Abstract
Background
Growing evidence suggests that immunotherapy and radiation therapy can be synergistic in the treatment of cancer. We sought to determine the effect of the relative timing and type of immune checkpoint therapy on response of melanoma brain metastases to treatment with stereotactic radiosurgery (SRS).
Methods
75 melanoma patients with 566 brain metastases were treated with both SRS and immunotherapy between 2007 and 2015 at a single institution. Immunotherapy and radiosurgery treatment to any single lesion was considered concurrent if SRS was administered within four weeks of immunotherapy. The impact of timing and type of immunotherapy on lesional response was determined using the Wilcoxon rank sum test to compare median percent lesion volume change at 1.5 months, 3 months, and 6 months after SRS treatment, with significance determined by p=0.0167, per the Bonferroni correction for multiple comparisons.
Results
Concurrent use of immunotherapy and SRS resulted in significantly greater median percent reduction in lesion volume at 1.5 months (−63.1% vs −43.2%, p<0.0001), 3 months (−83.0% vs −52.8%, p<0.0001), and 6 months (−94.9% vs −66.2%, p<0.0001) compared to non-concurrent therapy. Median percent reduction in lesion volume was also significantly greater for anti-PD-1 than for anti-CTLA-4 at 1.5 months (−71.1% vs −48.2%, p<0.0001), 3 months (−89.3% vs −66.2%, p<0.0001), and 6 months (−95.1% vs −75.9%, p=0.0004).
Conclusions
Administration of immunotherapy within four weeks of SRS results in improved lesional response of melanoma brain metastases compared to treatment separated by greater than four weeks. Anti-PD-1 therapy also results in greater lesional response than anti-CTLA-4 after SRS.
Keywords: stereotactic radiosurgery, brain metastases, melanoma, anti-CTLA-4, anti-PD-1, immunotherapy
Precis: For melanoma brain metastases, immune checkpoint therapy administered within 4 weeks of SRS is more effective than treatment separated by more than 4 weeks. In addition, anti-PD-1 therapy increases lesional response to SRS compared to anti-CTLA-4.
Introduction
Brain metastases (BrMets) historically develop in 10-40% of all cancer patients with metastatic disease.1 As survival is increasing in duration with the use of new systemic therapies such as targeted agents and immunotherapies, the incidence of brain metastases is increasing also.1 Understanding the efficacy of treatments for BrMets and their toxicities is therefore becoming increasingly important. Despite data that shows that both targeted agents and immunotherapy agents can have therapeutic effect in the central nervous system (CNS),2-4 the long-term control rates for these drugs remain unknown. Because of this, whole brain radiation therapy (WBRT), stereotactic radiosurgery (SRS), and/or surgical resection remain standard treatments for BrMets given their high rate of local treatment success.5
Radiation therapy, historically thought to be immunosuppressive because of its lymphotoxicity,6 has more recently been shown to induce pro-inflammatory responses secondary to modulation of antigen presentation and immune signaling pathways.7 In the setting of systemic use of immune checkpoint inhibitors such as ipilimumab, pembrolizumab, and nivolumab against a variety of tumor types,8 it remains unknown if combining these agents with standard local treatment modalities might result in synergistic efficacy or toxicity. Given what could be synergistic mechanisms of action between immunotherapy and radiation therapy, it is reasonable to hypothesize that the combination could result in improved treatment outcomes.9 Further, it is unknown what the best timing might be for achieving maximal synergism, and if anti-CTLA-4 versus anti-PD-1 agents have different interactions with focal therapy.
Because of the increased efficacy of SRS in comparison to fractionated radiation, the majority of melanoma brain metastases at our institution are treated primarily with radiosurgery whenever possible.10 To explore the possible interaction between immunotherapy and radiation, we therefore performed a retrospective review of our melanoma BrMets patients who received both immune checkpoint therapy and SRS during their disease course, with a focus on how the timing and type of immunotherapy affected lesional response.
