Abstract
Background
The purpose of this analysis is to evaluate the impact of RT among women ≥70 with T1-2N0 ER negative breast cancer using Surveillance Epidemiology and End Results (SEER)-Medicare linked data.
Methods
The study included 3,432 women who received (n=2850) and did not receive (n=582) RT after BCS. Outcomes were estimated by the Cummulative Incidence (CI) method and compared with Gray's test. Fine and Gray's subdistribution hazard regression models were used to assess the impact of RT and other variables.
Results
Women who received RT were more commonly <75 years-old (42% vs. 16%), had T1 tumors (78% vs. 65%), ductal carcinoma histology (91% vs. 88%), a comorbidity index of zero (41% vs. 25%), and received chemotherapy (29% vs. 12%). The 5-year CI of mastectomy and breast cancer-specific death for patients who received versus did not receive adjuvant radiation was 4.9% and 8.3% versus 10.8% and 24.1% (p <0.001). On multivariable analysis, the omission of RT was an independent predictor for increased mastectomy risk (HR=2.33; 95% CI 1.56, 3.49). Among women aged ≥ 80 years or with T1N0 tumors, the mastectomy incidence with or without receipt of RT was 3.4% vs. 6.9%, and 5.3% vs 7.7%, respectively.
Conclusions
The use of adjuvant radiation after BCS in older women with T1-2N0 ER negative breast cancer is associated with a reduced incidence of future mastectomy and breast cancer death. The magnitude of benefit may be small for women ≥80 or with T1 tumors.
Keywords: Breast neoplasms, Radiotherapy, Estrogen receptor, Aged, Mastectomy
Introduction
A meta-analysis of seventeen randomized trials testing adjuvant breast radiotherapy (RT) versus observation following breast conserving surgery (BCS) demonstrated a significant improvement in local-regional control, disease free survival, and breast cancer-specific survival with the use of RT 1. Adjuvant RT following BCS is therefore standard of care for the majority of women with early stage breast cancer. While the improvement in local-regional control with RT is well recognized in nearly all patients, the magnitude of this benefit and the resulting effect on breast cancer-specific survival depends on multiple prognostic factors, defining a patient's individual risk of disease recurrence.
Advanced age is a well-recognized prognostic factor for a decreased risk of tumor recurrence 2-4, which suggests there may be a diminished benefit of adjuvant radiation therapy in elderly patients. The Cancer and Leukemia Group B (CALGB) and PRIME II randomized trials each demonstrated a modest reduction in local recurrence, but no difference in breast-cancer specific survival with the addition of RT in elderly women with stage I estrogen receptor (ER) positive tumors receiving tamoxifen 5. As a result, the National Comprehensive Cancer Network adopted the omission of RT as a standard of care option for this subset of patients 6. However, it remains unknown whether RT may also be safely omitted from a population of elderly women with ER negative tumors.
The magnitude of benefit with adjuvant RT for elderly women with early stage ER negative tumors is poorly understood, since this population is highly underrepresented in the literature. Among the Early Breast Cancer Trialists Collaborative Group (EBCTCG) meta-analysis of individual patient data from 17 randomized trials of adjuvant RT after BCS, only 23 women > 70 years old with T1-T2N0 ER negative tumors were included. This group of women comprised <1% of the total population studied 1.
Despite the association between ER negative tumors and an increased risk for tumor recurrence demonstrated in this analysis 1, other data from both prospective and retrospective studies suggest that patients with ER negative tumors may experience a reduced benefit with adjuvant RT when compared with ER positive patients 1, 7. Thus, the increased risk of tumor recurrence recognized in patients with ER negative tumors may not necessarily equate to a greater benefit from adjuvant RT.
The purpose of this analysis is to evaluate the benefit of adjuvant radiotherapy following BCS among women ≥70 years old with T1-2 N0 ER negative breast cancer using Surveillance Epidemiology and End Results data linked to Medicare claims (SEER-Medicare). The effect of adjuvant RT on mastectomy incidence and breast cancer specific death has been assessed in relationship to multiple prognostic factors to explore whether RT may also be safely omitted from a population of early stage, elderly women with ER negative tumors.
Materials and Methods
Data Source
SEER-Medicare data link two large population-based data sources within the United States (US). The SEER Program of the National Cancer Institute is the authoritative source of long-term cancer incidence and survival in the US with current coverage of approximately 28% of the population. Medicare is the federally funded insurance program in the US that provides health insurance to individuals over the age of 65. Medicare data include both fee-for-service and managed care plans but only the fee-for-service claims are included with the SEER-Medicare dataset. For this study, the analytic dataset comprised eligible breast cancer cases diagnosed between 1993 and 2007 with follow-up through 2010. Clinical, demographic and cause of death information were derived from SEER, while covered health services from the time of Medicare eligibility until death were derived from Medicare claims. SEER Registries conduct both active and passive follow-up activities to longitudinally trace a patient's vital status from the point of a cancer diagnosis forward. This protocol includes linking with both state vital records and the National Death Index to ascertain date and cause of death when applicable. Institutional review board approval was obtained for this analysis.
