Abstract
Background
The incidence of melanoma in situ is rising, but little is known about its characteristics.
Objective
To determine trends in diagnosis and clinical features of melanoma in situ.
Methods
Incident cases of melanoma were collected prospectively from the Nurses’ Health Study from 1976–2010 and Health Professionals Follow-up Study from 1986–2010.
Results
MIS incidence increased from 2 to 42 per 100,000-person-year (100KPY) among women, and from 11 to 73 per 100KPY among men, exceeding the rate of increase of invasive melanomas. Melanoma mortality initially increased during the follow-up period then plateaued. Men were more likely than women to develop in situ melanomas on upper half of the body (p<0.001). Invasive melanomas were diagnosed at a younger age than melanoma in situ (p<0.001), and were more likely to be found on the lower extremities than in situ melanomas (p<0.001).
Limitations
This is a strictly descriptive study without examination into mechanisms.
Conclusion
We found epidemiologic and clinical differences in in situ and invasive melanomas, which support further examination into the variations in etiologic pathways. The lack of improvement in mortality despite increase in detection of in situ relative to invasive lesions further highlight the need to improve invasive melanoma-specific clinical screening features.
Keywords: Melanoma, melanoma in situ, lentigo maligna, invasive malignant melanoma, incidence, anatomic sites, cancer registry, epidemiology, gender, age, melanoma screening
INTRODUCTION
In situ melanomas, characterized by malignant melanocytes limited to the epidermis during a noninvasive radial growth phase, is believed to be a biologic precursor to invasive melanomas.1 Over the past thirty years, multiple epidemiologic studies have shown that incidence of melanoma in situ is increasing at a faster rate than invasive melanomas.2–4 Despite the increase in detection of pre-invasive lesions, the distribution of melanoma thickness and mortality due to melanoma in the United States has not improved.2,5–9 These epidemiological observations have led some to conclude that the increase in melanoma in situ incidence may not be exclusively due to earlier detection,5 while others believe that the stabilization in mortality in the setting of increase in incidence support that screening efforts have been sufficient in preventing further rise in mortality.2 Around the world, primary and secondary prevention efforts for melanoma have produced conflicting results. While some groups have shown that screening was effective in increasing the proportion of thin tumors,10–13 stabilizing the incidence of thick tumors,11,14 and improving survival,13,15 others have shown that the proportion of thick IMMs and fatal incidence has remained unaffected by screening efforts.3,12,14,16–19
While understanding the clinical features of melanoma in situ over time will provide important information on the biologic properties of these tumors, understanding the epidemiological trends of melanoma in situ over time will also provide important information on primary and secondary prevention of invasive malnomas. In the U.S., there are currently no large-scale studies evaluating the epidemiological and clinical features of melanoma in situ. This is partially because epidemiologic studies on melanomas have been largely supported by national registries missing information on melanoma in situ.2,20 This study circumvented this and other issues of other large national registries21–24 by using information from two prospective cohorts of healthcare professionals with consistent follow-up and validated reporting25,26
MATERIALS AND METHODS
Data Source
Nurses’ Health Study (NHS) is an ongoing prospective cohort of 121,700 female registered nurses established in 1976. At enrollment, study participants were 30 to 55 years of age. No restrictions were made on the basis of ethnicity or race, however, the participants were 97% Caucasian, reflecting the ethnic background of women trained as registered nurses in 1976. Health Professional’s Follow-up Study (HPFS) is an ongoing prospective cohort study, established in 1986 to complement the all-female NHS. HPFS is comprised of 51,529 men in the healthcare professions, ages 40 to 75. Similarly, the participants were 97% Caucasian. This study was approved by the Institutional Review Board of Brigham and Women's Hospital. The participants' completion and return of the self-administered questionnaires were considered as informed consent.
Data Ascertainment
Participants reported new cases of melanoma in each biennial cycle. Skin cancer confirmation was carried out routinely through review of primary pathological records. Melanoma pathology records were reviewed for tumor depth (in situ, Clark’s Level, Breslow thickness), affected skin type (cutaneous, mucosal or oral, vulvar, or anal), pathological subtype (in situ not-otherwise-specified or non-lentigo maligna, lentigo maligna, superficial spreading, nodular, lentigo maligna melanoma, ocular, acral lentiginous, or invasive not-otherwise-specified), and affected anatomical site (head/neck, trunk, thigh/buttock, upper extremity, leg/ankle, knee/popliteal, anal/vulvar, ocular, or site unknown). Only pathologically confirmed cases were included as eligible outcomes.
