Abstract
Naoxintong (NXT) is an empirical formula based on the principle of traditional Chinese medicine, which has been approved by China Food and Drug Administration (CFDA) and is widely used for treatment of patients with cerebrovascular and cardiovascular diseases in China. The aim of this study is to investigate the protective mechanism of NXT on H9c2 cells (cardiogenic cell line) in response to H2O2. MTT, Western blot, and flow cytometry (FCM) methods were used to identify the protective effect of NXT extract on H2O2-induced H9c2 cells. Here we found that NXT extract significantly increased H9c2 cell viability and reduced H2O2-induced cell apoptosis and autophagy. More importantly, NXT inhibited H2O2-induced H9c2 cell apoptosis and autophagy by increasing PPARα protein levels. In contrast, silenced PPARα terminated NXT protective effect on H2O2-induced H9c2 cells. These findings suggest that NXT/PPARα signaling suppressed H2O2-induced H9c2 cell apoptosis and autophagy.
1. Introduction
Naoxintong (NXT) is an empirical formula based on the principle of traditional Chinese medicine, which has been approved by China Food and Drug Administration (CFDA, Z20025001) and widely used for treatment of patients with cerebrovascular and cardiovascular diseases in China. NXT contains sixteen kinds of traditional Chinese medicines including Astragalus membranaceus (Fish.) Bge. (Huangqi), Radix Paeoniae (Chishao), Salviae miltiorrhizae radix et rhizoma (Danshen), Radix Angelicae Sinensis (Danggui), Radix Paeoniae Rubra (Chishao), Rhizoma Ligustici Chuanxiong (Chuanxiong), Semen Persicae (Taoren), Achyranthes bidentata (Niuxi), Spatholobus stem (Jixueteng), Mulberry Twig (Sangzhi), Cassia Twig (Guizhi), Boswellia carteri (Ruxiang), and Commiphora myrrha Eng1 (Moyao) and animal medicines including hirudo nipponica Whitman (Shuizhi), Scorpio (Quanxie), and Pheretima (Dilong). NXT alleviates atherosclerosis involved in inhibition of iNOS expression and dendritic cell maturation [1, 2]. Clinical observation shows that NXT can increase antiplatelet effect and decrease subsequent major adverse cardiovascular events (MACE) in patients with cytochrome P450 2C19∗2 polymorphism undergoing percutaneous coronary intervention (PCI) [3]. Moreover, NXT reduces the development of diabetic retinopathy [4] and cardiomyocyte damage in response to reactive oxygen species (ROS) [5]; however, the mechanism of NXT on the cardiomyocyte apoptosis and autophagy in response to oxidative stress is still unclear.
Oxidative stress plays an important role in the pathogenesis of cardiovascular diseases. ROS is a main oxidative stress in cardiovascular damage. ROS is a byproduct of the normal metabolism of oxygen and has important roles in the cell signaling and homeostasis. But too much ROS production leads to oxidative stress [6]. The myocardial ischemia-reperfusion pathophysiologically produces ROS upon the restoration of blood flow [7]. ROS can be induced by hyperglycemia or H2O2 in vitro. At present, extracellular H2O2-induced H9c2 cell injury model is well documented [8]. Our previous study shows that the extract of Angelica sinensis and Ligusticum chuanxiong has protective effect against H2O2-induced endothelial cell damage [9] and inhibits rat vascular smooth muscle cell proliferation [10]. PPARα plays a protective role in cardiomyocyte damage [11], while the mechanism of NXT water extract protecting cardiomyocyte from H2O2-induced damage is not well known. Here we found that NXT activated PPARα signaling decreased H2O2-induced H9c2 cell apoptosis and autophagy.
2. Materials and Methods
2.1. NXT Water Extract
NXT was kindly provided by Xianyang Buchang Pharmaceutical Co. Ltd. (Shanxi, China). The NXT water extract was isolated from 5 g NXT powder incubating in 15 mL H2O 60°C for 6 hs, and then the solution was evaporated to 1 mL and filtered. The composition of NXT water extract was identified by UHPLC-Q-TOF Tandem Mass Spectrometry.
2.2. Cell Culture
The rat embryonic-heart derived H9c2 cell line (ATCC, CRL-1446). Cells were cultured at 37°C and 5% CO2 in 25 mL cell culture flask containing Dulbecco's Modified Eagle Medium (DMEM) (Gibco) supplemented with 10% (v/v) Fetal Bovine Serum (FBS) (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin sulfate. Cells were maintained in exponential phase of growth and were subcultured when they reached about 80% confluence at a split ratio of 1 : 3.
2.3. Cell Viability Assay
MTT assay was used to screen the cytotoxic or protective activity of the water extract compound from NXT. The cell viability was determined with MTT Cell Proliferation and Cytotoxicity Assay Kit (Sangon) according to the manufacturer's instructions. Cells (1 × 103 cells/well) were seeded in 96-well plates. After the corresponding treatment, cells were washed twice with PBS and then incubated with the MTT solution for 1.5 h at 37°C. Cells were then dissolved in 200 μL DMSO in 96-well plates. The absorbance of the reaction solution at 570 nm was measured with an ELISA-plate reader, Multidetection Microplate Reader Synergy H4 (BioTek).
