Abstract
It is well established that stress impacts the underlying processes of learning and memory. The effects of stress on memory are thought to involve, at least in part, effects on the hippocampus, which is particularly vulnerable to stress. Chronic stress induces hippocampal alterations, including but not limited to dendritic atrophy and decreased neurogenesis, which are thought to contribute to chronic stress-induced hippocampal dysfunction and deficits in learning and memory. Changes in synaptic transmission, including changes in GABAergic inhibition, have been documented following chronic stress. Recently, our laboratory demonstrated shifts in EGABA in CA1 pyramidal neurons following chronic stress, compromising GABAergic transmission and increasing excitability of these neurons. Interestingly, here we demonstrate that these alterations are unique to CA1 pyramidal neurons, since we do not observe shifts in EGABA following chronic stress in dentate gyrus granule cells. Following chronic stress, there is a decrease in the expression of the GABAA receptor (GABAAR) δ subunit and tonic GABAergic inhibition in dentate gyrus granule cells; whereas, there is an increase in the phasic component of GABAergic inhibition, evident by an increase in the peak amplitude of spontaneous inhibitory postsynaptic currents (sIPSCs). Given the numerous changes observed in the hippocampus following stress, it is difficult to pinpoint the pertinent contributing pathophysiological factors. Here we directly assess the impact of a reduction in tonic GABAergic inhibition of dentate gyrus granule cells on learning and memory using a mouse model with a decrease in GABAAR δ subunit expression specifically in dentate gyrus granule cells (Gabrd/Pomc mice). Reduced GABAAR δ subunit expression and function in dentate gyrus granule cells is sufficient to induce deficits in learning and memory. Collectively, these findings suggest that the reduction in GABAAR δ subunit-mediated tonic inhibition in dentate gyrus granule cells contributes, at least in part, to deficits in learning and memory associated with chronic stress. These findings have significant implications regarding the pathophysiological mechanisms underlying impairments in learning and memory associated with stress and suggest a role for GABAAR δ subunit containing receptors in dentate gyrus granule cells.
Keywords: GABA, stress, hippocampus, learning and memory
Introduction
Stress has deleterious effects on cognition and impairs learning and memory. However, the effects of stress on learning and memory are complex, with differences observed depending upon the source of stress, stressor duration, stressor intensity, timing of the stressor in relation to the learning and memory phase, and learning type (for review see (Sandi and Pinelo-Nava, 2007)). Despite this complex relationship, numerous studies have demonstrated that stress impairs performance on learning and memory tasks in both humans and experimental animals (for review see (McEwen and Sapolsky, 1995;Kim and Diamond, 2002)).
Stress-induced impairments in learning and memory are thought be mediated, at least in part, by glucocorticoids (for review see (Roozendaal, 2002)), given the evidence that learning impairments are observed in patients with hypercortisolism and can also be induced by administration of cortisol (Newcomer et al., 1999) (for review see (Kim and Diamond, 2002)). Further, experimentally, chronic stress causes deficits in learning and memory, which can be mimicked by administration of exogenous corticosterone (for review see (Kim and Diamond, 2002;McEwen and Sapolsky, 1995)). In addition, stress impairs the neurophysiological correlate of memory, long-term potentiation (LTP) in the hippocampus (Howland and Wang, 2008). Important for the current study, stress-induced alterations in LTP have been demonstrated specifically in the dentate gyrus (Shors and Dryver, 1994).
The hippocampus is particularly vulnerable to stress ((Conrad, 2008;Mcewen and Magarinos, 1997;Mcewen, 1999;Mcewen and Magarinos, 2001)). Stress induces alterations in the hippocampus, including but not limited to dendritic atrophy, alterations in both excitatory and inhibitory synaptic transmission, and impaired neurogenesis (for review see (McEwen and Sapolsky, 1995)). Vulnerability of the hippocampus to stress is thought to involve the action of glucocorticoids, since the hippocampus is enriched in both mineralocorticoid and glucocorticoid receptors (Reul and de Kloet, 1985). Given the well-established role for the hippocampus in learning and memory (for review see (Preston and Eichenbaum, 2013)), stress-induced alterations in hippocampal function likely contribute to the stress-induced deficits in learning and memory.
A large body of work has focused on the importance of stress-induced changes in excitatory synaptic transmission associated with impairments in learning and memory (for review see (Popoli et al., 2012)). However, alterations in GABAergic inhibition have also been demonstrated in the hippocampus following stress (for review see (Mody and Maguire, 2011)). Tonic inhibition has specifically been noted to affect learning and memory (Caraiscos et al., 2004;Collinson et al., 2002). Mice lacking the GABAAR α5 subunit, which mediates tonic inhibition in CA1 pyramidal neurons (Caraiscos et al., 2004), exhibit enhanced learning and memory (Caraiscos et al., 2004;Collinson et al., 2002), and inverse agonists of α5-containing receptors have been shown to enhance cognition (Chambers et al., 2003;Collinson et al., 2006;Dawson et al., 2006); although another study demonstrated that a reduction in the expression of the α5 subunit of the GABAAR resulted in impairments in the memory for the location of objects (Prut et al., 2010) and mice lacking the α5 subunit specifically in dentate gyrus granule cells exhibit impairments in high-interference cognitive tasks (Engin et al., 2015). Mice lacking the GABAAR δ subunit, which mediates tonic GABAergic inhibition in dentate gyrus granule cells (Stell et al., 2003), have deficits in learning and memory (Cushman et al., 2014). In addition, prolonged pharmacological enhancement of GABAAR δ subunit-mediated inhibition enhances learning and memory (Whissell et al., 2013b); however, interestingly, acute potentiation of GABAAR δ subunit-mediated inhibition impairs learning and memory (Whissell et al., 2013a). Collectively, these studies suggest that tonic GABAergic inhibition in the hippocampus may impact learning and memory. However, the contribution of tonic GABAergic inhibition to the stress-induced impairments in learning and memory remains to be determined.
Stress has previously been demonstrated to alter GABAAR δ subunit-mediated tonic inhibition of dentate gyrus granule cells (Holm et al., 2011;Maguire and Mody, 2007). The goal of the current study is to investigate whether alterations in tonic GABAergic control of dentate gyrus granule cells contribute to the chronic stress-induced impairments in learning and memory. Here we demonstrate alterations in the GABAergic control of dentate gyrus granule cells following chronic stress, including a reduction in tonic GABAergic inhibition, which is associated with impairments in learning and memory. Mice generated which have a modest reduction in the expression of the GABAAR δ subunit and a decrease in tonic inhibition specifically in dentate gyrus granule cells (Gabrd/Pomc mice) mimic the deficits in learning and memory associated with chronic stress. These findings implicate alterations of tonic inhibition in the pathophysiology underlying the chronic stress-induced deficits in learning and memory.
Materials and Methods
Animal handling
Adult (8-12 weeks of age) male and female C57BL/6J mice obtained from The Jackson Laboratory and housed at the Tufts University School of Medicine, Division of Laboratory Animal Medicine and were handled according to protocols approved by the Institutional Animal Care and Use Committee (IACUC). Animals were group housed with a maximum of 5 mice per cage with ad libitum access to food and water. Mice were provided with a normal 12 hour light/dark cycle (lights on at 7 a.m.) and all experiments were performed during the light phase.
Restraint stress was performed as previously described (MacKenzie and Maguire, 2015). Briefly, mice were restrained in a modified clear polypropylene tube (50 ml, 30 × 115 mm) which were drilled with air holes to allow for ample ventilation. The chronic stress paradigm consisted of a single 30 minute restraint episode once daily for 14 consecutive days. Following the final bout of restraint stress, the mice were either sacrificed immediately for the electrophysiology experiments or allowed to recover for 30 mins in their home cage prior to experimentation. Minimally handled mice were used as controls and allowed to acclimate to new surroundings in their home cage for a minimum of 1 hour prior to experimentation.
Generation of mouse models
Gabrd/Pomc mice
The Gabrd/Pomc mice were generated in-house by crossing floxed Gabrd mice with Pomc-Cre mice obtained from Jackson Laboratory (Stock #010714). The floxed Gabrd mice were previously generated and characterized by our laboratory (Lee and Maguire, 2013). The Gabrd/Pomc mice were genotyped using the following primer sets: floxed Gabrd 5′: GACTCCAGTTGCCAAGCCTTTAATTCC floxed Gabrd 3′: CATCTGCCTGTACCTCCAATGCCTG Pomc-Cre 5′: GCATTACCGGTCGATGCAACGAGTGATGAG Pomc-Cre 3′: GAGTGAACGAACCTGGTCGAAATCAGTGCG
The Pomc-Cre mice (at least 20 weeks of age) have been employed previously to specifically knockout genes from the dentate gyrus (McHugh et al., 2007). Pomc-Cre is also expressed in the arcuate nucleus as well as the dentate gyrus; however, the Gabrd gene is not expressed in the arcuate nucleus (Pirker et al., 2000) and therefore is not likely to impact the current study. Cre−/− littermate controls were used as the comparison group for all experiments using this mouse line.
