Abstract
We present the assessment of predictions submitted in the template-based modeling (TBM) category of CASP11 (Critical Assessment of Protein Structure Prediction). Model quality was judged on the basis of global and local measures of accuracy on all atoms including side chains. The top groups on 39 human-server targets based on model 1 predictions were LEER, Zhang, LEE, MULTICOM, and Zhang-Server. The top groups on 81 targets by server groups based on model 1 predictions were Zhang-Server, nns, BAKER-ROSETTASERVER, QUARK, and myprotein-me. In CASP11, the best models for most targets were equal to or better than the best template available in the Protein Data Bank, even for targets with poor templates. The overall performance in CASP11 is similar to the performance of predictors in CASP10 with slightly better performance on the hardest targets. For most targets, assessment measures exhibited bimodal probability density distributions. Multi-dimensional scaling of an RMSD matrix for each target typically revealed a single cluster with models similar to the target structure, with a mode in the GDT-TS density between 40 and 90, and a wide distribution of models highly divergent from each other and from the experimental structure, with density mode at a GDT-TS value of ~20. The models in this peak in the density were either compact models with entirely the wrong fold, or highly non-compact models. The results argue for a density-driven approach in future CASP TBM assessments that accounts for the bimodal nature of these distributions instead of Z-scores, which assume a unimodal, Gaussian distribution.
Keywords: protein structure prediction, CASP, template-based modeling, homology modeling, structural biology
Introduction
Template-based modeling is the most basic and widespread task in protein structure prediction and modeling. Papers describing such programs as Modeller,1 Swiss-Model,2 I-TASSER,3 and SCWRL4 have been cited 1000s of times each, the vast majority of which are applications of these programs in molecular and cellular biology. The basic protocol established in the 1970s and 1980s5,6 has remained the same: identifying one or more proteins of known structure homologous to the target protein; aligning the sequence of the target to the sequences and structures of these proteins; building backbone and side-chain coordinates according to this alignment, while filling in insertions and repairing deletions due to gaps in the sequence alignment(s); refinement of the coordinates of the model to account for potential differences in orientation or distance between substructures in the target compared with the template(s); and assessment of model quality. The rapidly increasing number of sequences homologous to any target and the development of powerful database search programs based on sequence profiles or HMMs7 have made the identification and alignment steps robust and relatively straightforward, although ambiguous alignments are sometimes encountered resulting in substantial errors in models. Accurate loop modeling and refinement remain challenging,8 particularly the building of larger substructures (some including secondary structure) present in the target but not present in any of the available templates.
Template-based modeling has been a component in the CASP experiments since CASP1, and the relevant assessment papers provide a historical progression of the state of the field over the last 20 years.9–23 In the early years, the main challenge was identifying whether there was in fact a template homologous (or even analogous – with the same fold but not related by evolution) to the target protein and producing a reasonable alignment. This challenge was referred to as “fold recognition”24 and was a distinct category from the targets with obvious homologues in the PDB (“comparative modeling”); targets without any similar structures in the PDB were put in the “new fold” category.25–28 Since CASP7, there have been two structure prediction categories, “template-based modeling” targets with both easy and hard-to-identify templates in the PDB (mostly homologues)19 and “free modeling” targets (mostly without homologous templates).29
Numerous measures have been developed over the CASP experiments to assess the template-based modeling categories. Alignment accuracy was used as a measure through CASP821 (determined by calculating a target/prediction sequence alignment that results from sequence-independent structure alignment). In recent years, however, all of the assessment criteria have been based on either sequence-dependent structure alignment or comparing atom-atom distances within the prediction to the same atom pairs in the target experimental structure.30 The former category includes the global measures GDT-TS,31 GDT-HA (a high-resolution version of GDT-TS), GDC-SC (the ends of side chains are compared), and GDC-ALL (all atoms are compared), while the latter category includes the local structure assessment measures such as SphereGrinder (SpG),32 LDDT,33 and RPF.23 The global measures are highly related to each other as are the local measures, while the local and global measures are less well correlated. Thus, the two sets of measures are complementary measures of structure prediction accuracy.
In this paper, we present an assessment of template-based modeling in the CASP11 experiment. In CASP11, there were 39 target domains in the TBM category available to all predictors (both servers and human groups) and 42 target domains available to server groups alone (a total of 81 targets for servers). As with earlier CASPs,34 some targets were broken into two or more assessment units (AUs), usually based on domain boundaries. This generally occurred when few groups were able to predict the relative orientation and distance of the domains with respect to each other.35 This might occur because no template contained both domains, the arrangement of the domains was not the same in templates that contained them together, or the protein contained both TBM and free-modeling target domains.
In the current assessment, all prediction accuracy measures and Z-scores were calculated by the Structure Prediction Center at the University of California Davis directed by Krzysztof Fidelis.30 We experimented with a number of combinations of different measures and settled on a combination of Z-scores of two global distance measures (GDT-HA and GDC-ALL), Z-scores of two local distance measures (SpG and LDDT), and a scaled Z-score of the MolProbity scores36 (which was also used in CASP10). The MolProbity scores have the affect of urging predictors to attain statistically reasonable Ramachandran map positions, good rotamers and Cα positions, and few steric clashes.37 Such factors make the models more useful for starting molecular dynamics simulations for instance. The MolProbity Z-scores were multiplied by 0.2, since they were spread over a much larger range than the global and local prediction accuracy Z-scores.
We observed a bimodal distribution of each measure for most of the targets. Multi-dimensional scaling of an RMSD-based distance matrix demonstrated that one peak in each distribution consisted of a cluster of models resembling the native structure (to a greater or lesser degree, depending on the target), while the lower peak was in fact a broad distribution of models not resembling each other or the native structure.
Methods
Measures of model quality
Root mean square deviation (RMSD)
RMSD is the most commonly used metric for structure comparison. It is calculated as root mean of the sum of squared distances between corresponding atoms of two structures. CASP reports the RMSD calculated by the program LGA31 which uses a sequence-dependent algorithm to maximize the number of Cα atoms of the model that are within 4 Å of the target structure. We utilized the program Theseus38,39 to calculate RMSDs. Theseus uses a maximum likelihood, sequence-dependent superposition method that aligns the most similar regions of two (or more) structures and allows greater variance in divergent regions, such as loops. We used these RMSD values for multi-dimensional scaling with the R Project program (http://www.r-project.org), using the R command cmdscale. The top 10 eigenvectors and eigenvalues (representing the greatest variance in the data) were generated.
Global distance test scores
There are four related Global distance test scores routinely used for assessment of models in CASP. Global distance test - total score (GDT-TS) was first used in CASP4 to overcome the limitations of RMSD. It is computed by performing four different superpositions using LGA, each maximizing the number of Cα atom pairs (one from the model and one from the target at the same position in the sequence) within different cutoff distances. GDT-TS is the average percentage of Cα-atoms in the target within 1, 2, 4, and 8 Å after LGA superpositions with these cutoffs:
where GDT _ Pd denotes percent of residues under distance cutoff ≤ d Å. Global distance test – high accuracy (GDT-HA) is computed in the same manner as GDT-TS but with smaller cut-off values of 0.5, 1, 2, 4 Å:
It is more sensitive to smaller changes in the structure of the model. Global distance calculation for side chains (GDC-SC) is GDT-like metric which uses a characteristic atom near the end of each side chain type (instead of Cα atoms) for the evaluation of residue-residue distance deviations:
where GDC_Pk denotes percent of residues under distance cutoff <= 0.5k Å. Similarly Global distance calculation for all atoms (GDC-ALL) is calculated for all atoms of the protein.
Local distance difference test (LDDT)
The LDDT measure evaluates the quality of the model in terms of preserving local interactions in the structure.33 The atom-atom distances in the model are compared with the corresponding distances in the native structure. An interaction is presumed to be preserved if the difference between the corresponding atom-atom distances of prediction and native is below a specific cutoff. The final score is calculated by averaging the number of correct interactions in the model for four different difference cutoffs of 0.5, 1, 2, and 4 Å.
Sphere Grinder score (SpG)
SpG is an all-atom score evaluating the quality of the model based on its similarity to local substructures of the native structure.40 For Cα atom of every residue in the experimental structure the residues within a radius of 6Å are identified. RMSD is calculated only for this set of atoms between the model and native structure. The mean percentage of number of Cα atoms below two different cutoffs for which this RMSD is ≤ 2.0 Å and ≤ 4.0 Å is defined as the SpG score.
Recall, Precision, and F-measure score (RPF)
RPF is conceptually similar to LDDT score and was first introduced in CASP10.23 First, the pairwise atom-atom distances are calculated in the model and native structure. Then a graph is constructed where the atoms of the structure represent the vertices and the interactions (if shorter than a fixed cutoff) between them represent the edges. The distances which are under a specific cutoff in both the structures are considered to be true positives (TP). On the other hand, the atom pairs which are below the cutoff in native but above it in the model are considered to be false negatives (FN). However, for the opposite case the atom pairs are considered to be false positives (FP) when they are below the cutoff in model and above it in the native structure. The cutoff used in CASP is 9 Å. From these numbers, a value of F is calculated from the True-positive rate (TPR=TP/(TP+FN)) and the Positive predictive value (PPV=TP/(TP+FP)):
where Fmodel and Frandom are the F scores comparing the native structure with the model or with a random polypeptide respectively. In essence, it reports the similarity of atom-atom contacts in the model and target.