2.4. Western Blot
Cells were seeded in 6-well plates and cultured as mentioned above. Cells were lysed in lysis buffer containing protease inhibitors. The lysate was sonicated (10 s) and then centrifuged at 13,000 ×g at 4°C for 10 min. Protein concentration in the supernatant was determined by the Pierce BCA Protein Assay Kit (Thermo). Samples were diluted (1 : 4) in protein loading buffer (67 mM Tris-HCl, pH 6.8, 30% glycerol, 2% SDS, and 0.01% bromophenol blue) and boiled for 5 min. The proteins were separated by SDS-PAGE using 10% acrylamide gels and transferred to a nitrocellulose membrane (PALL). After being blocked with 5% (w/v) nonfat dry milk dissolved in TBST buffer (10 mM Tris-HCl pH 7.5, 150 mM NaCl, and 0.1% Tween 20), the membranes were incubated with primary antibodies, anti-cascas3/9, anti-PARP-1, Bcl-2, Bax, Bad, and beta-actin (Santa Cruz Biotechnology) and LC3b antibody (Novus Biologicals). The membranes were washed three times with TBST buffer at room temperature and then incubated with HRP-conjugated secondary antibody for 1~2 h at room temperature. The secondary antibody was removed by washing three times with TBST buffer. The immunoreactive bands were visualized with ECL reagent.
2.5. Plasmids and Transfection
PPARα shRNA plasmids (GV248 vector) were purchased from GeneCHEM (China). Plasmids were transfected by turboFect transfection reagent according to the manufacturer's instructions (Thermo Scientific).
2.6. Flow Cytometry (FCM) Assay
Cells were harvested by trypsinization and centrifuged at 400 ×g at 4°C for 10 min. For each sample, 106 cells were collected. The cells were treated with Annexin V-FITC Apoptosis Detection Kit according to the manufacturer's instructions (BD Biosciences). The cells flow through the FCM at about 100–1000 cells per second.
2.7. Statistical Analysis
Data are expressed as the mean ± SEM. Statistical comparison was carried out with Student's t-test or one-way analysis of variance (ANOVA).
3. Results
3.1. Determination of Composition in NXT Water Extract by UHPLC-Q-TOF
The UHPLC-Q-TOF results show that the main composition of NXT water extract is paeoniflorin (80.4%), salvianolic acid B (10.1%), trihydroxybenzoic acid (4.6%), chlorogenic acid (1.8%), and ferulic acid (1.5%). The relative concentration of the main compositions was calculated (Table 1).
Table 1.
Diverse composition in NXT water extract identified by UHPLC-Q-TOF.
| Number | Peak number | Identification | Formula | MS | Relative concentration (%) |
|---|---|---|---|---|---|
| 1 | 35 | Paeoniflorin | C23H28O11 | 480.1632 | 80.42 |
| 2 | 55 | Salvianolic acid B | C36H30O16 | 718.1534 | 10.13 |
| 3 | 11 | Gallic acid | C7H6O5 | 170.0215 | 4.59 |
| 4 | 22 | Chlorogenic acid | C16H18O9 | 354.0951 | 1.79 |
| 5 | 39 | Ferulic Acid | C10H10O4 | 194.0579 | 1.53 |
| 6 | 25 | Hydroxysafflor yellow A | C27H32O16 | 612.1690 | 0.80 |
| 7 | 49 | Rosmarinic acid | C18H16O8 | 360.0845 | 0.22 |
| 8 | 16 | 5-Hydroxymethylfurfural | C6H6O3 | 126.0317 | 0.17 |
| 9 | 47 | 1,5-Di-O-caffeoylquinic acids | C25H24O12 | 516.1268 | 0.14 |
| 10 | 46 | 3,5-Di-O-caffeoylquinic acids | C25H24O12 | 516.1268 | 0.11 |
| 11 | 48 | Kaempferol-3-O-rutinoside | C27H30O15 | 594.1585 | 0.07 |
| 12 | 51 | Lithospermate | C27H22O12 | 538.1111 | 0.03 |
3.2. NXT Increases Cell Viability in Response to H2O2
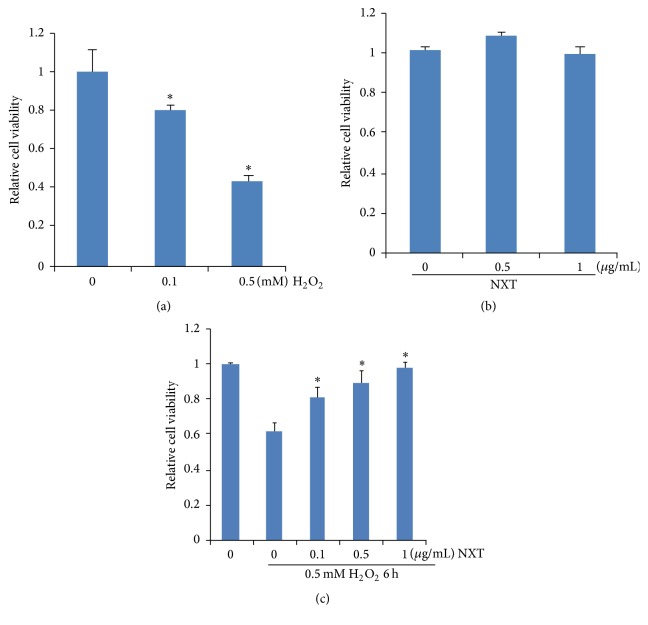
H9c2 cells were treated with H2O2. The results show that H2O2 dose dependently reduced cell viability (Figure 1(a)). Further analysis shows that the NXT extract had no cytotoxicity effect on H9c2 cells (Figure 1(b)). To assay the protective effect of NXT on H9c2 cells, cells were pretreated with different dose (0.1~1.0 μg/mL) of NXT for 12 h. After that cells were induced with H2O2 for 6 h. The results show that NXT significantly increased cell survival in response to H2O2 (Figure 1(c)).
