Abstract
Introduction
Despite a lack of demonstrated efficacy, potassium and magnesium supplementation are commonly thought to prevent postoperative atrial fibrillation (POAF) after cardiac surgery. Our aim was to evaluate the natural time course of electrolyte level changes after cardiac surgery and their relationship to POAF occurrence.
Methods
Data were reviewed from 2041 adult patients without preoperative AF who underwent CABG and/or valve surgery between 2009 and 2013. In patients with postoperative AF, the plasma potassium and magnesium levels nearest to the first AF onset time were compared to time-matched electrolyte levels in patients without AF.
Results
POAF occurred in 752 patients (36.8%). At the time of AF onset or the matched time point, patients with POAF had higher potassium (4.30 vs. 4.21 mmol/L, p<0.001) and magnesium (2.33 vs. 2.16 mg/dL, p<0.001) levels than controls. A stepwise increase in AF risk occurred with increasing potassium or magnesium quintile (p<0.001). On multivariate logistic regression analysis, magnesium level was an independent predictor of POAF (OR 4.26, p<0.001), in addition to age, Caucasian race, preoperative beta blocker use, valve surgery, and postoperative pneumonia. Prophylactic potassium supplementation did not reduce the POAF rate (37 vs. 37%, p=0.813), while magnesium supplementation was associated with increased POAF (47 vs. 36%, p=0.005).
Conclusion
Higher serum potassium and magnesium levels were associated with increased risk for POAF after cardiac surgery. Potassium supplementation was not protective against POAF, while magnesium supplementation was even associated with increased POAF risk. These findings help explain the poor efficacy of electrolyte supplementation in POAF prophylaxis.
Keywords: Atrial fibrillation, Electrophysiology, Postoperative care, Pharmacology
Although hypokalemia and hypomagnesemia are commonly thought to increase risk for postoperative atrial fibrillation (POAF) after cardiac surgery (1, 2), no large studies have evaluated the actual postoperative changes in electrolyte levels or their true clinical relevance to POAF risk.
The relationship of potassium and magnesium levels with atrial fibrillation (AF), while widely assumed, has not been well characterized. Longitudinal community studies have demonstrated an increased long-term risk of AF in patients with lower potassium or magnesium levels at baseline (3-5), although these observations have little clinical relevance to postoperative cardiac surgery patients. In one study of 1033 hospitalized patients with acute myocardial infarction (MI), lower serum potassium concentrations were related to ventricular but not atrial arrhythmias (6). The largest study to investigate serum electrolyte levels in cardiac surgery patients (n=500) showed that electrolyte disturbances are common on ICU admission, with 34% of patients exhibiting hypokalemia and 46% exhibiting hypomagnesemia, however subsequent postoperative electrolyte levels and arrhythmia occurrence were not examined (2). Several smaller studies in cardiac surgery patients have demonstrated conflicting results: low potassium levels have been associated with episodes of ventricular tachycardia (7) but not atrial arrhythmias (8), while low magnesium levels have been associated with an increase (9) or no change (8, 10) in POAF risk.
Despite these conflicting data and the fact that most large studies on risk factors for POAF have not investigated postoperative electrolyte levels (11-13), electrolyte supplementation is commonly used as a therapy for POAF prevention and treatment. The few existing studies of intensive care unit (ICU) electrolyte repletion protocols have demonstrated tighter control of postoperative potassium levels but no effect on the occurrence of POAF (14, 15). Intraoperative and postoperative magnesium administration has also been evaluated as an AF prophylaxis agent in many small and heterogeneous studies, with conflicting but overall limited efficacy (16, 17).
The purpose of this study was to characterize the natural time course of plasma potassium and magnesium levels after cardiac surgery, and to evaluate the effect of electrolyte levels and electrolyte supplementation on the occurrence of POAF.
Patients and Methods
All adult patients undergoing coronary artery bypass grafting (CABG), valve surgery, or CABG plus valve surgery at our institution over a five-year period (Jan 1, 2009 to Dec 31, 2013) were retrospectively reviewed. Patients with a preoperative history of atrial fibrillation or atrial flutter were excluded. The study was approved by the Institutional Review Board of the Washington University School of Medicine, with a waiver of patient consent. Demographic and perioperative clinical data were collected from our institutional Society of Thoracic Surgeons (STS) database, while laboratory results, medication administration data, and arrhythmia onset times were obtained by query of our electronic medical record database (Sunrise Clinical Manager, Allscripts Healthcare Solutions Inc, Chicago, IL).
Postoperative atrial fibrillation
The study cohort was divided into two groups based on the occurrence of new-onset postoperative atrial fibrillation, which was defined as new atrial fibrillation or atrial flutter of at least 30 seconds duration occurring between the times of OR exit and hospital discharge and documented in the inpatient nursing flowsheets. The control group consisted of patients with no AF documented for the duration of their postoperative hospital stay.
