Abstract
Melanocytic nevi are a strong phenotypic marker of cutaneous melanoma risk. Changes in nevi during childhood and adolescence make these prime periods for studying nevogenesis. Insights gained by the study of nevi in childhood have implications for melanoma detection in both adults and children. A more comprehensive understanding of the morphologic characteristics of nevi in different anatomic locations, in association with the patient’s age and pigmentary phenotype may aid in the identification of melanomas. When monitoring melanocytic lesions over time, it is essential to differentiate normal from abnormal change. In this review, we summarize the rapidly expanding body of literature, particularly in the context of our experience with the Study of Nevi in Children (SONIC) Project.
Keywords: Nevi, Nevogenesis, Melanoma, Detection, Dermoscopy, Childhood, Adolescence
Introduction
In 2010, more than 49,000 individuals were estimated to have died from melanoma worldwide.1 Nevi are among the strongest phenotypic markers of melanoma risk2 .iagnosis often relies on differentiating a melanoma from the patient’s many nevi. This can be challenging because nevi are heterogeneous and their morphology varies depending on the patient’s age, pigmentary phenotype, ultraviolet radiation exposure, and genotype, as well as the anatomic location and potential for change.3–14 While current aids for melanoma detection, such as ABCDE criteria [A (asymmetry), B (border irregularity), C (color variegation), D (diameter>6 mm), E (evolving)] and dermoscopy,15–19 rely on morphology to distinguish nevi from melanomas, patient-related factors are not included in diagnostic algorithms. A more comprehensive understanding of the natural history of nevi and variations in their patterns is needed to improve the melanoma detection.20
The first 2 decades of life are notable for appearance and growth of nevi, making this an important period for studying nevogenesis.21–23 Insights gained by the study of nevi in childhood have implications for melanoma detection in adults and children. The Study of Nevi in Children (SONIC), a cohort in Framingham, Massachusetts11–13,24–36 is a population-based study that documents the dermoscopic and clinical evolution of nevi in childhood and adolescence over time. Herein, we summarize a rapidly expanding body of literature, particularly in the context of our experience with SONIC, regarding patient-related factors that contribute to nevus phenotype and their implications for melanoma diagnosis.
Age related prevalence of dermoscopic patterns of nevi
The predominant dermoscopic pattern can be predicted based on the patient’s age. In early childhood, a globular nevus pattern is most prevalent, while in adulthood, a reticular pattern predominates (Table 1).10 In the SONIC cohort we observed a gradual decline in the percentage of globular nevi and an increase in that of reticular nevi during the 2nd decade of life.12,31,37 A similar trend was observed in a pediatric cohort study from Italy.38 Cross-sectional analysis of the dermoscopic patterns of nevi on the trunk by age is consistent with these longitudinal observations (Table 1).
Table 1.
Prevalence of dermoscopic pattern of nevi by age
| Study (ref) | Design | No. of nevi (No. of patients) | Age of patients, years | Prevalence of dermoscopic pattern by age | |
|---|---|---|---|---|---|
| Globular No. (%) | Reticular No. (%) | ||||
| Scope A et al 2008 (12) | Cross-sectional | 1198 (443) | 11 | 449 (37%) | 155 (13%) |
| Fonsesca M et al 2015 (31) | Cross-sectional | 2,320 (509) | 14 | 357 (15%) | 868 (38%) |
| Fortina AB et al 2012 (38) | Longitudinal | 717 (160) | Baseline 3–16; up to 7 years FU* | Baseline - 144 (20%) | Baseline - 336 (47%) |
| FU -80 (11%) | FU -374 (52%) | ||||
| Zalaudek I et al 2011 (10) | Cross sectional | 5481 (480) | |||
| 363 | 3–10 | 212 (58%) | 58 (16%) | ||
| 902 | 11–20 | 380 (42%) | 318 (35%) | ||
| 1019 | 21–30 | 325 (32%) | 501 (32%) | ||
| 1057 | 31–40 | 215 (20%) | 646 (61%) | ||
| 832 | 41–50 | 182 (22%) | 498 (60%) | ||
| 677 | 51–60 | 120 (18%) | 461 (68%) | ||
| 446 | 61–75 | 64 (14%) | 318 (71%) | ||
| 185 | >75 | 14 (8%) | 133 (72%) | ||
FU – follow-up
Another dermoscopic pattern with an age-dependent prevalence is the peripheral globular pattern. This nevus morphology is associated with growth,39 showing large junctional nests at the periphery on examination by histopathology and reflectance confocal microscopy.40 Among SONIC participants aged 14 (n=345), this pattern was observed in approximately 3% of back nevi (n=1,152), but was rare (<0.5%) in leg nevi (n=290) (unpublished data). Zalaudek et al10 showed the relative frequency of nevi with peripheral globules on the trunk among patients seen in pigmented lesion clinics to be 9% in the 1st decade, 5% in the 2nd decade, 4% in the 3rd decade, and 1.5% in the 4th decade, after which the frequency became <1%.
Implications for clinical practice
A new globular nevus is less common with advanced age (Fig 1). Recently, a subtype of melanoma termed “nested melanoma of the elderly” has been reported.41–44 These melanomas have irregularly distributed globules on dermoscopic examination, corresponding to a “clod” pattern of large compact nests on confocal microscopy, and to large intra-epidermal nests on histopathologic examination.41–44 Early recognition is important given the observation of Beer et al45 that melanomas harboring a predominantly globular pattern, with large nests and aggregates on histopathology, grow faster than melanomas that show a predominantly reticular pattern, with lentiginous or small-nested pattern on histopathology.