Figure 1.
NXT extract increases cell viability. (a) H9c2 cells were treated with H2O2 as indicated for 6 h. Cell viability was assayed by MTT. Results are expressed as means ± SEM (n = 5). ∗ P < 0.05 versus no H2O2 treatment. (b) H9c2 cells were treated with NXT extract as indicated for 12 h. Cell viability was assayed by MTT. (c) H9c2 cells were pretreated with or without NXT extract for 12 h, and then cells were treated with 0.5 mM H2O2 for 6 h as indicated. Cell viability was assayed by MTT. Results are expressed as means ± SEM (n = 5). ∗ P < 0.05 versus no NXT treatment.
3.3. NXT Increases Antiapoptotic Protein Expression in Response to H2O2
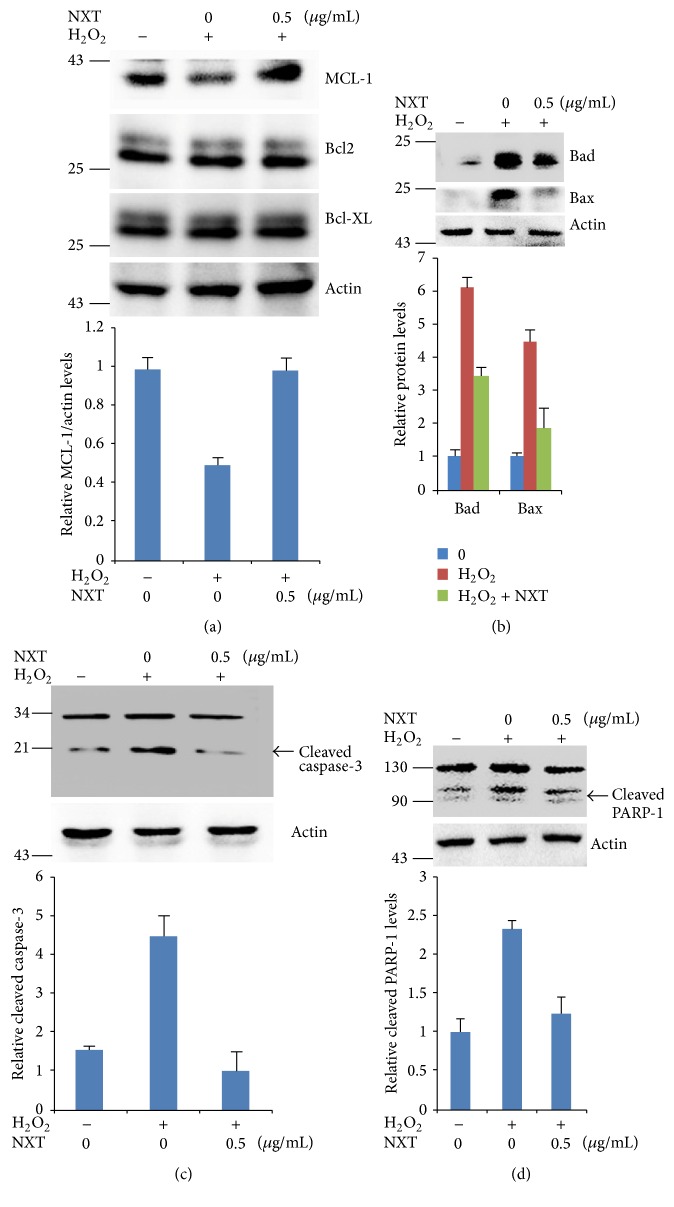
The antiapoptotic MCL-1 protein prevents the release of mitochondrial contents against caspase-3 activation [12, 13]. We next detected the effect of NXT on the cell apoptotic signaling. The results show that NXT extract significantly increased antiapoptotic MCL-1 protein levels (Figure 2(a)). In contrast, NXT decreased Bad and Bax proapoptotic protein levels (Figure 2(b)). H2O2 induces cell apoptosis through activate caspase-3 [12, 13]. Our results show that NXT extract reversed this apoptotic signaling by inhibiting caspase-3 activation (Figure 2(c)); subsequently, cleaved PARP-1 was decreased in H9c2 cells treated with NXT extract (Figure 2(d)). These findings suggest that NXT inhibited the H9c2 cell apoptosis through inhibiting activation of caspase-3/PARP-1 signaling pathway.