Patient Selection
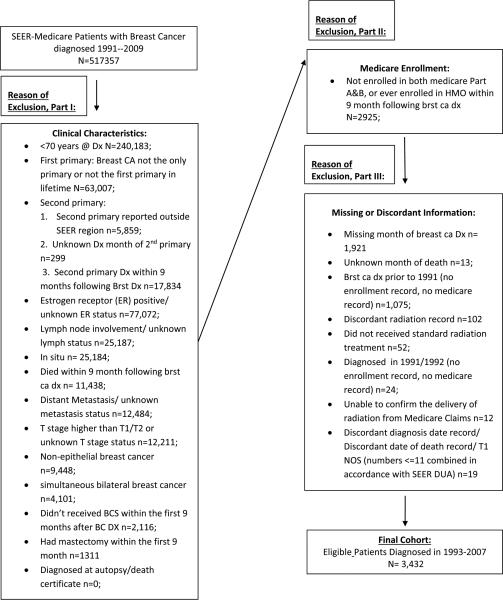
Women ≥70 years old registered in the SEER-Medicare database with a first primary diagnosis of T1-2, lymph node (LN) negative, ER negative invasive breast cancer who were treated with breast conserving surgery during the first 9 months after diagnosis were selected for inclusion. Ninety-seven percent of all women in the study underwent BCS within 3 months of diagnosis. The 9 month period following breast cancer diagnosis was defined as the treatment window for measuring the initiation of all cancer related treatment in accordance with the work from Smith et al8. Women were excluded for the following: simultaneous bilateral breast cancer, non-epithelial histology, distant metastasis, initial treatment with a mastectomy, a second cancer diagnosis, death within the treatment window, a diagnosis date of any subsequent primary was not known, no continuous enrollment in Medicare Parts A and B or enrollment in a HMO during the treatment window, missing or unknown information regarding any component of stage, receptor status, month of diagnosis or month of death.
Independent Variables
The Medicare codes used to define BCS, mastectomy, chemotherapy and external beam RT are listed in Table 1. Receipt of BCS or initiation of chemotherapy and RT were defined by having the corresponding Medicare claims filed within the 9-month treatment window. Patients with both BCS and mastectomy claims within this window were considered to have undergone mastectomy and were excluded. Patients with Medicare claims for radiation planning only or for having less than 15 radiation treatments were considered to have had non-standard or incomplete treatment and were also excluded from analysis. The period for assessing the delivery of radiation treatment was extended beyond the treatment window as long as RT was initiated within the 9-month window. The receipt of radiation for all patients as defined by SEER was confirmed by Medicare claims. The receipt of chemotherapy was defined by the presence of claims with any of the chemotherapy codes listed in Table 1 in conjunction with a corresponding diagnosis code for breast cancer.
Table 1.
Patient, Tumor and Treatment Characteristics
| Breast Radiation |
|||||
|---|---|---|---|---|---|
| Characteristic | No | Yes | P-valuea | ||
| N | % | N | % | ||
| Age | |||||
| 70–74 | 93 | 16% | 1204 | 42% | <0.0001 |
| 75–79 | 116 | 20% | 921 | 32% | |
| ≥ 80 | 373 | 64% | 725 | 25% | |
| Year of Diagnosis | |||||
| 1993–1999 | 135 | 23% | 647 | 23% | 0.54 |
| 2000–2004 | 219 | 38% | 1018 | 36% | |
| 2005–2009 | 228 | 39% | 1185 | 42% | |
| Derived AJCC T stage | |||||
| T1mic+T1a | 33 | 5.7% | 270 | 9.5% | <0.0001 |
| T1b | 111 | 19% | 644 | 23% | |
| T1c | 233 | 40% | 1306 | 46% | |
| T2 | 205 | 35% | 630 | 22% | |
| Progesterone receptor status | |||||
| Positive | >32 | >5.7% | 209 | 7.3% | 0.64 |
| Negative | 538 | 92% | 2613 | 92% | |
| Indeterminate+Not Done+Unknown | <11 | <1.9% | 28 | 0.98% | |
| Tumor Histology | |||||
| Ductal Carcinoma | 511 | 88% | 2600 | 91% | 0.03 |
| Lobular Carcinoma | 24 | 4.1% | 88 | 3.1% | |
| Other | 47 | 8.1% | 162 | 5.7% | |
| Histologic grade | |||||
| Well differentiated | 41 | 7.0% | 180 | 6.3% | 0.84 |
| Intermediate/moderate | 174 | 30% | 823 | 29% | |
| Poorly-/Un- differentiated | 325 | 57% | 1608 | 59% | |
| Not determined | 35 | 6.0% | 175 | 6.1% | |
| Comorbidity Indexb | |||||
| 0 | 144 | 25% | 1163 | 41% | <0.0001 |
| 1 | 91 | 16% | 444 | 16% | |
| ≥ 2 | 347 | 60% | 1243 | 43% | |
| Chemotherapy | |||||
| Yes | 69 | 12% | 822 | 29% | <0.0001 |
| No | 513 | 88% | 2028 | 71% | |
| Race | |||||
| White | 485 | 83% | 2477 | 87% | 0.02 |
| Black | 69 | 12% | 245 | 8.6% | |
| Hispanic | <11 | <1.9% | 33 | 1.2% | |
