Summary
Background
WHO recommends combinations of an artemisinin derivative plus an antimalarial drug of longer half-life as treatment options for uncomplicated Plasmodium falciparum infection. In Africa, artemether–lumefantrine is the most widely used artemisinin-based combination therapy, whereas artesunate–mefloquine is used infrequently because of a perceived poor tolerance to mefloquine. WHO recommends reconsideration of the use of artesunate–mefloquine in Africa. We compared the efficacy and safety of fixed-dose artesunate–mefloquine with that of artemether–lumefantrine for treatment of children younger than 5 years with uncomplicated P falciparum malaria.
Methods
We did this multicentre, phase 4, open-label, non-inferiority trial in Burkina Faso, Kenya, and Tanzania. Children aged 6–59 months with uncomplicated malaria were randomly assigned (1:1), via a computer-generated randomisation list, to receive 3 days' treatment with either one or two artesunate–mefloquine tablets (25 mg artesunate and 55 mg mefloquine) once a day or one or two artemether–lumefantrine tablets (20 mg artemether and 120 mg lumefantrine) twice a day. Parasitological assessments were done independently by two microscopists who were blinded to treatment allocation. The primary outcome was the PCR-corrected rate of adequate clinical and parasitological response (ACPR) at day 63 in the per-protocol population. Non-inferiority was shown if the lower limit of the 95% CI for the difference between groups was greater than −5%. Early vomiting was monitored and neuropsychiatric status assessed regularly during follow-up. This study is registered with ISRCTN, number ISRCTN17472707, and the Pan African Clinical Trials Registry, number PACTR201202000278282.
Findings
945 children were enrolled and randomised, 473 to artesunate–mefloquine and 472 to artemether–lumefantrine. The per-protocol population consisted of 407 children in each group. The PCR-corrected ACPR rate at day 63 was 90·9% (370 patients) in the artesunate–mefloquine group and 89·7% (365 patients) in the artemether–lumefantrine group (treatment difference 1·23%, 95% CI −2·84% to 5·29%). At 72 h after the start of treatment, no child had detectable parasitaemia and less than 6% had fever, with a similar number in each group (21 in the artesunate–mefloquine group vs 24 in the artemether–lumefantrine group). The safety profiles of artesunate–mefloquine and artemether–lumefantrine were similar, with low rates of early vomiting (71 [15·3%] of 463 patients in the artesunate–mefloquine group vs 79 [16·8%] of 471 patients in the artemether–lumefantrine group in any of the three dosing days), few neurological adverse events (ten [2·1%] of 468 vs five [1·1%] of 465), and no detectable psychiatric adverse events.
Interpretation
Artesunate–mefloquine is effective and safe, and an important treatment option, for children younger than 5 years with uncomplicated P falciparum malaria in Africa.
Funding
Agence Française de Développement, France; Department for International Development, UK; Dutch Ministry of Foreign Affairs, Netherlands; European and Developing Countries Clinical Trials Partnership; Fondation Arpe, Switzerland; Médecins Sans Frontières; Swiss Agency for Development and Cooperation, Switzerland.
Introduction
Since 2000, there has been substantial progress in the worldwide effort to control, and in some regions eliminate, malaria.1 However, the disease still caused an estimated 438 000 deaths worldwide in 2015, mostly in Africa (90%) and in children younger than 5 years (70%).1, 2 The widespread deployment of artemisinin-based combination therapies (ACTs) for treating malaria is recognised as one key public health initiative for reducing malaria morbidity and mortality. Five ACTs are currently recommended by WHO for the treatment of uncomplicated Plasmodium falciparum infection,3 the predominant cause of malaria in Africa and, when untreated, the most deadly form of malaria worldwide.2
Research in context.
Evidence before this study
A systematic search of PubMed was done on Nov 25, 2014, without date or language restrictions, with the terms “artesunate” and “mefloquine” and “malaria, falciparum” and “ACPR” and “non-inferiority”. We found no randomised trials that tested the efficacy and safety of fixed-dose artesunate–mefloquine in young children in Africa according to the WHO recommendations.
WHO recommends “adequate clinical and parasitological response (ACPR)” (corrected for possible re-infections and a per-protocol analysis) as the primary outcome and a non-inferiority trial design with a follow-up of adequate duration, which is 63 days to allow for the prolonged clearance rate of mefloquine. We found two trials (Smithuis et al, 2010; Ashley et al, 2006) of the fixed-dose artesunate–mefloquine tablet with ACPR at day 63 of follow-up as the primary outcome. These studies were done in Asia in populations of mixed age. Four trials (Faye et al, 2007; Agomo et al, 2008; Sagara et al, 2008; Bhatt et al, 2006) of fixed-dose artesunate–mefloquine were done in Africa in populations of mixed age but with ACPR at 28 days as the primary outcome.
Added value of this study
As far as we are aware, our trial is the first to be reported on a fixed-dose artesunate–mefloquine formulation and done in young African patients with a trial design that fully complies with WHO guidelines. It is the largest randomised, controlled trial providing efficacy and safety data for artesunate–mefloquine in children in Africa. Our findings showed that artesunate–mefloquine is non-inferior to artemether–lumefantrine for the primary endpoint of PCR-corrected ACPR rate at day 63. We also found that the two treatments had similarly good safety profiles, with low rates of early vomiting and few recorded neurological adverse events. Our efficacy and safety data are consistent with the findings of large trials (Smithuis et al, 2010; Ashley et al, 2006) of the same fixed-dose tablet, which were done in Asia in mixed-aged patients with uncomplicated malaria. Additionally, we obtained evidence that artesunate–mefloquine might delay re-infection for longer than artemether–lumefantrine does, which might be explained by the much slower rate of clearance of mefloquine compared with lumefantrine.
Implications of all the available evidence
Artesunate–mefloquine is one of five artemisinin-based combination therapies (ACTs) that are currently recommended by WHO as antimalarial treatments. However, this combination was not registered in Africa at the time of the start of the trial. WHO recommends deployment of multiple ACTs to reduce the risk of the development of drug resistance. Our data suggest that fixed-dose artesunate–mefloquine is safe and effective in treating young children with uncomplicated malaria in Africa and is as effective as artemether–lumefantrine. These findings should have important implications for health policy in Africa, where malaria remains a major public health problem, particularly in young children.
Antimalarial drugs are used in combinations to prevent or delay the development of drug resistance and are now recommended in most malaria-endemic countries.4 Monotherapy with artemisinin or its derivatives is now strongly discouraged,3 especially after the emergence of artemisinin resistance in some regions.5 Deployment of multiple ACTs is regarded to be a further means to reduce development of drug resistance.6 Several effective ACTs are now available, many as fixed-dose formulations,3 which offer improved patient adherence. Artemether–lumefantrine was the first fixed-dose ACT to become available and is currently the most extensively used ACT.2
The Drugs for Neglected Diseases initiative (DNDi), in partnership with the TDR (the Special Programme for Research and Training in Tropical Diseases) consortium, has collaborated with industry and academia for the development of two fixed-dose ACTs.7 This partnership formed the FACT (Fixed-dose Artesunate-based Combination Therapy) project consortium, which is a model needs-driven initiative aiming to increase availability and reliable supply of fixed-dose ACTs of required quality.