Electrolyte levels and supplementation
The collection times and results for one baseline (most recent preoperative) and all postoperative plasma potassium, magnesium, and creatinine levels were obtained from the hospital electronic medical record. The administration times and dosing of all potassium and magnesium supplementations given during the hospital admission, including intraoperative administrations, were also obtained. Patients received postoperative supplementation of potassium and magnesium based on provider-dependent practices. While in the ICU, a standardized repletion protocol was used for potassium levels <4.0 mEq/dL, but not for magnesium.
Time-matching algorithm
A time-matching algorithm was used to select the most accurate estimation possible for each patient's true plasma potassium, magnesium, and creatinine level at the exact time of AF onset. For patients with POAF, the time of AF onset was defined as the first postoperative documentation of atrial fibrillation or atrial flutter in the nursing flowsheets, and was converted to postoperative hours by subtracting the date and time of OR exit. For the control patients, the median AF onset time in the POAF group (50.9 postoperative hours) was used as a surrogate time point for the time-matching algorithm. For potassium, magnesium, and creatinine separately, the nearest laboratory result to the patient's AF onset time (or surrogate time point for the control patients) was then selected. The absolute nearest laboratory result was selected regardless of whether it occurred prior to or after the onset of AF. Laboratory results were only used for this analysis if they fell within ±12 hr from the AF onset time for POAF patients or within the interquartile range of AF onset times (35.6-76.4 postoperative hours) for control patients. Laboratory results were excluded if an intervening potassium or magnesium dose, respectively, had been administered between the time of blood collection and the time of AF onset. In this scenario, the next nearest laboratory result was used as long as it abided by all of the above criteria.
Statistical analysis
Demographic, perioperative, and laboratory data were compared between the POAF and control groups. Continuous variables were expressed as mean ± standard deviation or as median with interquartile range. Means were compared using the two sample t-test with Dunn-Sidak adjustment for multiple comparisons. Medians were compared using the Mann-Whitney U statistic. Categorical variables were expressed as frequencies and percentages, and were compared using chi-squared analysis or Fisher's exact test. Kaplan-Meier survival curves of freedom from POAF were constructed, and were compared with the log-rank test.
Multivariate logistic regression analysis was used to analyze the effect of postoperative electrolyte levels on POAF occurrence while controlling for known clinical risk factors for POAF. All clinical and laboratory variables were first evaluated in a univariate analysis to identify potential predictors of POAF. Significant covariates on univariate analysis (p<0.1) were entered into a multivariate stepwise logistic regression analysis and receiver operating characteristic (ROC) curves were constructed. Data analyses were performed using Systat 13 (Systat Software Inc, Chicago, IL) and SPSS 23.0 (IBM Software, Armonk, NY) statistical software.
Results
Demographics and postoperative AF
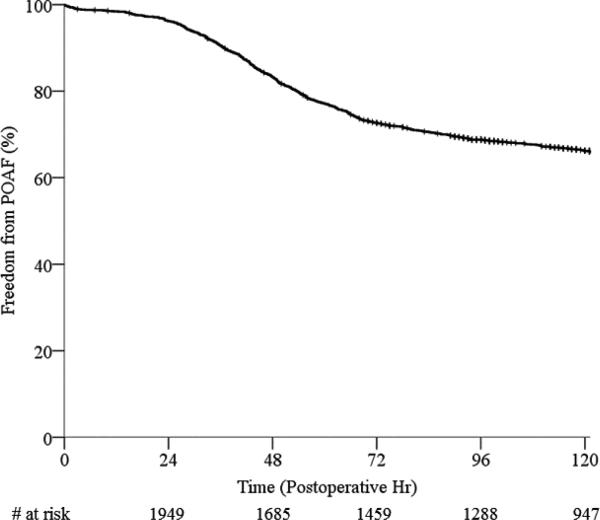
A total of 2041 patients were included in the study cohort. The overall incidence of AF during the postoperative course was 36.8% (752/2041). The median time to AF onset was 50.9 postoperative hours (35.6-76.4 hr). Demographic and perioperative clinical data for the POAF and control groups are shown in Table 1. The overall postoperative time course of AF onset is illustrated by Kaplan-Meier analysis of freedom from POAF through the first 120 postoperative hr (5 days, Figure 1). This time period encompasses 90% of all first AF onset times in the dataset (90th percentile for AF onset time = 123 hr).
Table 1.
