Abstract
Probiotics which do not result in the development and spread of microbial resistance are among the candidate replacements for antibiotics previously used as growth promotors. In this study the effect of in-feed supplementation of the butyrate producing Butyricicoccus pullicaecorum strain 25-3T on performance, intestinal microbiota and prevention of necrotic enteritis (NE), a disease caused by Clostridium perfringens was evaluated in broilers. For the performance study, day old Ross 308 chicks were randomly allocated into two treatment groups and fed either a non-supplemented diet or a diet supplemented with 109 cfu lyophilized B. pullicaecorum per kg feed for 40 days. On day 40 broilers administered B. pullicaecorum had a significant lower bodyweight (2675 g vs. 2762 g; p = 0.0025) but supplementation of B. pullicaecorum decreased the feed conversion ratio significantly (1.518 vs. 1.632; p < 0.0001). Additionally, ingestion of the Butyricicoccus strain significantly lowered the abundance of Campylobacter spp. in the caecum and Enterococcus and Escherichia/Shigella spp. in the ileum at day 40. In feed supplementation of B. pullicaecorum in the NE trials resulted in a significant decrease in the number of birds with necrotic lesions compared with the untreated control group. These studies show that supplementation of B. pullicaecorum is able to improve feed conversion, to reduce the abundance of some potentially important pathogens in the caeca and ileum and to contribute to the prevention of NE in broilers, making the strain a potential valuable probiotic.
Keywords: Butyricicoccus, butyrate, broiler, microbiota, FCR
Introduction
Since the 1970’s, broilers have substantially improved in growth rate, breast-meat yield and efficiency of feed conversion (Dawkins and Layton, 2012). Feed conversion ratio (FCR), calculated as the ratio of feed consumed to weight gained, is a widely used performance measure, representing how efficiently the feed is utilized and converted into body mass (Stanley et al., 2012). The extraction of energy and nutrients from feed requires interplay between the biochemical functions provided by the chicken and the intestinal microbiota (Stanley et al., 2014a). The chicken gut harbors a very diverse microbiota that for many years has been kept stable by the use of sub-therapeutic dosages of antimicrobial growth promoters (AGPs) in the feed. The ban on the use of AGPs in the EU has resulted in an increase of intestinal health problems in broiler flocks and thus reduced profitability for poultry farmers. A disease that has emerged after the AGP ban is necrotic enteritis (NE), caused by Clostridium perfringens.
Prebiotics, probiotics, essential oils, plant extracts, and organic acids have been proposed as potential alternatives to antibiotics (Alloui et al., 2013). Probiotics or direct-fed microbials (DFM) are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (Hill et al., 2014). This beneficial effect can be achieved indirectly by improving the properties of the indigenous microbiota, resulting in the protection from enteric pathogen infections (Fuller, 1989). Most probiotic strains that are used in livestock are members of the bacterial genera Bacillus, Enterococcus, Lactobacillus or the yeast genus Saccharomyces. They are predominantly used to improve performance parameters, including market-aged body weight and FCR (Gaggia et al., 2010). Probiotics display multiple modes of action, including modification of gut pH via secretion of short chain fatty acids (SCFA), inhibition of the proliferation and colonization of pathogens and control of enteric diseases (van Der Wielen et al., 2000). In feed supplementation of the SCFA butyrate, which is an important end-product of anaerobic bacterial fermentation of carbohydrates, has been shown to decrease pro-inflammatory cytokine expression in broiler challenge models and to induce expression of antimicrobial host defense components in the gut (Sunkara et al., 2011; Zhang et al., 2011). Moreover, it stimulates proliferation, differentiation and function of mucosal epithelial cells and immune cells. Butyrate mediated beneficial effects on growth and intestinal integrity have been demonstrated in piglets (Kotunia et al., 2004; Le Gall et al., 2009) and broiler chickens (Adil et al., 2010; Mountzouris et al., 2010; Ahmed et al., 2014; Ritzi et al., 2014). In all these studies, butyric acid was added to the feed (Guilloteau et al., 2010). A valuable alternative strategy would consist of stimulation of in situ production of butyrate through administration of a selected bacterium or substrates that are converted to butyrate.
The objectives of the present study were to assess the effect of dietary supplementation of Butyricicoccus pullicaecorum, a butyrate-producing chicken isolate, on animal performance, on the intestinal microbiota composition and on the resistance against C. perfringens induced NE.
Materials and Methods
Strains
Butyricicoccus pullicaecorum strain 25-3T (LMG 24109), isolated from the caecal content of a chicken (Eeckhaut et al., 2008), member of Clostridium cluster IV and shown to produce 15.3 mM butyrate under lab conditions, was grown overnight at 37°C in M2GSC medium at pH6 (Miyazaki et al., 1997) in an anaerobic (84% N2, 8% CO2, and 8% H2) workstation (Ruskinn Technology, Bridgend, UK). After centrifugation (6400 × g, 37°C, 15 min) of the bacterial culture, the pellet was resuspended and lyophilized in lyoprotectant consisting of 750 ml horse serum and 250 ml distilled water supplemented with 7.5% sucrose (Sigma–Aldrich, St Louis, MO, USA), 0.63% nutrient broth (Sigma–Aldrich, St Louis, MO, USA) and 1 mg/ml cysteine HCl. The number of surviving cells after freeze drying was counted using the agar plate count method and expressed as cfu/g lyophilized powder.
