Abstract
Methamphetamine (meth) is one of the most abused substances worldwide. Chronic use has been associated with repeated relapse episodes that may be exacerbated by cognitive impairments during drug abstinence. Growing evidence demonstrates that meth compromises prefrontal cortex activity, resulting in persisting attentional and memory impairments. After summarizing recent studies of meth-induced cognitive dysfunction using a translationally relevant model of self-administered meth, this review emphasizes the cortical brain changes contributing to cognitive dysregulation during abstinence. Finally, we propose the use of cognitive enhancers during abstinence that may promote a drug-free state by reversing cortical dysfunction linked with prolonged meth abuse.
Keywords: Addiction, methamphetamine, cognitive deficits, prefrontal, perirhinal, glutamate, attention, object recognition
1. Background
Methamphetamine addiction, as any addiction with other abused substances, is a chronic relapsing disorder meriting the need for effective treatment strategies. The development of such strategies relies on concerted efforts by researchers to integrate findings from basic animal scientists and human clinical scientists with those of practitioners conducting clinical trials. Despite gains in our knowledge of meth, the effects of repeated meth abuse, and the severity of this problem, no treatments to date have proven to be consistently effective for alleviating the symptoms of meth addiction, particularly in regards to cognitive deficits and drug-seeking [1, 2]. These seemingly intractable problems can dramatically reduce quality of life for addicts and contribute to sustained drug taking and relapse.
Executive function includes the domains of decision-making, attentional control, and working memory. The most pronounced and lasting deficits experienced by psychostimulant addicts occur in executive function, as well as in episodic memory and information processing [3]. These cognitive domains require intact cortical function and meth addicts often show impairments of working memory and memory recall [4], psychomotor function [5], response inhibition [6, 7], strategy shifting tasks [8], and risky decision-making [9]. These deficits likely contribute, at least in part, to continued drug taking and poor unhealthy decision-making underlying addiction.
Although there are multiple reports suggesting that abused drugs impact these cognitive domains, the relationship between impaired executive function, addiction, and relapse is not well understood. Several reviews of the human literature have already been written summarizing the effects of meth on human cognitive dysfunction and the clinical relevance of these deficits [3, 10, 11]. In general, the majority of data indicates that chronic meth causes cognitive decline in some people, particularly during early-to-middle adulthood [11]. Importantly, the high incidence of cognitive dysfunction that occurs in chronic pathological meth addiction is distinct from acute or low dose effects of meth [12]. Under these low dose conditions, meth may act to enhance cognition, even after repeated use. Indeed, meth has a long history of being prescribed as a pharmacological therapy for attentional disorders [13]. Here we review the existing literature demonstrating the existence of cortically mediated cognitive dysfunction following chronic exposure to self-administered meth with a focus on animal models.
2. Models of meth administration used to study cognitive dysfunction
Although there is a presumed relationship between drug-seeking, cognitive deficits, and neural dysfunction, it is difficult to establish whether these deficits are caused by meth or a cognitive predisposition, since human subjects undergo evaluation well after they have become addicted. A major strength of preclinical research is the ability to study these relationships in a controlled manner using translationally relevant animal models.
Experimenter-administered meth, consisting primarily of sensitization or a repeated single day treatment regimen, has typically been used to assess meth induced neurotoxic consequences (e.g., loss of dopamine transporters, glial fibrillary acidic protein, tyrosine hydroxylase). Thorough reviews of meth-induced neurotoxicity have recently been provided [13, 14]. While useful, these models do not use contingent drug delivery and so cannot account for motivational and cognitive facets of drug use, which constitute core features of meth addiction. Additionally, overt markers of striatal toxicity do not always occur with adverse cognitive consequences (discussed further in section 5, 15]. As such, drug self-administration (SA) procedures allow rats to regulate drug intake by eliciting a behavioral output such as a lever press or nose poke into a hole. This benefit increases face and construct validity of the self-administration model relative to systemic drug administration. Additionally, the predictive validity of self-administration models of psychostimulant abuse liability is robust [16, 17]. Finally, self-administration has greater translational relevance due to the flexibility of self-administration to develop protocols assessing various dimensions of the addiction spectrum [18].
As an exemplar, increasing the amount of time drug is available during daily sessions further extends the translational impact. Standard session lengths during acquisition are typically conducted in the 1–2 hour range [i.e., short access to the drug (ShA)] resulting in a stabilization of daily drug intake. In contrast, rats given 6 or more hours of daily drug SA [i.e., long access to the drug, (LgA)] display an escalation of drug intake over time reminiscent of human compulsive drug taking, thereby inducing a long-lasting increase in set point for the drug in LgA as compared to ShA rats [19–21]. Moreover, recent evidence indicates that LgA meth rats exhibit enhanced drug seeking and cognitive impairments relative to control rats or ShA. These impairments observed in LgA rats include attentional and impulsivity deficits [22], higher vulnerability to relapse and deficits in episodic memory [23], and persistent alterations of monoamine systems in prefrontal and striatal brain areas [24], which constitute several potent examples related to the construct validity of this model. These findings suggest that increasing drug access provides a clinically relevant paradigm that can assess drug taking during acquisition as well as motivational and cognitive processes when the drug has been discontinued. The next sections will therefore focus on studies that identify deficits during periods of withdrawal or abstinence (i.e., when the drug has been discontinued) from chronic meth SA.
3. Attention, inhibitory control, and cognitive flexibility
The ability to adapt behavior according to current environmental or situational demands requires cognitive control by attending to task relevant information over extended periods of time, regardless of attentional interference [25]. These dimensions are particularly relevant to dissociate recreational from compulsive drug intake in addiction. For instance, deficits in response inhibition could account for the inability of a subject to resist the urge of drug taking when being in the presence of drug-associated cues, while deficits in strategy-shifting task could explain the difficulties of an addict in selecting alternatives to drug taking from a non-pathological behavioral repertoire. Based on the accumulation of 20 years of research, separate dimensions of impulsivity and attention shifting have been shown to be mediated by different cortical subregions [26, 27], which are disrupted by meth exposure.
3.1 Attentional set-shifting task
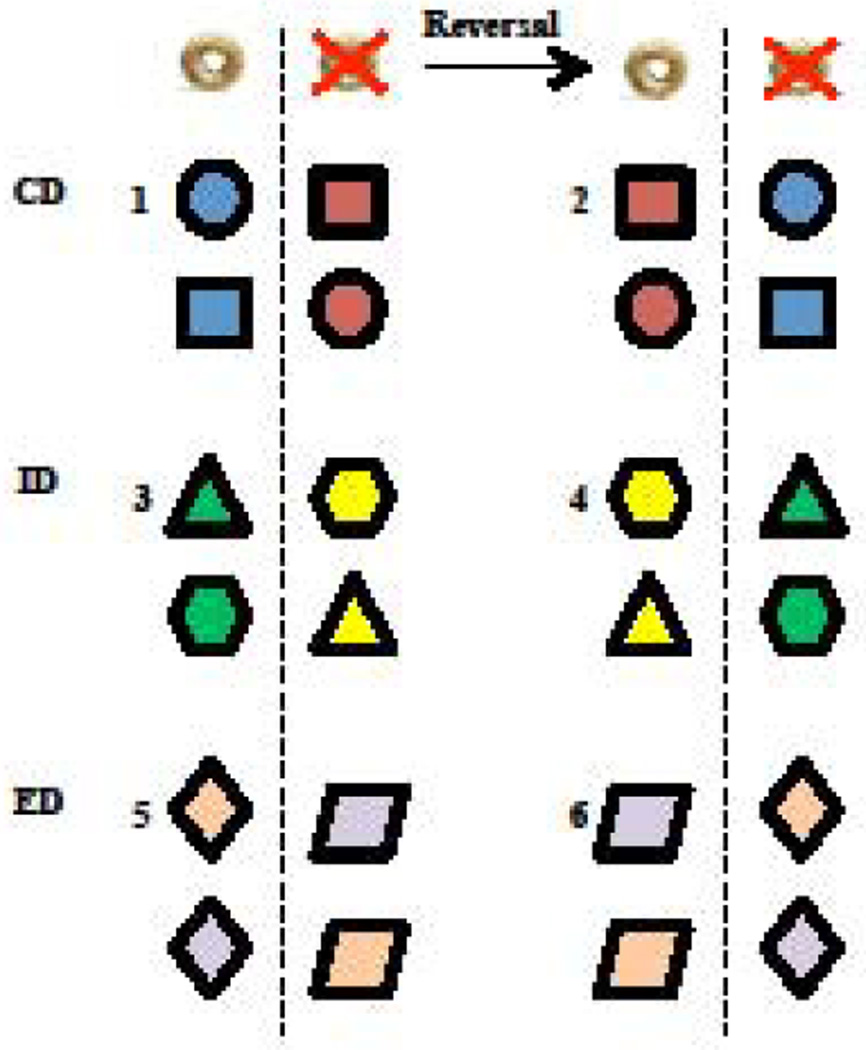
In humans, the Wisconsin Card Sorting Task can identify specific deficits in attention and cognitive flexibility that correspond to prefrontal cortex function. This task assesses the adaptability to rule shift between a previously learned strategy and a new one, and is modelled in rodents by the Attentional Set-Shifting Task (ASST). In the most well characterized protocol [28–32], rats are trained to dig in bowls for a food reward and then to discriminate between a baited and non-baited bowl based on a variety of dimensions (e.g., odor, digging media, and bowl texture). It must be noted that instead of not baiting the other bowl, crushed reinforcers are sometimes used as an alternative in order to avoid olfactory bias that would guide the rats to the only reinforced location [33]. Over the course of a single session, rats are presented with a series of discrimination phases that assess various components of learning (Figure 1), with advancement through the phases occurring after subjects reach the predetermined criteria. During the compound discrimination (CD), only one stimulus dimension reliably predicts food reward, while the other dimension is considered irrelevant. As illustrated in Figure 1, if a particular odor constantly predicts reward location (represented in blue color), it would be paired with different irrelevant digging media or bowl textures (represented by different shapes). Rats then undergo a reversal phase, in which the stimulus previously associated with the reward now predicts the absence of a reward, without changes in the relevant or irrelevant dimension. Then, intra- and extradimensional (ID and ED) shifts and reversals occur successively, assessing the adaptability of rats in novel learning paradigms. The ID shift requires the animal to learn a new discrimination between two novel stimuli of the same relevant dimension used for the CD phase (Figure 1, new odors represented with green and yellow colors); whereas, the ED introduces a novel discrimination between two stimuli among a dimension that was previously irrelevant (Figure 1, relevant digging media/bowl texture represented with the diamond shape), while the previously relevant dimension (i.e., odor) now becomes irrelevant. Correctly adjusting a learning strategy to perform an ED shift requires additional components beyond just working memory and procedural learning. Specifically, the rats must attend to the fact that previous strategies no longer apply and must implement an alternative behavioral strategy, thus avoiding unsuccessful "perseverative" responding.
