Abstract
The objective was to evaluate the prognostic impact of pre-transplant minimal residual disease (MRD) as determined by real-time quantitative polymerase chain reaction in 67 adult NPM1-mutated acute myeloid leukemia patients receiving allogeneic hematopoietic stem cell transplantation (HSCT). Twenty-eight of the 67 patients had a FLT3-ITD (42%). Median age at transplantation was 54.7 years, median follow-up for survival from time of allografting was 4.9 years. At transplantation, 31 patients were in first, 20 in second complete remission (CR) and 16 had refractory disease (RD). Pre-transplant NPM1 MRD levels were measured in 39 CR patients. Overall survival (OS) for patients transplanted in CR was significantly longer as compared to patients with RD (P=0.004), irrespective of whether the patients were transplanted in first or second CR (P=0.74). There was a highly significant difference in OS after allogeneic HSCT between pre-transplant MRD-positive and MRD-negative patients (estimated 5-year OS rates of 40 vs 89% P=0.007). Multivariable analyses on time to relapse and OS revealed pre-transplant NPM1 MRD levels >1% as an independent prognostic factor for poor survival after allogeneic HSCT, whereas FLT3-ITD had no impact. Notably, outcome of patients with pre-transplant NPM1 MRD positivity >1% was as poor as that of patients transplanted with RD.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is considered to be the treatment strategy with the highest anti-leukemic efficacy for acute myeloid leukemia (AML) patients.1 Nevertheless, relapse remains the major cause of treatment failure even after allogeneic HSCT in complete remission (CR),1, 2, 3 suggesting that the sensitivity of morphological remission assessment is too low to allow for the detection of clinically relevant residual leukemia left behind after conventional chemotherapy. Besides achievement of morphological CR as pre-requisite for cure, the term ‘molecular remission' has been introduced for the first time in the 2003 International Working Group guidelines to refine treatment response in AML.4 There is increasing evidence that in AML, levels of submicroscopic amounts of leukemia cells (minimal residual disease, MRD) persisting after standard induction therapy are independently associated with increased risk of relapse and poor survival.5
Over the last two decades, several methods, particularly multiparameter flow cytometry (MFC) and quantitative real-time polymerase chain reaction (RT-qPCR) have been developed that enable the sensitive detection and monitoring of MRD in AML.5 When comparing both methods, RT-qPCR offers the highest level of sensitivity (10−4 to 10−6), depending on the AML-specific fusion gene or gene mutation measured.5, 6
Since frameshift mutations of the NPM1 gene are one of the most frequent molecular abnormalities in AML and are relatively stable over time,7, 8, 9 they represent an ideal target for RT-qPCR MRD monitoring. To date, more than 50 different NPM1 mutations have been reported; however, the subtypes A, B, and D comprise 90% of all variants.10 These three mutation subtypes have been shown to be reliable markers for MRD detection with high sensitivity.5, 11 The same assay can be adapted for cases with rare NPM1 mutation variants by replacing mutation-specific primers, but case-specific RT-qPCRs need to be carefully established to avoid non-specific background amplification from the wild-type NPM1 allele.12 The presence of NPM1 MRD has consistently been shown to be associated with an adverse outcome in patients treated with chemotherapy alone.11, 12, 13, 14, 15 In contrast, data based on NPM1 RT-qPCR pertaining to allogeneic HSCT are still scarce. Schnittger and colleagues reported on 252 NPM1-mutated AML patients, of whom 53 underwent allogeneic HSCT.12 However, their analyses were primarily focused on the correlation of outcome with MRD levels after chemotherapy. In a subgroup analysis they reported that a 100-fold increase of NPM1 MRD levels in samples taken between day 61 and 365 after allogeneic HSCT was associated with a significantly inferior event-free survival. Krönke et al. evaluated the prognostic impact of MRD levels in 245 NPM1-mutated AML patients, of whom 45 patients received allogeneic HSCT.13 Again, NPM1 MRD levels were a significant prognostic marker for remission duration and overall survival (OS). However, a subgroup analysis on MRD levels exclusively pertaining to allogeneic HSCT was not presented.
Pre-transplant MFC-MRD has been shown to be predictive for post-transplant outcome with high relapse rates of 60 to 70% after two or three years in MRD-positive patients as compared to only 8 to 21% in MRD-negative patients, respectively.16, 17, 18 The aim of this study was to evaluate the prognostic impact of pre-transplant NPM1 MRD levels determined by RT-qPCR in correlation to clinical characteristics and genetic abnormalities assessed at initial diagnosis in a cohort of adult AML patients receiving allogeneic HSCT.
Patients and Methods
Patients and Treatment
Between 2005 and 2013, 238 AML patients (median age at time of allogeneic HSCT, 53.5 years; range, 17–73 years) received an allogeneic HSCT at the University of Heidelberg. Diagnosis of AML was based on standard criteria.4 All patients gave written informed consent in accordance with the Declaration of Helsinki. Data collection and analysis were approved by the Institutional Review Board.
Chromosome banding was performed using standard techniques, and karyotypes were described according to the International System for Human Cytogenetic Nomenclature.19 Based on material availability, the mutational status of NPM1 and FLT3-ITD was analyzed in 208 and 215 of the 238 patients, respectively as previously described.20, 21 For this study, the criterion used to include patients was the presence of an NPM1 mutation (n=67). In patients with a concurrent FLT3-ITD the allelic ratio was quantified by GeneScan-based fragment-length analysis; in cases with more than one ITD mutation, all FLT3-ITDs were summed-up.