Clostridium perfringens strain 56, originally isolated from the intestine of a chicken with severe necrotic gut lesions, was used to induce NE in the in vivo broiler model. This strain has been characterized as a NetB toxin positive type A strain (no β2 or enterotoxin genes) and a producer of moderate amounts of alpha toxin in vitro (Gholamiandekhordi et al., 2006). Before inoculation of the chickens, the bacteria were cultured in brain heart infusion broth (Oxoid, Basingstoke, UK) for 24 h at 37°C in the anaerobic cabinet.
Performance Trial
A total of 504 1-day-old (252 males and 252 females) Ross 308 broiler chicks obtained from a commercial hatchery were weighed and randomly allocated to one of two treatment groups. Each treatment group consisted of three replicates with 42 male and three replicates with 42 female chickens per pen. There was no significant difference in body weight between the two groups at the start of the trial. The birds were raised on concrete floor covered with wood shavings. Temperature was maintained at 33°C for the 1st week and then gradually reduced at a rate of 3°C per week to a final temperature of 22°C. The lighting schedule provided 18 h of light per day and proper ventilation was ensured by means of exhaust fans. The animals had ad libitum access to water and feed in a mash form that was formulated for a starter (days 1–13), grower (days 14–26), and finisher (days 27–40) period. Lyophilized probiotic bacteria were mixed thoroughly in a small amount of feed (1 kg). The resultant mixture was then mixed with the rest of the feed in a mechanical blender until a thorough and consistent mixture was obtained containing 109 cfu B. pullicaecorum/kg feed. All birds were given the same basal diet. The treatment was as follows: basal diet without addition (control) and basal diet containing 109 cfu B. pullicaecorum/kg feed.
Growth Performance, Feed Conversion Ratio, and Sample Collection
All chickens were weighed individually on d 14, 26, and 40. In addition, feed consumption for each pen between weighing was determined by measuring leftovers of the feed on the same days as the birds were weighed. FCR was calculated.
On days 26 and 40, three chickens per pen were randomly selected and euthanized by an intravenous overdose of sodium pentobarbital 20% (Kela, Hoogstraten, Belgium). The ileal and cecal content was collected yielding 18 digesta samples per treatment and time point. The samples were preserved at -80°C until 100 mg was weighed and subjected to total DNA extraction using hexadecyltrimethylammonium bromide (CTAB) based on the protocol described by Boon et al. (2003). Isolated DNA was used as template for further analyses.
Library Construction and Sequencing of the V4 Region of 16S Ribosomal DNA
The V4 region of the bacterial 16S rRNA gene was amplified using PCR primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) together with flanking molecular identifier (MID) and Illumina sequencing adapter as described by Kozich et al. (2013). PCR amplification was performed in a 50 μl reaction volume containing 25 μl 2X Taq Master Mix (Thermo Scientific, Aalst, Belgium), 0.2 μM of each forward and reverse primer and 20 ng DNA template. The reaction conditions consisted of an initial 94°C for 3 min, followed by 35 cycles at 94°C for 45 s, 50°C for 60 s and 72°C for 90 s, as well as a final extension at 72°C for 10 min. Next, amplified products with an expected band size of 300–350 bp were checked by 2% agarose gel electrophoresis and ethidium bromide staining. Amplicons were purified using the MoBio UltraClean PCR Clean-Up kit (Mo Bio, Carlsbad, CA, USA) according to the manufacturer’s instructions. Libraries purified from agarose gel electrophoresis were applied for sequencing using Illumina MiSeq sequencer and MiSeq v2 sequencing kit to produce 250 bp pair-end reads. The raw fastq sequences were merged using FLASH software (Magoc and Salzberg, 2011) and quality-filtered using the FASTX-toolkit1 Chimeras were removed using UCHIME v6.0.307 (Edgar et al., 2011) and each sample was downsampled to 10,000 reads using random selection of reads. The taxonomy of the reads was determined using RDP classifier (Wang et al., 2007), and species-level OTUs (taxonomical bins of sequences with >97% similarities) were clustered de novo using USEARCH v6.0 (Edgar, 2010). All taxonomy tables were created using Perl scripts for the follow-up analysis. Statistical analysis of alpha-diversity and beta-diversity were carried out using Vegan package (Dixon, 2003) in R.
Real-Time PCR Quantification of Campylobacter spp.
Members of the genus Campylobacter were quantified using the CFX384 Bio-Rad detection system (Bio-Rad, Nazareth-Eke, Belgium). Amplification and detection were carried out in a 384-well plate using 2X SensiMixTM SYBR No-ROX mix (Bioline, Nazareth-Eke, Belgium). Each reaction was done in triplicate in a 12 μl total reaction mixture using 2 μl of appropriate dilutions of the DNA sample and 0.5 μM final qPCR primer concentration (Table 1). The reaction conditions used were 1 cycle at 95°C for 10 min, followed by 40 cycles at 90°C for 30 s and 60°C for 45 s. A stepwise increase of the temperature from 65 to 95°C was added and melting curve data were analyzed to confirm specificity of the reaction. For construction of the standard curve, a 812 bp long PCR product was generated using standard PCR primers listed in Table 1 and genomic DNA from Campylobacter jejuni. After purification (Invitek, Berlin, Germany) and determination of the DNA concentration with a Nanodrop ND 1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), the volume of the linear dsDNA standard was adjusted to 108 copies/μl assuming an average molecular weight of 660 per nucleotide pair. This stock solution was serially diluted to obtain a standard series from 108 to 1 copies/μl with each step differing by 10-fold. The copy numbers of samples were determined by reading off the standard series with the cycle threshold (Ct) values of the samples. Gene copy numbers were expressed as log10 values per gram wet weight of intestinal content.
Table 1.