Statistical Analysis
The main analysis was based on 2656 cases with invasive (n=1609, including 1114 in NHS and 495 in HPFS) or in situ melanomas (n=1047, including 726 in NHS and 321 in HPFS). We conducted several sets of statistical descriptions and comparisons for the epidemiologic and clinical features of invasive and in situ melanomas. First, we calculated the age-adjusted incidence of in situ and invasive melanomas and compared the ratio of in situ versus invasive melanomas during the follow-up in the women’s (NHS, 1976–2010) and men’s (HPFS, 1986–2010) cohort. Next, we calculated the age-adjusted incidence of in situ melanomas by subtypes. As lentigo maligna (LM) occurs on sun-damaged skin, we examined the trend in LM to elucidate whether the increase in melanoma incidence can be attributed to cumulative sun exposure in the aging cohorts. We subsequently compared the distribution in anatomic sites of in situ and invasive melanomas in women and men. Chi-square test or Fisher exact probability test was used to compare the distribution in anatomic sites between in situ and invasive melanomas, and between women and men. For melanoma in situ, we additionally compared the head and neck to trunk ratio by age of diagnosis. Head and neck was chosen to represent an area of chronic sun exposure over other body sites to eliminate the potential confounder of clothing style and climate. We chose to compare head and neck to trunk ratios with the lowest age group of <50 year of age instead of comparing percentage of head and neck MIS among different age groups given the low number of cases of melanoma in our cohort that developed below the age of 45. Finally, we calculated the melanoma mortality during the follow-up for women and men. Analyses were carried out by using SAS (version 9.2; SAS Institute Inc, Cary, NC). All statistical tests were two-sided. P-values <0.05 were considered significant.
RESULTS
Trends in Incidence over Time
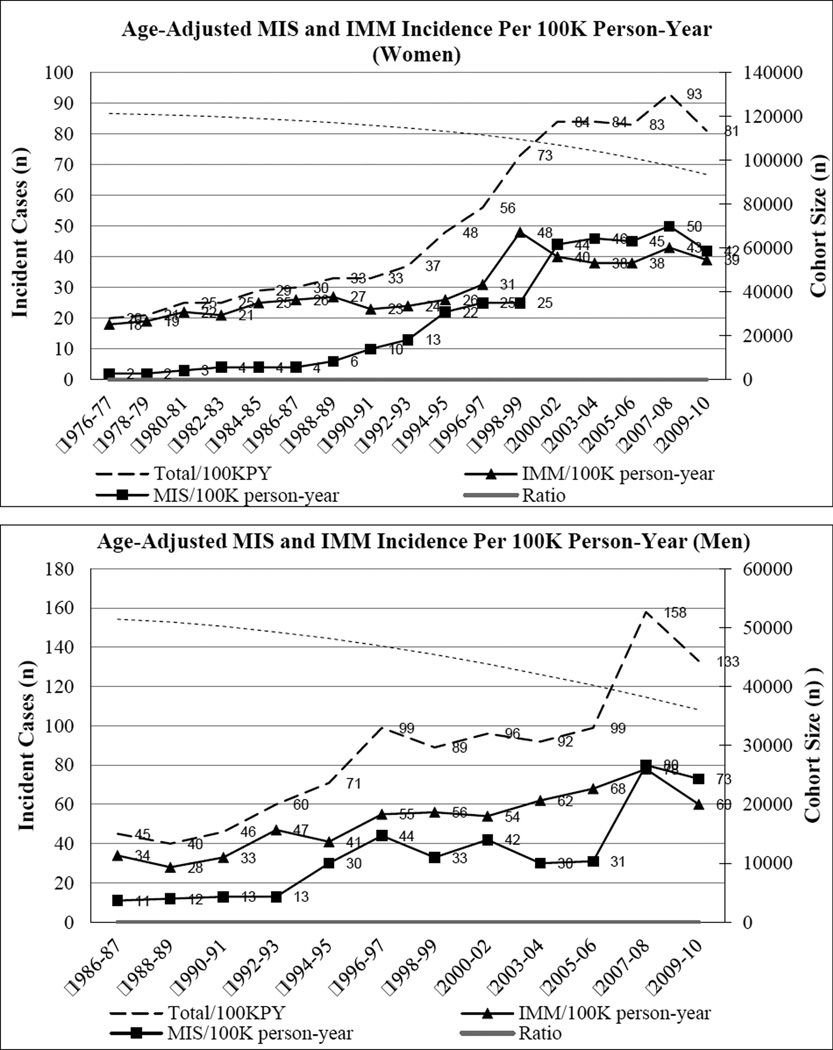
There were 1609 incident cases of invasive melanomas and 1047 incident cases of melanomas in situ during the follow-up. Age-adjusted incidence per 100K person-years is presented according to year of diagnosis in Figure 1. For women, the ratio of incident cases of in situ to invasive melanomas increased from 0.1 to 1.1 from 1976–77 to 2008–10. The absolute incident cases of melanoma in situ began to exceed that of IMM during 2001–02. Among men, the ratio of absolute in situ to invasive incident cases increased from 0.3 to 1.2 from 1986–87 to 2008–10. The absolute incident cases of in situ began to exceed that of invasive during 2007–08. The increase in incident cases of in situ was not driven by increase in melanoma on sun-damaged skin or lentigo maligna over the cohort followup period (Table 1). The mortality from IMM increased over time but plateaued towards the end of the follow-up (shown in Supplementary Figure 1), while Breslow thickness of invasive melanomas did not show a definitive trend (Supplementary Figure 2).