All patients received intensive treatment either within clinical trials (n=48) or according to our local institutional standard (n=19). Reasons for allogeneic HSCT in first CR were: i) FLT3-ITD positivity (n=13), ii) requirement of the respective trial protocol (availability of an HLA-matched sibling donor in case of intermediate-risk patients; n=9), iii) secondary/therapy-related AML (n=5), iv) cytogenetically high-risk abnormalities (n=2), and v) raising NPM1 MRD levels (n=2). Induction regimens included intensive chemotherapy according to the ‘7+3' scheme (cytarabine (Ara-C) 100 mg/m2, d1-7 plus daunorubicin 60 mg/m2, d3-5) or Ara-C 1 g/m2 bid, d1,3,5,7 plus mitoxantrone (mito) 10 mg/m2, d1-3 and pegfilgrastim 6 mg s.c., d10. Consolidation therapy consisted of age-adapted high-dose Ara-C (3 g/m2, bid, d1,3,5 for patients age ⩽60 years and 1 g/m2, bid, d1,3,5 for patients >60 years) or age-adapted mito (10 mg/m2, d4-6 for patients ⩽60 years and 10 mg/m2, d1+2 and pegfilgrastim 6 mg s.c., d8 for patients >60 years) plus age-adapted Ara-C (1 g/m2, bid, d1-6 for patients ⩽60 years and Ara-C 500 mg/m2 bid, d1,3,5, for patients >60 years) or mito 10 mg/m2, d4-6 with amsacrine 100 mg/m2, d1-5 and Ara-C 1 g/m2, bid, d1-5. In refractory or relapsed patients either mito and high-dose Ara-C; mito 10 mg/m2, d1-5 and etoposide 100 mg/m2, d1-5 (NOVE);22 fludarabine 30 mg/m2 plus high-dose Ara-C 2 g/m2 and amsacrine 100 mg/m2 for four days (FLAMSA);23 clofarabine 40 mg/m2, d2-6 and Ara-C 1 g/m2, d1-524 or fludarabine/Ara-C/granulocyte colony-stimulating factor/idarubicin (Flag-IDA)25 have been used as salvage therapy.
In total 67 patients with NPM1-mutated AML were included into the analysis and data pertaining to these patients were collected from electronic patient records with follow-up until July 27, 2015.
Detection of MRD
Collection of bone marrow (BM) samples for MRD analysis was recommended at diagnosis, during aplasia within induction therapy, after each treatment cycle and every three months after completion of therapy. RT-qPCR analysis was performed at diagnosis and follow-up on cDNA obtained from BM (n=406) specimens as described previously.11, 13, 26 MRD levels were expressed as a ratio of the NPM1 mutation normalized to the housekeeping gene ABL1 to adjust for variations in mRNA quality and efficiencies of cDNA synthesis. To increase external validity and to be consistent with a previous report indicating a poor survival of AML patients after chemotherapy26 we used a cut-point of 1% (less than 100 copies of mutated NPM1/104 ABL1 copies) to define MRD-negativity prior to allogeneic HSCT. The sensitivity level was 10−5 to 10−6.
Statistical analyses
CR and survival endpoints such as OS, relapse-free survival (RFS), time to relapse (TTR) and time to non-relapse mortality (NRM) were defined as recommended.27 All event times were measured from date of allogeneic HSCT. For OS all 67 patients were considered. The analysis of RFS and competing risk analysis of TTR vs NRM was restricted to NPM1-mutated patients transplanted in CR (n=51). Cytogenetic categorization into favorable-, intermediate- and adverse-risk groups followed recommended criteria.28 Pairwise comparisons between patient characteristics (covariates) were performed by the Mann-Whitney U test for continuous variables and by Fisher's exact test for categorical variables. The follow-up distribution was computed using the reverse Kaplan-Meier estimate.29 The Kaplan-Meier method was used to estimate the distribution of RFS and OS.30 Confidence interval (CI) estimation for survival curves was based on the cumulative hazard function using Greenwood's formula for variance estimation. Logrank tests were employed to compare survival curves between groups. Cumulative incidence of relapse (CIR) and cumulative incidence of death in remission (CID) were computed using the Aalen-Johansen estimator31 and included only patients attaining CR. A Cox proportional hazards regression model was used to identify prognostic variables for OS and RFS.32 For competing risks analyses a cause-specific Cox model was used. The following variables were included in the Cox models: achievement of CR prior to allogeneic HSCT in combination with pre-transplant MRD status (for OS only), age, percentage of BM blasts, lactate dehydrogenase (LDH), FLT3-ITD and MRD positivity prior to allogeneic HSCT (for TTR only). All statistical analyses were performed with the statistical software environment R, version 3.1.3, using the R packages rms, version 4.3-1, prodlim, version 1.5.1, coxphf, version 1.11, and survival, version 2.38-1.33
Results
Pretreatment characteristics and factors pertaining to allogeneic HSCT
Genetic risk category was intermediate in 62 of the 67 NPM1-mutated patients based on revised Medical Research Council/National Cancer Research Institute criteria28 and most of them (n=52) had a normal karyotype. Three patients belonged to the adverse risk category28 and two patients had no evaluable metaphases. The FLT3 status was measured in all NPM1-mutated patients. A FLT3-ITD was present in 28 of the 67 patients (42%); the allelic ratio could be measured in 24 (86%) of the FLT3-ITD patients, with a median of 0.58 (range 0.03-14.3). Source of donor was matched-related in 20, matched-unrelated in 45 and haplo-identical in 2 of the 67 patients, respectively. The majority of patients (n=61) received reduced-intensity conditioning (RIC), consisting of either melphalan/fludarabine (n=27),34 treosulfan/fludarabine (n=11),35 busulfan/fludarabine (n=4), busulfan/fludarabine plus amsacrine/Ara-C (n=6), fludarabine/TBI, 2–8 Gy (n=12) and cyclophosphamide/TBI, 4 Gy (n=1).36, 37, 38, 39 Disease status at allogeneic HSCT was CR1 in 31, CR2 in 20 and refractory in 16 patients, without significant differences for other baseline characteristics between the three groups (Table 1). Similarly, there was no difference between MRD positive (n=22) and MRD negative (n=17) patients allografted in CR (Table 2).