MolProbity
MolProbity validates the physical feasibility of the predicted structure. A model with good prediction of overall backbone orientation might still have clashes or bad rotamers in the structure. Molprobity penalizes such instances and hence is an important tool in assessing the geometrical quality of models. This is a knowledge-based metric which is derived from analysis of a large number of high resolution protein structures. The score is composed of four components which include a clash score (clashscore, the number of all-atom steric overlaps > 0.4Å per 1000 atom), a rotamer outlier score (rota_out, the percentage of side chain conformations classified as rotamer outliers, from those side chains that can be evaluated), and a Ramachandran outlier score (rama_out, the percentage of residues with a ϕ,ψ angles outside of the favored regions of the Ramachandran maps as determined by kernel density estimates).
Z-Scores
Predictors were allowed to submit up to 5 models and requested to rank them from 1 to 5 in decreasing order of preference. The Z-scores were calculated separately for model_1s and all models and also for server groups and all groups. When calculated for model 1s, it is a measure of the quality of a model 1 submitted by a group with respect to the entire set of model 1s submitted for that particular target by all the groups. In the first step, Z-scores using a certain measure were calculated for each model 1 of every target from the average and standard deviation of all the model 1s submitted by all the groups. In the next step, all the models that were worse than two standard deviations from the mean are removed. The mean and standard deviation were then recalculated on the remaining set, and new Z-scores were then calculated on the entire set. All the models with a new Z-score less −2.0 were assigned a value of −2.0. The final Z-scores summed over targets were then used to rank the performance of participating groups. The same procedure was performed on the set of all models for each target.
Results
A total of 143 groups participated in the template-based modeling (TBM) category of CASP11. This includes 99 human-curated groups and 44 prediction servers. There were a total of 26,028 submissions from all the groups. The Structure Prediction Center allows the groups to submit up to five predictions for every target. We assessed model 1 from each group for each target, and separately the best model from each group for each target. The model 1 results are considered the fairer assessment, since the best model assessment favors groups that provide 5 models for every target over those that provide fewer than 5.
All the scores for quality assessment of predictions were computed by the Protein Structure Prediction Center.30 A total of 81 domains from 69 target proteins were available in CASP11 which included 39 human-server (HS) and 42 server only (S) domains, listed in Tables I and II respectively. The Structure Prediction Center determined which target proteins were made available for servers or for both human and server groups. The tables include the Uniprot code, the PDB code (if available), the likely biological assembly (if available), and the median and maximum GDT-HA scores of the predictions. The maximum GDT-HA (per target) varied from 23.91 to 95.11 for HS targets and 34.12 to 87.96 for S targets. Moreover, the sequence identity of the most closely related template varied from 5.9 to 99.7 for HS targets and from 11.4 to 65.1 for S targets, suggesting inclusion of structures with varying level of difficulty.
Table I.
Targets predicted by human-servers
| Target | Domain | UniProt | PDB | Taxon | GDT- HA- Median* |
Best Model |
GDT- HA- Max |
Seq identity |
PDBBA | PISABA |
|---|---|---|---|---|---|---|---|---|---|---|
| T0826 | D2 | Q7DD94_NEIMB | 4KAV | Bacteria | 90.13 | TS008_1 | 95.11 | 99.7 | A | A |
| T0759 | D1 | PEPL_HUMAN | 4Q28 | Human | 77.21 | TS044_1 | 91.91 | 14.7 | A | A |
| T0773 | D1 | designed | 2N2U | Synthetic | 50.37 | TS336_2 | 85.45 | 14.1 | A | A |
| T0816 | D1 | Y1502_ARCFU | 5A1Q | Archaea | 28.68 | TS428_1 | 74.64 | 19.6 | A2 | A2 |
| T0759 | D2 | PEPL_HUMAN | 4Q28 | Human | 28.63 | TS328_5 | 71.37 | 20.0 | A | A |
| T0769 | D1 | designed | 2MQ8 | Synthetic | 48.45 | TS120_5 | 69.59 | 17.1 | A | A |
| T0765 | D1 | MZRA_KLEP7 | 4PWU | Bacteria | 39.47 | TS403_5 | 67.76 | 17.9 | A2 | A4 |
| T0820 | D2 | (unknown marine phage) | - | Virus | 31.25 | TS328_1 | 63.89 | 67.8 | - | - |
| T0799 | D4 | Q5DMH0_BPT5 | 4UW8 | Virus | 4.90 | TS204_2 | 60.44 | 22.6 | A3 | A3 |
| T0828 | D2 | A4TUL6_9PROT | 4Z29 | Bacteria | 29.76 | TS204_5 | 60.41 | 20.3 | A | A |
| T0795 | D1 | F4MI11_9ADEN | - | Virus | 52.75 | TS340_5 | 59.93 | 18.1 | - | - |
| T0848 | D1 | YCDA_BACSU | 4R4Q | Bacteria | 43.12 | TS184_3 | 58.88 | 22.1 | A | A |
| T0783 | D1 | ISPD_HUMAN | 4CVH | Human | 51.65 | TS346_1 | 58.64 | 30.5 | A2 | A2 |
| T0810 | D2 | Q6MI90_BDEBA | - | Bacteria | 50.11 | TS439_1 | 57.45 | 34.5 | - | - |
| T0794 | D1 | VNN1_HUMAN | 4CYF | Human | 46.44 | TS044_3 | 56.34 | 24.2 | A | A |
| T0828 | D1 | A4TUL6_9PROT | 4Z29 | Bacteria | 15.18 | TS425_1 | 50.59 | 16.4 | A | A |
| T0853 | D2 | Q99T58_STAAM | 2MQB | Bacteria | 24.82 | TS296_4 | 49.31 | 13.8 | A | (NMR) |
| T0808 | D1 | A5ZGP5_9BACE | 4QHW | Bacteria | 41.79 | TS333_3 | 48.66 | 14.8 | A | A |
| T0767 | D1 | Q931R5_STAAM | 4QPV | Bacteria | 22.37 | TS347_4 | 48.35 | 5.9 | A | A |
| T0793 | D4 | G7ZLR1_STAAU | - | Bacteria | 22.06 | TS499_5 | 47.06 | 7.1 | - | - |
| T0827 | D1 | B8H2Q8_CAUCN | - | Bacteria | 14.12 | TS328_2 | 46.37 | 10.2 | - | - |
| T0783 | D2 | ISPD_HUMAN | 4CVH | Human | 29.64 | TS358_1 | 42.15 | 12.7 | A2 | A2 |
| T0803 | D1 | U3USG4_PEPDI | 4OGM | Bacteria | 27.61 | TS357_1 | 40.48 | 25.0 | A | A |
| T0838 | D1 | R7NY94_9BACE | - | Bacteria | 28.77 | TS042_1 | 38.30 | 11.1 | - | - |
| T0830 | D2 | Q1LDT6_CUPMC | 5EZM | Bacteria | 20.05 | TS347_1 | 37.62 | 9.2 | A | A |
| T0853 | D1 | Q99T58_STAAM | 2MQB | Bacteria | 28.94 | TS144_2 | 37.50 | 20.7 | A | (NMR) |
| T0822 | D1 | A0A011VZQ5_RUMAL | - | Bacteria | 24.34 | TS216_4 | 35.75 | 14.0 | - | - |
| T0835 | D1 | A9R6D9_YERPG | - | Bacteria | 24.75 | TS333_5 | 34.59 | 12.0 | - | - |
| T0831 | D1 | SHPRH_HUMAN | 4QN1 | Human | 23.15 | TS153_2 | 32.58 | 15.6 | A | A |
| T0814 | D3 | A7AEG3_9PORP | 4R7F | Bacteria | 28.91 | TS204_3 | 31.08 | 23.4 | A2 | A2 |
| T0830 | D1 | Q1LDT6_CUMPC | 5EZM | Bacteria | 23.77 | TS044_4 | 28.12 | 17.8 | A | A |
| T0774 | D1 | A6L3B5_BACV8 | 4QB7 | Bacteria | 21.32 | TS216_3 | 27.33 | 19.7 | A | A4 |
| T0793 | D3 | G7ZLR1_STAAU | - | Bacteria | 25.13 | TS038_5 | 27.26 | 16.1 | - | - |
| T0818 | D1 | B0MNI9_9FIRM | 4R1K | Bacteria | 21.08 | TS281_1 | 26.68 | 13.8 | A | A |
| T0800 | D1 | J7TBR0_CLOSG | 4QRK | Bacteria | 21.40 | TS173_2 | 26.53 | 12.1 | A | A |
| T0781 | D2 | A7B4B4_RUMGN | 4QAN | Bacteria | 21.71 | TS044_4 | 26.29 | 17.2 | A | A |
| T0799 | D3 | Q5DMH0_BPT5 | 4UW8 | Virus | 17.16 | TS360_1 | 25.49 | 18.6 | A3 | A3 |
| T0848 | D2 | YCDA_BACSU | 4R4Q | Bacteria | 18.72 | TS333_5 | 24.32 | 15.2 | A | A |
| T0812 | D1 | LAMA2_HUMAN | 4YEQ | Human | 22.20 | TS067_3 | 23.91 | 12.2 | A | A |
GDT-HA median computed from Model 1s
PDBBA is the first biological assembly given by the PDB (A2 = homodimer, etc.)
