Abstract
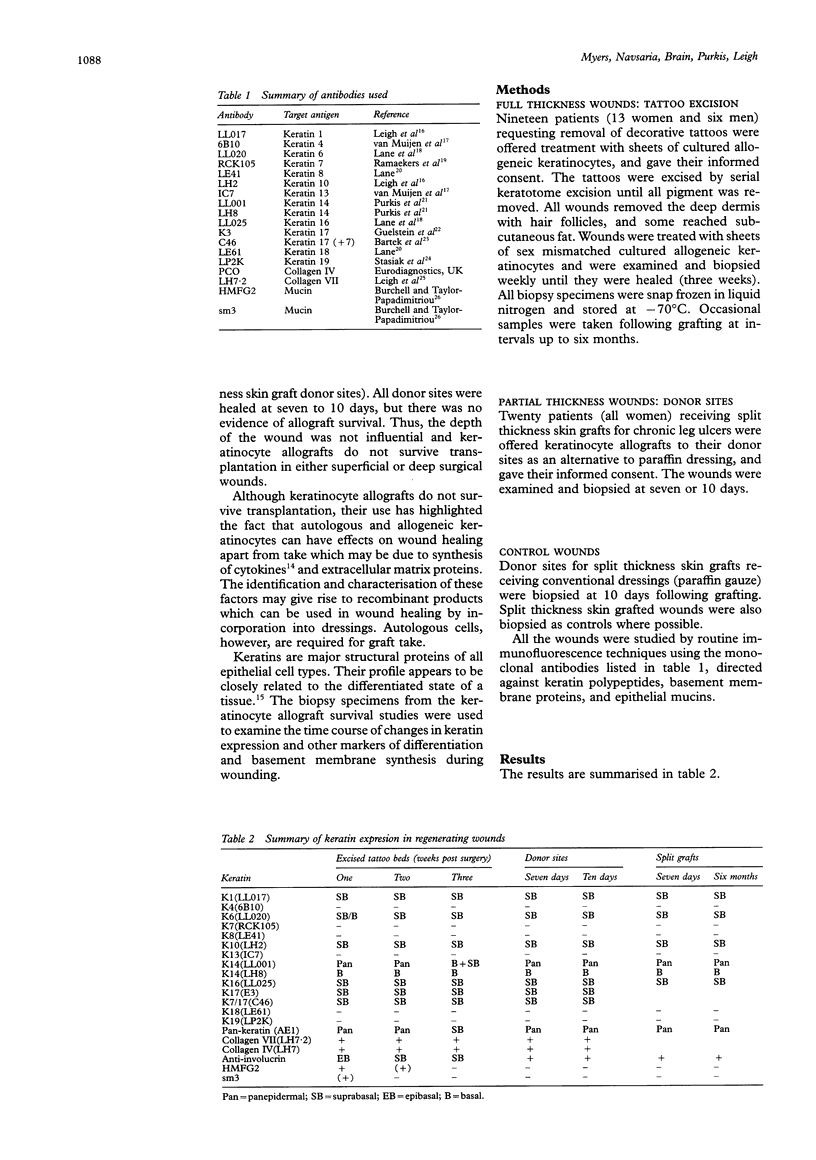
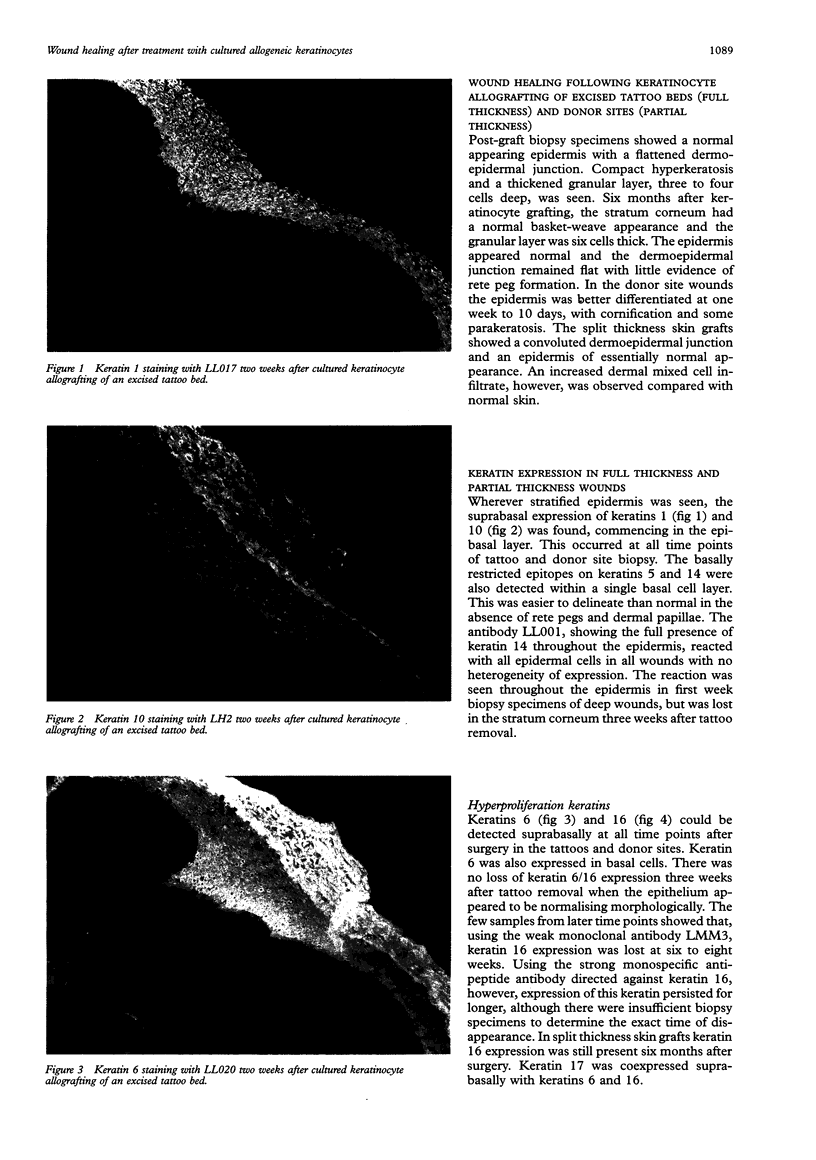
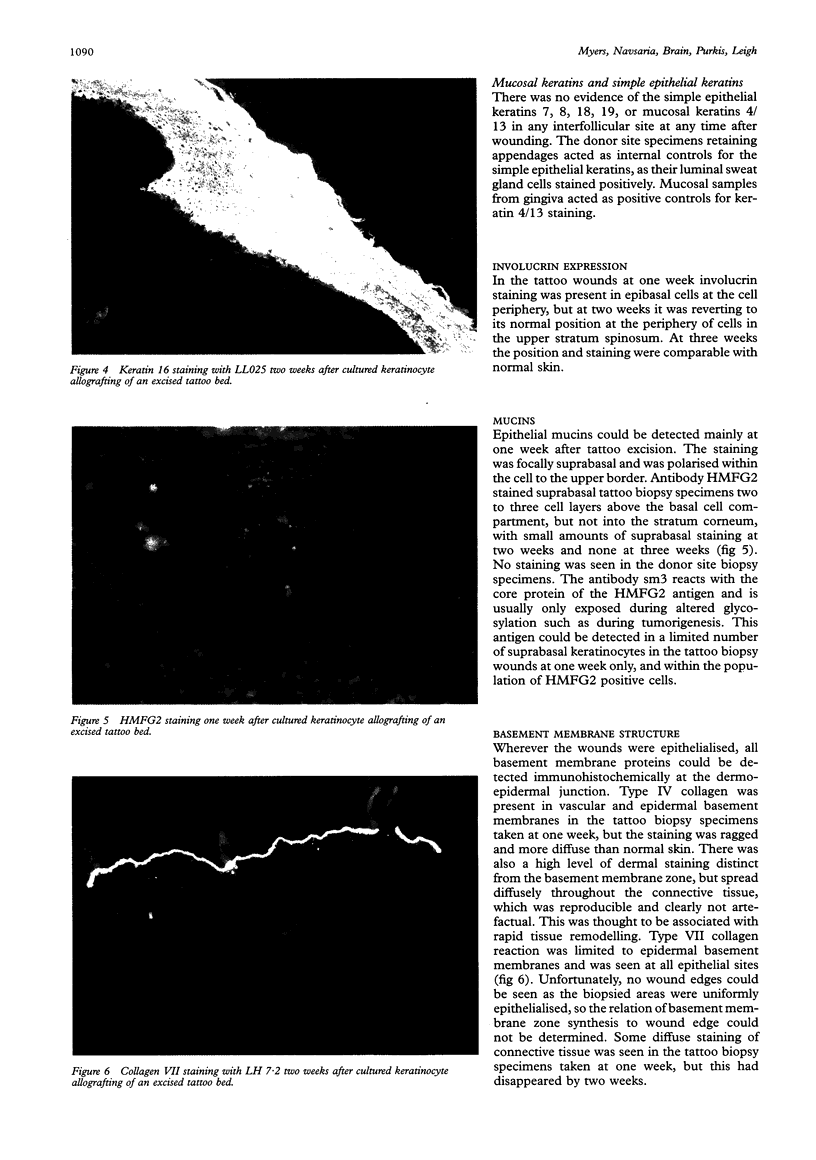
AIMS--To establish the structural changes that occur in deep surgical wounds engrafted with allogeneic sheets, their time course and inter-relation. METHODS--Deep surgical wounds following shave excision of tattoos (down to deep dermis/subcutaneous fat) were treated with sheets of sex mismatched allogeneic keratinocytes in 19 patients and then biopsied weekly until wound healing was complete. More superficial surgical wounds--that is, 20 standard skin graft donor sites, were biopsied at seven to 10 days (all healed) following application of keratinocyte allografts. All biopsy specimens were examined with a large panel of monoclonal antibodies to keratins, envelope proteins, basement membrane components, and to extracellular matrix components. RESULTS--The hyperproliferative keratin pair K6/16 was expressed in all wounds, for up to six weeks in keratinocyte grafted deep wounds, and up to six months in split thickness skin grafted wounds. CONCLUSIONS--Keratins 6 and 16 have not been detected in normal skin, although the relevant mRNA has. This raises the possibility of regulation at a post-transcriptional level allowing a rapid response to injury with cytoskeletal changes that may aid cell migration. This keratin pair offers the most sensitive marker for altered epidermis following wounding.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden P. E., Wood E. J., Cunliffe W. J. Comparison of prekeratin and keratin polypeptides in normal and psoriatic human epidermis. Biochim Biophys Acta. 1983 Feb 28;743(1):172–179. doi: 10.1016/0167-4838(83)90431-4. [DOI] [PubMed] [Google Scholar]
- Brain A., Purkis P., Coates P., Hackett M., Navsaria H., Leigh I. Survival of cultured allogeneic keratinocytes transplanted to deep dermal bed assessed with probe specific for Y chromosome. BMJ. 1989 Apr 8;298(6678):917–919. doi: 10.1136/bmj.298.6678.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell J., Taylor-Papadimitriou J. Antibodies to human milk fat globule molecules. Cancer Invest. 1989;7(1):53–61. doi: 10.3109/07357908909038267. [DOI] [PubMed] [Google Scholar]