Table 1. Patient characteristics by disease status prior to allogeneic hematopoietic stem cell transplantation in NPM1-mutated patients.
| Remission status prior to allogeneic HSCT |
1st CR (n=31) |
2nd CR (n=20) |
RD (n=16) |
P-value | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Median age (at time of SCT; years) | 57.1 | 48.0 | 57.0 | ||||
| Range | 21.0–70.8 | 29.2–70.1 | 43.3–63.4 | 0.13 | |||
| Gender | |||||||
| Male | 13 | 41.9 | 14 | 70 | 7 | 43.8 | 0.13 |
| Female | 18 | 58.1 | 6 | 30 | 9 | 56.2 | |
| Type of AML | |||||||
| de novo | 26 | 83.9 | 19 | 95 | 14 | 87.5 | 0.51 |
| secondary | 2 | 6.5 | 1 | 5 | 2 | 12.5 | |
| therapy-related | 3 | 9.7 | - | - | - | - | |
| Hemoglobin, g/dl | |||||||
| Median | 9.6 | 8.7 | 10.4 | 0.22 | |||
| Range | 6.2–12.2 | 4.6–13.5 | 5.8–12.5 | ||||
| No. missing | - | - | - | ||||
| WBC, x109/l | |||||||
| Median | 52.3 | 46.2 | 28.3 | 0.77 | |||
| Range | 1.4–348.5 | 4.0–160.4 | 2.1–290 | ||||
| No. missing | - | - | - | ||||
| Platelet count, x109/l | |||||||
| Median | 77 | 83 | 103.5 | 0.63 | |||
| Range | 4–369 | 28–379 | 23–242 | ||||
| No. missing | - | - | - | ||||
| LDH value, U/l | |||||||
| Median | 540 | 539 | 446.5 | 0.67 | |||
| Range | 144–2741 | 128–2083 | 139–2573 | ||||
| No. missing | - | 1 | - | ||||
| Percentage of BM blasts | |||||||
| Median | 80 | 76 | 80 | 0.82 | |||
| Range | 25–100 | 27–95 | 47–90 | ||||
| No. missing | - | - | - | ||||
| Cytogenetic risk categorya | |||||||
| intermediate | 29 | 93.5 | 20 | 100 | 13 | 92.9 | 0.42 |
| high | 2 | 6.5 | - | - | 1 | 7.1 | |
| No. missing | - | - | 2 | ||||
| FLT3-ITD mutated | 13 | 41.9 | 6 | 30 | 9 | 56.2 | 0.29 |
| No. missing | - | - | - | ||||
| Donor type | |||||||
| MUD | 18 | 58.1 | 14 | 70 | 13 | 81.2 | 0.10 |
| MRD | 13 | 41.9 | 4 | 20 | 3 | 18.8 | |
| Haplo | - | 2 | 10 | - | |||
Abbreviations: AML, acute myeloid leukemia; BM, bone marrow; CR, complete remission; HSCT, hematopoietic stem cell transplantation; ITD, internal tandem duplication; LDH, lactate dehydrogenase; RD, refractory disease; WBC, white blood count.
According to reference.28
Percentages may not add to 100 because of rounding.
Table 2. Comparison of clinical and laboratory findings according to minimal residual disease status prior to allogeneic hematopoietic stem cell transplantation in NPM1-mutated patients in complete remission.
| MRD status prior to allogeneic HSCT |
MRD-positive (n=22) |
MRD-negative (n=17) |
P-value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Median age (at time of SCT; years) | 51.1 | 52.5 | 0.49 | ||
| Range | 21.0–70.1 | 29.2–70.8 | |||
| Gender | |||||
| Male | 13 | 59.1 | 7 | 41.2 | 0.34 |
| Female | 9 | 40.9 | 10 | 58.8 | |
| Type of AML | |||||
| de novo | 20 | 90.9 | 14 | 82.3 | 0.78 |
| secondary | 1 | 4.5 | 2 | 11.8 | |
| therapy-related | 1 | 4.5 | 1 | 5.9 | |
| Hemoglobin, g/dL | |||||
| Median | 9.2 | 9.7 | 0.54 | ||
| Range | 6.2–13.3 | 4.6–12.7 | |||
| No. missing | - | - | |||
| WBC, x109/L | |||||
| Median | 62.0 | 31.6 | 0.07 | ||
| Range | 1.9–348.5 | 1.4–153.0 | |||
| No. missing | - | - | |||
| Platelet count, x109/L | |||||
| Median | 85 | 77 | 0.98 | ||
| Range | 4–210 | 14–369 | |||
| No. missing | - | - | |||
| LDH value, U/L | |||||
| Median | 667 | 420 | 0.06 | ||
| Range | 203–2449 | 144–1112 | |||
| No. missing | - | - | |||
| Percentage of BM blasts | |||||
| Median | 82.5 | 75 | 0.31 | ||
| Range | 41–100 | 40–96 | |||
| No. missing | - | - | |||
| Cytogenetic risk categorya | |||||
| intermediate | 21 | 95.5 | 16 | 94.1 | 1.00 |
| high | 1 | 4.5 | 1 | 5.9 | |
| No. missing | - | - | |||
| FLT3-ITD mutated | 11 | 50 | 4 | 23.5 | 0.11 |
| No. missing | - | - | |||
| Donor type | |||||
| MUD | 15 | 68.2 | 9 | 52.9 | 0.16 |
| MRD | 5 | 22.7 | 8 | 47.1 | |
| Haplo | 2 | 9.1 | - | ||
| Achievement of CR | |||||
| CR1 | 14 | 63.6 | 11 | 64.7 | 1.00 |
| CR2 | 8 | 36.4 | 6 | 35.3 | |
Abbreviations: AML, acute myeloid leukemia; BM, bone marrow; CR, complete remission; HSCT, hematopoietic stem cell transplantation; ITD, internal tandem duplication; LDH, lactate dehydrogenase; WBC, white blood count.
According to reference. Percentages may not add to 100 because of rounding.
