Abstract
We studied 190 patients with smoldering multiple myeloma (SMM) at our institution between 1973 and 2014. Evolving change in monoclonal protein level (eMP) was defined as ⩾10% increase in serum monoclonal protein (M) and/or immunoglobulin (Ig) (M/Ig) within the first 6 months of diagnosis (only if M-protein ⩾3 g/dl) and/or ⩾25% increase in M/Ig within the first 12 months, with a minimum required increase of 0.5 g/dl in M-protein and/or 500 mg/dl in Ig. Evolving change in hemoglobin (eHb) was defined as ⩾0.5 g/dl decrease within 12 months of diagnosis. A total of 134 patients (70.5%) progressed to MM over a median follow-up of 10.4 years. On multivariable analysis adjusting for factors known to predict for progression to MM, bone marrow plasma cells ⩾20% (odds ratio (OR)=3.37 (1.30–8.77), P=0.013), eMP (OR=8.20 (3.19–21.05), P<0.001) and eHb (OR=5.86 (2.12–16.21), P=0.001) were independent predictors of progression within 2 years of SMM diagnosis. A risk model comprising these variables was constructed, with median time to progression of 12.3, 5.1, 2.0 and 1.0 years among patients with 0–3 risk factors respectively. The 2-year progression risk was 81.5% in individuals who demonstrated both eMP and eHb, and 90.5% in those with all three risk factors.
Introduction
Smoldering multiple myeloma (SMM) is a plasma cell disorder characterized by serum monoclonal protein (M) ⩾3 g/dl and/or 10–60% clonal bone marrow plasma cells (BMPCs), with no evidence of myeloma-defining events or amyloidosis.1 It is found in 14% of people with newly diagnosed multiple myeloma (MM),2 a disease predicted to account for >2% of all cancer deaths in the United States this year.3
Defining the risk of progression from SMM to MM has been heavily studied, with several factors found to predict for high-risk SMM (defined as ⩾50% risk of progression to MM within 2 years).4 These include serum M-protein ⩾3 g/dl,5 a higher extent of BMPC involvement,5 immunoglobulin A (IgA) SMM,5 immunoparesis in uninvolved Igs,6 increased circulating plasma cells,7 cytogenetic abnormalities,8 a serum involved/uninvolved free light chain ratio ⩾89 and abnormalities on magnetic resonance imaging10 and positron emission tomography–computed tomography.11 Identification of such risk factors is important as a randomized trial conducted by the Spanish Myeloma Group showed that early treatment with lenalidomide and dexamethasone delayed progression and improved overall survival in high-risk SMM patients compared with observation, which remains the current standard of care.12
There has been some interest in evaluating the impact of changes in disease biomarkers on progression to symptomatic disease. An ‘evolving' pattern in serum M-protein independently predicted for progression to MM in a single-center cohort, with a 2-year progression risk of 45%,13 and a similar risk was observed in a SWOG study among patients in whom there was a change in M-protein from <3 to ⩾3 g/dl over a period of 4 months.14 However, post hoc analysis from the observation arm of the Spanish trial suggested that progression risk was comparable between patients with and without evolving changes in M-protein.15
Based on these considerations, we sought to evaluate the impact of evolving changes in SMM disease biomarkers on risk of progression to MM. We specifically aimed to try and use such changes to identify a cohort of ultra-high-risk SMM with ⩾80% risk of progression to MM within 2 years (that is, early progression), a threshold that has been deemed by the International Myeloma Working Group (IMWG) to meet diagnostic criteria for MM.1
Materials and methods
Study cohort
We identified all patients diagnosed with SMM at our institution between 1973 and 2014 (n=1253) from the Mayo Clinic institutional review board-approved electronic database. Only those meeting the revised 2014 IMWG diagnostic criteria for SMM1 were included, and those who received myeloma-specific therapy as part of a therapeutic strategy to delay progression to MM were excluded. Patients who did not have adequate follow-up (that is, a minimum of two data points before confirmed progression to MM) to permit study of evolving changes in at least one SMM-specific biomarker (see below) were also excluded.