Figure 1. Age-adjusted incidence of melanoma overall, MIS and IMM in (A) women (NHS) and (B) men (HPFS) during the follow-up period.
Figure 1 shows that MIS incidence increased over the follow-up period among both women and men; the incidence of MIS increased at a greater rate than that of IMM. For women, the absolute incident cases of MIS first exceeded that of IMM during 2001–02. Among men, the absolute incident cases of MIS first exceeded that of IMM during 2007–08.
*MIS, melanoma in situ; IMM, invasive malignant melanoma
Table 1.
Age-adjusted incidence of MIS by subtypes in women (NHS) and men (HPFS) during the follow-up period
| Women |
Non- LM*/100KPY |
LM/100KPY |
| 1976–1980 | 1 | 1 |
| 1981–1984 | 2 | 1 |
| 1985–1988 | 3 | 1 |
| 1989–1992 | 5 | 2 |
| 1993–1996 | 13 | 4 |
| 1997–2000 | 20 | 5 |
| 2001–2004 | 33 | 12 |
| 2005–2008 | 32 | 11 |
| 2009–2010 | 63 | 10 |
| Men |
Non- LM/100KPY |
LM/100KPY |
| 1986–1990 | 4 | 2 |
| 1991–1994 | 10 | 1 |
| 1995–1998 | 26 | 1 |
| 1999–2002 | 33 | 1 |
| 2003–2006 | 18 | 1 |
| 2007–2010 | 51 | 12 |
LM, lentigo maligna
Anatomic Distribution of in situ and invasive melanomas
Anatomic distribution of invasive and in situ melanomas is presented in Figure 2. The anatomic distribution of MIS and IMM did not change over the follow-up period (Supplementary Figure 3). We compared the age of diagnosis and found that invasive melanomas were diagnosed at a younger age compared to melanomas in situ (p<0.001, Supplementary Figure 4). As shown in Figure 2, women more likely to have melanoma in situ on the lower extremities compared to men (p<0.001). Invasive melanoma was significantly more likely than melanoma in situ to be found on the lower extremities for both women (34% of IMM vs. 21% of MIS, p<0.001) and men (12% of IMM vs. 6% of MIS, p<0.001). Compared to women diagnosed at ≥71, women age ≤50 showed different distribution in melanoma in situ (p<0.001), with older women twice the ratio of head and neck to trunk melanoma in situ than younger women (Table 2). Similarly among men, the distribution of melanoma in situ was different between younger (age ≤50) and older men (age ≥71) (p<0.001). Older men were 3-times the ratio of in situ melanomas on the head and neck versus the trunk (Table 2).