Evaluation of MRD
Of the 51 NPM1-mutated patients who were in CR at allogeneic HSCT, pre-transplant MRD was assessed in 39 patients. MRD was measured in BM within one month prior to allogeneic HSCT in 28 (72%) and within two months in 11 of the 39 (28%) patients, respectively. Twenty-two of the 39 (56%) patients were MRD-positive and 17 (44%) MRD-negative. MRD was not measured in RD patients.
Survival analysis
The estimated median follow-up for survival of the 67 NPM1-mutated patients was 4.9 years (95%-CI, 3.8 to 6.2 years); the estimated 5-year OS rate was 57% (95%-CI, 44% to 68%). For patients in first and second CR the estimated 5-year OS rate was 60% (95%-CI, 40% to 75%) and 68% (95%-CI, 41% to 84%), respectively. Patients with refractory disease (RD) had an estimated 5-year OS rate of 38% (95%-CI, 15% to 60%). OS for patients transplanted in CR was significantly longer as compared to patients with RD (P=0.004), irrespective of whether the patients were transplanted in first or second CR (P=0.74). The estimated CIR and CID at 5 years for patients transplanted in first as compared to second CR were 32% (95%-CI, 16% to 49%) and 11% (95%-CI, 0% to 23%) for first CR, and 34% (95%-CI, 11% to 56%) and 5% (95%-CI, 0% to 15%) for second CR patients, respectively.
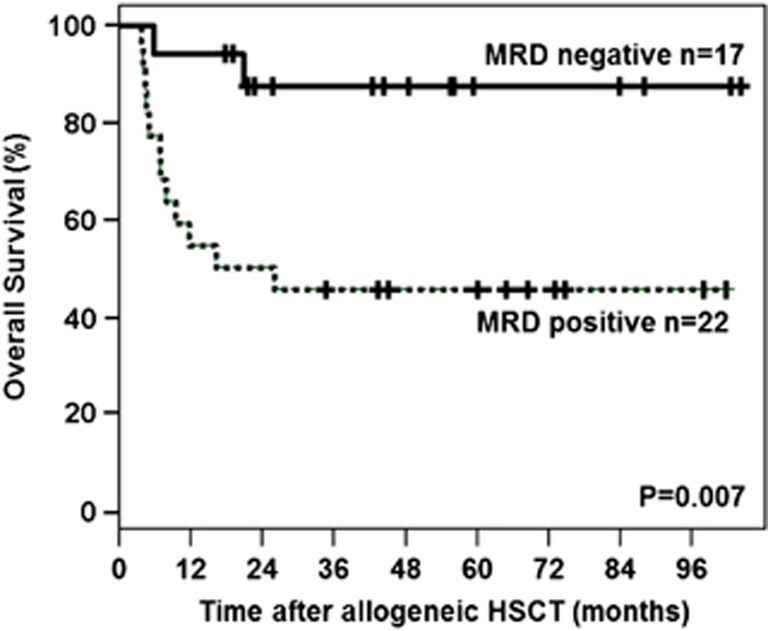
There was no difference in outcome for CR patients with (n=39) or without (n=12) MRD measurement (P=0.84 for OS). Yet, there was a highly significant difference in OS after allogeneic HSCT between pre-transplant MRD-positive and MRD-negative patients, with estimated 5-year OS rates of 40 vs 89%, respectively (P=0.007; Figure 1).
Figure 1.
Kaplan-Meier plot illustrating the impact of pre-transplant NPM1 minimal residual disease on overall survival of patients with complete remission.
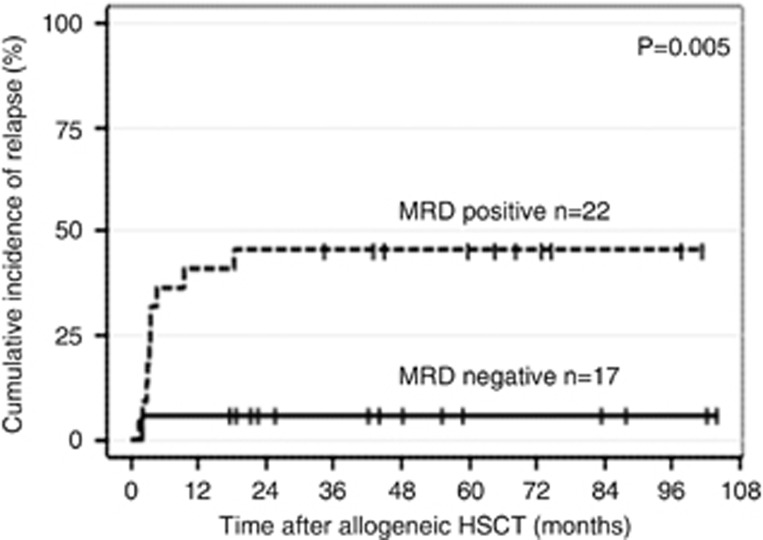
Considering MRD-positive patients (n=22) the estimated CIR at 5 years was 46% (95%-CI, 25% to 66%) as compared to 6% (95%-CI, 0% to 17%) for MRD-negative patients (n=17; Figure 2). CID at 5 years was comparable between the two groups (MRD-positive: 9% 95%-CI, 6% to 21% MRD-negative: 7% 95%-CI, 0% to 19%).
Figure 2.
Cumulative incidence of relapse according to minimal residual disease status in NPM1-mutated AML patients transplanted in complete remission.
In total, 16 relapses occurred in patients transplanted in CR: nine of 32 (28%) NPM1-mutated/FLT3-ITD negative patients and seven of 19 (37%) NPM1-mutated/FLT3-ITD positive patients relapsed. With regard to the pre-transplant MRD status of NPM1-mutated/FLT3-ITD negative patients, seven of 11 (64%) MRD-positive patients relapsed, whereas none of 13 MRD-negative patients experienced disease recurrence so far. With respect to NPM1-mutated/FLT3-ITD positive patients three of 11 (27%) MRD-positive patients and one of four MRD-negative patients relapsed after allogeneic HSCT. The latter patient experienced MRD negativity for only three months prior to allogeneic HSCT and relapsed with the same NPM1 subtype already two months after myeloablative allogeneic HSCT.
