Abstract
Subcortical structures, which include the basal ganglia and parts of the limbic system, have key roles in learning, motor control and emotion, but also contribute to higher-order executive functions. Prior studies have reported volumetric alterations in subcortical regions in schizophrenia. Reported results have sometimes been heterogeneous, and few large-scale investigations have been conducted. Moreover, few large-scale studies have assessed asymmetries of subcortical volumes in schizophrenia. Here, as a work completely independent of a study performed by the ENIGMA consortium, we conducted a large-scale multisite study of subcortical volumetric differences between patients with schizophrenia and controls. We also explored the laterality of subcortical regions to identify characteristic similarities and differences between them. T1-weighted images from 1680 healthy individuals and 884 patients with schizophrenia, obtained with 15 imaging protocols at 11 sites, were processed with FreeSurfer. Group differences were calculated for each protocol and meta-analyzed. Compared with controls, patients with schizophrenia demonstrated smaller bilateral hippocampus, amygdala, thalamus and accumbens volumes as well as intracranial volume, but larger bilateral caudate, putamen, pallidum and lateral ventricle volumes. We replicated the rank order of effect sizes for subcortical volumetric changes in schizophrenia reported by the ENIGMA consortium. Further, we revealed leftward asymmetry for thalamus, lateral ventricle, caudate and putamen volumes, and rightward asymmetry for amygdala and hippocampal volumes in both controls and patients with schizophrenia. Also, we demonstrated a schizophrenia-specific leftward asymmetry for pallidum volume. These findings suggest the possibility of aberrant laterality in neural pathways and connectivity patterns related to the pallidum in schizophrenia.
Introduction
Patients with schizophrenia have volumetric abnormalities in both cortical and subcortical brain regions, which are closely related to characteristic symptoms and behaviors.1, 2 Patients with schizophrenia demonstrate both positive and negative symptoms as well as cognitive impairment, and many of these characteristic symptoms have been related to structural brain alterations and disrupted interregional connections.3 Subcortical structures, which include the basal ganglia and parts of the limbic system, are integrally involved in learning and memory, as well as many primitive functions such as motor control, attention and emotion.4, 5 Further, they also have important roles in higher-order executive functions including inhibitory control and working memory through their structural and functional connectivity with prefrontal cortices.6, 7
Prior studies have revealed volumetric alterations in the subcortical regions in schizophrenia. For example, many studies report bilateral hippocampal volume reductions in patients with schizophrenia.8, 9, 10 Likewise, on average, individuals with schizophrenia demonstrate lower volumes in the left,8 right11 or both thalamic regions,12, 13, 14 and decreased left thalamic volume has even been reported in individuals experiencing first-episode psychosis.14 On the other hand, results of prior studies on basal ganglia volume in schizophrenia have been somewhat heterogeneous. Stegmayer et al.15 reported decreased gray matter density in the right ventral striatum in patients with schizophrenia having severe emotional dysregulation, while Ha et al.16 showed increased gray matter concentrations in the right striatum in schizophrenia. Perhaps owing to differences in antipsychotic medications or duration of illness, the right putamen volume has been reported as being increased in schizophrenia in one study,8 while another study demonstrated decreased gray matter volume in the left putamen in recurrently ill patients with schizophrenia.14 Thus, individual studies may reach different conclusions, suggesting added value of multi-cohort meta-analyses.
Brain lateralization is considered highly related to human psychological and behavioral characteristics.17 Initially, it was widely believed that the left hemisphere supported language and logical thinking, while the right hemisphere was involved to a greater extent in creativity and intuition.18 In recent years, anatomical and functional brain lateralization has been assessed using brain-mapping approaches,17 and various investigations have revealed the lateralization of subcortical regional volumes in healthy subjects. For example, rightward asymmetry has been commonly reported for the hippocampus19, 20, 21, 22 and amygdala.20, 21, 22 In contrast, results on lateralization of regional volumes for the thalamus,21, 23 lateral ventricles,24, 25 caudate nuclei,26, 27, 28, 29, 30, 31 putamen,30, 31 globus pallidus30, 31 and nucleus accumbens21 are so far controversial and non-conclusive.