Figure 2.
NXT increases antiapoptotic protein levels. (a) H9c2 cells were pretreated with or without 0.5 μg/mL NXT for 12 h, and then the cells were treated with 200 μM H2O2 for 6 h. Cell lysates were subjected to Western blot. (b) H9c2 cells were pretreated with or without 0.5 μg/mL NXT for 12 h, and then the cells were treated with 200 μM H2O2 for 6 h. Cell lysates were subjected to Western blot as indicated. (c) H9c2 cells were pretreated with or without 0.5 μg/mL NXT for 12 h, and then the cells were treated with 200 μM H2O2 for 6 h. Cell lysates were subjected to Western blot as indicated. (d) H9c2 cells were pretreated with or without 0.5 μg/mL NXT for 12 h, and then the cells were treated with 200 μM H2O2 for 6 h. Cell lysates were subjected to Western blot. Data are triplicates from three independent experiments.
3.4. NXT Decreases Cell Apoptosis
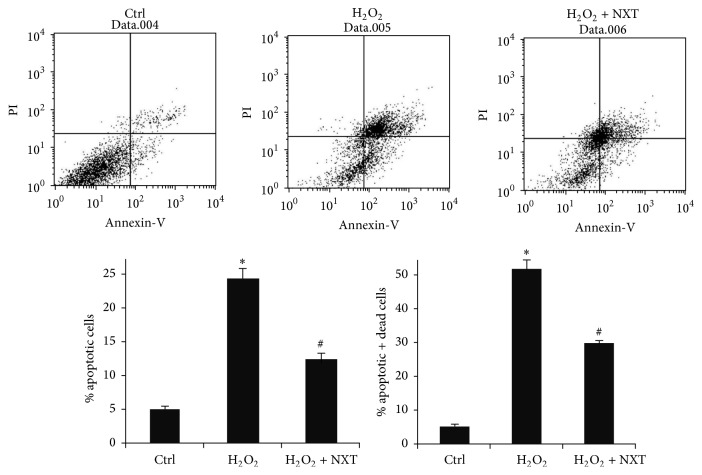
To detect NXT protective effect on H9c2 cells in response to H2O2, flow cytometry analysis was performed to detect the effect of NXT on H2O2-induced H9c2 cell apoptosis. The results show that H2O2 significantly induced cell apoptosis, but NXT reversed this event (Figure 3), suggesting that NXT extract inhibited H2O2-induced H9c2 cell apoptosis associated with increased antiapoptotic protein levels.
Figure 3.
NXT extract inhibits H2O2-induced cell apoptosis. H9c2 cells were pretreated with or without 0.5 μg/mL NXT for 12 h, and then the cells were treated with 200 μM H2O2 for 6 h. Cell apoptosis was assayed by FCM. Apoptotic cells or apoptotic and death cells were quantified. ∗ P < 0.05 Ctrl versus H2O2 only treatment; # P < 0.05 H2O2 versus NXT treatment. Data are triplicates from three independent experiments.