PISABA is the biological assembly given by PISA for crystal structures
Table II.
Targets predicted by servers.
| Target | Domain | UniProt | PDB | Taxon | GDT-HA- Median* |
Best Model |
GDT- HA- Max |
Seq identity |
PDBA | PISAA |
|---|---|---|---|---|---|---|---|---|---|---|
| T0766 | D1 | A7V9L7_BACUN | 4Q53 | Bacteria | 84.72 | TS008_5 | 87.96 | 65.1 | A | A |
| T0784 | D1 | A7M5D7_BACO1 | 4QEY | Bacteria | 72.10 | TS008_3 | 84.80 | 58.7 | A | A |
| T0854 | D1 | D7AL49_GEOSK | 4RN3 | Bacteria | 70.83 | TS184_3 | 83.14 | 29.8 | A | A |
| T0811 | D1 | TPIS2_RHIME | - | Bacteria | 72.46 | TS335_4 | 78.58 | 37.9 | - | - |
| T0815 | D1 | A0A077HY11_RHIML | 4U13 | Bacteria | 68.40 | TS479_3 | 76.89 | 16.5 | A2 | A2 |
| T0762 | D1 | I6L927_STRMU | 4Q5T | Bacteria | 67.31 | TS156_5 | 71.69 | 38.8 | A | A |
| T0768 | D1 | A6NQU6_9FIRM | 4OJU | Bacteria | 47.90 | TS216_1 | 71.68 | 38.1 | A4 | A4 |
| T0801 | D1 | RFFA_ECOLI | 4PIW | Bacteria | 63.20 | TS184_4 | 70.35 | 31.6 | A2 | A2 |
| T0807 | D1 | V3S9R5_KLEPN | 4WGH | Bacteria | 58.30 | TS335_2 | 66.52 | 27.0 | A | A |
| T0819 | D1 | Q92R63_RHIME | 4WBT | Bacteria | 55.82 | TS184_4 | 66.42 | 23.8 | A2 | A2 |
| T0817 | D1 | Q92YH7_RHIME | 4WED | Bacteria | 57.21 | TS156_1 | 66.32 | 27.1 | A | A |
| T0817 | D2 | Q92YH7_RHIME | 4WED | Bacteria | 59.64 | TS184_2 | 66.07 | 28.3 | A | A |
| T0782 | D1 | A7V1A8_BACUN | 4QRL | Bacteria | 36.59 | TS184_3 | 65.23 | 12.9 | A | A |
| T0776 | D1 | R6WSE2_9PORP | 4Q9A | Bacteria | 60.50 | TS184_2 | 64.50 | 33.7 | A2 | A2 |
| T0813 | D1 | Q92MG1_RHIME | 4WJI | Bacteria | 54.92 | TS008_1 | 63.41 | 34.7 | A2 | A2 |
| T0833 | D1 | A6LGW3_PARD8 | 4R03 | Bacteria | 46.87 | TS420_3 | 62.27 | 20.9 | A | A2 |
| T0856 | D1 | HERC1_HUMAN | 4QT6 | Human | 52.59 | TS277_1 | 62.26 | 17.7 | - | A |
| T0764 | D1 | A6LCA7_PARD8 | 4Q34 | Bacteria | 56.85 | TS011_1 | 61.37 | 34.5 | A2 | A2 |
| T0854 | D2 | D7AL49_GEOSK | 4RN3 | Bacteria | 50.72 | TS184_3 | 60.36 | 12.5 | A | A |
| T0805 | D1 | W6H7D8_MYCTX | - | Bacteria | 49.87 | TS184_4 | 59.77 | 22.5 | - | - |
| T0843 | D1 | Q0H2X1_9ACTO | 4OCA | Bacteria | 54.98 | TS110_3 | 59.42 | 29.6 | A2 | A2 |
| T0780 | D2 | Q97PP3_STRPN | 4QDY | Bacteria | 36.72 | TS184_5 | 59.37 | 20.3 | A2 | A2 |
| T0851 | D1 | Q8KNF6_MICEC | 4XRR | Bacteria | 46.80 | TS479_3 | 59.30 | 29.7 | A2 | A2 |
| T0792 | D1 | OSKA_DROME | 5A49 | Drosophila | 45.99 | TS184_4 | 58.98 | 30.4 | A2 | A2 |
| T0847 | D1 | FA83A_HUMAN | 4URJ | Human | 53.10 | TS184_3 | 58.58 | 20.6 | A | A2 |
| T0858 | D1 | Q8A2J3_BACTN | - | Bacteria | 53.22 | TS452_4 | 58.39 | 26.7 | - | - |
| T0839 | D1 | SLA2_SCHPO | - | Fungi | 38.92 | TS420_1 | 58.10 | 17.2 | - | - |
| T0760 | D1 | A5ZGW9_9BACE | 4PQX | Bacteria | 44.83 | TS041_1 | 57.71 | 29.2 | A | A |
| T0829 | D1 | Q1CIG2_YERPN | 4RGI | Bacteria | 36.20 | TS277_4 | 56.34 | 11.4 | A | A |
| T0780 | D1 | Q97PP3_STRPN | 4QDY | Bacteria | 50.52 | TS499_1 | 54.47 | 21.4 | A2 | A2 |
| T0852 | D1 | C7M590_CAPOD | 4W9R | Bacteria | 48.82 | TS184_1 | 54.06 | 19.1 | A2 | A2 |
| T0845 | D1 | Q8A7N7_BACTN | 4R5O | Bacteria | 34.41 | TS251_4 | 52.58 | 15.5 | A | A3 |
| T0772 | D1 | A6LIT1_PARD8 | 4QHZ | Bacteria | 43.69 | TS381_4 | 49.18 | 30.6 | A4 | A4 |
| T0786 | D1 | Q73EW7_BACC1 | 4QVU | Bacteria | 41.13 | TS184_5 | 49.08 | 17.2 | A4 | A4 |
| T0821 | D1 | R6X2D1_9PORP | 4R7S | Bacteria | 35.98 | TS216_3 | 49.02 | 16.4 | A | A |
| T0770 | D1 | C6IS27_9BACE | 4Q69 | Bacteria | 41.55 | TS492_1 | 48.30 | 23.5 | A2 | A2 |
| T0849 | D1 | D0LNW1_HALO1 | 4W66 | Bacteria | 38.45 | TS073_2 | 46.29 | 22.3 | A2 | A2 |
| T0823 | D1 | B8H047_CAUCN | - | Bacteria | 37.16 | TS184_2 | 42.10 | 19.7 | - | - |
| T0852 | D2 | C7M590_CAPOD | 4W9R | Bacteria | 22.42 | TS277_2 | 40.08 | 10.8 | A2 | A2 |
| T0845 | D2 | Q8A7N7_BACTN | 4R5O | Bacteria | 31.16 | TS216_2 | 36.78 | 18.9 | A | A |
| T0796 | D1 | A7IZR5_BACTU | 4PKM | Bacteria | 29.48 | TS210_1 | 36.65 | 15.9 | A2 | A2 |
| T0857 | D1 | A6KYV6_BACV8 | 2MQC | Bacteria | 17.97 | TS454_1 | 34.12 | 14.5 | A | (NMR) |
GDT-HA median computed from Model 1s
PDBBA is the first biological assembly given by the PDB (A2 = homodimer, etc.)
PISABA is the biological assembly given by PISA for crystal structures
Quality of predictions based on sequence identity
The sequence identity of the most closely related template to the target (as determined by structure alignment of the target structure to homologues in the PDB35) is a simple measure of target difficulty, one that can be measured prior to structure determination. We first analyzed the relationship between the sequence identity and the structure prediction accuracy of the best models. Figure 1 shows the maximum value (over all models) of GDT-HA (Figure 1A), GDC-ALL (Figure 1B), LDDT (Figure 1C), and SpG (Figure 1D) versus the sequence identity of the template. The S targets (solid circles in Figure 1) generally had higher sequence identity to the template than the HS targets (open circles in Figure 1). The maximum GDT-HA and GDC-ALL values are highly correlated with sequence identity, although there are (not surprisingly) significant outliers since sequence identity is not a perfect measure of target difficulty. Both of GDT-HA and GDC-ALL are global measures of quality assessment suggesting, as expected, that better backbone orientation is predicted if the template is more closely related to the model. However, with the local measures like SpG and LDDT, the relationship with sequence identity is only roughly linear indicating that although good sequence identity leads to better overall fold, in some cases the local substructures can still be poorly defined. The maximum SpG values are the least correlated with sequence identity, suggesting that despite good templates, accurate local packing is difficult to achieve for many targets. Overall, the quality of prediction with respect to sequence identity with the template is strongly correlated with global measures and less strongly with local ones.
Figure 1.
Relationship between sequence identity of the closest template to the target experimental structure and the maximum structure prediction accuracy for each target across all models for different global and local quality assessment measures: A) GDT-HA, B) GDC-ALL, C) LDDT, D) SpG. The line of regression is computed by robust linear regression fitting over all the data points and is shown as a solid line. The dotted line represents x=y.