Demographic and perioperative data for the POAF and control groups. POAF, postoperative atrial fibrillation.
| POAF | Control | p value | |
|---|---|---|---|
| N | 752 (36.8%) | 1289 (63.2%) | |
| Age (yr) | 68.1 ± 11.4 | 59.8 ± 14.6 | <0.001 |
| Male | 472 (62.8%) | 833 (64.6%) | 0.399 |
| White/Caucasian | 682 (90.7%) | 1083 (84.0%) | <0.001 |
| Black/African American | 62 (8.2%) | 178 (13.8%) | <0.001 |
| Smoker (within 1 year) | 158 (21.0%) | 375 (29.1%) | <0.001 |
| Comorbidities | |||
| Hypertension | 647 (86.0%) | 988 (76.6%) | <0.001 |
| Diabetes Mellitus | 300 (39.9%) | 462 (35.8%) | 0.068 |
| Dyslipidemia | 633 (84.2%) | 1033 (80.1%) | 0.023 |
| Renal Failure (dialysis-dependent) | 40 (5.3%) | 55 (4.3%) | 0.276 |
| Chronic Lung Disease (moderate-severe) | 85 (11.3%) | 96 (7.4%) | 0.001 |
| Peripheral Vascular Disease | 227 (30.2%) | 326 (25.3%) | 0.016 |
| Cerebrovascular Disease | 158 (21.0%) | 235 (18.2%) | 0.124 |
| Prior Myocardial Infarction | 302 (40.2%) | 491 (38.1%) | 0.355 |
| LV Ejection Fraction | 53 ± 15 | 55 ± 13 | 0.348 |
| Heart Failure (within 2 weeks) | 497 (66.1%) | 714 (55.4%) | <0.001 |
| NYHA Class III-IV | 364 (73.2%) | 497 (69.6%) | 0.23 |
| Cardiogenic Shock | 43 (5.7%) | 40 (3.1%) | 0.004 |
| Endocarditis | 45 (6.0%) | 79 (6.1%) | 0.895 |
| Preoperative Medications | |||
| Aspirin | 573 (76.2%) | 935 (72.5%) | 0.192 |
| Beta Blocker | 499 (66.4%) | 788 (61.1%) | 0.027 |
| ACE Inhibitor or ARB | 153 (53.5%) | 292 (51.8%) | 0.067 |
| Statin | 424 (77.4%) | 692 (78.6%) | 0.065 |
| Steroids | 49 (6.5%) | 64 (5.0%) | 0.139 |
| Valve Disease (moderate-severe) | |||
| Aortic Insufficiency | 90 (16.2%) | 144 (15.8%) | 0.826 |
| Aortic Stenosis | 305 (55.4%) | 373 (41.2%) | <0.001 |
| Mitral Insufficiency | 218 (35.6%) | 263 (26.3%) | <0.001 |
| Mitral Stenosis | 43 (7.3%) | 45 (4.7%) | 0.03 |
| Tricuspid Insufficiency | 47 (8.0%) | 65 (6.7%) | 0.362 |
| Procedure Details | |||
| Any CABG | 419 (55.7%) | 733 (56.9%) | 0.614 |
| Any Valve Surgery | 483 (64.2%) | 665 (51.6%) | <0.001 |
| CABG Only | 265 (35.3%) | 616 (47.9%) | <0.001 |
| Valve Only | 328 (43.7%) | 551 (42.8%) | 0.711 |
| CABG + Valve | 153 (20.4%) | 114 (8.9%) | <0.001 |
| Reoperation | 127 (16.9%) | 221 (17.1%) | 0.903 |
| Urgent/Emergent Status | 112 (14.9%) | 145 (11.3%) | 0.019 |
| Cardiopulmonary Bypass Time (min) | 128 ± 46 | 117 ± 40 | <0.001 |
| Aortic Cross-clamp Time (min) | 88 ± 34 | 84 ± 30 | 0.26 |
| Intraoperative Transfusion (any product) | 566 (75.3%) | 817 (63.4%) | <0.001 |
| RBC (# units) | 2.6 ± 3.1 | 1.8 ± 2.6 | <0.001 |
| FFP (#units) | 1.2 ± 2.7 | 0.7 ± 1.6 | <0.001 |
| Platelets (# units) | 0.8 ± 1.0 | 0.5 ± 0.8 | <0.001 |
| Cryoprecipitate (# units) | 0.4 ± 1.0 | 0.2 ± 0.7 | <0.001 |
| Intra-aortic Balloon Pump | 96 (12.8%) | 92 (7.1%) | <0.001 |
| Postoperative Details | |||
| Postoperative Transfusion (any product) | 522 (69.4%) | 747 (58.0%) | <0.001 |
| RBC (# units) | 3.0 ± 6.5 | 1.6 ± 2.8 | <0.001 |
| FFP (#units) | 0.6 ± 2.4 | 0.3 ± 1.7 | 0.007 |
| Platelets (# units) | 0.4 ± 1.6 | 0.2 ± 0.9 | 0.02 |
| Cryoprecipitate (# units) | 0.1 ± 0.6 | 0.1 ± 0.6 | 0.096 |
| Reoperation for Bleeding | 38 (5.1%) | 44 (3.4%) | 0.079 |
| Deep Sternal Infection/Mediastinitis | 3 (0.4%) | 4 (0.3%) | 0.713 |
| Stroke | 17 (2.5%) | 15 (3.8%) | 0.207 |
| Prolonged Ventilation (>24 hr) | 194 (25.8%) | 147 (11.4%) | <0.001 |
| Pneumonia | 74 (9.8%) | 34 (2.6%) | <0.001 |
| Renal Failure (all) | 54 (7.2%) | 20 (1.6%) | <0.001 |
| Renal Failure (dialysis required) | 30 (4.0%) | 13 (1.0%) | <0.001 |
| ICU Length of Stay (initial, median, hr) | 66 (27-123) | 43 (24-73) | <0.001 |
| ICU Readmission | 60 (8.0%) | 24 (1.9%) | <0.001 |
| Hospital Length of Stay (median, days) | 8.1 (6.1-12.8) | 6.0 (4.9-7.9) | <0.001 |
| Hospital Readmission (30 days) | 101 (13.4%) | 169 (13.1%) | 0.692 |
| Operative Mortality | 36 (4.8%) | 32 (2.5%) | 0.007 |
Figure 1.
