Abstract
Interventricular septal hematoma is a rare complication of retrograde chronic total occlusion (CTO) percutaneous coronary interventions (PCI) with a typically benign course. Here we report two cases of interventricular septal hematoma and coronary-cameral fistula development after right coronary artery (RCA) CTO-PCI using a retrograde approach. Both were complicated by development of ST-segment elevation and chest pain. One case was managed actively and the other conservatively, both with a favorable outcome.
1. Introduction
Interventricular septal hematoma is a rare complication of retrograde chronic total occlusion (CTO) percutaneous coronary interventions (PCI) with a typically benign course [1]. Here we report two cases of interventricular septal hematoma and coronary-cameral fistula development after right coronary artery (RCA) CTO-PCI using a retrograde approach. Both cases were complicated by the development of ST-segment elevation and chest pain. One case was managed actively with exclusion of the hematoma and the other conservatively, both with a favorable outcome. These cases with clues from seven other case reports have led us to a suggested observation and treatment algorithm for this unique procedural complication.
2. Case 1
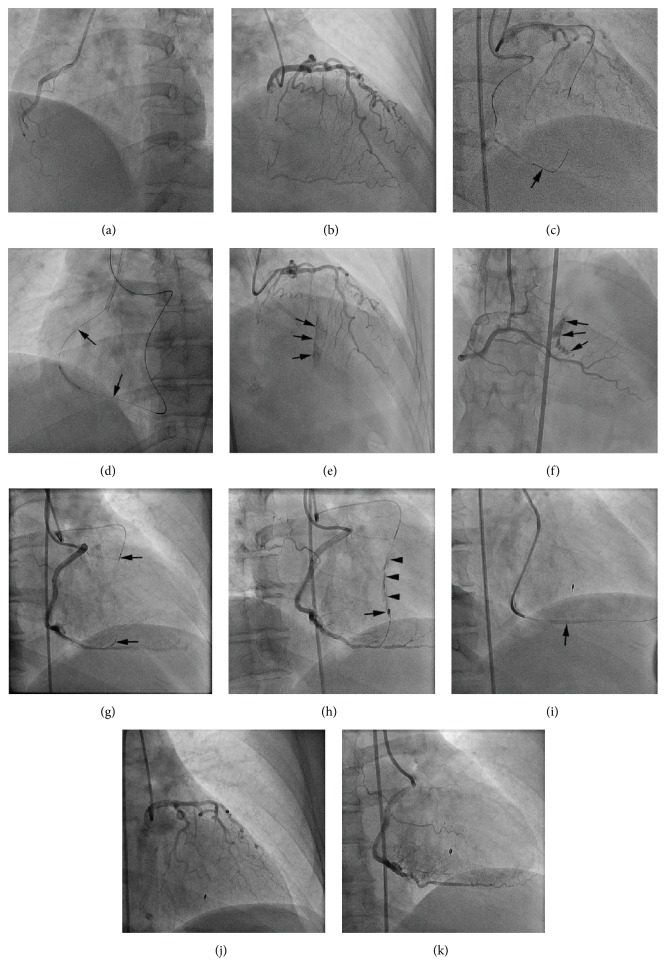
A 46-year-old female with Canadian Cardiovascular Society (CCS) class IV angina symptom despite maximal medical therapy was referred for coronary angiogram and was found to have right coronary artery (RCA) chronic total occlusion (CTO) (Figure 1(a)).
Figure 1.
(a) Right coronary artery (RCA) chronic total occlusion (CTO). (b) Left anterior descending artery (LAD) supplying Werner's grade 1 septal collaterals to RCA CTO. (c) Fielder FC (arrow) advanced from LAD through second septal perforator into distal RCA. (d) After successful retrograde wire crossing, externalization was achieved with guidewire (arrow). (e and f) Angiogram of LAD and RCA, respectively, reveals Ellis Type III septal collateral perforation (arrows) into the right ventricle. (g) Negative suction applied to Corsairs (arrow heads) that were advanced from LAD and RCA into the perforated septal collateral. (h) Detachable coil delivered (arrow) but persistent of perforation seen (arrow head). (i) Covered stent (arrow) delivered across perforated septal vessel. (j and k) Final angiogram of LAD and RCA, respectively, confirmed resolution of septal perforation.