Based on previous reports showing a differential impact of FLT3-ITD according to its allelic ratio40, 41, 42 we performed additional exploratory subgroup analyses. Considering a dichotomized allelic ratio with a cutoff of 0.540, 41, 42 no significant prognostic impact was evident for OS (P=0.36), albeit based on a small subgroup analysis (n=24) only.
In a multivariable cause-specific Cox model on TTR, the hazard ratio for pre-transplant MRD was 9.0 (95%-CI: 1.1–75.9; P=0.04) in the subset of patients in CR, whereas the FLT3-ITD status measured at diagnosis had no impact (P=0.92; Table 3a). The same held true in a Cox model on OS (Table 3b). Additional significant and trendwise important variables were achievement of CR in combination with pre-transplant MRD status (on OS) as well as LDH value (on TTR and OS) whereas age at the time of allogeneic HSCT and percentage of BM blasts at diagnosis had no impact (Tables 3a and 3b). Again, there was no significant difference in outcome for patients in first or second CR (data not shown). A model on NRM was not performed due to a very low event number for this endpoint (3 out of 39 patients).
Table 3a. Multivariable cause-specific Cox model on time to relapse in NPM1-mutated patients with acute myeloid leukemia allografted in complete remission (n=39).
| HR (95%-CI) | P-value | |
|---|---|---|
| FLT3-ITD | 0.91 (0.16–5.26) | 0.92 |
| Log10(LDH) (at diagnosis) | 7.87 (0.93–66.4) | 0.06 |
| Percentage of BM blasts at diagnosis; 10% increase | 1.05 (0.67–1.66) | 0.82 |
| Age at the time of allogeneic HSCT; 10 years increase | 1.67 (0.82–3.39) | 0.16 |
| Pre-transplant MRD positivity | 9.03 (1.07–75.9) | 0.04 |
Abbreviations: BM, bone marrow; CI, confidence interval; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; ITD, internal tandem duplication; LDH, lactate dehydrogenase; MRD, minimal residual disease.
Table 3b. Multivariable Cox model on overall survival in 55 NPM1-mutated patients with acute myeloid leukemia (including patients in complete remission with measurement of minimal residual disease and patients with refractory disease).
| HR (95%-CI) | P-value | |
|---|---|---|
| FLT3-ITD | 0.58 (0.17–1.93) | 0.37 |
| Log10(LDH) (at diagnosis) | 6.65 (1.25–35.3) | 0.03 |
| Percentage of BM blasts at diagnosis; 10% increase | 1.24 (0.90–1.72) | 0.19 |
| Age at the time of allogeneic HSCT; 10 years increase | 1.56 (0.92–2.64) | 0.10 |
| Achievement of CR and pre-transplant MRD status (reference RD) | ||
| CR, MRD-positive | 0.70 (0.29–1.70) | 0.02 |
| CR, MRD-negative | 0.11 (0.02–0.50) | |
Abbreviations: BM, bone marrow; CI, confidence interval; CR, complete remission; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; ITD, internal tandem duplication; LDH, lactate dehydrogenase; MRD, minimal residual disease; RD, refractory disease.
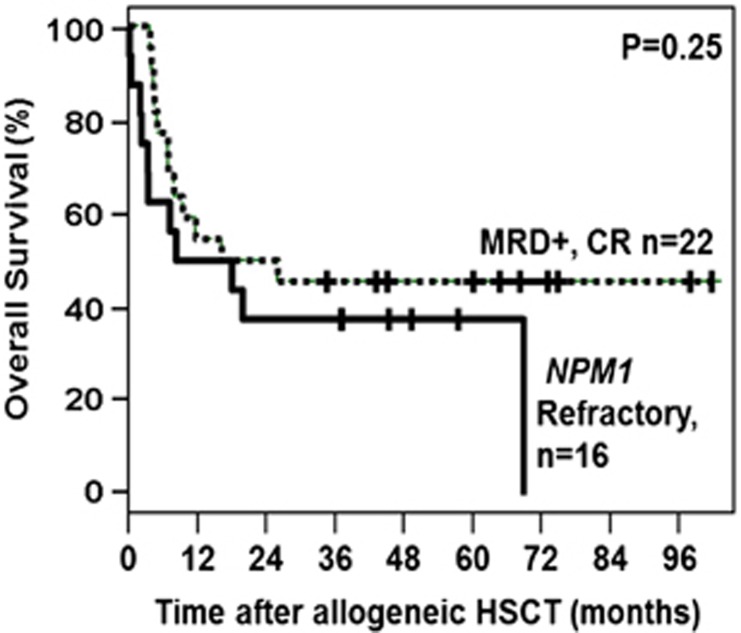
The 5-year OS rate of patients receiving an allogeneic HSCT in refractory disease was 38% and comparable to that observed in pre-transplant MRD-positive patients (40% P=0.42; Figure 3).
Figure 3.
Overall survival of pre-transplant NPM1 minimal residual disease positive patients as compared to NPM1-mutated patients transplanted with refractory disease.
Discussion
The focus of our study was to assess the prognostic impact of NPM1 MRD prior to allogeneic HSCT on TTR and OS. Albeit performed on a limited number of patients, in our cohort of NPM1-mutated patients pre-transplant NPM1 MRD positivity was a significant predictor of poor outcome after allogeneic HSCT independent from other variables, including FLT3-ITD status, BM blast count at diagnosis and age which adds to recently published data.12, 13 Of note, prognosis of MRD-positive patients was not better than that of patients transplanted in RD.