Figure 2. Distribution in anatomic sites of MIS and IMM in women (NHS) and men (HPFS).
Figure 2 shows that among men diagnosed with MIS, 39% of lesions were found on the head and neck, 40% on the trunk, 15% on the upper extremities, and 6% on lower extremities, whereas in men diagnosed with IMM, 23% were found on the head and neck, 51% on the trunk, 14% on the upper extremities, 12% on the lower extremities. Among women, 25% of MIS were found on the head and neck, 29% on the trunk, 26% on the upper extremities, 21% on lower extremities, compared to IMM, where 10% were found on the head and neck, 31% on the trunk, 25% on the upper extremities, 34% on the lower extremities. (Illustrations by Iris Fung)
*MIS, melanoma in situ; IMM, invasive malignant melanoma
Table 2.
Head and neck to trunk ratio by age of diagnosis of MIS
| Age of Diagnosis |
Women | Men |
|---|---|---|
| <50 | 0.6 | 0.6 |
| 51–60 | 1 | 0.5 |
| 61–70 | 1.3 | 0.8 |
| 71+ | 1.2 | 1.8 |
| P-value* | P<0.001 | P<0.001 |
P value for the distribution of head and neck to trunk ratio according to age of diagnosis was calculated by using chi-square test for men and women respectively.
CONCLUSIONS
The present study revealed interesting epidemiologic and clinical features of melanoma in situ First, the increase in melanoma in situ detection relative to invasive melanoma over 30 years (women) and 20 years (men) of cohort follow-up did not correspond to a reduction in melanoma mortality. Additionally, invasive and in situ melanomas showed distinct anatomic distribution, which may have greater implications on biologic behavior in distinct anatomic sites. Invasive melanomas are diagnosed at a younger age than in situ lesions; invasive melanomas were more likely than in situ melanomas to be found on the lower extremities in both men and women. Overall, an overwhelming percentage of invasive melanomas were found above the waist.
Although a greater proportion of lesions detected in later cohort years were in situ relative to invasive melanomas, it did not translate to a reduction in melanoma mortality in later years. One reason for this observation may be a lag-time between increased screening and decrease in mortality on a cohort level. However, a lack of significant reduction in melanoma mortality further support that many of the biologically aggressive lesions are still being missed, despite increased screening and biopsy. These results may also reflect, in part, a trend of over-diagnosis of lesions that have little or no invasive potential. Indirectly supporting this, most of the in situ lesions diagnosed in later years were not of the lentigo melanoma (i.e. melanoma on sun damaged skin), which is contrary to what would be expected given increase in melanoma in situ incidence as the cohorts aged.27,28 Assuming that melanoma in situ diagnoses are accurate, these findings call in to question the dogma that all in situ lesions are precursors for invasive lesions, and point to a new idea that at least some of the melanoma in situ cases may not in themselves be precursor lesions, but perhaps are instead markers for increased risk of development invasive melanomas. This is the case for lobular carcinoma in situ of the breast which is a significant risk factor for the development of breast cancer, but itself does not transforms into cancer, as opposed to ductal carcinoma in situ of the breast, which does transform into invasive cancer.29
Melanocyte density in the skin increases with age,30 which may in part explain why melanomas are more common in older individuals. However, in this study, we found that melanoma in situ had a significantly higher age of diagnosis than invasive melanomas. This is contrary to what would be expected based on previous studies on invasive melanomas where tumor depth increases with age of diagnosis,31,32 and based on what is known of biological decline in host immunity with age.33 This observation may support the notion that genetically protected individuals who develop melanoma at a more advanced age tend to develop slow growing tumors that are caught in the early stage, whereas more genetically predisposed individuals who develop melanomas at a younger age may also develop more aggressive tumors with higher potential for local metastasis.34 Another possible explanation for this finding may be increased surveillance in older individuals resulting in earlier capturing of potentially malignant lesions, but the differences in in anatomic distribution of melanoma in situ versus invasive melanomas speaks for a true biologic difference in development instead of screening bias. Again, this finding may suggest that not all melanoma in situ are biologically equivalent. An additional factor that could contribute to this observation is over-diagnosis during later cohort follow-up years due to changes in practice patterns of dermatologists and dermatopathologists in the current geo-political climate.