Performance of groups based on single and pairwise measures
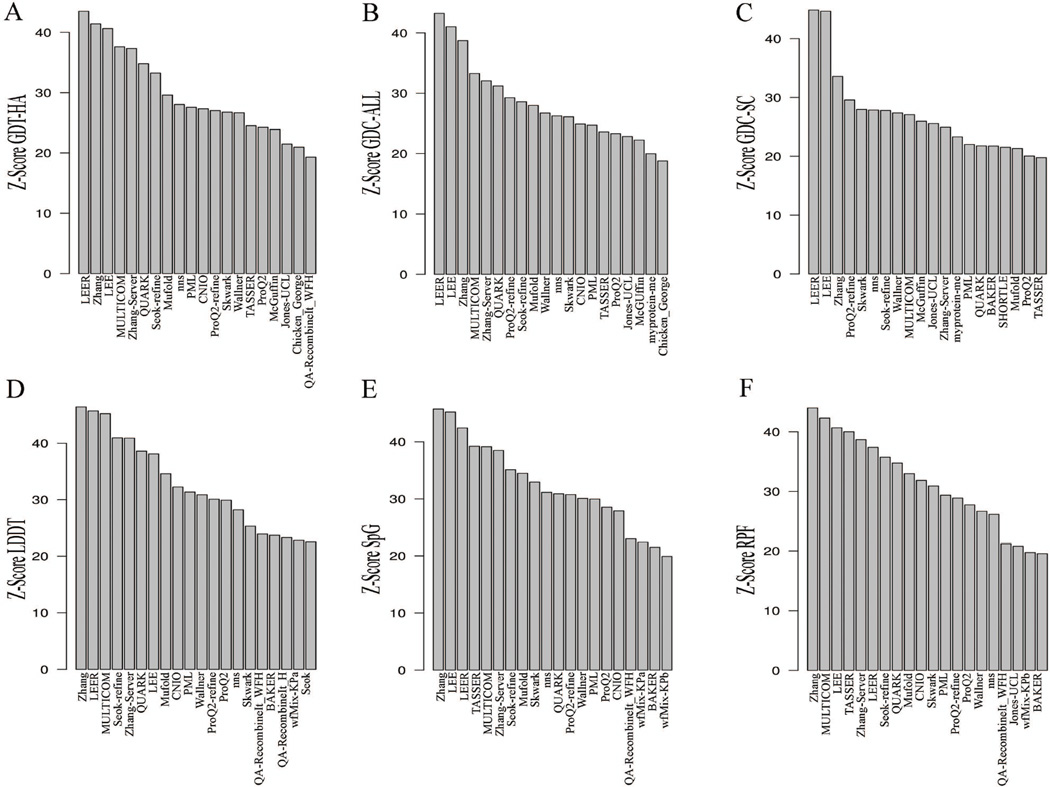
Z-scores for each measure were provided by the Structure Prediction Center along with a means for testing out various combinations of measures and their weights. The performance of different groups on the 39 HS targets based on the Z-score of six different single measures, three global and three local, is shown in Figure 2. Based on the global scores (GDT-HA, GDC-ALL and GDC-SC) LEE, LEER, and Zhang are the top performing groups. The Z-scores of GDT-HA (Figure 2A) and GDC-ALL (Figure 2B) of the three top groups are comparable but in the case of GDC-SC (Figure 2C), LEER and LEE outperform Zhang and other groups by a considerable margin. The assessment from Z-scores of local measure like LDDT, SpG and RPF also display leading performances by the same three groups, joined by MULTICOM in third place in LDDT and second place in RPF. While the top performing groups exhibit good performance for all the measures, a few other groups display excellent performance in some scores but not others. For instance, TASSER exhibits good performance in local scores like SpG and RPF but performs less well in overall backbone prediction scores like GDT-HA and GDC-ALL. On the other hand, there are groups who perform better with GDT-HA and GDC-ALL but do not do as well in GDC-SC and SpG.
Figure 2.
Summary of Z-scores summed over targets for single metrics of model 1 of top 20 groups for each measure. The x-axis displays the groups names. The y-axis is the summed Z-score for each single metric: A) GDT-HA, B) GDC-ALL, C) GDC-SC, D) LDDT, E) SpG and F) RPF.
Next, we analyzed the performance of groups by summing up Z-scores for global and local measures (Figure 3). In this assessment also LEE, Zhang and LEER outperform the other predictors in most of the combinations, accompanied by MULTICOM and Zhang-Server who also exhibit consistent performance across different combinations of quality metrics. Since every measure exploits a different feature of the model, the ranking of groups change slightly with different combinations of measures used. A fair numerical assessment of groups therefore requires a sum of certain measures which reflects a balanced assessment of both global and local features of the predictions. Therefore to find a suitable combination we have tried to understand the relationship between different metrics.
Figure 3.
Summary of sum of Z-scores of metrics of model 1 of top 20 groups for various combinations of global and local metrics. The x-axis displays the group names. The y-axis is the Z-score for sum of metrics: A) GDT-HA+SpG, B) GDT-HA+LDDT, C) GDT-HA+RPF, D) GDT-HA+GDC-ALL+LDDT+SpG, E) GDT-HA+GDC-ALL+LDDT+RPF, and F) GDT-HA+GDC-ALL+LDDT+RPF+SpG.
Relationship between different global and local measures
To evaluate correlations between the different metrics of assessment, we plotted the various metrics against each other for the models with the best value for each metric. This exercise helped us to identify a suitable combination of measures for assessment of groups. The relationship of GDT-HA with GDT-TS (Figure 4A) and GDC-ALL (Figure 4B) is nearly linear, which is expected. These metrics are superposition-based global measurements of quality. Figure 4C shows the relationship between the best MolProbity score (lower is better) and the best GDT-HA score for each target. As expected, there is no correlation since it is possible to achieve dihedral angle values close to known statistical distributions and few clashes within a model that is not at all similar to the experimental structure of the target. Generally, much better MolProbity scores are achieved when the human groups are included (the HS targets) than with the server groups alone (S targets).
Figure 4.
Relationship between different global and local quality assessment measures. Pairwise comparison of maximum GDT-HA for every target is done with A) Maximum GDT-TS, B) Maximum GDC-ALL, C) Minimum (best) MolProbity score, D) Maximum LDDT, E) Maximum RPF and F) Maximum SpG. Pairwise comparison of maximum LDDT for every target is done with G) Maximum RPF, H) Maximum SpG and I) Maximum SpG. The line of regression is computed by robust linear regression fitting over all the data points and is shown in a solid line.
However, the correlations between GDT-HA and the local metrics, LDDT (Figure 4D), RPF (Figure 4E), and especially SpG (Figure 4F) are relatively weak. The comparisons demonstrate that the local and global metrics provide distinctly different pieces of information. Plotting RPF against LDDT indicates that these measures are highly correlated (Figure 4G). This redundancy is also observed when both of these measures are plotted against SpG (Figures 4H and 4I). This high correlation of LDDT and RPF is probably because both are distance matrix-based measures. Both of them in essence represent the similarity of atom-atom contacts between the predicted and native structure.
Performance of groups based on sum of different metrics
It is pertinent that the performance of different groups is assessed by both local and global features while remaining non-redundant. Therefore, we have used the sum of Z-scores for five measures (GDT-HA+GDC-ALL+LDDT+SpG+0.2MolP) that are related to each other in a useful and non-redundant way. While GDT-HA depends heavily on the accuracy in Cα-atom positions, GDC-ALL shows the confidence in prediction of all atoms. On the other hand LDDT reports the preservation of residue-residue contacts, and SpG examines the packing of local regions. The last quality measure used is MolProbity to assess whether the predicted model is physically feasible or not. The factor 0.2 in front of the MolProbity term is somewhat arbitrary, but the summed Z-scores per group for MolProbity had more than twice the range of the other metrics. Almost any model could be improved in its MolProbity score with some regularization of the structure, so we reduced the factor to 0.2.
The ranking based on sum of Z-scores (GDT-HA+GDC-ALL+LDDT+SpG+0.2MolP) for model 1 submitted by all the groups shows that LEER, Zhang, and LEE are the top ranked groups (Figure 5A). They are followed by MULTICOM, Zhang-Server, and Seok-refine. The pairwise bootstrap values for the ranking are provided in Table S1 of the Supplemental Material. Bootstrap values were determined by randomly picking (with replacement) N target domains from the N target domains that two groups have in common, and comparing the total sum of Z-scores. This procedure was repeated 1000 times, and the table provides the fraction of times that the higher ranked group had a higher sum than the lower ranked group. LEER has a bootstrap value with Zhang of 0.918, but values >0.99 with all other groups. Zhang has bootstrap values with three of the next four groups below 0.95 (except Zhang-server), and >0.99 with all subsequent groups. Values above 0.95 may be used to show that the relative ranking is statistically significant at a 5% level. Thus LEER and Zhang are quite similar, while their ranks over most of the other groups are statistically significant.
Figure 5.
Final ranking of TBM predictor groups using a summed Z-score consisting of GDT-HA+GDC-ALL+LDDT+SpG+0.2MolP. The x-axis displays the group names. The y-axis is the sum of Z-scores for computed for A) Model 1 and, B) Best model.