Outcome data of NPM1-mutated patients without FLT3-ITD transplanted in first as compared to second CR are still scarce. At least until recently, those patients were not transplanted in first CR. Regarding our patient cohort our results are in line with the data published by Alan Burnett et al. who reported on a 5 years CIR in NPM1-mutated/FLT3-ITD negative patients of 39% and a 5-year survival from CR of 68% when the patients who received salvage treatment were considered.43
In several studies it has been shown that the prognostic impact of NPM1 should be interpreted in the context of a cooperating FLT3-ITD mutation, which is present in approximately 45% of this patient population with normal karyotype.21, 44, 45 In particular, in younger adult NPM1-mutated patients with high FLT3-ITD allelic ratio (⩾0.5)40, 41, 42 the favorable prognostic effect of NPM1 is mitigated or even abolished as compared to patients with a low allelic ratio.21, 41, 42 Nevertheless, in none of these publications the relative impact of the FLT3-ITD allelic ratio on the background of NPM1 MRD has been evaluated. In our study MRD positivity prior to allogeneic HSCT turned out to be a stronger predictor of relapse than FLT3-ITD at initial diagnosis, which adds to recently published data reporting an increased relapse risk associated with raising NPM1 MRD levels of >1% despite of having achieved CR after completion of chemotherapy.26 In line with our data, in the paper by Shayegi et al. FLT3-ITD had no further prognostic information after conventional chemotherapy and autologous HSCT if NPM1 MRD level was considered.26 Moreover, in the recently published paper by Ivey et al. the presence of NPM1 MRD was the only significant prognostic factor in multivariable analysis for relapse and death, whereas the presence of a FLT3-ITD did not provide additional prognostic information.15
Data regarding MRD and specific FLT3-ITD characteristics, such as the allelic ratio and ITD insertion site in the FLT3 gene, are still scarce. In line with our data, Ivey et al. could not find a difference in the allelic burden according to NPM1 MRD level.15
Considering a cutoff value of 0.5,40, 41, 42 the FLT3-ITD ratio had no impact on OS in our analyses, albeit based on a small subgroup analysis only. Based on availability of material, we could neither address the impact of ITD insertion site nor evaluate the FLT3-ITD mutational status at the time-point of allogeneic HSCT. Therefore, one possibility why FLT3-ITD seemed not to be associated with outcome after allogeneic HSCT could be that the clone present at the time of allogeneic HSCT was ITD negative.
RFS and OS for NPM1 MRD-positive patients transplanted in CR was identical in our cohort (data not shown), suggesting that once relapse occurred, further treatment with tapering of immunosuppression, chemotherapy or even a second allogeneic HSCT had no major impact on survival. A major benefit of allogeneic HSCT performed in CR was only present in patients who had NPM1 MRD levels below 1% prior to allogeneic HSCT. Hence, current practice to recommend allogeneic HSCT without considering MRD levels has to be called into question. Currently it is unclear, if MRD-positive patients would benefit from additional cycles of pre-transplant high-dose Ara-C or other intensification. Several retrospective studies have suggested that standard Ara-C-based consolidation chemotherapy before allogeneic HSCT for AML patients of all risk-groups in first CR does not improve post-transplant outcomes.46, 47, 48, 49 However, in none of these trials information on MRD was available, and it is unknown whether the subset of MRD-positive patients would benefit from additional post-remission therapy prior to allogeneic HSCT.
Another strategy to overcome MRD could be increasing conditioning intensity. Several retrospective analyses as well as one prospective study reported comparable outcomes after myeloablative vs RIC in AML,50, 51, 52, 53 whereas current data by the Blood and Marrow Transplant Clinical Trials Network suggest a beneficial impact of myeloablative over RIC in patients with myelodysplastic syndromes or AML.54 Yet again, in none of these studies data on MRD were available. Since most of our patients have received RIC, the impact of myeloablative conditioning on MRD levels should be addressed further.
Numerous studies have convincingly demonstrated that MRD positivity before allogeneic HSCT, determined by MFC, is independently associated with a significantly increased risk of relapse and inferior survival.16, 17, 18, 55, 56, 57 Assuming that a further reduction of MRD levels optimizes outcome after allogeneic HSCT, this relationship would justify risk-stratified treatment allocation, including the use of additional pre-transplant chemotherapy. However, as MRD might simply reflect reduced sensitivity of leukemia cells to chemotherapy, the presence of residual disease might only mark those patients who are unlikely to be cured with subsequent similar-type therapies, even if disease levels are brought temporarily below the level of detection. Therefore, another approach could be pre-emptive immunotherapy in MRD-positive patients,58 which has successfully been demonstrated in childhood AML with mixed chimerism after allogeneic HSCT,59 or by post-transplant application of demethylating agents, such as azacitidine, to prevent imminent relapse in MRD-positive patients.60
In summary, our data provide clinically relevant information that may allow to improve post-transplant outcome in MRD-positive patients with NPM1-mutated AML. In addition, pre-transplant NPM1 MRD levels seem to outperform the prognostic information provided by FLT3-ITD with regard to outcome after allogeneic HSCT. Nonetheless, this observation warrants confirmation in further studies.
Acknowledgments
The authors thank Dr Richard F Schlenk, MD, for critical reading of an earlier version of the manuscript and helpful comments. Financial support: SK gratefully acknowledges to have received a grant from the University of Heidelberg.
Author contributions
SK and AK were responsible for the concept of this paper, contributed to the literature search data collection, analyzed and interpreted data, and wrote the manuscript. AB analyzed and interpreted data and wrote the manuscript. PD analyzed and interpreted data and critically revised the manuscript. CT and JWGJ performed research and critically revised the manuscript. JH and PS collected data and critically revised the manuscript. UM, CR, MJU, TB, UH, GE, and ADH contributed patients and critically revised the manuscript. All authors reviewed and approved the final manuscript.
Footnotes
Presented in part at the 57th Annual Meeting of the American Society of Hematology in Orlando, Florida, December 5th, 2015.