Figure 1. Attentional set-shifting task protocol.
Each bowl is characterized by a specific digging medium (shape) and a specific odor (shape’s color). Rats undergo several phases in sequential order (here noted from 1 to 6), progressing from one phase to another after reaching predetermined criteria. The different phases are the compound discrimination (CD, top panel), the intradimensional shift (ID, mid panel), the extradimensional shift (ED, low panel), and each of these phases are followed by reversal testing (right side of panels). In this example, CD consists of associating reward location with a particular odor, regardless of digging medium; the ID consists of associating reward location with another odor, still regardless of the digging medium; and the ED consists of associating reward location with a specific digging medium, regardless of the odors presented. In every choice, only one bowl is baited (left of dotted line), and pairs of stimuli among each bowl are pseudo randomly assigned. Adapted from [28].
Consistently, performance in this task relies on intact prefrontal cortex function in humans and animals. Lesions of the dorsolateral prefrontal cortex (primates) or medial prefrontal cortex (rodents) produce deficits shifting from one stimulus dimension to another (i.e., extra-dimensional shift), while similar damage to the orbital frontal cortex impairs reversal learning [28–31, 34]. Recently, using an automated variant of the task, data has shown that dorsal striatal neurons are involved in encoding rule representations during ASST [35]. Given the substantial amount of PFC efferents to dorsal striatal areas [36], together with the role of the dorsal striatum in encoding stimulus-response relations [37, 38], frontostriatal connectivity plays a key role in regulating set-shifting performance. Further indication that prefrontal dopamine levels constitute a key regulator of set-shifting performance comes from studies showing that infusions of a dopamine D1 [39] or D2 receptor antagonist [40] into the prelimbic cortex impaired ED set-shifting performance consisting of shifts from a response-based to a visual-based strategy.
3.2 Five-Choice Serial Reaction Time Task
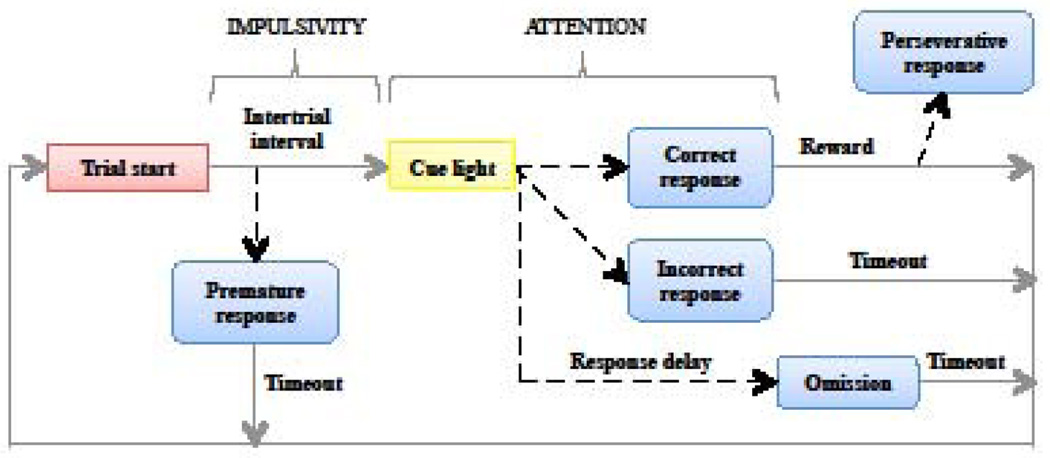
The 5-CSRTT is an automated operant procedure for measuring sustained visual attention and impulsivity in rats (see [41, 42] for review and detailed methodology). Briefly, to perform this task, a rat is placed in an apparatus with five holes equipped with photobeams to record head entries open for exploration. A stimulus light above each hole signals the hole that a rat is supposed to explore. Upon a correct head entry, a sucrose pellet is delivered, and collection of the reward in the food magazine initiates the next trial. After several phases of training, attentional demand is progressively enhanced by decreasing the duration of the visual stimulus indicating the relevant hole. As shown in Figure 2, several types of responses are recorded in the test phase, namely incorrect, omission, premature, or perseverative responses. Correct responses are defined as a nose entry into the hole below visual stimulus, and lead to a reward delivery. Incorrect responses are defined as nose entries into a hole without the visual stimulus. An accuracy ratio of responding is calculated between correct and incorrect responses, reflecting the attentional performance of rats. Omissions are a lack of a response per trial. Premature responses, the main indicator of impulsive behavior in this task, occur when a rat makes a nose entry during the inter-trial interval, (i.e. before the light stimulus predicting reward delivery appears). These responses are evident with a constant inter-trial interval, and increasing this interval (usually from 5 to 7 seconds) enhances the propensity of premature responding. Perseverative responses are recorded when rats persist in responding into a hole after making a correct response, which is considered as a form of compulsive behavior.
Figure 2. Five-choice serial reaction time task.
In a classical protocol, rats initiate a trial by poking their nose into the food magazine, which triggers the illumination of a cue light located in one of the 5 holes. A nose poke into the previously illuminated hole results in reward delivery, while nose entries into non-illuminated holes or no response are punished by a timeout period. Impulsive behaviors are determined by the number of nose pokes occurring before presentation of the cue light and are termed premature responses. Attentional performance is determined by the accuracy of responding before the end of the response delay, inferred by the ratio of correct over incorrect responses.
The 5-CSRTT requires corticostriatal functional integrity, as well as cortically projecting dopamine, norepinephrine, acetylcholine, and serotonin brain neurotransmitter systems [26, 42, 43]. Interestingly, distinct cortical areas mediate the specific type of response errors. Accuracy involves the anterior cingulate and prelimbic cortices [44]. More specifically, attentional selectivity encompasses the pregenual region of the anterior cingulate while impulsive premature responses depend upon the post-genual anterior cingulate and the infralimbic cortex [44–46]. Further, compulsive-like responding as assessed by perseverative errors involves the prelimbic and orbital frontal cortex [45, 46]. Taken together, these studies show specificity to a certain degree with some overlap of cortical areas that account for response errors in the 5-CSRTT. The striatum has also been identified as the main subcortical area underlying performance in the 5-CSRTT, primarily through its connection with the frontal cortex [47]. Lesions of the medial and lateral striatum produce global deficits in every parameter of the 5-CSRTT, with even an inability of lateral striatum-lesioned animals to perform this task [48]. This section reviewed the importance of corticostriatal circuitry in regards to 5-CSRTT. Additionally this circuitry underlies reward-guided decision-making [49]; therefore, this circuitry is also a critical component in the transition from recreational to compulsive drug use [16, 27]. A discussion of chronic meth’s impact on this task is described in section 3.3.2.
3.3 Chronic meth SA disrupts cortical control of attention and cognitive flexibility
3.3.1. Attentional set-shifting task
Chronic meth addicts exhibit deficits in attentional set shifting akin to patients with prefrontal damage or disorders associated with frontal cortical pathology, and these impairments correspond with alterations of prefrontal white and grey matter [50, 51]. In rats, LgA meth caused selective impairments in the ED, but not in the ID shift, that also corresponded with deficits found following excitotoxic lesions to the mPFC [32]. This meth-induced impairment indicates that LgA meth rats were less efficient in executing a new behavioral strategy. Further, this specific finding suggests that LgA meth does not cause global deficits in ASST, but suggests selectivity within a specific domain within the task. Similar results have been reported after a chronic five-week amphetamine treatment that induced drug sensitization, though amphetamine further induced some reversal deficits [52, 53]. In contrast, a single day high dose meth treatment (4 × 2 mg/kg meth) produced reversal deficits during the reversal component of both ID and ED shifts, while attentional set-shifting before reversal remained intact [54]. As such, discrepancies exist in the degree to which meth disrupts set-shifting and the cortical structures involved.