Methods
Study design and participants
All patients with melanoma BrMets treated with Gamma Knife stereotactic radiosurgery (SRS) between 2007 and 2015 who also received either anti-CTLA-4 or anti-PD-1 immunotherapy were identified from an IRB-approved institutional database. Patients were excluded if they had leptomeningeal disease or no follow-up imaging after SRS. Individual lesions were also excluded within each patient’s data if they were post-operative resection cavities or if the lesions were associated with extensive extralesional hemorrhage. In patients who underwent SRS treatment more than once, each new lesion was studied independently and included in this study.
All patients were treated using the Leksell Perfexion Gamma Knife (Elekta Medical Systems, Inc.). Lesions were treated to a median of 20 Gy (range, 12-24 Gy) to the tumor margin, with doses individualized using institutional standardized modifications of RTOG 90-05,11 which take into account both tumor volume and number of lesions. Lower doses were prescribed for both increasing tumor volume and increasing number of lesions to be treated. Most patients treated with anti-CTLA-4 therapy received up to four doses of ipilimumab at either 3 mg/kg or 10 mg/kg; several of these patients later received a re-induction course. Patients treated with anti-PD-1 therapy received pembrolizumab, at doses of either of 2 mg/kg or 10 mg/kg every 2 or 3 weeks, or nivolumab, at doses of 3 mg/kg every 2 or 3 weeks. Examination of the number of days elapsed between SRS and either the first or last dose of immunotherapy for each lesion (with lesions treated during immunotherapy assigned a value of 0) demonstrated a cluster of lesions around +/− 4 weeks (Supplemental Figure 1). On this basis, immunotherapy and radiosurgery treatment to any single lesion was considered concurrent if SRS was administered within 4 weeks of the start or end of immunotherapy; all other lesions were defined as having had non-concurrent treatment.
3D MPRAGE, T1-weighted gadolinium enhanced MR images with 1 mm slice thickness of the whole brain were obtained on the day of SRS treatment and at each follow-up, as described in a previous publication from this institution.12 To determine lesional response, the maximal diameter of the T1 contrast-enhancing portion of each SRS-treated lesion was measured in three orthogonal planes at the time of treatment and at each follow-up by a single individual, to reduce inter-reader measuring errors. Lesion volumes were calculated using the formula (length × width × height)/2, as previously published.12 Data collection was censored for any single lesion if it required local intervention, such as surgery, laser thermocoagulation, or salvage radiation or if the patient received bevacizumab therapy. In addition, data collection was also terminated if the patient was switched from anti-CTLA-4 therapy to anti-PD-1 therapy during follow-up or vice versa. Volume changes at each follow-up were normalized to the baseline treatment volume. For a descriptive graphical analysis of temporal changes in volume, scans were grouped into intervals clustered at 1.5 months, 3 months, 6 months, 9 months, 12 months, 18 months, 24 months, and 36 months.
Statistical analysis
Statistical analyses were performed using STATA (Version 13.0, StataCorp, College Station, Texas). Baseline characteristics were compared using chi-square tests (for categoric variables) or ANOVA (for continuous variables). To determine the impact of the relative timing of therapies (concurrent vs non-concurrent) and the type of immunotherapy (anti-CTLA-4 vs anti-PD-1) on early lesional response, we used the Wilcoxon rank-sum test to compare the median percent volume change at follow-up between treatment cohorts at 1.5 months, 3 months, and 6 months, with significance determined by p=0.0167, per the Bonferroni correction for multiple comparisons. These intervals were chosen a priori for clinical relevance and based on our anecdotal experience that differences in treatment response between types of immunotherapy during those months may be most significant. We also used Kaplan-Meier methods to estimate overall survival (OS) per patient, from the time of first SRS treatment, and the log rank test was used to compare median survival between different treatment groups.
Results
Patient demographics
A total of 75 patients with 566 SRS-treated melanoma brain metastases were included in this study. Baseline patient, treatment, and lesion characteristics are listed in Table 1. The mean age at the time of treatment was 62.5 years, and 68% of the patients were male. Median KPS of the patients was 90 (range 50-100) and median melanoma-specific GPA was 3.0 (range, 0 to 4.0). 81% of the patients had active extra-cerebral metastases at the time of first SRS treatment. Median time from initial diagnosis of primary melanoma to the development of BrMets was 37.5 months (range, 0-318 months). The median lesion size for the entire cohort was 105.6 mm3 (range, 4-27,482 mm3), and the median marginal dose for each lesion was 20 Gy (range 12-24 Gy). Median length of imaging follow-up per lesion was 6 months (range 1-93 months).