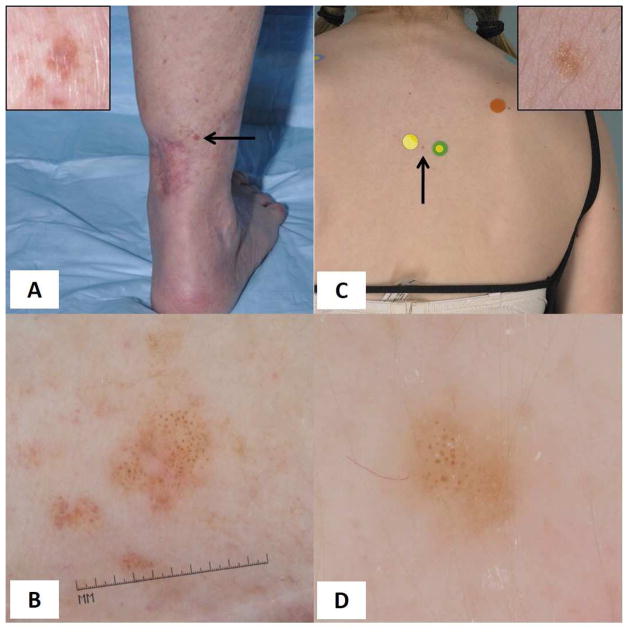
Figure 1. Melanoma and Nevus.
Age-dependent evaluation of globular lesions. (A) An 84-year-old female patient with history of 3 prior melanomas presented with a 6 mm light brown flat papule adjacent to a previous melanoma scar on the leg. (B) On dermoscopy, the lesion presented a diffuse globular pattern. While banal-appearing, a new globular-patterned nevus in an older individual is unlikely. The lesion was excised and diagnosed as melanoma 0.8mm in thickness. (C) Clinical image of a nevus presenting as 4 mm macule on the upper back of a 14 year-old child. (D) On dermoscopy, this nevus shows a globular pattern. Despite the morphologic resemblance of the nevus to the melanoma shown in (B), the young age and anatomic location are typical for a globular nevus.
The age-dependent prevalence of nevi with peripheral globules has clinical implications (Fig 2). In the 2nd–3rd decades,10 nevi with a peripheral rim of globules do not require specific intervention. In patients >30 years, nevi with a peripheral rim of globules without any other melanoma-specific criteria should be digitally monitored to ensure symmetric enlargement. The growth rate of nevi with peripheral globules is comparable to the growth rate of some melanomas.33,45 Unlike melanoma, nevi with peripheral globules grow symmetrically and do not develop any new structures or colors as they grow, lack melanoma-specific structures, and the peripheral globules become smaller and eventually disappear once nevus growth ceases. In patients >50 years, nevi with peripheral globules are rare, the incidence of melanoma among new or changing lesions is relatively high, and this dermoscopic pattern should prompt consideration of biopsy.10,46
Figure 2. Nevus and Melanoma.
Age-dependent evaluation of lesions with peripheral globules. (A) A growing nevus on the back of a 17-year-old female patient with family history of melanoma. The dermoscopic pattern, showing symmetric rim of peripheral globules, is commonly encountered in growing nevi in young patients. (B) A 5 mm brown macule on the right upper abdomen of a 60-year-old male patient without personal or family history of melanoma. Dermoscopy shows a reticular pattern with peripheral globules and streaks, and although these are distributed around the entire perimeter, the pattern indicates a growing melanocytic neoplasm in an individual >50 years. The lesion was excised and proved melanoma 0.5mm in thickness.
Anatomic site-related prevalence of dermoscopic patterns of nevi
In SONIC, we observed that globular-patterned nevi occur more frequently on the upper part of the body, where they tend to be larger in diameter; on the lower trunk and extremities, the predominant nevus pattern becomes increasingly reticular and nevi tend to be smaller.12,31 At age 11, nevi were more likely to be globular on the upper than on the lower back (OR=2, 95% CI 1.6–2.6, p<0.001).12 At age 14, compared to referent homogeneous nevi, globular nevi were more commonly observed on the back than the legs (OR=29.4, 95% CI 9.5–90.7, p<0.001), whereas reticular nevi were less likely to be observed on the back than the legs (OR=0.7, 95% CI 0.5–0.8, p=0.001).31,37
Similarly, congenital melanocytic nevi with a globular pattern are observed more commonly on the trunk than on the extremities.47,48 These findings are consistent with histopathologic data that bulkier, predominantly dermal nevi, which correlate dermoscopically with globular-patterned nevi, are more common in cephalad than in caudal locations.49 Based on the distribution of globular nevi by anatomic site, which is compatible with the embryonic cephalad-to-caudal and axial-to-peripheral melanoblast migration sequence, we and others9,12 speculate that nevi showing a globular dermoscopic pattern may be congenital in origin. This hypothesis is supported by the observation that globular nevi are more likely to appear in children than later in life.
Implications for clinical practice
A pigmented lesion showing a globular pattern on the lower extremities in adults is concerning in the absence of a history of long-standing stability (Fig 3). Although the prevalence of melanomas displaying a predominantly globular pattern at diagnosis is unknown (but likely uncommon), most melanomas will show a multicomponent pattern often with atypical dots and/or globules.50 The subtype of melanoma termed “nested melanoma of the elderly” 41–44 is uniquely characterized by a globular pattern on dermoscopic examination. Of 24 melanomas reported with this pattern, 15 (63%) have occurred on the extremities.41–44
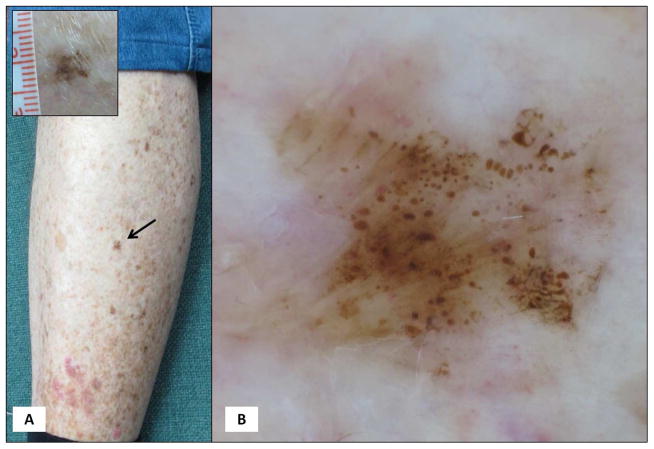
Figure 3. Melanoma.