Pomc-GFP mice
Reporter mice expressing GFP under the control of Cre recombinase driven by the Pomc promoter were generated by crossing Pomc-Cre mice obtained from Jackson Laboratory (Stock #010714) with floxed mTomato-GFP mice obtained from Jackson Laboratory (Stock #007676).
Western Blotting
Western blotting was performed as previously described (MacKenzie and Maguire, 2015;O'Toole et al., 2013). Mice were euthanized under isoflurane anesthesia by rapid decapitation and the hippocampi were removed and placed into ice cold lysis buffer containing (in mM): 10 NaPO4, 10 sodium pyrophosphate, 100 NaCl, 25 NaF, 2% Triton X-100, 0.5% deoxycholate, 1 sodium vanadate, 5 EDTA, 5 EGTA, pH 7.4 and protease inhibitors (Complete Mini, Roche, and 1 mM fresh PMSF). The samples were briefly sonicated and the lysate was incubated on ice for 30 minutes before centrifugation (14,000 rpm for 5 minutes). The supernatant was collected and used to measure total protein concentrations, determined using the DC protein assay kit (BioRad) as per the manufacturer’s instructions. Total protein (25 μg) was separated by gel electrophoresis (12% SDS polyacrylamide gels) and transferred to an Immobilin-P membrane (Millipore). The membrane was blocked in 10% non-fat milk and then probed with a polyclonal antibody against the GABAAR γ2 subunit (AbCam, ab16213, 1:1000), a polyclonal GABAAR δ subunit antibody (PhosphoSolutions, 868-GDN, 1:500) or a monoclonal β-tubulin antibody (Sigma, T8328, 1:10,000). The blots were incubated with either peroxidase labeled anti–rabbit IgG (GE Healthcare, NA934, 1:2,000) or peroxidase labeled anti–mouse IgG (GE Healthcare, NA931, 1:2,000) and immunoreactive proteins were visualized using enhanced chemiluminescence (Amersham). Optical density measurements were performed using NIH ImageJ software.
Corticosterone measurements
To verify that the chronic stress group exhibited elevated corticosterone levels, mice were euthanized by anesthetized decapitation 30 mins following the last bout of restraint stress and trunk blood was collected in serum collection tubes (Terumo, MD, USA). The serum was then isolated by high speed centrifugation (14,000 rpm, 5 minutes) and supernatant transferred to a new tube. Corticosterone concentrations were measured in duplicate using an enzyme immunoassay as described previously (MacKenzie and Maguire, 2015;Sarkar et al., 2011) and compared to a standard curve of known corticosterone concentrations as per the manufacturer’s instructions (Enzo Life Sciences). All samples from each complete experiment were stored at −80°C before testing in parallel to minimize variability.
Electrophysiological recordings
The brain was rapidly removed following decapitation under isoflurane anesthesia and immersed in ice cold artificial cerebral spinal fluid (aCSF) containing (in mM) 126 NaCl, 26 NaHCO3, 1.5 NaH2PO4, 5 KCl, 2 CaCl2, 10 dextrose (300–310 mOsm) and 3 mM kynurenic acid. Coronal sections (350 μm) were cut using a Leica vibratome and incubated at 33°C in normal aCSF for at least one hour prior to transferring to the recording chamber. The recording chamber was maintained at 33°C (in line heater, Warner Instruments) and continuously perfused at a rate of ≥ 4 ml/minute with aCSF throughout the experiment. Solutions were continuously bubbled with 95% O2 and 5% CO2. GABA (5 μM), kynurenic acid (3 mM), SR95531 (≥200 μM) and picrotoxin (100 nM) were added to the extracellular solution where indicated.
Patch clamp measurements from visually identified dentate gyrus granule cells were made using a 200B Axopatch amplifier (Molecular Devices) and recorded using PowerLab Hardware and LabChart 7 data acquisition software (AD Instruments). Borosilicate glass micropipettes (World Precision Instruments) with DC resistance of 5–8 MΩ were backfilled with internal solution containing (in mM): 140 CsCl, 1 MgCl2, 10 HEPES, 4 NaCl, 0.1 EGTA, 2 Mg-ATP, 0.3 Na-GTP (pH = 7.25, 280–290 mOsm/LH2O). Phasic and tonic GABAergic responses were recorded in dentate gyrus granule cells voltage clamped at −70 mV in the whole cell configuration. The bath solution contained 3 mM kynurenic acid to block the glutamatergic synaptic responses. Synaptic events were analyzed using winEDR (version 3.3.1) and winWCP software (version 4.5.8, kindly provided by John Dempster, University of Strathclyde, UK) as described previously (MacKenzie and Maguire, 2015). Amplitude threshold crossing was used to detect spontaneous IPSCs. Averaged IPSCs were constructed from events which exhibited a monotonic rise and an uninterrupted decay phase and were aligned on their initial rising phases. Events were selected over a 120 s epoch (a minimum of 50 events). The 5 ms epoch immediately prior to the detected event defined the baseline with the peak current calculated relative to this value. The integral of the averaged IPSCs divided by the peak current defined the decay time constant. The tonic current was measured as previously described (Maguire and Mody, 2007;Maguire and Mody, 2008;Maguire et al., 2005). The distribution of the average holding current, measured over a 60s period before and after the addition of SR95531, was plotted and fit with a Gaussian function. The difference in the peak of the Gaussian before and after the addition of SR95531 is defined as the tonic GABAergic current.
Perforated patch recordings
EGABA was estimated using gramicidin perforated patch recordings as described previously (MacKenzie and Maguire, 2015;O'Toole et al., 2013;Sarkar et al., 2011). Briefly, the internal solution contained ( in mM): 140 KCl, 4 NaCl, 10 HEPES, 0.1 EGTA, 2 Mg-ATP, 0.3 Na-GTP, 2 QX314-Br (pH = 7.25, 280–290 mOsm) with 50 μg/ml gramicidin (ABCD, Sigma) which was vortexed prior to experimentation. Following generation of a seal > 1GΩ, perforation was monitored by the decrease in input resistance and the gradual increase in the capacitive transient following a −5mV voltage step and Vpipette reached a value close to the RMP similar to previous studies (Zhu et al., 2008). Recordings were immediately rejected if the membrane potential and/or input resistance suddenly changed. In addition, due to the high intracellular chloride concentration in the electrode, rupture of the patch led to a sudden switch to inwardly directed IPSCs, and when this occurred the data were discarded from analysis.
Glutamatergic signaling was blocked by addition of 3 mM kynurenic acid to the aCSF containing 5 μM GABA. Recordings from dentate gyrus granule cells were rejected if their RMP (measured in I = 0) was greater than −50 mV or less than −100 mV. The current generated in response to 5 mV voltage steps (2 s duration) from −90 mV to −40 mV was used to plot the current-voltage (I-V) relationship (Song et al., 2011;MacKenzie and Maguire, 2015). The GABAA receptor-mediated current was then determined from the difference in the I-V relationship in the presence or absence of SR95531 (≥400 μM applied directly to the bath) which was fitted with a 2nd order polynomial equation to determine EGABA (Song et al., 2011). Data were excluded if the GABAA mediated I-V relationship failed to cross the x-axis between −90 and −40 mV.
For all electrophysiological recordings, series resistance and whole-cell capacitance were continually monitored and compensated throughout the course of the experiment. Recordings were eliminated from data analysis if series resistance increased by > 20%.
Behavioral Tests
The mice were acclimated to the testing facility for at least one hour prior to testing. The equipment was cleaned with 70% ethanol in between each animal being tested.
Fear conditioning
Fear conditioning was performed in a rectangular box with a steel grid floor (Coulbourn Instruments; H10-11R-TC, 12”Wx10”Dx12”H). The experimental design is diagrammed in Figure 1a. For the fear conditioning training, the mice were placed into the center of the arena and allowed to acclimate for 3 mins before being presented with three 20 sec tones (2800 Hz, 80 dB) which ended simultaneously with foot shocks (2s, 0.7 mA) separated by a 1 min interval. Following the training phase, the mice were returned to their home cage. The following day, the mice were returned to the same rectangular box for 3 min, in the absence of tone or shock. The percent time freezing was calculated during this 3 min period as a measure of contextual fear memory. After a minimum of a 3 hour gap spent in their home cage, the mice were placed into a new environment, a rectangular plastic container with black and white stripes along the sides and bedding scented with 1% acetic acid. In this new context, the mice were presented with the same tone protocol as they were during the training phase (3 minute baseline and three 20 s tones separated by a 60 s gap), but were not subjected to any foot shocks. The percent time freezing for 40 secs after each tone was calculated as a measure of cued fear memory. Freezing behavior was analyzed using Actimetrics FreezeFrame software (Coulbourn Instruments, bout length 1s). The freezing behavior during the training phase was also measured to assess any potential baseline differences in freezing behavior unrelated to memory.