Time course of POAF onset after cardiac surgery by Kaplan-Meier survival analysis for freedom from POAF. POAF, postoperative atrial fibrillation.
Postoperative electrolyte levels
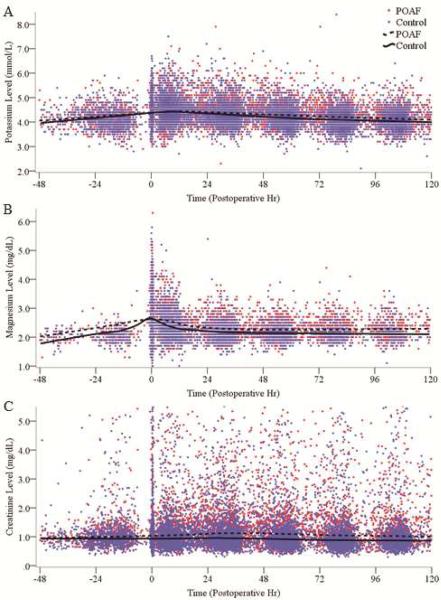
The overall trends in plasma potassium, magnesium, and creatinine levels from baseline (preoperative) through the first 120 postoperative hr (5 days) are graphically depicted in Figure 2. The moving average (Loess curve) throughout this time period was fit for each electrolyte, which varied within the respective reference ranges postoperatively (at our institution, 3.5-4.9 mmol/L for potassium and 1.4-2.5 mg/dL for magnesium).
Figure 2.
Scatter plots of all baseline and postoperative plasma potassium (A), magnesium (B), and creatinine (C) levels through 120 postoperative hours (5 days). Time 0 represents time of exit from operating room. Trend lines represent moving average (Loess curve) throughout the time period. Compared to controls, patients with POAF had higher postoperative potassium (p<0.001), magnesium (p<0.001), and creatinine (p<0.001) levels. POAF, postoperative atrial fibrillation.
Intraoperative potassium was administered in only 4% (90/2041) of patients, at a mean dose of 26 ± 14 mEq. Intraoperative magnesium was administered in 40% (810/2041) of patients, at a mean dose of 2.5 ± 1.2 g. Intraoperative magnesium dosing is reflected by a rise in the plasma magnesium level from 2.0 mg/dL to a peak of 2.6 mg/dL immediately following surgery, which quickly returned to a steady state level of about 2.2 mg/dL (Figure 2B). Severe hypokalemia (<2.5 mmol/L) was extremely rare in the dataset, occurring in only 2 of 24,566 total potassium results (0.008%), while severe hypomagnesemia (<1.0 mg/dL) was not observed. The plasma potassium, magnesium, and creatinine curves over the first 120 postoperative hr were all significantly higher in the POAF group compared to controls (p<0.001 for each).
Time-matched analysis
After application of the time-matching algorithm described above, 1800 patients with a qualifying potassium and/or magnesium result remained in the cohort for further analysis (N=1688 for potassium, N=925 for magnesium). For the selected potassium and creatinine results, the mean time interval from lab draw to AF onset (or matched time point) was 4.2 ± 3.4 hr (range 0-12 hr) in the POAF group and 5.9 ± 3.6 hr (range 0-23 hr) in the control group. For the selected magnesium results, the mean time interval from lab draw to AF onset (or matched time point) was 4.1 ± 3.6 hr (range 0-12 hr) in the POAF group and 5.5 ± 4.4 hr (range 0-25.4 hr) in the control group.
At the time of AF onset or at the matched time point, patients with POAF had higher mean potassium (4.30 ± 0.44 vs. 4.21 ± 0.43 mmol/L, p=0.001) and magnesium (2.33 ± 0.39 vs. 2.16 ± 0.29 mg/dL, p<0.001) levels than controls. Creatinine levels at this time point, in contrast, were not different between the two groups (1.33 ± 1.02 vs. 1.21 ± 1.17 mg/dL, p=0.291).