Melanocyte density has been reported to vary with anatomic site, with highest densities observed on the back or shoulders and upper limbs.30 In general, melanomas developing from distinct anatomic locations are pathologically distinct,35 may show distinct patterns of tumor suppressor gene variation and ability for invasion.36–38 In concordance with anatomic-dependent biologic melanocyte behavior, we also found that the MIS that developed in older versus younger individuals differed in anatomic distribution. Younger individuals had a tendency to develop in situ lesions in areas of intermittent sun exposure, while older individuals developed in situ melanomas in areas of chronic sun exposure. Other studies have shown that truncal melanomas are associated with intermittent sun exposure, while head and neck melanomas are associated with chronic sun exposure.39,40 These differences in sun exposure pattern have been thought to reflect the distinct pathway of malignant transformation of melanocytes in different anatomic areas.41–43
With regards to gender distinctions in melanoma in situ development, these data show that women were more likely to develop melanoma in situ on the lower extremities as compared to men. These findings are similar to several previous studies on anatomic distribution of invasive melanomas, with men showing predilection for the upper half and women with propensity to develop invasive melanomas on the lower extremities.37,38,44,45 These gender differences in melanoma in situ development may reflect differences in carcinogenic exposures in clothing/hair style, indoor versus outdoor occupation, and sun-seeking behavior between men and women. Previous studies have hinted at the possibility of gender-specific melanocyte susceptibility to malignant transformation.46 These differences further point to gender differences in tumor-host interaction as suggested in worse melanoma survival in men regardless of age when compared to women.47–48
To our knowledge, this is the first study of this magnitude describing clinical and epidemiologic features of melanoma in situ. Further, the use of occupational cohorts with high quality data validated by objective measures25,26 and consistently high rate of follow-up removes a potential reporting bias. Finally, the use of healthcare professionals as the study population eliminates a potential confounder of differential access to care. We also acknowledge limitations. Our study is a purely descriptive study, in which we did not attempt to examine the related mechanisms for the differential trends in diagnosis and clinical features between in situ and invasive melanoma. As most of the study participants are Caucasians, extrapolation of our findings to other race/ethnicities should be approached with caution. Our study participants were health professionals, with higher socioeconomic status than the general population. Actually, the age-adjusted incident cases found in this study differed than that found by SEER database. SEER estimated the new cases of melanoma of the skin was 21.8 per 100,000 men and women per year (2009–2013), compared to 81 and 133 within our cohorts for women and men from 2009–2010, respectively27 This difference can be attributed to our study population being comprised higher proportion of at-risk individuals for melanomas than the general population, such as a predominance of Caucasian race (97%) and high socioeconomic status49 of the study participants.
The lack of improvement in melanoma mortality despite increase in detection of in situ relative to invasive melanomas suggest that many of the features used for screening for melanoma are not specific to detect invasive lesions, and may catch many slow-growing, intraepidermal proliferations that may or may not become invasive over time. These findings highlight the need to improve invasive melanoma-specific clinical screening. Based on our findings, clinicians should be vigilant of new or changing lesions on lower extremities in both men and women, given higher relatively percentage of invasive versus in situ lesions in this area.
Supplementary Material
Capsule Summary.
Incidence of melanoma is rising in the U.S. and around the world.
Incidence of melanoma in situ is rising at a faster rate than that of invasive malignant melanoma.
In situ and invasive melanomas demonstrate distinct epidemiologic and clinical features, suggesting divergent etiologic pathways of development.
Acknowledgments
Funding Source: This work was supported by the National Institute of Health grants (UM1 CA186107 and R01 CA87969 to support Nurses’ Health Study and UM1 CA167552 to support Health Professionals’ Follow-up Study).
Abbreviations used
- MIS
melanoma in situ
- IMM
invasive malignant melanoma
- LM
lentigo maligna
- NHS
Nurses’ Health Study
- HPFS
Health Professionals Follow-up Study
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Authors Disclosure
The authors involved with this journal based CME activity have reported no relevant financial relations with commercial interest(s)
Learning Objective
After completing this learning activity, participants should have gained improved understanding of epidemiology and clinical features of melanoma in situ.