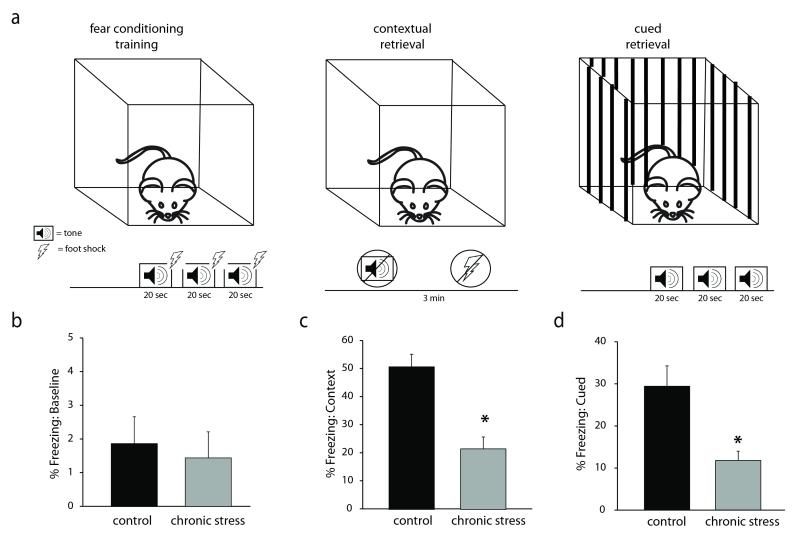
Figure 1. Chronic restraint stress impairs learning and memory.
a, A diagram of the experimental design of the fear conditioning paradigm. Minimally-handled controls or mice subjected to chronic stress underwent fear conditioning training in which they were exposed to three separate 20 sec tones paired with a foot shock. Twenty-four hours later, the percent time freezing as a measure of fear learning was measured in the context in which the foot shocks occurred or in response to the tone cue presented in a novel environment. b, There was no baseline difference in freezing behavior pre-tone during the training session. However, mice subjected to chronic stress exhibit impairments in both contextual and cued memory, evident by a decrease in the percent time freezing in the same context in which the foot shock occurred (c) or in response to the auditory cue (d). n = 12– 22 mice per experimental group. * denotes significance of p<0.01 using a Student’s t-test.
Open field test
The open field test was performed as previously described (Lee et al., 2014). Mice were individually placed into the center of a 40 cm × 40 cm open field apparatus with 16 × 16 equally spaced photocells (Hamilton-Kinder). The number of entries, the total distance traveled, and the amount of time spent in the center of the open field as well as the total number of beam breaks were measured during the 10 min test using MotorMonitor software (Hamilton-Kinder).
Light/dark box test
The light/dark box test was performed as previously described (Lee et al., 2014). Briefly, mice were individually into a 22 cm × 43 cm light/dark box apparatus with 4 × 8 equally spaced photocells (Hamilton-Kinder), where one half is an open, illuminated compartment and the other half is an enclosed, dark compartment. The number of entries into the light box and the total distance traveled in the light compartment were measured during the 10 min test using MotorMonitor software (Hamilton-Kinder).
Tail suspension test
The tail suspension test was performed as previously described (Lee et al., 2014). Mice were individually suspended by the tip of their tail from a bar at a height of 36 cm above a table top. The latency to the first bout of immobility and the cumulative time spent immobile during the 6 min test was measured. All trials were videotaped and subsequently scored with the investigator blind to the experimental group.
Forced swim test
The forced swim test was performed as previously described (Lee et al., 2014). Mice were placed individually into cylinders (21 cm diameter) containing 15 cm of water at room temperature (22–25°C). The latency to immobility and the cumulative time spent immobile during the 6 min test was measured. All trials were videotaped and subsequently scored with the investigator blind to the experimental group.
Statistical analyses
Statistical significance between two experimental groups (minimally-handled controls and chronic stress mice) was determined using an unpaired Student’s t-test. All statistical calculations were performed using Graphpad Prism 6. Data in the text are presented as the Mean ± the Standard Error of the Mean (SEM).
Results
Chronic stress leads to contextual memory impairment
To determine whether the chronic restraint stress protocol affects learning and memory in our hands, we subjected mice to 30 mins of restraint stress daily for 14 days and assessed learning and memory using the fear conditioning paradigm. We measured circulating corticosterone levels to ensure that the chronic stress paradigm was evoking a physiological response to stress. Corticosterone levels are elevated in mice subjected to chronic restraint stress (97.78 ± 26.46 ng/ml) compared to minimally-handled controls (20.23 ± 3.01 ng/ml) (data not shown) (n = 4 – 5 mice per experimental group; p < 0.05 using a Student’s unpaired t-test). Our data demonstrate that this chronic stress paradigm impairs both contextual and cued memory. Mice subjected to chronic stress exhibit a reduction in the percent time freezing (21.52 ± 4.20 %) in the same context in which the fear conditioning training occurred compared to unstressed controls (50.61 ± 4.48 %) (Figure 1c) (n = 12 – 22 mice per experimental group; p < 0.001 using a Student’s unpaired t-test). In addition, mice subjected to chronic stress exhibit a decrease in the percent time freezing in response to the auditory cue previously paired with the foot shock (11.81 ± 2.22 %) compared to minimally-handled controls (29.45 ± 2.22 %) (Figure 1d) (n = 12 – 22 mice per experimental group; p < 0.01 using a Student’s unpaired t-test). To ensure that the chronic stress procedure did not alter baseline freezing behavior, we measured the percent time freezing during the first minute of the fear conditioning training trial before presentation of the conditioned or unconditioned stimulus, in which we observed no difference between the experimental groups (control: 1.31 ± 0.65 %; stress: 0.26 ± 0.26 %) (Figure 1b) (n = 12 – 22 mice per experimental group). These data suggest that chronic stress leads to deficits in learning and memory.
Reduced tonic but increased phasic inhibition in DGGCs of the hippocampus
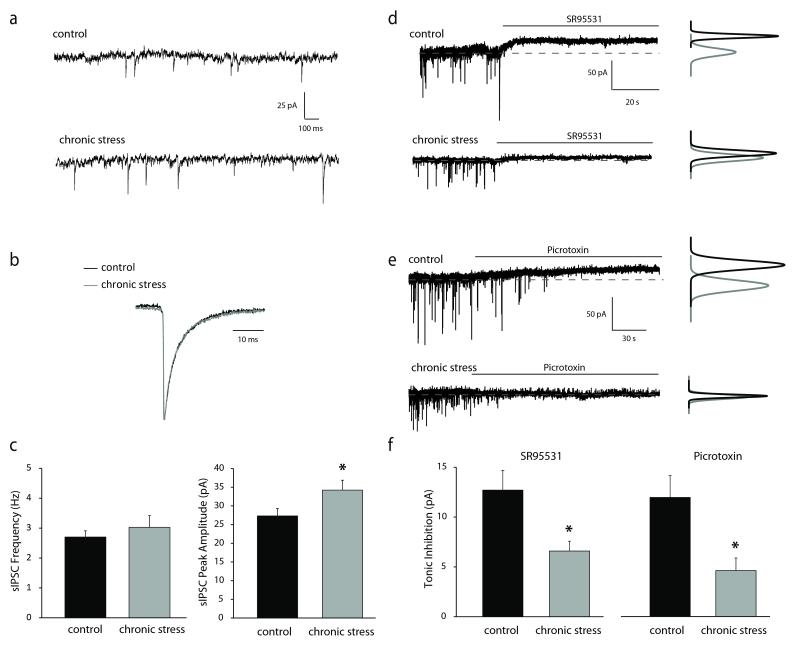
To determine if the chronic stress-induced impairments in learning and memory are due to alterations in GABAergic inhibition, we performed whole cell patch clamp recordings in dentate gyrus granule cells of the hippocampus. No difference in spontaneous inhibitory postsynaptic (sIPSC) frequency was observed following chronic stress (2.70 ± 0.21 Hz) compared to control (3.03 ± 0.39 pA) (Figure 2a,c) (n = 18 – 27 cells, 10 – 11 mice per experimental group). However, there was an increase in the peak amplitude of sIPSCs following stress (34.23 ± 2.63 pA) compared to minimally-handled controls (27.32 ± 1.98 pA), with no difference in decay kinetics (control: 4.86 ± 0.26 ms; stress: 4.61 ± 0.31 ms) (Figure 2a-c) (n = 18 – 27 cells, 10 – 11 mice per experimental group; statistical significance defined as p < 0.05 using a Student’s unpaired t-test). These data are consistent with enhanced phasic GABAergic inhibition in dentate gyrus granule cells. However, tonic inhibition is reduced in dentate gyrus granule cells following chronic stress (7.06 ± 0.94 pA) compared with slices from minimally-handled controls (12.70 ± 1.97 pA) (Figure 2d,f) (n = 13 – 14 cells, 7 mice per experimental group; p < 0.05 using a Student’s unpaired t-test) as measured in the absence of exogenous GABA by the difference in the baseline following the addition of the competitive antagonist GABAzine. A similar reduction was observed with picrotoxin (control = 13.04 ± 2.08 pA, stress = 4.88 ± 1.33 pA) (Figure 2e-f) (n = 11 – 13 cells, 4 – 7 mice per experimental group; p < 0.01 using a Student’s unpaired t-test).