Altered structural lateralization in schizophrenia has recently been investigated. Many studies have shown differences in hemispheric asymmetries of cortical volume between patients with schizophrenia and controls.32 In addition, changes in subcortical structural asymmetry in schizophrenia have also been demonstrated. For example, exaggerated rightward asymmetry of the thalamus,13 similar but exaggerated rightward asymmetry of the hippocampus19 and reduced rightward asymmetry of the amygdala21 have been reported. However, few comprehensive studies have been conducted and little is known on the lateralization of subcortical structures in schizophrenia.
Conventional meta-analyses have been used to assess the overall evidence for structural alterations in schizophrenia.12, 33, 34, 35 However, this method has some disadvantages. Studies included in a conventional meta-analysis have varying designs, and often have different populations, conditions and analysis methods.36 Thus, the overall effects may be weakened.
Multi-centered large-scale research is very important for elucidating the neural basis of psychiatric disorders such as schizophrenia, as some single studies may have only small effects, or the confidence interval on the magnitude of the effects may be wide in smaller cohorts. In this context, the Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) Consortium was organized by neuroscience and genetics researchers from worldwide, to work together on imaging genetics research for psychiatric disorders. Several reports have already been published by the consortium.37, 38, 39, 40, 41, 42, 43 Recently, van Erp et al.44 published an original article about the analysis of brain morphology in schizophrenia. They identified subcortical regional volumes that are different between patients with schizophrenia and controls, across 15 cohorts, and ordered them by effect sizes. Patients with schizophrenia showed smaller-than-normal volumes in the hippocampus, amygdala, thalamus and accumbens as well as smaller-than-normal intracranial volume (ICV). On the other hand, these patients showed larger-than-normal pallidum and lateral ventricle volumes.
In our current study, as a research project by a Japanese consortium, the COCORO (Cognitive Genetics Collaborative Research Organization), we conducted a multisite large-scale cross-sectional investigation of subcortical regional volumetric differences between patients with schizophrenia and healthy subjects by using meta-analytic methods similar to those of the van Erp et al.44 study. As a new line of work, we also separately assessed group differences in each hemispheric regional volume. Further, we explored the asymmetry of subcortical regional volumes both in healthy subjects and in patients with schizophrenia, and investigated possible alterations of subcortical asymmetry in schizophrenia. The first aim of our study was to investigate whether the results of the work by van Erp et al. could be replicated. The second aim was to elucidate characteristic similarities and differences in subcortical volumetric lateralization between patients with schizophrenia and healthy individuals.
Materials and methods
Sample subjects and imaging
A total of 3208 individuals took part in the current large-scale cross-sectional cohort project—the overall cohort consisted of 2091 healthy controls and 1117 patients with schizophrenia from 11 sites in COCORO. Participants did not overlap between the van Erp et al.44 study and our current study. Subject inclusion and exclusion criteria by site are described in Supplementary Method 1. Written informed consent was obtained from each subject before participation. This procedure was approved by each local institutional review board. Each participating site conducted magnetic resonance imaging (MRI) scanning and obtained T1-weighted images with one or more scanner(s) and imaging protocol(s). A combination of one scanner and one imaging protocol was defined as one ‘protocol', and 26 protocols were registered in the current study. Detailed imaging parameters for each protocol are shown in Supplementary Table 1. For accurate exploration of brain hemispheric characteristics including lateralization, we investigated the possibility of left–right errors derived from MRI data format conversion. The details are described in Supplementary Method 2.
