ms increase.
Adjusted for age, sex, race/ethnicity, education, and income.
Adjusted for Model 1 covariates plus heart rate, SBP, smoking, DM, BMI, cholesterol, HDL‐C, aspirin, statins, antihypertensive medications, and LVH.
Interactions tested using Model 2.
Stratified by the median age for study participants.
Of the 217 HF cases, 194 (108 systolic cases, 86 diastolic cases) had available data on HF subtype. When we separated the analysis by HF subtype, the relationship between maximum time to ID (per 10‐ms increase) and HF was limited to systolic cases (HR: 1.56, 95% CI: 1.17‐2.09) and not diastolic cases (HR: 1.37, 95% CI: 0.99‐1.89).
When maximum time to ID was included in the Framingham HF risk score, it improved the predictive ability of the original risk score beyond original covariates (Table 4). The categorical NRI showed that the addition of time to ID did improve the predictive ability of the risk score. The reclassification of participants who did and did not develop HF is shown in Supporting Information, Table, in the online version of this article.
Table 4.
Reclassification of HF Risk
| C‐Index (95% CI) | IDI (95% CI) | Relative IDI (95% CI) | NRI (95% CI)b | |
|---|---|---|---|---|
| Framinghama | 0.773 (0.744‐0.803) | — | — | — |
| Framinghama + maximum time to ID | 0.780 (0.752‐0.809) | 0.34% (0.10%‐0.59%) | 8.9% (2.5%‐16%) | 6.9% (1.5%‐13%) |
Abbreviations: BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; HF, heart failure; HR, hazard ratio; ID, intrinsicoid deflection; IDI, integrated discrimination improvement; max, maximum; NRI, net reclassification improvement; SBP, systolic blood pressure.
Adjusted for age, heart rate, SBP, BMI, and DM.
Presented for the following risk categories: <2.5%, 2.5%–5.0%, >5.0%.
Peak R wave to QRS end duration was not predictive of HF events (Table 2). The HF risk associated with delayed time to ID (>45 ms: HR: 1.77, 95% CI: 1.21‐2.59) was greater than the risk observed with intraventricular conduction delay (QRS >100 ms: HR: 1.44, 95% CI: 1.04‐1.98). Also, the HF risk in participants with both intraventricular conduction delay and delayed time to ID (HR: 2.12, 95% CI: 1.27‐3.53) was greater than the risk observed with intraventricular conduction delay in isolation.
4. DISCUSSION
In this analysis from MESA, time to ID was significantly associated with an increased risk for future HF events among a population of adults without clinically apparent cardiovascular disease or major ventricular conduction delays. This measure was shown to improve the predictive ability of the Framingham HF risk score beyond original covariates. Additionally, time to ID, and not peak R wave to QRS end duration, was associated with incident HF. We also observed a higher HF risk in participants with the combined presence of intraventricular conduction delay and delayed time to ID than with intraventricular conduction delay in isolation. In aggregate, our data suggest that time to ID is a useful marker to identify individuals who are high risk for future HF events, and it is able to provide additional prognostic information beyond traditional markers of global ventricular depolarization (eg, QRS duration).
The association between global measures of delayed ventricular activation (eg, QRS duration) and HF has been examined extensively.3, 4, 5, 6 Additionally, an analysis from MESA has shown that the risk of HF increases considerably with intraventricular conduction delay (QRS duration >100 ms).7 In contrast, the current analysis showed that delayed time to ID is associated with future HF events among participants without evidence of major ventricular conduction delays, and that the HF risk associated with intraventricular conduction delay is greater when accounting for delayed time to ID. The relationship between delayed time to ID and HF was limited to systolic cases, but we acknowledge that our study possibly was underpowered to detect a significant result for diastolic events. We also demonstrated that the risk of HF associated with the QRS complex is limited to the beginning of the QRS complex to the peak of the R wave (eg, time to ID). This suggests that additional prognostic information is gained with time to ID beyond QRS duration, a marker of global ventricular conduction. Therefore, time to ID possibly is able to identify subclinical structural abnormalities that increase one's risk for future HF events rather than the characteristic delayed‐conduction abnormalities detected with QRS duration. Our data also show that time to ID is a useful tool for HF risk prediction, as this measure was able to improve the discriminatory capacity of the Framingham HF risk score beyond covariates included in the original model. Overall, our findings provide evidence that time to ID is a novel ECG marker that identifies persons who possibly will benefit from evaluation for underlying structural abnormalities that predispose to HF.
Delayed time to ID is thought to represent conduction delay secondary to increases in LV cavity size rather than delayed conduction observed in bundle branch blocks. This is supported by a recent examination of 146 adults with aortic insufficiency that found delayed time to ID to be highly predictive of a reduced ejection fraction (<50%) with a specificity of 89.1%.16 Additionally, delayed time to ID has been shown to suggest volume overload (eg, increased left ventricular end‐diastolic volume) in patients with chronic mitral regurgitation.17 These findings that link delayed time to ID with LV dysfunction suggest that time to ID is able to detect subclinical anatomical abnormalities that predispose to HF. Our findings support this argument, as the association between QRS complex and HF was limited to time to ID and the association between intraventricular delay and HF strengthened when we accounted for delayed time to ID.