Figure 2. Alterations in GABAergic inhibition in dentate gyrus granule cells following chronic stress.
a, Representative traces of sIPSCs in dentate gyrus granule cells from minimally-handled controls or mice subjected to chronic restraint stress. b, Superimposed traces of average sIPSCs normalized to the peak amplitude from control and chronic stress mice showing no change in the decay time constant. c, There is no difference in the frequency of sIPSCs between controls and mice subjected to chronic stress. The average peak amplitude of sIPSCs recorded in dentate gyrus granule cells is increased in chronic stress mice compared to controls. d-e, Representative traces of tonic currents in dentate gyrus granule cells from minimally-handled controls or mice subjected to chronic restraint stress recorded by blocking GABAARs with either SR95531 (d) or picrotoxin (e). f, Tonic inhibition measured using either SR95531 or picrotoxin is decreased in dentate gyrus granule cells in slices from mice subjected to chronic stress compared to minimally-handled controls. n = 13 – 27 cells, 7 – 11 mice per experimental group. * denotes significance of p<0.05 using a Student’s t-test.
Altered GABAAR subunit expression in the hippocampus following chronic stress
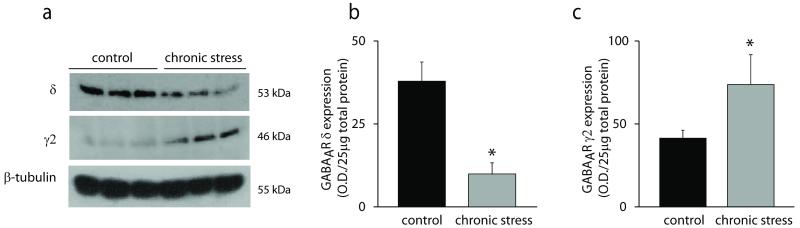
Consistent with the functional alterations in GABAergic inhibition in dentate gyrus granule cells, we observed changes in the expression of subunits that mediate the tonic and phasic GABAergic inhibition in these neurons. An increase in the expression of the GABAAR γ2 subunit is observed in total protein isolated from the hippocampus of mice subjected to chronic stress (69.79 ± 17.08 O.D. units/25μg total protein) compared to controls (39.18 ± 4.53 O.D. units/25μg total protein) (Figure 3a,c) (n = 11 – 19 mice per experimental group; p < 0.05 using an unpaired Student’s t-test), which is consistent with the increase in the amplitude of the sIPSCs recorded in dentate gyrus granule cells. A decrease in the expression of the GABAAR δ subunit is observed in the hippocampus following chronic stress (9.48 ± 3.41 O.D units/25μg total protein) compared with the control group (39.18 ± 4.53 O.D. units/25μg total protein) (Figure 3a-b) (n = 8 mice per experimental group; p < 0.01 using a Student’s unpaired t-test), which is consistent with the reduction in the tonic GABAergic inhibition following chronic stress in dentate gyrus granule cells, in which tonic inhibition is mediated by this receptor subtype.
Figure 3. Chronic stress-induced decrease in the expression of the GABAAR δ subunit in the dentate gyrus.
a, Representative Western blots of GABAAR δ and γ2 subunit expression in minimally-handled controls and mice subjected to chronic restraint stress. b, The average optical density of GABAAR δ subunit expression is decreased in mice subjected to chronic stress compared to controls. n = 8 mice per experimental group; p < 0.01 using a Student’s unpaired t-test. c, The average optical density of GABAAR γ2 subunit expression is increased in mice subjected to chronic stress compared to controls. n = 11 – 19 mice per experimental group; p < 0.05 using an unpaired Student’s t-test.
No shift in EGABA in the dentate gyrus following chronic stress
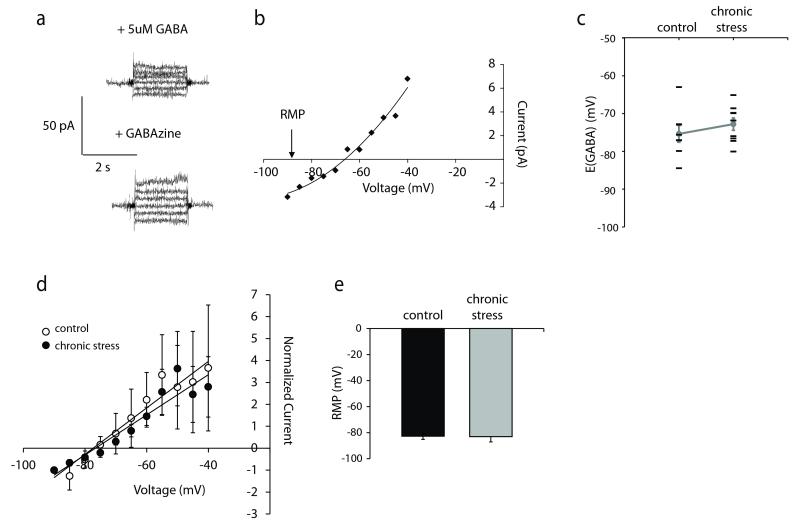
Recently, we have observed compromised GABAergic inhibition in CA1 pyramidal neurons following chronic stress involving the dephosphorylation and downregulation of the K+/Cl− co-transporter 2 (KCC2) and a shift in EGABA (MacKenzie and Maguire, 2015). Therefore, we sought to determine whether a similar shift in EGABA occurs in dentate gyrus granule cells following chronic stress. To measure EGABA in dentate gyrus granule cells, gramicidin perforated patch recordings were performed to prevent disruption of the native chloride gradients and the GABAAR mediated current at each potential was determined by subtracting the current-voltage relationships in the presence and absence of the GABAAR antagonist SR95331 ((Song et al., 2011) (Figure 4a-b). Consistent with previous data (Chiang et al., 2012;Sauer et al., 2012), the GABA reversal potential of dentate gyrus granule cells (EGABA = −75.36 ± 2.21 mV) (Figure 4c-d) is depolarized compared to the resting membrane potential (RMP) (−82.76 ± 2.33 mV) (Figure 4e). However, no shift in EGABA or the RMP was observed in the dentate gyrus granule cells following chronic stress (EGABA = −72.79 ± 1.63 mV, RMP = −83.03 ± 4.09 mV) (Figure 4c-e) (n = 8 – 9 cells, 6 – 9 mice per experimental group; statistical significance defined as p < 0.05 using a Student’s unpaired t-test).
Figure 4. No shift in EGABA in dentate gyrus granule cells following chronic stress.
a, Representative current traces at different voltages in the presence of 5μM GABA, with or without SR95531,. The voltage-current relationships generated were subtracted and plotted to measure EGABA. A representative example is shown (b). No difference in the average EGABA values (c-d) or resting membrane potential (e) were observed in dentate gyrus granule cells from minimally-handled controls or mice subjected to chronic restraint stress. d, Normalized, averaged voltage-current relationships in dentate gyrus granule cells from control and chronic stress mice demonstrate equivalent EGABA values. n = 8 – 9 cells, 6-9 mice per experimental group; * denotes significance of p<0.05 using a Student’s t-test.