Imaging processing, quality control and protocol selection
The procedures described in this section are summarized in Figure 1. In the first quality control step, original T1-weighted images were checked by visual inspection. We excluded images with insufficient brain coverage (field-of-view problem), those with low signal-to-noise ratios or any artifacts (for example, motion artifacts and magnetic susceptibility artifacts) and those with any abnormal organic findings (for example, large cerebellar cysts and cavum septum pellucidum). Through this process, 306 healthy subjects and 167 patients with schizophrenia were excluded. Next, T1-weighted imaging data that had passed the first quality control step were processed with FreeSurfer software version 5.3 (http://surfer.nmr.mgh.harvard.edu), as described previously.44 Through this procedure, we obtained images of subcortical segmentation and regional volumes (for the bilateral lateral ventricles, thalamus, caudate, putamen, globus pallidus, hippocampus, amygdala, accumbens and the ICV). Fourteen healthy controls and three patients with schizophrenia were rejected owing to the failure of FreeSurfer processing. After that, two independent researchers visually inspected each segmentation image to exclude images with poor parcellation. One patient with schizophrenia was excluded by this approach. A total of 14 healthy controls and four patients with schizophrenia were excluded in the second quality control step (the quality control of FreeSurfer analysis). After the two quality control steps (inspection of original MRI data and screening for successful FreeSurfer processing), 1771 healthy subjects and 946 patients with schizophrenia remained for possible analysis in the study (available data remained as shown in Supplementary Table 2). Among potential candidates, protocols with less than 50 total participants and those with less than 10 participants in either diagnostic group were excluded before the following meta-analysis, to minimize undue effects of very small groups. Finally, 1680 healthy individuals and 884 patients with schizophrenia from 15 protocols remained for inclusion (Table 1).
Figure 1.
Flow chart of protocol selection into the current meta-analytic study. HC, healthy control; MRI, magnetic resonance imaging; SZ, schizophrenia.
Table 1. Basic characteristics of the included protocols.
| Protocol name |
HC |
SZ |
Vendor | MFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Age |
Age |
|||||||||||
| N | Male | Female | Mean | s.d. | N | Male | Female | Mean | s.d. | |||
| 01. Osaka_A | 404 | 187 | 217 | 35.4 | 12.6 | 136 | 80 | 56 | 36.4 | 12.6 | GE | 1.5 T |
| 02. Tokyo_A | 233 | 143 | 90 | 34.4 | 11.5 | 102 | 57 | 45 | 33.3 | 9.5 | GE | 1.5 T |
| 03. Osaka_B | 237 | 131 | 106 | 31.4 | 13.2 | 79 | 35 | 44 | 33.1 | 12.0 | GE | 3.0 T |
| 04. Toyama_A | 118 | 63 | 55 | 25.9 | 6.3 | 117 | 60 | 57 | 26.7 | 6.3 | Siemens | 1.5 T |
| 05. Kyoto | 111 | 63 | 48 | 31.9 | 10.6 | 85 | 45 | 40 | 35.9 | 9.2 | Siemens | 3.0 T |
| 06. Hokkaido | 35 | 15 | 20 | 48.3 | 12.0 | 117 | 43 | 74 | 35.1 | 12.4 | Siemens | 1.5 T |
| 07. Tokyo_B | 83 | 57 | 26 | 28.7 | 5.5 | 45 | 30 | 15 | 31.2 | 9.0 | GE | 3.0 T |
| 08. Yamaguchi | 91 | 18 | 73 | 49.5 | 16.1 | 28 | 5 | 23 | 55.5 | 8.0 | Siemens | 1.5 T |
| 09. Nagoya_A | 68 | 38 | 30 | 37.7 | 9.8 | 43 | 26 | 17 | 43.6 | 10.6 | Siemens | 3.0 T |
| 10. Kyushu_A | 78 | 36 | 42 | 33.2 | 11.9 | 31 | 9 | 22 | 39.4 | 9.5 | Philips | 3.0 T |
| 11. Kanazawa-med | 53 | 32 | 21 | 35.9 | 11.7 | 34 | 14 | 20 | 35.9 | 9.5 | Siemens | 3.0 T |
| 12. UOEH | 54 | 36 | 18 | 36.6 | 12.0 | 15 | 6 | 9 | 28.0 | 13.4 | GE | 3.0 T |
| 13. Yaesu_A | 47 | 17 | 30 | 38.8 | 9.0 | 11 | 6 | 5 | 38.4 | 4.2 | Philips | 3.0 T |
| 14. Tokyo_C | 41 | 25 | 16 | 28.8 | 7.5 | 15 | 8 | 7 | 30.5 | 12.7 | GE | 3.0 T |
| 15. Kyushu_B | 27 | 11 | 16 | 34.6 | 13.8 | 26 | 14 | 12 | 35.5 | 11.0 | Philips | 3.0 T |
| Total | 1680 | 872 | 808 | 34.5 | 12.7 | 884 | 438 | 446 | 34.8 | 11.7 | ||
Abbreviations: HC, healthy control; MFS, magnetic field strength; SZ, schizophrenia.