Biomarkers studied, definitions of evolving changes and outcomes
For each patient, baseline levels of various SMM parameters (serum M-protein, percentage clonal BMPCs, Igs, involved/uninvolved free light chain ratio, creatinine, calcium, hemoglobin (Hb), lactate dehydrogenase and β2-microglobulin) at diagnosis were abstracted from the electronic medical record. Levels of SMM-specific biomarkers (serum M-protein, involved Ig, Hb, calcium and creatinine) at each follow-up for the first 3 years after diagnosis were also abstracted to permit study of evolving changes. Immunoparesis was defined as reduction below the lower limit of normal in two uninvolved Igs.
Evolving change in monoclonal protein level (eMP) was defined as ⩾10% increase in serum M-protein and/or Ig within the first 6 months of diagnosis (only if M-protein was ⩾3 g/dl) and/or ⩾25% increase in serum M-protein and/or Ig within the first 12 months of diagnosis (for any level of M-protein), with a required minimum increase of either 0.5 g/dl in M-protein or 500 mg/dl in Ig (or both). We used changes in Ig conjunctively with M-protein to study evolving change in monoclonal disease burden given that both of them represent outputs from a clonal plasma cell population. Evolving change in hemoglobin level (eHb) was defined as ⩾0.5 g/dl decrease within the first 12 months of diagnosis. We also studied changes in calcium and creatinine, but were unable to find consistent patterns to produce a candidate definition of an evolving change in these two variables.
Time to progression (TTP) was defined as duration between confirmed diagnosis of SMM (as per revised 2014 IMWG criteria) and diagnosis of MM or commencement of myeloma-directed therapy for symptomatic disease by the treating physician, or censored at last follow-up. Overall survival was defined as duration between SMM diagnosis and death, or censored at the last follow-up date.
Statistical analysis
Univariate logistic regression analyses were used to test the association between variables of interest and the risk of early progression to MM. Variables achieving a significance level of P<0.2 on univariable analysis were included in the multivariable logistic regression analysis. Survival and TTP analyses were performed using the Kaplan–Meier method, and the log-rank test was used to make comparisons between groups. All tests were two sided with P-values of <0.05 considered to be significant. Statistical analysis was performed using SPSS v.20 (IBM Corp., Armonk, NY, USA).
Results
A total of 190 patients were included in the analysis (Table 1). Median age at SMM diagnosis was 64 years, and the majority of patients (55%) were male. Of the cohort, 66.3% had a serum M-protein of <3 g/dl at diagnosis and BMPC was ⩾10% in the vast majority (95.3%) of patients. Of the patients, 75.8% had IgG SMM, with most of the remainder having IgA disease; immunoparesis was observed in 61.6% of patients.
Table 1. Baseline characteristics of 190 patients at SMM diagnosis.
| N | |
|---|---|
| Age, median (range) | 64 (30–83) |
| Sex (%) | |
| Male | 104 (54.7) |
| Female | 86 (45.3) |
| Serum M-protein (%) | |
| <3 g/dl | 126 (66.3) |
| ⩾3g/dl | 52 (27.4) |
| Not available | 12 (6.3) |
| Clonal bone marrow plasma cells (%) | |
| <10 | 7 (3.7) |
| 10–19 | 90 (47.4) |
| 20–59 | 91 (47.9) |
| Not available | 2 (1.1) |
| Immunoglobulin subtype (%) | |
| IgG | 144 (75.8) |
| IgA | 35 (18.4) |
| IgM | 2 (1.1) |
| IgD | 2 (1.1) |
| Biclonal | 1 (0.5) |
| Unknown | 6 (3.2) |
| Immunoparesis (%) | |
| Present | 117 (61.6) |
| Absent | 33 (17.4) |
| Not available | 40 (21.1) |
| Hemoglobin (g/dl), median (range) | 12.7 (7.2–17.1) |
| Involved/uninvolved FLC ratio (%) | |
| <8 | 45 (23.7) |
| ⩾8 | 42 (22.1) |
| Not available | 103 (54.2) |
| LDH, median (range), units/l | 143 (3–451) |
| β2-microglobulin, median (range), μg/ml | 2.4 (1–17.6) |
| Progression to multiple myeloma (%) | 134 (70.5) |
| Median, years (95% CI) | 3.9 (2.8–5.1) |
| ⩽2 Years | 61 (45.5) |
| >2 Years | 73 (54.5) |
| Progression events (%) | |
| Anemia | 59 (44.0) |
| Bone lesions | 49 (36.6) |
| Renal failure | 16 (11.9) |
| Other | 10 (7.5) |
Abbreviations: CI, confidence interval; FLC, free light chain; Ig, immunoglobulin; LDH, lactate dehydrogenase; SMM, smoldering multiple myeloma.