Anatomic site-dependent evaluation of a globular lesion. (A) A 77-year-old male patient with 2 prior melanomas, was found to have a 5 mm brown macule on the leg. (B) Dermoscopy reveals a globular pattern with globules non-uniform in shape and spacing. A globular patterned lesion on the leg of an older individual, without history of long-standing stability is highly suspicious; indeed, the lesion proved melanoma 0.1mm in thickness.
A solitary pigmented lesion that breaks the cephalad-to-caudal size and pattern gradient should be closely examined. For example, an acquired reticular-patterned melanocytic lesion located on the upper torso and significantly larger than the patient’s other nevi is an “ugly duckling”51–54 that should be carefully analyzed with dermoscopy for melanoma-specific criteria (Fig 4) such as those seen in melanomas on sun-damaged skin.55
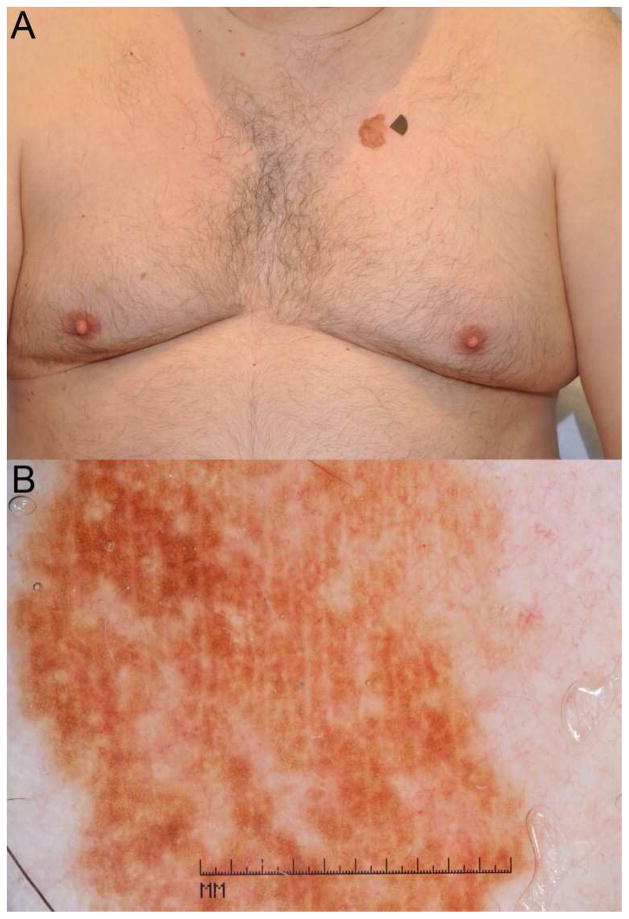
Figure 4. Melanoma.
A solitary lesion “breaking” the cephalad-caudal distribution gradient of nevi. (A) A 74-year-old male patient without personal or family history of melanoma, was found to have a >20mm brown patch on the chest. The patient denied having this lesion from childhood and noticed this growing lesion on his chest for many years. (B) Dermoscopy revealed a seemingly uniform reticular pattern, but without criteria for the diagnosis of solar lentigo. A solitary, large, acquired reticular-patterned melanocytic neoplasm on the torso of an adult is suspicious for melanoma, and indeed, the lesion proved to be melanoma 0.15mm in maximal thickness.
Pigment phenotype-related prevalence of dermoscopic patterns of nevi
There is a predictable association between a patient’s skin color, and the dermoscopic pattern and size of nevi. In SONIC, we observed that at age 11, a darker pigment phenotype was associated with presence of reticular nevi (p<0.0001) and smaller nevus size (p<0.0001 for trend).12 Similarly, at age 14, reticular-patterned nevi were twice as likely to be associated with darker than with lighter skin color (p<0.001).31 Globular, homogeneous, and complex dermoscopic patterns were more frequently observed in nevi of children with a lighter pigment phenotype.12 In 680 white adult patients, Zalaudek et al found that the frequency of reticular patterned nevi on dermoscopy increased with darker pigment phenotype (OR=2.8 for skin type IV v. type I, p=0.03).5 Skin color has been associated with variation in the melanocortin-1 receptor (Mc1R).56 Quint et al tested the association of dermoscopic patterns and structures by the number of Mc1R red-hair-color (RHC) variants, the premise being that the more variants one has, the lighter the pigmentary phenotype of that individual: in 876 clinically atypical nevi from 111 patients, they found that pigment network and dark-brown color were more frequently observed in atypical nevi from individuals without RHC variants, while structureless areas were more often observed in individuals with two RHC variants.57
Implications for clinical practice
Melanoma presents as an “ugly duckling”51–54 when it deviates from the expected dermoscopic pattern of the patient’s other nevi,53,58 based on pigmentary phenotype. Hence, a darkly pigmented lesion with dermoscopic criteria for melanocytic neoplasia and without a long-standing history of stability in an individual with fair skin should be examined carefully (Fig 5). The converse is similarly true: a new non-pigmented papule in a patient with skin type ≥ III may be concerning for amelanotic melanoma. Puig et al59 found that early melanomas from patients with two MC1R RHC variants may be difficult to diagnose because they harbor few dermoscopic colors and structures. They recommended sequential dermoscopic monitoring in patients with this genotype, with particular attention to the presence of subtle atypical vessels.