The mean and standard deviation of age across protocols for healthy controls were 34.5±12.7 and for patients were 34.8±11.7 years. As for sex ratio, 52% of the controls and 50% of the patients were male. Supplementary Figures 1a–q and 2a–q demonstrate regional volume histograms by site in healthy subjects and in patients with schizophrenia, respectively. Subgroup sample sizes of the van Erp et al. study44 and the current study are compared in Supplementary Table 3.
Alterations of subcortical regional volumes in schizophrenia
All linear regression analyses were conducted using SPSS version 19.0.0 (SPSS, Chicago, IL, USA), and all meta-analyses were performed using Review Manager version 5.3 (The Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark) and Metasoft software.45 For definition of statistical significance, we set the type-I error rate (P-value) at 0.05. Moreover, a Bonferroni correction was applied to the statistical results to reduce type-I errors generated by multiple comparisons. First, means and standard deviations of subcortical regional volumes and ICV were calculated for each protocol for each diagnostic group. Second, we examined group differences in regional volumes within each protocol. Group differences in subcortical regional volumes were investigated using a univariate linear regression analysis including sex, age and ICV as nuisance covariates. For group differences in ICV, only sex and age were included as nuisance covariates in the regression analysis. Finally, we performed meta-analysis of group differences in each regional volume. The group differences and standard errors for each protocol were entered into a random-effect model meta-analysis, and overall group differences and standard errors were obtained. For each brain region, Cohen's d effect sizes were calculated from the overall group contrast. The analytical methods used in the van Erp et al. study from the ENIGMA Schizophrenia Working Group (ENIGMA-SZ) were followed in this analysis.44
Laterality of subcortical regional volumes
To assess laterality for each regional volume, we used a laterality index (LI), defined as the ratio [(left−right)/(left+right)] this is commonly used to evaluate brain structural asymmetry.19, 30, 46 LIs can range from −1 to 1 and a positive LI means a leftward asymmetry.
First, the means and standard deviations of LIs of subcortical regional volumes were calculated for each protocol for each diagnostic group. One-sample tests were conducted to evaluate whether mean LIs were significantly different from zero. Second, we performed a separate meta-analysis for LIs in each group. Mean LIs and standard errors in each protocol were entered into a random-effect model meta-analysis, and overall mean LIs in each group were obtained. LIs have similar characteristics to effect sizes; that is, both of them are referenced and indexed to zero (thus, LIs themselves can be meta-analyzed). For each group and each brain region, Cohen's d effect sizes for LIs were calculated from the overall average.
Third, group differences in LIs within each protocol were examined using a univariate linear regression, which included sex and age as nuisance covariates, as LIs may not distribute normally. Fourth, we performed a meta-analysis of group differences in LIs. Group differences in LIs and standard errors in each protocol were included into a random-effect model meta-analysis, and overall group differences in LIs were obtained. Cohen's d effect sizes for differences in LIs between groups were calculated from the overall group contrast. Using a similar method as used in the van Erp et al.44 study, we also conducted a power analysis to estimate the sample sizes required to detect the given effect sizes at a power of 0.80 and a one-tailed significance level of 0.05, with G*Power version 3.1.9.2.47
Finally, as an additional analysis, we performed a meta-analysis of group differences in LIs using FMRIB's Integrated Registration and Segmentation Tool (FIRST) in the FMRIB Software Library (FSL) version 5.0.8 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST), as LIs for subcortical volumes calculated with FreeSurfer can be different from those calculated using FSL.48 Of the 2564 participants (1680 healthy controls and 884 patients with schizophrenia) included in the main analyses, 29 subjects (18 controls and 11 patients) failed to be processed with FSL. After that, each segmentation image was visually checked for exclusion of poor parcellation. Fifty-eight subjects (35 controls and 23 patients) were excluded by this approach. Thus, a total of 2477 participants (1627 controls and 850 patients) were entered into the additional analysis.