Institutional Review Board (IRB) Approval: This study was approved by the Institutional Review Board of Brigham and Women's Hospital. The participants' completion and return of the self-administered questionnaires were considered as informed consent.
Acknowledgement Statement
We are indebted to participants and staff of the NHS and HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
References
- 1.Mihm MC, Jr, Fitzpatrick TB, Brown MM, Raker JW, Malt RA, Kaiser JS. Early detection of primary cutaneous malignant melanoma. A color atlas. The New England journal of medicine. 1973;289(19):989–996. doi: 10.1056/NEJM197311082891901. [DOI] [PubMed] [Google Scholar]
- 2.Criscione VD, Weinstock MA. Melanoma thickness trends in the United States, 1988–2006. The Journal of investigative dermatology. 2010;130(3):793–797. doi: 10.1038/jid.2009.328. [DOI] [PubMed] [Google Scholar]
- 3.Coory M, Baade P, Aitken J, Smithers M, McLeod GR, Ring I. Trends for in situ and invasive melanoma in Queensland, Australia, 1982–2002. Cancer causes & control : CCC. 2006;17(1):21–27. doi: 10.1007/s10552-005-3637-4. [DOI] [PubMed] [Google Scholar]
- 4.Thorn M, Ponten F, Johansson AM, Bergstrom R. Rapid increase in diagnosis of cutaneous melanoma in situ in Sweden, 1968–1992. Cancer detection and prevention. 1998;22(5):430–437. doi: 10.1046/j.1525-1500.1998.00052.x. [DOI] [PubMed] [Google Scholar]
- 5.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. The Journal of investigative dermatology. 2009;129(7):1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A, Devesa SS, Fears TR, Hartge P. Cancer surveillance series: changing patterns of cutaneous malignant melanoma mortality rates among whites in the United States. J Natl Cancer Inst. 2000;92(10):811–818. doi: 10.1093/jnci/92.10.811. [DOI] [PubMed] [Google Scholar]
- 7.Swerlick RA, Chen S. The melanoma epidemic: more apparent than real? Mayo Clin Proc. 1997;72(6):559–564. doi: 10.4065/72.6.559. [DOI] [PubMed] [Google Scholar]
- 8.Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132(8):881–884. doi: 10.1001/archderm.132.8.881. [DOI] [PubMed] [Google Scholar]
- 9.Wolff T, Tai E, Miller T. Screening for skin cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150(3):194–198. doi: 10.7326/0003-4819-150-3-200902030-00009. [DOI] [PubMed] [Google Scholar]
- 10.Garbe C, McLeod GR, Buettner PG. Time trends of cutaneous melanoma in Queensland, Australia and Central Europe. Cancer. 2000;89(6):1269–1278. doi: 10.1002/1097-0142(20000915)89:6<1269::aid-cncr11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Ahn SK, Lee HS, Han SK, Lee SH, Lee S. Multiple basal cell carcinoma associated with keratoacanthoma. Yonsei medical journal. 1992;33(3):277–280. doi: 10.3349/ymj.1992.33.3.277. [DOI] [PubMed] [Google Scholar]
- 12.Baade P, Meng X, Youlden D, Aitken J, Youl P. Time trends and latitudinal differences in melanoma thickness distribution in Australia, 1990–2006. International journal of cancer. Journal international du cancer. 2012;130(1):170–178. doi: 10.1002/ijc.25996. [DOI] [PubMed] [Google Scholar]
- 13.Montella A, Gavin A, Middleton R, Autier P, Boniol M. Cutaneous melanoma mortality starting to change: a study of trends in Northern Ireland. European journal of cancer. 2009;45(13):2360–2366. doi: 10.1016/j.ejca.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Murray CS, Stockton DL, Doherty VR. Thick melanoma: the challenge persists. The British journal of dermatology. 2005;152(1):104–109. doi: 10.1111/j.1365-2133.2005.06409.x. [DOI] [PubMed] [Google Scholar]
- 15.Schneider JS, Moore DH, 2nd, Mendelsohn ML. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. Journal of the American Academy of Dermatology. 2008;58(5):741–749. doi: 10.1016/j.jaad.2007.10.648. [DOI] [PubMed] [Google Scholar]
- 16.Tejera-Vaquerizo A, Mendiola-Fernandez M, Fernandez-Orland A, Herrera-Ceballos E. Thick melanoma: the problem continues. Journal of the European Academy of Dermatology and Venereology : JEADV. 2008;22(5):575–579. doi: 10.1111/j.1468-3083.2007.02517.x. [DOI] [PubMed] [Google Scholar]