RCA CTO percutaneous intervention was performed using right radial and right femoral artery dual catheter approach. The RCA was engaged with 6 French Judkins right guide catheter; the right femoral artery was cannulated with a 6 French sheath and the left main was engaged with a 6 French XB 3.5 guide catheter. With standard antegrade wire escalation technique, the CTO could not be crossed [2]. The treatment strategy was changed to the retrograde approach utilizing septal collaterals from the left anterior descending artery (LAD) (Figure 1(b)). A fielder FC guidewire (Asahi Intecc, Nagoya, Japan) was advanced through the second septal perforator and then into the distal RCA (Figure 1(c)). This was followed by advancement of a Corsair catheter (Asahi Intecc, Nagoya, Japan) into the distal RCA. A Pilot 200 (Abbott Vascular, Abbott Park, Illinois) was advanced through the Corsair and crossed the CTO segment into the antegrade guide catheter followed by Corsair advancement into the guide. An R350 guidewire (Vascular Solutions, Minneapolis, Minnesota) was used for externalization (Figure 1(d)) and three drug-eluting stents were delivered.
After removal of retrograde Corsair and guidewire, angiogram of the LAD and RCA revealed Ellis Type III cavity spilling perforation of the second septal branch into the right ventricle (RV) (Figures 1(e) and 1(f)). The patient was asymptomatic and hemodynamically stable and therefore transferred to a monitored bed. Subsequently, she complained of intermittent chest pain throughout the night and had sinus tachycardia with normal blood pressure.
The next morning, she was taken back to the cardiac catheterization lab and repeat coronary angiography appeared unchanged. Given the patient's symptom and tachycardia, the decision to proceed with exclusion of the septal artery to RV perforation was made. Bilateral femoral access was obtained and similar guide catheters were used to engage the left and right coronary systems. The perforated collateral septal branch was accessed with workhorse type coronary guidewires from the LAD and RCA. Corsairs were then advanced into the proximal segment of the collateral vessel and negative suction was applied for approximately ten minutes (Figure 1(g)). This maneuver was successful in resolving the perforation from the LAD but not from the RCA side. Therefore, a Renegade Microcatheter (Boston Scientific, Natick, Massachusetts) was advanced from the RCA into the perforated septal branch and a 2 × 3 × 23 mm complex helical Interlock detachable coil (Boston Scientific) was delivered but was ineffective (Figure 1(h)). An attempt to deliver another coil failed due to poor microcatheter support and, therefore, a 2.8 × 19 mm Graftmaster covered stent (Abbott Vascular) was deployed across the perforated collateral vessel in the posterior descending artery of the RCA (Figure 1(i)). Final angiograms of the left and right coronary system confirmed complete resolution of the perforation (Figures 1(j) and 1(k)). The patient's chest pain and tachycardia resolved after the procedure and she was subsequently discharged home.
3. Case 2
A 66-year-old male with known coronary artery disease and a chronically occluded mid right coronary stent presented with non-ST-segment elevation myocardial infarction. The culprit lesion was the RCA proximal to the remotely deployed and chronically occluded bare metal stent resulting in right ventricular ischemia. An attempt to recanalize the RCA was unsuccessful. He was treated medically but had persistent Canadian Cardiovascular Society (CCS) class III angina and was referred for RCA CTO-PCI.
The antegrade approach was unsuccessful because the wire repeatedly tracked beside the stent; therefore, the retrograde approach was used. The in-stent lesion was successfully crossed with reverse cart technique in the proximal RCA [8]. The RCA was then stented with 2.5 × 38 mm, 3.0 × 38 mm, 3.5 × 38 mm, and 3.5 × 8 mm drug-eluting stents from distal to proximal, respectively. TIMI 3 flow was achieved after procedure.