Sequence of used primers.
| Target | Forward primer | Reverse primer | Analysis | Reference |
|---|---|---|---|---|
| 16S rRNA gene from Campylobacter spp. | CATTGTAGCACGTGTGTC | GGATGACACTTTTCGGAGC | Standard PCR | This study |
| nt∗ 380–398 | nt 1191–1174 | |||
| GCGTAGGCGGATTATCAAGT | CGGATTTTACCCCTACACCA | qPCR | Bui et al., 2012 | |
| nt 526–545 | nt 628–647 | |||
∗nt = nucleotide sequences are presented from 5′ to 3′ and their position in the 16S rRNA gene is given based on the sequences of Campylobacter jejuni NCTC 11351.
Necrotic Enteritis Trials
Ross 308 broiler chickens were obtained as 1-day-old chicks from a commercial hatchery and reared in cages with a density of 30 birds/m2 on wood shavings. All cages were separated by solid walls to prevent contact between birds from different treatment groups. Four trials were performed. For each experiment the chickens were randomly allocated into groups of 30 birds. A 23 h/1h light/darkness program was applied. The chickens received drinking water and feed ad libitum. The feed was based on wheat/rye as described by Gholamiandehkordi et al. (2007) with soybean meal as protein source. A one and twofold dose of Poulvac Bursa Plus (Zoetis, Zaventem, Belgium) was given in the drinking water on days 4 and 9, respectively, to all groups. On day 11, all birds were orally inoculated with a 10-fold dose of Hipracox (Hipra, Girona, Spain) and on day 16 they received a 10-fold dose of Paracox-8TM (Schering-Plough Animal Health, Brussels, Belgium). Both vaccines were diluted in water and each chicken received 1ml orally. From day 17 onwards, the soybean in the feed was replaced by fishmeal (30%) as the protein source. All chickens were orally challenged (three times a day) on days 18, 19, 20, and 21 with 1 ml of a fresh broth culture containing 5 × 108 cfu C. perfringens per milliliter. On day 22 all birds were euthanized by an intravenous overdose of sodium pentobarbital 20% (Kela, Hoogstraten, Belgium). Necrotic lesions in the small intestine (duodenum to ileum) were scored as described by Keyburn et al. (2006). Briefly, the scoring was as follows: 0 = no gross lesions; 1 = congested intestinal mucosa; 2 = small focal necrosis or ulceration (1–5 foci); 3 = focal necrosis or ulceration (6–15 foci); 4 = focal necrosis or ulceration (16 or more foci); 5 = patches of necrosis 2–3 cm long; 6 = diffuse necrosis typical of field cases. Birds with lesions score of two or more were classified as NE-positives.
In the first trial, from day 1 onwards, lyophilized B. pullicaecorum bacteria were mixed in the feed of two groups at a concentration of 109 cfu per kg, while the control group received unsupplemented feed. The second, third, and fourth trial both comprised two groups with one group receiving feed supplemented with 109 cfu lyophilized B. pullicaecorum per kg and one control group. All the bird experiments were carried out according to the recommendations of, and following approval by the Ethical Committee of the Faculty of Veterinary Medicine, Ghent University (EC2009/098, EC2010/099, EC2012/101, EC2014/50).
Statistical Analysis
The data for body weight and FCR were statistically analyzed with GraphPad Prism version 5.0 (GraphPad, La Jolla, CA, USA) using a non-parametric unpaired t-test. A one-tailed Wilcoxon test for each genus was applied to test for differences in relative abundancy. A non-parametric Mann–Whitney U test was performed to compare the mean number Campylobacter bacteria as measured with qPCR in the treated and untreated group. The data from the NE trials were analyzed using one-tailed Wilcoxon signed-rank tests. In all cases, a probability level of p ≤ 0.05 was considered statistically significant.
Results
Performance Trial
Daily Weight Gain, Growth Performance, and FCR
The effects of dietary supplementation with B. pullicaecorum on animal performance of all broilers and for both sexes separately during different periods are shown in Table 2.
Table 2.
The effect of Butyricicoccus pullicaecorum supplementation on the growth performance of the chickens.
| Body weight (g)a | Feed conversion ratioa | ||||||
|---|---|---|---|---|---|---|---|
| D14 | D26 | D40 | D0-14 | D0-26 | D0-40 | ||
| Male | Ctrl | 497.1 ± 4.5 | 1405 ± 14.3 | 2949 ± 31.8 | 1.125 ± 0.013 | 1.422 ± 0.059 | 1.629 ± 0.024 |
| Bp | 484.5 ± 4.8 | 1328 ± 11.2 | 2789 ± 23.6 | 1.103 ± 0.006 | 1.310 ± 0.046 | 1.521 ± 0.011 | |
| P-value | 0.0573 | <0.0001 | <0.0001 | 0.1935 | 0.2043 | 0.0148 | |
| Female | Ctrl | 466.1 ± 4.7 | 1250 ± 10.0 | 2575 ± 23.8 | 1.111 ± 0.011 | 1.384 ± 0.008 | 1.635 ± 0.017 |
| Bp | 463.0 ± 3.9 | 1268 ± 9.3 | 2564 ± 18.6 | 1.100 ± 0.004 | 1.295 ± 0.002 | 1.516 ± 0.001 | |
| P-value | 0.6107 | 0.1980 | 0.7061 | 0.4129 | 0.0005 | 0.0120 | |
| All | Ctrl | 481.6 ± 3.4 | 1328 ± 10.2 | 2762 ± 23.8 | 1.118 ± 0.008 | 1.403 ± 0.028 | 1.632 ± 0.013 |
| Bp | 473.6 ± 3.2 | 1298 ± 7.5 | 2675 ± 16.7 | 1.102 ± 0.003 | 1.302 ± 0.021 | 1.518 ± 0.011 | |
| P-value | 0.0860 | 0.0155 | 0.0025 | 0.0936 | 0.0157 | <0.0001 | |
Body weight and feed conversion ratio (FCR) were measured at three time intervals. aValues are the mean of three replicates of 42 chickens ± standard error of the mean. Ctrl: feed without probiotic supplementation, Bp: feed supplemented with 109 cfu B. pullicaecorum per kg feed. Bolded values are the ones that are significantly different.
