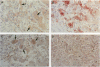
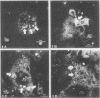
Abstract
AIMS--To investigate the effects of transforming growth factor beta 1 (TGF-beta 1) on regeneration and induction of apoptosis of liver cell and bile duct in various liver diseases. METHODS--Formalin fixed paraffin wax sections of 18 liver tissue samples were obtained by needle biopsy, surgery, or necropsy; these included six liver cirrhosis, three obstructive jaundice; five fulminant hepatitis, one subacute hepatitis, and three normal liver. Expression of TGF-beta 1, apoptosis related Le(y) antigen, Fas antigen, a receptor for tumour necrosis factor, and biotin nick end labelling with terminal deoxynucleotidyl transferase mediated dUTP (TUNEL) for locating DNA fragmentation, was investigated histochemically. RESULTS--TGF-beta 1 was expressed in areas of atypical bile duct proliferation, where bile duct continuously proliferated from liver cells. In occlusive jaundice and fulminant hepatitis, TUNEL was positive in nuclei and cytoplasm of metaplastic cells which formed incomplete bile ducts, and these cells appeared to extend from TGF-beta 1 expressing liver cells. Fas antigen was found only on the cell membrane of proliferated bile duct in fulminant hepatitis, which differed from TGF-beta 1 and TUNEL positive areas. Le(y) antigen was expressed in liver cell and bile duct at the areas with atypical bile duct proliferation, but its coexpression with TUNEL was rare. CONCLUSIONS--TGF-beta 1 plays a role in the arrest of liver cell regeneration and atypical bile duct proliferation, and in areas of rapidly progressing atypical bile duct proliferation, such as in fulminant hepatitis or bile retention. Apoptosis appears to be induced by TGF-beta 1. This phenomenon may account for the inadequate hepatic regeneration that occurs with liver disease.
Full text
PDF

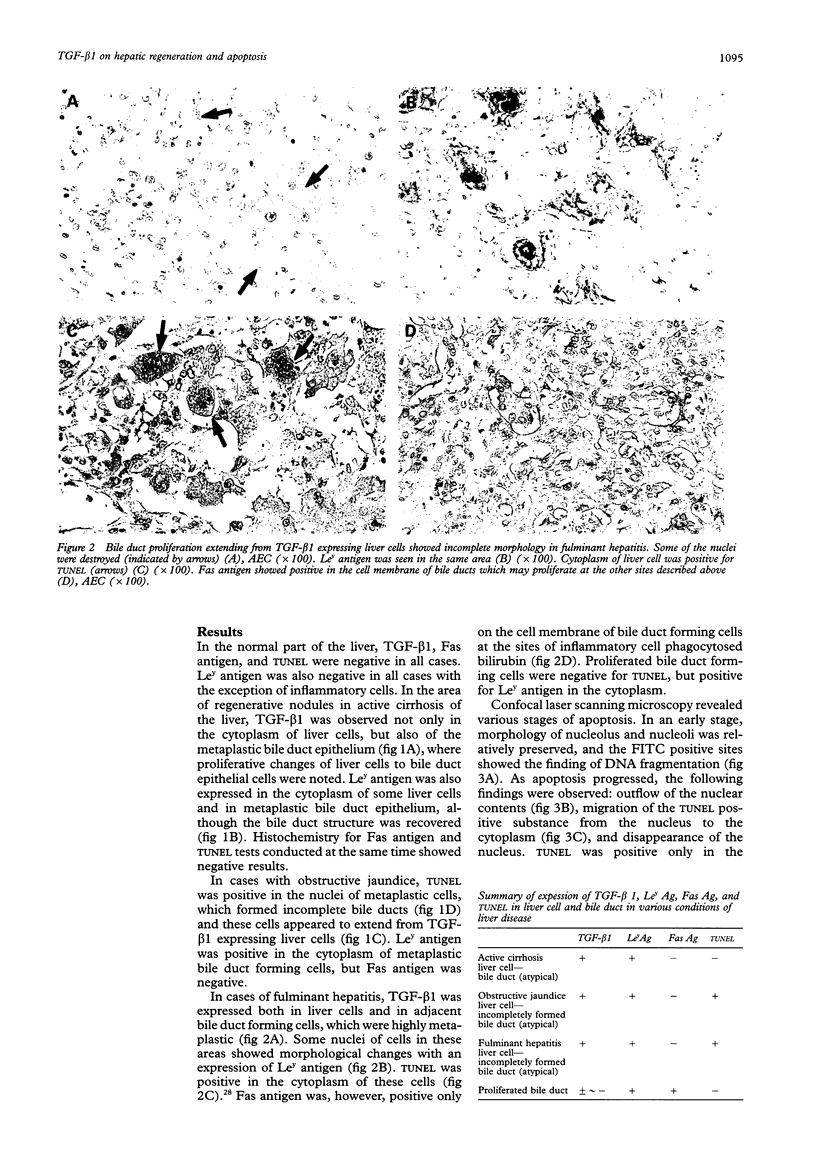
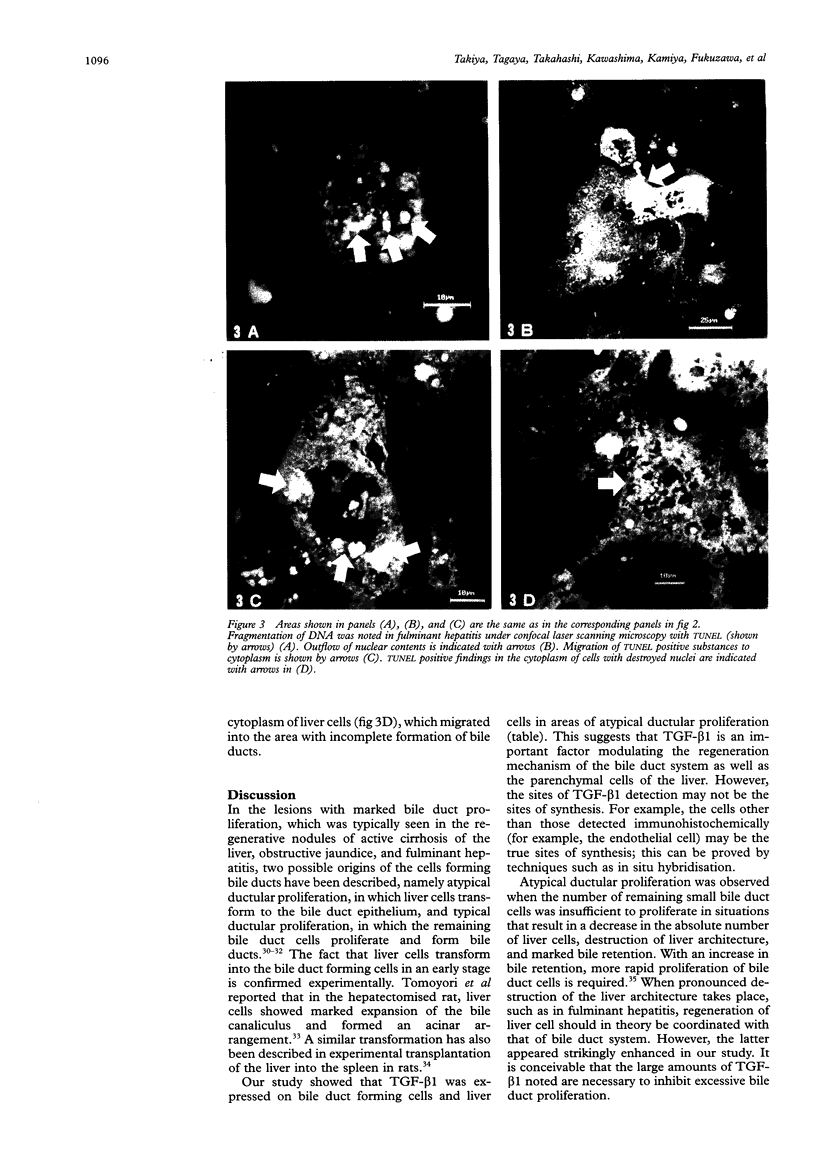

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., McKibbin J. M., Hakomori S. The monoclonal antibody directed to difucosylated type 2 chain (Fuc alpha 1 leads to 2Gal beta 1 leads to 4[Fuc alpha 1 leads to 3]GlcNAc; Y Determinant). J Biol Chem. 1983 Oct 10;258(19):11793–11797. [PubMed] [Google Scholar]
- Adachi M., Hayami M., Kashiwagi N., Mizuta T., Ohta Y., Gill M. J., Matheson D. S., Tamaoki T., Shiozawa C., Hakomori S. Expression of Ley antigen in human immunodeficiency virus-infected human T cell lines and in peripheral lymphocytes of patients with acquired immune deficiency syndrome (AIDS) and AIDS-related complex (ARC). J Exp Med. 1988 Feb 1;167(2):323–331. doi: 10.1084/jem.167.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annoni G., Weiner F. R., Zern M. A. Increased transforming growth factor-beta 1 gene expression in human liver disease. J Hepatol. 1992 Mar;14(2-3):259–264. doi: 10.1016/0168-8278(92)90168-o. [DOI] [PubMed] [Google Scholar]