| Asian | <11 | <1.9% | 44 | 1.5% | |
| Other/ Native Amerciand | <11 | <1.9% | 51 | 1.8% | |
| Marital status | |||||
| Married | 142 | 24% | 1320 | 46% | <0.0001 |
| Unmarried | 409 | 70% | 1453 | 51% | |
| Unknown | 31 | 5.3% | 77 | 2.7% | |
| Demographic Region | |||||
| West | 258 | 44% | 1193 | 42% | 0.63 |
| Midwest | 82 | 14% | 412 | 15% | |
| South | 110 | 19% | 538 | 19% | |
| Northeast | 133 | 23% | 712 | 25% | |
| Urban residence | |||||
| Metro | 503 | 86% | 2535 | 89% | 0.08 |
| Urban+Rural | 79 | 14% | 315 | 11% | |
| Census Track Level Povertyc | |||||
| 0.00%–9.99% | 345 | 59% | 1921 | 67% | 0.0003 |
| 10.00%–19.99% | 150 | 26% | 628 | 22% | |
| 20.00%+ | 87 | 15% | 301 | 11% | |
P-value is calculated by Chi-square Test except for Race, which is calculated by Fisher's Exact.
69 patients’ Medicare Enrollment in the 12 months prior to Breast Cancer Diagnosis were not continuous, CCI were calculated from all the claims existed in Medicare for these 69 patients.
Zip code poverty level were used for 30 patients with missing census track poverty level
This category also contains a very small number of unknown race subgroup
Most of the patient and tumor characteristics (e.g. diagnosis, age, race, marital status, region, histology, grade, year of diagnosis, and receptor status) were taken directly from SEER data. Since SEER does not directly collect information on patient socioeconomic status, an area-based measure of poverty was derived from SEER census tract data and was included with the SEER-Medicare dataset. Tumor stage was defined by the AJCC 6th edition TNM stage for all cases. AJCC staging data were directly available in SEER for cases diagnosed between 2004 and 2007 and were derived from SEER EOD 3rd edition codes for cases diagnosed from 1993 to 2003. A measure of comorbidity for each patient was defined according to the Deyo adaptation of the Charlson comorbidity index 9, 10, modified to exclude incident breast cancer diagnosis. This index used Medicare inpatient, carrier, and outpatient claims during the 12 month period before breast cancer diagnosis, excluding the month of diagnosis.
Statistical Analysis
Study follow-up began at month 10 from diagnosis for all individuals. The primary outcome variable, cumulative incidence (CI) of mastectomy, was defined as the occurrence of mastectomy, or was censored at the time of contralateral breast cancer, loss to follow-up, or the end of the designated period for which follow-up was being assessed (e.g. 5 years). The secondary outcome variable, cumulative incidence of breast cancer death, was defined as the incidence of death due to breast cancer. Censored events were similarly defined. The CI of mastectomy and breast cancer death were generated using cumulative incidence function and using death without the event of interest as the competing risk. CI functions were adjusted with other covariates (all the demographic and clinical characteristics listed in table 1,) at average levels. Chi-square and Fisher's exact tests were used to compare patient, tumor and treatment characteristics between groups. The effect of RT and other demographic and clinical characteristics on the outcome variables was assessed through univariate Gray's test and multivariate Fine and Gray's subdistribution hazard regression. Further sub-group analysis was also performed to assess the effect of RT across strata according to age and T stage. Overall survival was analyzed using the Kaplan-Meier method and compared for patients who did and not receive RT using the log-rank test. All statistics were calculated using SAS software version 9.3 (SAS Institute., Inc., Cary, NC, USA) and all statistical tests were two sided using an α = 0.05 Type I error rate.