The male birds fed the B. pullicaecorum supplemented diet had a lower (p < 0.05) body weight on days 26 and 40 than the male birds in the control group. Supplementation of the probiotic had no significant effect on the body weight of the females at the three different time points. Significant differences between treatments were noted in the FCR. The FCR for B. pullicaecorum treated females was significantly lower during grower (1.295 ± 0.002 vs. 1.384 ± 0.008) and finisher (1.516 ± 0.001 vs. 1.635 ± 0.017) period than for the control group. A significantly lower FCR for the B. pullicaecorum treated male birds (1.521 ± 0.011 vs. 1.629 ± 0.024) was observed only during the finisher period.
Characterization of Microbial Communities
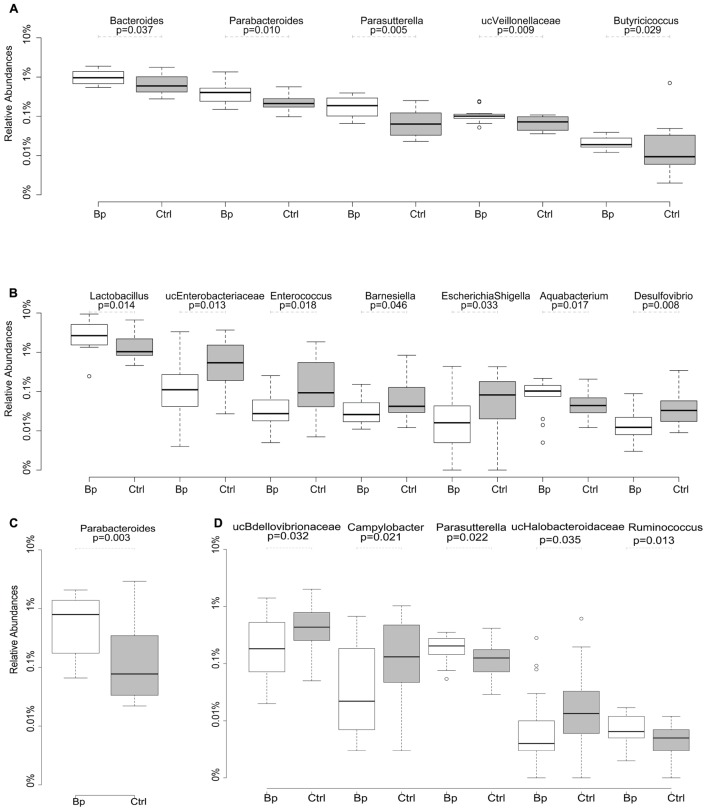
After quality trimming, a total of 745,182 and 980,262 sequencing reads were obtained from the ileal and caecal samples, respectively. Rarefaction of all samples to 10,000 reads resulted in the identification of a total of 7490 and 4996 OTUs in the ileum and caecum, respectively. Firmicutes, Bacteroidetes, and Proteobacteria were the most abundant bacterial phyla in the ileal and caecal samples, regardless age or group. Overall, major separation was observed between the microbiome from different sampling sites (ileum vs. caecum, 21.5% variation, p = 0.001); minor differences were noted between samples taken at different time points (26 days vs. 40 days, 6.9% variation, p = 0.001). Neither gender nor administration of B. pullicaecorum had a significant impact on microbial community differences (both <1% variation, p > 0.05). On both days 26 and 40 the proportions of a number of genera representing at least 1% of sequences were altered significantly in the Butyricicoccus treated group compared with the control group. On day 26, significantly higher proportions of Bacteroides, Butyricicoccus, Parabacteroides, Parasutterella, and unclassified Veillonellaceae were detected in the ileum of the probiotic group as compared to control chickens (Figure 1A). The numbers of Lactobacillus and Aquabacterium spp. were significantly higher in the ileum of the treated chickens on day 40, while the abundances of Enterococcus, Escherichia/Shigella, Desulfovibrio, Barnesiella, and unclassified Enterobacteriaceae were significantly lower in the ileum compared to the control animals (Figure 1B). Differences in the caecum on day 26 were observed for Parabacteroides (Figure 1C) and on day 40 for Parasutterella and Ruminococcus, with each genus being significantly more abundant in the animals that received Butyricicoccus. Numbers of unclassified Bdellovibrionaceae, unclassified Halobacteroidaceae, and Campylobacter were significantly lower in treated vs. control chickens (Figure 1D).
FIGURE 1.
Relative abundance (percent) of major bacterial genera determined using Ilumina MiSeq sequencing, that showed significant differences in ileum at day 26 (A) and day 40 (B), and in caecum at day 26 (C) and day 40 (D) between the ctrl (received unsupplemented feed) and the Bp (received feed supplemented with 109 cfu Butyricicoccus pullicaecorum per kg feed) group. Abundances are expressed in log scale. o, outliers.
Quantification of Campylobacter spp.