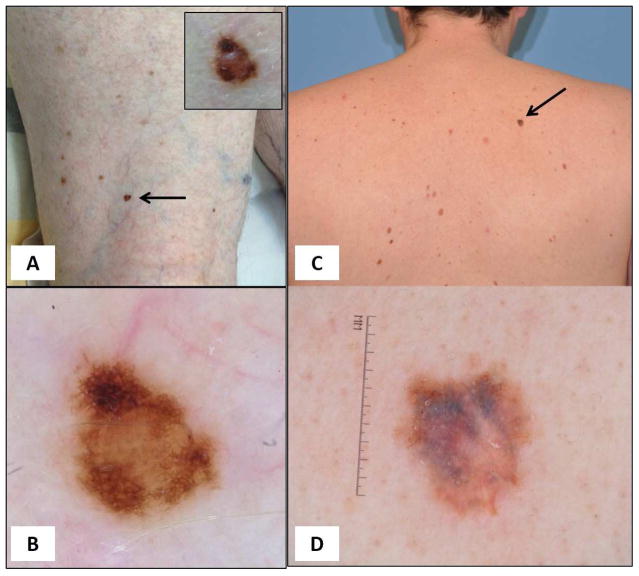
Figure 5. Melanoma.
Lesions “too dark” for the patient’s pigmentary phenotype. (A) An 80-year-old male patient presented with multiple nevi, blue eyes and skin phototype II, and reported blonde hair in his youth. A 5-mm papule (arrow) stands out as an ugly duckling based on its dark brown color. (B) Dermoscopy reveals a central homogenous peripheral reticular pattern with focal thickened network. This was a melanoma 0.45mm in thickness. (C) A 43-year-old male patient, with multiple atypical nevi and without previous history of melanoma, presented for routine examination. A 9-mm macule (arrow) stands out as an ugly duckling based on its dark brown color. (D) Dermoscopy shows a homogenous reticular pattern with variegate colors. This was a melanoma 0.95mm in thickness.
Changes in nevi during childhood and adolescence
Cross-sectional and longitudinal studies have shown that nevus counts increase during childhood and adolescence.60–65 A 3-year prospective study of 164 white children (87 aged 6–7 years; 77 aged 9–10 years) living in Vancouver, Canada showed that median whole-body nevus counts increased from 68 to 96 nevi; however, a few children were noted to have a lower nevus count at follow-up.60 Among SONIC participants, many new nevi appeared, but existing nevi also decreased in size, faded, or completely disappeared. Among 366 children followed from ages 11 to 14, 75% developed new nevi with an average increase in back nevus count of 2; 28% of participants had at least 1 nevus that disappeared in this time period.11 These findings of dynamic turnover among melanocytic nevi in early life are supported by a longitudinal study of facial and neck nevi in 20 adolescents aged 12–14 years old in Queensland, Australia.61 After 3-years, 190 new nevi were observed and 61 of the 230 baseline nevi (26.5%) had disappeared.66 In SONIC a majority of new nevi detected at age 14 and followed through age 17 (n=121) either increased in size (43%), showed no change (50%), disappeared (5%) or decreased in size (2%).67 Children with the highest baseline nevus counts were those with the highest nevus volatility, i.e., a proclivity for new and disappearing nevi.11,13 Sunburns were associated with an increase in nevus counts in these children. Among 936 nevi followed over a 3-year period (age 11 to 14), nearly 31% of nevi showed a change in global dermoscopic pattern.11 The most frequent change was from a patterned (i.e., reticular, globular, complex) nevus to a homogenous nevus and vice versa; this type of pattern volatility was most frequent among children with a light pigmentary phenotype and tendency to sunburn.11
Change in size of nevi in adolescence was common in the SONIC cohort. Of 404 back nevi followed from ages 11 to 17, >70% changed in surface area by at least 50%; 59% increased, 3% decreased, and 10% disappeared.67 Nevus growth differed by global dermoscopic pattern: compared to reticular nevi, globular nevi were significantly more likely to increase in size (65% v. 52%), and less likely to decrease (7% v. 18%) (p= 0.02).67 This growth characteristic difference by dermoscopic pattern was most pronounced for nevi with the greatest surface area at baseline (p=0.001).67
In SONIC, we observed that the vast majority of disappearing nevi in childhood had no evidence of a clinical halo phenomenon or dermoscopic regression structures (i.e., white scar-like areas and multiple blue-grey granules). Among 945 back nevi followed from ages 11 to 14 (n=336 participants), 9 (2.7%) were noted to completely disappear without displaying a halo or regression structures in preceding images.11 Preliminary analysis of 945 nevi from the back and legs followed from age 14 to 17 (n=213 participants) showed that 22 nevi (2.3%) completely disappeared; all but one disappeared without a halo or regression (Fig 6); unpublished data). In one nevus, a clinical halo and dermoscopic blue-grey granularity were distributed symmetrically around and throughout the entire nevus, respectively. Disappearance of nevi was more common among children with higher nevus counts, suggesting that volatility may have clinical implications for melanoma risk.68
Figure 6. Nevi.
Patterns of nevus disappearance in childhood. Among SONIC participants we observed 2 patterns of disappearance of nevi. The overwhelming majority of disappearing nevi showed only gradual fading of pigmentation pattern (A, upper panel), in contrast to the halo-nevus phenomenon with blue-grey granularity (B, Lower panel), which was infrequently observed.