Results
Alterations of subcortical regional volumes in schizophrenia
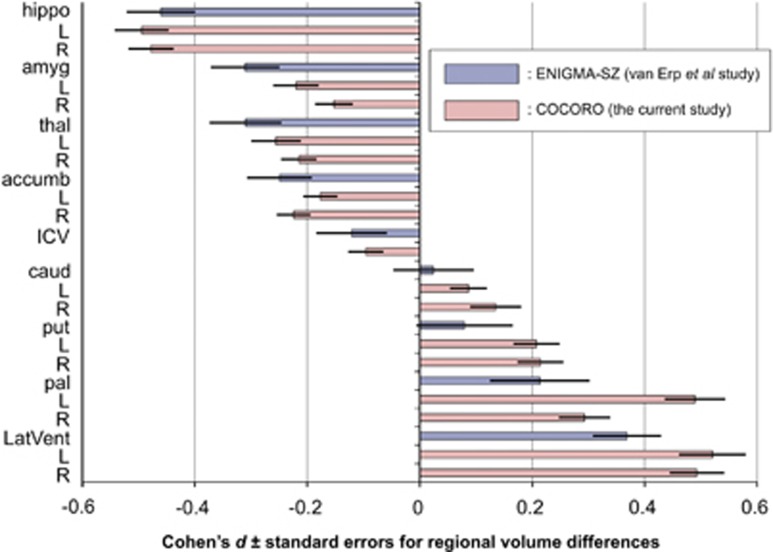
Means and standard deviations of regional volumes for each protocol for each diagnostic group can be found in Supplementary Table 4. Group differences in regional volumes within each protocol were examined and listed in Supplementary Table 5. We performed a meta-analysis of group differences in regional volumes. Compared with controls, patients with schizophrenia demonstrated significantly smaller bilateral hippocampus, amygdala, thalamus and accumbens volumes as well as smaller ICVs; however, patients exhibited larger bilateral caudate, putamen, pallidum and lateral ventricle volumes (Supplementary Table 6). The largest decreasing patient–control effect size was found for left hippocampal volume (d=−0.49, P=7.2 × 10−25) and the largest increase was seen for left lateral ventricle volume (d=0.52, P=2.1 × 10−18). Each of these brain structures studied, excluding the left caudate volume (d=0.09, P=8.5 × 10−3) and right caudate volume (d=0.13, P=3.0 × 10−3), had significant group differences even at a conservative Bonferroni-corrected threshold of P<2.9 × 10−3 (0.05/17). Results for left and right hemispheric volumes of each region demonstrated similar tendencies. The effect sizes and standard errors for subcortical regional volume differences are depicted in Figure 2, where the results of the van Erp et al.44 study from ENIGMA-SZ and those of the current study from COCORO are merged. The I2 index, which represented the heterogeneity of effect sizes, varied from zero to moderate (range: 0–56%). Group differences for each protocol are provided in Supplementary Figures 3a–q.
Figure 2.
Meta-analytic overall effect sizes (Cohen's d)±standard errors for subcortical regional volume differences between patients with schizophrenia and healthy controls. Results of the van Erp et al. study from ENIGMA-SZ (blue-colored bars) and those of the current study from COCORO (pink-colored bars) are merged. Between-group differences in subcortical volumes adjusted for age, sex and intracranial volume, as well as differences in intracranial volume adjusted for age and sex, were included into the meta-analysis and overall differences were obtained. The corresponding forest plots are described in Supplementary Figures 3a–q. An overall effect size was calculated as the ratio of the overall difference to the pooled standard deviation. A positive effect size means that patients with schizophrenia had larger volumes than healthy controls. accumb, accumbens; amyg, amygdala; caud, caudate; COCORO, Cognitive Genetics Collaborative Research Organization; ENIGMA, Enhancing Neuro Imaging Genetics through Meta-Analysis; ENIGMA-SZ, ENIGMA Schizophrenia Working Group; hippo, hippocampus; ICV, intracranial volume; L, left; LatVent, lateral ventricle; pal, pallidum; put, putamen; R, right; thal, thalamus.