- 17.Hiatt RA, Fireman B. The possible effect of increased surveillance on the incidence of malignant melanoma. Preventive medicine. 1986;15(6):652–660. doi: 10.1016/0091-7435(86)90070-8. [DOI] [PubMed] [Google Scholar]
- 18.MacKie RM, Hole D, Hunter JA, et al. Cutaneous malignant melanoma in Scotland: incidence, survival, and mortality, 1979–94. The Scottish Melanoma Group. Bmj. 1997;315(7116):1117–1121. doi: 10.1136/bmj.315.7116.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLennan R, Green AC, McLeod GR, Martin NG. Increasing incidence of cutaneous melanoma in Queensland, Australia. Journal of the National Cancer Institute. 1992;84(18):1427–1432. doi: 10.1093/jnci/84.18.1427. [DOI] [PubMed] [Google Scholar]
- 20.Swetter SM, Boldrick JC, Jung SY, Egbert BM, Harvell JD. Increasing incidence of lentigo maligna melanoma subtypes: northern California and national trends 1990–2000. J Invest Dermatol. 2005;125(4):685–691. doi: 10.1111/j.0022-202X.2005.23852.x. [DOI] [PubMed] [Google Scholar]
- 21.Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 22.Erickson C, Driscoll MS. Melanoma epidemic: Facts and controversies. Clin Dermatol. 28(3):281–286. doi: 10.1016/j.clindermatol.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Rigel DS, Friedman RJ, Kopf AW. The incidence of malignant melanoma in the United States: issues as we approach the 21st century. J Am Acad Dermatol. 1996;34(5 Pt 1):839–847. doi: 10.1016/s0190-9622(96)90041-9. [DOI] [PubMed] [Google Scholar]
- 24.Purdue MP, Freeman LE, Anderson WF, Tucker MA. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol. 2008;128(12):2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. American journal of epidemiology. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 26.Joshipura KJ, Pitiphat W, Douglass CW. Validation of self-reported periodontal measures among health professionals. Journal of public health dentistry. 2002;62(2):115–121. doi: 10.1111/j.1752-7325.2002.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 27.Cohen LM. Lentigo maligna and lentigo maligna melanoma. Journal of the American Academy of Dermatology. 1995;33(6):923–936. doi: 10.1016/0190-9622(95)90282-1. quiz 937–940. [DOI] [PubMed] [Google Scholar]
- 28.Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Archives of pathology & laboratory medicine. 2011;135(7):838–841. doi: 10.5858/2011-0051-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 29.To T, Wall C, Baines CJ, Miller AB. Is Carcinoma in Situ a Precursor Lesion of Invasive Breast Cancer? International journal of cancer. Journal international du cancer. 2014 doi: 10.1002/ijc.28803. [DOI] [PubMed] [Google Scholar]
- 30.Whiteman DC, Parsons PG, Green AC. Determinants of melanocyte density in adult human skin. Archives of dermatological research. 1999;291(9):511–516. doi: 10.1007/s004030050446. [DOI] [PubMed] [Google Scholar]
- 31.Chao C, Martin RC, 2nd, Ross MI, et al. Correlation between prognostic factors and increasing age in melanoma. Annals of surgical oncology. 2004;11(3):259–264. doi: 10.1245/aso.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Austin PF, Cruse CW, Lyman G, Schroer K, Glass F, Reintgen DS. Age as a prognostic factor in the malignant melanoma population. Annals of surgical oncology. 1994;1(6):487–494. doi: 10.1007/BF02303614. [DOI] [PubMed] [Google Scholar]
- 33.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Current opinion in immunology. 2010;22(4):507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balch CM, Thompson JF, Gershenwald JE, et al. Age as a Predictor of Sentinel Node Metastasis among Patients with Localized Melanoma: An Inverse Correlation of Melanoma Mortality and Incidence of Sentinel Node Metastasis Among Young and Old Patients. Annals of surgical oncology. 2014;21(4):1075–1081. doi: 10.1245/s10434-013-3464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Micu E, Baturaite Z, Juzeniene A, Bruland OS, Moan JE. Superficial-spreading and nodular melanomas in Norway: a comparison by body site distribution and latitude gradients. Melanoma research. 2012;22(6):460–465. doi: 10.1097/CMR.0b013e3283599cc3. [DOI] [PubMed] [Google Scholar]