One of the five WHO-recommended ACTs is artesunate with mefloquine. Available in loose or fixed-dose combination, artesunate–mefloquine showed high efficacy in treating uncomplicated P falciparum malaria8, 9, 10 and has been used extensively over 20 years, mostly in Asia and Latin America.11 This combination is less commonly used in Africa, because of the availability of other affordable and already registered ACTs.3 Reports of mefloquine resistance in Asia,12 shortly after the drug's introduction as monotherapy in the 1990s, had a negative effect on the introduction of artesunate–mefloquine in Africa. Furthermore, excessive vomiting associated with mefloquine seems the main reason for the restricted use of artesunate–mefloquine in African children. Early vomiting, shortly after treatment, is a reported cause of treatment failure in children.13 However, WHO has recommended that artesunate–mefloquine be reconsidered for treatment of uncomplicated malaria in Africa, particularly highlighting the paucity of data for this combination in children younger than 5 years.3
A fixed-dose artesunate–mefloquine (containing 100 mg artesunate and 220 mg mefloquine hydrochloride) was, therefore, developed in Brazil by Farmanguinhos, through the FACT consortium, and registered there in 2008. A pivotal study in Thailand, involving 500 patients with uncomplicated malaria, including children (aged <15 years),14 showed this fixed-dose combination to be better tolerated, with a lower incidence of vomiting, than equivalent loose tablet combinations. A randomised comparative trial in Burma showed that this fixed-dose artesunate–mefloquine had, compared with other ACTs (loose artesunate–mefloquine, artemether–lumefantrine, artesunate–amodiaquine, dihydroartemisinin–piperaquine), the highest cure rate, the lowest rate of gametocyte carriage, and the most effective suppression of Plasmodium vivax malaria,9 and a large phase 4 trial (23 845 patients) in Brazil confirmed its effectiveness as a treatment of uncomplicated P falciparum infection.8
We did a randomised, multicentre, phase 4 trial with the aim of obtaining the most definitive evidence so far on the efficacy and safety of fixed-dose artesunate–mefloquine in children younger than 5 years with uncomplicated P falciparum malaria in Africa. The trial design was based on WHO guidelines15 and compared the efficacy and safety of fixed-dose artesunate–mefloquine with that of fixed-dose dispersible artemether–lumefantrine. Because of the long half-life of mefloquine, there was a prolonged follow-up (63 days), with an extensive safety assessment.
Methods
Study design and participants
This phase 4, multicentre, open-label, randomised, non-inferiority trial was done in six medical research centres across three African countries: Kilosa, Bagamoyo, and Korogwe (Tanzania); Balonghin and Banfora (Burkina Faso); and Ahero-Kisumu (Kenya).
Children were eligible for inclusion if they were aged 6–59 months, had an axillary temperature of 37·5°C or more, and had uncomplicated P falciparum monoinfection (2000–200 000 asexual parasites per μL). Exclusion criteria were signs and symptoms of severe or complicated malaria; bodyweight less than 5 kg; inability to tolerate oral medication; mixed Plasmodium species infection; fever caused by non-malarial disease; hypersensitivity to mefloquine, quinine, quinidine, artesunate, or other artemisinins; antimalarial treatment within the previous 2 weeks (4 weeks for mefloquine or piperaquine); and participation in a clinical intervention trial within the previous 3 months. Informed consent was obtained from the child's parent or legal guardian.
The trial was done in accordance with the Declaration of Helsinki and international and local national laws for protecting human rights and welfare. Ethical and regulatory approvals were obtained separately by each study centre: in Burkina Faso from the Ethics Review Committee of the Ministry of Health; in Tanzania from the National Health Research Ethics Review Committee and from the Tanzania Food and Drugs Authority; and in Kenya from the KEMRI Institutional Review Board and from the Kenyan Expert Committee on Clinical Trials/Pharmacy and Poisons Board.
An independent data safety monitoring committee was established to advise the sponsor if it was of the opinion that the ongoing trial had provided evidence that all or a specific subgroup(s) could either benefit from or be contraindicated for artesunate–mefloquine, on the basis of difference in the primary endpoint; evidence of drug-related toxic effects that outweighs the benefits of artesunate–mefloquine; or evidence that might reasonably be expected to influence patient management by clinicians who have become aware of any of the main trial results. The basis of any decision of the data safety monitoring committee was the content of any serious adverse event reports, all of which were received from the investigators without delay, and reviews of the study database done at regular intervals during the study.
Randomisation and masking
Eligible children were randomly assigned (1:1) to receive artesunate–mefloquine or artemether–lumefantrine. Treatment allocation was made using a computer-generated randomisation list and, for each patient, the allocated treatment was transmitted in a sealed envelope to the nurse or physician administering the treatment. This study was open label; however, laboratory technicians who did the parasitological assessments were masked to treatment allocation.
Procedures
The paediatric fixed-dose artesunate–mefloquine tablet (25 mg artesunate and 55 mg mefloquine hydrochloride, containing no flavouring [Farmanguinhos, Rio de Janeiro, Brazil]), is a round, smooth, biconvex, blue-coated tablet of 6·0 mm diameter; it was given once a day for 3 days, either as one tablet (for children aged 6–11 months) or two tablets (for children aged 12–59 months). The fixed-dose artemether–lumefantrine dispersible tablet (20 mg artemether and 120 mg lumefantrine, containing flavouring [Novartis Pharmaceuticals, Basel, Switzerland]), was dispersed in 200 mL milk (or breastmilk); it was given twice a day for 3 days, either as one tablet (for bodyweight ≥5 kg to <15 kg) or two tablets (for bodyweight ≥15 kg to <25 kg). Treatment was supervised and children observed for 60 min after treatment for possible vomiting. Test drug was readministered if vomiting occurred within 60 min. Patients with persistent vomiting and requiring more than a single repeat dose were excluded from the study and referred for further clinical management.
Children were followed up to day 63 after the start of treatment or to the first recurrence (first follow-up period). Patients with parasitaemia during follow-up were then switched to the alternative test treatment (except in Burkina Faso, where quinine was used for parasitaemia occurring within 28 days of the start of initial treatment, according to a national recommendation) and another 63 days of follow-up was started (second follow-up period), to collect efficacy and safety data, because artesunate–mefloquine was not a registered medicine in the participating countries.
Clinical signs, including neuropsychological observations, were assessed and recorded before and during the first 3 days post treatment, and on follow-up visits on days 7, 14, 21, 28, 35, 42, 49, 56, and 63 (of first follow-up) and on days 7, 28, and 63 post recurrence (of second follow-up), and whenever required. Adverse events and concomitant drug treatment were systematically recorded at each visit, with haematological and biochemical analyses done on samples taken on days 0, 7, 28, and 63, and whenever clinically required. Investigators recorded whether results of patient examinations and sample analyses were normal or abnormal (with detailed description of any abnormalities). Concomitant drug treatment was recorded, with paracetamol allowed for fever. Intake of any drug with antimalarial properties led to study withdrawal.
Parasitological assessments (thick and thin blood films) were done independently by two microscopists (blinded to treatment allocation) at least once every 24 h or until parasite clearance (defined as two consecutive negative readings 8–24 h apart), and then, on days 7, 14, 21, 28, 35, 42, 49, 56, and 63, and whenever required. Parasite density was expressed as number of asexual parasites per μL blood, calculated from a count relative to 200 or 300 counted white blood cells and the blood cell density; a negative count was 1000 white blood cells counted without detecting any asexual parasites.
Recrudescence and re-infection were differentiated by characterising malaria strains of initial infection and of recurrence, using internationally prescribed procedures16 for PCR genotypic analysis of extensively diverse genes (msp1, msp2, glurp) in blood collected immediately before treatment (baseline) and on day of recurrence.
In the first amendment of the original study protocol, the study was extended to include sites in Kenya and Burkina Faso, the sampling sequence of the pharmacokinetic part of the study was increased to be consistent with the product's half-life, and an additional sampling for PCR analysis was included at day 7, in accordance with WHO recommendations. The second amendment included addition of the second 63-day follow-up. In the third amendment, the major change was the addition of new sites (Korogwe and Bagamoyo) in Tanzania, to reach the required number of patients.