Laterality of subcortical regional volumes
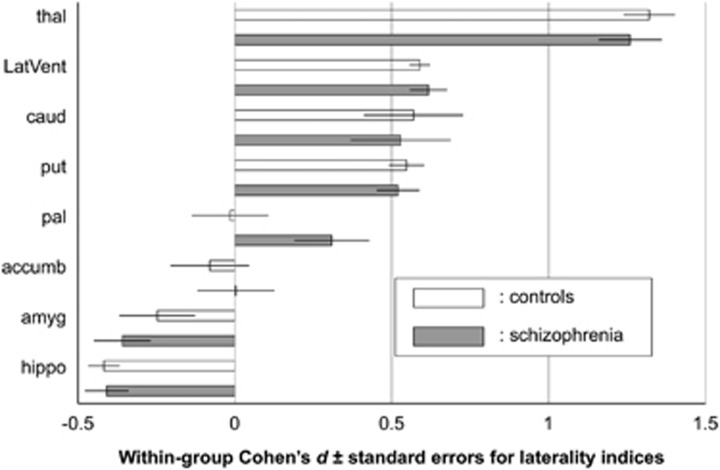
Means and standard deviations of LIs of regional volumes for each protocol for each group are listed in Supplementary Table 7. We performed a meta-analysis of LIs in each group (Figure 3 and Supplementary Table 8). Healthy controls and patients with schizophrenia showed significantly positive LIs in the thalamus (d=1.32, d=1.26, respectively), lateral ventricles (d=0.59, d=0.62, respectively), caudate (d=0.57, d=0.53, respectively) and putamen (d=0.55, d=0.52, respectively), as well as significantly negative LIs in the hippocampus (d=−0.42, d=−0.41, respectively) and amygdala (d=−0.25, d=−0.36, respectively). LIs of these regions in both groups survived a Bonferroni correction with a threshold of P<6.3 × 10−3 (0.05/8), except that of the amygdala in healthy subjects. LIs of the nucleus accumbens in both groups were not significantly different from zero (d=−0.08, d=0.00, respectively). The LI of the globus pallidus in controls did not differ from zero (d=−0.02, P=0.90), while in patients with schizophrenia it was positive with a trend towards significance (d=0.31, P=1.0 × 10−2). LIs for each protocol in controls and in patients with schizophrenia are provided in Supplementary Figures 4a–h and 5a–h, respectively.
Figure 3.
Within-group meta-analytic overall effect sizes (Cohen's d)±standard errors for laterality indices (LIs) of subcortical regional volumes in healthy controls (white bars) and in patients with schizophrenia (gray bars). An LI was defined as the ratio [(left−right)/(left+right)]. In each group, mean LIs and their standard errors were entered into the meta-analysis and an overall mean was obtained. The corresponding forest plots are described in Supplementary Figures 4a–h and 5a–h. An overall effect size for LIs was calculated as the ratio of the overall mean LIs to the overall standard deviation. A positive effect size demonstrates a leftward asymmetry. accumb, accumbens; amyg, amygdala; caud, caudate; hippo, hippocampus; LatVent, lateral ventricle; pal, pallidum; put, putamen; thal, thalamus.
Group differences in LIs within each protocol were examined (Supplementary Table 9). We performed a meta-analysis of group differences in LIs (Supplementary Figure 6 and Supplementary Table 10). The LI of the globus pallidus in patients with schizophrenia was significantly higher than in the healthy controls (mean difference=0.021, d=0.30, P=2.8 × 10−11). However, LIs of the other regions were not significantly different between groups. Group differences in LIs for each protocol are provided in Supplementary Figures 7a–h. According to a power analysis, the estimated sample sizes required for each group to reach a power of 0.80 (P<0.05, one-tailed) across regions ranged from 141 to 1 016 462.