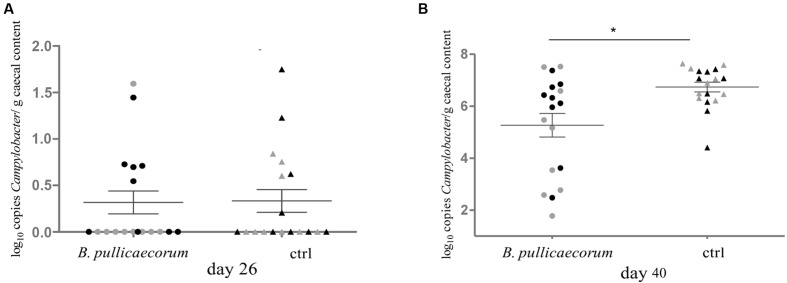
To confirm the decrease in abundance of Campylobacter in the caeca of chickens fed a B. pullicaecorum supplemented feed, as measured by pyrosequencing, we quantified the number of this human enteropathogen in the chicken intestinal microbiota by qPCR. We found that the average number of bacteria belonging to the genus Campylobacter on day 40 was significantly (p = 0.0169) lower (5.27 ± 0.46 vs. 6.74 ± 0.19 in log number of 16S rRNA gene copy number/g caecal content) in the caeca of chickens that were administered B. pullicaecorum supplemented feed compared to control animals (Figure 2).
FIGURE 2.
Quantification using qPCR of Campylobacter bacteria in caecal content from nine male ( ) and nine female (
) and nine female ( ) B. pullicaecorum treated and nine male (
) B. pullicaecorum treated and nine male ( ) and nine female (
) and nine female ( ) untreated chickens on day 26 (A) and day 40 (B). Bacterial density is expressed as log10 copy number of 16S rRNA genes of Campylobacter per g of caecal content. ∗p = 0.0169.
) untreated chickens on day 26 (A) and day 40 (B). Bacterial density is expressed as log10 copy number of 16S rRNA genes of Campylobacter per g of caecal content. ∗p = 0.0169.
Necrotic Enteritis Trials
Table 3 shows the percentage of birds with necrotic lesions in their small intestine in all trials. The NE challenge was effective in inducing gross lesions, presented as multiple necrotic foci in the duodenum and jejunum. No marked clinical signs or mortality were seen during the experiments. The percentage of birds presenting lesions in the infected untreated control group varied between 14.8 and 56.6. When the results of all trials were combined and evaluated for statistical differences, supplementation of B. pullicaecorum resulted in a significant (p = 0.0313) reduction in the number of birds developing necrotic lesions in comparison with the untreated control group.
Table 3.
Percentage of chickens with macroscopic necrotic enteritis (NE) lesions (lesion score ≥2) per trial after challenge with Clostridium perfringens.
| Percentage animals with necrotic lesions | ||
|---|---|---|
| Trial | Ctrl | B. pullicaecorum |
| 1 | 56.6 | 40.0 and 39.3 |
| 2 | 36.0 | 7.69 |
| 3 | 53.6 | 25 |
| 4 | 14.8 | 3.6 |
Ctrl: feed without probiotic supplementation, B. pullicaecorum: feed supplemented with 109 cfu B. pullicaecorum per kg feed.
Discussion
Recently, 16S rRNA gene-based pyrosequencing analysis identified the Ruminococcaceae family, harboring a range of highly oxygen-sensitive butyrate-producing species, to be associated with desirable productivity outcomes since they were found highly abundant in the caecal (Stanley et al., 2016) and fecal (Singh et al., 2012) microbiota of chickens with low FCR. In our study supplementation of B. pullicaecorum, a member of the Ruminococcaceae family, resulted in a significantly lower body weight which was compensated by a significantly lower FCR. Rapid growth and heavy body weight of broilers often results in metabolic diseases such as ascites and sudden death syndrome. Also skeletal disorders resulting in immobility of the birds are often observed (Brickett et al., 2007). Therefore, slowing down body weight gain may be advantageous. This can only be of practical relevance when the improvement in feed conversion outweighs the reduced body weight in terms of economic benefit.
The subclinical form of C. perfringens associated- NE can adversely affects growth rate, feed conversion, and flock uniformity (Lovland and Kaldhusdal, 2001) resulting in serious economic losses on the poultry industry (Timbermont et al., 2011). In addition to the etiological agent C. perfringens, predisposing factors such as Eimeria-induced mucosal damage and diets containing high levels of proteins are required to elicit the clinical signs and lesions of NE in poultry (Fernandes da Costa et al., 2013). Those predisposing factors are hypothesized to change the gastrointestinal microbiota, providing a disrupted intestinal ecosystem in which C. perfringens can proliferate and cause disease (Wu et al., 2014). Pyrosequencing of 16S rRNA amplicons showed that the predominant families Ruminococcaceae and Lachnospiraceae, linked with gut health through butyrate production (Biddle et al., 2013) were the most affected bacterial phylotypes by the predisposing treatments for NE (Perez et al., 2011; Stanley et al., 2014b; Wu et al., 2014). Leeson et al. (2005) showed that birds previously fed butyrate were better in withstanding the stress of coccidial challenge (Leeson et al., 2005). Moreover butyrate was shown to induce synthesis of a subset of host defense peptides (cathelicidins) in jejunal and caecal explants from chicken origin, with immunomodulatory properties and a natural broad antimicrobial spectrum important as first line of defense (Sunkara et al., 2011). This butyrate-induced mechanism of innate host defense may account for the suppressive effect on potential pathogenic and performance-related species as observed in the present performance trial. Because butyrate-producing bacteria are depleted in the gut of chickens with NE, and butyrate has anti-inflammatory and epithelial barrier-strengthening effects (Place et al., 2005; Wang et al., 2012), administrating a strain such as B. pullicaecorum can restore the unbalanced microbiota composition and the butyrate production and thus counteract NE.