Clearly, methodological difference regarding meth administration could account for the discrepancies regarding set-shifting deficits. The absence of ED shift deficits after the single-day injection protocol might be linked with an absence of prefrontal alterations, though not assessed in that particular study. Indeed, alterations of medial prefrontal cortex functioning have been linked with LgA meth-induced deficits in ED attentional set-shifting [32]. Therefore, a single-day meth injection regimen might not be enough to induce neuronal alterations in this particular prefrontal region. In parallel, reversal deficits with binge regimen might indicate a specific regulation of the orbitofrontal cortex, a key region involved in behavioral flexibility and reversal learning [55, 56]. Such discrepancies merit future studies on the relationship between the degree of meth exposure, attentional flexibility and its neurobiological underpinnings, especially regarding the contribution of the prefrontal and orbitofrontal cortex.
3.3.2. Five-choice serial reaction time task
Meth, and several related compounds (amphetamine, methylenedioxy-methamphetamine (MDMA), or methylphenidate) impact responding on the 5-CSRTT in multiple ways. An acute meth injection enhanced attention on a two lever version of the CSRTT as indexed by reduced choice reaction time and increased general responding [57]. Similar improvements of attentional performance have been observed after acute non-contingent injections of d-amphetamine or methylphenidate [58–60]. In contrast, an escalating dose of amphetamine (3 injections per week for 5 weeks at 1–5 mg/kg per week) reduced accuracy and increased omissions when rats were tested during withdrawal on the 5-CSRTT [61]. MDMA self-administration also impaired accuracy and increased omissions when rats performed the 5-CSRTT the day after the last self-administration session, though these effects diminished after the first week of abstinence [62]. Most relevant to this discussion are the studies by Dalley et al, [22, 63], which used extended daily access SA procedures followed by assessment of attention and impulsivity with the 5-CSRTT. In a first set of experiments, rats were given repeated cycles of amphetamine SA (5 × 5 days, 8 hrs) and tested on the 5-CSRTT for nine consecutive days between self-administration cycles. Impairments were found mostly on the attentional component, illustrated by decreased speed and accuracy of responding as well as an increase in omissions, while other parameters were not affected [63]. These impairments typically recovered 4–5 days after withdrawal with no evidence of lasting impairments. Nevertheless, this recovery of function is quite fragile, because subsequent amphetamine challenges with 0.2–1.6 mg/kg (ip) revealed deficits in rats with previous self-administration experience. Specifically, amphetamine experienced animals had increased omissions, slower response times, and decreased impulsivity at the higher end of the dose range.
In regards to meth, an even more pronounced attentional deficit exists based on 5-CSRTT performance. Dalley et al [22] directly compared MDMA, d-amphetamine, and meth. Rats were initially trained on the attentional task before self-administration training with amphetamine, meth, or MDMA for 3 weeks (8 hrs daily). During withdrawal from meth, attention was impaired as evidenced by decreased accuracy, together with increased omission response latencies, while impulsive premature responding was transiently increased [22]. Although amphetamine and MDMA also resulted in impairments, deficits by these drugs were only apparent for a few days, consistent with other findings [62].
Altogether, it appears that dose, contingency and length of drug administration constitute critical factors to understand discrepancies in meth-induced effects on attentional performance, in both ASST and 5-CSRTT. Further studies are needed to elucidate the specific impairments of meth compared to other amphetamine-like drugs, as well as the structures involved. Indeed, while most studies investigating the effects of drugs of abuse on the 5-CSRTT have focused on impulsivity and inhibitory control (see [47] for a review), less interest has been accorded to attentional deficits induced by drugs of abuse in this task. Prefrontal corticostriatal connections and dopaminergic regulation, largely implicated in impulsivity, are also involved in attentional performance in the 5-CSRTT, as reviewed by Robbins [42]. In addition to dopamine, dysregulation of other neurotransmitter systems following meth may also contribute to performance on the task. Meth alters serotonin levels [64, 65] and most studies agree that the serotoninergic system mediates impulsivity rather than the attentional aspect of the 5-CSRTT [66–71] (but see [72, 73]). In contrast, acetylcholine and norepinephrine levels are changed during the 5-CSRTT [74], and both locus coeruleus [75] and basal forebrain nuclei [76] have been shown to constitute critical brain components driving attention in the 5-CSRTT. The impact of meth self-administration on these systems and brain areas are not completely known, but it is clear that meth impinges on cholinergic [77, 78] and noradrenergic [79, 80] structures, so these systems might further underlie meth-induced attentional deficits. It would be therefore interesting to test the role of these structures and their cortical outputs after acute or prolonged meth administration.
4. Recognition Memory
In humans, episodic memory is a form of declarative memory that implies the use of previously acquired autobiographic information for conscious recall [81]. Meth impacts this particular type of memory [3], and this impairment has been proposed to contribute to relapse in meth addicts [82]. For example, addicts that had just relapsed for meth had higher deficits in episodic memory than abstinent meth addicts, and also when compared to individuals who persisted in meth use during the course of the study. Since the possible mechanisms establishing the link between episodic memory deficits and relapse are not yet understood, rodent models constitute a useful tool to investigate to what extent meth impairs episodic memory, and the possible link between these impairments and features of addiction. Although tests of declarative memory are not possible with rodents in the same manner as humans, several rodent models have been proposed to mirror components of episodic memory, including object recognition tasks.
4.1 Object Recognition Memory
Object recognition tasks can be used to evaluate different aspects of working and/or episodic memory depending on the specifics of the task. These tasks are based on the natural tendency of a rat to spend more time interacting with novel stimuli compared to familiar ones and have the advantages of being one-trial memory tests that do not involve learning a rule or changes in motivational state [83]. The most widely studied protocols are illustrated in Figure 3. Typically, these tasks are conducted in three parts: First, rats are generally acclimated to the testing apparatus before the familiarization phase, since environmental exposure before sampling objects can directly impact the interaction with objects [84]. Then rats go through familiarization with objects (sampling phase) and test for recognition memory.
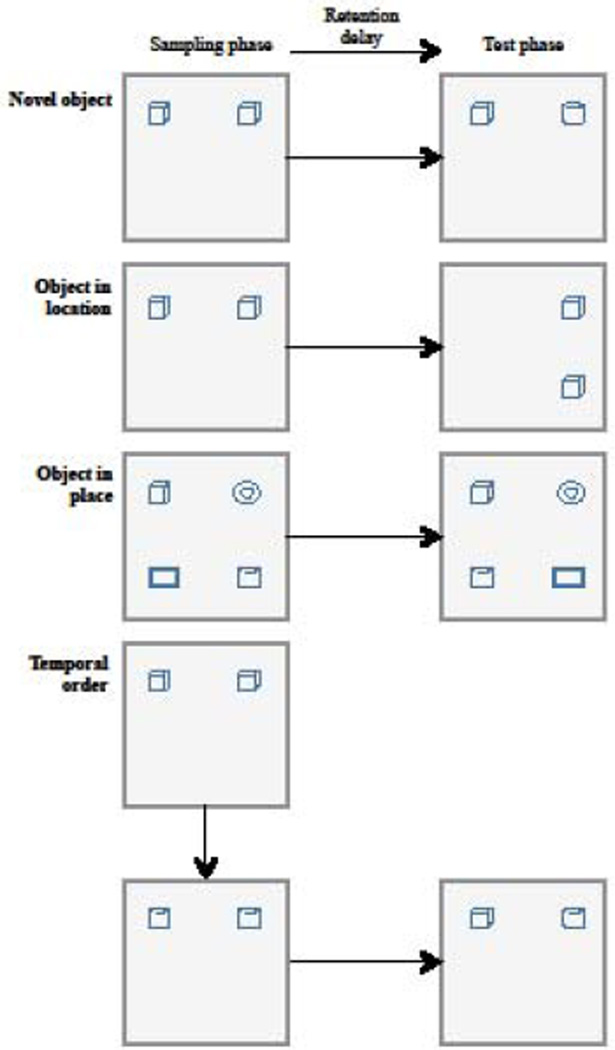
Figure 3. Object recognition memory protocols.
After habituation to the arena, animals explore the objects during an initial sampling phase. After a retention delay, memory is inferred by the amount of time spent interacting with a novel object (first panel), an object in a new location (second panel), or pair of objects that place has been switched (third panel), compared to the unchanged objects. During temporal order recognition (fourth panel), two sampling phases with distinct pairs of objects are followed by a recognition test with both objects. For this latter protocol, memory is inferred by the exploration time of the oldest object. Adapted from [199].
In the novel object recognition task, during the sampling phase, rats are placed into the environment with two identical objects and are allowed to interact with both objects. Rats are then returned to the home cage for a predetermined retention interval. After this time, testing occurs in which rats are placed back into the apparatus with an object from the sampling phase and a novel object. Memory for the sample object is inferred by the amount of time rats spend with the novel object. Other variants of recognition memory protocols assess object location, object-in-place, or temporal order memory (See Figure 3). During object location testing, both objects remain the same between sampling and testing phase, but the location of one of these objects is changed prior to the test. Regarding object-in-place, four distinct objects are presented during the sampling phase; during the test phase, position of two objects is switched, while the two other ones remain in the same place. Memory in these variants is inferred by the amount of time rats explore the object that have changed location or place during the test phase. In the temporal version, a pair of objects is presented during the first sampling phase, followed by a second sampling phase of a different pair of objects. After the retention interval, rats are tested simultaneously with one object from each sampling phase. Here, although none of the objects are completely unfamiliar, memory is assessed through the exploration time of the object presented during the first sampling phase (the oldest one) compared to the object that has been explored more recently.
Recognition memory relies on functional connectivity between the PFC, perirhinal cortex (PRH) and hippocampus, a neurocircuit identified through anatomical tracing studies (for an in depth review see [85]). The PRH cortex has a reciprocal connection to the PFC [86, 87] and projects to the hippocampus, relaying information reciprocally via the entorhinal cortex [88, 89]. Further, there are anatomical connections between the PFC and the hippocampus [90, 91]. The subiculum and CA1 region of the hippocampus are the major output structures projecting to the prefrontal cortex [90, 92]. This connection is not reciprocal in the rat [93, 94], but instead the medial PFC indirectly projects back to the hippocampus via the entorhinal cortex [95].