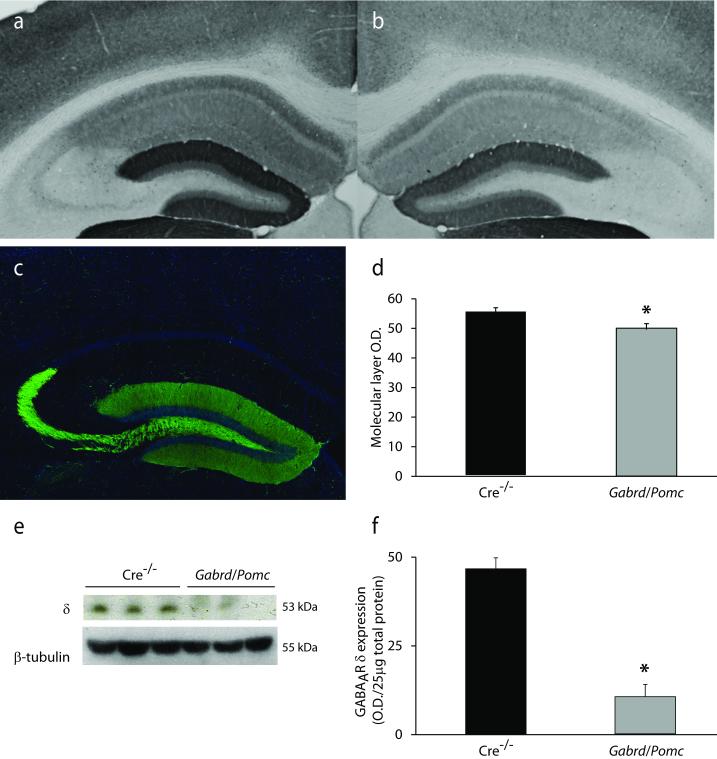
A reduction in GABAAR δ subunit expression in Gabrd/Pomc mice
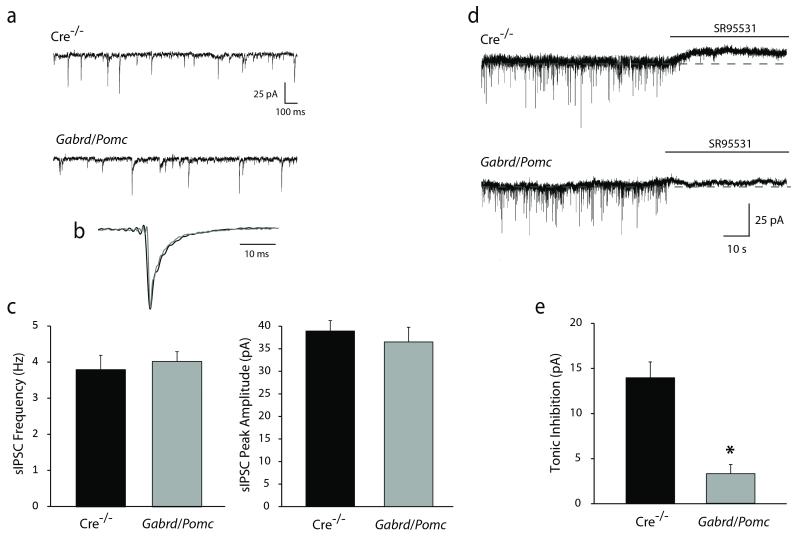
To determine whether deficits in GABAAR δ subunit expression and function in the dentate gyrus contribute to the learning and memory deficits following chronic stress, we generated mice with a modest reduction in the expression of the GABAAR δ subunit specifically in dentate gyrus granule cells by crossing floxed Gabrd mice previously generated and characterized by our laboratory (Lee and Maguire, 2013) with Pomc-Cre mice. The restricted expression of Cre in Pomc-Cre mice was assessed in Pomc-GFP mice. Reporter expression in the hippocampus is restricted to dentate gyrus granule cells and mossy fiber projections to CA3 (Figure 5c), as previously described (McHugh et al., 2007). Crossing Pomc-Cre mice with floxed Gabrd mice resulted in a reduction in the expression of the GABAAR δ subunit in the dentate gyrus of Gabrd/Pomc mice (49.98 ± 1.91 O.D. units) compared to Cre−/− littermate controls (55.24 ± 1.68 O.D. units) (Figure 5a-b,d) (n = 3 mice per experimental group; p < 0.05 using a Student’s unpaired t-test). A decrease in the expression of the GABAAR δ subunit in Gabrd/Pomc mice can be appreciated by Western blot analysis (9.93 ± 3.26 O.D units/25μg total protein) compared to Cre−/− controls (44.08 ± 2.93 O.D. units/25μg total protein) (Figure 5e-f) (n = 6 mice per experimental group; p < 0.01 using a Student’s unpaired t-test). The reduction in the expression of the GABAAR δ subunit in dentate gyrus granule cells also resulted in a functional decrease in tonic inhibition in dentate gyrus granule cells (3.28 ± 1.01 pA) compared to Cre−/− littermate controls (13.92 ± 1.75 pA) (Figure 6d-e) (n = 12 – 25 slices, 3 – 8 mice per experimental group; p < 0.05 using a Student’s unpaired t-test). However, we did not observe any compensatory change in the frequency (4.03 ± 0.27 Hz) or peak amplitude (36.50 ± 3.26 pA) of sIPSCs in Gabrd/Pomc mice compared to Cre−/− littermates (frequency: 3.80 ± 0.40 Hz; amplitude: 38.93 ± 2.30 pA) (Figure 6a-c) (n = 12 – 13 cells, 3 – 8 mice per experimental group; p < 0.05 using a Student’s unpaired t-test).
Figure 5. Reduced expression of the GABAAR δ subunit in the dentate gyrus of Gabrd/Pomc mice.
Representative images of immunoreactivity for the GABAAR δ subunit in Cre−/− littermates (a) and Gabrd/Pomc mice (b). c, Representative image of GFP reporter expression in Pomc-GFP mice, demonstrating the specificity of Cre expression in dentate gyrus granule cells. d, The average optical density of GABAAR δ subunit immunoreactivity is decreased in Gabrd/Pomc mice compared to Cre−/− littermates. n = 3 mice per experimental group. e, Representative Western blot of GABAAR δ subunit expression in Gabrd/Pomc mice and Cre−/− littermates. f, The average optical density of GABAAR δ subunit expression, quanitified using Western blot analysis, is decreased in Gabrd/Pomc mice Gabrd/Pomc mice compared to Cre−/− littermates. n = 6 mice per experimental group; * denotes significance of p<0.05 using a Student’s t-test.
Figure 6. Decreased tonic inhibition of dentate gyrus granule cells in Gabrd/Pomc mice.
a, Representative traces of sIPSCs in dentate gyrus granule cells from Gabrd/Pomc mice and Cre−/− littermates. b, Superimposed traces of average sIPSCs normalized to the peak amplitude from Gabrd/Pomc mice and Cre−/− littermates showing no change in the decay time constant. c, There is no difference in the frequency or peak amplitude of sIPSCs between in Gabrd/Pomc mice or Cre−/− littermates. d, Representative traces of tonic currents in dentate gyrus granule cells from Gabrd/Pomc mice and Cre−/− littermates. e, The average tonic current is decreased in dentate gyrus granule cells from Gabrd/Pomc mice compared to Cre−/− littermates. n = 12 – 25 slices, 3 – 8 mice per experimental group. * denotes significance of p<0.05 using a Student’s t-test.