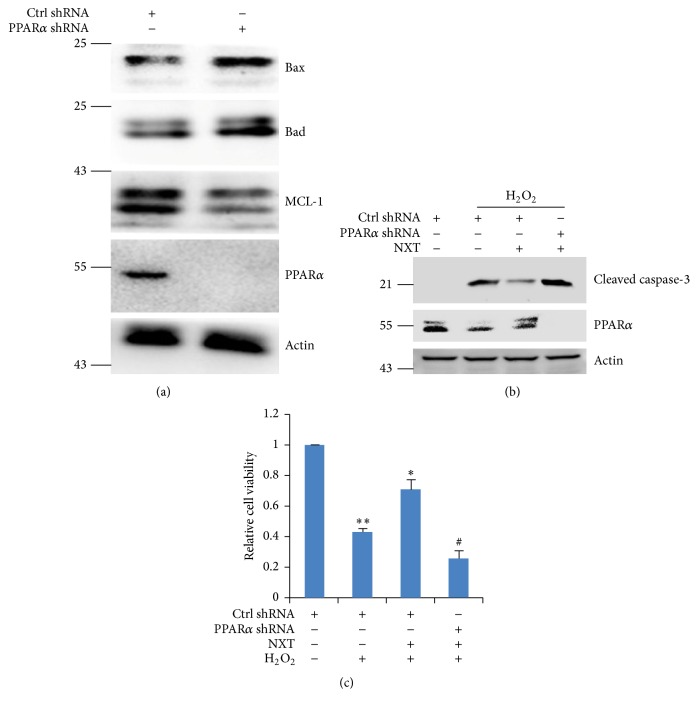
3.5. NXT Inhibits Cell Apoptosis by Increasing PPARα Expression
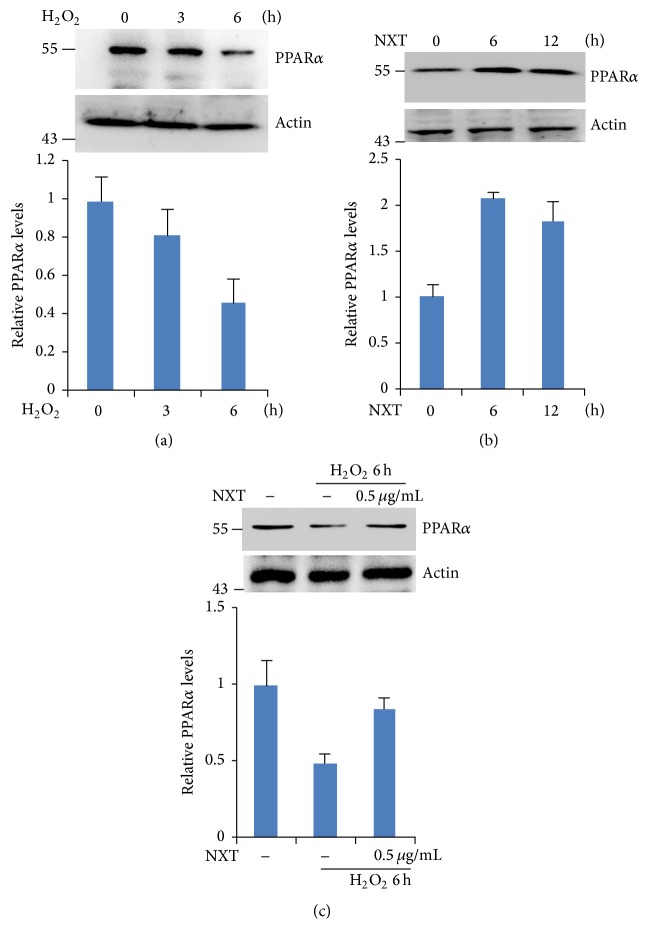
Our data have demonstrated that NXT significantly decreased H9c2 cell apoptosis in response to H2O2, which was involved in increasing antiapoptotic protein expression. As nuclear receptor, PPAR is a critical regulator of inflammation, adipocyte differentiation, and glucose homeostasis [3, 14–17]. Other reports show that PPARα protects against cardiomyocyte damage [11, 18]. Here we found that H2O2 significantly reduced PPARα protein levels (Figure 4(a)), but NXT increased PPARα protein expression levels (Figure 4(b)). More importantly, cells pretreated with NXT did not reduce PPARα protein levels in response to H2O2 (Figure 4(c)). Further analysis shows that PPARα silenced H9c2 cells increased proapoptotic protein levels (Bax and Bad) and decreased antiapoptotic protein MCL-1 protein levels (Figure 5(a)). Consistent with this, PPARα silenced H9c2 cells led to increased activation of caspase-3 in response to H2O2 (Figure 5(b)). As expected, PPARα silence in H9c2 cells did not increase cell survival in response to H2O2 (Figure 5(c)). These findings show that NXT activated PPARα singling leading to inhibition of H2O2-induced H9c2 cell damage.
Figure 4.
NXT extract increases PPARα expression. (a) H9c2 cells were treated with 200 μM H2O2 as indicated time. Cell lysates were subjected to Western blot. (b) H9c2 cells were treated with 0.5 μg/mL NXT as indicated time. Cell lysates were subjected to Western blot. (c) H9c2 cells were treated with or without 0.5 μg/mL NXT for 12 h. After that, cells were treated with 200 μM H2O2 for 6 h. Cell lysates were subjected to Western blot. Data are triplicates from three independent experiments.
Figure 5.
Silenced PPARα decreases NXT protective effect on H9c2 cells. (a) H9c2 cells were transfected with control shRNA or PPARα shRNA for 24 h. Cell lysates were subjected to Western blot. Data are triplicates from three independent experiments. (b) H9c2 cells were transfected with control shRNA or PPARα shRNA for 24 h. Cells were pretreated with or without 0.5 μg/mL NXT for 12 h, and then cells were treated with 200 μM H2O2 for 6 h. Cell lysates were subjected to Western blot. Data are triplicates from three independent experiments. (c) H9c2 cells were transfected with control shRNA or PPARα shRNA for 24 h. Cells were pretreated with or without 0.5 μg/mL NXT for 12 h, and then cells were treated with 200 μM H2O2 for 6 h. Cell viability was assayed by MTT. Results are expressed as means ± SEM (n = 5). ∗∗ P < 0.05 versus control (no treatment); ∗ P < 0.05 versus only H2O2 treatment. # P < 0.05 versus only H2O2 treatment.