Lesion and inactivation studies confirm that these brain areas are uniquely involved in recognition memory depending on specific task parameters. Lesions to the PRH cortex impair object recognition memory [96–99], object-in-place recognition memory, and temporal order recognition memory [100, 101], while object location memory is largely unaffected [101, 102]. In contrast, PFC lesions have no effect on object recognition memory [101–104], but do impair temporal order and object-in-place recognition memory [101]. Hippocampal lesions impair object location, object-in-place, and temporal order memory, but not novel object recognition [105]. In summary, the PRH cortex is crucial for judgment of a prior occurrence or object, but when the task involves multiple items and/or contextual associations, recognition depends on interactions between the PRH, PFC, and hippocampus.
4.2 Chronic meth SA disrupts cortical control of recognition memory
Meth impairs object recognition under several different drug administration procedures. For example, an experimenter-administered binge regimen (4 injections of 4 mg/kg at 2 hr intervals) of meth severely impaired long and short-term recognition memory in rats after 1 or 3 weeks of withdrawal [106]. Additionally, rats sensitized to meth (3 mg/kg on alternating days for a total of 10 injections) had impaired object recognition memory one week later. The deficit was not complete, as both meth-sensitized rats and control subjects had significantly more interaction with the novel object; however, control subjects interacted with the novel object significantly more than meth-treated rats.
Our laboratory has found that LgA meth SA impaired novel object recognition [23]. In the first demonstration, a non-traditional object recognition was used to assess multiple components of recognition memory. In that task, rats were familiarized to 5 objects to incorporate both a test of object-in-place and a subsequent introduction of a novel object in a final test session. Tests were conducted both pre- and post-meth self-administration. Spatial reconfiguration remained unaffected; however, novel object interaction decreased depending on cumulative daily meth exposure (1, 2, or 6-hr/day), with 6-hr/day subjects showing significant deficits relative to saline controls. This study was the first to demonstrate that a translationally relevant meth SA procedure impaired object recognition memory. In subsequent studies, we have consistently found that LgA meth SA impairs short and long-term recognition memory in a traditional two-item novel object recognition task in both males and females [107, 108], as well as in an object-in-place memory [15, 109]. Given the extensive schedule of drug self-administration in LgA rats, it is possible that LgA meth rats have elevated anxiety during withdrawal that could interfere with expression of object memory [110, 111]. Enthusiasm for an anxiety account is, however, dampened because in our studies, LgA meth rats have not differed from control rats on thigmotaxis or motor activity when introduced to the novel environment (unpublished data from our lab), nor do they differ in object exploration during sampling phase [107, 108, 109, 118].
We suggest that the PFC and PRH might be particularly vulnerable to damage by LgA meth SA. Chronic meth dysregulates serotonin, cannabinoids, and glutamate in the PRH. Among these changes are reduced levels of CB1 receptors [112] and reduced monoamine transporters [15, 113] in the PRH. Noteworthy, meth-induced reduction of monoamine transporters in this region has been correlated with object recognition deficits [114]. Regarding glutamate receptors, LgA meth decreased expression of mGluR5 and GluN2b in a crude membrane fraction of the PRH cortex after 2 weeks of abstinence [107, 118]. These receptors are important in recognition memory, as blockade of both mGluR5 and GluN2b containing NMDA receptors before sampling objects impairs memory after a 24-hour interval [115, 116]. Interestingly, interactions between mGluR5 and GluN2b form a unique type of long-term depression in the PRH [117]. Moreover, LgA meth SA disrupts this PRH long-term depression, which can be restored by PRH micro-infusion of D-cycloserine [118]. Thus, targeting meth-induced alterations of PRH glutamatergic receptors and the related neurophysiology may be a promising approach to treat working memory deficits following meth abuse.
In the PFC, chronic meth SA decreased gliogenesis, altered neuronal firing states, and reduced glutamate homeostasis [32, 119, 120]. Similar reductions in glutamate homeostasis in the nucleus accumbens have been reported after cocaine self-administration, and are attributed in part to a reduction in the cysteine-glutamate exchanger [121, 122]. This reduction could result in decreased basal extra synaptic glutamate levels, and subsequent reduced glutamatergic tone on presynaptic mGluR2/3 receptors [123]. A similar mechanism may occur in the PFC of meth SA rats, since mGluR2/3 receptors show downregulation following 14 days of abstinence [124]. mGluR2/3 receptors are one of the most abundant presynaptic glutamate receptors found on corticostriatal glutamatergic terminals [125], and their activation inhibits drug seeking [126, 127]. Conversely, a decrease in the function of this autoreceptor may contribute to increased striatal glutamate release and an enhancement of drug seeking [128, 129]. Further, prefrontal glutamatergic dysregulation may impact recognition memory given that PRH-PFC-hippocampal communications are necessary for some types of recognition memory [101]. Thus, the combined vulnerability of the PFC and PRH to repeated meth exposure could be considered as a prominent target to investigate cognitive deficits during abstinence and the propensity towards relapse.
5. Chronic meth SA neurotoxicity in subcortical areas
The cognitive impairments reviewed above focused on cortical dysfunction. However, extended meth access could also affect subcortical areas to induce cognitive sequelae. Standard markers of neurotoxicity after meth include marked reduction of dopamine and serotonin levels, reduction in striatal monoamine transporters, and gliosis. As reviewed by Krasnova and colleagues, repeated meth injections result in consistent signs of neurotoxicity in subcortical regions [14]. In particular, “binge” meth regimens have profound effects on dopaminergic striatal neuron integrity, including marked reduction of striatal dopamine transporters and monoamine levels [15, 54, 130–133]. However, some discrepant findings have emerged regarding LgA meth on subcortical toxicity. After LgA meth exposure, some studies report reduced dopamine transporters (DAT) levels in the striatum following protracted abstinence (between 7 and 30 days) [24, 134, 135], while other studies report no changes in neurotoxic markers in this region [15, 136]. Interestingly, increased DAT levels were reported after a short abstinence period (3 days) [137], suggesting dynamic regulation of striatal dopaminergic processing during withdrawal. Regarding signs of gliosis, increased levels of glial fibrillary acidic protein (GFAP) are thought to indicate neurotoxicity by reflecting the accumulation of glial filaments in response to neuronal injury [138]. Most studies report no change in striatal GFAP after LgA meth SA [15, 24, 135]. Although one group reports increased GFAP levels in the striatum [134], it should be noted that this study used a particularly extended LgA meth SA protocol (15h/day, compared to 6–8 hours in other studies). Interestingly, progressively increasing doses of meth protect against further meth-induced neurotoxicity, a phenomenon known as meth-preconditioning [139, 140]. This effect may account for discrepancies in subcortical neurotoxicity found after “binge” regimens of meth but not after SA protocols during which meth intake might be progressively developed.
Several lines of evidence report striatal-dependent impairments after a systemic neurotoxic meth regimen (7.5 to 10 mg/kg, 4 injections separated by 2h each), including shifts from action-outcome to stimulus-response strategies [133] or sequential motor learning in a radial maze [132]. Reduced striatal DAT levels are also found with lower meth doses (4 × 2 mg/kg) along with impairments in reversal learning [54]. Since these deficits have been attributed to the neurotoxic effect of meth, it remains to be determined whether similar cognitive alteration could be found following meth SA protocols, in which neurotoxicity might not occur.
LgA meth SA also interferes with hippocampal neurogenesis in the dentate gyrus [141, 142], which seems restricted to dorsal areas of the hippocampus [143]. However, to date, only one study has investigated the behavioral consequences of meth-induced reduction of hippocampal neurogenesis by comparing ShA and LgA meth rats [144]. In this study, rats were assessed for spatial and working memory using a Y-maze and T-maze, respectively, following ShA or LgA meth SA. These rats were further injected with BrdU, a substance used to label neuronal progenitors in the hippocampus, after the last SA session and were killed either 2 hours or 28 days after the injection. Both spatial and working memory was impaired in LgA, but not ShA rats, and these deficits were negatively correlated with 2-hour labelling of neuronal progenitors. Additionally, this study reported higher meth-primed reinstatement in LgA rats, associated with increased Fos expression in the dentate gyrus, which could suggest a direct implication of the hippocampus in addiction-like behaviors. Given the emerging implication of hippocampal neurogenesis in psychostimulant addiction [145], further investigations of LgA meth-induced alterations in the hippocampus are merited.
6. Medications that reduce cognitive deficits without abuse potential
Some cognitive and neuronal deficits induced by meth appear to recover with time [6, 146]. However, in the interim, cognitive dysfunction may distract users from treatment compliance as addicts often report continued use of meth to “make them feel normal” [147]. Unfortunately, other cognitive impairments seem to persist even after protracted period of abstinence. Besides displaying even more cognitive impairments than opiate abusers, meth addicts did not differ from 1-year abstinent meth users on cognitive tasks assessing visual memory and executive function [148]. Moreover, a longitudinal study of meth users seeking outpatient treatment reported that both abstinent meth users (6 months) or those that have recently relapsed were even more impaired on memory tests than continuous meth users [82].