Implications for clinical practice in children and risk of pediatric melanoma
Use of total body photography in children and adolescents to identify new or changing nevi, and surgical removal based on change, are likely to have poor specificity given the volatility of nevi in this age group (Fig 7). Recent estimates in the United States and Europe suggest that between 594 and 982 benign nevi are removed for every one melanoma detected in individuals aged ≤ 19 years.69,70 Banky et al46 have shown the likelihood of melanoma detection among new or changing lesions identified with total body photography is age dependent, ranging from 0.4% of new lesions at age <50 years to 30% of new lesions at age >50. Similarly, Menzies et al71 found that dermoscopic change in melanocytic lesions undergoing short-term follow-up (2.5–4.5 months) had poor specificity for the diagnosis of melanoma in children and adolescents. Pediatric melanomas often fail to exhibit conventional ABCDE criteria but change remains an important diagnostic clue despite its poor specificity. Change was present in nearly 100% of cases from a recent pediatric melanoma cohort (n=70)72; thus, a changing lesion that differs from the child’s other nevi (i.e., an ugly duckling) should be carefully examined, with the understanding that childhood melanoma may present alternate ABCDE features72 [A (amelanosis), B (bleeding, bumps), C (uniform color), D (small diameter, de novo), and E (evolution)]. However, the clinical features described as ABCDE for pediatric melanoma are not specific for melanoma and could have a low predictive value. To avoid unnecessary biopsy, of common benign conditions in children with a similar description (e.g., arthropod bite), lesions without clinical or dermoscopic features suggestive of melanoma could be closely observed. Because pediatric melanomas can be amelanotic, subtle dermoscopic clues in blood vessels may be pertinent. Menzies et al73 evaluated 497 amelanotic and hypomelanotic skin lesions and identified predominantly central vessels, hairpin vessels, milky red-pink areas, more than one shade of pink, a combination of dotted and linear irregular vessels, and a predominance of linear irregular vessels, as the most important clues to the diagnosis of melanoma. Disappearance and fading of nevi are not rare in childhood and adolescence and should not trigger alarm. Most of these nevi will disappear via gradual global fading of pigmentation, with transition of dermoscopic appearance from patterned to homogenous to eventual complete disappearance (Fig 6).
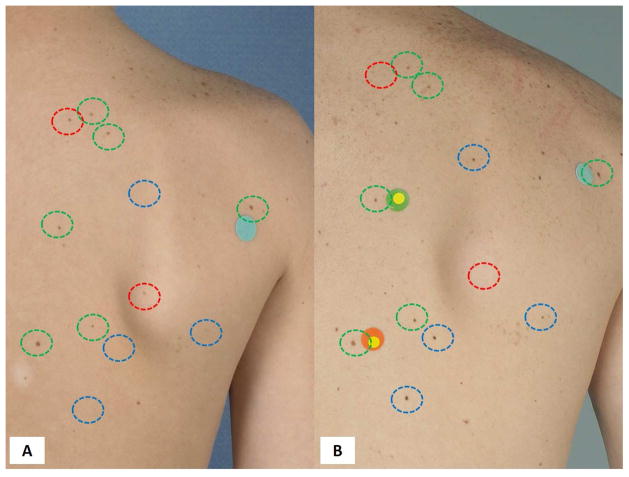
Figure 7. Nevi.
Nevus volatility in childhood. Overview images of the back at age 11 (A) and 17 (B) showing multiple new (blue circles), disappearing / fading (red circles) and stable nevi (green circles).
Additional research is required to develop useful criteria for recognizing high-risk lesions, to more clearly identify normal patterns of change in nevi during childhood and adolescence, and to determine how non-invasive technologies, such as sequential digital dermoscopic imaging, can be used to improve diagnostic accuracy in this age range.
Conclusions
The SONIC project has contributed to a more comprehensive understanding of the distribution, morphology, and host-associations of melanocytic nevi. This knowledge may help in the detection of melanomas that are difficult to diagnose from appearance alone, based on their deviation from what is expected in benign nevi. Conversely, familiarity with the morphologic variations of nevi may reduce unnecessary biopsies in children and adolescents. With advances in non-invasive imaging technologies and molecular studies, future studies will better define which nevi are stronger markers of melanoma risk, the effects of genetic-environmental interactions in nevogenesis, and the role of prevention strategies.
Acknowledgments
Funding Sources: Research reported in this publication was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01-AR049342. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- SONIC
Study of Nevi in Children
- OR
odds ratio
- CI
confidence interval
- RHC
red hair color
- Mc1R
Melanocortin-1 receptor
Footnotes
Conflicts of Interest: The authors have no conflict of interest to declare.
Human Rights: The study of Nevi in Children has been approved by the authors’ Institutional Review Board (Harvard University).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41(1):28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Marchetti MA, Kiuru MH, Busam KJ, et al. Melanocytic Naevi with Globular and Reticular Dermoscopic Patterns Display Distinct BRAF V600E Expression Profiles and Histopathologic Patterns. Br J Dermatol. 2014 doi: 10.1111/bjd.13260. [DOI] [PubMed] [Google Scholar]
- 4.Argenziano G, Zalaudek I, Ferrara G, Hofmann-Wellenhof R, Soyer HP. Proposal of a new classification system for melanocytic naevi. Br J Dermatol. 2007;157(2):217–227. doi: 10.1111/j.1365-2133.2007.07972.x. [DOI] [PubMed] [Google Scholar]
- 5.Zalaudek I, Argenziano G, Mordente I, et al. Nevus type in dermoscopy is related to skin type in white persons. Arch Dermatol. 2007;143(3):351–356. doi: 10.1001/archderm.143.3.351. [DOI] [PubMed] [Google Scholar]
- 6.Zalaudek I, Grinschgl S, Argenziano G, et al. Age-related prevalence of dermoscopy patterns in acquired melanocytic naevi. Br J Dermatol. 2006;154(2):299–304. doi: 10.1111/j.1365-2133.2005.06973.x. [DOI] [PubMed] [Google Scholar]