A total of 134 individuals (70.5%) progressed to MM over a median follow-up of 10.4 years, with a median TTP of 3.9 years (95% confidence interval 2.8–5.1). Of those who progressed, 61 patients (45.5%) did so within 2 years of diagnosis, and progression events were primarily due to anemia (44.0%) or bony lesions (36.6%). Median overall survival in the entire cohort was 10.0 years (8.9–11.1).
Evolving changes in monoclonal protein and hemoglobin
Table 2 provides the definition used for eMP and eHb and Table 3 shows the numbers of patients who met these criteria. A total of 58 patients (30.5%) demonstrated eMP—of these, 24 (41.4%) met criteria for evolving change in M-protein, 15 (25.9%) for evolving change in Ig and 19 (32.8%) meeting criteria for evolving changes in both M-protein and Ig. In the vast majority of patients (84.5%), confirmation of the change or progression to MM was seen at the next follow-up. In addition, 48 patients (25.3%) displayed eHb, and the change was either confirmed, or progression to MM determined, at next follow-up in all of these individuals.
Table 2. Definitions of evolving changes in monoclonal protein (eMP) and hemoglobin (eHb).
| Criteria | Definition |
|---|---|
| Evolving monoclonal protein level (eMP) | ⩾10% Increase in serum M-protein and/or involved immunoglobulin level within the first 6 months of diagnosis (only if baseline M-protein was ⩾3 g/dl) and/or ⩾25% increase in serum M-protein and/or involved immunoglobulin level within the first 12 months of diagnosis (for any level of M-protein) plus minimum increase of 0.5 g/dl in M-protein or 500 mg/dl in immunoglobulin or both |
| Evolving hemoglobin level (eHb) | ⩾0.5 g/dl Decrease in hemoglobin within the first 12 months of diagnosis |
Table 3. Evolving changes in monoclonal protein (eMP) and hemoglobin (eHb).
| Parameter | N |
|---|---|
| eMP (%) | |
| Present | 58 (30.5) |
| Evolving M-protein | 24 (41.4) |
| Evolving Ig | 15 (25.9) |
| Evolving M-protein and Ig | 19 (32.8) |
| Confirmed on next measurementa | 49 (84.5) |
| Absent | 122 (64.2) |
| Not evaluable | 10 (5.3) |
| eHb (%) | |
| Present | 48 (25.3) |
| Confirmed on next measurementa | 48 (100) |
| Absent | 127 (66.8) |
| Not evaluable | 15 (7.9) |
Abbreviation: Ig, involved immunoglobulin.
Including patients who progressed at next follow-up date.
Predictors of early (⩽2 years) progression to MM
Table 4 shows the results of uni- and multivariable logistic regression analyses assessing predictors of progression to MM within 2 years of SMM diagnosis. On univariable analysis, male sex (odds ratio (OR)=2.39 (1.26–4.54), P=0.008), BMPC ⩾20% (OR=4.41 (2.26–8.57), P<0.001), eMP (OR=9.55 (4.62–19.73), P<0.001) and eHb (OR=8.25 (3.92–17.36), P<0.001) predicted for early progression. All four variables remained independent predictors of early progression on multivariable analysis (male sex: OR=3.51 (1.32–9.29), P=0.012; BMPC ⩾20%: OR=3.37 (1.30–8.77), P=0.013; eMP: OR=8.20 (3.19–21.05), P<0.001; eHb: OR=5.86 (2.12–16.21), P=0.001).