- Bhathal P. S., Gall J. A. Deletion of hyperplastic biliary epithelial cells by apoptosis following removal of the proliferative stimulus. Liver. 1985 Dec;5(6):311–325. doi: 10.1111/j.1600-0676.1985.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch W., Oberhammer F., Jirtle R. L., Askari M., Sedivy R., Grasl-Kraupp B., Purchio A. F., Schulte-Hermann R. Transforming growth factor-beta 1 as a signal for induction of cell death by apoptosis. Br J Cancer. 1993 Mar;67(3):531–536. doi: 10.1038/bjc.1993.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla A., Prieto J., Fausto N. Transforming growth factors beta 1 and alpha in chronic liver disease. Effects of interferon alfa therapy. N Engl J Med. 1991 Apr 4;324(14):933–940. doi: 10.1056/NEJM199104043241401. [DOI] [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Flanders K. C., Giambrone M. A., Wind R., Biempica L., Zern M. A. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989 Jun;108(6):2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebata H., Onodera K., Sawa M., Mito M. A study of liver regeneration using fetal rat liver tissue transplanted into the spleen. Jpn J Surg. 1988 Sep;18(5):540–547. doi: 10.1007/BF02471488. [DOI] [PubMed] [Google Scholar]
- Gall J. A., Bhathal P. S. Origin and involution of hyperplastic bile ductules following total biliary obstruction. Liver. 1990 Apr;10(2):106–115. doi: 10.1111/j.1600-0676.1990.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraishi K., Suzuki K., Hakomori S., Adachi M. Le(y) antigen expression is correlated with apoptosis (programmed cell death). Glycobiology. 1993 Aug;3(4):381–390. doi: 10.1093/glycob/3.4.381. [DOI] [PubMed] [Google Scholar]
- Itoh N., Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993 May 25;268(15):10932–10937. [PubMed] [Google Scholar]
- Maher J. J., McGuire R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Nagy P., Schaff Z., Lapis K. Immunohistochemical detection of transforming growth factor-beta 1 in fibrotic liver diseases. Hepatology. 1991 Aug;14(2):269–273. [PubMed] [Google Scholar]
- Nakamura T., Arakaki R., Ichihara A. Interleukin-1 beta is a potent growth inhibitor of adult rat hepatocytes in primary culture. Exp Cell Res. 1988 Dec;179(2):488–497. doi: 10.1016/0014-4827(88)90286-8. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Ichihara A. Control of growth and expression of differentiated functions of mature hepatocytes in primary culture. Cell Struct Funct. 1985 Mar;10(1):1–16. doi: 10.1247/csf.10.1. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Tomita Y., Ichihara A. Density-dependent growth control of adult rat hepatocytes in primary culture. J Biochem. 1983 Oct;94(4):1029–1035. doi: 10.1093/oxfordjournals.jbchem.a134444. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa H., Nagy P., Evarts R. P., Hsia C. C., Marsden E., Thorgeirsson S. S. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990 Jun;85(6):1833–1843. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F. A., Pavelka M., Sharma S., Tiefenbacher R., Purchio A. F., Bursch W., Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhammer F., Bursch W., Parzefall W., Breit P., Erber E., Stadler M., Schulte-Hermann R. Effect of transforming growth factor beta on cell death of cultured rat hepatocytes. Cancer Res. 1991 May 1;51(9):2478–2485. [PubMed] [Google Scholar]
- Oberhammer F., Bursch W., Tiefenbacher R., Fröschl G., Pavelka M., Purchio T., Schulte-Hermann R. Apoptosis is induced by transforming growth factor-beta 1 within 5 hours in regressing liver without significant fragmentation of the DNA. Hepatology. 1993 Nov;18(5):1238–1246. [PubMed] [Google Scholar]
- Oberhammer F., Fritsch G., Pavelka M., Froschl G., Tiefenbacher R., Purchio T., Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in the regressing liver by transforming growth factor-beta 1 occurs without activation of an endonuclease. Toxicol Lett. 1992 Dec;64-65 Spec No:701–704. doi: 10.1016/0378-4274(92)90250-n. [DOI] [PubMed] [Google Scholar]
- Ogasawara J., Watanabe-Fukunaga R., Adachi M., Matsuzawa A., Kasugai T., Kitamura Y., Itoh N., Suda T., Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993 Aug 26;364(6440):806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Russell W. E., Coffey R. J., Jr, Ouellette A. J., Moses H. L. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama Y. Factors producing bile infarction and bile duct proliferation in biliary obstruction. J Pathol. 1990 Jan;160(1):57–62. doi: 10.1002/path.1711600112. [DOI] [PubMed] [Google Scholar]
- Tomoyori T., Ogawa K., Mori M., Onoé T. Ultrastructural changes in the bile canaliculi and the lateral surfaces of rat hepatocytes during restorative proliferation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;42(2):201–211. doi: 10.1007/BF02890383. [DOI] [PubMed] [Google Scholar]
- Uchida T., Peters R. L. The nature and origin of proliferated bile ductules in alcoholic liver disease. Am J Clin Pathol. 1983 Mar;79(3):326–333. doi: 10.1093/ajcp/79.3.326. [DOI] [PubMed] [Google Scholar]
- Weiner F. R., Giambrone M. A., Czaja M. J., Shah A., Annoni G., Takahashi S., Eghbali M., Zern M. A. Ito-cell gene expression and collagen regulation. Hepatology. 1990 Jan;11(1):111–117. doi: 10.1002/hep.1840110119. [DOI] [PubMed] [Google Scholar]
- Wijsman J. H., Jonker R. R., Keijzer R., van de Velde C. J., Cornelisse C. J., van Dierendonck J. H. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993 Jan;41(1):7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnegar R., Michalopoulos G. Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res. 1989 Jun 15;49(12):3314–3320. [PubMed] [Google Scholar]