- 7.Zalaudek I, Guelly C, Pellacani G, et al. The dermoscopical and histopathological patterns of nevi correlate with the frequency of BRAF mutations. J Invest Dermatol. 2011;131(2):542–545. doi: 10.1038/jid.2010.332. [DOI] [PubMed] [Google Scholar]
- 8.Zalaudek I, Hofmann-Wellenhof R, Kittler H, et al. A dual concept of nevogenesis: theoretical considerations based on dermoscopic features of melanocytic nevi. J Dtsch Dermatol Ges. 2007;5(11):985–992. doi: 10.1111/j.1610-0387.2007.06384.x. [DOI] [PubMed] [Google Scholar]
- 9.Zalaudek I, Hofmann-Wellenhof R, Soyer HP, Ferrara G, Argenziano G. Naevogenesis: new thoughts based on dermoscopy. Br J Dermatol. 2006;154(4):793–794. doi: 10.1111/j.1365-2133.2006.07152.x. [DOI] [PubMed] [Google Scholar]
- 10.Zalaudek I, Schmid K, Marghoob AA, et al. Frequency of dermoscopic nevus subtypes by age and body site: a cross-sectional study. Arch Dermatol. 2011;147(6):663–670. doi: 10.1001/archdermatol.2011.149. [DOI] [PubMed] [Google Scholar]
- 11.Scope A, Dusza SW, Marghoob AA, et al. Clinical and dermoscopic stability and volatility of melanocytic nevi in a population-based cohort of children in Framingham school system. J Invest Dermatol. 2011;131(8):1615–1621. doi: 10.1038/jid.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scope A, Marghoob AA, Dusza SW, et al. Dermoscopic patterns of naevi in fifth grade children of the Framingham school system. Br J Dermatol. 2008;158(5):1041–1049. doi: 10.1111/j.1365-2133.2008.08510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveria SA, Scope A, Satagopan JM, et al. Factors Associated with Nevus Volatility in Early Adolescence. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piliouras P, Gilmore S, Wurm EM, Soyer HP, Zalaudek I. New insights in naevogenesis: number, distribution and dermoscopic patterns of naevi in the elderly. Australas J Dermatol. 2011;52(4):254–258. doi: 10.1111/j.1440-0960.2011.00794.x. [DOI] [PubMed] [Google Scholar]
- 15.Bafounta ML, Beauchet A, Aegerter P, Saiag P. Is dermoscopy (epiluminescence microscopy) useful for the diagnosis of melanoma? Results of a meta-analysis using techniques adapted to the evaluation of diagnostic tests. Arch Dermatol. 2001;137(10):1343–1350. doi: 10.1001/archderm.137.10.1343. [DOI] [PubMed] [Google Scholar]
- 16.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3(3):159–165. doi: 10.1016/s1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 17.Rigel DS, Friedman RJ, Kopf AW, Polsky D. ABCDE--an evolving concept in the early detection of melanoma. Arch Dermatol. 2005;141(8):1032–1034. doi: 10.1001/archderm.141.8.1032. [DOI] [PubMed] [Google Scholar]
- 18.Friedman RJ, Rigel DS, Kopf AW. Early detection of malignant melanoma: the role of physician examination and self-examination of the skin. CA Cancer J Clin. 1985;35(3):130–151. doi: 10.3322/canjclin.35.3.130. [DOI] [PubMed] [Google Scholar]
- 19.Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159(3):669–676. doi: 10.1111/j.1365-2133.2008.08713.x. [DOI] [PubMed] [Google Scholar]
- 20.Zalaudek I, Docimo G, Argenziano G. Using dermoscopic criteria and patient-related factors for the management of pigmented melanocytic nevi. Arch Dermatol. 2009;145(7):816–826. doi: 10.1001/archdermatol.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luther H, Altmeyer P, Garbe C, et al. Increase of melanocytic nevus counts in children during 5 years of follow-up and analysis of associated factors. Arch Dermatol. 1996;132(12):1473–1478. [PubMed] [Google Scholar]
- 22.Bauer J, Buttner P, Wiecker TS, Luther H, Garbe C. Risk factors of incident melanocytic nevi: a longitudinal study in a cohort of 1,232 young German children. Int J Cancer. 2005;115(1):121–126. doi: 10.1002/ijc.20812. [DOI] [PubMed] [Google Scholar]
- 23.Milne E, Simpson JA, English DR. Appearance of melanocytic nevi on the backs of young Australian children: a 7-year longitudinal study. Melanoma Res. 2008;18(1):22–28. doi: 10.1097/CMR.0b013e3282f20192. [DOI] [PubMed] [Google Scholar]
- 24.Scope A, Marghoob AA, Chen CS, Lieb JA, Weinstock MA, Halpern AC. Dermoscopic patterns and subclinical melanocytic nests in normal-appearing skin. Br J Dermatol. 2009;160(6):1318–1321. doi: 10.1111/j.1365-2133.2009.09073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dusza SW, Halpern AC, Satagopan JM, et al. Prospective study of sunburn and sun behavior patterns during adolescence. Pediatrics. 2012;129(2):309–317. doi: 10.1542/peds.2011-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dusza SW, Oliveria SA, Geller AC, Marghoob AA, Halpern AC. Student-parent agreement in self-reported sun behaviors. J Am Acad Dermatol. 2005;52(5):896–900. doi: 10.1016/j.jaad.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 27.Geller AC, Oliveria SA, Bishop M, Buckminster M, Brooks KR, Halpern AC. Study of health outcomes in school children: key challenges and lessons learned from the Framingham Schools’ Natural History of Nevi Study. J Sch Health. 2007;77(6):312–318. doi: 10.1111/j.1746-1561.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 28.LaVigne EA, Oliveria SA, Dusza SW, Geller AC, Halpern AC, Marghoob AA. Clinical and dermoscopic changes in common melanocytic nevi in school children: the Framingham school nevus study. Dermatology. 2005;211(3):234–239. doi: 10.1159/000087017. [DOI] [PubMed] [Google Scholar]
- 29.Oliveria SA, Geller AC, Dusza SW, et al. The Framingham school nevus study: a pilot study. Arch Dermatol. 2004;140(5):545–551. doi: 10.1001/archderm.140.5.545. [DOI] [PubMed] [Google Scholar]