The performance assessed by using the best models is shown in Figure 5B. LEER and Zhang remain the top two groups, followed by ProQ2 and ProQ2-refine, which exhibit a drastically improved performance over model 1 assessments (Figure 5A). This is likely because both of these groups selected server models which were made available to predictors prior to the human group deadlines. As a result their best models are the same as the best models submitted by some of the top groups. The reduction in the total score across the top 10 groups is more gradual when calculated for best models than it is for model 1s. This is reflected in the bootstrap values (Table S2), which show that the differences in rank are not significant at the 95% level for groups at least 5 apart in rank. This indicates that the best models submitted by many of the groups are of comparable quality.
Performance of servers on HS+S targets
We have also separately assessed the performance of server groups on the combined Human-Server and Server-only target domains, using the same sum of Z-scores (GDT-HA+GDC-ALL+LDDT+SpG+0.2MolP) as displayed in Figure 6. Mean values and standard deviations used to calculate the Z-scores were based only on the raw scores of the models from the servers, even on the HS targets. Zhang-Server, nns, BAKER-ROSETTASERVER, QUARK, and myprotein-me are the top five groups when assessed using model 1 for all targets (Figure 6A), followed by a significant drop off after the fifth-ranked group This is confirmed by the bootstrap values in Table S3, which show values of ≥0.95 only from the sixth-ranked group and onwards. Another significant drop off occurs after the eighth-ranked group.
Figure 6.
Final ranking of TBM server groups over the HS+S targets. The x-axis displays the group names. The y-axis is the sum of Z-scores for GDT-HA+GDC-ALL+LDDT+SpG+0.2MolP computed for A) Model 1 and, B) Best model.
When the best models are scored for the server groups (Figure 6B), BAKER-ROSETTASERVER is ranked at the top by a significant margin as demonstrated by bootstrap values >0.95 for all subsequently ranked groups (Table S4). The next four groups, nns, Zhang-Server, QUARK, and myprotein-me are all quite similar. We observe that in the case of the best models for servers on the HS+S targets, there is again a steep decline in the total score after the fifth group and again after the eighth group. The distribution of Z-scores for models 1, 2, 3, 4, and 5 for BAKER-ROSETTASERVER were all quite similar to each other (not shown), indicating that the difference in ranks for model 1s and best models was not due to distinct protocols for each of the 5 models.
Performance based on MolProbity
MolProbity is calculated from statistical analysis of a large number of protein crystal structures.20,36 It gives negative scores to clashes, unfavorable Ramachandran dihedrals, bad Cβ positions, and bad rotamers—thereby identifying models that are not physically reasonable irrespective of the model’s similarity to the native experimental structure. A higher MolProbity score represents a lower quality model. MolProbity Z-scores were therefore calculated on inverted values. Physical reasonableness is an important component of structure prediction, and therefore we have examined the performance of different groups based on MolProbity separately (Figure 7). We analyzed the separate components of the MolProbity score for the top-ranked groups (Figure 5), with the addition of the STAP group, which had the highest sum of MolProbity Z-scores. The results are shown in Figure 8, and the groups are ordered by their total average MolProbity score (smaller values are better) (Figure 8A). The separate components include percent Cβ outliers (Figure 8B), percent Ramachandran outliers (Figure 8C), percent bad rotamers (Figure 8D), and the number of clashes per 100 residues (Figure 8E). Several groups have up to 8% Cβ outliers, 10% Ramachandran outliers, nearly 15% bad rotamers, and up to 8 clashes per 100 residues. The groups differ in which components of the MolProbity score they performed well or poorly in. Seok-refine, PML, Zhang, Zhang-Server, QUARK, McGuffin, and TASSER have higher percentages of Cβ outliers than other groups; Zhang, Zhang-Server, QUARK, and TASSER have higher percentage of Ramachandran outliers than other groups; PML, Zhang-Server, QUARK, and McGuffin have poor side-chain rotamer scores. A different set of groups performed worse than average on the clashscore (Figure 8E), including Skwark, CNIO, nns, LEE, MULTICOM, Mufold, and TASSER. Since the clashscore (Figure 8E) is weighted more heavily than the other MolProbity components (see Methods), even groups with moderate clashscores do not rank well in overall MolProbity scores (Figure 8A). The individual MolProbity scores can be utilized by predictors to focus their efforts on improving some aspects of their models in reference to the statistically probable values of bond angles, dihedral angles, and atom-atom distances.
Figure 7.
Summed Z-score of MolProbity for model 1 of top 20 groups for all the targets.
Figure 8.
Assessment of different groups on the basis of MolProbity metric. The x-axis displays the group names and y axis shows the score. The groups included are those that ranked highly in the model 1 assessments with the inclusion of the group with top MolProbity score (STAP). A) Total MolProbity score (lower is better); B) Percent Cβ outliers, C) Percent Ramachandran outliers, D) Percent bad rotamers and E) Number of clashes per 100 residues. In each panel, the groups are ordered by total MolProbity score.
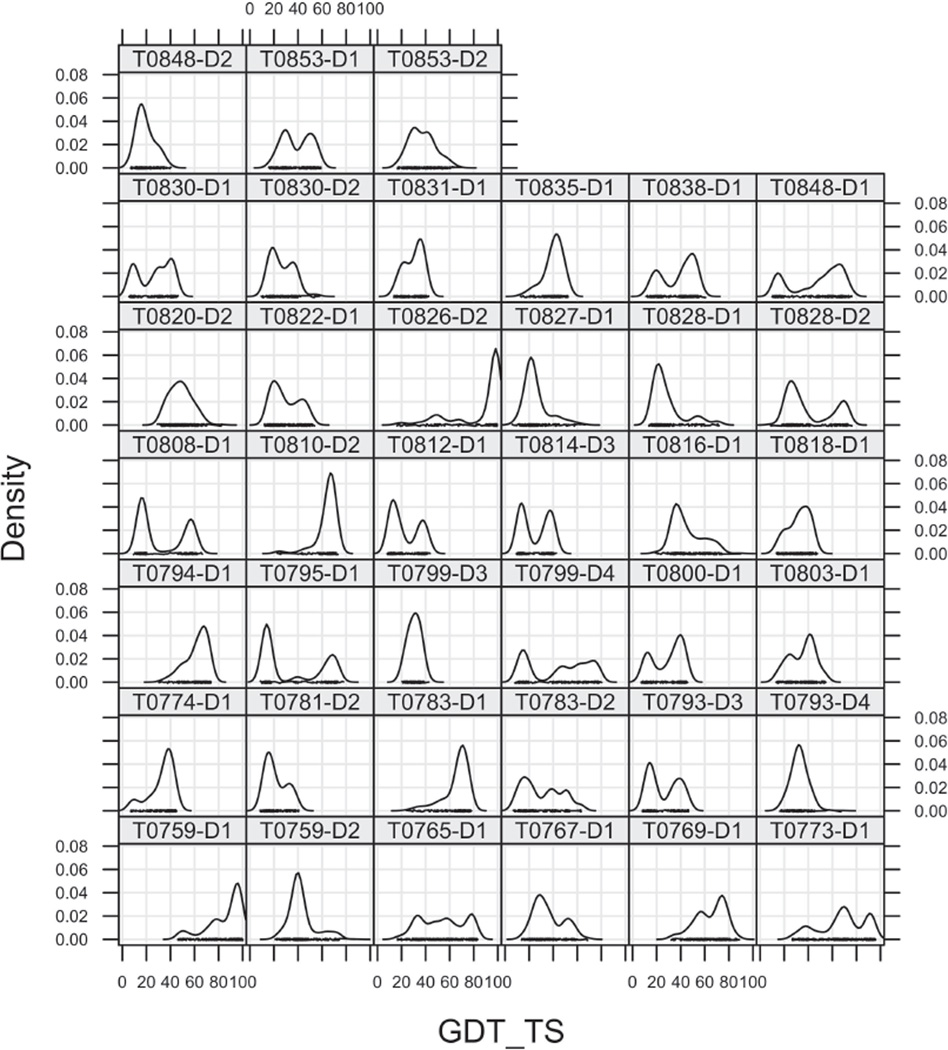
Distribution of scores in predictions of all targets
Model-quality assessment programs have had some success in picking out accurate models from an ensemble of models generated by other programs,41 and some groups using this method performed well in our assessment of the TBM models in CASP11.42 We therefore were interested in the analyzing the ensemble of models for each target to determine how the models relate to one another, in addition to how they relate to the target experimental structure. Kernel density estimates of GDT-TS and RMSD (calculated with the program Theseus38,39) to native for all 39 human-server targets are shown in Figures 9 and 10 respectively. What is striking is that nearly all of the distributions are bimodal. The same is true of other accuracy measures, including GDC_ALL, LDDT, and SphereGrinder, and it is also true for the scores of most of the server-only target domains (not shown).
Figure 9.
Kernel density estimates of the probability distribution of GDT-TS for 39 human-server targets.
Figure 10.
Kernel density plots of backbone RMSD to native for 39 human-server targets. The pairwise RMSD between models and native structure of each target is calculated by doing maximum likelihood superposition using the Theseus program.