PD has received research support from Riemser Pharma and Neovii, and has worked as consultant for Riemser Pharma. UH has received honoraria from Janssen. AK has received research support from Merck and Bayer. SK gratefully acknowledges to have received a grant from the University of Heidelberg. CT is part owner of AgenDix GmbH. All other authors declare no competing conflict of interest.
References
- Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhäuser M, Juliusson G et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol 2012; 9: 579–590. [DOI] [PubMed] [Google Scholar]
- Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011; 29: 487–494. [DOI] [PubMed] [Google Scholar]
- Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet 2013; 381: 484–495. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003; 21: 4642–4649. [DOI] [PubMed] [Google Scholar]
- Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for "prime time"? Blood 2014; 124: 3345–3355. [DOI] [PubMed] [Google Scholar]
- Kayser S, Walter RB, Stock W, Schlenk RF. Minimal residual disease in acute myeloid leukemia-current status and future perspectives. Curr Hematol Malig Rep 2015; 10: 132–144. [DOI] [PubMed] [Google Scholar]
- Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005; 352: 254–266. [DOI] [PubMed] [Google Scholar]
- Krönke J, Bullinger L, Teleanu V, Tschürtz F, Gaidzik VI, Kühn MW et al. Clonal evolution in relapsed NPM1-mutated acute myeloid leukemia. Blood 2013; 122: 100–108. [DOI] [PubMed] [Google Scholar]
- Kristensen T, Møller MB, Friis L, Bergmann OJ, Preiss B. NPM1 mutation is a stable marker for minimal residual disease monitoring in acute myeloid leukaemia patients with increased sensitivity compared to WT1 expression. Eur J Haematol. 2011; 87: 400–408. [DOI] [PubMed] [Google Scholar]
- Falini B, Sportoletti P, Martelli MP. Acute myeloid leukemia with mutated NPM1: diagnosis, prognosis and therapeutic perspectives. Curr Opin Oncol. 2009; 21: 573–581. [DOI] [PubMed] [Google Scholar]
- Gorello P, Cazzaniga G, Alberti F, Dell'Oro MG, Gottardi E, Specchia G et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia 2006; 20: 1103–1108. [DOI] [PubMed] [Google Scholar]
- Schnittger S, Kern W, Tschulik C, Weiss T, Dicker F, Falini B et al. Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood 2009; 114: 2220–2231. [DOI] [PubMed] [Google Scholar]
- Krönke J, Schlenk RF, Jensen KO, Tschürtz F, Corbacioglu A, Gaidzik VI et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011; 29: 2709–2716. [DOI] [PubMed] [Google Scholar]
- Hubmann M, Köhnke T, Hoster E, Schneider S, Dufour A, Zellmeier E et al. Molecular response assessment by quantitative real-time polymerase chain reaction after induction therapy in NPM1-mutated patients identifies those at high risk of relapse. Haematologica 2014; 99: 1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A et al. Assessment of Minimal Residual Disease in Standard-Risk AML. N Engl J Med. 2016; 374: 422–433. [DOI] [PubMed] [Google Scholar]
- Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood 2013; 122: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos-Oreiro M, Perez-Corral A, Martínez-Laperche C, Bento L, Pascual C, Kwon M et al. Prognostic impact of minimal residual disease analysis by flow cytometry in patients with acute myeloid leukemia before and after allogeneic hemopoietic stem cell transplantation. Eur J Haematol. 2014; 93: 239–246. [DOI] [PubMed] [Google Scholar]
- Anthias C, Dignan FL, Morilla R, Morilla A, Ethell ME, Potter MN et al. Pre-transplant MRD predicts outcome following reduced-intensity and myeloablative allogeneic hemopoietic SCT in AML. Bone Marrow Transplant. 2014; 49: 679–683. [DOI] [PubMed] [Google Scholar]
- Mitelman F. ISCN: An International System for Human Cytogenetic Nomenclature. Basel, Switzerland: S. Karger, 1995. [Google Scholar]
- Thiede C, Steudel C, Mohr B, Schaich M, Schäkel U, Platzbecker U et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335. [DOI] [PubMed] [Google Scholar]
- Thiede C, Koch S, Creutzig E, Steudel C, Illmer T, Schaich M et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood 2006; 107: 4011–4020. [DOI] [PubMed] [Google Scholar]
- Knauf WU, Berdel WE, Ho AD, Kreuser ED, Thiel E. Combination of mitoxantrone and etoposide in the treatment of myelodysplastic syndromes transformed into acute myeloid leukemia. Leuk Lymphoma. 1994; 12: 421–425. [DOI] [PubMed] [Google Scholar]
- Schmid C, Schleuning M, Hentrich M, Markl GE, Gerbitz A, Tischer J et al. High antileukemic efficacy of an intermediate intensity conditioning regimen for allogeneic stem cell transplantation in patients with high-risk acute myeloid leukemia in first complete remission. Bone Marrow Transplant. 2008; 41: 721–727. [DOI] [PubMed] [Google Scholar]
- Faderl S, Gandhi V, O'Brien S, Bonate P, Cortes J, Estey E et al. Results of a phase 1-2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood 2005; 105: 940–947. [DOI] [PubMed] [Google Scholar]
- Parker JE, Pagliuca A, Mijovic A, Cullis JO, Czepulkowski B, Rassam SM et al. Fludarabine, cytarabine, G-CSF and idarubicin (FLAG-IDA) for the treatment of poor-risk myelodysplastic syndromes and acute myeloid leukaemia. Br J Haematol. 1997; 99: 939–944. [DOI] [PubMed] [Google Scholar]
- Shayegi N, Kramer M, Bornhäuser M, Schaich M, Schetelig J, Platzbecker U et al. The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood 2013; 122: 83–92. [DOI] [PubMed] [Google Scholar]
- Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115: 453–474. [DOI] [PubMed] [Google Scholar]
- Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010; 116: 354–365. [DOI] [PubMed] [Google Scholar]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996; 17: 343–346. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- Aalen OO, Johansen S. An Empirical Transition Matrix for Non- Homogeneous Markov Chains Based on Censored Observations. Scandinavian Journal of Statistics. 1978; 5: 141–150. [Google Scholar]