B. pullicaecorum treatment resulted in a depletion of sequences related to the genera Enterococcus, Escherichia/Shigella, Barnesiella, Desulfovibrio, and Campylobacter. All, except for Enterococcus, are Gram negative organisms and thus the primary source for endotoxin, also referred to as lipopolysaccharide (LPS), able to stimulate localized or systemic inflammation resulting in attenuated growth performance (Mani et al., 2012). Depending on the strain, enterococci are considered probiotic (such as Enterococcus faecalis; Christoffersen et al., 2012) or pathogenic organisms (such as E. cecorum). The latter may adversely affect performance, as it is associated with arthritis, osteomyelitis, and femoral head necrosis (Stalker et al., 2010). Campylobacter is considered the most common bacterial cause of human gastroenteritis and poultry has often been implicated as source (Sheppard and Maiden, 2015). Intestinal colonization of Campylobacter in chickens causes a negative effect on growth performance (Cisek and Binek, 2014) but, more importantly, plays an important role in carcass contamination at slaughter. In the present study, B. pullicaecorum supplementation was associated with a 1.47 log reduction of Campylobacter at slaughter age. It has been calculated that a reduction of 2 log units would reduce the public health risk by more than 90% (EFSA, 2011).
Sequences related to the genera Lactobacillus and Bacteroides were enriched in the gut of chickens treated with B. pullicaecorum. Lactobacilli have been shown to beneficially affect performance in broilers (Peng et al., 2016) by inducing immunomodulation and protection of the intestinal barrier via antagonistic activities against pathogens (Koenen et al., 2004; Brisbin et al., 2011). Although Gram-negative, and thus a source of endotoxin, organisms belonging to the genus Bacteroides are thought to play an important role in the development of the immune system, gut homeostasis and metabolism in humans (Round and Mazmanian, 2009). In infants, a low Bacteroides signature is associated with a decreased microbial diversity (Yassour et al., 2016) that has been associated with decreased intestinal health in general. In the chicken, as in other species, members of the Bacteroides genus are involved in hydrolyzing polysaccharides from plant fibers (Koropatkin et al., 2012). As such, they may contribute to the improved feed conversion.
Conclusion
The continuous feeding of B. pullicaecorum suppressed the growth of undesirable microorganisms in the gut, prevented NE and improved the feed conversion in broilers. Considering the relatively small quantities of Butyricicoccus that were added to the feed and the relatively low abundance of the strain in the intestine, the beneficial effect of performance of Butyricicoccus probiotic supplementation most probably is not only due to the direct effect of its butyrate production but also due to its modulating effects on the microbiome.
Author Contributions
FVI, RD, and FH designed the study, VE and AVP conducted the animal trials. VE performed the qPCR analysis and JW, MJ, GF, and JR carried out bioinformatics analysis of the data. VE drafted the manuscript. All authors edited the manuscript and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LB and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
The authors want to thank the Ph.D. students from the Department of Pathology, Bacteriology and Avian Diseases who assisted in the sampling during the in vivo trial and Leen Rymenans from KU Leuven for preparing the sequencing library.
Funding. This work was supported by the Institute of Science and Technology, Flanders (IWT), under contract no SBO-100016. MJ is postdoctoral fellow of the Research Foundation - Flanders (FWO).
References
- Adil S., Banday T., Bhat G. A., Mir M. S., Rehman M. (2010). Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 2010:479485 10.4061/2010/479485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S. T., Islam M., Mun H. S., Sim H. J., Kim Y. J., Yang C. J. (2014). Effects of bacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult. Sci. 93 1963–1971. 10.3382/ps.2013-03718 [DOI] [PubMed] [Google Scholar]
- Alloui M. N., Szczurek W., Swiatkiewicz S. (2013). The usefulness of prebiotics and probiotics in modern poultry nutrition: a review. Ann. Anim. Sci. 13 17–32. [Google Scholar]
- Biddle A., Stewart L., Blanchard J., Leschine S. (2013). Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity 5 627–640. 10.3390/d5030627 [DOI] [Google Scholar]
- Boon N., Top E. M., Verstraete W., Siciliano S. D. (2003). Bioaugmentation as a tool to protect the structure and function of an activated-sludge microbial community against a 3-chloroaniline shock load. Appl. Environ. Microbiol. 69 1511–1520. 10.1128/AEM.69.3.1511-1520.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickett K. E., Dahiya J. P., Classen H. L., Annett C. B., Gomis S. (2007). The impact of nutrient density, feed form, and photoperiod on the walking ability and skeletal quality of broiler chickens. Poult. Sci. 86 2117–2125. 10.1093/ps/86.10.2117 [DOI] [PubMed] [Google Scholar]
- Brisbin J. T., Gong J., Orouji S., Esufali J., Mallick A. I., Parvizi P., et al. (2011). Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clin. Vaccine Immunol. 18 1447–1455. 10.1128/CVI.05100-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui X. T., Wolff A., Madsen M., Bang D. D. (2012). Reverse transcriptase real-time pcr for detection and quantification of viable Campylobacter jejuni directly from poultry faecal samples. Res. Microbiol. 163 64–72. 10.1016/j.resmic.2011.10.007 [DOI] [PubMed] [Google Scholar]