Table 4. Univariable and multivariable logistic regression analyses of predictors of progression to MM within 2 years of SMM diagnosis.
|
Univariable |
Multivariable (n=156) |
|||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | 1.01 (0.98–1.04) | 0.626 | — | — |
| Male sex | 2.39 (1.26–4.54) | 0.008 | 3.51 (1.32–9.29) | 0.012 |
| BMPC ⩾20% | 4.41 (2.26–8.57) | <0.001 | 3.37 (1.30–8.77) | 0.013 |
| M-protein ⩾3 g/dl | 1.68 (0.86–3.30) | 0.129 | 0.41 (0.13–1.31) | 0.132 |
| IgA SMM | 1.69 (0.80–3.58) | 0.171 | 1.09 (0.36–3.32) | 0.883 |
| Immunoparesis | 1.59 (0.63–3.99) | 0.328 | — | — |
| FLC ⩾8 | 1.57 (0.60–4.10) | 0.358 | — | — |
| eMP | 9.55 (4.62–19.73) | <0.001 | 8.20 (3.19–21.05) | <0.001 |
| eHb | 8.25 (3.92–17.36) | <0.001 | 5.86 (2.12–16.21) | 0.001 |
| LDH>ULN | 0.30 (0.04–2.49) | 0.266 | — | — |
| β2-microglobulin >3.5 μg/ml | 0.86 (0.34–2.22) | 0.760 | — | — |
Abbreviations: BMPC, clonal bone marrow plasma cell; 95% CI, 95% confidence interval; OR, odds ratio; eHb, evolving hemoglobin; eMP, evolving monoclonal protein; FLC, free light chain; IgA, involved immunoglobulin A; LDH, lactate dehydrogenase; MM, multiple myeloma; SMM, smoldering multiple myeloma; ULN, upper limit of normal. Bold values indicate statistically significant on multivariate analysis.
Risk model for early progression, and 2-year progression risks
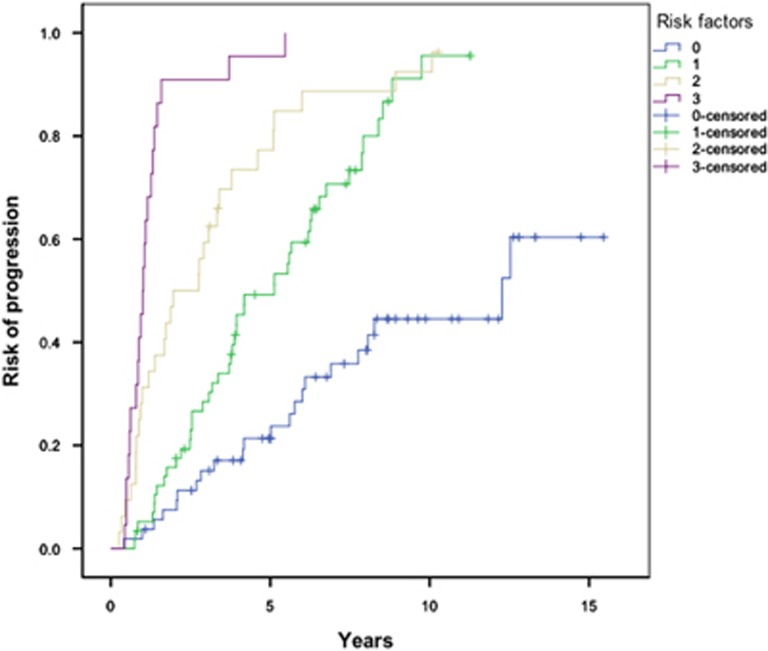
Based upon the three disease-specific variables independently predicting for early progression (BMPC ⩾20%, eMP and eHb), a risk model for progression was constructed based upon the number of risk factors (0–3) for each patient at diagnosis (Figure 1). Median TTP in patients with none (n=54), one (n=58), two (n=32) and three (n=22) risk factors was 12.3 (7.1–17.5), 5.1 (3.3–6.9), 2.0 (0.5–3.4) and 1.0 years (0.8–1.2) respectively (P<0.001).