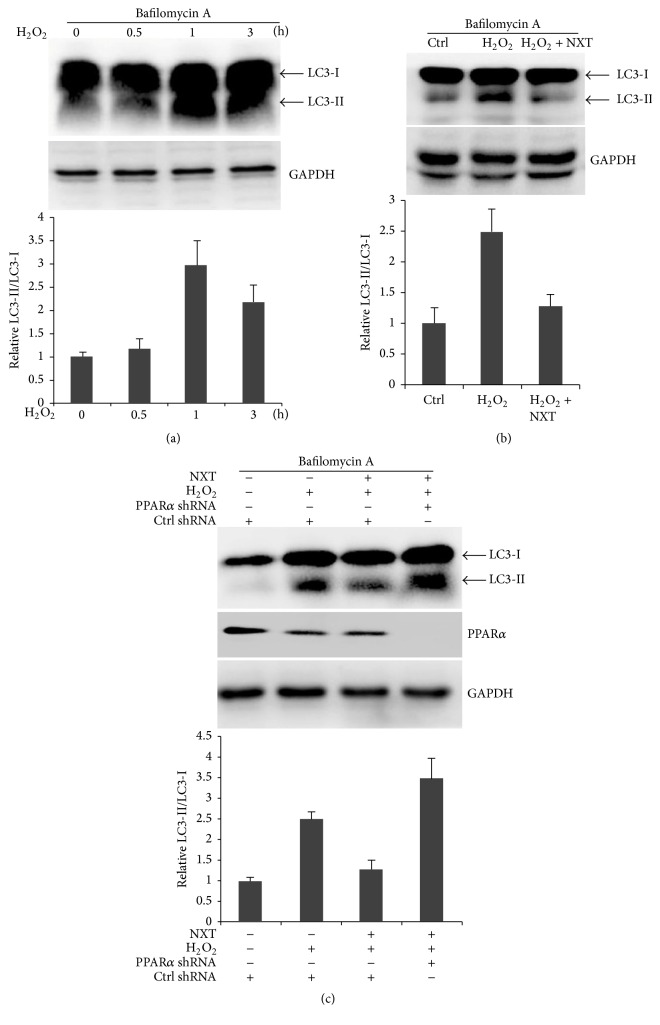
3.6. NXT Reduces Cell Autophagy in Response to H2O2
Mammalian cell death has three types: apoptosis, necrosis, and autophagy [12]. We further detected whether H2O2 would induce cardiomyocyte autophagy. As expected, H2O2 significantly increased processing of LC3-I to LC3-II conversion (Figure 6(a)), suggesting that H2O2 induced H9c2 cell autophagy. Further analysis shows that H2O2-induced cell autophagy was reduced by NXT treatment (Figure 6(b)), suggesting that NXT decreased H2O2-induced H9c2 cell autophagy. As PPARα inhibited H2O2-induced cell apoptosis, further analysis shows that PPARα silence led to increased H9c2 cell autophagy (Figure 6(c)), suggesting that PPARα suppressed cell autophagy in response to H2O2.
Figure 6.
NXT/PPARα signaling inhibits cell autophagy. (a) H9c2 cells were treated with 200 μM H2O2 as indicated time. Cell lysates were subjected to Western blot. (b) H9c2 cells were treated with or without 0.5 μg/mL NXT for 12 h. After that, cells were treated with 200 μM H2O2 for 6 h. Cell lysates were subjected to Western blot. (c) H9c2 cells were transfected with control shRNA or PPARα shRNA for 24 h. Cells were pretreated with or without 0.5 μg/mL NXT for 12 h, and then cells were treated with 200 μM H2O2 for 6 h. Cell lysates were subjected to Western blot. Cells were treated with 50 μM bafilomycin A for 1 h before cell lysis. Data are triplicates from three independent experiments.
4. Discussions
Naoxintong (NXT) is an empirical formula based on the principle of traditional Chinese medicine, which has been approved by China Food and Drug Administration (CFDA) and is widely used for treatment of patients with cerebrovascular and cardiovascular diseases in China. Increasing evidences show that NXT alleviates atherosclerosis involved in reducing expression of iNOS and NO level in the vessel wall [2]. In the mice model of atherosclerosis, NXT suppresses atherosclerosis through lipid-lowering and inhibition of dendritic cell maturation [1]. Clinical observations show that NXT can increase the antiplatelet effect and decrease subsequent major adverse cardiovascular events (MACE) in patients with cytochrome P450 2C19∗2 polymorphism undergoing percutaneous coronary intervention (PCI) [3]. In addition to that, NXT reduces the development of diabetic retinopathy involved in inhibition of the expression of CAS-3, MMP-2/9, and TNF-α [4]. Moreover, NXT increases the effect on the catalytic activities of drug-metabolising CYP2C19 enzyme [19]. Although ethanol extraction of NXT reduces cardiomyocyte cell damage [5], the water soluble components of NXT on H2O2-induced cardiomyocyte cell apoptosis are still unclear. Here we found that the NXT water extract has significantly protective effect on H9c2 cells in response to H2O2 treatment. To detect the possible component of the NXT water extract, the UHPLC-Q-TOF analysis was performed. Interestingly, the main composition of NXT water extract is paeoniflorin (80.4%), salvianolic acid B (10.1%), trihydroxybenzoic acid (4.6%), chlorogenic acid (1.8%), and ferulic acid (1.5%) (Table 1). These findings suggest that paeoniflorin may be the main or critical effective component for cardiovascular diseases, which needs to be further determined.