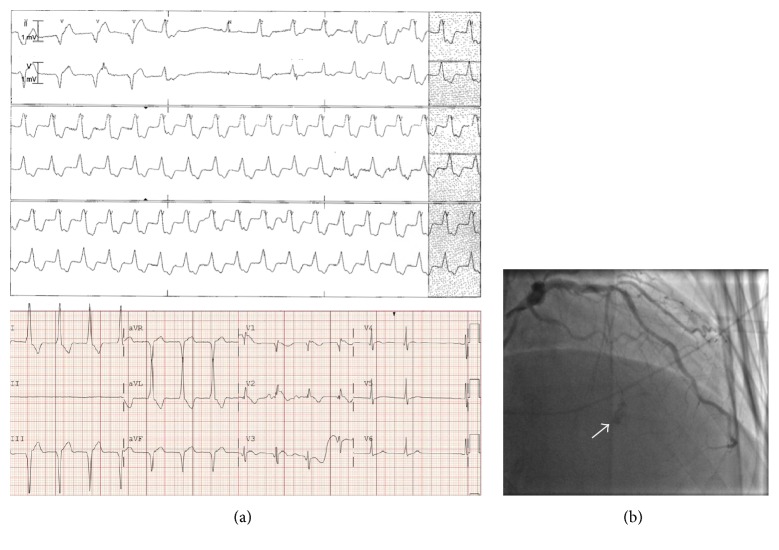
On the night after his PCI, he developed severe chest pain, anterior ST-segment elevation, and nonsustained ventricular tachycardia (Figure 2(a)). Repeat angiography showed patent RCA stents and a perforation with large septal hematoma formation in the first septal artery branch territory (Figure 2(b)). He was treated conservatively with beta blockers and analgesics.
Figure 2.
(a) A cardiac telemetry monitor strip and an electrocardiogram showing nonsustained ventricular tachycardia and anterior ST-segment elevation after RCA CTO-PCI using the retrograde approach, which was complicated by perforation of first septal artery branch (arrow, (b)).
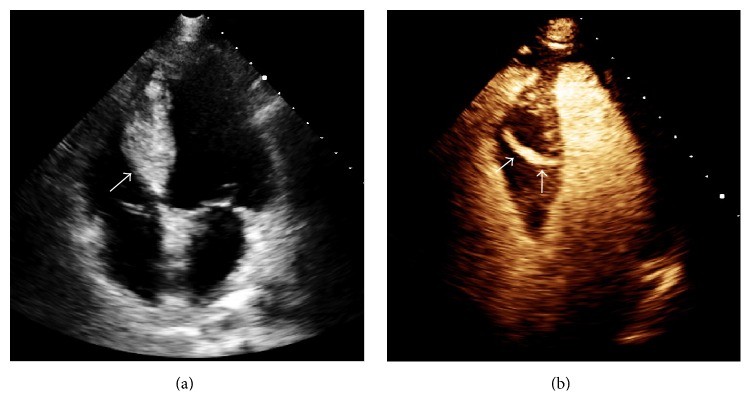
The following day, a contrast enhanced transthoracic echocardiogram confirmed a new large interventricular hematoma. The end systolic dimensions were 5.3 × 2.6 cm (Figure 3(a)). There was a 5 mm diameter fistulous tract from the left ventricle that appeared to extend to within a few millimeters of the right ventricular cavity (Figure 3(b)). Serial echocardiography revealed no appreciable change in the hematoma and fistula. There was no further arrhythmia and right heart catheterization with oximetry showed a Qp/Qs of 1.1 : 1. The patient was discharged on hospital day four.
Figure 3.
Interventricular septal hematoma (5.3 × 2.6 cm) (arrow, (a)), with coronary-ventricular fistula (5 mm in diameter) (arrows, (b)). (Videos 1 and 2 show baseline echocardiographic findings and are included in Supplementary Material available online at http://dx.doi.org/10.1155/2016/8750603.)
Ten days after PCI he was clinically stable and angina-free. An echocardiogram revealed a smaller intramyocardial septal hematoma (3.6 × 1.3 cm) with a fistulous tract in the septum that entered the apical right ventricle. At 3 months a subsequent echocardiogram showed resolution of the interventricular septal hematoma (Figure 4) and the patient remained symptom-free.
Figure 4.