- Christoffersen T. E., Jensen H., Kleiveland C. R., Dørum G., Jacobsen M., Lea T. (2012). In vitro comparison of commensal, probiotic and pathogenic strains of enterococcus faecalis. Br. J. Nutr. 108 2043–2053. 10.1017/S0007114512000220 [DOI] [PubMed] [Google Scholar]
- Cisek A. A., Binek X. (2014). Chicken intestinal microbiota function with a special emphasis on the role of probiotic bacteria. Pol. J. Vet. Sci. 17 385–394. [DOI] [PubMed] [Google Scholar]
- Dawkins M. S., Layton R. (2012). Breeding for better welfare: genetic goals for broiler chickens and their parents. Anim. Welf. 21 147–155. 10.7120/09627286.21.2.147 [DOI] [Google Scholar]
- Dixon P. (2003). Vegan, a package of r functions for community ecology. J. Veg. Sci. 14 927–930. 10.1111/j.1654-1103.2003.tb02228.x [DOI] [Google Scholar]
- Edgar R. C. (2010). Search and clustering orders of magnitude faster than blast. Bioinformatics 26 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Edgar R. C., Haas B. J., Clemente J. C., Quince C., Knight R. (2011). Uchime improves sensitivity and speed of chimera detection. Bioinformatics 27 2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhaut V., Van Immerseel F., Teirlynck E., Pasmans F., Fievez V., Snauwaert C., et al. (2008). Butyricicoccus pullicaecorum gen. Nov., sp. Nov., an anaerobic, butyrate-producing bacterium isolated from the caecal content of a broiler chicken. Int. J. Syst. Evol. Microbiol. 58 2799–2802. 10.1099/ijs.0.65730-0 [DOI] [PubMed] [Google Scholar]
- EFSA (2011). Scientific opinion on campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 9:141. [Google Scholar]
- Fernandes da Costa S. P., Mot D., Bokori-Brown M., Savva C. G., Basak A. K., Van Immerseel F., et al. (2013). Protection against avian necrotic enteritis after immunisation with netb genetic or formaldehyde toxoids. Vaccine 31 4003–4008. 10.1016/j.vaccine.2013.05.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. (1989). Probiotics in man and animals. J. Appl. Bacteriol. 66 365–378. 10.1111/j.1365-2672.1989.tb05105.x [DOI] [PubMed] [Google Scholar]
- Gaggia F., Mattarelli P., Biavati B. (2010). Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 141(Suppl. 1), S15–S28. 10.1016/j.ijfoodmicro.2010.02.031 [DOI] [PubMed] [Google Scholar]
- Gholamiandekhordi A. R., Ducatelle R., Heyndrickx M., Haesebrouck F., Van Immerseel F. (2006). Molecular and phenotypical characterization of clostridium perfringens isolates from poultry flocks with different disease status. Vet. Microbiol. 113 143–152. 10.1016/j.vetmic.2005.10.023 [DOI] [PubMed] [Google Scholar]
- Gholamiandehkordi A. R., Timbermont L., Lanckriet A., Van Den Broeck W., Pedersen K., Dewulf J., et al. (2007). Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathol. 36 375–382. 10.1080/03079450701589118 [DOI] [PubMed] [Google Scholar]
- Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., Van Immerseel F., et al. (2010). From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr. Res. Rev. 23 366–384. 10.1017/S0954422410000247 [DOI] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G. R., Merenstein D. J., Pot B., et al. (2014). Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11 506–514. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- Keyburn A. L., Sheedy S. A., Ford M. E., Williamson M. M., Awad M. M., Rood J. I., et al. (2006). Alpha-toxin of clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 74 6496–6500. 10.1128/IAI.00806-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen M. E., Kramer J., van der Hulst R., Heres L., Jeurissen S. H., Boersma W. J. (2004). Immunomodulation by probiotic lactobacilli in layer- and meat-type chickens. Br. Poult. Sci. 45 355–366. 10.1080/00071660410001730851 [DOI] [PubMed] [Google Scholar]
- Koropatkin N. M., Cameron E. A., Martens E. C. (2012). How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 10 323–335. 10.1038/nrmicro2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotunia A., Woliński J., Laubitz D., Jurkowska M., Romé V., Guilloteau P., et al. (2004). Effect of sodium butyrate on the small intestine development in neonatal piglets fed [correction of feed] by artificial sow. J. Physiol. Pharmacol. 55(Suppl. 2), 59–68. [PubMed] [Google Scholar]
- Kozich J. J., Westcott S. L., Baxter N. T., Highlander S. K., Schloss P. D. (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl. Environ. Microbiol. 79 5112–5120. 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall M., Gallois M., Sève B., Louveau I., Holst J. J., Oswald I. P., et al. (2009). Comparative effect of orally administered sodium butyrate before or after weaning on growth and several indices of gastrointestinal biology of piglets. Br. J. Nutr. 102 1285–1296. 10.1017/S0007114509990213 [DOI] [PubMed] [Google Scholar]
- Leeson S., Namkung H., Antongiovanni M., Lee E. H. (2005). Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 84 1418–1422. 10.1093/ps/84.9.1418 [DOI] [PubMed] [Google Scholar]
- Lovland A., Kaldhusdal M. (2001). Severely impaired production performance in broiler flocks with high incidence of clostridium perfringens-associated hepatitis. Avian Pathol. 30 73–81. 10.1080/03079450020023230 [DOI] [PubMed] [Google Scholar]