Table 1.
Baseline patient, treatment, and lesion characteristics.
| Patient Characteristic | Patient Cohort (n=75) |
|---|---|
| Mean age at first SRS (yrs) | 62.5 |
| Sex | |
| Male | 51 (68%) |
| Female | 24 (32%) |
| Median KPS | 90 (50-100) |
| Median melanoma-specific GPA | 3 (0-4) |
| History of WBRT before SRS | 5 (7%) |
| Active systemic disease | 61 (81%) |
| Median time from initial melanoma diagnosis to development of BrMets (months) |
37.5 (0-318) |
| BRAF | |
| Mutated | 22 (29%) |
| Wild-type | 30 (40%) |
| Unknown/not tested | 23 (31%) |
| Prior chemotherapy | 18 (24%) |
| BRAF inhibitor | 15 (20%) |
| Immunotherapy type | |
| Anti-CTLA-4 | 54 (72%) |
| Anti-PD-1 | 21 (28%) |
| Lesion Characteristic | Lesion Cohort (n=566) |
| Median lesion volume (mm3) | 105.6 (4-27482) |
| Median dose (Gy) | 20 (12-24) |
| Timing of SRS | |
| Concurrent | 313 (55%) |
| Non-concurrent | 253 (45%) |
| Median length of f/u after SRS (mo) | 6 (1-93) |
Abbreviations: KPS = Karnofsky performance status; GPA = graded prognostic analysis.
33 patients with 193 lesions had concurrent treatment with immunotherapy and SRS; 9 of these patients had multiple SRS treatments that were all concurrent with immunotherapy. 22 patients with 91 lesions had non-concurrent treatment; 9 of these patients had multiple SRS treatments that were all non-concurrent. The remaining 20 patients with 282 lesions had both concurrent and non-concurrent SRS treatments, with 120 (43%) of these lesions treated concurrently and 162 (57%) of these lesions treated non-concurrently. In total, 313 lesions in 53 patients were treated concurrently, and 253 lesions in 42 patients were treated non-concurrently. Baseline characteristics for these groups are shown in Table 2. For the non-concurrent group, median time between SRS and immunotherapy was 7.3 months (range 1.5-41.6 months), with 195 lesions (77%) receiving immunotherapy before SRS and 58 lesions (23%) receiving immunotherapy after SRS.
Table 2.
Baseline patient and lesion characteristics by timing of immunotherapy.
| Patient characteristic | Concurrent only (n=33) |
Non- concurrent only (n=22) |
Both concurrent and non- concurrent SRS (n=20) |
p value |
|---|---|---|---|---|
| Mean age at first SRS (yrs) | 64.1 | 61.4 | 61.4 | 0.6765 |
| Sex | ||||
| Male | 24 (73%) | 13 (59%) | 14 (70%) | 0.555 |
| Female | 9 (27%) | 9 (41%) | 6 (30%) | |
| Median KPS | 90 (70-100) | 90 (60-100) | 100 (50-100) | 0.218 |
| Median melanoma-specific GPA | 2 (1-4) | 3 (0-4) | 3 (0-4) | 0.580 |
| History of WBRT before SRS | 3 (9%) | 2 (9%) | 0 (0%) | 0.378 |
| Active systemic disease | 29 (88%) | 16 (73%) | 16 (80%) | 0.363 |
| Median time from initial melanoma diagnosis to development of BrMets (months) |
26.1 (0-229) | 66.2 (0-318) | 51.5 (0-287) | 0.0128 |
| BRAF status* | ||||
| Mutated | 10 (30) | 5 (23) | 7 (35) | 0.601 |
| Wild-type | 17 (52) | 7 (32) | 6 (30) | |
| Prior chemotherapy | 7 (21%) | 11 (50%) | 0 (0%) | <0.0001 |
| BRAF inhibitor | 6 (18%) | 3 (14%) | 6 (30%) | 0.392 |
| Type of immunotherapy | ||||
| Anti-CTLA-4 | 19 (58%) | 19 (86%) | 16 (80%) | 0.043 |
| Anti-PD-1 | 14 (42%) | 3 (14%) | 4 (20%) | |
| Median number of BrMets treated per SRS session |
3 (1-23) | 3 (1-20) | 4 (1-21) | 0.4981 |
| Lesion characteristic | n=313 | n=253 | - | |
| Median lesion volume (mm3) | 112 (4- 10370) |
97.5 (4- 27482) |