Results
Patient Population
Of the 516,260 women registered within the SEER-Medicare database and diagnosed with breast cancer between 1993 to 2007, 3,432 women met all eligibility criteria for inclusion (Figure 1). Patient, tumor and treatment characteristics for patients who received (n= 2850) and did not receive (n=582) radiation therapy are presented in Table 1. Patients who received RT were more often younger, of white race, married, and living in areaswith lower poverty rates. Patients who received RT were also more likely to have lower T stage tumors, and a lower comorbidity index, and less likely to have ductal carcinoma histology, and receive chemotherapy. There were no substantial differences in year of diagnosis, demographic region, progesterone receptor status or histologic grade between patients who received and did not receive radiation therapy. Women ≥75 were more likely to have a comorbidity index ≥2 (52% vs. 37%, p<0.001) and less likely to receive chemotherapy (18% vs. 41%, p<0.001) compared with patients age 70–74. There was no substantial difference in the distribution of histological grade among the varying age groups.
Figure 1.
Exclusion Flow Chart of SEER-Medicare Cases Selected for Analysis
Incidence of Mastectomy
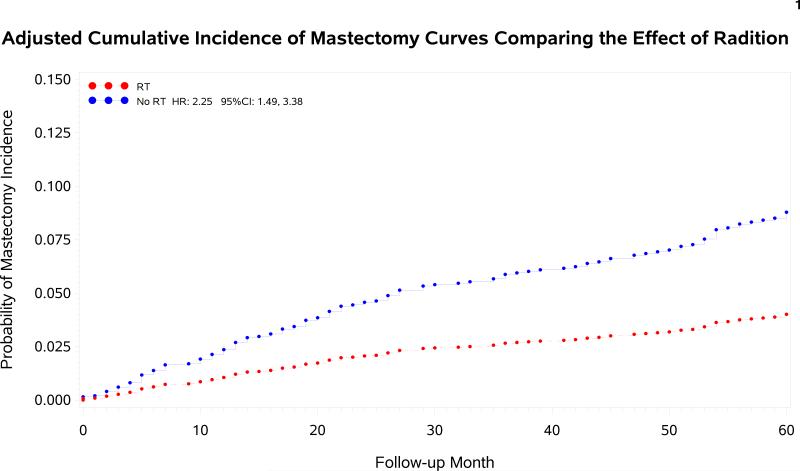
The median follow-up period for mastectomy assessment for the patient population is 45 months (IQR 20–81). The 5-year CI of mastectomy for patients who received vs. did not receive adjuvant radiation were 4.9% (95% CI 4.0%, 5.9%) versus 10.8% (95% CI 7.7%, 14.6%), p<.0001.
On multivariate analysis (Table 2), women treated with BCS alone had a significantly higher incidence of future mastectomy at 5 years compared with women treated with BCS and adjuvant RT, with a HR of 2.3; 95% CI 1.6, 3.5. Multivariate analysis for CI of mastectomy is listed in Table 3. Additional variables associated with MFS were tumor stage and urban residence; less substantial associations also existed between MFS and progesterone receptor status, cormorbidity index, and SEER region.
Table 2.
Associations with 5-Year Mastectomy Cumulative Incidence
| Characteristic | Gray's Test P-value | Number of Outcomes | Person-Year | HR (95%CI) | P-value | |
|---|---|---|---|---|---|---|
| Breast radiation | <.0001 | |||||
| Yes | 97 | 9793.08 | 1 (Ref) | |||
| No* | 40 | 1509.5 | 2.33 | (1.56, 3.49) | <.0001 | |
| Age | 0.93 | |||||
| 70 - 74 | 53 | 4558.17 | 1.41 | (0.86, 2.34) | 0.18 | |
| 75 - 79 | 44 | 3527.75 | 1.41 | (0.89, 2.24) | 0.14 | |
| ≥ 80 | 40 | 3216.67 | 1 (Ref) | |||
| T Stage | 0.01 | |||||
| T1a + T1mic | 13 | 1004.67 | 1 (Ref) | |||
| T1b | 22 | 2639.58 | 0.63 | (0.31, 1.28) | 0.20 | |
| T1c* | 57 | 5257.17 | 0.75 | (0.40, 1.40) | 0.36 | |
| T2* | 45 | 2401.17 | 1.01 | (0.52, 1.96) | 0.97 | |
| Progesterone receptor status | 0.08 | |||||
| Positive | 5 | 942.5 | 1 (Ref) | |||
| Negative | 129 | 10230.5 | 2.01 | (0.82, 4.92) | 0.12 | |
| Indeterminate | 3 | 129.58 | 3.43 | (0.80, 14.73) | 0.10 | |
| Tumor Histology | 0.89 | |||||
| Ductal Carcinoma | 122 | 10172.42 | 1 (Ref) | |||
| Lobular Carcinoma | 5 | 415.33 | 1.47 | (0.60, 3.63) | 0.40 | |