Outcomes
Treatment outcomes (classified using standard definitions and adjusted to the 63 days of follow-up) were adequate clinical and parasitological response (ACPR), early treatment failure, late parasitological failure, and late clinical failure. At intervals after the start of treatment, we calculated the gametocyte carriage (percentage of patients with gametocytes) and the parasite reduction ratio (baseline parasite count/parasite count at the specific interval).
The primary efficacy endpoint was PCR-corrected ACPR rate at day 63 (in the per-protocol population). The secondary efficacy endpoints were non-PCR-corrected ACPR rate at day 63; Kaplan-Meier analysis of number of patients with ACPR after PCR correction; proportion of patients with early treatment failure, late treatment failure, and late parasitological failure; proportion of patients with recrudescence or re-infection; PCR-corrected and non-PCR-corrected ACPR rates on days 28 and 42; proportion of patients with parasitaemia on days 1, 2, and 3; rate of gametocyte carriage; and proportion of patients with fever on days 1, 2, and 3. Pharmacokinetic analyses were also included as a secondary endpoint and will be presented elsewhere. The safety endpoints were proportion and severity of adverse events, time of any vomiting relative to and within 60 min of treatment, and proportions of serious adverse events and adverse events that led to treatment discontinuation.
Statistical analysis
The main features of the statistical methods were described in the protocol and detailed in the statistical analysis plan. The final version of the statistical analysis plan was signed before database lock and any analyses of results. The statistical analyses were done independently (Venn Life Sciences, Paris, France). The primary analysis tested the non-inferiority of artesunate–mefloquine over artemether–lumefantrine, using the per-protocol analysis of PCR-adjusted ACPR at day 63. The per-protocol population consisted of all patients without a major protocol deviation, who were fully treatment compliant (defined as ≥80% and <120% intake of study drug), who had a primary endpoint at day 63, and who did not withdraw from study treatment (except for those who withdrew because of adverse events or an absence of efficacy). Patients who withdrew because of adverse events were initially excluded from the per-protocol population according to the protocol, but, during the development of the statistical analysis plan, it was decided they be included in the per-protocol population and imputed as failures because withdrawals due to adverse events could potentially be linked to treatment. Non-inferiority was shown if the lower limit of the 95% CI for the difference between groups was greater than −5%. A further analysis of PCR-adjusted ACPR at day 63 (with the same method as for the per-protocol analysis) was done in the intention-to-treat population, which consisted of all randomly allocated patients who received at least one dose of study drug. No adjustment on any baseline covariate was performed. For the intention-to-treat analysis, we imputed missing primary endpoint data. We did an additional Kaplan-Meier analysis (log-rank test) of the rate of PCR-adjusted ACPR using a modified intention-to-treat population defined as all intention-to-treat patients except for those with undetermined or missing PCR results. This analysis, not planned in the protocol, was added to the statistical analysis plan to follow the 2009 WHO guidelines.17 A sample size of 399 patients per treatment was calculated to be required for 90% power with 2·5% significance for the one-sided comparison of rate of treated patients attaining a PCR-adjusted ACPR at day 63; this calculation was based on the assumptions that 95% of patients treated with artemether–lumefantrine would achieve the primary endpoint, an expected difference of 0%, and a clinically relevant non-inferiority margin of 5%. To allow for an expected loss to follow-up of 15%, target recruitment was 470 patients in each treatment group.
Wilcoxon-Mann-Whitney or Kruskal-Wallis tests were used for comparison of non-normally distributed continuous variables. The differences between treatments in the parasite reduction ratios, measured at intervals during the first 3 days after the start of treatment, were analysed using a linear mixed effects model, with parasite reduction ratio as the dependent variable and treatment group, baseline parasite count, and site as independent variables, and the time interval as random effect (the parasite count was derived from thick smear counts; counts of 0 were assigned a value of 1 for the analysis). Parasite clearance parameters were calculated using the Worldwide Antimalarial Resistance Network (WWARN) parasite clearance estimator.18
The safety analyses included all randomised patients who took at least one dose of study drug and had at least one exploitable safety measure. Analyses were done with SAS version 9.2. This trial is registered with ISRCTN, number ISRCTN17472707, and the Pan African Clinical Trials Registry, number PACTR201202000278282.
Role of funding source
DNDi was responsible for the study design, collection of data, interpretation of results, and reviewing the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
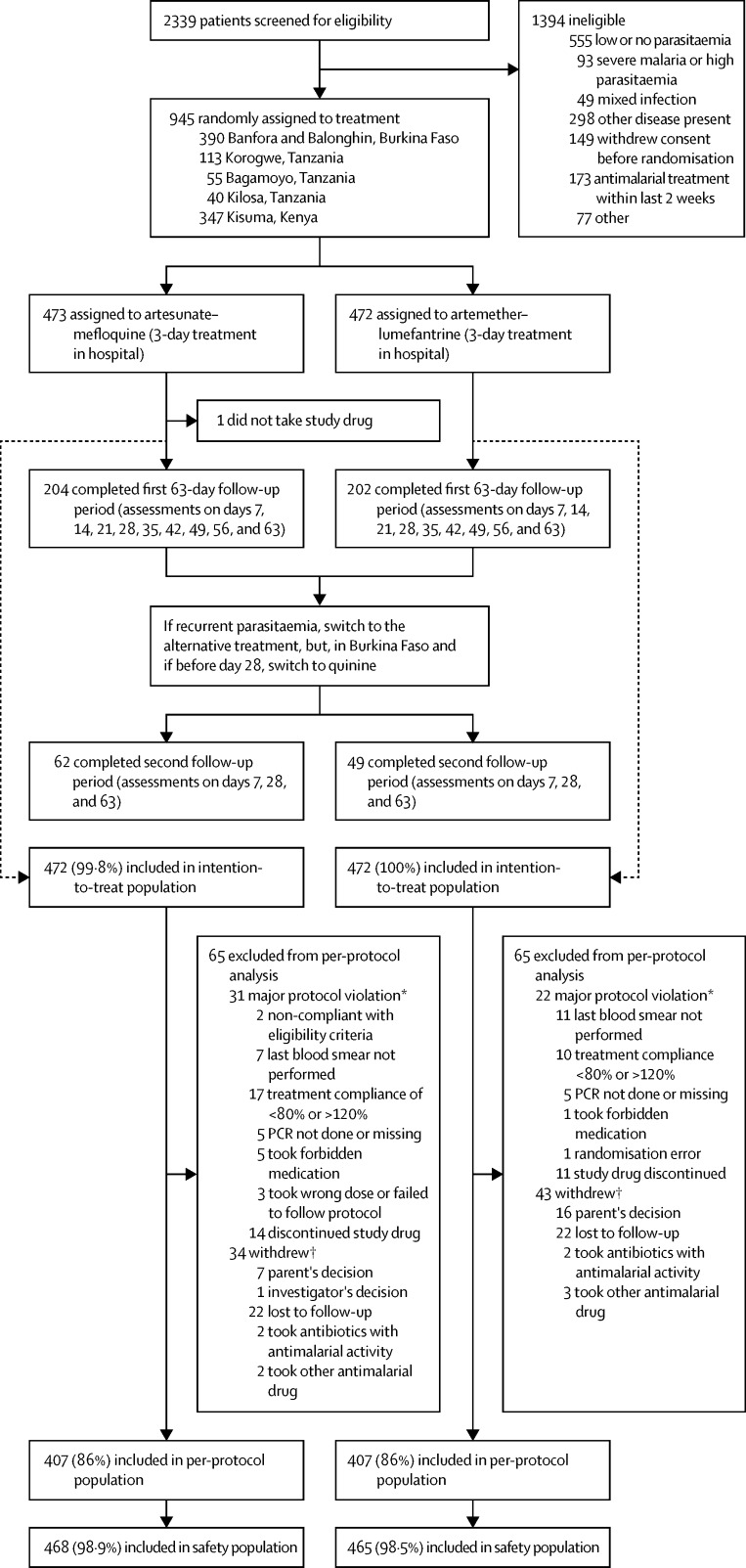
2339 children were screened, of whom 945 were recruited and entered randomisation, with 944 receiving either of the study treatments (figure 1). The most common reason for non-inclusion was low or absent parasitaemia. The treatment groups had similar baseline demographic and clinical characteristics (table 1); however, vomiting at baseline was more frequent in the artesunate–mefloquine group (15% of children) than in the artemether–lumefantrine group (10%). The first patient entered the trial on Dec 13, 2010, and the last patient completed the trial on Oct 1, 2013.