- 30.Oliveria SA, Satagopan JM, Geller AC, et al. Study of Nevi in Children (SONIC): baseline findings and predictors of nevus count. Am J Epidemiol. 2009;169(1):41–53. doi: 10.1093/aje/kwn289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fonseca M, Marchetti MA, Chung E, et al. Cross-sectional analysis of the dermoscopic patterns and structures of melanocytic naevi on the back and legs of adolescents. Br J Dermatol. 2015;173(6):1486–1493. doi: 10.1111/bjd.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchetti MA, Fonseca M, Dusza SW, et al. Dermatoscopic imaging of skin lesions by high school students: a cross-sectional pilot study. Dermatol Pract Concept. 2015;5(1):11–28. doi: 10.5826/dpc.0501a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajaj S, Dusza SW, Marchetti MA, et al. Growth-Curve Modeling of Nevi With a Peripheral Globular Pattern. JAMA Dermatol. 2015 doi: 10.1001/jamadermatol.2015.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satagopan JM, Oliveria SA, Arora A, et al. Sunburn, sun exposure, and sun sensitivity in the Study of Nevi in Children. Ann Epidemiol. 2015;25(11):839–843. doi: 10.1016/j.annepidem.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: Mother and teenage daughters in discussion. J Health Psychol. 2014 doi: 10.1177/1359105314551621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlow I, Satagopan JM, Berwick M, et al. Genetic factors associated with naevus count and dermoscopic patterns: preliminary results from the Study of Nevi in Children (SONIC) Br J Dermatol. 2014 doi: 10.1111/bjd.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fonseca M, Burnett M, SD, et al. Dermoscopic patterns of nevi have a distinct anatomical distribution in adolescents. Abstract #315 presented at the 2014 Society for Investigative Dermatology Meeting in Albuquerque, New Mexico. J Invest Dermatol. 2014;134(Suppl 1):S49–60. [Google Scholar]
- 38.Fortina AB, Zattra E, Bernardini B, Alaibac M, Peserico A. Dermoscopic changes in melanocytic naevi in children during digital follow-up. Acta Derm Venereol. 2012;92(4):427–429. doi: 10.2340/00015555-1306. [DOI] [PubMed] [Google Scholar]
- 39.Kittler H, Seltenheim M, Dawid M, Pehamberger H, Wolff K, Binder M. Frequency and characteristics of enlarging common melanocytic nevi. Arch Dermatol. 2000;136(3):316–320. doi: 10.1001/archderm.136.3.316. [DOI] [PubMed] [Google Scholar]
- 40.Pellacani G, Scope A, Ferrari B, et al. New insights into nevogenesis: in vivo characterization and follow-up of melanocytic nevi by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61(6):1001–1013. doi: 10.1016/j.jaad.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Casari A, Cinotti E, Cesinaro AM, Pellacani G, Longo C. Dermatoscopy and reflectance confocal microscopy aspects of a “nested melanoma of the elderly”. Ann Dermatol Venereol. 2014;141(6–7):458–461. doi: 10.1016/j.annder.2014.04.118. [DOI] [PubMed] [Google Scholar]
- 42.Longo C, Zalaudek I, Piana S, et al. Dermoscopy and confocal microscopy of nested melanoma of the elderly: recognizing a newly defined entity. JAMA Dermatol. 2013;149(8):941–945. doi: 10.1001/jamadermatol.2013.321. [DOI] [PubMed] [Google Scholar]
- 43.Pennacchia I, Garcovich S, Gasbarra R, Leone A, Arena V, Massi G. Morphological and molecular characteristics of nested melanoma of the elderly (evolved lentiginous melanoma) Virchows Arch. 2012;461(4):433–439. doi: 10.1007/s00428-012-1293-0. [DOI] [PubMed] [Google Scholar]
- 44.Kutzner H, Metzler G, Argenyi Z, et al. Histological and genetic evidence for a variant of superficial spreading melanoma composed predominantly of large nests. Mod Pathol. 2012;25(6):838–845. doi: 10.1038/modpathol.2012.35. [DOI] [PubMed] [Google Scholar]
- 45.Beer JXL, Tschandl P, Kittler H. Growth rate of melanoma in vivo and correlation with dermatoscopic and dermatopathologic findings. Dermatology Practical & Conceptual. 2011;1(1):13. doi: 10.5826/dpc.dp0101a13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banky JP, Kelly JW, English DR, Yeatman JM, Dowling JP. Incidence of new and changed nevi and melanomas detected using baseline images and dermoscopy in patients at high risk for melanoma. Arch Dermatol. 2005;141(8):998–1006. doi: 10.1001/archderm.141.8.998. [DOI] [PubMed] [Google Scholar]
- 47.Seidenari S, Pellacani G, Martella A, et al. Instrument-, age- and site-dependent variations of dermoscopic patterns of congenital melanocytic naevi: a multicentre study. Br J Dermatol. 2006;155(1):56–61. doi: 10.1111/j.1365-2133.2006.07182.x. [DOI] [PubMed] [Google Scholar]
- 48.Changchien L, Dusza SW, Agero AL, et al. Age- and site-specific variation in the dermoscopic patterns of congenital melanocytic nevi: an aid to accurate classification and assessment of melanocytic nevi. Arch Dermatol. 2007;143(8):1007–1014. doi: 10.1001/archderm.143.8.1007. [DOI] [PubMed] [Google Scholar]
- 49.Krengel S. Nevogenesis--new thoughts regarding a classical problem. Am J Dermatopathol. 2005;27(5):456–465. doi: 10.1097/01.dad.0000175532.27368.3f. [DOI] [PubMed] [Google Scholar]
- 50.Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48(5):679–693. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 51.Grob JJ, Bonerandi JJ. The ‘ugly duckling’ sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998;134(1):103–104. doi: 10.1001/archderm.134.1.103-a. [DOI] [PubMed] [Google Scholar]