Meth users are a heterogeneous population meriting individualized treatment strategies [149]. Several reviews have detailed the cognitive deficits observed in meth addicts [3, 150] and other stimulant abusers [151, 152]. A general consensus is that prefrontal mediated cognitive deficits interfere with treatment programs aimed at increasing abstinence duration, and measures directed at normalizing these deficits should therefore be implemented in treatment programs [153, 154]. In regards to cognitive deficits, cognitive-behavioral therapies designed to improve attention, memory, problem solving, and spatial skills have had some success in both inpatient and outpatient settings [151]. However, development of pharmacotherapies aimed specifically at improving cognitive deficits during abstinence from meth is currently in the nascent stages.
To date, there are no medications that have been approved for psychostimulant abuse, let alone pharmacotherapies that target specific aspects of relapse such as the attenuation of drug reward or cognitive impairments [155]. Many drugs have been suggested and reviewed for the treatment of meth addiction [156–158], but most of the suggested compounds have only been assessed off-label. For example, bupropion blocks dopamine, serotonin, and norepinephrine reuptake, and is approved for smoking cessation, attention deficit hyperactivity disorder, and depression. In regards to meth, bupropion increased days of abstinence in mild to moderate users [159], and decreased meth intake in rodents [160]. Mirtazapine, another antidepressant acting on norepinephrine and serotonin systems [161], has shown some potential in clinical trials [162]. A recent review promoted cholinergic and noradrenergic-based pharmacotherapies, due to the cognitive enhancing properties of many of these compounds [152, 153, 163]. Cholinesterase inhibitors are generally well tolerated [163] and attenuated some of meth’s subjective effects [164, 165], but have not been studied systematically in abstinent meth users. Norepinephrine reuptake inhibitors such as atomoxetine enhance synaptic norepinephrine and dopamine levels in the PFC [165]. To date, this drug has not been tested in clinical trials of psychostimulant abusers [153]. However, although the use of atomoxetine in stimulant-dependent patients does not pose any untoward health risks, the usefulness of this treatment on meth’s subjective effects or abstinence promotion have been called into question [166].
Another drug, modafinil, shows clear and growing promise as a pharmacotherapy for meth addiction [167, 168]. Despite sharing a similar mechanism with amphetamine via inhibition of dopamine reuptake through its transporter [169], the effects of modafinil are more complex, involving a global regulation of all the main neurotransmitter systems in the brain [170], and may further have some antioxidative and neuroprotective effects [171]. The clinical data shows modafinil to be safe for use in meth addicts [167, 172] and suggests some efficacy for treating meth addiction. Selective populations show more promising results including subjects with access to counseling [168], baseline high-frequency meth use, and low cognitive behavioral therapy attendance [173]. Consistently, modafinil alleviates working memory deficits in meth users demonstrating relatively poor baseline performance [174].
In rodents, acute modafinil decreased responding on a drug-associated lever following reinstatement conditions induced by either context, conditioned cue and meth prime, without reinstating meth seeking when tested alone [175, 176], suggesting little or no abuse liability. Modafinil also restored memory function during abstinence from LgA meth SA, by reversing meth-induced deficits in an object-in-place task that requires intact function between the PFC and PRH [109]. Modafinil may therefore rectify impaired pre-frontal cortical ability to integrate object and location information in meth-experienced rats. As such, modafinil is one of the first cognitive enhancing drugs to alleviate both cognitive impairments and relapse in animal models [109, 175, 176]. Further, based on data using a cocaine SA model, this combined effect may be due to the ability of modafinil to increase extracellular glutamate during abstinence and interactions with mGluR2/3 receptors [177].
Glutamate has been well implicated in relapse to multiple types of addictive drugs [178, 179], with the most extensive evidence having been found in regards to cocaine addiction. Cocaine withdrawal causes reductions in basal extracellular glutamate concentrations [122]. Additionally, the glutamate/cystine antiporter, which regulates basal glutamate levels, shows downregulation during withdrawal [180, 181]. N-acetylcysteine (NAC) is a cystine prodrug that restores basal glutamate levels, blocks cocaine-primed reinstatement of drug seeking, and attenuates increased glutamate levels during reinstatement by reversing glutamate dysregulation due to cocaine self-administration in rats [123, 128, 180]. This drug further has antioxidant properties that have been shown to prevent neurotoxicity and improves memory performance in rodents [182, 183]. Although not tested within a meth SA procedure, NAC protects against experimenter-delivered methamphetamine-induced toxicity in primates and rodents [184, 185]. In humans, NAC reduces cocaine desire [186], and a reduction of oxidative stress in human brain endothelial cells following NAC treatment confirms preclinical evidence on possible neuroprotective effects [187]. Therefore, one could propose that protection from meth-induced neurotoxicity could help alleviate its cognitive sequelae, and possibly maintain abstinence. However, NAC was administered in these studies before meth injections, an approach not relevant to the human drug-taking situation whereby treatment occurs after years of repeated meth intoxication. Until now, the treatment efficacy of NAC has been mixed regardless of drug class [188]. For example NAC treatment combined with naltrexone had no effect on meth abusers [189], but a recent study by Mousavi and colleagues (2015) found that NAC reduced craving for meth [190]. Within that study, meth addicts benefited from a weekly cognitive and psychological approach according to the Matrix model [191], thus highlighting the importance of combining different therapeutic approaches to achieve potent results in the treatment of addictive disorders.
7. Concluding remarks
The studies reviewed here highlight some of the key cognitive domains as a result of extended access to methamphetamine self-administration. The impairments in attentional processing (5-choice serial reaction time task), cognitive set-shifting, and object recognition memory pinpoint a particular vulnerability in cortical areas to meth-induced dysregulation (Figure 4). The use of contingent models of meth self-administration show that long access, but not short access to meth, is more likely to produce such deficits. Extended access to meth self-administration may therefore represent a good framework to further evaluate drug-induced cortical dysfunction and cognitive impairments in the context of drug addiction. It is also likely that subcortical areas are involved in many of these cognitive tasks, notably through cortical-subcortical connections. Subcortical structural integrity and function may also be affected by extended meth self-administration, although only one study has so far investigated the role of subcortical alterations in drug-induced cognitive dysfunction [144]. Future studies should therefore address how key structures such as the dorsal and ventral striatum are affected by extended access to self-administered meth. Although both contingent and non-contingent meth administration could sometimes result in similar cognitive consequences (e.g., object recognition), some distinct neurochemical alterations between these two models suggest that striatal neurotoxicity may not be sufficient to explain meth-induced cognitive deficits. Noteworthy, the schedule and duration of meth SA may drive specific neurochemical alterations and related cognitive impairments that deserve future investigations.
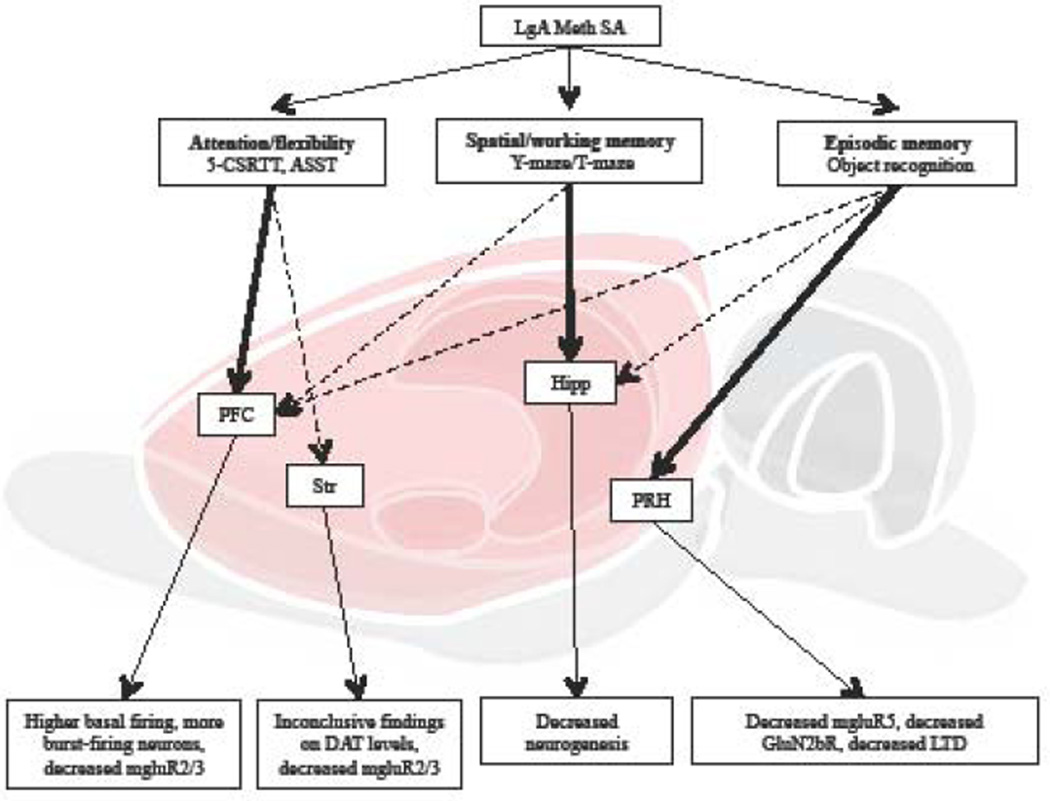
Figure 4. Summary of cognitive deficits after extended meth self-administration.
Among the few studies investigating cognitive alterations induced by LgA meth SA, some vulnerable structures have been reported (bold arrows), while other related structures have not been fully explored (dotted arrows). The lower level depicts neurophysiological alterations that have been corroborated with LgA meth-induced cognitive impairments, in the PFC [32, 124], striatum [15, 24, 124], hippocampus [142–144] and perirhinal cortex [107, 109, 118]. 5-CSRTT: five-choice serial reaction time task; ASST: Attention set-shifting task; PFC: prefrontal cortex; Str: striatum; Hipp: hippocampus; PRH: perirhinal cortex; DAT: dopamine active transporter; LTD: long term depression.