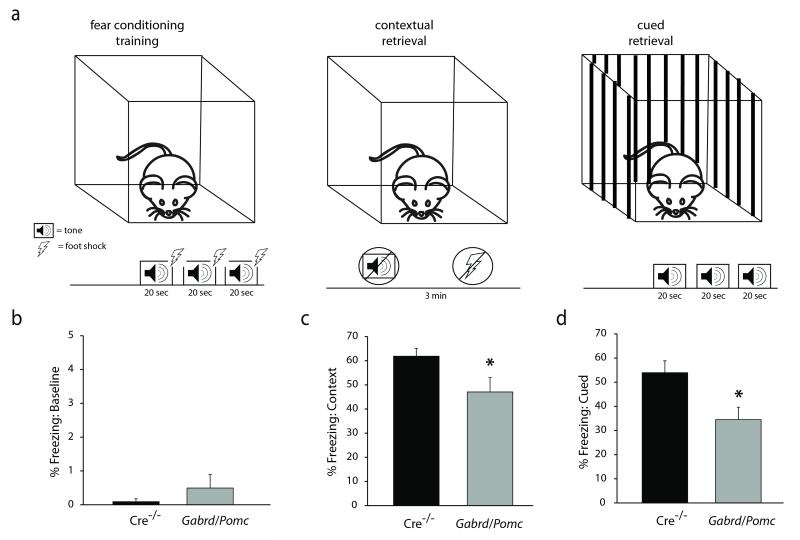
Gabrd/Pomc mice exhibit impairments in learning and memory
Our data demonstrate that there are deficits in both cued and contextual memory in Gabrd/Pomc mice. Gabrd/Pomc mice exhibit a reduction in the percent time freezing (47.06 ± 5.90 %) in the same context in which the fear conditioning training occurred compared to Cre−/− littermate controls (61.86 ± 3.23 %) (Figure 7c) (n = 19 – 20 mice per experimental group; p < 0.05 using a Student’s unpaired t-test). Similarly, Gabrd/Pomc mice exhibit a reduction in the percent time freezing in response to the auditory cue in a novel context following fear conditioning training (34.53 ± 5.14 %) compared to Cre−/− littermate controls (53.95 ± 4.93 %) (Figure 7d) (n = 19 – 20 mice per experimental group; p < 0.05 using a Student’s unpaired t-test). To determine whether there were baseline changes in freezing behavior in Gabrd/Pomc mice compared to Cre−/− littermates, we measured the percent time freezing during the first minute of the fear conditioning training trial before presentation of the conditioned or unconditioned stimulus. There was no difference in baseline freezing behavior between Gabrd/Pomc mice (0.49 ± 0.41 %) and Cre−/− littermates (0.09 ± 0.09 %) (Figure 7b) (n = 19 – 20 mice per experimental group; statistical significance defined as p < 0.05 using a Student’s unpaired t-test). These data suggest that a reduction in GABAAR δ subunit expression and function in dentate gyrus granule cells is sufficient to induce deficits in learning and memory.
Figure 7. Impairments in learning and memory in Gabrd/Pomc mice.
a, A diagram of the experimental design of the fear conditioning paradigm. Minimally-handled controls or mice subjected to chronic stress underwent fear conditioning training in which they were exposed to three separate 20 sec tones paired with a foot shock. Twenty-four hours later, the percent time freezing as a measure of fear learning was measured in the context in which the foot shocks occurred or in response to the tone cue presented in a novel environment. b, There was no baseline difference in freezing behavior pre-tone during the training session. However, Gabrd/Pomc mice exhibit impairments in both contextual and cued memory, evident by a decrease in the percent time freezing in the same context in which the foot shock occurred (c) or in response to the auditory cue (d). n = 19 – 20 mice per experimental group. * denotes significance of p<0.05 using a Student’s t-test.
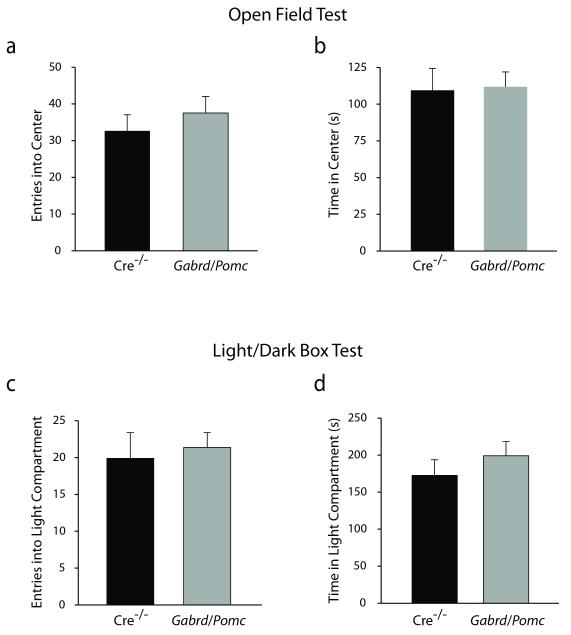
Learning and memory deficits in Gabrd/Pomc mice appear to be specific behavioral deficits in these animals since we do not observe any changes in anxiety- or depression-like behaviors. Anxiety-like behavior was assessed in Gabrd/Pomc mice compared to Cre−/− littermates using the light/dark box and open field tests. There is no difference in the number of entries (37.53 ± 4.49) or amount of time spent (111.84 ± 10.12 sec) in the center of the open field in Gabrd/Pomc mice compared to Cre−/− littermate controls (entries: 32.65 ± 4.41; time spent: 109.50 ± 14.92 sec) (Figure 8a-b) (n = 19 – 20 mice per experimental group; statistical significance defined as p < 0.05 using a Student’s unpaired t-test). Similarly, Gabrd/Pomc mice make a similar number of entries (21.37 ± 2.01) and spend a similar amount of time (199.22 ± 18.92 sec) in the light compartment of the light/dark box compared to Cre−/− littermates (entries: 19.90 ± 3.49; time in light: 172.56 ± 21.11 sec) (Figure 8c-d) (n = 19 – 20 mice per experimental group; statistical significance defined as p < 0.05 using a Student’s unpaired t-test). There is no difference in the locomotor behavior of Gabrd/Pomc mice in either the open field test (2231.26 ± 132.24 beam breaks) or the light/dark box (1101.64 ± 65.58 beam breaks) compared to Cre−/− littermates (open field: 1972.55 ± 154.16 beam breaks; light/dark box: 964.11 ± 39.49 beam breaks) (data not shown). These data suggest that the loss of the GABAAR δ subunit in the dentate gyrus does not alter anxiety-like behaviors.
Figure 8. No difference in anxiety-like behaviors in Gabrd/Pomc mice.
There is no difference in the number of entries (a) or the amount of time spent (b) in the center of open field in Gabrd/Pomc mice compared to Cre−/− littermates. Similarly, there is no difference in the number of entries (c) or the amount of time (d) that Gabrd/Pomc mice spend in the light compartment of the light/dark box compared to Cre−/− littermates. n = 19 – 20 mice per experimental group. * denotes significance of p<0.05 using a Student’s t-test.
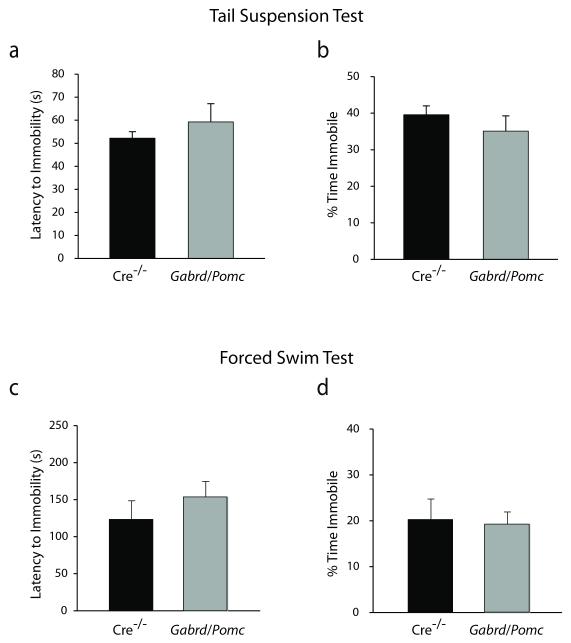
Depression-like behaviors in Gabrd/Pomc mice were assessed using the forced swim and tail suspension tests. Gabrd/Pomc mice do not exhibit any differences in the latency to immobility (59.29 ± 3.86 sec) or the percent time spent immobile (35.09 ± 3.22 %) in the tail suspension test compared to Cre−/− littermates (latency: 52.21 ± 3.80 sec; percent time: 39.58 ± 2.65 %) (Figure 9a-b) (n = 19 – 20 mice per experimental group; statistical significance defined as p < 0.05 using a Student’s unpaired t-test). Similarly, Gabrd/Pomc mice do not exhibit any differences in the latency to immobility (155.50 ± 21.55 sec) or the percent time spent immobile (19.78 ± 2.81 %) in the forced swim test compared to Cre−/− littermates (latency: 124.75 ± 25.09 sec; percent time: 20.78 ± 4.68 %) (Figure 9c-d) (n = 19 – 20 mice per experimental group; statistical significance defined as p < 0.05 using a Student’s unpaired t-test). The results of this study suggest that the loss of the GABAAR δ subunit in the dentate gyrus does not alter depression-like behaviors. Collectively, these data demonstrate that a reduction in GABAAR δ subunit expression in dentate gyrus granule cells results in specific behavioral deficits in learning and memory.
Figure 9. No difference in depression-like behaviors in Gabrd/Pomc mice.
There is no difference in the latency (a) or total time spent immobile (b) during the tail suspension test between Gabrd/Pomc mice and Cre−/− littermates. Similarly, Gabrd/Pomc mice and Cre−/− littermates exhibit an equivalent latency to immobility (c) and total time spent immobile (d) in the forced swim test. n = 19 – 20 mice per experimental group. * denotes significance of p<0.05 using a Student’s t-test.