Figure 1.
Trial profile
The intention-to-treat population consisted of all randomly allocated patients who received at least one dose of study drug. The per-protocol population consisted of all patients without a major protocol deviation, who were fully treatment compliant (defined as ≥80% and <120% intake of study drug), who had a primary endpoint at day 63, and who did not withdraw from study treatment (except for those who withdrew because of adverse events or an absence of efficacy). The safety analyses included all randomly allocated patients who took at least one dose of study drug and had at least one exploitable safety measure. *Some patients had more than one protocol violation. †Reason for withdrawal was other than recurrences or adverse event during follow-up.
Table 1.
Baseline characteristics of the two treatment groups for intention-to-treat and per-protocol populations
|
Intention to treat |
Per protocol |
||||
|---|---|---|---|---|---|
| Artesunate–mefloquine (n=472) | Artemether–lumefantrine (n=472) | Artesunate–mefloquine (n=407) | Artemether–lumefantrine (n=407) | ||
| Age (months), mean (range; SD) | 30·35 (6·0–59·8; 14·76) | 29·72 (6·0–59·7; 14·33) | 30·49 (6·0–59·8; 14·78); | 29·93 (6·0–59·7; 14·36) | |
| Sex | |||||
| Female | 242 (51%) | 218 (46%) | 209 (51%) | 188 (46%) | |
| Male | 230 (49%) | 254 (54%) | 198 (49%) | 219 (54%) | |
| Ratio (male:female) | 0·95 | 1·16 | 0·95 | 1·16 | |
| Plasmodium falciparum parasite count (per μL) | |||||
| Mean (SD) | 66 205·5 (55 126·32) | 66 078·92 (53 420·44) | 65 299·39 (53 532·58) | 66 520·83 (54 718·61) | |
| Median (range) | 47 368 (237–268 787) | 50 925 (1935–199 928) | 48 187·0 (2034·0–268 787·0) | 49 892·0 (1935·0–199 928·0) | |
| Axillary temperature (°C) | |||||
| Mean (SD) | 38·62 (0·82) | 38·61 (0·82) | 38·65 (0·82) | 38·62 (0·82) | |
| Median (range) | 38·5 (37·5–40·7) | 38·5 (37·5–41·0) | 38·5 (37·5–40·7) | 38·5 (37·5–41·0) | |
| Gametocytaemia | 22 (5%) | 25 (5%) | 21 (5%) | 22 (5%) | |
| Concentration (per μL), range | 0–1380 | 0–384 | 0·00–280·0 | 0·00–370·0 | |
| Missing samples | 1 | 3 | 1 | 3 | |
| Haemoglobin (g/L) | 95·1 (17·2) | 94·7 (19·0) | 94·9 (17·2) | 94·7 (19·1) | |
| Missing samples | 0 | 1 | 0 | 0 | |
| Neutrophils (× 103 per μL) | 5·15 (2·85) | 5·18 (2·76) | 5·16 (2·87) | 5·13 (2·70) | |
| Missing samples | 12 | 15 | 10 | 11 | |
| Platelets (× 103 per μL) | 176·25 (102·78) | 174·30 (102·77) | 179·18 (105·00) | 174·95 (104·05) | |
| Missing samples | 0 | 1 | 0 | 0 | |
| Leucocytes (× 103 per μL) | 9·70 (3·90) | 9·80 (3·93) | 9·76 (4·00) | 9·68 (3·82) | |
| Headache | 59 (13%) | 65 (14%) | 52 (13%) | 58 (14%) | |
| Missing samples | 1 | 1 | 0 | 1 | |
| Vomiting | 71 (15%) | 46 (10%) | 63 (15%) | 37 (9%) | |
| Missing samples | 1 | 0 | 0 | 0 | |
| Gastrointestinal disorders | 2 (<1%) | 3 (1%) | 1 (<1%) | 3 (1%) | |
| Loss of appetite or anorexia | 139 (30%) | 146 (31%) | 122 (30%) | 115 (28%) | |
| Missing samples | 1 | 0 | 0 | 0 | |
Data are n (%), mean (SD), or median (range), unless otherwise indicated. For some parameters, patient samples were missing; number of missing samples is only indicated for parameters for which samples were missing.
The analysis populations are listed and defined in the appendix. In the per-protocol population, the PCR-corrected ACPR rate at day 63 was 370 (90·9%) of 407 patients in the artesunate–mefloquine group compared with 365 (89·7%) of 407 in the artemether–lumefantrine group (treatment difference 1·23%, 95% CI −2·84% to 5·29%; table 2), showing non-inferiority of artesunate–mefloquine for the primary endpoint. Findings were similar when the intention-to-treat population was used in the analysis (difference in PCR-corrected ACPR rate at day 63 of 1·91%, 95% CI −3·31% to 7·13%; table 2).
Table 2.
Rates of adequate clinical and parasitological response (ACPR)
| Artesunate–mefloquine (per-protocol n=407; intention-to-treat n=472) | Artemether–lumefantrine (per-protocol n=407; intention-to-treat n=472) | Difference, %(95% CI) | ||
|---|---|---|---|---|
| Day 63 | ||||
| Per-protocol population | ||||
| PCR-corrected ACPR | 370 (90·9%) | 365 (89·7%) | 1·23 (−2·84 to 5·29) | |
| Non-PCR-corrected ACPR | 201 (49·4%) | 200 (49·1%) | 0·25 (−6·62 to 7·11) | |
| Intention-to-treat population | ||||
| PCR-corrected ACPR | 376 (79·7%) | 367 (77·8%) | 1·91 (−3·31 to 7·13) | |
| Non-PCR-corrected ACPR | 203 (43·0%) | 201 (42·6%) | 0·42 (−5·89 to 6·74) | |
| Day 42 | ||||
| Per-protocol population | ||||
| PCR-corrected ACPR | 381 (93·6%) | 375 (92·1%) | 1·47 (−2·06 to 5·01) | |
| Non-PCR-corrected ACPR | 253 (62·2%) | 234 (57·5%) | 4·67 (−2·06 to 11·4) | |
| Intention-to-treat population | ||||
| PCR-corrected ACPR | 397 (84·1%) | 384 (81·4%) | 2·75 (−2·06 to 7·57) | |
| Non-PCR-corrected ACPR | 266 (56·4%) | 243 (51·5%) | 4·87 (−1·48 to 11·22) | |
| Day 28 | ||||
| Per-protocol population | ||||
| PCR-corrected ACPR | 397 (97·5%) | 385 (94·6%) | 2·95 (0·29 to 5·61) | |
| Non-PCR-corrected ACPR | 331 (81·3%) | 289 (71·0%) | 10·32 (4·51 to 16·13) | |
| Intention-to-treat population | ||||
| PCR-corrected ACPR | 422 (89·4%) | 402 (85·2%) | 4·24 (−0·00 to 8·48) | |
| Non-PCR-corrected ACPR | 355 (75·2%) | 306 (64·8%) | 10·38 (4·57 to 16·19) | |
Data are n (%), unless otherwise indicated. The non-inferiority of artesunate–mefloquine over artemether–lumefantrine is shown when the lower limit of the 95% CI for the difference between groups is greater than −5%.