- Bártek J., Vojtesek B., Stasková Z., Bártková J., Kerekés Z., Rejthar A., Kovarík J. A series of 14 new monoclonal antibodies to keratins: characterization and value in diagnostic histopathology. J Pathol. 1991 Jul;164(3):215–224. doi: 10.1002/path.1711640306. [DOI] [PubMed] [Google Scholar]
- Faure M., Mauduit G., Schmitt D., Kanitakis J., Demidem A., Thivolet J. Growth and differentiation of human epidermal cultures used as auto- and allografts in humans. Br J Dermatol. 1987 Feb;116(2):161–170. doi: 10.1111/j.1365-2133.1987.tb05807.x. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O., Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelstein V. I., Tchypysheva T. A., Ermilova V. D., Litvinova L. V., Troyanovsky S. M., Bannikov G. A. Monoclonal antibody mapping of keratins 8 and 17 and of vimentin in normal human mammary gland, benign tumors, dysplasias and breast cancer. Int J Cancer. 1988 Aug 15;42(2):147–153. doi: 10.1002/ijc.2910420202. [DOI] [PubMed] [Google Scholar]
- Hancock K., Leigh I. M. Cultured keratinocytes and keratinocyte grafts. BMJ. 1989 Nov 11;299(6709):1179–1180. doi: 10.1136/bmj.299.6709.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefton J. M., Amberson J. B., Biozes D. G., Weksler M. E. Loss of HLA-DR expression by human epidermal cells after growth in culture. J Invest Dermatol. 1984 Jul;83(1):48–50. doi: 10.1111/1523-1747.ep12261671. [DOI] [PubMed] [Google Scholar]
- Hefton J. M., Madden M. R., Finkelstein J. L., Shires G. T. Grafting of burn patients with allografts of cultured epidermal cells. Lancet. 1983 Aug 20;2(8347):428–430. doi: 10.1016/s0140-6736(83)90392-6. [DOI] [PubMed] [Google Scholar]
- Lane E. B., Bártek J., Purkis P. E., Leigh I. M. Keratin antigens in differentiating skin. Ann N Y Acad Sci. 1985;455:241–258. doi: 10.1111/j.1749-6632.1985.tb50415.x. [DOI] [PubMed] [Google Scholar]