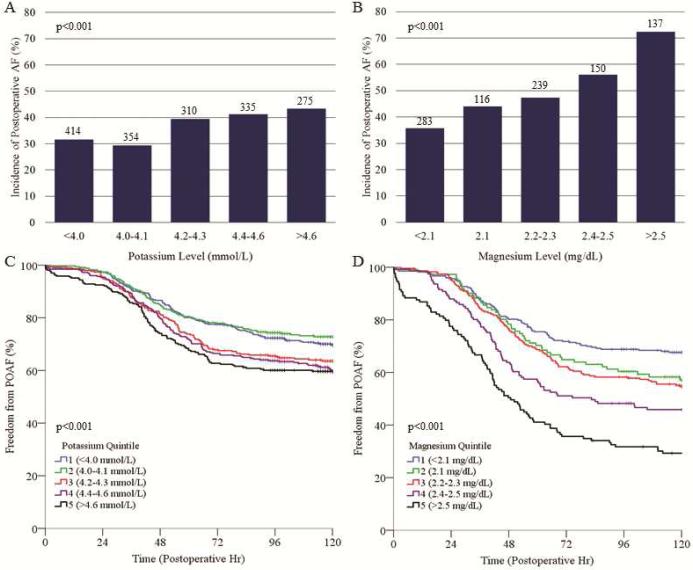
The relationship between electrolyte levels and AF risk was further analyzed by dividing the study cohort into quintiles based on the selected potassium or magnesium levels at AF onset or the matched time point. For both potassium and magnesium, the incidence of POAF was higher in patients belonging to higher electrolyte level quintiles (p<0.001 for both, Figure 3). This relationship was especially notable for magnesium, for which the incidence of POAF in the highest quintile was more than double that of the lowest quintile (72.3 vs. 35.7%, respectively).
Figure 3.
Incidence of POAF (A, B) and Kaplan-Meier analysis of freedom from POAF (C, D) based on quintile grouping of plasma potassium level (A, C) or magnesium level (B, D) at AF onset or matched time point. Numbers above bars represent number of patients per respective quintile group. A higher potassium or magnesium quintile was associated with a higher probability of POAF occurrence. POAF, postoperative atrial fibrillation; +, patient censored.
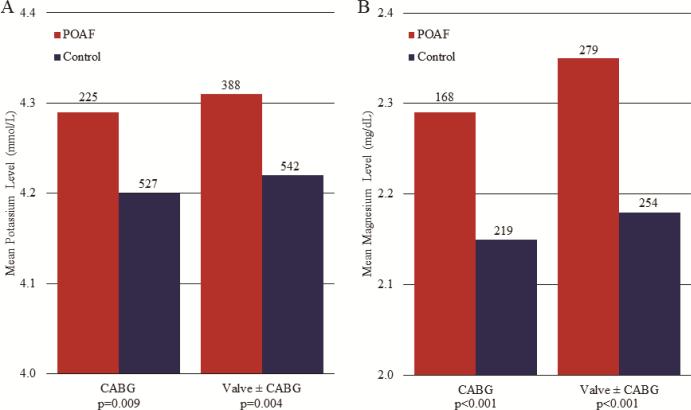
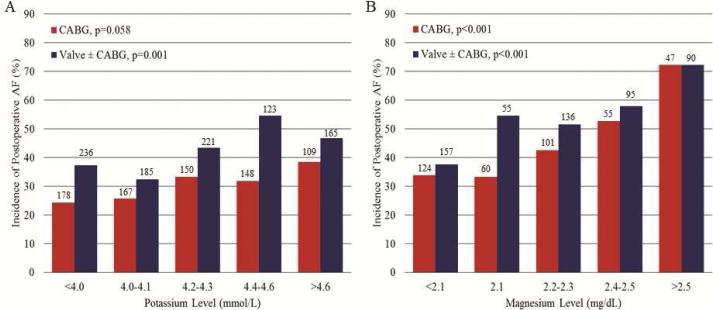
These data were also analyzed with subdivision by procedure type into isolated CABG and valve ± CABG groups, in which similar trends to the overall group were observed. Regardless of procedure type, mean time-matched potassium and magnesium levels were higher in patients with POAF (Figure 4), and an increased incidence of POAF occurred with increased electrolyte level quintile (Figure 5).
Figure 4.
Mean potassium (A) and magnesium (B) levels at AF onset or matched time point were higher in patients with POAF vs. controls, regardless of procedure type (CABG or valve ± CABG). Numbers above bars represent number of patients per respective group, p values represent POAF vs. control within respective procedure type. POAF, postoperative atrial fibrillation.
Figure 5.
Incidence of POAF based on quintile grouping of plasma potassium level (A) or magnesium level (B) at AF onset or matched time point, subdivided by procedure type (CABG or valve ± CABG). Numbers above bars represent number of patients per respective group. A higher potassium or magnesium quintile was associated with a higher probability of POAF occurrence regardless of procedure type. POAF, postoperative atrial fibrillation.