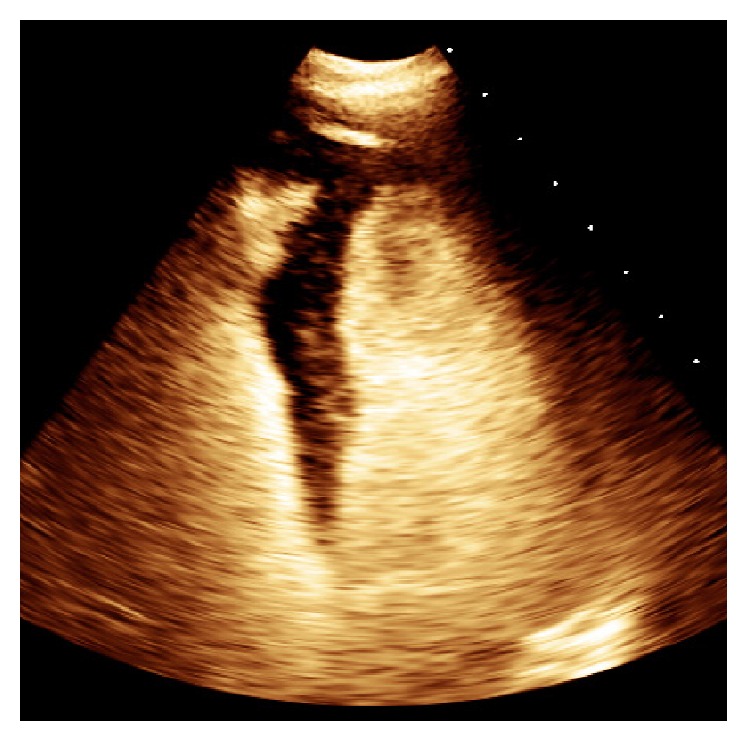
Complete resolution of interventricular septal hematoma at 3 months after PCI. (Video 3 shows follow-up echocardiographic findings and is included as supplementary material online.)
4. Discussion
CTO-PCI frequency is increasing [9]. In a recent meta-analysis including 26 studies, use of the retrograde approach was associated with a technical success rate of 74.5% (overall CTO-PCI procedural success rate was 83.3%). Typical procedural major adverse cardiovascular events (MACE) including mortality (0.7%), urgent CABG (0.7%), and stroke (0.5%) were described. CTO specific complications such as collateral perforation occurred in 6.9% of cases with tamponade occurring in 1.4% [10].
Septal collateral perforation and hematoma formation is a rare complication after retrograde CTO-PCI [1] and believed to have a typically benign course especially among asymptomatic cases. Here we present two cases where clinical deterioration led to further evaluation and subsequent intervention in one but not the other. In both cases there were no adverse sequelae but aggressive management led to additional treatment with coils and a covered stent while conservative management led to prolonged inpatient observation and additional imaging. Other individual case reports of septal hematoma after retrograde CTO-PCI exist (Table 1). In most of the reported cases, cardiac imaging such as contrast and Doppler two-dimensional echocardiography, computed tomography, and cardiac magnetic resonance imaging played a crucial role in early detection and monitoring of these complications.
Table 1.
Other individual case reports of septal hematoma after retrograde CTO-PCI.
| Author | Publication year | CTO location and approach | Signs and symptoms | Complications | Intervention | Imaging modality used |
|---|---|---|---|---|---|---|
| Fairley et al. [1] | 2010 | LAD, retrograde | Asymptomatic, ventricular bigeminy | Ventricular Septal Defect (VSD) | Spontaneous resolution at 5 weeks | Echocardiography |
|
| ||||||
| Murthy et al. [3] | 2014 | LAD in-stent restenosis, antegrade | Contrast stain during catheterization, asymptomatic | Coronary-cameral fistula | Covered stent | Coronary angiography and Intravascular Ultrasound (IVUS) |
|
| ||||||
| Lin et al. [4] | 2006 | LAD, retrograde | Fever and chest pain | Myocardial infarction and septal hematoma | Spontaneous resolution at 1 month | Echocardiography and computed tomography |
|
| ||||||
| Hashidomi and Saito [5] | 2011 | LAD in-stent restenosis, retrograde | Contrast stain, immediate hypotension and tachycardia | Cardiac tamponade | Pericardiocentesis and coil embolization | Echocardiography |
|
| ||||||
| Higuchi et al. [6] | 2015 | LAD, antegrade | Contrast stain, cardiogenic shock | Large expanding subepicardial hematoma, tamponade, and death | Attempted pericardial drainage | Echocardiography |
|
| ||||||
| Araki et al. [7] | 2016 | RCA, retrograde | Contrast stain, asymptomatic | None | Spontaneous resolution at 6 weeks | Echocardiography and cardiac magnetic resonance imaging |
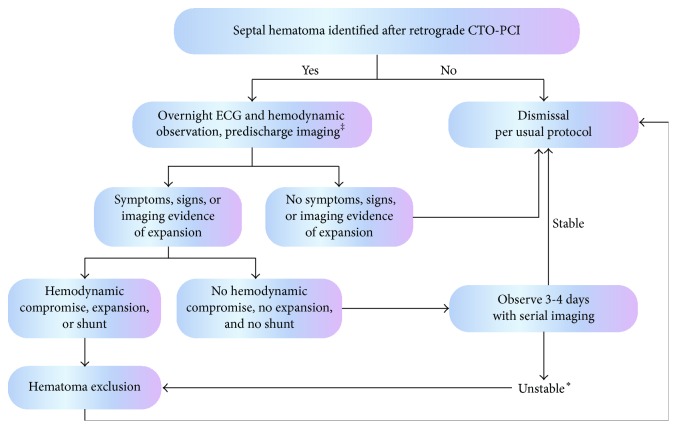
While the management of these cases remains controversial and undefined by large experience some guidance is needed as the frequency of CTO-PCI and the retrograde approach increases. Taking our observations together with previous reports we suggest the following when retrograde CTO-PCI results in septal hematoma formation (Figure 5). Inpatient telemetry, hemodynamic, and Creatine Kinase (CK) and CK-MB levels monitoring of these patients for symptoms and signs of hematoma expansion is prudent.