4.1. Study Limitations
The current study should be interpreted in the context of several limitations. Incident HF cases were identified by subsequent study visits, follow‐up phone calls, and the examination of hospitalization data, including International Classification of Diseases codes. Despite this effort to account for all HF cases, incident HF cases possibly were missed. However, we do not know of any reason to suggest that the resulting bias, if any, would have been differential in nature, rather than merely reducing effect estimates toward the null (eg, diminishing power to detect a statistically significant result). Also, several potential confounders were included in our multivariable models, but similar to other epidemiological studies, we acknowledge that residual confounding remains a possibility.
5. CONCLUSION
We have shown that delayed time to ID is associated with the development of future HF events before major ventricular conduction delays (eg, bundle branch block) are evident. Additionally, this ECG measure improves the discriminatory capacity of the Framingham HF risk score and is able to provide additional prognostic information beyond traditional markers of global ventricular depolarization (eg, QRS duration). Further research is needed to explore the potential usefulness of this marker to reduce the future burden that HF will place on the health care system.
Supporting information
Table S1. Reclassification of Participants with and without Heart Failure
Figure S1. Cumulative Incidence of Heart Failure by Delayed Intrinsicoid Deflection
ACKNOWLEDGMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa‐nhlbi.org.
O'Neal WT, Qureshi WT, Nazarian S, Kawel‐Boehm N, Bluemke DA, Lima JAC, Soliman EZ. Electrocardiographic Time to Intrinsicoid Deflection and Heart Failure: The Multi‐Ethnic Study of Atherosclerosis, Clin Cardiol 2016. DOI: 10.1002/clc.22561
Dr. Nazarian is a consultant and principal investigator for research funding awarded to Johns Hopkins University from Biosense Webster Inc. This research was supported by contracts N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040 and UL1‐TR‐001079 from the National Center for Research Resources.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
REFERENCES
- 1. Prineas RJ, Crow RS, Zhang ZM. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. 2nd ed. London, UK: Springer; 2010. [Google Scholar]
- 2. Del Carpio Munoz F, Powell BD, Cha YM, et al. Delayed intrinsicoid deflection onset in surface ECG lateral leads predicts left ventricular reverse remodeling after cardiac resynchronization therapy. Heart Rhythm. 2013;10:979–987. [DOI] [PubMed] [Google Scholar]
- 3. Fahy GJ, Pinski SL, Miller DP, et al. Natural history of isolated bundle branch block. Am J Cardiol. 1996;77:1185–1190. [DOI] [PubMed] [Google Scholar]
- 4. Bibbins‐Domingo K, Lin F, Vittinghoff E, et al. Predictors of heart failure among women with coronary disease. Circulation. 2004;110:1424–1430. [DOI] [PubMed] [Google Scholar]
- 5. Zhang ZM, Rautaharju PM, Prineas RJ, et al. Ventricular conduction defects and the risk of incident heart failure in the Atherosclerosis Risk in Communities (ARIC) Study. J Card Fail. 2015;21:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang ZM, Rautaharju PM, Soliman EZ, et al. Different patterns of bundle‐branch blocks and the risk of incident heart failure in the Women's Health Initiative (WHI) study. Circ Heart Fail. 2013;6:655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ilkhanoff L, Liu K, Ning H, et al. Association of QRS duration with left ventricular structure and function and risk of heart failure in middle‐aged and older adults: the Multi‐Ethnic Study of Atherosclerosis (MESA). Eur J Heart Fail. 2012;14:1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bild DE, Bluemke DA, Burke GL, et al. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 9. Devereux RB, Casale PN, Eisenberg RR, et al. Electrocardiographic detection of left ventricular hypertrophy using echocardiographic determination of left ventricular mass as the reference standard. Comparison of standard criteria, computer diagnosis and physician interpretation. J Am Coll Cardiol. 1984;3:82–87. [DOI] [PubMed] [Google Scholar]
- 10. Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the Multi‐Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62:511.e1–517.e1. [DOI] [PubMed] [Google Scholar]
- 12. Kannel WB, D'Agostino RB, Silbershatz H, et al. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. [DOI] [PubMed] [Google Scholar]
- 13. Harrell FE Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 14. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 15. Kerr KF, McClelland RL, Brown ER, et al. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol. 2011;174:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Recke SH. Left ventricular function‐conduction impairment as reflected by the ECG in chronic aortic regurgitation. Wien Klin Wochenschr. 2011;123:502–507. [DOI] [PubMed] [Google Scholar]
- 17. Recke S, Gansser R, Marienhagen J, et al. R peak time prolongation and R peak delay in leads I, V5, or V6: diagnostic values as signs of myocardial dysfunction in chronic mitral incompetence. J Electrocardiol. 1994;27:129–136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Reclassification of Participants with and without Heart Failure
Figure S1. Cumulative Incidence of Heart Failure by Delayed Intrinsicoid Deflection