MCL-1 is the antiapoptotic proteins, but Bad and Bax are proapoptotic proteins, and MCL-1 inhibits caspase-3-mediated cell apoptotic signaling pathway [20, 21]. It is well known that activated caspase-3 results in cleavage of PARP-1 leading to cell apoptosis [22]. Some natural compounds from traditional medicine increase Bcl-2 family protein levels to inhibit cell apoptosis [23]. Our data show that NXT increased MCL-1 protein levels and decreased activation of caspase-3 and PARP-1 in H2O2-treated H9c2 cells. These results show that NXT protected the cells against apoptosis by reducing caspase-3/PARP-1 signaling pathway. The increased antiapoptotic Bcl-2 family proteins (MCL-1) and decreased proapoptotic proteins (Bad and Bax) suggest that NXT extract reduced cell apoptosis in response to H2O2. FCM assay further demonstrated that NXT extract decreased H9c2 cell apoptosis. MCL-1 is transmembrane protein in the mitochondria; however, Bax and Bak are two nuclear-encoded proteins that are able to pierce the mitochondrial outer membrane to induce apoptosis [24]. Based on our findings, mitochondria may be one of the targets of NXT water extract.
As nuclear receptor, PPARs are the critical regulator of inflammation, adipocyte differentiation, and glucose homeostasis [3, 14–17]. Other reports show that PPARα protects against cardiomyocyte damage [11, 18]. Here we found that NXT significantly increased PPARα expression levels in response to H2O2. In contrast, silenced PPARα terminated the inhibition of NXT on H2O2-induced H9c2 cell damage involved in PPARα mediated the MCL-1 antiapoptotic protein expression.
Autophagy delivers cytoplasmic materials or organelles into lysosomes for degradation, which is also a progress of nutrient recycling [25]. Especially in response to starvation stress, autophagy increases cell survival; however, long-term periods autophagy without new nutrients replenishment leads to digestion of all available substrates and death (autophagy-associated cell death) [12, 25]. Here we found that H2O2 significantly induced H9c2 cell autophagy. In contrast, NXT reversed this event. More importantly, NXT reduced H9c2 cell autophagy in a PPARα dependent manner, suggesting that NXT/PPARα signaling suppressed H9c2 cell autophagy.
5. Conclusion
NXT water extract inhibited H9c2 cell apoptosis and autophagy by increasing PPARα expression leading to increased H9c2 cell viability.
Acknowledgments
The authors acknowledge the financial support from the project supported by the National Natural Science Foundation of China (81173592), Program for New Century Excellent Talents in University of Ministry of Education of China (NCET-13-0935), Program of International S&T Cooperation project of China (2015DFA30430), and the Program for Changjiang Scholars and Innovative Research Team in University, PCSIRT (IRT1276).
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Huimin Xu and Jianhua Jin equally contributed to this work.