While this review focused on the consequences of extended meth use in preclinical models, individual predispositions before the onset of drug use also represent a critical factor in the etiology of drug addiction. For instance, several endophenotypes have been proposed to explain interindividual variability to drug addiction [192–194]. On the other hand, these pre-existing individual specificities may also account for the beneficial effects on cognition provided by some psychostimulants. For instance, amphetamine-like substances such as methylphenidate produce opposite brain activation pattern between ADHD and healthy children [195], and the effectiveness of this treatment has further been corroborated with striatal DAT bioavailability [196, 197]. Similarly in rats, methylphenidate increased impulsivity in rats characterized as low-impulsive in the 5-CSRTT, but decreased impulsivity of pre-established high-impulsive rats [198]. To our knowledge, no study has yet investigated how attentional performance or recognition memory could constitute predisposing factors for drug abuse. Nonetheless, it would be important to assess to what extent drug-induced cortical alterations occur in relation with specific traits present before the onset of drug abuse.
Although meth abuse in humans has been correlated with cognitive decline, it has been questioned to what extent these cognitive deficits might fall outside the range of significant clinical impairment [10]. Another recent review highlighted the important of inter-individual variability in meth users undergoing psychological assessment, suggesting that cognitive deficits should be highly attributable to meth abuse in vulnerable individuals [11]. This heterogeneity emphasizes an emerging area of research to focus on inter-individual variability of meth-induced deficits. Ultimately, newer studies focused on the ability of meth to disrupt the entire circuits involved in attention, impulsivity, and memory will help inform clinical researchers aimed at using cognitive enhancers as treatments for relapse.
Highlights.
-
-
Human methamphetamine abusers exhibit cognitive deficits to varying degrees
-
-
Extended meth access in rats impairs attentional processing and recognition memory
-
-
These deficits result from cortical alterations following extended meth access
-
-
Other cognitive dimensions and related structures are largely unexplored
-
-
Medication restoring these deficits may help to maintain abstinence in meth addicts
Acknowledgments
Support for this review was from NIH/NIDA grant R01DA033049.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. The American Journal of Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 2.Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. Journal of Pharmacy Practice. 2011;24:541–550. doi: 10.1177/0897190011426557. [DOI] [PubMed] [Google Scholar]
- 3.Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 4.Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive Impairment in Individuals Currently Using Methamphetamine. The American Journal on Addictions. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. The American Journal of Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 6.Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetamine-dependent individuals. Journal of Substance Abuse Ttreatment. 2009;37:292–297. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- 8.Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, et al. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- 9.Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry. 2014;71:812–820. doi: 10.1001/jamapsychiatry.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology. 2012;37:586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013;38:259–274. doi: 10.1038/npp.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood S, Sage JR, Shuman T, Anagnostaras SG. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol Rev. 2014;66:193–221. doi: 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, et al. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug and Alcohol Dependence. 2013;129:167–179. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Research Reviews. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reichel CM, Ramsey LA, Schwendt M, Mcginty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62:1119–1126. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morein-Zamir S, Robbins TW. Fronto-striatal circuits in response-inhibition: Relevance to addiction. Brain Res. 2014 doi: 10.1016/j.brainres.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura O, Wee S, Specio S, Koob G, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 22.Dalley JW, Lääne K, Theobald DE, Peña Y, Bruce CC, Huszar AC, et al. Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacology. 2007;32:1195–1206. doi: 10.1038/sj.npp.1301220. [DOI] [PubMed] [Google Scholar]
- 23.Rogers J, Santis S, See R. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology. 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwendt M, Rocha A, See R, Pacchioni A, Mcginty J, Kalivas P. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. Journal of Pharmacology And Experimental Therapeutics. 2009;331:555. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 26.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends in Neurosciences. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 30.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 31.Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from "on-line" processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsegian A, Glen WB, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman LA, McGaughy J. Attentional effects of lesions to the anterior cingulate cortex: how prior reinforcement influences distractibility. Behav Neurosci. 2011;125:360–371. doi: 10.1037/a0023250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Bissonette GB, Roesch MR. Rule encoding in dorsal striatum impacts action selection. Eur J Neurosci. 2015;42:2555–2567. doi: 10.1111/ejn.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 37.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. The Journal of Neuroscience. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- 39.Ragozzino ME. The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn Mem. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- 41.Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nature Protocols. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- 42.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 43.Carli M, Invernizzi RW. Serotoninergic and dopaminergic modulation of cortico-striatal circuit in executive and attention deficits induced by NMDA receptor hypofunction in the 5-choice serial reaction time task. Frontiers in Neural Circuits. 2014:8. doi: 10.3389/fncir.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cerebral Cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- 45.Passetti F, Chudasama Y, Robbins TW. The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cerebral Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- 46.Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behavioural Brain Research. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 48.Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behavioral Neuroscience. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- 49.Burton AC, Nakamura K, Roesch MR. From ventral-medial to dorsal-lateral striatum: neural correlates of reward-guided decision-making. Neurobiol Learn Mem. 2015;117:51–59. doi: 10.1016/j.nlm.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, et al. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10:765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- 51.Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, et al. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- 52.Fletcher PJ, Tenn CC, Rizos Z, Lovic V, Kapur S. Sensitization to amphetamine, but not PCP, impairs attentional set shifting: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Psychopharmacology (Berl) 2005;183:190–200. doi: 10.1007/s00213-005-0157-6. [DOI] [PubMed] [Google Scholar]
- 53.Featherstone RE, Rizos Z, Kapur S, Fletcher PJ. A sensitizing regimen of amphetamine that disrupts attentional set-shifting does not disrupt working or long-term memory. Behav Brain Res. 2008;189:170–179. doi: 10.1016/j.bbr.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 54.Izquierdo A, Belcher A, Scott L, Cazares V, Chen J, O'Dell S, et al. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- 57.Mishima K, Fujii M, Aoo N, Yoshikawa T, Fukue Y, Honda Y, et al. The pharmacological characterization of attentional processes using a two-lever choice reaction time task in rats. Biol Pharm Bull. 2002;25:1570–1576. doi: 10.1248/bpb.25.1570. [DOI] [PubMed] [Google Scholar]
- 58.Slezak JM, Katz JL. An influence of delayed reinforcement on the effectiveness of psychostimulants to enhance indices of attention under a five-choice serial reaction time procedure in male rats. Exp Clin Psychopharmacol. 2013;21:355–362. doi: 10.1037/a0033726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puumala T, Ruotsalainen S, Jakala P, Koivisto E, Riekkinen P, Jr, Sirvio J. Behavioral and pharmacological studies on the validation of a new animal model for attention deficit hyperactivity disorder. Neurobiol Learn Mem. 1996;66:198–211. doi: 10.1006/nlme.1996.0060. [DOI] [PubMed] [Google Scholar]
- 60.Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA., Jr Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol Psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 61.Fletcher PJ, Tenn CC, Sinyard J, Rizos Z, Kapur S. A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacology. 2007;32:1122–1132. doi: 10.1038/sj.npp.1301221. [DOI] [PubMed] [Google Scholar]
- 62.Bird J, Schenk S. Contribution of impulsivity and novelty-seeking to the acquisition and maintenance of MDMA self-administration. Addict Biol. 2013;18:654–664. doi: 10.1111/j.1369-1600.2012.00477.x. [DOI] [PubMed] [Google Scholar]
- 63.Dalley JW, Theobald DE, Berry D, Milstein JA, Lääne K, Everitt BJ, et al. Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology. 2005;30:525–537. doi: 10.1038/sj.npp.1300590. [DOI] [PubMed] [Google Scholar]
- 64.Bakhit C, Morgan ME, Peat MA, Gibb JW. Long-term effects of methamphetamine on the synthesis and metabolism of 5-hydroxytryptamine in various regions of the rat brain. Neuropharmacology. 1981;20:1135–1140. doi: 10.1016/0028-3908(81)90053-8. [DOI] [PubMed] [Google Scholar]
- 65.Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- 66.Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- 67.Robinson ES, Dalley JW, Theobald DE, Glennon JC, Pezze MA, Murphy ER, et al. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology. 2008;33:2398–2406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- 68.Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- 69.Harrison AA, Everitt BJ, Robbins TW. Doubly dissociable effects of median- and dorsal-raphe lesions on the performance of the five-choice serial reaction time test of attention in rats. Behav Brain Res. 1997;89:135–149. doi: 10.1016/s0166-4328(97)00053-3. [DOI] [PubMed] [Google Scholar]