- | 0.3176 |
| Median dose (Gy) | 20 (12-24) | 20 (12-24) | - | <0.0001† |
not all patients were tested for BRAF status
concurrently treated lesions had a lower distribution of marginal doses
Fifty-four patients (72%) received anti-CTLA-4 immunotherapy, for a median number of 4 doses (range, 1-17 doses). Twenty-one patients (28%) received anti-PD-1 immunotherapy, for a median number of 12 doses, (range, 1-40 doses). Of note, 12 of these patients had previously received anti-CTLA-4 therapy. No patients received concurrent anti-CTLA-4 and anti-PD-1 therapy. Baseline characteristics for both groups are shown in Table 3. While many of the standard demographics were similar between the 2 groups, lesions in the anti-PD-1 group tended to have larger baseline tumor volumes (median of 229.6 mm3 vs 85.7 mm3, p<0.0001), were prescribed lower SRS doses (median of 18 Gy vs 20 Gy, p<0.0001), and were more likely to have concurrent SRS treatment (85% of lesions treated concurrently for anti-PD-1 vs 47% for anti-CTLA-4, p<0.0001).
Table 3.
Baseline patient and lesion characteristics by type of immunotherapy.
| Patient characteristic | Anti-CTLA-4 (n=54) |
Anti-PD-1 (n=21) | p value |
|---|---|---|---|
| Mean age at first SRS (yrs) | 62.9 | 61.9 | 0.7579 |
| Sex | |||
| Male | 34 (63%) | 17 (81%) | 0.134 |
| Female | 20 (37%) | 4 (19%) | |
| Median KPS | 100 (50-100) | 90 (70-100) | 0.259 |
| Median melanoma-specific GPA | 3 (0-4) | 3 (1-4) | 0.331 |
| History of WBRT before SRS | 5 (9%) | 0 (0%) | 0.149 |
| Active systemic disease | 46 (85%) | 15 (71%) | 0.170 |
| Median time from initial melanoma diagnosis to development of BrMets (months) |
38.9 (0-318) | 27.8 (0-204) | 0.5225 |
| BRAF status* | |||
| Mutated | 15 (28) | 7 (33) | 0.399 |
| Wild-type | 17 (31) | 13 (62) | |
| Prior chemotherapy | 16 (30%) | 2 (10%) | 0.067 |
| BRAF inhibitor | 8 (15%) | 7 (33%) | 0.072 |
| Median number of BrMets treated per SRS session |
3 (1-20) | 2 (1-23) | 0.1472 |
| Lesion characteristic | n=438 | n=128 | |
| Median lesion volume (mm3) | 85.7 (4-27482) | 229.6 (4-10370) | <0.0001 |
| Median dose (Gy) | 20 (12-24) | 18 (12-22) | <0.0001 |
| Timing of SRS | |||
| Concurrent | 204 (47%) | 109 (85%) | <0.0001 |
| Non-concurrent | 234 (53%) | 19 (15%) |
not all patients were tested for BRAF status
Early lesional response relative to timing of treatment
As shown in Figure 1a, median percent reduction in lesion volume was significantly greater for the concurrent group than for the non-concurrent group at 1.5 months (−63.1% vs −43.2%, p<0.0001), 3 months (−83.0% vs −52.8%, p<0.0001), and 6 months (−94.9% vs −66.2%, p<0.0001). Because of the differences between the groups in prior treatment with chemotherapy, immunotherapy type, time to development of BrMets from initial melanoma diagnosis, and SRS doses, for sensitivity we analyzed lesions in the subset of 20 patients with both concurrent and non-concurrent SRS treatments, as these patients could serve as their own controls. As expected, there were no longer differences in any baseline characteristics within this subset. Results are shown in Figure 1b where median percent reduction in lesion volume remained significantly greater for the concurrent group than for the non-concurrent group at 1.5 months (−62.8% vs −46.1%, p=0.0057), 3 months (−83.6% vs −57.7%, p<0.0001), and 6 months (−90.4% vs −71.4%, p=0.0046).