Figure 1.
Risk of progression in SMM patients, stratified by the number of risk factors (eMP, eHb and BMPC ⩾20%) at diagnosis. P<0.001.
Table 5 shows the rates of early progression among patients with different disease-specific risk factors. The 2-year progression risks were 63.8% and 64.6% in patients displaying eMP and eHb respectively; among individuals who displayed both eMP and eHb, the early progression risk was 81.5%, and this increased to 90.5% in patients with all 3 risk factors (BMPC ⩾20%, eMP and eHb).
Table 5. Risk of early progression (⩽2 years) in SMM patients with disease-specific risk factors.
| Risk factor | Proportion of patients progressing within 2 years (%) |
|---|---|
| BMPC ⩾20% | 44/91 (48.4) |
| eMP only | 37/58 (63.8) |
| eHb only | 31/48 (64.6) |
| Both eMP and eHb | 22/27 (81.5) |
| eMP, eHb and BMPC ⩾20% | 19/21 (90.5) |
Abbreviations: BMPC, clonal bone marrow plasma cells; eHb, evolving hemoglobin; eMP, evolving monoclonal protein; SMM, smoldering multiple myeloma.
Discussion
The natural history of SMM has been well defined, with a risk of progression to MM of 10% per year for the first 5 years, 3% per year for the next 3 years and 1% per year thereafter.5 Several factors predicting for a higher risk of progression have been identified, leading to the development of risk models,6, 9 although there remains discordance in their predictive accuracy.16 The need to improve the risk stratification of SMM patients has arisen as a result of the development of more effective and less toxic treatments in myeloma that may allow SMM patients the opportunity to be treated before the herald of end-organ damage. Indeed, randomized data have shown a progression and survival benefit in high-risk SMM patients treated with lenalidomide and dexamethasone compared with observation,12 and additional trials utilizing novel agents are currently underway in high-risk patients.
In 2014, the IMWG updated the diagnostic criteria for MM by removing the requirement for end-organ damage to define malignancy, and called for the development of additional biomarkers associated with a risk of progression of SMM to MM of at least 80% within 2 years, to enable their addition to diagnostic criteria for MM.1 In this study, we have shown that SMM patients with evolving changes in their monoclonal protein burden (defined by changes in M-protein and/or involved Ig) and Hb are at >80% risk of progressing to MM within the first 2 years of diagnosis, and that patients with both evolving changes and a BMPC of ⩾20% have a 2-year progression risk of >90%. Although these findings require validation, these data provide the first step toward incorporating evolving changes in commonly measured disease biomarkers in diagnostic definitions for myeloma or as an indication for treatment in SMM.