Figure 5.
Suggested algorithm for observation and management of septal hematoma after retrograde CTO-PCI. ‡Imaging can be performed with catheterization, contrast echocardiography (our preferred method), or perhaps cardiac magnetic resonance imaging. ∗Unstable includes hemodynamic compromise, continued symptoms, development of large shunt, persistent or recurrent life threatening arrhythmia, effusion, or tamponade.
Patients who remain asymptomatic can be managed conservatively and dismissed 24–48 hours after CTO-CPI. Among those with symptoms of hematoma expansion, those without hemodynamic compromise can also be monitored with serial echocardiography and dismissed after 3-4 days of additional observation in the absence of additional evidence of hematoma expansion or significant shunt formation. We recommend more aggressive management with hematoma exclusion for those with hemodynamic compromise, evidence of additional hematoma expansion, pericardial effusion, tamponade, or significant shunt development (Qp/Qs > 1.5 : 1). Early dismissal after definitive management is not unreasonable and may be useful in avoiding prolonged inpatient observation, but the long term outcome of this approach is unknown. After successful CTO-PCI, exclusion of septal hematoma can be accomplished by local occlusion of the contributing septal perforator or occlusion of the origin of the septal with a covered stent. Both the donor and recipient vessel side of the collateral must be occluded to prevent flow into the hematoma. After unsuccessful CTO-PCI only the donor limb of the collateral needs to be occluded. Local occlusion of either limb of the septal can be accomplished with aspiration (as in Case 1) through a microcatheter or coiling with a bailout strategy of covered stenting. We preferentially use aspiration and coils in an effort to avoid the long term risk of covered stent restenosis or thrombosis which is as high as 30% in some series [11]. Additionally, coil occlusion of the septal hematoma space is possible. Caution should be used with microsphere, thrombus, gelfoam, or thrombin injection thrombosis of the hematoma space as many of these communicate with a ventricular cavity and extrusion of these materials into the cavity is a potentially catastrophic possibility.
5. Conclusion
Septal hematoma formation is an unusual but potentially dangerous complication of the increasingly used technique of retrograde CTO-PCI. The case reports to date provide some clues as to the best management of this event. We propose an observation and treatment algorithm and recommend serial imaging with contrast echocardiography to assist in the decision-making during these rare but important events.
Supplementary Material
RCA CTO PCI was complicated by large interventricular septal hematoma “5.3 × 2.6 cm” at end systole (video 1), with coronary-ventricular fistula (5 mm in diameter) (Video 2). At 3 months post PCI a subsequent echocardiogram showed resolution of the hematoma (Video 3).
Abbreviations
- CTO:
Chronic total occlusion
- PCI:
Percutaneous coronary interventions
- RCA:
Right coronary artery
- LAD:
Left anterior descending
- CCS:
Canadian Cardiovascular Society
- RV:
Right ventricle
- Qp/Qs:
Pulmonary flow/systemic flow
- MACE:
Major adverse cardiovascular events
- CABG:
Coronary artery bypass graft
- VSD:
Ventricular Septal Defect
- NSVT:
Nonsustained ventricular tachycardia
- EKG:
Electrocardiogram.