| Other | 10 | 714.83 | 1.40 | (0.71, 2.74) | 0.34 | |
| Histologic grade | 0.11 | |||||
| Well differentiated | 4 | 796.83 | 1 (Ref) | |||
| Intermediate/moderate | 35 | 3386.83 | 2.21 | (0.78, 6.28) | 0.14 | |
| Poorly differentiated/Undifferentiated | 89 | 6352.25 | 2.62 | (0.95, 7.23) | 0.07 | |
| Not determined | 9 | 766.67 | 2.18 | (0.66, 7.16) | 0.20 | |
| Chemotherapy received | 0.34 | |||||
| Yes | 40 | 2885.92 | 1 (Ref) | |||
| No | 97 | 8416.67 | 0.90 | (0.60, 1.36) | 0.62 | |
| Comorbidity Index | 0.14 | |||||
| 0 | 41 | 4299.58 | 1 (Ref) | |||
| 1 | 26 | 1823.83 | 1.47 | (0.89, 2.41) | 0.13 | |
| ≥ 2 | 70 | 5179.17 | 1.32 | (0.88, 2.00) | 0.18 | |
| Race | 0.45 | |||||
| White | 120 | 9807.83 | 1 (Ref) | |||
| Black | 9 | 975.17 | 0.66 | (0.32, 1.36) | 0.26 | |
| Hispanic | 1 | 155.92 | 0.52 | (0.07, 3.68) | 0.52 | |
| Other/ Unknown | 7 | 363.67 | 1.52 | (0.67, 3.44) | 0.31 | |
| Marital Status | 0.23 | |||||
| Married | 64 | 4902.42 | 1 (Ref) | |||
| Unmarried | 72 | 6046.33 | 0.81 | (0.57, 1.16) | 0.23 | |
| Unknown | 1 | 353.83 | 0.17 | (0.02, 1.22) | 0.08 | |
| Year of Diagnosis | 0.12 | |||||
| 1993-1999 | 27 | 3124.92 | 1 (Ref) | |||
| 2000-2004* | 68 | 4935.75 | 1.58 | (1.00, 2.50) | 0.05 | |
| 2005-2009 | 42 | 3241.92 | 1.32 | (0.80, 2.18) | 0.27 | |
| SEER Region | 0.07 | |||||
| West | 59 | 4824.67 | 1 (Ref) | |||
| Midwest | 25 | 1724.42 | 1.40 | (0.85, 2.31) | 0.18 | |
| Northeast | 22 | 2777.58 | 0.69 | (0.42, 1.13) | 0.14 | |
| South | 31 | 1975.92 | 1.24 | (0.78, 1.99) | 0.36 | |
| Urban Residence | 0.03 | |||||
| Metro | 114 | 10054.08 | 1 (Ref) | |||
| Urban/ Rural | 23 | 1248.5 | 1.24 | (0.77, 2.02) | 0.38 | |
| Census Track Level Poverty | 0.01 | |||||
| 0.00%-9.99% | 80 | 7612.67 | 1 (Ref) | |||
| 10.00%-19.99% | 45 | 2472.08 | 1.51 | (1.00, 2.30) | 0.05 | |
| 20.00%+ | 12 | 1217.83 | 0.81 | (0.43, 1.55) | 0.53 | |
Table 3.
Stratified 5 Year Cumulative Incidences
| 5 Year Cumulative Incidence of Mastectomy CI (95% Confidence Interval) | 5 year Cumulative Incidence of Breast cancer death CI (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|---|
| Age Group | N | RT | No RT | P | RT | No RT | P |
| 70-74 | 93 | 4.0% (2.8%- 5.8% | 12.6% (6.6%- 23.9%) | 0.002 | 5.6% (4.0%- 7.7%) | 14.8% (9.3%- 23.6%) | 0.001 |
| 75-79 | 116 | 3.8% (2.5%- 5.8%) | 10.2% (5.2%- 20.1%) | 0.003 | 7.3% (5.6%- 9.5%) | 17.2% (10.6%- 27.6%) | 0.001 |
| 80+ | 373 | 3.4% (2.1%- 5.7%) | 6.9% (4.0%- 12.1%) | 0.05 | 9.5% (7.3%- 12.4%) | 21.4% (15.9%- 28.9%) | <0.001 |
| T Stage | |||||||
| T1 | 377 | 5.3% (3.1%- 5.2%) | 7.7% (5.1%- 11.6%) | 0.01 | 5.3% (4.2%- 6.5%) | 12.5% (9.1%- 17.2%) | <0.001 |
| T2 | 205 | 4.24% 95% CI: 2.64%-6.81% | 9.92% 95% CI: 6.15%-15.98% | 0.01 | 14.2% (11.2%- 18.0%) | 28.9% (22.9%- 36.5%) | <0.001 |
In further exploratory analysis evaluating MSF among subgroups according to patient age and tumor stage (Table 3), RT was associated with a reduced risk of mastectomy among all subcohorts, though the magnitude of benefit was small in women ≥80 or with T1 tumors. Mastectomy incidence with vs. without RT was 3.4% vs. 6.9% (p=0.05) for women ≥80 and 5.3% vs. 7.7% (p=0.01) for women with T1N0 tumors.