- 70.Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT2A and 5-HT2C receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology. 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- 71.Passetti F, Dalley JW, Robbins TW. Double dissociation of serotonergic and dopaminergic mechanisms on attentional performance using a rodent five-choice reaction time task. Psychopharmacology. 2003;165:136–145. doi: 10.1007/s00213-002-1227-7. [DOI] [PubMed] [Google Scholar]
- 72.Carli M, Samanin R. The 5-HT1A receptor agonist 8-OH-DPAT reduces rats’ accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: role of presynaptic 5-HT1A receptors. Psychopharmacology. 2000;149:259–268. doi: 10.1007/s002139900368. [DOI] [PubMed] [Google Scholar]
- 73.Jakala P, Sirvio J, Jolkkonen J, Riekkinen P, Jr, Acsady L, Riekkinen P. The effects of p-chlorophenylalanine-induced serotonin synthesis inhibition and muscarinic blockade on the performance of rats in a 5-choice serial reaction time task. Behav Brain Res. 1992;51:29–40. doi: 10.1016/s0166-4328(05)80309-2. [DOI] [PubMed] [Google Scholar]
- 74.Dalley JW, McGaughy J, O'Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cole BJ, Robbins TW. Forebrain norepinephrine: role in controlled information processing in the rat. Neuropsychopharmacology. 1992;7:129–142. [PubMed] [Google Scholar]
- 76.McGaughy J, Dalley JW, Morrison CH, Everitt BJ, Robbins TW. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. J Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siegel JA, Craytor MJ, Raber J. Long-term effects of methamphetamine exposure on cognitive function and muscarinic acetylcholine receptor levels in mice. Behav Pharmacol. 2010;21:602–614. doi: 10.1097/FBP.0b013e32833e7e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siegel JA, Park BS, Raber J. Methamphetamine exposure during brain development alters the brain acetylcholine system in adolescent mice. J Neurochem. 2011;119:89–99. doi: 10.1111/j.1471-4159.2011.07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cook JD, Schanberg SM. Effect of methamphetamine on norepinephrine metabolism in various regions of brain. J Pharmacol Exp Ther. 1975;195:87–93. [PubMed] [Google Scholar]
- 80.Wang Y, Chou J, Jeng CH, Morales M, Wang JY. Chronic methamphetamine exposure decreases high affinity uptake function in norepinephrine afferents in the cerebellar cortex: an electrophysiological and electrochemical study. Neuropharmacology. 2000;39:2112–2123. doi: 10.1016/s0028-3908(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 81.Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- 82.Simon SL, Dacey J, Glynn S, Rawson R, Ling W. The effect of relapse on cognition in abstinent methamphetamine abusers. Journal of Substance Abuse Treatment. 2004;27:59–66. doi: 10.1016/j.jsat.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 83.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 84.Besheer J, Bevins RA. The role of environmental familiarization in novel-object preference. Behav Processes. 2000;50:19–29. doi: 10.1016/s0376-6357(00)00090-5. [DOI] [PubMed] [Google Scholar]
- 85.Kealy J, Commins S. The rat perirhinal cortex: A review of anatomy, physiology, plasticity, and function. Progress in Neurobiology. 2011;93:522–548. doi: 10.1016/j.pneurobio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 86.Deacon TW, Eichenbaum H, Rosenberg P, Eckmann KW. Afferent connections of the perirhinal cortex in the rat. The Journal of Comparative Neurology. 1983;220:168–190. doi: 10.1002/cne.902200205. [DOI] [PubMed] [Google Scholar]
- 87.Delatour B, Witter MP. Projections from the parahippocampal region to the prefrontal cortex in the rat: evidence of multiple pathways. Eur J Neurosci. 2002;15:1400–1407. doi: 10.1046/j.1460-9568.2002.01973.x. [DOI] [PubMed] [Google Scholar]
- 88.Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- 89.van Strien NM, Cappaert NLM, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 90.Naber PA, Witter MP. Subicular efferents are organized mostly as parallel projections: a double-labeling, retrograde-tracing study in the rat. J Comp Neurol. 1998;393:284–297. [PubMed] [Google Scholar]
- 91.Thierry AM, Gioanni Y, Dégénétais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 92.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. The Journal of Comparative Neurology. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 93.Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- 94.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. The Journal of Comparative Neurology. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 95.Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. The Journal of Comparative Neurology. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 96.Mumby DG, Pinel JP. Rhinal cortex lesions and object recognition in rats. Behavioral Neuroscience. 1994;108:11–18. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- 97.Ennaceur A, Aggleton JP. The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res. 1997;88:181–193. doi: 10.1016/s0166-4328(97)02297-3. [DOI] [PubMed] [Google Scholar]
- 98.Bussey TJ, Muir JL, Aggleton JP. Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J Neurosci. 1999;19:495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Norman G, Eacott MJ. Impaired object recognition with increasing levels of feature ambiguity in rats with perirhinal cortex lesions. Behav Brain Res. 2004;148:79–91. doi: 10.1016/s0166-4328(03)00176-1. [DOI] [PubMed] [Google Scholar]
- 100.Hannesson DK, Howland JG, Phillips AG. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci. 2004;24:4596–4604. doi: 10.1523/JNEUROSCI.5517-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barker GRI, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. Journal of Neuroscience. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 103.Warburton EC, Brown MW. Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia. 2009;48:2262–2272. doi: 10.1016/j.neuropsychologia.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 104.Mitchell JB, Laiacona J. The medial frontal cortex and temporal memory: tests using spontaneous exploratory behaviour in the rat. Behavioural Brain Research. 1998;97:107–113. doi: 10.1016/s0166-4328(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 105.Barker GRI, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marshall JF, O'Dell SJ. Methamphetamine influences on brain and behavior: unsafe at any speed? Trends in Neurosciences. 2012;35:536–545. doi: 10.1016/j.tins.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology. 2012;223:371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reichel CM, Gilstrap MG, Ramsey LA, See RE. Modafinil restores methamphetamine induced object-in-place memory deficits in rats independent of glutamate N-methyl-d-aspartate receptor expression. Drug and Alcohol Dependence. 2014;134:115–122. doi: 10.1016/j.drugalcdep.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. PNAS. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning & Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bortolato M, Frau R, Bini V, Luesu W, Loriga R, Collu M, et al. Methamphetamine neurotoxicity increases brain expression and alters behavioral functions of CB(1) cannabinoid receptors. Journal of Psychiatric Research. 2010 doi: 10.1016/j.jpsychires.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 113.Belcher A, O'dell S, Marshall J. Impaired Object Recognition Memory Following Methamphetamine, but not p-Chloroamphetamine- or d-Amphetamine-Induced Neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- 114.Belcher AM, Feinstein EM, O'Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- 115.Barker G. A temporally distinct role for group I and group II metabotropic glutamate receptors in object recognition memory. Learning & Memory. 2006;13:178–186. doi: 10.1101/lm.77806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barker G, Warburton E. NMDA Receptor Plasticity in the Perirhinal and Prefrontal Cortices Is Crucial for the Acquisition of Long-Term Object-in-Place Associative Memory. Journal of Neuroscience. 2008;28:2837–2844. doi: 10.1523/JNEUROSCI.4447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cho K, Kemp N, Noel J, Aggleton JP, Brown MW, Bashir ZI. A new form of long-term depression in the perirhinal cortex. Nat Neurosci. 2000;3:150–156. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- 118.Scofield MD, Trantham-Davidson H, Schwendt M, Leong KC, Peters J, See RE, et al. Failure to recognize novelty after extended methamphetamine self-administration results from loss of long-term depression in the perirhinal cortex. Neuropsychopharmacology. 2015;40:2526–2535. doi: 10.1038/npp.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mandyam C, Wee S, Eisch A, Richardson H, Koob G. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. Journal of Neuroscience. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology. 2014;39:811–822. doi: 10.1038/npp.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baker DA, McFarland K, Lake RW, Shen H, Tang X-C, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 122.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 124.Schwendt M, Reichel CM, See RE. Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self-administration in rats. PLoS One. 2012;7:e34299. doi: 10.1371/journal.pone.0034299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 126.Li X, Li J, Gardner EL, Xi Z-X. Activation of mGluR7s inhibits cocaine-induced reinstatement of drug-seeking behavior by a nucleus accumbens glutamate-mGluR2/3 mechanism in rats. Journal of Neurochemistry. 2010;114:1368–1380. doi: 10.1111/j.1471-4159.2010.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. Journal of Neuroscience. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xi Z-X, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. The Journal of Pharmacology and Experimental Therapeutics. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- 130.Son JH, Kuhn J, Keefe KA. Perseverative behavior in rats with methamphetamine-induced neurotoxicity. Neuropharmacology. 2013;67:95–103. doi: 10.1016/j.neuropharm.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pastuzyn ED, Chapman DE, Wilcox KS, Keefe KA. Altered learning and arc-regulated consolidation of learning in striatum by methamphetamine-induced neurotoxicity. Neuropsychopharmacology. 2012;37:885–895. doi: 10.1038/npp.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 133.Son JH, Latimer C, Keefe KA. Impaired formation of stimulus-response, but not action-outcome, associations in rats with methamphetamine-induced neurotoxicity. Neuropsychopharmacology. 2011;36:2441–2451. doi: 10.1038/npp.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, et al. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, et al. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J Pharmacol Exp Ther. 2012;340:295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shepard JD, Chuang DT, Shaham Y, Morales M. Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacology (Berl) 2006;185:505–513. doi: 10.1007/s00213-006-0316-4. [DOI] [PubMed] [Google Scholar]