- Lane E. B. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane E. B., Wilson C. A., Hughes B. R., Leigh I. M. Stem cells in hair follicles. Cytoskeletal studies. Ann N Y Acad Sci. 1991 Dec 26;642:197–213. doi: 10.1111/j.1749-6632.1991.tb24388.x. [DOI] [PubMed] [Google Scholar]
- Leigh I. M., Purkis P. E., Whitehead P., Lane E. B. Monospecific monoclonal antibodies to keratin 1 carboxy terminal (synthetic peptide) and to keratin 10 as markers of epidermal differentiation. Br J Dermatol. 1993 Aug;129(2):110–119. doi: 10.1111/j.1365-2133.1993.tb03511.x. [DOI] [PubMed] [Google Scholar]
- Madden M. R., Finkelstein J. L., Staiano-Coico L., Goodwin C. W., Shires G. T., Nolan E. E., Hefton J. M. Grafting of cultured allogeneic epidermis on second- and third-degree burn wounds on 26 patients. J Trauma. 1986 Nov;26(11):955–962. doi: 10.1097/00005373-198611000-00001. [DOI] [PubMed] [Google Scholar]
- McKay I. A., Leigh I. M. Epidermal cytokines and their roles in cutaneous wound healing. Br J Dermatol. 1991 Jun;124(6):513–518. doi: 10.1111/j.1365-2133.1991.tb04942.x. [DOI] [PubMed] [Google Scholar]
- Morhenn V. B., Benike C. J., Cox A. J., Charron D. J., Engleman E. G. Cultured human epidermal cells do not synthesize HLA-DR. J Invest Dermatol. 1982 Jan;78(1):32–37. doi: 10.1111/1523-1747.ep12497875. [DOI] [PubMed] [Google Scholar]
- Phillips T. J., Kehinde O., Green H., Gilchrest B. A. Treatment of skin ulcers with cultured epidermal allografts. J Am Acad Dermatol. 1989 Aug;21(2 Pt 1):191–199. doi: 10.1016/s0190-9622(89)70160-2. [DOI] [PubMed] [Google Scholar]
- Purkis P. E., Steel J. B., Mackenzie I. C., Nathrath W. B., Leigh I. M., Lane E. B. Antibody markers of basal cells in complex epithelia. J Cell Sci. 1990 Sep;97(Pt 1):39–50. doi: 10.1242/jcs.97.1.39. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Schaart G., Moesker O., Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987 May;170(1):235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Schweizer J., Winter H., Hill M. W., Mackenzie I. C. The keratin polypeptide patterns in heterotypically recombined epithelia of skin and mucosa of adult mouse. Differentiation. 1984;26(2):144–153. doi: 10.1111/j.1432-0436.1984.tb01388.x. [DOI] [PubMed] [Google Scholar]