Additional by-site analysis prespecified in the statistical analysis plan showed that the PCR-corrected ACPR rates at day 63 (per-protocol population) were similar between groups at each of the individual trial centres (Kilosa, Tanzania: 15 [88·2%] of 17 patients in the artesunate–mefloquine group vs 17 [89·5%] of 19 patients in the artemether–lumefantrine group; Bagamoyo, Tanzania: 21 [100%] of 21 vs 18 [85·7%] of 21; Korogwe, Tanzania: 41 [100%] of 41 vs 50 [98·0%] of 51; Kisumu, Kenya: 121 [81·8%] of 148 vs 108 [79·4%] of 136; Balonghin, Burkina Faso: 120 [97·6%] of 123 vs 117 [95·9%] of 122; Banfora, Burkina Faso: 52 [91·2%] of 57 vs 55 [94·8%] of 58). The small sample sizes prevented meaningful statistical analysis.
The non-PCR-corrected ACPR rates at day 63 were very similar in the two treatment groups, in both the per-protocol and intention-to-treat analyses (table 2), and were non-significant in non-inferiority analysis (insufficient power for statistical analysis). Non-inferiority of artesunate–mefloquine to artemether–lumefantrine was shown for the PCR-corrected ACPR rates at days 28 and 42 in both the per-protocol and intention-to-treat analyses (table 2).
The Kaplan-Meier analysis (see appendix; modified intention-to-treat population) showed no difference in efficacy between artesunate–mefloquine and artemether–lumefantrine (p=0·8153, log-rank test), with higher PCR-corrected ACPR rates for both treatments than in the per-protocol non-inferiority analysis. There were 13 ACPR failures with artesunate–mefloquine and 16 with artemether–lumefantrine in the per-protocol non-inferiority analysis, and 17 and 18, respectively, in the Kaplan-Meier modified intention-to-treat analysis (appendix).
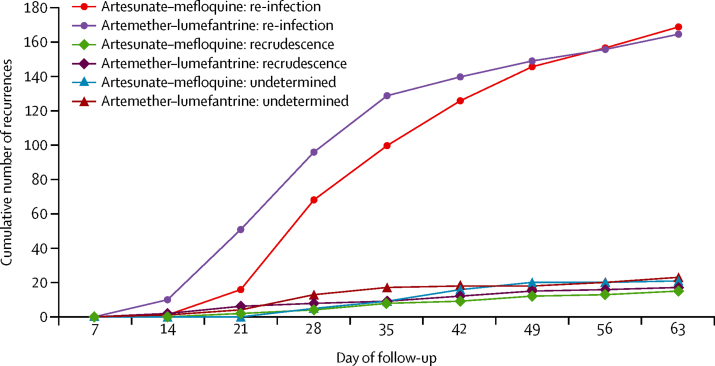
A similar number of patients in the artesunate–mefloquine (n=204) and artemether–lumefantrine (n=202) groups completed the 63-day study period (table 3). There were two early treatment failures, both in the artesunate–mefloquine group (0·4% of 472 patients); both patients developed danger signs of severe malaria with parasitaemia on the first day of treatment and switched treatment to intravenous quinine. There were similar numbers of late treatment failures in the artesunate–mefloquine (n=214) and artemether–lumefantrine (n=211) groups; these were similarly divided, within treatment groups, between late clinical and late parasitological failures (table 3). The PCR analysis showed 15 cases of recrudescence in the artesunate–mefloquine group and 17 cases in the artemether–lumefantrine group, in the per-protocol population (15 vs 18 in the intention-to-treat population; appendix). Thus, most recurrences were re-infections, with similar proportions in the two treatment groups in both per-protocol (169 [43·8%] of 386 patients in the artesunate–mefloquine group vs 165 [43·0%] of 384 in the artemether–lumefantrine group) and intention-to treat (173 [38·8%] of 446 vs 166 [37·4%] of 444) populations (denominators exclude patients with missing PCR analysis; appendix). Comparison of cumulative weekly rates of re-infections, recrudescences, and indeterminate recurrences during follow-up in the per-protocol population shows a delay of about 7 days, up to day 49, with artesunate–mefloquine relative to artemether–lumefantrine (figure 2).
Table 3.
Study withdrawals and reasons (intention-to-treat population)
| Artesunate–mefloquine (n=472) | Artemether–lumefantrine (n=472) | |||
|---|---|---|---|---|
| Completed study | 204 (43·2%) | 202 (42·8%) | ||
| Withdrawal from study | 268 (56·8%) | 270 (57·2%) | ||
| Reasons for withdrawal* | ||||
| Early treatment failure | ||||
| Parasitaemia with sign/danger of severe malaria on days 0, 1, or 2 | 2 (0·7%) | 0 | ||
| Higher parasitaemia on day 2 than day 0 | 0 | 0 | ||
| Parasitaemia on day 3 with axillary temperature of ≥37·5°C | 0 | 0 | ||
| Parasitaemia count on day 3 ≥25% than on day 0 | 0 | 0 | ||
| Late treatment failure | ||||
| Late clinical failure | 61 (22·8%) | 69 (25·6%) | ||
| Late parasitological failure | 153 (57·1%) | 142 (52·6%) | ||
| Adverse event | 8 (3·0%) | 4 (1·5%) | ||
| Serious adverse event | 0 | 2 (0·7%) | ||
| Parent or guardian's decision | 12 (4·5%) | 21 (7·8%) | ||
| Intake of other antimalarial drugs | 2 (0·7%) | 0 | ||
| Intake of antibiotics with antimalarial activity | 2 (0·7%) | 2 (0·7%) | ||
| Investigator's decision | 1 (0·4%) | 0 | ||
| Lost to follow-up | 25 (9·3%) | 24 (8·9%) | ||
| Other reason | 2 (0·7%) | 6 (2·2%) | ||
Data are n (%).
Percentage values indicate proportion of withdrawals.
Figure 2.
Cumulative number of recurrences at weekly intervals during follow-up
24 h after the start of treatment, 266 (65·4%) of 407 patients in the artesunate–mefloquine group and 297 (73·2%) of 406 in the artemether–lumefantrine group had parasitaemia (per-protocol population; p=0·0160, Pearson χ2 test). At 48 h, the proportions were 20 (6·3%) of 320 in the artemether–lumefantrine group and 24 (7·2%) of 332 in the artemether–lumefantrine group. At 72 h, no patients in either group had parasitaemia (none of 324 in the artesunate–mefloquine group and none of 329 in the artemether–lumefantrine group; per-protocol population). In both the per-protocol and intention-to-treat analyses, the mean time to parasite clearance did not differ between treatment groups (analyses exclude those with missing data).