Such a phenomenon could occur because there are two (or more) distinct clusters of models, perhaps based on different templates, or it could occur if there is a single cluster of models that are relatively close to the target and a distribution of poor models that are not similar to the target nor similar to each other. We performed multi-dimensional scaling (MDS) which represents higher dimensional data in a smaller number of dimensions, while approximately preserving the pairwise distances among all data points. In the current context, the conformational variation of the protein models is a space of 3Natoms-6. We applied MDS to visualize the structural variation among the models in two dimensions. Structurally distant models are separated from each other and similar models are clustered together on the plot. This requires calculation of pairwise RMSD matrix for all the models of a target. For this purpose we have performed pairwise structure alignments with the program Theseus38,39 for each of the 39 TBM human-server targets in CASP11. Theseus uses a maximum-likelihood method that includes realistic assumptions that Cα-Cα distances nearby in sequence are correlated and that the variance of Cα-Cα distances along the chain are not uniform.
The results are shown for three representative targets in Figure 11 (T0795-D1), 12 (T0828-D2), and 13 (T0838-D1). In panel A of each figure, a scatterplot of the first two MDS coordinates is colored by RMSD to native (from blue to red) and four structures are marked – the native (panel C), the best model (panel D), a model near the upper mode in the GDT-TS density for the target (panel E), and a model near the lower mode in the GDT-TS density (panel F). The same structures are indicated in a scatterplot of GDT-TS vs RMSD (panel B). The symbols are shown next to the protein structure images.
Figure 11.
Assessment of predictions for the target T0795-D1. A) A scatter plot of the first two coordinates of multidimensional scaling computed from pairwise RMSD distance matrix including all the models submitted by all the groups. The RMSD to native of every model is represented by the color gradient of blue (lowest RMSD) to red (highest RMSD). B) A scatter plot of the RMSD to native of all the models against GDT-TS. C) The experimentally determined target structure for T0795-D1. D) The model with highest GDT-TS submitted by Handl (TS340_5). E) The model with median GDT-TS of the upper peak (closer to native) in the bimodal distribution of GDT-TS of T0795-D1 in Figure 9. F) The model with median GDT-TS of the lower peak in the bimodal distribution of GDT-TS of T0795-D1 in Figure 9. The structures in C, D, E and F are highlighted in the graphs in panel A and B with different symbols as shown.
It is evident that the second scenario explains the bimodal nature of the GDT-TS and RMSD densities in most cases, i.e. that there is a single cluster of models relatively similar to the target and to each other, and a broad distribution of other models that are not similar to one another or to the target. The best models for these targets have GDT-TS values of 77.8, 76.2, and 60.5 respectively (Figure 11D, 12D, 13D), the models at the mode of the upper peak are at 68.6, 69.0, and 48.8 (Figure 11E, 12E, 13E), and those of the lower peak are at 17.5, 26.5, and 19.4 (Figures 11F, 12F, 13F). The structures at the mode of the lower peak have very high RMSD (>14 Å) and are all in a widely scattered group of models in the MDS plots (square symbol, Figure 11A, 12A, 13A). A value of GDT-TS of around 20 appears easy to achieve with a reasonably compact model, even if the fold is entirely incorrect, and explains the presence of the lower peak for most of the targets in Figure 9.
Figure 12.
Assessment of predictions for the target T0828-D2. A) A scatter plot of the first two coordinates of multidimensional scaling computed from pairwise RMSD distance matrix including all the models submitted by all the groups. The RMSD to native of every model is represented by the color gradient of blue (lowest RMSD) to red (highest RMSD). B) A scatter plot of the RMSD to native of all the models against GDT-TS. C) The experimentally determined target structure for T0828-D2. D) The model with highest GDT-TS submitted by Zhang (TS204_5). E) The model with median GDT-TS of the upper peak (closer to native) in the bimodal distribution of GDT-TS of T0828-D2 in Figure 9. F) The model with median GDT-TS of the lower peak in the bimodal distribution of GDT-TS of T0828-D2 in Figure 9. The structures in C, D, E and F are highlighted in the graphs with different symbols as shown.
Figure 13.
Assessment of predictions for the target T0838-D1. A) A scatter plot of the first two coordinates of multidimensional scaling computed from pairwise RMSD distance matrix including all the models submitted by all the groups. The RMSD to native of every model is represented by the color gradient of blue (lowest RMSD) to red (highest RMSD). B) A scatter plot of the RMSD to native of all the models against GDT-TS. C) The experimentally determined target structure for T0838-D1. D) The model with highest GDT-TS submitted by wfHHPred-PTIGRESS (TS034_5). E) The model with median GDT-TS of the upper peak (closer to native) in the bimodal distribution of GDT-TS of T0838-D1 in Figure 9. F) The model with median GDT-TS of the lower peak in the bimodal distribution of GDT-TS of T0838-D1 in Figure 9. The structures in C, D, E and F are highlighted in the graphs with different symbols as shown.
We note that in each case, the proportion of variance in the first two components (eigenvectors) of the multi-dimensional scaling was low – 34%, 22%, and 29%. We checked additional dimensions and it is evident that additional clusters do not form. The central cluster spreads out, which correlates with the observation that the native structure appears too close to models than it is in the original distance matrix.
CASP11 compared to previous CASPs
The assessment of progress in CASP in comparison with previous experiments has always been difficult due to two main reasons: a suitable metric for target difficulty is challenging, and the growth of sequence and structure databases means that methods may improve simply because of larger sequence alignments or the existence and effective utilization of multiple templates.
However, to make a broad comparison, we chose as a measure of target difficulty the GDT-TS of a model based on copying the coordinates of the best template according to a sequence alignment resulting from template-target structure alignment. Predictors may do better than this template in a number of different ways. They may be able to build regions of the structure for which there is no information in the template; even if they do this poorly, GDT-TS may improve because it gives credit for Cα atoms placed with in 1, 2, 4, and 8 Å in a sequence-dependent structure alignment. Predictors may also do better than the best template if they combine multiple templates, and/or they are able to refine the model successfully from the initial alignment-based model towards the template. Predictors may do worse than the best template if they choose a less suitable template and/or they align the sequence of the target to the template incorrectly. Since about two thirds of predictors do not list templates they use in their submitted predictions, it is difficult to tell the difference.
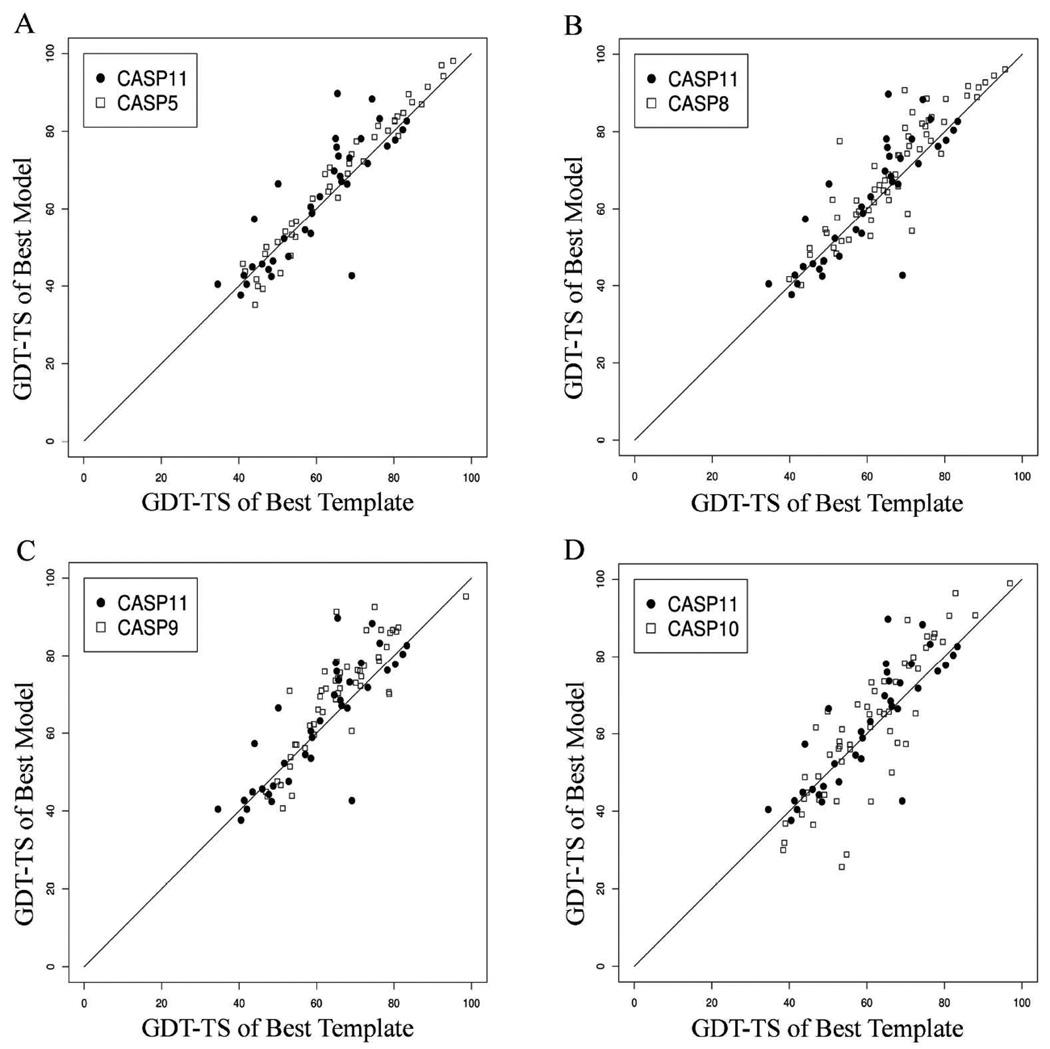
We have plotted the GDT-TS of models built directly from the best template and a structure-based sequence alignment versus the GDT-TS of the best predictor models of CASP11 with CASP5, CASP8, CASP9 and CASP10 (Figure 14). Density estimates of the best template GDT-TS and the differences between model and template GDT-TS are plotted in Figures 15A and 15B respectively (where positive values mean the model is better than the single best template). Note that the point with best-template value of GDT-TS of 69 and best-model GDT-TS of 43 is a small target (T0799-D3) of three β sheet strands from a viral spike protein with numerous structural repeats of β strands. It is very difficult to find the best template given the large sequence divergence of the target and the templates.