Discussion
The hippocampus undergoes numerous alterations following stress (for review see (McEwen and Sapolsky, 1995)), many of which have been implicated in the stress-induced impairments in learning and memory. However, it is difficult to directly determine the contribution of these changes to stress-induced impairments in learning and memory. Here, we demonstrate that chronic stress decreases the tonic GABAergic inhibition of dentate gyrus granule cells and we directly demonstrate that this reduction in tonic inhibition in dentate gyrus granule cells is capable of altering learning and memory. Using Gabrd/Pomc mice, we demonstrate that a reduction in GABAAR δ subunit-mediated tonic inhibition in dentate gyrus granule cells is sufficient to induce deficits in learning and memory. These data suggest that chronic stress-induced decreases in tonic inhibition in dentate gyrus granule cells may contribute to the learning and memory impairments associated with chronic stress. However, there are likely numerous contributing factors to the stress-induced alterations in learning and memory, some of which may be convergent mechanisms and others which may independently modulate the effects of stress on learning and memory.
For example, there is ample evidence that glucocorticoids play a role in mediating the effects of stress on learning and memory (for review see (Roozendaal, 2002)). Our data suggest that alterations in the GABAergic control of dentate gyrus granule cells may also contribute to stress-induced deficits in learning and memory. It is possible that glucocorticoids may play a role in the changes in GABAAR δ subunit expression and function in dentate gyrus granule cells following chronic stress. In fact, neurosteroids derived from glucocorticoids have been shown to alter the surface expression of extrasynaptic GABAARs, including extrasynaptic GABAA receptors (Abramian et al., 2014). This could be one mechanism whereby these pathways may converge and contribute to the stress-induced impairments in learning and memory. Consistent with this proposed mechanism, stress-induced alterations in tonic GABAergic inhibition in the amygdala have been shown to be mediated by glucocorticoids (Liu et al., 2014). These findings also demonstrate that chronic stress-induced impairments in tonic GABAergic inhibition are not restricted to the hippocampus. However, the reduction in GABAAR δ subunit expression and function appears to be subregion specific, since we observed a reduction in tonic inhibition in dentate gyrus granule cells (Figure 2), but an increase in CA1 pyramidal neurons (MacKenzie and Maguire, 2015). Further, we observed a shift in EGABA in CA1 pyramidal neurons (MacKenzie and Maguire, 2015), but not in dentate gyrus granule cells (Figure 4), consistent with a recent study demonstrating chronic stress-induced downregulation of KCC2 in the CA1 and CA3 subregions of the hippocampus, but not in the dentate gyrus (Tsukahara et al., 2015). We previously speculated that an increase in tonic inhibition could contribute to the depolarizing shift in EGABA in CA1 pyramidal neurons (MacKenzie and Maguire, 2015) in combination with the observed reduction in KCC2 expression (Tsukahara et al., 2015). In dentate gyrus granule cells, tonic inhibition is reduced which may explain why we do not observe a shift in EGABA in this subregion.
Alterations in GABAAR subunit expression and GABAergic inhibition have been documented in many different brain regions, including the hippocampus, following many different stress paradigms (for review see (Maguire, 2014)). In contrast to the current results, previous studies have demonstrated an increase the expression of the α4 and δ subunits of the GABAAR in the hippocampus (Serra et al., 2006) and an increase in tonic GABAergic inhibition in dentate gyrus granule cells (Holm et al., 2011) following chronic stress, which is mediated by these receptors (Stell et al., 2003). The discrepancy in these findings may be due to numerous factors, such as the type or duration of the stressor, which has been shown to exert differential effects on stress-induced plasticity changes and behavioral changes associated with stress (for review see (Maguire, 2014)).
Tonic GABAergic inhibition, mediated by extrasynaptic GABAARs, has been shown to convey a larger charge transfer than synaptic GABAergic transmission (for review see (Farrant and Nusser, 2005)), and is thought to regulate inhibitory tone and modulate the gain of inhibitory synaptic transmission (for review see (Semyanov et al., 2004)). Thus, the stress-induced reduction in GABAAR δ subunit-mediated tonic control of dentate gyrus granule cells will likely have a robust impact on the excitability of these neurons. In fact, the dentate gyrus has been shown to “gate” the activity of the hippocampus and tonic inhibition has been demonstrated to have a substantial role in maintaining this dentate gate (Coulter and Carlson, 2007). Therefore, the changes in GABAergic inhibition documented in the current study are likely to impact hippocampal function, which may contribute to changes in hippocampal-dependent learning and memory.
GABAergic inhibition has long been thought to impact learning and memory, at least in part due to the observation that drugs that enhance GABAergic inhibition, such as anesthetics, barbiturates, and benzodiazepines, impair learning and memory. Tonic inhibition mediated by GABAAR δ subunit-containing receptors has been proposed to constrain hippocampal neuronal excitability and learning and memory. Enhanced tonic GABAergic inhibition through long-term pharmacological potentiation of δ subunit-containing GABAARs impairs learning and memory (Whissell et al., 2013b) and increased expression of the GABAAR δ subunit in the CA1 subregion of the hippocampus during puberty in mice impairs LTP and spatial learning (Shen et al., 2010). In addition, increased tonic inhibition of dentate gyrus granule cells has implicated in contributing to the learning and memory deficits in a mouse model of Alzheimer’s disease (Wu et al., 2014). Thus, it may seem counterintuitive that the current findings demonstrate deficits in learning and memory associated with a reduction in the GABAAR δ subunit in the dentate gyrus. However, there is a precedent for deficits in learning and memory in mice lacking the GABAAR δ subunit. Mice with a global loss of the GABAAR δ subunit (Gabrd−/− mice) exhibit deficits in learning and memory (Cushman et al., 2014), impaired recognition memory, contextual discrimination, and fear extinction (Whissell et al., 2013b). However, in contrast to the current findings, Gabrd−/− mice exhibit enhanced fear acquisition (Whissell et al., 2013b). Mice with a global loss in the GABAAR α5 subunit, which mediates tonic inhibition in CA1 pyramidal neurons (Caraiscos et al., 2004), exhibit enhanced hippocampal-dependent learning and memory (Collinson et al., 2002). However, a reduction in GABAAR α5 subunit expression results in impairments in the memory for the location of objects (Prut et al., 2010) and a recent study demonstrated that mice lacking the α5 subunit in dentate gyrus granule cells exhibit impairments in high-interference cognitive tasks (Engin et al., 2015), although they do not exhibit changes in baseline learning and memory. These findings, along with the evidence presented in the current manuscript, suggest that the GABAAR δ subunit plays a complex role in learning and memory.
The data summarized here demonstrate a complex role for tonic GABAergic inhibition in the hippocampus on learning and memory; wherein enhancing or diminishing tonic GABAergic inhibition may impair learning and memory. It is thought that enhancing tonic GABAergic inhibition likely impairs learning and memory via constraining neuronal excitability, plasticity, and long-term potentiation. A disruption in neurogenesis associated with a reduction in tonic GABAergic inhibition, mediated by GABAAR δ subunit-containing receptors, in dentate gyrus granule cells has been implicated in inducing deficits in learning and memory (Whissell et al., 2013b). These data suggest two independent mechanisms whereby alterations in tonic GABAergic inhibition in the hippocampus may impair learning and memory. Future studies are required to determine whether the impairments in learning and memory associated with a loss of tonic GABAergic inhibition in the dentate gyrus following chronic stress or in Gabrd/Pomc mice involve a disruption in neurogenesis.
These data implicate alterations in GABAAR δ subunit-containing receptors and tonic GABAergic regulation of dentate gyrus granule cells in contributing, at least in part, to the stress-induced impairments in learning and memory. This study is the first to directly investigate the contribution of tonic inhibition in dentate gyrus granule cells to learning and memory. These discoveries also raise the possibility that targeting these receptors may be therapeutic for impairments in learning and memory. In fact, neurosteroids have been implicated in learning and memory processes and associated with deficits in aging (for review see (Reddy, 2010;Vallee et al., 2001)). Further studies are required to fully appreciate the role of GABAAR δ subunit-containing receptors in the processes underlying learning and memory.
Acknowledgements
The authors would like to thank Dr. Leon Reijmers for consultation on the fear conditioning paradigm. J. Maguire was supported by NIH Grant R01 NS073574 and a Research Grant from the Epilepsy Foundation. V. Lee was supported, in part, by the Tufts Medical Scientist Training Program T32 GM008448 and F31 NS078815. G. MacKenzie was supported, in part, by a postdoctoral fellowship from the American Epilepsy Society and Sunovion Pharmaceuticals, Inc.. A. Hooper was supported by a predoctoral fellowship from the Epilepsy Foundation and the Dean’s Award from Tufts University School of Medicine. The behavioral experiments were conducted in the Circuits and Behavior Core and Imaging Core facilities within the Tufts Center for Neuroscience Research, P30 NS047243.