Breast Cancer Specific Survival
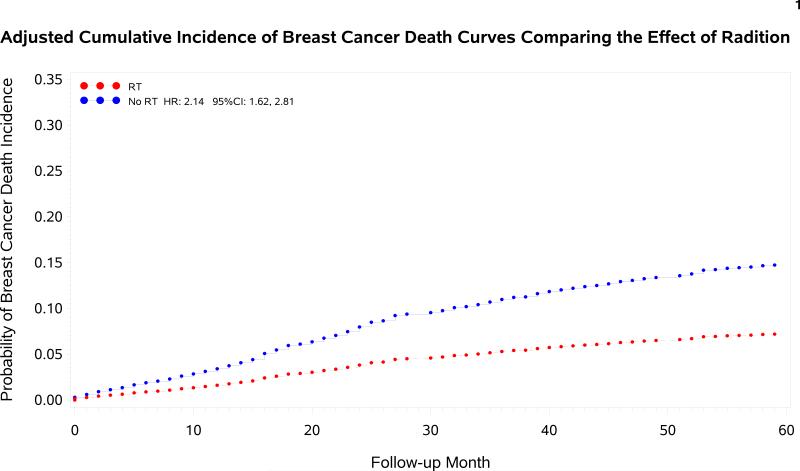
The 5-year CI of breast cancer death (BCD) for patients who received versus did not receive adjuvant radiation were 8.3% (95% CI 7.2%, 9.5%) versus 24.1% (95% CI 19.7%, 28.7%), p<.0001. On multivariable analysis (Table 4), women treated with BCS alone had a significantly increased risk of BCD at 5 years compared with women treated with BCS and adjuvant RT, with a HR of 2.2 (95% CI 1.6, 2.8). The associations between all patient and tumor characteristics and CI of BCD are listed in Table 4. Additional variables associated with an increased risk of breast cancer specific death were older age, greater T stage, histologic grade and comorbidity index. Cumulative incidence of BCD with and without RT according to T stage and age group are shown in Table 3.
Table 4.
Associations with 5-Year Breast Cancer Specific Death Cumulative Incidence
| Characteristic | Gray's Test P-value | Number of Outcomes | Person-Year | HR (95%CI) | P-value | |
|---|---|---|---|---|---|---|
| Breast radiation | <.0001 | |||||
| Yes | 188 | 10417.17 | 1 (Ref) | |||
| No* | 97 | 1636.33 | 2.15 | (1.63, 2.82) | <.0001 | |
| Age | <.0001 | |||||
| 70 - 74* | 80 | 4910 | 0.73 | (0.53, 1.01) | 0.06 | |
| 75 - 79 | 80 | 3744.67 | 0.86 | (0.63, 1.16) | 0.32 | |
| ≥ 80 | 125 | 3398.83 | 1 (Ref) | |||
| T Stage | <.0001 | |||||
| T1a + T1mic | 10 | 1078.83 | 1 (Ref) | |||
| T1b | 28 | 2796.25 | 1.01 | (0.49, 2.08) | 0.99 | |
| T1c* | 113 | 5574.67 | 1.85 | (0.98, 3.55) | 0.07 | |
| T2* | 134 | 2603.75 | 3.77 | (2.12, 7.26) | <.0001 | |
| Progesterone receptor status | 0.07 | |||||
| Positive | 13 | 986.83 | 1 (Ref) | |||
| Negative | 270 | 10928.5 | 1.35 | (0.77, 2.35) | 0.30 | |
| Indeterminate | 2 | 138.17 | 1.01 | (0.23, 4.31) | 0.99 | |
| Tumor Histology | 0.07 | |||||
| Ductal Carcinoma | 267 | 10834 | 1 (Ref) | |||
| Lobular Carcinoma | 9 | 437.25 | 1.11 | (0.59, 2.11) | 0.75 | |
| Other | 9 | 782.25 | 0.51 | (0.27, 1.05) | 0.07 | |
| Histologic grade | <.0001 | |||||
| Well differentiated | 3 | 843 | 1 (Ref) | |||
| Intermediate/moderate | 63 | 3580.92 | 3.95 | (1.24, 12.56) | 0.02 | |
| Poorly differentiated/Undifferentiated | 207 | 6804.25 | 5.81 | (1.84, 18.35) | 0.00 | |
| Not determined | 12 | 825.33 | 3.64 | (1.03, 12.90) | 0.05 | |
| Chemotherapy received | 0.58 | |||||
| Yes | 69 | 3083.17 | 1 (Ref) | |||
| No | 216 | 8970.33 | 1.11 | (0.81, 1.52) | 0.52 | |
| Comorbidity Index | 0.02 | |||||
| 0 | 86 | 4592.17 | 1 (Ref) | |||
| 1 | 47 | 1945.67 | 1.11 | (0.77, 1.59) | 0.59 | |
| ≥ 2 | 152 | 5515.67 | 1.10 | (0.83, 1.45) | 0.51 | |
| Race in General | 0.96 | |||||
| White | 248 | 10426.75 | 1 (Ref) | |||
| Black | 24 | 1070.08 | 0.91 | (0.56, 1.51) | 0.73 | |
| Hispanic | 3 | 164.08 | 0.68 | (0.22, 2.15) | 0.52 | |
| Other/ Unknown | 10 | 392.58 | 1.23 | (0.62, 2.45) | 0.55 | |
| Marital Status | 0.13 | |||||
| Married | 107 | 5243.92 | 1 (Ref) | |||
| Unmarried | 167 | 6441.17 | 0.95 | (0.74, 1.23) | 0.69 | |