P falciparum gametocytes were detected (thick blood smears) in few patients at baseline (intention-to-treat population: 22 [4·7%] of 471 patients in the artesunate–mefloquine group and 25 [5·3%] of 469 in the artemether–lumefantrine group; per-protocol population: 21 [5·2%] of 406 vs 22 [5·4%] of 404; analyses exclude those with missing data). At 48 h and 72 h (approximately 20% with missing data), the gametocyte carriage rates were 14 (4·4%) of 318 patients and seven (2·2%) of 319 patients in the artesunate–mefloquine group and 17 (5·2%) of 329 patients and 12 (3·8%) of 317 patients in the artemether–lumefantrine group, respectively (per-protocol population). No patient at days 28, 42, and 63 had detectable gametocytes.
Between 24 h and 48 h after the start of treatment, axillary temperatures of more than 37·5°C were significantly less frequent in patients in the artesunate–mefloquine group (45 [11·1%] of 406) than in patients in the artemether–lumefantrine group (85 [20·9%] of 407; per-protocol population; p=0·0002; Fisher's exact test). After 48 h, the proportions declined and were similar in both treatment groups. The mean time to attain an axillary temperature of 37·5°C or lower was slightly shorter with artesunate–mefloquine (6·5 h) than with artemether–lumefantrine (7·5 h). These findings were unaffected by the number of patients taking paracetamol, which was similar between groups (between 24 h and 48 h: 302 patients in the artesunate–mefloquine group vs 300 in the artemether–lumefantrine group). 21 (4·8%) of 436 patients in the artesunate–mefloquine group had fever 72 h after the start of treatment versus 24 (5·4%) of 444 in the artemether–lumefantrine group.
Table 4 lists adverse events that occurred in more than 5% of patients. The frequencies of adverse events were similar in the two treatment groups and were similarly high for anaemia, cough, upper respiratory tract infection, vomiting (early and late), bronchitis, and diarrhoea. In the second follow-up period, fewer adverse events had an incidence of more than 5% of patients and, notably, less than 5% of patients in each treatment group reported anaemia or vomiting.
Table 4.
Adverse events occurring in more than 5% of patients, by study period
| Artesunate–mefloquine | Artemether–lumefantrine | ||
|---|---|---|---|
| First 63-day follow-up | |||
| Number of patients | 468 | 465 | |
| Blood and lymphatic system disorders | |||
| Anaemia | 130 (27·8%) | 104 (22·4%) | |
| Gastrointestinal disorders | |||
| Diarrhoea | 57 (12·2%) | 43 (9·2%) | |
| Vomiting | 69 (14·7%) | 77 (16·6%) | |
| General disorders and administration site conditions | |||
| Pyrexia | 34 (7·3%) | 22 (4·7%) | |
| Infections and infestations | |||
| Bronchitis | 56 (12·0%) | 52 (11·2%) | |
| Gastroenteritis | 27 (5·8%) | 19 (4·1%) | |
| Pneumonia | 24 (5·1%) | 21 (4·5%) | |
| Rhinitis | 41 (8·8%) | 48 (10·3%) | |
| Tinea capitis | 38 (8·1%) | 19 (4·1%) | |
| Upper respiratory tract infection | 93 (19·9%) | 87 (18·7%) | |
| Metabolism and nutrition disorders | |||
| Decreased appetite | 34 (7·3%) | 23 (4·9%) | |
| Respiratory, thoracic, and mediastinal disorders | |||
| Cough | 98 (20·9%) | 92 (19·8%) | |
| Rhinorrhoea | 40 (8·5%) | 30 (6·5%) | |
| Skin and subcutaneous tissue disorders | |||
| Rash | 32 (6·8%) | 22 (4·7%) | |
| Second 63-day follow-up after treatment with the alternative test treatment | |||
| Number of patients | 192 | 171 | |
| General disorders and administration site conditions | |||
| Pyrexia | 10 (5·2%) | 4 (2·3%) | |
| Infections and infestations | |||
| Bronchitis | 6 (3·1%) | 9 (5·3%) | |
| Rhinitis | 5 (2·6%) | 12 (7·0%) | |
| Upper respiratory tract infection | 19 (9·9%) | 16 (9·4%) | |
| Respiratory, thoracic, and mediastinal disorders | |||
| Cough | 18 (9·4%) | 15 (8·8%) | |
| Second 63-day follow-up after treatment with antimalarial other than artesunate–mefloquine or artemether–lumefantrine | |||
| Number of patients | 33 | 48 | |
| Blood and lymphatic system disorders | |||
| Anaemia | 2 (6·1%) | 1 (2·1%) | |
| Gastrointestinal disorders | |||
| Diarrhoea | 1 (3·0%) | 3 (6·3%) | |
| Infections and infestations | |||
| Malaria | 4 (12·1%) | 3 (6·3%) | |
Data are n (%). Data show adverse events occurring in more than 5% of patients during the first follow-up (up to day 63 or until the day before start of rescue treatment); the second follow-up of patients receiving, as rescue treatment, the alternative investigated drug; and the second follow-up of patients receiving, as rescue treatment, an antimalarial drug other than the alternative investigated drug.
During the first follow-up period, nervous system disorders were infrequent overall but more frequent in the artesunate–mefloquine group than in the artemether–lumefantrine group (ten [2·1%] of 468 patients vs five [1·1%] of 465). Six patients in the artesunate–mefloquine group had convulsions or febrile convulsions and two patients in the artemether–lumefantrine group had convulsions, with two patients in each treatment group having such events during the 3 days of treatment. The frequencies of the other CNS adverse events, headache and lethargy, were similar between groups: three (0·6%) patients in the artesunate–mefloquine group and two (0·4%) patients in the artemether–lumefantrine group had headache; one (0·2%) patient in each group had lethargy. However, none of the CNS adverse events were deemed related to study treatment by the investigator or led to study treatment discontinuation or were serious adverse events. No psychiatric disorders were reported in any patient.
The proportion of patients presenting with early vomiting was similar between groups (71 [15·3%] of 463 patients in the artesunate–mefloquine group vs 79 [16·8%] of 471 in the artemether–lumefantrine group in any of the three dosing days), when considering the two artemether–lumefantrine daily doses together (appendix). The overall proportion per day decreased from 10·5% (98 of 930 patients) to 3·5% (32 of 903 patients) over the 3 days and most (>90%) children who vomited within 1 h of treatment did not vomit following treatment readministration.
The frequency and descriptions of reported adverse events are further summarised in the appendix. During the first 63-day follow-up period, similar proportions of patients in the artesunate–mefloquine and artemether–lumefantrine groups had at least one adverse event; a non-significant greater proportion of patients in the artemether–lumefantrine group had an adverse event during treatment (165 [35·5%] of 465 patients in the artesunate–mefloquine group vs 138 [29·5%] of 468 in the artemether–lumefantrine group; p=0·0590). For both treatments, about 10% of patients (46 of 468 in the artesunate–mefloquine group vs 49 of 465 in the artemether–lumefantrine group) had an adverse event that could be at least possibly related to treatment (according to the investigator). Treatment was discontinued because of an adverse event in ten (2·1%) patients in the artesunate–mefloquine group and in four (0·9%) patients in the artemether–lumefantrine group (p=0·1763), with vomiting the reason for treatment discontinuation in eight and four patients, respectively. A similar number of serious adverse events (mostly anaemia or infection) occurred with artesunate–mefloquine (n=16) as with artemether–lumefantrine (n=14), with one life-threatening adverse event and no fatalities. The life-threatening adverse event was anaemia, reported on day 1 of artesunate–mefloquine treatment, and led to early treatment failure caused by severe malaria and suspected sepsis, and recorded as possibly related to treatment. Overall, 18 (1·9%) of 933 patients had at least one serious adverse event, mostly infection (in seven patients in the artesunate–mefloquine group and five in the artemether–lumefantrine group; pneumonia being most common with three cases in each group) or anaemia (two vs four patients). Of these patients, five in the artesunate–mefloquine group and two in the artemether–lumefantrine group had the serious adverse event during treatment.