Figure 14.
Comparison of predictions in CASP11 with previous CASP experiments. The GDT-TS of the best structural template is plotted against GDT-TS of the best predicted model (highest GDT-TS) for A) CASP11 vs. CASP5. B) CASP11 vs. CASP8. C) CASP11 vs. CASP9. D) CASP11 vs. CASP10. The GDT-TS of the model built from the best template is derived by copying coordinates of the template according to a sequence alignment obtained from a structure alignment of the template and the experimental structure of the target.
Figure 15.
Kernel density plots of A) GDT-TS of best templates and B) Difference between GDT-TS of best model and template for all human-server targets in CASP5, CASP8, CASP9, CASP10 and CASP11.
The later CASPs (9, 10, and 11) appear to have a few more points substantially above the diagonal (Figure 14) with ΔGDT-TS>15 (Figure 15B) than CASP8 and particularly CASP5. Most of the relatively easy targets (with GDT-TS of the best structural template greater than 55) were consistently improved in each of the CASP experiments, probably because alignments were accurate and missing loops in the template were filled in, which automatically improves GDT-TS if the loops are within 8 Å of the native. Similarly, CASP11 has lower density than the other CASPs at values of ΔGDT-TS worse than −15 even though CASP10 and CASP11 had a larger number of targets where the GDT-TS of the best structural template was below 55 (relatively hard targets, Figure 15A), compared to CASP8 and CASP9. The number of targets is small in both categories and we do not want to make too much of the differences or caterogically say there is progress in modeling in recent CASPs. The differences in performance over the CASPs has been analyzed in significantly greater detail in another paper in this issue (Moult et al.).43
Discussion
We have presented the assessment results for the template-based modeling category of CASP11. After examining the relationships among the different scoring metrics provided by the Structure Prediction Center, we chose a set of four metrics equally balanced between global (GDT-HA and GDC-ALL) and local (LDDT and SphereGrinder) measures with the addition of the MolProbity score to favor or penalize models with realistic or unrealistic features of bond and dihedral angles and atom-atom clashes. Since the MolProbity score is relatively easy to improve with energy minimization with a molecular mechanics or knowledge-based scoring function without significantly altering the global and local accuracy measures, we scaled the MolProbity Z-scores by 0.2.
By analyzing the resulting Z-score sums of individual metrics and pairs of metrics, we observed that the top five groups differ somewhat with individual metrics since some groups perform better with local or global metrics or vice versa. When local and global metrics are combined pairwise, the top five groups are always the same with some reordering. In the final scoring function, the top five groups are LEER, Zhang, LEE, MULTICOM, and Zhang-Server. The LEER and LEE methods44 are based on a selection of models from the CASP11 server models (including their own server, nns), which were made publicly available 48 hours after the release of the targets, followed by a conformational space annealing refinement with a backbone-dependent rotamer library for side-chain conformations45 supplemented with a “residue-specific rotamer library.”44 The LEER method supplemented the LEE method by a final refinement step with molecular dynamics simulations. Zhang and Zhang-Server combine threading methods and a free-modeling approach to assemble the three-dimensional structure46, side chains built with the program REMO,47 which utilizes SCWRL3,48 followed by “fragment-dependent molecular dynamics simulations.” MULTICOM ranked server models with model-quality assessment scores and combined the models with a “model combination approach” and side-chain modeling with SCWRL, and further refinement with an energy-minimization approach implemented in 3Drefine.49 When taking the best model submitted by each group, according to a comparison with the native structure, the top 5 groups include LEER, Zhang, ProQ2, ProQ2-refine, and MULTICOM. ProQ2 selects the best model from the CASP11 server models with a model-quality assessment approach, while ProQ2-refine repacks the side chains of these models with Rosetta.50
With the same assessment scoring function, we evaluated the CASP11 servers on 81 targets. The top 5 groups were separated by the remaining groups by a significant drop in score. The top 5 groups when only model 1 was considered were Zhang-Server, nns (from the LEE group), BAKER-ROSETTASERVER, QUARK (from the Zhang group), and myprotein-me. The top 5 groups when the best model was considered were BAKER-ROSETTASERVER, nns, Zhang-Server, QUARK (from the Zhang group), and myprotein-me.
By comparing the GDT-TS of the best predicted model for each target and a model for each target built from the best template in the PDB (by copying coordinates according to a sequence alignment from a structure alignment of the template with the experimental structure of the target), we showed that the performances of the best predictors in recent CASPs (8, 9, 10, and 11) has been relatively steady with a small increase in the number of models with ΔGDT-TS>15 (i.e., models improved from the best template) in CASP10 and CASP11 compared to the earlier CASPs.
We analyzed the distribution of evaluation metrics for each target, and identified a bimodal probability density for most targets and measures. Multi-dimensional scaling of the RMSD distance matrix revealed that this was due to a single cluster of models reasonably close to the experimental structure and a broad distribution of models with very high RMSD to each other and to the native structure. The latter group appears in a separate mode in the probability distribution of each measure.
The data illustrate an important difference between evaluation parameters such as GDT-TS and LDDT, which measure accuracy as a percentage of the total structure, and thus bounded in value on both the left (low accuracy, a value of 0) and right (high accuracy, a value of 100) and a distance metric such as RMSD, which is bounded on the left (high accuracy, RMSD=0.0) but unbounded on the right. The boundedness of low accuracy models in GDT-TS results in a piling up of low-accuracy structures in a Gaussian-shaped peak at GDT-TS of about 20 in most of the targets. This peak is spread out in RMSD density and in the MDS plots of the RMSD values, since the RMSD value is unbounded and the models do not resemble each other.
We conclude that perhaps assessment methods should take account of the bimodal nature of these distributions, rather than assessment methods that depend on Z-scores, which presume a single, normal distribution of scores.
Supplementary Material
Acknowledgments
We thank Peter Huwe for comments on the manuscript. This work was funded by NIH Grant R01 GM084453.