- 52.Gachon J, Beaulieu P, Sei JF, et al. First prospective study of the recognition process of melanoma in dermatological practice. Arch Dermatol. 2005;141(4):434–438. doi: 10.1001/archderm.141.4.434. [DOI] [PubMed] [Google Scholar]
- 53.Scope A, Dusza SW, Halpern AC, et al. The “ugly duckling” sign: agreement between observers. Arch Dermatol. 2008;144(1):58–64. doi: 10.1001/archdermatol.2007.15. [DOI] [PubMed] [Google Scholar]
- 54.Inskip M, Magee J, Weedon D, Rosendahl C. When algorithms falter: a case report of a very small melanoma excised due to the dermatoscopic “ugly duckling” sign. Dermatol Pract Concept. 2013;3(2):59–62. doi: 10.5826/dpc.0302a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaimes N, Marghoob AA, Rabinovitz H, et al. Clinical and dermoscopic characteristics of melanomas on nonfacial chronically sun-damaged skin. J Am Acad Dermatol. 2015;72(6):1027–1035. doi: 10.1016/j.jaad.2015.02.1117. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez-Laorden BL, Sanchez-Mas J, Martinez-Alonso E, Martinez-Menarguez JA, Garcia-Borron JC, Jimenez-Cervantes C. Dimerization of the human melanocortin 1 receptor: functional consequences and dominant-negative effects. J Invest Dermatol. 2006;126(1):172–181. doi: 10.1038/sj.jid.5700036. [DOI] [PubMed] [Google Scholar]
- 57.Quint KD, van der Rhee JI, Gruis NA, et al. Melanocortin 1 receptor (MC1R) variants in high melanoma risk patients are associated with specific dermoscopic ABCD features. Acta Derm Venereol. 2012;92(6):587–592. doi: 10.2340/00015555-1457. [DOI] [PubMed] [Google Scholar]
- 58.Argenziano G, Catricala C, Ardigo M, et al. Dermoscopy of patients with multiple nevi: Improved management recommendations using a comparative diagnostic approach. Arch Dermatol. 2011;147(1):46–49. doi: 10.1001/archdermatol.2010.389. [DOI] [PubMed] [Google Scholar]
- 59.Cuellar F, Puig S, Kolm I, et al. Dermoscopic features of melanomas associated with MC1R variants in Spanish CDKN2A mutation carriers. Br J Dermatol. 2009;160(1):48–53. doi: 10.1111/j.1365-2133.2008.08826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gallagher RP, Rivers JK, Lee TK, Bajdik CD, McLean DI, Coldman AJ. Broad-spectrum sunscreen use and the development of new nevi in white children: A randomized controlled trial. Jama. 2000;283(22):2955–2960. doi: 10.1001/jama.283.22.2955. [DOI] [PubMed] [Google Scholar]
- 61.Siskind V, Darlington S, Green L, Green A. Evolution of melanocytic nevi on the faces and necks of adolescents: a 4 y longitudinal study. J Invest Dermatol. 2002;118(3):500–504. doi: 10.1046/j.0022-202x.2001.01685.x. [DOI] [PubMed] [Google Scholar]
- 62.Gallagher RP, McLean DI, Yang CP, et al. Anatomic distribution of acquired melanocytic nevi in white children. A comparison with melanoma: the Vancouver Mole Study. Arch Dermatol. 1990;126(4):466–471. [PubMed] [Google Scholar]
- 63.Kelly JW, Rivers JK, MacLennan R, Harrison S, Lewis AE, Tate BJ. Sunlight: a major factor associated with the development of melanocytic nevi in Australian schoolchildren. J Am Acad Dermatol. 1994;30(1):40–48. doi: 10.1016/s0190-9622(94)70005-2. [DOI] [PubMed] [Google Scholar]
- 64.Harrison SL, MacKie RM, MacLennan R. Development of melanocytic nevi in the first three years of life. J Natl Cancer Inst. 2000;92(17):1436–1438. doi: 10.1093/jnci/92.17.1436. [DOI] [PubMed] [Google Scholar]
- 65.Darlington S, Siskind V, Green L, Green A. Longitudinal study of melanocytic nevi in adolescents. J Am Acad Dermatol. 2002;46(5):715–722. doi: 10.1067/mjd.2002.120931. [DOI] [PubMed] [Google Scholar]
- 66.Chang YM, Newton-Bishop JA, Bishop DT, et al. A pooled analysis of melanocytic nevus phenotype and the risk of cutaneous melanoma at different latitudes. Int J Cancer. 2009;124(2):420–428. doi: 10.1002/ijc.23869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu X, Fonseca M, Marchetti MA, et al. Longitudinally followed nevi in children and adolescents show significant size changes. Abstract #300 presented at the 2014 Society of Investigative Dermatology in Albuquerque, New Mexico. J Invest Dermatol. 2014;134(Suppl 1):S49–60. [Google Scholar]
- 68.Oliveria SA, Scope A, Satagopan JM, et al. Factors associated with nevus volatility in early adolescence. J Invest Dermatol. 2014;134(9):2469–2471. doi: 10.1038/jid.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oliveria SA, Selvam N, Mehregan D, et al. Biopsies of Nevi in Children and Adolescents in the United States, 2009 Through 2013. JAMA Dermatol. 2014 doi: 10.1001/jamadermatol.2014.4576. [DOI] [PubMed] [Google Scholar]
- 70.Moscarella E, Zalaudek I, Cerroni L, et al. Excised melanocytic lesions in children and adolescents - a 10-year survey. Br J Dermatol. 2012;167(2):368–373. doi: 10.1111/j.1365-2133.2012.10952.x. [DOI] [PubMed] [Google Scholar]
- 71.Menzies SW, Stevenson ML, Altamura D, Byth K. Variables predicting change in benign melanocytic nevi undergoing short-term dermoscopic imaging. Arch Dermatol. 2011;147(6):655–659. doi: 10.1001/archdermatol.2011.133. [DOI] [PubMed] [Google Scholar]
- 72.Cordoro KM, Gupta D, Frieden IJ, McCalmont T, Kashani-Sabet M. Pediatric melanoma: results of a large cohort study and proposal for modified ABCD detection criteria for children. J Am Acad Dermatol. 2013;68(6):913–925. doi: 10.1016/j.jaad.2012.12.953. [DOI] [PubMed] [Google Scholar]
- 73.Menzies SW, Kreusch J, Byth K, et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch Dermatol. 2008;144(9):1120–1127. doi: 10.1001/archderm.144.9.1120. [DOI] [PubMed] [Google Scholar]