During the second 63-day follow-up period with artesunate–mefloquine or artemether–lumefantrine as rescue treatment, the frequency of adverse events was generally lower than in the first 63-day follow-up period and proportions were similar between the two treatment groups (appendix), possibly influenced by the lower frequency of visits during the second follow-up period. During this follow-up, the relation of adverse events to rescue treatment was only recorded in the centres in Burkina Faso, where no patient had an adverse event that was at least possibly related to treatment. The frequency of serious adverse events was also lower in the second 63-day follow-up period than in the first 63-day follow-up. Five patients treated with artemether–lumefantrine as rescue treatment and two patients treated with artesunate–mefloquine as rescue treatment had at least one serious adverse event, which were all infections. Four and two patients in the respective groups of patients receiving rescue treatment other than artesunate–mefloquine or artemether–lumefantrine had at least one serious adverse event (either anaemia or infection).
Discussion
Our study provides important findings on the use of artesunate–mefloquine in the treatment of children younger than 5 years with uncomplicated P falciparum infection in Africa. It included a 63-day follow-up and an extensive safety assessment, and provides much-needed additional data on the use of artesunate–mefloquine in young African children.19 To our knowledge, this is the first trial of artesunate–mefloquine with a design compliant with current WHO guidelines in this especially vulnerable population.2
The primary outcome, the rate of PCR-corrected ACPR at day 63 in the per-protocol population, was very similar in the artesunate–mefloquine and artemether–lumefantrine groups, with a percentage difference that had a lower bound of the 95% CI (−2·84%) greater than −5%. Thus, the criterion for the non-inferiority of artesunate–mefloquine to artemether–lumefantrine was met in young African children. The cure rates were similar to, although a little below, published rates for the two ACTs in mixed-aged populations in non-African countries, including trials with the fixed-dose artesunate–mefloquine tablet.9, 14 We found no published data on 63-day cure rates for artesunate–mefloquine and artemether–lumefantrine in young children. All our patients were admitted to hospital for treatment and compliance was 100%, or just less, at all sites during the 3 days of treatment.
Previously, in studies in populations of mixed age in Senegal,20 Nigeria,21 Mali,22 and Kenya,23 artesunate–mefloquine was reported to be effective and well tolerated. These trials each used a follow-up of just 28 days, which might be too short to fully assess accurate cure rates and safety of artesunate–mefloquine because of mefloquine's slow elimination. The low deployment of artesunate–mefloquine in Africa might result from the early vomiting and neuropsychiatric effects reported with mefloquine monotherapy,24 and also from the absence of a fixed-dose paediatric formulation. In this study we tested a fixed-dose artesunate–mefloquine tablet, designed to optimise the compliance of a 3-day combination treatment and to improve the tolerability of mefloquine by dividing the dose into three equal daily doses. We found that the fixed-dose artesunate–mefloquine tablet has a good safety profile that is similar to that of artemether–lumefantrine, with a low risk of repeated early vomiting during the treatment period and a low incidence of neuropsychiatric adverse events, which were deemed unrelated to study treatment.
The parasite clearance rate was rapid with both ACTs, with most children showing complete parasite clearance within 2 days. The parasite reduction ratios for both artesunate–mefloquine and artemether–lumefantrine are high compared with published values.25 Artesunate–mefloquine was marginally superior to artemether–lumefantrine in the rapidity of parasite clearance and the slightly more rapid decline in fever. Although these differences might be chance findings, the high parasite reduction ratios serve to strengthen our evidence of the efficacy of artesunate–mefloquine in African children by comparison with artemether–lumefantrine. Rapid parasite clearance and fever reduction are essential to ensure compliance with antimalarial treatment.
There were only two early treatment failures overall. By day 63, the rates of late treatment failure were similar for artesunate–mefloquine and artemether–lumefantrine, and mostly caused by re-infection. We detected that, up to day 49, the cumulative rates of recrudescence and of re-infection were each delayed by about 7 days with artesunate–mefloquine relative to those with artemether–lumefantrine. A similar finding was reported by Sagara and colleagues22 in their trial of artesunate–mefloquine versus artemether–lumefantrine in Mali.22 These findings suggest that artesunate–mefloquine provides longer protection against malaria than artemether–lumefantrine, possibly because of the longer half-life of mefloquine compared with lumefantrine, with reported median terminal clearance half-lives of about 20 days26 and 5 days,27 respectively. By day 63, the comparative efficacy is unaffected by the different clearance rates.
We found the safety profiles of artesunate–mefloquine and artemether–lumefantrine to be similar, with low frequencies of nervous system disorders and no psychiatric disorders. Our investigators were instructed to look for possible neuropsychiatric disorders, but no active neurological testing was done. Relevant neuropsychiatric adverse events are difficult to identify in young children and are consequently under-reported. Another study of artesunate–mefloquine (fixed dose of 50 mg artesunate and 125 mg mefloquine per day for 3 days) in 213 young children in Africa,28 with a 63-day follow-up, actively tested for neuropsychiatric effects (using standard infant neurological tests) and reported mild-to-moderate, spontaneously resolving neuropsychiatric adverse events, with sleeping disorders being the most common (2·3% of patients). Notably, because of the risk of serious psychiatric side-effects, the latest WHO antimalarial treatment guideline3 recommends an interval of 60 days between consecutive periods of mefloquine treatment.
In children younger than 5 years, mefloquine has been associated with early vomiting causing impaired absorption,29 but studies, including in young children, show that the incidence of early vomiting is substantially reduced by splitting the mefloquine dose across successive days and by coadministration with artesunate. In a mixed-aged population, early vomiting was reported in only 3% of patients treated with fixed-dose artesunate–mefloquine.13 Dividing the mefloquine dose across 2 or 3 days does not affect efficacy because of its slow clearance. In our study population, about 10% had early vomiting but less than 10% of these patients vomited after readministration. Lower rates were measured in mixed-aged populations in large trials in Brazil8 and in Burma,9 with the same fixed-dose artesunate–mefloquine tablet that we tested here.
Our study has limitations. It is an open-labelled study and was not powered for safety. However, to our knowledge, it is the largest randomised controlled trial providing efficacy and safety data for artesunate–mefloquine in children in Africa. We conclude that fixed-dose artesunate–mefloquine is safe and effective in treating young African children with uncomplicated malaria. This combination is shown to be as effective as artemether–lumefantrine, which is recognised as a safe and efficacious antimalarial drug in children.19 Our data are derived from a large trial, optimally designed for testing an ACT, such as artesunate–mefloquine, with a slowly cleared active component. We believe our results support the deployment of fixed-dose artesunate–mefloquine in young children in Africa. Our findings should have important implications for health policy in sub-Saharan Africa.
Acknowledgments
Acknowledgments
Artemether–lumefantrine was provided by Farmanguinhos (Rio de Janeiro, Brazil). We thank the participating children, their guardians, and the local health personnel who contributed to study execution; the data monitoring committee, Piero Olliaro, Elizabeth Ashley, Tsiri Agbenyega, and Michel Vaillant; the study monitors, Moses N Waweru, Marguerite Crato Cissé, and Daniel Ansong, for their constant support; Patrice Piola and Isabela Ribeiro for advice on the study protocol; Yap Boum, Dan Nyehangane from Epicentre Mbarara Research Base for support with parasite genotyping and QC of malaria blood smears; Kasia Stepniewska and Philippe Guerin from Worldwide Antimalarial Resistance Network (WWARN) for the parasite clearance estimator analyses. We thank Gerard McGregor (Omniscience SA), who provided medical writing services on behalf of the Drugs for Neglected Diseases initiative (DNDi), and Jean-René Kiechel and Graciela Diap for providing an editorial contribution to the clinical study report and this manuscript. DNDi acknowledges the following for their financial support of the study: Agence Française de Développement (AFD, France); Department for International Development (DFID, UK); Dutch Ministry of Foreign Affairs (DGIS, Netherlands); European and Developing Countries Clinical Trials Partnership (EDCTP, EU); Fondation Arpe (Switzerland); Médecins Sans Frontières (MSF International); Swiss Agency for Development and Cooperation (SDC, Switzerland).