Predictors of postoperative AF
To determine independent predictors of POAF, logistic regression analysis was performed based on all available clinical variables and the time-matched laboratory data. Only patients with qualifying time-matched data for all three laboratory tests (potassium, magnesium, and creatinine) could be included in this analysis (N=899). On univariate analysis, increased potassium, magnesium, and creatinine levels were each associated with increased risk for postoperative AF occurrence (OR 1.57, 95% CI 1.25-1.97, p<0.001 for potassium; OR 4.56, 95% CI 2.97-7.0, p<0.001 for magnesium; OR 1.1, 95% CI 1.01-1.2, p<0.001 for creatinine).
On multivariate logistic regression analysis, magnesium level was a strong independent predictor of AF occurrence (OR 4.26, CI 2.69-6.72, p<0.001). The final multivariate model also included age, Caucasian race, preoperative beta blocker use, valve surgery, and postoperative pneumonia (Table 2). This model was moderately predictive of POAF occurrence, with an area under the ROC curve of 0.71.
Table 2.
Multivariate logistic regression model for independent predictors of postoperative atrial fibrillation (POAF). CI, confidence interval.
| Variable | Odds Ratio | 95% CI | p value |
|---|---|---|---|
| Age | 1.04 | 1.03-1.05 | <0.001 |
| Caucasian race | 1.8 | 1.17-2.75 | 0.007 |
| Preoperative beta blocker | 1.45 | 1.05-2.0 | 0.023 |
| Valve surgery | 1.39 | 1.03-1.88 | 0.033 |
| Magnesium level | 4.26 | 2.69-6.72 | <0.001 |
| Postoperative pneumonia | 2.32 | 1.42-3.8 | 0.001 |
Postoperative electrolyte administration
Overall, 90% (1831/2041) of patients received potassium administration at any point in their postoperative course, including 95% (712/752) of patients in the POAF group and 87% (1119/1289) of patients in the control group (p<0.001). Postoperative magnesium administration was much less common, with 22% (447/2041) of patients receiving magnesium at any time point, including 31% (234/752) of patients in the POAF group and 17% (213/1289) of patients in the control group (p<0.001).
To assess the impact of postoperative prophylactic electrolyte administration on the prevention of POAF, the rates of AF occurrence were then analyzed only in patients who had received the respective electrolyte prior to the onset of AF or the matched time point. The rate of AF was equivalent when comparing patients that did or did not receive potassium supplementation prior to this time point [37% (467/1275) vs. 37% (285/766), p=0.813]. In contrast, the rate of AF was higher in patients that received magnesium supplementation prior to AF onset or the matched time point compared to those that did not [47% (81/173) vs. 36% (671/1868), p=0.005].
Comment
In this large observational study, the changes in postoperative potassium and magnesium levels after cardiac surgery and their relationship to POAF risk were examined. Key findings of our study were: 1) plasma potassium and magnesium levels mostly varied within the normal range after cardiac surgery, and severe electrolyte deficiencies were very rare; 2) potassium and magnesium levels were higher in patients with POAF in a time-matched analysis, and higher magnesium levels were independently predictive of POAF in a multivariate analysis; and 3) potassium supplementation was associated with no effect on POAF rates, while magnesium supplementation was associated with higher rates of POAF.
Potassium and magnesium are both intracellular cations and play multiple roles within the cardiac myocyte, including maintenance of the resting membrane potential and regulation of calcium handling (2, 18). The effect of abnormal potassium and magnesium levels on ventricular electrophysiology is well characterized. Hypokalemia is known to predispose medical patients to ventricular arrhythmias, including ventricular tachycardia, ventricular fibrillation, and torsade de pointes, especially in the setting of myocardial ischemia, heart failure, or hypertension (19-21). Hypomagnesemia may exacerbate the arrhythmogenic effects of hypokalemia or cause ventricular arrhythmias itself (18, 22), and magnesium sulfate is a first-line therapy in digitalis toxicity and torsade de pointes with prolonged QT interval (23).
In contrast and despite prevailing clinical wisdom (1, 2), the link between atrial fibrillation and electrolyte deficiencies has not been well established. Studies of standardized potassium repletion protocols have not demonstrated a reduction in atrial tachyarrhythmias (14, 15), while multiple trials of magnesium supplementation for POAF prophylaxis have demonstrated no clear benefit (16, 17, 24). Our findings help to explain the lack of efficacy of potassium and magnesium supplementation in these other reports, in that the maintenance of near-normal electrolyte levels was not sufficient to reduce the rate of POAF. Surprisingly, patients with POAF actually had higher potassium and magnesium levels at the time of AF onset, and patients who were supplemented with magnesium were more likely to experience POAF. These findings suggest that current electrolyte repletion practices do not reduce, and may actually increase, the risk for POAF in postoperative cardiac surgery patients. A randomized trial comparing current practice to a standardized electrolyte repletion protocol is necessary to elucidate the true effect of potassium and magnesium supplementation on POAF risk. Assuming a POAF rate of 37% (as was observed in this study), an α level of 0.05, and a β level of 0.8, such a trial would require a sample size of about 300 patients per group to demonstrate non-inferiority at a margin of 10%.