- 36.Straume O, Akslen LA. Alterations and prognostic significance of p16 and p53 protein expression in subgroups of cutaneous melanoma. International journal of cancer. Journal international du cancer. 1997;74(5):535–539. doi: 10.1002/(sici)1097-0215(19971021)74:5<535::aid-ijc10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Egger ME, Tabler BL, Dunki-Jacobs EM, et al. Clinicopathologic and survival differences between upper and lower extremity melanomas. The American surgeon. 2012;78(7):779–787. [PubMed] [Google Scholar]
- 38.Shaw HM, McGovern VJ, Milton GW, Farago GA, McCarthy WH. Malignant melanoma: influence of site of lesion and age of patient in the female superiority in survival. Cancer. 1980;46(12):2731–2735. doi: 10.1002/1097-0142(19801215)46:12<2731::aid-cncr2820461232>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Whiteman DC, Stickley M, Watt P, Hughes MC, Davis MB, Green AC. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24(19):3172–3177. doi: 10.1200/JCO.2006.06.1325. [DOI] [PubMed] [Google Scholar]
- 40.Anderson WF, Pfeiffer RM, Tucker MA, Rosenberg PS. Divergent cancer pathways for early-onset and late-onset cutaneous malignant melanoma. Cancer. 2009;115(18):4176–4185. doi: 10.1002/cncr.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green A. A theory of site distribution of melanomas: Queensland, Australia. Cancer Causes Control. 1992;3(6):513–516. doi: 10.1007/BF00052747. [DOI] [PubMed] [Google Scholar]
- 42.Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95(24):1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 43.Greene VR, Johnson MM, Grimm EA, Ellerhorst JA. Frequencies of NRAS and BRAF mutations increase from the radial to the vertical growth phase in cutaneous melanoma. J Invest Dermatol. 2009;129(6):1483–1488. doi: 10.1038/jid.2008.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carli P, Borgognoni L, Biggeri A, Carli S, Reali UM, Giannotti B. Incidence of cutaneous melanoma in the centre of Italy: anatomic site distribution, histologic types and thickness of tumour invasion in a registry-based study. Melanoma research. 1994;4(6):385–390. doi: 10.1097/00008390-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Cho E, Rosner BA, Colditz GA. Risk factors for melanoma by body site. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2005;14(5):1241–1244. doi: 10.1158/1055-9965.EPI-04-0632. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Gomez B, Aragones N, Gustavsson P, Lope V, Lopez-Abente G, Pollan M. Do sex and site matter? Different age distribution in melanoma of the trunk among Swedish men and women. The British journal of dermatology. 2008;158(4):766–772. doi: 10.1111/j.1365-2133.2007.08429.x. [DOI] [PubMed] [Google Scholar]
- 47.Joosse A, de Vries E, Eckel R, et al. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. The Journal of investigative dermatology. 2011;131(3):719–726. doi: 10.1038/jid.2010.354. [DOI] [PubMed] [Google Scholar]
- 48.Stidham KR, Johnson JL, Seigler HF. Survival superiority of females with melanoma. A multivariate analysis of 6383 patients exploring the significance of gender in prognostic outcome. Archives of surgery. 1994;129(3):316–324. doi: 10.1001/archsurg.1994.01420270094020. [DOI] [PubMed] [Google Scholar]
- 49.Pion IA, Rigel DS, Garfinkel L, Silverman MK, Kopf AW. Occupation and the risk of malignant melanoma. Cancer. 1995;75(2 Suppl):637–644. doi: 10.1002/1097-0142(19950115)75:2+<637::aid-cncr2820751404>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 50.National Cancer Institute, Surveillance, Epidermiology, and End Results Program. [Accessed Apr 15, 2016];Cancer Stat Fact Sheet. 2013 Available from http://seer.cancer.gov/statfacts/html/melan.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.