Contributors
SBS, BO, JPAL, AM, and ZM supervised the execution of the study at the different sites. AO, JBY, KOO, SG, EM, and JSN supported the field trial coordination and were involved in data collection. IA coordinated the data management and data analyses activities. FA supported the data analyses plan, data analyses, was involved in the interpretation of the results, and co-authored the clinical study report. JV participated in the database cleaning and data analyses plan, and contributed to the clinical study report and the manuscript. NS participated in study design and protocol, data analyses plan, and contributed to the clinical study report and the manuscript. GC coordinated the overall trial, participated in study design and protocol, data analyses plan, study documents, in the drafting of the manuscript, and co-authored the clinical study report. All authors reviewed and approved the final version.
Declaration of interests
BO received a grant from the Kenyan Medical Research Institute for conducting this trial. FA states that his institute received payment for electronic data capture, data management, statistical analysis, and clinical study report writing from DNDi for the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO . World malaria report 2015. World Health Organization; Geneva: 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ (accessed April 5, 2016). [Google Scholar]
- 2.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet. 2014;383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Guidelines for the treatment of malaria. Third edition. World Health Organization; Geneva: 2015. http://www.who.int/malaria/publications/atoz/9789241549127/en/ (accessed April 5, 2016). [Google Scholar]
- 4.Bosman A, Mendis KN. A major transition in malaria treatment: the adoption and deployment of artemisinin-based combination therapies. Am J Trop Med Hyg. 2007;77(suppl):193–197. [PubMed] [Google Scholar]
- 5.Ashley EA, Dhorda M, Fairhurst RM. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hastings I. How artemisinin-containing combination therapies slow the spread of antimalarial drug resistance. Trends Parasitol. 2011;27:67–72. doi: 10.1016/j.pt.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Kameda K. Needs-driven versus market-driven pharmaceutical innovation: the consortium for the development of a new medicine against malaria in Brazil. Dev World Bioeth. 2014;14:101–108. doi: 10.1111/dewb.12056. [DOI] [PubMed] [Google Scholar]
- 8.Santelli AC, Ribeiro I, Daher A. Effect of artesunate-mefloquine fixed-dose combination in malaria transmission in Amazon basin communities. Malar J. 2012;11:286. doi: 10.1186/1475-2875-11-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smithuis F, Kyaw MK, Phe O. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis. 2010;10:673–681. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valecha N, Srivastava B, Dubhashi NG. Safety, efficacy and population pharmacokinetics of fixed-dose combination of artesunate-mefloquine in the treatment of acute uncomplicated Plasmodium falciparum malaria in India. J Vector Borne Dis. 2013;50:258–264. [PubMed] [Google Scholar]
- 11.Wells S, Diap G, Kiechel JR. The story of artesunate-mefloquine (ASMQ), innovative partnerships in drug development: case study. Malar J. 2013;12:68. doi: 10.1186/1475-2875-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontanet AL, Johnston DB, Walker AM. High prevalence of mefloquine-resistant falciparum malaria in eastern Thailand. Bull World Health Organ. 1993;71:377–383. [PMC free article] [PubMed] [Google Scholar]
- 13.ter Kuile FO, Nosten F, Luxemburger C. Mefloquine treatment of acute falciparum malaria: a prospective study of non-serious adverse effects in 3673 patients. Bull World Health Organ. 1995;73:631–642. [PMC free article] [PubMed] [Google Scholar]
- 14.Ashley EA, Lwin KM, McGready R. An open label randomized comparison of mefloquine-artesunate as separate tablets vs. a new co-formulated combination for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. Trop Med Int Health. 2006;11:1653–1660. doi: 10.1111/j.1365-3156.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 15.WHO . Guidelines for the treatment of malaria. 1st edn. World Health Organization; Geneva: 2006. [Google Scholar]
- 16.WHO. Malaria for Medicines Venture Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. 2007. http://www.who.int/malaria/publications/atoz/9789241596305/en/ (accessed April 5, 2016).
- 17.WHO . Methods for surveillance of antimalarial drug efficacy. World Health Organization; Geneva: 2009. [Google Scholar]
- 18.Flegg JA, Guerin PJ, White NJ, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J. 2011;10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egunsola O, Oshikoya KA. Comparative safety of artemether-lumefantrine and other artemisinin-based combinations in children: a systematic review. Malar J. 2013;12:385. doi: 10.1186/1475-2875-12-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faye B, Ndiaye JL, Ndiaye D, Dieng Y, Faye O, Gaye O. Efficacy and tolerability of four antimalarial combinations in the treatment of uncomplicated Plasmodium falciparum malaria in Senegal. Malar J. 2007;6:80. doi: 10.1186/1475-2875-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agomo PU, Meremikwu MM, Watila IM. Efficacy, safety and tolerability of artesunate-mefloquine in the treatment of uncomplicated Plasmodium falciparum malaria in four geographic zones of Nigeria. Malar J. 2008;7:172. doi: 10.1186/1475-2875-7-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagara I, Diallo A, Kone M. A randomized trial of artesunate-mefloquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am J Trop Med Hyg. 2008;79:655–661. [PubMed] [Google Scholar]
- 23.Bhatt KM, Samia BM, Bhatt SM, Wasunna KM. Efficacy and safety of an artesunate/mefloquine combination, (artequin) in the treatment of uncomplicated P falciparum malaria in Kenya. East Afr Med J. 2006;83:236–242. doi: 10.4314/eamj.v83i5.9428. [DOI] [PubMed] [Google Scholar]
- 24.Sowunmi A, Salako LA, Oduola AM, Walker O, Akindele JA, Ogundahunsi OA. Neuropsychiatric side effects of mefloquine in Africans. Trans R Soc Trop Med Hyg. 1993;87:462–463. doi: 10.1016/0035-9203(93)90037-q. [DOI] [PubMed] [Google Scholar]
- 25.White NJ. The parasite clearance curve. Malar J. 2011;10:278. doi: 10.1186/1475-2875-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karbwang J, White NJ. Clinical pharmacokinetics of mefloquine. Clin Pharmacokinet. 1990;19:264–279. doi: 10.2165/00003088-199019040-00002. [DOI] [PubMed] [Google Scholar]
- 27.Salman S, Page-Sharp M, Griffin S. Population pharmacokinetics of artemether, lumefantrine, and their respective metabolites in Papua New Guinean children with uncomplicated malaria. Antimicrob Agents Chemother. 2011;55:5306–5313. doi: 10.1128/AAC.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambler MT, Dubowitz LM, Arunjerdja R. The neurological assessment in young children treated with artesunate monotherapy or artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria. Malar J. 2009;8:207. doi: 10.1186/1475-2875-8-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slutsker LM, Khoromana CO, Payne D. Mefloquine therapy for Plasmodium falciparum malaria in children under 5 years of age in Malawi: in vivo/in vitro efficacy and correlation of drug concentration with parasitological outcome. Bull World Health Organ. 1990;68:53–59. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.