Figure 1.
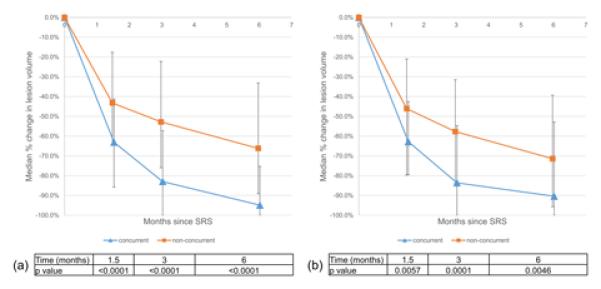
Early lesional response with respect to timing of immunotherapy for (a) the entire cohort as well as (b) a subset of patients with both concurrent and non-current SRS treatments. Error bars denote the interquartile range of volume change at each time point.
We additionally examined if the sequence of therapies affected the results for concurrently treated lesions. 122 lesions were treated with SRS before starting immunotherapy, whereas 191 lesions were treated with SRS after having started immunotherapy. There was no difference at 1.5 months (−64.9% vs −62.8%, p=0.53), 3 months (−82.0% vs −83.9%, p=0.93), or 6 months (−92.0% vs −96.2%, p=0.23).
Early lesional response by treatment type
As shown in Figure 2a, median percent reduction in lesion volume was significantly greater for anti-PD-1 than for anti-CTLA-4 at 1.5 months (−71.1% vs −48.2%, p<0.0001), 3 months (−89.3% vs −66.2%, p<0.0001), and 6 months (−95.1% vs −75.9%, p=0.0004). Given the differences in baseline tumor volumes and SRS doses between the two groups, for sensitivity we analyzed a subset of lesions that were 10 mm or larger in diameter and received at least 16 Gy (Figure 2b). 105 lesions met these criteria, 76 in the anti-CTLA-4 group and 29 in the anti-PD1 group. With subset analysis, median percent reduction in lesion volume remained significantly greater for anti-PD-1 than for anti-CTLA-4 at 1.5 months (−67.4% vs −39.4%, p<0.0001), 3 months (−75.4% vs −48.4%, p=0.0080), and 6 months (−88.4% vs −71.3%, p=0.0154).
Figure 2.
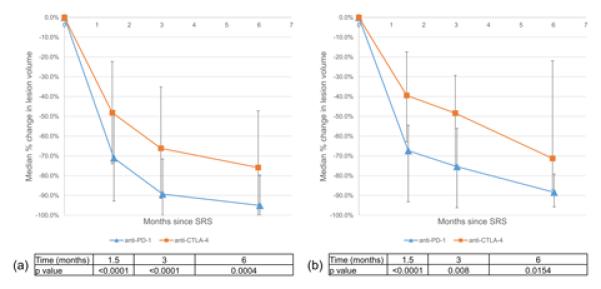
Early lesional response with respect to type of immunotherapy for (a) the entire cohort as well as (b) a subset of lesions ≥10 mm and treated with at least 16 Gy. Error bars denote the interquartile range of volume change at each time point.
We additionally examined if prior therapy with anti-CTLA-4 affected the results for the anti-PD-1 patients. 12 patients with 69 lesions had previously received anti-CTLA-4 whereas 9 patients with 59 lesions were anti-CTLA-4 naïve. There was no consistent difference over time for median percent reduction in lesion volume at 1.5 months (−70.4% vs −72.3%, p=0.68), 3 months (−90.0% vs −78.7% p=0.0088), and 6 months (−92.3% vs −96.6%, p=0.26).