References
- 1.Zhao J., Zhu H., Wang S., et al. Naoxintong protects against atherosclerosis through lipid-lowering and inhibiting maturation of dendritic cells in LDL receptor knockout mice fed a high-fat diet. Current Pharmaceutical Design. 2013;19(33):5891–5896. doi: 10.2174/1381612811319330008. [DOI] [PubMed] [Google Scholar]
- 2.Zhong X.-N., Wang H.-H., Lu Z.-Q., et al. Effects of naoxintong on atherosclerosis and inducible nitric oxide synthase expression in atherosclerotic rabbit. Chinese Medical Journal. 2013;126(6):1166–1170. doi: 10.3760/cma.j.issn.0366-6999.20122895. [DOI] [PubMed] [Google Scholar]
- 3.Xu H., You M., Shi H., Hou Y. Ubiquitin-mediated NFκB degradation pathway. Cellular and Molecular Immunology. 2014;12(6):653–655. doi: 10.1038/cmi.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivennikov S., Karin E., Terzic J., et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang F., Huang B., Zhao Y., et al. BNC protects H9c2 cardiomyoblasts from H2O2-induced oxidative injury through ERK1/2 signaling pathway. Evidence-Based Complementary and Alternative Medicine. 2013;2013:12. doi: 10.1155/2013/802784.802784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Marchi E., Baldassari F., Bononi A., Wieckowski M. R., Pinton P. Oxidative stress in cardiovascular diseases and obesity: role of p66Shc and protein kinase C. Oxidative Medicine and Cellular Longevity. 2013;2013:11. doi: 10.1155/2013/564961.564961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zweier J. L., Talukder M. A. H. The role of oxidants and free radicals in reperfusion injury. Cardiovascular Research. 2006;70(2):181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Turner N. A., Xia F., Azhar G., Zhang X., Liu L., Wei J. Y. Oxidative stress induces DNA fragmentation and caspase activation via the c-Jun NH2-terminal kinase pathway in H9c2 cardiac muscle cells. Journal of Molecular and Cellular Cardiology. 1998;30(9):1789–1801. doi: 10.1006/jmcc.1998.0743. [DOI] [PubMed] [Google Scholar]
- 9.Hou Y. Z., Zhao G. R., Yang J., Yuan Y. J., Zhu G. G., Hiltunen R. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sciences. 2004;75(14):1775–1786. doi: 10.1016/j.lfs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Hou Y.-Z., Zhao G.-R., Yuan Y.-J., Zhu G.-G., Hiltunen R. Inhibition of rat vascular smooth muscle cell proliferation by extract of Ligusticum chuanxiong and Angelica sinensis . Journal of Ethnopharmacology. 2005;100(1-2):140–144. doi: 10.1016/j.jep.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Barreto-Torres G., Hernandez J. S., Jang S., et al. The beneficial effects of AMP kinase activation against oxidative stress are associated with prevention of PPARα-cyclophilin D interaction in cardiomyocytes. American Journal of Physiology—Heart and Circulatory Physiology. 2015;308(7):H749–H758. doi: 10.1152/ajpheart.00414.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotchkiss R. S., Strasser A., McDunn J. E., Swanson P. E. Cell death. The New England Journal of Medicine. 2009;361(16):1570–1583. doi: 10.1056/nejmra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou Y., Gao F., Wang Q., et al. Bcl2 impedes DNA mismatch repair by directly regulating the hMSH2-hMSH6 heterodimeric complex. The Journal of Biological Chemistry. 2007;282(12):9279–9287. doi: 10.1074/jbc.m608523200. [DOI] [PubMed] [Google Scholar]
- 14.Hou Y., Gao J., Xu H., et al. PPARγ E3 ubiquitin ligase regulates MUC1-C oncoprotein stability. Oncogene. 2014;33:5619–5625. doi: 10.1038/onc.2013.504. [DOI] [PubMed] [Google Scholar]
- 15.Hou Y., Moreau F., Chadee K. PPARγ is an E3 ligase that induces the degradation of NFκB/p65. Nature Communications. 2012;3, article 1300 doi: 10.1038/ncomms2270. [DOI] [PubMed] [Google Scholar]
- 16.You M., Yuan S., Shi J., Hou Y. PPARδ signaling regulates colorectal cancer. Current Pharmaceutical Design. 2015;21(21):2956–2959. doi: 10.2174/1381612821666150514104035. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z., Xu Y., Xu Q., Hou Y. PPARγ against tumors by different signaling pathways. Onkologie. 2013;36(10):598–601. doi: 10.1159/000355328. [DOI] [PubMed] [Google Scholar]
- 18.Yeh C.-H., Chen T.-P., Lee C.-H., Wu Y.-C., Lin Y.-M., Lin P. J. Cardiomyocytic apoptosis following global cardiac ischemia and reperfusion can be attenuated by peroxisome proliferator-activated receptor α but not γ activators. Shock. 2006;26(3):262–270. doi: 10.1097/01.shk.0000225863.56714.96. [DOI] [PubMed] [Google Scholar]
- 19.Chen H., Zhang Y., Wu X., Li C., Wang H. In vitro assessment of cytochrome P450 2C19 potential of Naoxintong. Evidence-Based Complementary and Alternative Medicine. 2012;2012:6. doi: 10.1155/2012/430262.430262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hata A. N., Engelman J. A., Faber A. C. The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discovery. 2015;5(5):475–487. doi: 10.1158/2159-8290.cd-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou F. F., Yang Y., Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. The FEBS Journal. 2011;278(3):403–413. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- 22.Gross A., McDonnell J. M., Korsmeyer S. J. BCL-2 family members and the mitochondria in apoptosis. Genes & Development. 1999;13(15):1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 23.Wu P.-F., Zhang Z., Wang F., Chen J.-G. Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacologica Sinica. 2010;31(12):1523–1531. doi: 10.1038/aps.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay J., Esposti M. D., Gilmore A. P. Bcl-2 proteins and mitochondria—specificity in membrane targeting for death. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research. 2011;1813(4):532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Kaur J., Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nature Reviews Molecular Cell Biology. 2015;16(8):461–472. doi: 10.1038/nrm4024. [DOI] [PubMed] [Google Scholar]