The first data on how changes in M-protein impact on progression came from the recognition of an evolving subtype among SMM patients treated at a single institution in Spain.17 Rosinol et al.17 found that TTP to MM was significantly higher among patients in whom serum M-protein increased in each of the first two consecutive follow-up visits after SMM diagnosis compared with patients who did not experience such a change; moreover, this evolving subtype was the only factor that independently predicted for shorter TTP. Subsequent work from the same institution showed that an evolving change in M-protein (defined as ⩾10% increase in the first 6 months if M-protein was ⩾3 g/dl, or a progressive increase in each of the annual consecutive measurements during a period of 3 years if M-protein was <3 g/dl) was an independent predictor of progression to MM, with an associated 2-year progression risk of 45%.13 Aside from these studies, the only other data on evolving changes in SMM come from a subgroup analysis within the SWOG S0120 prospective study on asymptomatic monoclonal gammopathies, with a 33.3% 2-year progression risk seen among the 6 patients in whom M-protein increased from <3 to ⩾3 g/dl in a 4-month period.14
In this regard, our study represents a valuable contribution to the literature by confirming that evolving changes in monoclonality (measured by M-spike and/or involved Ig) are an independent risk factor for progression to MM and, to our knowledge, being the first to show that evolving change in Hb is also predictive for early progression. In addition, our definitions of evolving change allow for both sensitivity and robustness in identifying patients with this phenotype, given that they can be measured within the first year of SMM diagnosis, can be achieved through either changes in M-protein or involved Igs (when the M-protein is too small) and have a minimum threshold to ensure that clinically insignificant findings (for example, from small rises meeting percentage thresholds in M-protein or Ig) are ignored.
The processes underlying progression of SMM to MM provide the biologic explanation that underpins our findings. Generation of a plasma cell clone arises from hyperdiploidy and translocation of the Ig heavy-chain locus that leads to cyclin D dysregulation and initiation of clonal plasma cell evolution.18 Further genetic, epigenetic and karyotypic abnormalities drive expansion of the plasma cell clone, allowing transformation from the precursor states of monoclonal gammopathy of undetermined significance and SMM toward symptomatic MM.19 It is therefore apparent that these events are associated with increasing production of M-protein and the involved Ig, with the expansion of clonal plasma cells leading to bone marrow suppression and giving rise to a progressive reduction in Hb. Consequently, identifying changes in these parameters provides the ability to identify patients with more aggressive disease biology, thereby allowing these individuals to avail from therapy before they suffer end-organ damage.
The main strength of our study lies in the relatively large number of patients included, representing the largest cohort on evolving changes in SMM that has been published to date, as well as the long follow-up available. In addition, we used the latest diagnostic criteria for SMM to ensure that patients with parameters such as BMPC ⩾60% and free light chain ⩾100, which have recently been categorized as being diagnostic for MM,1 were excluded. Furthermore, our definition of evolving change are simple and permit easy calculation for clinicians, as well as being in keeping with similar criteria for disease progression in MM20 and previous definitions of evolving changes.13
Nevertheless, there are certain limitations of this study in addition to those inherent to a single-center, observational, retrospective study. First, >70% of our cohort progressed to MM during the study period, with >30% progressing within 2 years of diagnosis. Given that high-risk SMM is defined as ⩾50% risk of progression within 2 years,4 this suggests our cohort was particularly high risk at baseline and may therefore limit the generalizability of our findings. Second, it may be argued that a reduction in Hb of ⩾0.5 g/dl, which constituted an evolving change, is too small to reflect a significant change in disease status, as well as potentially falling within the margin of error provided by laboratory variability and changes in hydration status. However, our intention was to balance sensitivity and specificity in producing a cutoff for evolving change; moreover, the validity of utilizing the ⩾0.5 g/dl threshold was shown by the change either being confirmed on next measurement or progression to MM being demonstrated at next follow-up in all 48 patients who met this criterion.
In summary, this study identified that evolving changes in Hb and M-protein and/or involved Ig, in addition to BMPC ⩾20%, were independent predictors of early progression in a large single-center cohort of patients with SMM. Individuals displaying evolving changes in both monoclonality and Hb, with or without a clonal BMPC of ⩾20%, had >80% risk of progression to MM within 2 years of diagnosis, meeting the IMWG threshold for a diagnosis of MM. We hope that following independent validation, our findings can be used to update the diagnostic criteria for MM, or to be considered as an indication for therapy of SMM.
Acknowledgments
This study was supported in part by Grants CA 107476, CA 168762 and CA186781 from the National Cancer Institute, Rockville, MD, USA. PR is supported by the American Society of Hematology HONORS Award.