- 137.D'Arcy C, Luevano JE, Miranda-Arango M, Pipkin JA, Jackson JA, Castaneda E, et al. Extended access to methamphetamine self-administration up-regulates dopamine transporter levels 72hours after withdrawal in rats. Behav Brain Res. 2015;296:125–128. doi: 10.1016/j.bbr.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O’Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert opinion on drug safety. 2005;4:433–442. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- 139.Johnson-Davis KL, Fleckenstein AE, Wilkins DG. The role of hyperthermia and metabolism as mechanisms of tolerance to methamphetamine neurotoxicity. Eur J Pharmacol. 2003;482:151–154. doi: 10.1016/j.ejphar.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 140.Cadet JL, Krasnova IN, Ladenheim B, Cai NS, McCoy MT, Atianjoh FE. Methamphetamine preconditioning: Differential protective effects on monoaminergic systems in the rat brain. Neurotox Res. 2009;15:252–259. doi: 10.1007/s12640-009-9026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yuan CJ, Quiocho JM, Kim A, Wee S, Mandyam CD. Extended access methamphetamine decreases immature neurons in the hippocampus which results from loss and altered development of neural progenitors without altered dynamics of the S-phase of the cell cycle. Pharmacol Biochem Behav. 2011;100:98–108. doi: 10.1016/j.pbb.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Galinato MH, Orio L, Mandyam CD. Methamphetamine differentially affects BDNF and cell death factors in anatomically defined regions of the hippocampus. Neuroscience. 2015;286:97–108. doi: 10.1016/j.neuroscience.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Recinto P, Samant AR, Chavez G, Kim A, Yuan CJ, Soleiman M, et al. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology. 2012;37:1275–1287. doi: 10.1038/npp.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Volkow ND, Chang L, Wang G-J, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bungay V, Malchy L, Buxton J, Johnson J, Macpherson D, Rosenfeld T. Life with jib: A snapshot of street youth's use of crystal methamphetamine. Addiction Research and Theory. 2006;14:235–251. [Google Scholar]
- 148.Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Newton TF, De La Garza R, Kalechstein AD, Tziortzis D, Jacobsen CA. Theories of addiction: methamphetamine users' explanations for continuing drug use and relapse. Am J Addict. 2009;18:294–300. doi: 10.1080/10550490902925920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- 151.Vocci FJ. Cognitive remediation in the treatment of stimulant abuse disorders: A research agenda. Experimental and Clinical Psychopharmacology. 2008;16:484–497. doi: 10.1037/a0014101. [DOI] [PubMed] [Google Scholar]
- 152.Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brady KT, Gray KM, Tolliver BK. Cognitive enhancers in the treatment of substance use disorders: clinical evidence. Pharmacology, biochemistry, and behavior. 2011;99:285–294. doi: 10.1016/j.pbb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jupp B, Lawrence AJ. New horizons for therapeutics in drug and alcohol abuse. Pharmacology & Therapeutics. 2010;125:138–168. doi: 10.1016/j.pharmthera.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 156.Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;(102 Suppl 1):96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- 157.Ling W, Rawson R, Shoptaw S, Ling W. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8:345–354. doi: 10.1007/s11920-006-0035-x. [DOI] [PubMed] [Google Scholar]
- 158.Rose ME, Grant JE. Pharmacotherapy for methamphetamine dependence: a review of the pathophysiology of methamphetamine addiction and the theoretical basis and efficacy of pharmacotherapeutic interventions. Ann Clin Psychiatry. 2008;20:145–155. doi: 10.1080/10401230802177656. [DOI] [PubMed] [Google Scholar]
- 159.Elkashef A, Rawson R, Anderson A, Li S, Holmes T, Smith E, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 160.Reichel CM, Murray JE, Grant KM, Bevins RA. Bupropion attenuates methamphetamine self-administration in adult male rats. Drug and Alcohol Dependence. 2009:1–9. doi: 10.1016/j.drugalcdep.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607–631. doi: 10.2165/00003495-199957040-00010. [DOI] [PubMed] [Google Scholar]
- 162.Cruickshank CC, Montebello ME, Dyer KR, Quigley A, Blaszczyk J, Tomkins S, et al. A placebo-controlled trial of mirtazapine for the management of methamphetamine withdrawal. Drug & Alcohol Review. 2008;27:326–333. doi: 10.1080/09595230801935672. [DOI] [PubMed] [Google Scholar]
- 163.Dunbar G, Boeijinga P, Demazières A, Cisterni C, Kuchibhatla R, Wesnes K, et al. Effects of TC-1734 (AZD3480), a selective neuronal nicotinic receptor agonist, on cognitive performance and the EEG of young healthy male volunteers. Psychopharmacology. 2007;191:919–929. doi: 10.1007/s00213-006-0675-x. [DOI] [PubMed] [Google Scholar]
- 164.De La Garza R, Mahoney JJ, Culbertson C, Shoptaw S, Newton TF. The acetylcholinesterase inhibitor rivastigmine does not alter total choices for methamphetamine, but may reduce positive subjective effects, in a laboratory model of intravenous self-administration in human volunteers. Pharmacol Biochem Behav. 2008;89:200–208. doi: 10.1016/j.pbb.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 166.Rush CR, Stoops WW, Lile JA, Glaser PEA, Hays LR. Physiological and subjective effects of acute intranasal methamphetamine during atomoxetine maintenance. Pharmacology Biochemistry and Behavior. 2011;100:40–47. doi: 10.1016/j.pbb.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McGaugh J, Mancino MJ, Feldman Z, Chopra MP, Gentry WB, Cargile C, et al. Open-label pilot study of modafinil for methamphetamine dependence. Journal of Clinical Psychopharmacology. 2009;29:488–491. doi: 10.1097/JCP.0b013e3181b591e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J, et al. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 169.Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mereu M, Bonci A, Newman AH, Tanda G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology (Berl) 2013;229:415–434. doi: 10.1007/s00213-013-3232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gerrard P, Malcolm R. Mechanisms of modafinil: A review of current research. Neuropsychiatr Dis Treat. 2007;3:349–364. [PMC free article] [PubMed] [Google Scholar]
- 172.De La Garza R, Zorick T, London E, Newton T. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug and Alcohol Dependence. 2009;106:173–180. doi: 10.1016/j.drugalcdep.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Heinzerling KG, Swanson A-N, Kim S, Cederblom L, Moe A, Ling W, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2010;109:20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kalechstein AD, De La Garza R, Newton TF. Modafinil administration improves working memory in methamphetamine-dependent individuals who demonstrate baseline impairment. Am J Addict. 2010;19:340–344. doi: 10.1111/j.1521-0391.2010.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology. 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Reichel CM, See RE. Chronic modafinil effects on drug-seeking following methamphetamine self-administration in rats. Int J Neuropsychopharmacol. 2011:1–11. doi: 10.1017/S1461145711000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mahler SV, Hensley-Simon M, Tahsili-Fahadan P, Lalumiere RT, Thomas C, Fallon RV, et al. Modafinil attenuates reinstatement of cocaine seeking: role for cystine-glutamate exchange and metabotropic glutamate receptors. Addiction Biology. 2012 doi: 10.1111/j.1369-1600.2012.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Current Opinion in Pharmacology. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Baker DA, Xi Z, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci. 2003;1003:349–351. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- 182.Prakash A, Kumar A. Effect of N-acetyl cysteine against aluminium-induced cognitive dysfunction and oxidative damage in rats. Basic Clin Pharmacol Toxicol. 2009;105:98–104. doi: 10.1111/j.1742-7843.2009.00404.x. [DOI] [PubMed] [Google Scholar]
- 183.Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 184.Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Shimizu E, et al. Protective effects of N-acetyl-L-cysteine on the reduction of dopamine transporters in the striatum of monkeys treated with methamphetamine. Neuropsychopharmacology. 2004;29:2018–2023. doi: 10.1038/sj.npp.1300512. [DOI] [PubMed] [Google Scholar]
- 185.Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, Iyo M. Effect of antioxidant N-acetyl-L-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Res. 2004;1016:90–95. doi: 10.1016/j.brainres.2004.04.072. [DOI] [PubMed] [Google Scholar]
- 186.LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, Mcrae A, et al. Is cocaine desire reduced by N-acetylcysteine? The American Journal of Psychiatry. 2007;164:1115–1157. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 187.Zhang X, Banerjee A, Banks WA, Ercal N. N-Acetylcysteine amide protects against methamphetamine-induced oxidative stress and neurotoxicity in immortalized human brain endothelial cells. Brain Res. 2009;1275:87–95. doi: 10.1016/j.brainres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Deepmala, Slattery J, Kumar N, Delhey L, Berk M, Dean O, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci Biobehav Rev. 2015;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 189.Grant JE, Odlaug BL, Kim SW. A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol. 2010;20:823–828. doi: 10.1016/j.euroneuro.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 190.Mousavi SG, Sharbafchi MR, Salehi M, Peykanpour M, Karimian Sichani N, Maracy M. The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: a double-blind controlled, crossover study. Arch Iran Med. 2015;18:28–33. [PubMed] [Google Scholar]
- 191.Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99:708–717. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 192.Jupp B, Dalley JW. Behavioral endophenotypes of drug addiction: Etiological insights from neuroimaging studies. Neuropharmacology. 2014;(76 Pt B):487–497. doi: 10.1016/j.neuropharm.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 193.Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proceedings of the National Academy of Sciences. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Krause J, la Fougere C, Krause K-H, Ackenheil M, St HD. Influence of striatal dopamine transporter availability on the response to methylphenidate in adult patients with ADHD. European Archives of Psychiatry and Clinical Neuroscience. 2005;255:428–431. doi: 10.1007/s00406-005-0602-x. [DOI] [PubMed] [Google Scholar]
- 197.Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neuroscience Letters. 2000;285:107–110. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- 198.Caprioli D, Jupp B, Hong YT, Sawiak SJ, Ferrari V, Wharton L, et al. Dissociable rate-dependent effects of oral methylphenidate on impulsivity and D2/3 receptor availability in the striatum. The Journal of Neuroscience. 2015;35:3747–3755. doi: 10.1523/JNEUROSCI.3890-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Brown MW, Barker GR, Aggleton JP, Warburton EC. What pharmacological interventions indicate concerning the role of the perirhinal cortex in recognition memory. Neuropsychologia. 2012;50:3122–3140. doi: 10.1016/j.neuropsychologia.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]