Differences in demographic and perioperative clinical characteristics between the POAF and control groups were consistent with prior studies of POAF predictors (11-13). These clinical factors were controlled for in the multivariate logistic regression analysis, in which magnesium levels remained as an independent predictor of POAF, along with age, Caucasian race, preoperative beta blocker use, valve surgery, and postoperative pneumonia. The higher prevalence of renal insufficiency in POAF patients may have contributed to abnormal potassium and magnesium levels after surgery, however neither renal failure nor the time-matched creatinine levels were independent predictors of POAF on multivariate analysis.
Although we demonstrated an independently predictive association between increased magnesium levels and POAF risk, caution should be taken in interpreting a causative relationship between these variables. An important caveat is that only patients with qualifying time-matched data for both potassium and magnesium could be included in the logistic regression analysis (n=899), and this group of patients was at higher clinical risk for POAF based on mean age (64.4 vs. 61.5 yr, p<0.001) and prevalence of valve surgery (58 vs. 53%, p=0.05). As such, the logistic regression analysis included a higher risk patient group and may not be entirely representative of the total study cohort. Similarly, while we found that the rate of POAF was higher in patients who were supplemented with magnesium (47 vs. 36%, p=0.005), patients who received magnesium supplementation were at higher clinical risk for POAF based on the prevalence of valve surgery (62 vs. 53%, p=0.002).
These considerations illustrate an important limitation of our retrospective analysis, which is that providers may have been biased to treat patients perceived as having higher clinical risk for POAF (based on factors such as older age, valve surgery, or atrial ectopy preceding the actual onset of AF (25)) with more aggressive prophylactic electrolyte supplementation. This may have led to higher electrolyte levels in patients already predisposed to develop POAF, which could have contributed to the observed association between higher electrolyte levels, magnesium supplementation, and POAF occurrence. We attempted to control for these confounders by performing a stringent time-matched analysis to estimate as closely as possible the true plasma electrolyte levels at AF onset time, by excluding inaccurate laboratory results due to intervening electrolyte administration, and by conducting a thorough multivariate analysis to account for contributing clinical factors. We also performed a sub-analysis by procedure type, which showed that the association between higher electrolyte quintiles and POAF incidence persisted regardless of the type of procedure performed (Figures 4, 5). Still, the potential for confounders cannot be completely removed, and additional data, such as postoperative administration of beta blockers and other medications, were not included in this analysis and deserve further study.
Although an argument that magnesium supplementation causes POAF may be inappropriate in light of the potential biases in this study, these data do suggest that potassium and magnesium supplementation are ineffective for the prevention of POAF. Further, they suggest that the mechanism of POAF is not related to electrolyte deficiency, as is commonly believed. Our findings help explain the limited efficacy of electrolyte supplementation for POAF prophylaxis, and confirm the need for more mechanistic studies into the specific causes and pathophysiology of POAF. A randomized trial comparing current practice to a standardized electrolyte repletion protocol would be helpful to elucidate the true effect of potassium and magnesium supplementation on POAF risk.
Finally, because of the prevailing clinical practice of routine electrolyte repletion, our study did not include a measurable group of patients with severe hypokalemia or hypomagnesemia, in whom the electrophysiologic effects of electrolyte deficiency might have been more apparent. Given the extensive cardiology literature supporting the maintenance of normal to high-normal potassium and magnesium levels for prevention of ventricular arrhythmias in patients with ischemia and heart failure, any changes to current electrolyte repletion practices should be cautiously implemented with consideration of the effect on ventricular ectopy in addition to atrial arrhythmias.
Acknowledgments
This work was supported by National Institutes of Health T32-HL-007776-19 (TSL, MRS) and the Barnes-Jewish Hospital Foundation (SJM). The authors wish to thank Dr. Michael Avidan for his help in collection of intraoperative electrolyte administration data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the poster session of the Fifty-second Annual Meeting of the Society of Thoracic Surgeons, Phoenix, AZ, Jan 23-27, 2016.