- Magoc T., Salzberg S. L. (2011). Flash: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27 2957–2963. 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani V., Weber T. E., Baumgard L. H., Gabler N. K. (2012). Growth and development symposium: endotoxin, inflammation, and intestinal function in livestock. J. Anim. Sci. 90 1452–1465. 10.2527/jas.2011-4627 [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Martin J. C., Marinsek-Logar R., Flint H. J. (1997). Degradation and utilization of xylans by the rumen anaerobe prevotella bryantii (formerly p. Ruminicola subsp. brevis) b(1)4. Anaerobe 3 373–381. 10.1006/anae.1997.0125 [DOI] [PubMed] [Google Scholar]
- Mountzouris K. C., Tsitrsikos P., Palamidi I., Arvaniti A., Mohnl M., Schatzmayr G., et al. (2010). Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 89 58–67. 10.3382/ps.2009-00308 [DOI] [PubMed] [Google Scholar]
- Peng Q., Zeng X. F., Zhu J. L., Wang S., Liu X. T., Hou C. L., et al. (2016). Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poult. Sci. 95 893–900. 10.3382/ps/pev435 [DOI] [PubMed] [Google Scholar]
- Perez V. G., Jacobs C. M., Barnes J., Jenkins M. C., Kuhlenschmidt M. S., Fahey G. C., Jr., et al. (2011). Effect of corn distillers dried grains with solubles and eimeria acervulina infection on growth performance and the intestinal microbiota of young chicks. Poult. Sci. 90 958–964. 10.3382/ps.2010-01066 [DOI] [PubMed] [Google Scholar]
- Place R. F., Noonan E. J., Giardina C. (2005). HDAC inhibition prevents NF-κB activation by suppressing proteasome activity: down-regulation of proteasome subunit expression stabilizes IκBα. Biochem. Pharmacol. 70 394–406. 10.1016/j.bcp.2005.04.030 [DOI] [PubMed] [Google Scholar]
- Ritzi M. M., Abdelrahman W., Mohnl M., Dalloul R. A. (2014). Effects of probiotics and application methods on performance and response of broiler chickens to an eimeria challenge. Poult. Sci. 93 2772–2778. 10.3382/ps.2014-04207 [DOI] [PubMed] [Google Scholar]
- Round J. L., Mazmanian S. K. (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9 313–323. 10.1038/nri2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard S. K., Maiden M. C. (2015). The evolution of Campylobacter jejuni and campylobacter coli. Cold Spring Harb. Perspect. Biol. 7:a018119 10.1101/cshperspect.a018119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K. M., Shah T., Deshpande S., Jakhesara S. J., Koringa P. G., Rank D. N., et al. (2012). High through put 16s rrna gene-based pyrosequencing analysis of the fecal microbiota of high fcr and low fcr broiler growers. Mol. Biol. Rep. 39 10595–10602. 10.1007/s11033-012-1947-7 [DOI] [PubMed] [Google Scholar]
- Stalker M. J., Brash M. L., Weisz A., Ouckama R. M., Slavic D. (2010). Arthritis and osteomyelitis associated with enterococcus cecorum infection in broiler and broiler breeder chickens in ontario, canada. J. Vet. Diagn. Invest. 22 643–645. 10.1177/104063871002200426 [DOI] [PubMed] [Google Scholar]
- Stanley D., Denman S. E., Hughes R. J., Geier M. S., Crowley T. M., Chen H., et al. (2012). Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl. Microbiol. Biotechnol. 96 1361–1369. 10.1007/s00253-011-3847-5 [DOI] [PubMed] [Google Scholar]
- Stanley D., Hughes R. J., Geier M. S., Moore R. J. (2016). Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: challenges presented for the identification of performance enhancing probiotic bacteria. Front. Microbiol. 7:187 10.3389/fmicb.2016.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D., Hughes R. J., Moore R. J. (2014a). Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 98 4301–4310. 10.1007/s00253-014-5646-2 [DOI] [PubMed] [Google Scholar]
- Stanley D., Wu S. B., Rodgers N., Swick R. A., Moore R. J. (2014b). Differential responses of cecal microbiota to fishmeal, eimeria and clostridium perfringens in a necrotic enteritis challenge model in chickens. PLoS ONE 9:e104739 10.1371/journal.pone.0104739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara L. T., Achanta M., Schreiber N. B., Bommineni Y. R., Dai G., Jiang W., et al. (2011). Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS ONE 6:e27225 10.1371/journal.pone.0027225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. (2011). Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 40 341–347. 10.1080/03079457.2011.590967 [DOI] [PubMed] [Google Scholar]
- van Der Wielen P. W., Biesterveld S., Notermans S., Hofstra H., Urlings B. A., van Knapen F., et al. (2000). Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 66 2536–2540. 10.1128/AEM.66.6.2536-2540.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-B., Wang P.-Y., Wang X., Wan Y.-L., Liu Y.-C. (2012). Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig. Dis. Sci. 57 3126–3135. 10.1007/s10620-012-2259-4 [DOI] [PubMed] [Google Scholar]
- Wang Q., Garrity G. M., Tiedje J. M., Cole J. R. (2007). Naive bayesian classifier for rapid assignment of rrna sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73 5261–5267. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. B., Stanley D., Rodgers N., Swick R. A., Moore R. J. (2014). Two necrotic enteritis predisposing factors, dietary fishmeal and eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 169 188–197. 10.1016/j.vetmic.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Yassour M., Vatanen T., Siljander H., Hämäläinen A. M., Härkönen T., Ryhänen S. J., et al. (2016). Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 8:343ra381 10.1126/scitranslmed.aad0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. H., Jiang Y., Zhu Q. F., Gao F., Dai S. F., Chen J., et al. (2011). Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br. Poult. Sci. 52 292–301. 10.1080/00071668.2011.578121 [DOI] [PubMed] [Google Scholar]