Combined effect of treatment timing and type on early lesional response
The effect of timing remained significant when analyzing only lesions in the anti-CTLA-4 group. Concurrent treatment again demonstrated significantly greater median percent reduction in lesion volume at 1.5 months (−58.3% vs −38.5%, p<0.0001), 3 months (−76.9% vs −52.5%, p<0.0001), and 6 months (−94.2% vs −65.8%, p<0.0001).
The effect of treatment type was diminished when analyzing only lesions treated concurrently, with a decrease in the magnitude of difference between anti-PD-1 and anti-CTLA-4 at 1.5 months (−70.0 % vs −58.3%, p=0.0761), 3 months (−89.8% vs −76.9%, p=0.0043), and 6 months (−95.1% vs −94.2%, p=0.8086).
Delayed lesional response
During follow up, 39 lesions in 24 patients demonstrated regrowth to greater than 120% of the baseline volume. Ultimately, 11 lesions in 8 patients required surgical management, with 6 lesions resected and 5 lesions treated with laser thermocoagulation. On pathology, all 11 lesions demonstrated features consistent with radiation necrosis; 3 lesions also contained some viable tumor. There were no significant differences in regrowth incidence when comparing treatment types and relative timing of treatment.
Overall survival
The median OS for all patients from first SRS treatment was 18.5 months (range, 2.1 to 96.1 months) (Figure 3). 23 of 75 patients (31%) are still alive at the time of analysis, with a median follow-up from first SRS treatment of 15.5 months (range, 3.7 to 96.1 months). Of the patients who started on anti-CTLA-4 and had either only non-concurrent (n=19) or only concurrent SRS treatment (n=19), median OS was 8.0 months (range 2.1 to 61.8 months) for non-concurrent treatment and 19.1 months (range 3.3 to 64.2) for concurrent treatment (p=0.0858). When both anti-CTLA-4 and anti-PD-1 patients were included in the analysis, median OS was 9.0 months (range 2.1 to 61.8 months) for the 22 non-concurrent only patients and 19.1 months (range 2.7 to 64.2 months) for the 33 concurrent only patients (p=0.0691).
Figure 3.
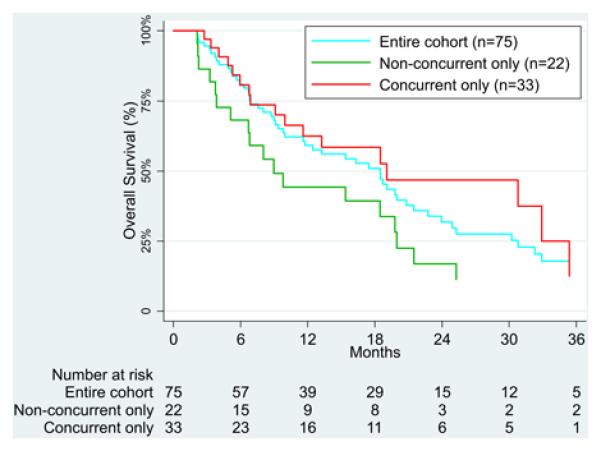
Kaplan-Meier curve illustrating survival of the entire cohort, as well as patients who had only non-concurrent or only concurrent SRS treatments.
Discussion
Little data is available in the literature regarding the effect of timing and type of immune checkpoint therapy on the outcome of patients undergoing radiosurgical treatment of melanoma brain metastases. Our study results suggest that (1) immunotherapy can have a synergistic effect with radiosurgery in the treatment of brain metastases, even in those not known to have PD-L1 expression, and (2) early lesional response is greater and more rapid with concurrent administration of immunotherapy and SRS. Of note, this timing effect remained significant even when we examined a subset of patients who had both concurrent and non-concurrent therapy.