References
- 1.Auer J, Weber T, Berent R, Lamm G, Eber B. Serum potassium level and risk of postoperative atrial fibrillation in patients undergoing cardiac surgery. J Am Coll Cardiol. 2004;44(4):938–939. doi: 10.1016/j.jacc.2004.05.035. author reply 939. [DOI] [PubMed] [Google Scholar]
- 2.Polderman KH, Girbes AR. Severe electrolyte disorders following cardiac surgery: A prospective controlled observational study. Crit Care. 2004;8(6):R459–466. doi: 10.1186/cc2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krijthe BP, Heeringa J, Kors JA, et al. Serum potassium levels and the risk of atrial fibrillation: The rotterdam study. Int J Cardiol. 2013;168(6):5411–5415. doi: 10.1016/j.ijcard.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 4.Khan AM, Lubitz SA, Sullivan LM, et al. Low serum magnesium and the development of atrial fibrillation in the community: The framingham heart study. Circulation. 2013;127(1):33–38. doi: 10.1161/CIRCULATIONAHA.111.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misialek JR, Lopez FL, Lutsey PL, et al. Serum and dietary magnesium and incidence of atrial fibrillation in whites and in african americans--atherosclerosis risk in communities (aric) study. Circ J. 2013;77(2):323–329. doi: 10.1253/circj.cj-12-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordrehaug JE, von der Lippe G. Serum potassium concentrations are inversely related to ventricular, but not to atrial, arrhythmias in acute myocardial infarction. Eur Heart J. 1986;7(3):204–209. doi: 10.1093/oxfordjournals.eurheartj.a062052. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RG, Shafique T, Sirois C, Weintraub RM, Comunale ME. Potassium concentrations and ventricular ectopy: A prospective, observational study in post-cardiac surgery patients. Crit Care Med. 1999;27(11):2430–2434. doi: 10.1097/00003246-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Svagzdiene M, Sirvinskas E. Changes in serum electrolyte levels and their influence on the incidence of atrial fibrillation after coronary artery bypass grafting surgery. Medicina (Kaunas) 2006;42(3):208–214. [PubMed] [Google Scholar]
- 9.Zaman AG, Alamgir F, Richens T, Williams R, Rothman MT, Mills PG. The role of signal averaged p wave duration and serum magnesium as a combined predictor of atrial fibrillation after elective coronary artery bypass surgery. Heart. 1997;77(6):527–531. doi: 10.1136/hrt.77.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vyvyan HA, Mayne PN, Cutfield GR. Magnesium flux and cardiac surgery. A study of the relationship between magnesium exchange, serum magnesium levels and postoperative arrhythmias. Anaesthesia. 1994;49(3):245–249. [PubMed] [Google Scholar]
- 11.Mathew JP, Parks R, Savino JS, et al. Atrial fibrillation following coronary artery bypass graft surgery: Predictors, outcomes, and resource utilization. Multicenter study of perioperative ischemia research group. JAMA. 1996;276(4):300–306. [PubMed] [Google Scholar]
- 12.Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94(3):390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 13.Shen J, Lall S, Zheng V, Buckley P, Damiano RJ, Jr., Schuessler RB. The persistent problem of new-onset postoperative atrial fibrillation: A single-institution experience over two decades. J Thorac Cardiovasc Surg. 2011;141(2):559–570. doi: 10.1016/j.jtcvs.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couture J, Letourneau A, Dubuc A, Williamson D. Evaluation of an electrolyte repletion protocol for cardiac surgery intensive care patients. Can J Hosp Pharm. 2013;66(2):96–103. doi: 10.4212/cjhp.v66i2.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoekstra M, Vogelzang M, Drost JT, et al. Implementation and evaluation of a nurse-centered computerized potassium regulation protocol in the intensive care unit--a before and after analysis. BMC Med Inform Decis Mak. 2010;10:5. doi: 10.1186/1472-6947-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arsenault KA, Yusuf AM, Crystal E, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;1:CD003611. doi: 10.1002/14651858.CD003611.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley D, Creswell LL, Hogue CW, Jr., et al. Pharmacologic prophylaxis: American college of chest physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128(2 Suppl):39S–47S. doi: 10.1378/chest.128.2_suppl.39s. [DOI] [PubMed] [Google Scholar]
- 18.Kolte D, Vijayaraghavan K, Khera S, Sica DA, Frishman WH. Role of magnesium in cardiovascular diseases. Cardiol Rev. 2014;22(4):182–192. doi: 10.1097/CRD.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43(2):155–161. doi: 10.1016/j.jacc.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 20.El-Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J. 2011;18(3):233–245. [PubMed] [Google Scholar]
- 21.Pezhouman A, Singh N, Song Z, et al. Molecular basis of hypokalemia-induced ventricular fibrillation. Circulation. 2015;132(16):1528–1537. doi: 10.1161/CIRCULATIONAHA.115.016217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb SS, Baruch L, Kukin ML, Bernstein JL, Fisher ML, Packer M. Prognostic importance of the serum magnesium concentration in patients with congestive heart failure. J Am Coll Cardiol. 1990;16(4):827–831. doi: 10.1016/s0735-1097(10)80329-8. [DOI] [PubMed] [Google Scholar]
- 23.Zipes DP, Camm AJ, Borggrefe M, et al. Acc/aha/esc 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the american college of cardiology/american heart association task force and the european society of cardiology committee for practice guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death): Developed in collaboration with the european heart rhythm association and the heart rhythm society. Circulation. 2006;114(10):e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 24.Klinger RY, Thunberg CA, White WD, et al. Intraoperative magnesium administration does not reduce postoperative atrial fibrillation after cardiac surgery. Anesth Analg. 2015;121(4):861–867. doi: 10.1213/ANE.0000000000000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong F, Yin Y, Dube B, Page P, Vinet A. Electrophysiological changes preceding the onset of atrial fibrillation after coronary bypass grafting surgery. PLoS One. 2014;9(9):e107919. doi: 10.1371/journal.pone.0107919. [DOI] [PMC free article] [PubMed] [Google Scholar]