- Stasiak P. C., Purkis P. E., Leigh I. M., Lane E. B. Keratin 19: predicted amino acid sequence and broad tissue distribution suggest it evolved from keratinocyte keratins. J Invest Dermatol. 1989 May;92(5):707–716. doi: 10.1111/1523-1747.ep12721500. [DOI] [PubMed] [Google Scholar]
- Stoler A., Kopan R., Duvic M., Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988 Aug;107(2):427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivolet J., Faure M., Demidem A., Mauduit G. Cultured human epidermal allografts are not rejected for a long period. Arch Dermatol Res. 1986;278(3):252–254. doi: 10.1007/BF00412936. [DOI] [PubMed] [Google Scholar]
- Thivolet J., Faure M., Demidem A., Mauduit G. Long-term survival and immunological tolerance of human epidermal allografts produced in culture. Transplantation. 1986 Sep;42(3):274–280. doi: 10.1097/00007890-198609000-00010. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. L., Dean D., Lane E. B., Dawber R. P., Leigh I. M. Keratinocyte differentiation in psoriatic scalp: morphology and expression of epithelial keratins. Br J Dermatol. 1994 Aug;131(2):191–200. doi: 10.1111/j.1365-2133.1994.tb08490.x. [DOI] [PubMed] [Google Scholar]
- Woodley D. T., Peterson H. D., Herzog S. R., Stricklin G. P., Burgeson R. E., Briggaman R. A., Cronce D. J., O'Keefe E. J. Burn wounds resurfaced by cultured epidermal autografts show abnormal reconstitution of anchoring fibrils. JAMA. 1988 May 6;259(17):2566–2571. [PubMed] [Google Scholar]
- van Muijen G. N., Ruiter D. J., Franke W. W., Achtstätter T., Haasnoot W. H., Ponec M., Warnaar S. O. Cell type heterogeneity of cytokeratin expression in complex epithelia and carcinomas as demonstrated by monoclonal antibodies specific for cytokeratins nos. 4 and 13. Exp Cell Res. 1986 Jan;162(1):97–113. doi: 10.1016/0014-4827(86)90429-5. [DOI] [PubMed] [Google Scholar]