Author contributions
PR and SVR designed the research, analyzed the data, wrote and edited the manuscript. All authors participated in data interpretation, reviewed the manuscript and provided critical comments. All authors reviewed and approved the final manuscript.
Footnotes
SK has obtained research support for clinical trials from Celgene, Millennium, Novartis, Janssen and Sanofi. AD has received research support for clinical trials from Pfizer, Janssen, Millennium, Alnylam and Celgene. MAG has received research support from ISIS and Prothena, and honoraria from Celgene, Millennium Pharmaceuticals and Novartis. PLB has obtained research support for clinical trials from Millennium, Novartis, Constellation Pharmaceuticals and MundiPharma, and honoraria from Amgen and Incyte. The other authors declare no conflict of interest.
References
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15: e538–e548. [DOI] [PubMed] [Google Scholar]
- Kristinsson SY, Holmberg E, Blimark C. Treatment for high-risk smoldering myeloma. N Engl J Med 2013; 369: 1762–1763. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- Mateos MV, San Miguel JF. Smoldering multiple myeloma: when to observe and when to treat? Am Soc Clin Oncol Educ Book 2015, e484–e492. [DOI] [PubMed]
- Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med 2007; 356: 2582–2590. [DOI] [PubMed] [Google Scholar]
- Perez-Persona E, Mateo G, Garcia-Sanz R, Mateos MV, de Las Heras N, de Coca AG et al. Risk of progression in smouldering myeloma and monoclonal gammopathies of unknown significance: comparative analysis of the evolution of monoclonal component and multiparameter flow cytometry of bone marrow plasma cells. Br J Haematol 2010; 148: 110–114. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Kyle RA, Larson DR, Witzig TE, Kumar S, Dispenzieri A et al. High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia 2013; 27: 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neben K, Jauch A, Hielscher T, Hillengass J, Lehners N, Seckinger A et al. Progression in smoldering myeloma is independently determined by the chromosomal abnormalities del(17p), t(4;14), gain 1q, hyperdiploidy, and tumor load. J Clin Oncol 2013; 31: 4325–4332. [DOI] [PubMed] [Google Scholar]
- Dispenzieri A, Kyle RA, Katzmann JA, Therneau TM, Larson D, Benson J et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood 2008; 111: 785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillengass J, Fechtner K, Weber MA, Bauerle T, Ayyaz S, Heiss C et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol 2010; 28: 1606–1610. [DOI] [PubMed] [Google Scholar]
- Siontis B, Kumar S, Dispenzieri A, Drake MT, Lacy MQ, Buadi F et al. Positron emission tomography-computed tomography in the diagnostic evaluation of smoldering multiple myeloma: identification of patients needing therapy. Blood Cancer J 2015; 5: e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos MV, Hernandez MT, Giraldo P, de la Rubia J, de Arriba F, Lopez Corral L et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med 2013; 369: 438–447. [DOI] [PubMed] [Google Scholar]
- Fernandez de Larrea C, Isola I, Ciberia MT, Rosinol L, Calvo X, Tovar N et al. Smoldering multiple myeloma: impact of the evolving pattern on early progression. Blood 2014; 124: 3363. [Google Scholar]
- Dhodapkar MV, Sexton R, Waheed S, Usmani S, Papanikolaou X, Nair B et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120). Blood 2014; 123: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood 2015; 125: 3069–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry BM, Korde N, Kwok M, Manasanch EE, Bhutani M, Mulquin M et al. Modeling progression risk for smoldering multiple myeloma: results from a prospective clinical study. Leuk Lymphoma 2013; 54: 2215–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinol L, Blade J, Esteve J, Aymerich M, Rozman M, Montoto S et al. Smoldering multiple myeloma: natural history and recognition of an evolving type. Br J Haematol 2003; 123: 631–636. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood 2003; 101: 4569–4575. [DOI] [PubMed] [Google Scholar]
- Korde N, Kristinsson SY, Landgren O. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM): novel biological insights and development of early treatment strategies. Blood 2011; 117: 5573–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011; 117: 4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]