As an additional analysis, we performed a meta-analysis of group differences in LIs using FSL (Supplementary Figure 8). The LI of the globus pallidus in patients with schizophrenia was significantly higher than in the healthy controls (mean difference=0.009, d=0.20, P=1.3 × 10−8). For the other regions, LIs were not significantly different between groups.
Discussion
In the current study, we showed smaller-than-normal volumes in the bilateral hippocampi, amygdala, accumbens and ICV, and larger-than-normal volumes in the bilateral caudate, putamen, pallidum and lateral ventricle in patients with schizophrenia. Supplementary Table 11 demonstrates sample sizes and results on subcortical volume alterations in schizophrenia both in previous studies and in the current study. We successfully replicated the previous study from the ENIGMA consortium44 and another large-scale meta-analytic study conducted by Haijma et al.,35 both of which showed smaller-than-normal volumes in the hippocampus, amygdala, thalamus and nucleus accumbens and ICV, as well as larger-than-normal volumes in the globus pallidus and lateral ventricles. In addition, as demonstrated in Figure 2, the rank order of effect sizes for group differences in the current study was similar to that in the van Erp et al.44 study. Our results revealing larger-than-normal volumes of the caudate and putamen in patients with schizophrenia were not in line with the two previous studies. This discrepancy might partly be because the samples included in the current study were relatively ethnically homogeneous, namely, most of the participants were Japanese people. The discrepancy might also be owing to the use of a single pipeline for preparation before the statistical analysis of the MRI data, which included quality control and preprocessing with FreeSurfer. In contrast to the two above-mentioned studies, our results of enlarged caudate and putamen volumes in patients with schizophrenia were consistent with a number of previous studies. For example, Hokama et al.49 reported enlarged volumes of the globus pallidus, putamen and caudate. Moreover, Glahn et al.50 reported enlarged volumes in the bilateral putamen and right head of the caudate. Another study reported an association between caudate volume and working memory, as well as putamen volume and verbal learning, vigilance and executive function in schizophrenia.51 Mamah et al.52 reported increased volume in the bilateral caudate and putamen and a positive correlation between attention/vigilance performance with caudate and putamen volume in schizophrenia. Finally, a review by Brandt and Bonelli53 demonstrated a decreased caudate volume in first-episode schizophrenia and increased caudate and putamen volume in chronic schizophrenia. Further research is needed to elucidate volumetric alterations of caudate and putamen in schizophrenia.
The current study revealed asymmetry in several subcortical structures. First, healthy controls demonstrated leftward asymmetry in the lateral ventricles, thalamus, caudate and putamen, while rightward asymmetry was found in the hippocampus and amygdala. For hippocampus and amygdala volumes, our results are consistent with previous studies,19, 20, 21, 22 one of which was a meta-analytic review.20 Although our results are discordant with those of some previous studies on volumetric lateralization for the thalamus,23 caudate27, 28, 29, 30 and putamen,31, 46 the current study is in accordance with other previous reports on volumetric lateralization of the lateral ventricle,24 putamen,27, 30 globus pallidus31 and nucleus accumbens.21 Except for Pedraza et al.,20 these previous studies had at most 150 participants, and may have been driven by sampling bias. Further, scanner differences (for example, vendor, model and magnetic field strength) and image processing biases may have also possibly influenced findings across the studies. Meta-analytic methods, such as the ones used in this study, could overcome such drawbacks.
Second, with the exception of the globus pallidus, volumetric asymmetries in the subcortical regions were not significantly different between controls and patients with schizophrenia. The current study is consistent with a prior study demonstrating similar patterns of rightward hippocampal asymmetry in both schizophrenia and control groups.19 However, it should be noted that our results are discordant with some previous reports. Csernansky et al.13 showed increased rightward asymmetry of the thalamus in patients with schizophrenia compared with healthy controls. Moreover, Qiu et al.21 revealed rightward amygdala asymmetry in controls and a reduction of this asymmetry in patients with schizophrenia. We assume that the differences in results across studies can be ascribed to scanner differences, imaging processing biases, different sample sizes and various ethnicities. Collectively, to our knowledge, our results are the first to comprehensively characterize laterality of brain subcortical regional volumes in schizophrenia.