Competing Interests
Abdul-rahman R. Abdel-karim has no competing interests. Minh Vo is a speaker and consultant for Boston Scientific and Abbott. Michael L. Main obtained research grants from Boston Scientific and Bracco and is a consultant for Boston Scientific. J. Aaron Grantham obtained significant grants from Medtronic, Boston Scientific, Abbott Vascular, and Asahi Intecc and is a consultant for Boston Scientific and Asahi Intecc and equity partner in Insysiv, LLC.
References
- 1.Fairley S. L., Donnelly P. M., Hanratty C. G., Walsh S. J. Images in cardiovascular medicine. Interventricular septal hematoma and ventricular septal defect after retrograde intervention for a chronic total occlusion of a left anterior descending coronary artery. Circulation. 2010;122(20):e518–e521. doi: 10.1161/circulationaha.110.976555. [DOI] [PubMed] [Google Scholar]
- 2.Brilakis E. S., Grantham J. A., Rinfret S., et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC: Cardiovascular Interventions. 2012;5(4):367–379. doi: 10.1016/j.jcin.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Murthy A., Singh A., Driesman M. H. Acquired coronary cameral fistula due to post stent balloon dilatation: dual coronary artery perforations into the left ventricle—what is the right treatment? World Journal of Cardiovascular Diseases. 2014;4(11):548–555. doi: 10.4236/wjcd.2014.411066. [DOI] [Google Scholar]
- 4.Lin T.-H., Wu D.-K., Su H.-M., et al. Septum hematoma: a complication of retrograde wiring in chronic total occlusion. International Journal of Cardiology. 2006;113(2):e64–e66. doi: 10.1016/j.ijcard.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Hashidomi H., Saito S. Dilation of the septal collateral artery and subsequent cardiac tamponade during retrograde percutaneous coronary intervention using a microcatheter for chronic total occlusion. Journal of Interventional Cardiology. 2011;24(1):73–76. doi: 10.1111/j.1540-8183.2010.00604.x. [DOI] [PubMed] [Google Scholar]
- 6.Higuchi Y., Fukamachi D., Fujii N., Nishida T., Hirayama A. Cardiogenic shock caused by a large sub-epicardial hematoma complicating percutaneous coronary intervention. Case Reports in Clinical Medicine. 2015;4(6):233–236. doi: 10.4236/crcm.2015.46045. [DOI] [Google Scholar]
- 7.Araki M., Murai T., Kanaji Y., et al. Interventricular septal hematoma after retrograde intervention for a chronic total occlusion of a right coronary artery: echocardiographic and magnetic resonance imaging—diagnosis and follow-up. Case Reports in Medicine. 2016;2016:6. doi: 10.1155/2016/8514068.8514068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joyal D., Thompson C. A., Grantham J. A., Buller C. E. H., Rinfret S. The retrograde technique for recanalization of chronic total occlusions: a step-by-step approach. JACC: Cardiovascular Interventions. 2012;5(1):1–11. doi: 10.1016/j.jcin.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Brilakis E. S., Banerjee S., Karmpaliotis D., et al. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry) JACC: Cardiovascular Interventions. 2015;8(2):245–253. doi: 10.1016/j.jcin.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 10.El Sabbagh A., Patel V. G., Jeroudi O. M., et al. Angiographic success and procedural complications in patients undergoing retrograde percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 3482 patients from 26 studies. International Journal of Cardiology. 2014;174(2):243–248. doi: 10.1016/j.ijcard.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Saeed B., Kandzari D. E., Agostoni P., et al. Use of drug-eluting stents for chronic total occlusions: a systematic review and meta-analysis. Catheterization and Cardiovascular Interventions. 2011;77(3):315–332. doi: 10.1002/ccd.22690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RCA CTO PCI was complicated by large interventricular septal hematoma “5.3 × 2.6 cm” at end systole (video 1), with coronary-ventricular fistula (5 mm in diameter) (Video 2). At 3 months post PCI a subsequent echocardiogram showed resolution of the hematoma (Video 3).