- Cox DR. Regression models and life tables (with discussion). J R Stat Soc 1972; 34: 187–220. [Google Scholar]
- R Development Core TeamR: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- van Besien K, Artz A, Smith S, Cao D, Rich S, Godley L et al. Fludarabine, melphalan, and alemtuzumab conditioning in adults with standard-risk advanced acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005; 23: 5728–5738. [DOI] [PubMed] [Google Scholar]
- Kroger N, Shimoni A, Zabelina T, Schieder H, Panse J, Ayuk F et al. Reduced-toxicity conditioning with treosulfan, fludarabine and ATG as preparative regimen for allogeneic stem cell transplantation (alloSCT) in elderly patients with secondary acute myeloid leukemia (sAML) or myelodysplastic syndrome (MDS). Bone Marrow Transplant. 2006; 37: 339–344. [DOI] [PubMed] [Google Scholar]
- Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763. [PubMed] [Google Scholar]
- Giralt S, Estey E, Albitar M, van Besien K, Rondón G, Anderlini P et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graftversus-leukemia without myeloablative therapy. Blood 1997; 89: 4531–4536. [PubMed] [Google Scholar]
- Shimoni A, Kroger N, Zabelina T, Ayuk F, Hardan I, Yeshurun M et al. Hematopoietic stem-cell transplantation from unrelated donors in elderly patients (age 45 years) with hematologic malignancies: older age is no longer a contraindication when using reduced intensity conditioning. Leukemia 2005; 19: 7–12. [DOI] [PubMed] [Google Scholar]
- Finke J, Nagler A. Viewpoint: What is the role of allogeneic haematopoietic cell transplantation in the era of reduced-intensity conditioning-is there still an upper age limit? A focus on myeloid neoplasia. Leukemia 2007; 21: 1357–1362. [DOI] [PubMed] [Google Scholar]
- Pratcorona M, Brunet S, Nomdedéu J, Ribera JM, Tormo M, Duarte R et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood 2013; 121: 2734–2738. [DOI] [PubMed] [Google Scholar]
- Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008; 111: 2776–2784. [DOI] [PubMed] [Google Scholar]
- Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood 2014; 124: 3441–3449. [DOI] [PubMed] [Google Scholar]
- Burnett AK, Goldstone A, Hills RK, Milligan D, Prentice A, Yin J et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol. 2013; 31: 1293–1301. [DOI] [PubMed] [Google Scholar]
- Döhner K, Schlenk RF, Habdank M, Scholl C, Rücker FG, Corbacioglu A et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood 2005; 106: 3740–3746. [DOI] [PubMed] [Google Scholar]
- Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008; 358: 1909–1918. [DOI] [PubMed] [Google Scholar]
- Tallman MS, Rowlings PA, Milone G, Zhang MJ, Perez WS, Weisdorf D et al. Effect of postremission chemotherapy before human leukocyte antigen-identical sibling transplantation for acute myelogenous leukemia in first complete remission. Blood 2000; 96: 1254–1258. [PubMed] [Google Scholar]
- Cahn JY, Labopin M, Sierra J, Blaise D, Reiffers J, Ferrant A et al. No impact of high-dose cytarabine on the outcome of patients transplanted for acute myeloblastic leukaemia in first remission. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Br J Haematol. 2000; 110: 308–314. [DOI] [PubMed] [Google Scholar]
- Warlick ED, Paulson K, Brazauskas R, Zhong X, Miller AM, Camitta BM et al. Effect of postremission therapy before reduced-intensity conditioning allogeneic transplantation for acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2014; 20: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun M, Labopin M, Blaise D, Cornelissen JJ, Sengeloev H, Vindelov L et al. Impact of postremission consolidation chemotherapy on outcome after reduced-intensity conditioning allogeneic stem cell transplantation for patients with acute myeloid leukemia in first complete remission: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer 2014; 120: 855–863. [DOI] [PubMed] [Google Scholar]
- Luger SM, Ringdén O, Zhang MJ, Pérez WS, Bishop MR, Bornhauser M et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012; 47: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 2005; 19: 2304–2312. [DOI] [PubMed] [Google Scholar]
- Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009; 27: 4570–4577. [DOI] [PubMed] [Google Scholar]
- Bornhäuser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012; 13: 1035–1044. [DOI] [PubMed] [Google Scholar]
- Scott BL, Pasquini MC, Logan B, Wu J, Devine S, Porter DL et al. Results of a phase III randomized, multi-center study of allogeneic stem cell transplantation after high versus reduced intensity conditioning in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML): Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0901. Blood 2015; 126, LBA-8 (abstract). [Google Scholar]
- Buccisano F, Maurillo L, Del Principe MI, Del Poeta G, Sconocchia G, Lo-Coco F et al. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood 2012; 119: 332–341. [DOI] [PubMed] [Google Scholar]
- Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011; 29: 1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Othus M, Araki D, Wood BL, Radich JP, Halpern AB et al. Pre- and post-transplant quantification of measurable ('minimal') residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia 2016, e-pub ahead of print 29 February 2016 doi:10.1038/leu.2016.46. [DOI] [PMC free article] [PubMed]
- Hofmann S, Götz M, Schneider V, Guillaume P, Bunjes D, Döhner H et al. Donor lymphocyte infusion induces polyspecific CD8(+) T-cell responses with concurrent molecular remission in acute myeloid leukemia with NPM1 mutation. J Clin Oncol. 2013; 31: e44–e47. [DOI] [PubMed] [Google Scholar]
- Rettinger E, Willasch AM, Kreyenberg H, Borkhardt A, Holter W, Kremens B et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood 2011; 118: 5681–5688. [DOI] [PubMed] [Google Scholar]
- Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia 2012; 26: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]