Reference List
- Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, Maguire JL, Terunuma M, Davies PA, Moss SJ. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABA(A) receptors. Proc Natl Acad Sci U S A. 2014;111:7132–7137. doi: 10.1073/pnas.1403285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha 5 subunit-containing, gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, Hobbs SC, Marshall G, Maubach KA, Pillai GV, Reeve AJ, MacLeod AM. Identification of a Novel, Selective GABAA α5 Receptor Inverse Agonist Which Enhances Cognition. J Med Chem. 2003;46:2227–2240. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- Chiang PH, Wu PY, Kuo TW, Liu YC, Chan CF, Chien TC, Cheng JK, Huang YY, Chiu CD, Lien CC. GABA Is Depolarizing in Hippocampal Dentate Granule Cells of the Adolescent and Adult Rats. The Journal of Neuroscience. 2012;32:62–67. doi: 10.1523/JNEUROSCI.3393-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, Atack JR, Laughton P, Dawson GR, Stephens DN. An inverse agonist selective for α5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology. 2006;188:619–628. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced Learning and Memory and Altered GABAergic Synaptic Transmission in Mice Lacking the α5 Subunit of the GABAAReceptor. The Journal of Neuroscience. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res. 2007;163:235–243. doi: 10.1016/S0079-6123(07)63014-3. [DOI] [PubMed] [Google Scholar]
- Cushman J, Moore M, Olsen R, Fanselow M. The Role of the δGABA(A) Receptor in Ovarian Cycle-Linked Changes in Hippocampus-Dependent Learning and Memory. Neurochem Res. 2014;39:1140–1146. doi: 10.1007/s11064-014-1282-6. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, Smith AJ, Sternfeld F, Tattersall FD, Wafford KA, Reynolds DS, Seabrook GR, Atack JR. An Inverse Agonist Selective for α5 Subunit-Containing GABAA Receptors Enhances Cognition. Journal of Pharmacology and Experimental Therapeutics. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- Engin E, Zarnowska ED, Benke D, Tsvetkov E, Sigal M, Keist R, Bolshakov VY, Pearce RA, Rudolph U. Tonic Inhibitory Control of Dentate Gyrus Granule Cells by alpha5-Containing GABAA Receptors Reduces Memory Interference. J Neurosci. 2015;35:13698–13712. doi: 10.1523/JNEUROSCI.1370-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nature Reviews Neuroscience. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm MM, Nieto-Gonzalez JL, Vardya I, Henningsen K, Jayatissa MN, Wiborg O, Jensen K. Hippocampal GABAergic dysfunction in a rat chronic mild stress model of depression. Hippocampus. 2011;21:422–433. doi: 10.1002/hipo.20758. [DOI] [PubMed] [Google Scholar]
- Howland JG, Wang YT. Chapter 8 Synaptic plasticity in learning and memory: Stress effects in the hippocampus. In: Wayne S, Sossin J-CLV, editors. Progress in Brain Research Essence of Memory. Elsevier; 2008. pp. 145–158. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Lee V, Maguire J. Impact of inhibitory constraint of interneurons on neuronal excitability. Journal of Neurophysiology. 2013 doi: 10.1152/jn.00047.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V, Sarkar J, Maguire J. Loss of Gabrd in CRH neurons blunts the corticosterone response to stress and diminishes stress-related behaviors. Psychoneuroendocrinology. 2014;41:75–88. doi: 10.1016/j.psyneuen.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZP, Song C, Wang M, He Y, Xu XB, Pan HQ, Chen WB, Peng WJ, Pan BX. Chronic stress impairs GABAergic control of amygdala through suppressing the tonic GABAA receptor currents. Mol Brain. 2014;7:32. doi: 10.1186/1756-6606-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie G, Maguire J. Chronic stress shifts the GABA reversal potential in the hippocampus and increases seizure susceptibility. Epilepsy Research. 2015;109:13–27. doi: 10.1016/j.eplepsyres.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Maguire J. Stress-induced plasticity of GABAergic inhibition. Front Cell Neurosci. 2014;8:157. doi: 10.3389/fncel.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcewen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Mcewen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci. 1997;821:271–84. 271–284. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- Mcewen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Current Opinion in Neurobiology. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li ZW, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Maguire J. The reciprocal regulation of stress hormones and GABA(A) receptors. Front Cell Neurosci. 2011;6:4. doi: 10.3389/fncel.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- O'Toole KK, Hooper A, Wakefield S, Maguire J. Seizure-induced disinhibition of the HPA axis increases seizure susceptibility. Epilepsy Research. 2013;108(1):29–43. doi: 10.1016/j.eplepsyres.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, Mcewen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston A, Eichenbaum H. Interplay of Hippocampus and Prefrontal Cortex in Memory. Current Biology. 2013;23:R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Prenosil G, Willadt S, Vogt K, Fritschy JM, Crestani F. A reduction in hippocampal GABAA receptor α5 subunits disrupts the memory for location of objects in mice. Genes, Brain and Behavior. 2010;9:478–488. doi: 10.1111/j.1601-183X.2010.00575.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and Memory: Opposing Effects of Glucocorticoids on Memory Consolidation and Memory Retrieval. Neurobiology of Learning and Memory. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and Memory: Behavioral Effects and Neurobiological Mechanisms. Neural Plast. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Wakefield S, Mackenzie G, Moss SJ, Maguire J. Neurosteroidogenesis Is Required for the Physiological Response to Stress: Role of Neurosteroid-Sensitive GABAA Receptors. J Neurosci. 2011;31:18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer JF, Str++ber M, Bartos M. Interneurons Provide Circuit-Specific Depolarization and Hyperpolarization. The Journal of Neuroscience. 2012;32:4224–4229. doi: 10.1523/JNEUROSCI.5702-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA(A) receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serra M, Mostallino MC, Talani G, Pisu MG, Carta M, Mura ML, Floris I, Maciocco E, Sanna E, Biggio G. Social isolation-induced increase in alpha and delta subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABA receptor function. J Neurochem. 2006;98:122–133. doi: 10.1111/j.1471-4159.2006.03850.x. [DOI] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A Critical Role for a4ßd GABA(A) Receptors in Shaping Learning Deficits at Puberty in Mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Dryver E. Effect of stress and long-term potentiation (LTP) on subsequent LTP and the theta burst response in the dentate gyrus. Brain Res. 1994;666:232–238. doi: 10.1016/0006-8993(94)90777-3. [DOI] [PubMed] [Google Scholar]
- Song I, Savtchenko L, Semyanov A. Tonic excitation or inhibition is set by GABA(A) conductance in hippocampal interneurons. Nat Commun. 2011;2:376. doi: 10.1038/ncomms1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA(A) receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Masuhara M, Iwai H, Sonomura T, Sato T. Repeated stress-induced expression pattern alterations of the hippocampal chloride transporters KCC2 and NKCC1 associated with behavioral abnormalities in female mice. Biochemical and Biophysical Research Communications. 2015;465:145–151. doi: 10.1016/j.bbrc.2015.07.153. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Koob GF, Le MM. Neurosteroids in learning and memory processes. Int Rev Neurobiol. 2001;46:273–320. doi: 10.1016/s0074-7742(01)46066-1. 273-320. [DOI] [PubMed] [Google Scholar]
- Whissell PD, Eng D, Lecker I, Martin LJ, Wang DS, Orser BA. Acutely increasing δGABA(A) receptor activity impairs memory and inhibits synaptic plasticity in the hippocampus. Front Neural Circuits. 2013a;7:146. doi: 10.3389/fncir.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whissell PD, Rosenzweig S, Lecker I, Wang DS, Wojtowicz JM, Orser BA. γ-aminobutyric acid type A receptors that contain the δ subunit promote memory and neurogenesis in the dentate gyrus. Ann Neurol. 2013b;74:611–621. doi: 10.1002/ana.23941. [DOI] [PubMed] [Google Scholar]
- Wu Z, Guo Z, Gearing M, Chen G. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer's [corrected] disease model. Nat Commun. 2014;5:4159. doi: 10.1038/ncomms5159.:4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Polley N, Mathews GC, Delpire E. NKCC1 and KCC2 prevent hyperexcitability in the mouse hippocampus. Epilepsy Res. 2008;79:201–212. doi: 10.1016/j.eplepsyres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]