References
- 1.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 2.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krivov GG, Shapovalov MV, Dunbrack RL., Jr Improved prediction of protein side-chain conformations with SCWRL4. Proteins. 2009;77:778–795. doi: 10.1002/prot.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browne WJ, North A, Phillips D, Brew K, Vanaman TC, Hill RL. A possible three-dimensional structure of bovine α-lactalbumin based on that of hen's egg-white lysozyme. J Mol Biol. 1969;42:65–86. doi: 10.1016/0022-2836(69)90487-2. [DOI] [PubMed] [Google Scholar]
- 6.Greer J. Model for haptoglobin heavy chain based upon structural homology. Proc Natl Acad Sci USA. 1980;77:3393–3397. doi: 10.1073/pnas.77.6.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Söding J. Protein homology detection by HMM-HMM comparison. Bioinformatics. 2005;21:951–960. doi: 10.1093/bioinformatics/bti125. [DOI] [PubMed] [Google Scholar]
- 8.Li Y. Conformational sampling in template-free protein loop structure modeling: an overview. Computational and structural biotechnology journal. 2013;5:e201302003. doi: 10.5936/csbj.201302003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosimann S, Meleshko R, James MN. A critical assessment of comparative molecular modeling of tertiary structures of proteins*. Proteins: Structure, Function, and Bioinformatics. 1995;23:301–317. doi: 10.1002/prot.340230305. [DOI] [PubMed] [Google Scholar]
- 10.Lemer CMR, Rooman MJ, Wodak SJ. Protein structure prediction by threading methods: evaluation of current techniques. Proteins: Structure, Function, and Bioinformatics. 1995;23:337–355. doi: 10.1002/prot.340230308. [DOI] [PubMed] [Google Scholar]
- 11.Martin AC, MacArthur MW, Thornton JM. Assessment of comparative modeling in CASP2. Proteins: Structure, Function and Genetics. 1997;(Suppl):14–28. doi: 10.1002/(sici)1097-0134(1997)1+<14::aid-prot4>3.3.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Jones TA, Kleywegt GJ. CASP3 comparative modeling evaluation. Proteins: Structure, Function and Genetics. 1999;37:30–46. doi: 10.1002/(sici)1097-0134(1999)37:3+<30::aid-prot6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Tramontano A, Leplae R, Morea V. Analysis and assessment of comparative modeling predictions in CASP4. Proteins: Structure, Function and Genetics. 2001;(Suppl 5):22–38. doi: 10.1002/prot.10015. [DOI] [PubMed] [Google Scholar]
- 14.Kinch LN, Wrabl JO, Krishna SS, Majumdar I, Sadreyev RI, Qi Y, Pei J, Cheng H, Grishin NV. CASP5 assessment of fold recognition target predictions. Proteins: Structure, Function and Genetics. 2003;53(Suppl 6):395–409. doi: 10.1002/prot.10557. [DOI] [PubMed] [Google Scholar]
- 15.Tramontano A, Morea V. Assessment of homology-based predictions in CASP5. Proteins: Structure, Function and Genetics. 2003;53(Suppl 6):352–368. doi: 10.1002/prot.10543. [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Jin Y, Dunbrack RL., Jr Assessment of fold recognition predictions in CASP6. Proteins: Structure, Function and Genetics. 2005 doi: 10.1002/prot.20721. [DOI] [PubMed] [Google Scholar]
- 17.Tress M, Ezkurdia I, Grana O, Lopez G, Valencia A. Assessment of predictions submitted for the CASP6 comparative modeling category. Proteins: Structure, Function and Genetics. 2005;61(Suppl 7):27–45. doi: 10.1002/prot.20720. [DOI] [PubMed] [Google Scholar]
- 18.Read RJ, Chavali G. Assessment of CASP7 predictions in the high accuracy template-based modeling category. Proteins. 2007;69(Suppl 8):27–37. doi: 10.1002/prot.21662. [DOI] [PubMed] [Google Scholar]
- 19.Kopp J, Bordoli L, Battey JN, Kiefer F, Schwede T. Assessment of CASP7 predictions for template-based modeling targets. Proteins. 2007;69(Suppl 8):38–56. doi: 10.1002/prot.21753. [DOI] [PubMed] [Google Scholar]
- 20.Keedy DA, Williams CJ, Headd JJ, Arendall WB, 3rd, Chen VB, Kapral GJ, Gillespie RA, Block JN, Zemla A, Richardson DC, Richardson JS. The other 90% of the protein: assessment beyond the Calphas for CASP8 template-based and high-accuracy models. Proteins. 2009;77(Suppl 9):29–49. doi: 10.1002/prot.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cozzetto D, Kryshtafovych A, Fidelis K, Moult J, Rost B, Tramontano A. Evaluation of template-based models in CASP8 with standard measures. Proteins. 2009;77(Suppl 9):18–28. doi: 10.1002/prot.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariani V, Kiefer F, Schmidt T, Haas J, Schwede T. Assessment of template based protein structure predictions in CASP9. Proteins. 2011;79(Suppl 10):37–58. doi: 10.1002/prot.23177. [DOI] [PubMed] [Google Scholar]
- 23.Huang YJ, Mao B, Aramini JM, Montelione GT. Assessment of template-based protein structure predictions in CASP10. Proteins. 2014;82(Suppl 2):43–56. doi: 10.1002/prot.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sippl MJ, Lackner P, Domingues FS, Koppensteiner WA. An attempt to analyse progress in fold recognition from CASP1 to CASP3. Proteins: Structure, Function and Genetics. 1999;(Suppl):226–230. doi: 10.1002/(sici)1097-0134(1999)37:3+<226::aid-prot29>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Orengo CA, Bray JE, Hubbard T, LoConte L, Sillitoe I. Analysis and assessment of ab initio three-dimensional prediction, secondary structure, and contacts prediction. Proteins: Structure, Function and Genetics. 1999;37:149–170. doi: 10.1002/(sici)1097-0134(1999)37:3+<149::aid-prot20>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Lesk AM, Lo Conte L, Hubbard TJ. Assessment of novel fold targets in CASP4: predictions of three-dimensional structures, secondary structures, and interresidue contacts. Proteins. 2001;5(Suppl):98–118. doi: 10.1002/prot.10056. [DOI] [PubMed] [Google Scholar]
- 27.Aloy P, Stark A, Hadley C, Russell RB. Predictions without templates: new folds, secondary structure, and contacts in CASP5. Proteins: Structure, Function and Genetics. 2003;53(Suppl 6):436–456. doi: 10.1002/prot.10546. [DOI] [PubMed] [Google Scholar]
- 28.Vincent JJ, Tai CH, Sathyanarayana BK, Lee B. Assessment of CASP6 predictions for new and nearly new fold targets. Proteins: Structure, Function and Genetics. 2005;61(Suppl 7):67–83. doi: 10.1002/prot.20722. [DOI] [PubMed] [Google Scholar]
- 29.Jauch R, Yeo HC, Kolatkar PR, Clarke ND. Assessment of CASP7 structure predictions for template free targets. Proteins. 2007;69(Suppl 8):57–67. doi: 10.1002/prot.21771. [DOI] [PubMed] [Google Scholar]
- 30.Kryshtafovych A, Monastyrskyy B, Fidelis K. CASP prediction center infrastructure and evaluation measures in CASP10 and CASP ROLL. Proteins: Structure, Function, and Bioinformatics. 2014;82(Suppl. 2):7–13. doi: 10.1002/prot.24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zemla A. LGA: A method for finding 3D similarities in protein structures. Nucleic Acids Res. 2003;31:3370–3374. doi: 10.1093/nar/gkg571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antczak PLM, Ratajczak T, Blazewicz J, Lukasiak P. IEEE International Conference on Bioinformatics and Biomedicine (BIBM) IEEE; 2015. SphereGrinder-reference structure-based tool for quality assessment of protein structural models; pp. 665–668. [Google Scholar]
- 33.Mariani V, Biasini M, Barbato A, Schwede T. LDDT: a local superposition-free score for comparing protein structures and models using distance difference tests. Bioinformatics. 2013;29:2722–2728. doi: 10.1093/bioinformatics/btt473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor TJ, Bai H, Tai CH, Lee B. Assessment of CASP10 contact-assisted predictions. Proteins. 2014;82(Suppl 2):84–97. doi: 10.1002/prot.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinch LN, Li W, Schaeffer RD, Dunbrack RL, Jr, Monastyrskyy B, Kryshtafovych A, Grishin NV. CASP11 target classification. Proteins. 2016 doi: 10.1002/prot.24982. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis IW, Murray LW, Richardson JS, Richardson DC. MOLPROBITY: structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic Acids Res. 2004;32:W615–W619. doi: 10.1093/nar/gkh398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovell SC, Davis IW, Arendall WB, 3rd, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Calpha geometry: phi, psi and Cbeta deviation. Proteins: Structure, Function and Genetics. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 38.Theobald DL, Wuttke DS. THESEUS: maximum likelihood superpositioning and analysis of macromolecular structures. Bioinformatics. 2006;22:2171–2172. doi: 10.1093/bioinformatics/btl332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theobald DL, Steindel PA. Optimal simultaneous superpositioning of multiple structures with missing data. Bioinformatics. 2012;28:1972–1979. doi: 10.1093/bioinformatics/bts243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukasiak P, Antczak M, Ratajczak T, Szachniuk M, Blazewicz J. Proceedings of the 25th European conference on operational research. Vilnius, Lithuania: 2012. Quality assessment methodologies in analysis of structural models; pp. 8–11. [Google Scholar]
- 41.Larsson P, Skwark MJ, Wallner B, Elofsson A. Assessment of global and local model quality in CASP8 using Pcons and ProQ. Proteins. 2009;77(Suppl 9):167–172. doi: 10.1002/prot.22476. [DOI] [PubMed] [Google Scholar]
- 42.Cao R, Bhattacharya D, Adhikari B, Li J, Cheng J. Massive integration of diverse protein quality assessment methods to improve template based modeling in CASP11. Proteins. 2015 doi: 10.1002/prot.24924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moult J, Fidelis K, Kryshtafovych A, Schwede T, Tramontano A. Critical Assessment of methods of protein structure prediction (CASP) -- progress and new directions in Round XI. Proteins. 2016 doi: 10.1002/prot.25064. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joo K, Joung I, Lee SY, Kim JY, Cheng Q, Manavalan B, Joung JY, Heo S, Lee J, Nam M, Lee IH, Lee SJ, Lee J. Template based protein structure modeling by global optimization in CASP11. Proteins. 2015 doi: 10.1002/prot.24917. [DOI] [PubMed] [Google Scholar]
- 45.Shapovalov MV, Dunbrack RL., Jr A smoothed backbone-dependent rotamer library for proteins derived from adaptive kernel density estimates and regressions. Structure. 2011;19:844–858. doi: 10.1016/j.str.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu D, Zhang Y. Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins: Structure, Function, and Bioinformatics. 2012;80:1715–1735. doi: 10.1002/prot.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Zhang Y. REMO: A new protocol to refine full atomic protein models from C-alpha traces by optimizing hydrogen-bonding networks. Proteins. 2009;76:665–676. doi: 10.1002/prot.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canutescu AA, Shelenkov AA, Dunbrack RL., Jr A graph-theory algorithm for rapid protein side-chain prediction. Protein Sci. 2003;12:2001–2014. doi: 10.1110/ps.03154503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhattacharya D, Cheng J. 3Drefine: Consistent protein structure refinement by optimizing hydrogen bonding network and atomic-level energy minimization. Proteins: Structure, Function, and Bioinformatics. 2013;81:119–131. doi: 10.1002/prot.24167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leaver-Fay A, Tyka M, Lewis SM, Lange OF, Thompson J, Jacak R, Kaufman K, Renfrew PD, Smith CA, Sheffler W. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 2011;487:545–574. doi: 10.1016/B978-0-12-381270-4.00019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.