| Unknown | 11 | 368.42 | 1.07 | (0.56, 2.03) | 0.85 | |
| Year of Diagnosis | 0.87 | |||||
| 1993-1999 | 74 | 3367.83 | 1 (Ref) | |||
| 2000-2004 | 124 | 5223.83 | 0.94 | (0.70, 1.27) | 0.68 | |
| 2005-2009 | 87 | 3461.83 | 0.83 | (0.59, 1.15) | 0.26 | |
| SEER Region | 0.09 | |||||
| West | 113 | 5127.67 | 1 (Ref) | |||
| Midwest | 35 | 1850.08 | 0.93 | (0.62, 1.40) | 0.73 | |
| Northeast* | 87 | 2943.58 | 1.37 | (1.01, 1.85) | 0.04 | |
| South | 50 | 2132.17 | 1.07 | (0.76, 1.52) | 0.70 | |
| Urban Residence | 0.33 | |||||
| Metro | 249 | 10750.75 | 1 (Ref) | |||
| Urban/ Rural | 36 | 1302.75 | 1.21 | (0.84, 1.74) | 0.32 | |
| Census Track Level Poverty | 0.81 | |||||
| 0.00%-9.99% | 187 | 8083.58 | 1 (Ref) | |||
| 10.00%-19.99% | 68 | 2673.08 | 1.05 | (0.77, 1.42) | 0.77 | |
| 20.00%+ | 30 | 1296.83 | 0.98 | (0.62, 1.55) | 0.92 | |
The 5-year observed overall survival for patients who received versus did not receive adjuvant radiation were 79% (95% CI 77%, 80%) versus 42% (95% CI 38%, 47%), p<0.001
Discussion
This study included a large population based cohort of elderly women ≥70 with T1-2N0 ER negative tumors, who are highly underrepresented in prospective phase III clinical trials evaluating the benefit of adjuvant RT following breast conserving surgery. While ER receptor negative status has previously been demonstrated as a negative prognostic factor for MFS in a SEER-Medicare analysis elderly women 11, the mastectomy rates among this population and the magnitude of benefit with adjuvant RT according to other disease variables has not previously been evaluated in this population. The results of this analysis demonstrate the use of adjuvant RT to be associated with a significantly lower incidence of mastectomy among women ≥70 years old with T1–2 ER negative tumors receiving breast conservation therapy.
The question remains whether there is a subpopulation of elderly women with early stage ER negative tumors who may not need adjuvant radiotherapy. Our results show that a reduced risk of mastectomy among all subgroups of patients according to age or T stage after breast conserving surgery with radiation; however, the magnitude of benefit at 5 years is smallest for women ≥ 80 or with T1N0 ER negative tumors. When analyzing patients ≥ 80 years old and with T1 tumors, the difference in mastectomy incidence with and without RT is no longer significant. This finding is consistent with previous data demonstrating that older age 2-4 and earlier T stage tumors 1, 11 are factors independently associated with a reduced risk of local regional recurrence In recent prospective trials evaluating the omission of adjuvant radiotherapy in women with early stage ER positive breast cancer, local regional recurrence at 5 years was approximately 1% with RT and 4% with BCS alone 5, 12. “Elderly” was defined as greater than 65 or 70 years in these trials. In comparison, our results show a mastectomy rate of approximately 5% with RT and 11% with BCS alone in women ≥70 with ER negative tumors.