Though much remains unknown about the effect of immunotherapy on brain metastases as well as its interaction with radiation therapy, our findings are consistent with current literature. Preclinical studies have suggested that concurrent treatment is most effective.7 To date, only a small number of retrospective clinical studies have examined timing in regards to the combination of SRS and immunotherapy for melanoma BrMets. While most of these studies had small patient numbers and failed to identify any significant effect of timing on outcomes,13-15 in 2015 Kiess et al. reported in 46 patients that those treated with SRS before or during ipilimumab had increased overall survival compared to those treated with SRS after ipilimumab.16 They also noted a trend towards higher rates of local control with concurrent treatment when compared to non-concurrent treatment. Schoenfeld et al. also found in a small series of 16 patients that SRS before ipilimumab was associated with increased survival compared to SRS after ipilimumab.17 Jiang et al. subsequently reported, in abstract form, on a larger cohort of 71 patients that those who received SRS within 5.5 months of their last dose of ipilimumab had significantly improved intracranial control compared to those who received SRS after 5.5 months; however, they did not find a difference in overall survival.18 While our study was not designed to look at survival, a sub-analysis of our data also suggests a trend towards increased survival with concurrent treatment, although this did not reach statistical significance.
Local control rates have reportedly been high for melanoma BrMets treated both with SRS and anti-CTLA-4 therapy as well as SRS and anti-PD-1 therapy.14-16 However, to our knowledge no study has attempted to compare anti-PD-1 and anti-CTLA-4 to each other directly in this setting. Our data suggest that, compared to anti-CTLA-4 and SRS, anti-PD-1 and SRS may result in greater and more rapid lesion shrinkage in the initial months after SRS, even after controlling for baseline lesion size and SRS dosing. However, these results may have been influenced by a disproportionate number of lesions in the anti-PD-1 group also having concurrent treatment with SRS. When we examined only lesions that were treated concurrently, the effect of anti-PD-1 on early lesional response was diminished, and remained significant only at the 3 month time point. It is possible however that the lower numbers of lesions and patients in this subgroup resulted in an analysis underpowered to detect smaller differences between anti-PD-1 and anti-CTLA-4.
Limitations of this study include its retrospective nature and relatively low number of patients, particularly in the non-concurrent subset of patients receiving anti-PD-1. Also, while we attempted to address several additional questions in secondary analyses, including the effect of treatment sequence and the effect of prior anti-CTLA-4 use on outcomes for anti-PD-1 and SRS, lower numbers of patients and lesions available for these subgroups may have produced underpowered analyses, obscuring potential differences. In addition, we recognize our use of early lesional response as a surrogate for treatment efficacy has limitations. Although some studies have suggested that significant early lesional response to SRS translates into prolonged local control,19, 20 ultimately our results still need to be correlated with more traditional measures of clinical outcome. Future studies could also examine treatment response based upon changes in other MR sequences looking at tumor hemorrhage, vascularity and cellularity, or perilesional edema. Finally, given the median survival of 18 months in our study patients, the role that immunotherapy may play in the development of radiation necrosis also needs to be determined.
In conclusion, we find that immune checkpoint therapy administered within 4 weeks of SRS (either prior to or after SRS) results in improved lesional response of melanoma BrMets compared to if immunotherapy and SRS are used more than 4 weeks apart. Anti-PD1 immunotherapy may also have a greater effect on lesional response than anti-CTLA-4 in this setting. While anti-CTLA-4 and anti-PD-1 have distinct mechanisms of action, comparison of monotherapies may become less relevant as oncologists move towards combination therapy.21 However, the mechanism by which concurrent immunotherapy increases the effect of radiation remains unknown, and it is unclear if this effect is isolated to melanoma or perhaps could be applied to other cancer types that also develop brain metastases. Further testing and validation of these results in larger prospective studies and in other cancer types is warranted. In addition, number of doses of immunotherapy concurrent with radiosurgery needs to be studied to determine if this paradigm could be used to improve the result of other radiotherapy treatments.
Supplementary Material
Supplemental Figure 1. Histogram examining number of days between immunotherapy and SRS, using a bin of 7 days. Negative values indicate immunotherapy given before SRS; positive values indicate immunotherapy given after SRS. Only lesions treated at +/− 180 days shown, in order to demonstrate clustering at +/− 28 days.