It should be noted that the incidence of mastectomy is analyzed as a surrogate for local tumor control, and data regarding the timing and pattern of disease recurrence cannot be recorded directly from SEER-Medicare data. Patients with local tumor failure may not have undergone mastectomy for several reasons, including patient preference for partial mastectomy at the time of recurrence, poor clinical or functional status that may have precluded surgical management, or the presence of simultaneous distant recurrence. Furthermore, it may be that mastectomies were performed for reasons other than disease recurrence. Biases that may have impacted treatment selection cannot be fully accounted for with this retrospective population based data. Additional factors—such as histologic grade, medical comorbidity, poverty level and urban residence—should be taken into account if considering a subpopulation of women to further investigate the effects of omitting adjuvant radiotherapy, as these variables were also associated with MFS. The incidence of mastectomy was lower among patients with T1 tumors and patients with well-differentiated histology, which is consistent with past literature establishing the association of these variables with improved local regional control 11.
Cause specific survival was poorer among women who did not receive adjuvant radiation. A meta-analysis of patient data from 17 randomized trials of adjuvant breast RT demonstrated a 5% absolute benefit in survival at 15 years with the use of radiotherapy 1. Thus, the approximate 15% absolute decrement in cumulative incidence of breast cancer specific death among women without adjuvant RT in this analysis is unlikely related to the lack of benefit from local regional RT alone. Other independent predictors of increased risk of breast cancer specific death in this analysis were older age, greater T stage, higher tumor grade, and increased comorbidity.
Our study is limited in that estrogen receptor status was the primary hormonal receptor used to categorize the population. Additional information about HER-2 receptor status and luminal type, which is also recognized to impact local regional recurrence 7, 13, 14 may have been of value, but was not available in this data set. Additional limitations include unknown surgical margin status, unknown use of endocrine therapy, bias in treatment selection that cannot be accounted for, and the lack of sufficient follow-up in this elderly population to adequately analyze outcomes beyond 5 years.
Conclusions
The use of adjuvant radiation is associated with a significant reduced incidence of mastectomy and breast cancer specific death among elderly women ≥70 with T1–2N0 ER negative tumors, though the magnitude of benefit at 5 years is small in the subgroups of women ≥80 or with T1 tumors.. These findings add to the sparse literate evaluating the benefit of RT in elderly women with ER negative receptor status.
Supplementary Material
Figure 2.
Adjusted cumulative incidence curves of mastectomy for patients with and without the receipt of adjuvant radiation therapy.
Figure 3.
Adjusted cumulative incidence curves of breast cancer specific death for patients with and without the receipt of adjuvant radiation therapy.
Acknowledgements
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development, and Information, CMS; Information Management Services, Inc; the Georgia Cancer Registry supported by NCI contract HHSN261201300015I; and the Surveillance, Epidemiology, and End Results (SEER) program tumor registries in the creation of the SEER-Medicare database.
Funding: This work is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Author Contributions: Bree R. Eaton: Conceptualization, methodology, formal analysis, investigation, resources, writing – original draft, writing – review and editing, and visualization. Renjian Jiang: Methodology, software, validation, formal analysis, data curation, writing – review and editing, and visualization. Mylin A. Torres: Conceptualization, validation, writing – original draft, writing – review and editing, and supervision. Shannon T. Kahn: Writing – original draft, writing – review and editing, and visualization. Karen Godette: Conceptualization, validation, writing – review and editing, and visualization. Timothy L. Lash: Conceptualization, methodology, and writing – review and editing. Kevin C. Ward: Conceptualization, methodology, validation, investigation, data curation, writing – review and editing, visualization, supervision, and project administration.
References
- 1.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Liljegren G, Holmberg L, Bergh J, et al. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999;17:2326–2333. doi: 10.1200/JCO.1999.17.8.2326. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 5.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. 2012:42. [Google Scholar]
- 7.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 8.Smith BD, Gross CP, Smith GL, Galusha DH, Bekelman JE, Haffty BG. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006;98:681–690. doi: 10.1093/jnci/djj186. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 11.Albert JM, Liu DD, Shen Y, et al. Nomogram to predict the benefit of radiation for older patients with breast cancer treated with conservative surgery. J Clin Oncol. 2012;30:2837–2843. doi: 10.1200/JCO.2011.41.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16:266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 13.Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis in women with small (T1mic,T1a,T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res Treat. 2011;127:713–720. doi: 10.1007/s10549-011-1465-7. [DOI] [PubMed] [Google Scholar]
- 14.Hattangadi-Gluth JA, Wo JY, Nguyen PL, et al. Basal subtype of invasive breast cancer is associated with a higher risk of true recurrence after conventional breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2012;82:1185–1191. doi: 10.1016/j.ijrobp.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.