Acknowledgments
Funding sources: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the NIH under award number TL1TR000141. The content is solely the responsibility of the authors and does not necessarily represented the official views of the National Institutes of Health.
Footnotes
Conflict of interest disclosures: JMQ reports funding support from the NIH-NCATS CTSA-TL1 Medical Student Research Fellowship. JBY reports funding from 21st Century Oncology. HMK reports receiving consulting fees from Regeneron, Alexion, and Prometheus. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Author Contributions: JMQ and VLC designed the study. JMQ collected the data. JMQ, JBY, and VLC analyzed and interpreted the data. JMQ and JBY did the statistical analysis. JMQ and VLC drafted the manuscript. All authors critically revised the manuscript and approved the final version.
References
- 1.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 2.Lin NU. Targeted therapies in brain metastases. Curr Treat Options Neurol. 2014;16:276. doi: 10.1007/s11940-013-0276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368–1379. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 5.Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Order SE. The effects of therapeutic irradiation on lymphocytes and immunity. Cancer. 1977;39:737–743. doi: 10.1002/1097-0142(197702)39:2+<737::aid-cncr2820390708>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 8.Kreamer KM. Immune Checkpoint Blockade: A New Paradigm in Treating Advanced Cancer. J Adv Pract Oncol. 2014;5:418–431. doi: 10.6004/jadpro.2014.5.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wargo JA, Reuben A, Cooper ZA, Oh KS, Sullivan RJ. Immune Effects of Chemotherapy, Radiation, and Targeted Therapy and Opportunities for Combination With Immunotherapy. Semin Oncol. 2015;42:601–616. doi: 10.1053/j.seminoncol.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramakrishna N, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma; local therapies for central nervous system metastases. Am Soc Clin Oncol Educ Book. 2013:399–403. doi: 10.14694/EdBook_AM.2013.33.399. [DOI] [PubMed] [Google Scholar]
- 11.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 12.Patel TR, McHugh BJ, Bi WL, Minja FJ, Knisely JP, Chiang VL. A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. AJNR Am J Neuroradiol. 2011;32:1885–1892. doi: 10.3174/ajnr.A2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117:227–233. doi: 10.3171/2012.5.JNS111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel KR, Shoukat S, Oliver DE, et al. Ipilimumab and Stereotactic Radiosurgery Versus Stereotactic Radiosurgery Alone for Newly Diagnosed Melanoma Brain Metastases. Am J Clin Oncol. 2015 doi: 10.1097/COC.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed KA, Stallworth DG, Kim Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol. 2015 doi: 10.1093/annonc/mdv622. [DOI] [PubMed] [Google Scholar]
- 16.Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92:368–375. doi: 10.1016/j.ijrobp.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoenfeld JD, Mahadevan A, Floyd SR, et al. Ipilmumab and cranial radiation in metastatic melanoma patients: a case series and review. J Immunother Cancer. 2015;3:50. doi: 10.1186/s40425-015-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W, Rodriguez Y, Kim BYS, et al. Temporally-Dependent Intracranial Control of Melanoma Brain Metastasis by Stereotactic Radiation Therapy in Patients Treated With Immune Checkpoint Blockade. Int J Radiat Oncol Biol Phys. 2015;93:S57. [Google Scholar]
- 19.Sharpton SR, Oermann EK, Moore DT, et al. The volumetric response of brain metastases after stereotactic radiosurgery and its post-treatment implications. Neurosurgery. 2014;74:9–15. doi: 10.1227/NEU.0000000000000190. discussion 16; quiz 16. [DOI] [PubMed] [Google Scholar]
- 20.Kim WH, Kim DG, Han JH, et al. Early significant tumor volume reduction after radiosurgery in brain metastases from renal cell carcinoma results in long-term survival. Int J Radiat Oncol Biol Phys. 2012;82:1749–1755. doi: 10.1016/j.ijrobp.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 21.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Histogram examining number of days between immunotherapy and SRS, using a bin of 7 days. Negative values indicate immunotherapy given before SRS; positive values indicate immunotherapy given after SRS. Only lesions treated at +/− 180 days shown, in order to demonstrate clustering at +/− 28 days.