Third, we revealed significant alterations of hemispheric asymmetry for globus pallidus volume in patients with schizophrenia when compared with controls. For this region, healthy subjects showed no asymmetry, while patients with schizophrenia demonstrated leftward asymmetry. Few previous studies have addressed globus pallidus volume laterality or made a direct comparison of left- and right-side volumes, although, contradictory to our results, some previous studies have reported enlarged globus pallidus volumes only on the right side in schizophrenia.52, 54 van Erp et al.44 demonstrated a bilaterally increased volume of the globus pallidus in schizophrenia with similar effect sizes for both left and right regions, while not investigating the volume lateralization of this region. We presume no lateralization differences between groups in their data set. Of note, our results were likely not owing to a technical pitfall or instability of the current imaging analysis technology, because the additional analysis using FSL found similar results to the main analysis using FreeSurfer. Thus, schizophrenia–control differences in the lateralization of globus pallidus volume could be robust.
There are several reports of left globus pallidus abnormalities in schizophrenia using imaging modalities other than structural MRI. A positron emission tomography study showed significantly higher blood flow in the left globus pallidus in never-medicated patients with schizophrenia than in controls.55 Another positron emission tomography study revealed a correlation between the severity of negative symptoms and increased left external globus pallidus activation in schizophrenia.56 A functional MRI study showed that patients with schizophrenia demonstrated greater activation during procedural learning in the left globus pallidus.57 Further, some previous investigations have shown altered connectivity involving the globus pallidus in schizophrenia. A resting-state functional MRI study revealed significantly aberrant interhemispheric connections in the globus pallidus, as well as a negative correlation with bilateral connections of the pallidum and duration of illness in schizophrenia.58 Finally, a diffusion tensor imaging study showed that patients with schizophrenia have lower-than-normal fractional anisotropy values in the bilateral globus pallidus, implying aberrant microstructures of the globus pallidus.59
The basal ganglia have important roles in motor function and the reward system,60, 61 and the globus pallidus externus is in the central position among the loop circuits of the basal ganglia.62 It has long been known that the basal ganglia use modulatory systems to regulate cerebral cortical activity via a projection to the thalamus.63 A recent mouse study described a direct GABAergic (gamma-aminobutyric acidergic) projection from the globus pallidus externus to the frontal cortex, which is exclusively ipsilateral and sensitive to antipsychotic drugs.64 These results imply the existence of altered structural and functional connectivity, especially ipsilateral connectivity, between basal ganglia and frontal cortices in schizophrenia. In patients with schizophrenia, altered GABAergic interneuron function resulting in dysfunction of the dorsolateral prefrontal cortex,65 which is closely associated with impairments in executive functioning such as working memory,66 may occur ipsilaterally. Thus, it is suggested that there may be aberrant laterality in neural pathways and connectivity patterns related to the globus pallidus in schizophrenia. Further research is required to elucidate this hypothesis.
A few limitations to the current study should be noted. First, possible effects of medically prescribed drugs on subcortical regional volumes in patients with schizophrenia cannot be overlooked. Although few studies reported associations between limbic volumes and antipsychotic medications,67 several studies have demonstrated increased basal ganglia volume after treatment with neuroleptics (especially typical neuroleptics).68, 69 A review by Ebdrup et al.,70 however, revealed that typical neuroleptics do not increase the volume of the basal ganglia, while atypical neuroleptics do. A recent meta-analytic study revealed no significant longitudinal differences in caudate volume related to antipsychotic treatment in patients with schizophrenia.71 Thus, results regarding medication-induced changes in subcortical regional volumes in schizophrenia remain controversial and heterogeneous. Second, the results of any meta-analysis must be interpreted cautiously, because of possible confounds such as a statistical paradox.72 The sample sizes of this study were unequal; therefore, the current meta-analytic results may be unstable. Third, we did not investigate correlations between subcortical region volumes and clinical characteristics. Only the Osaka site was able to provide such detailed clinical characteristics. The ENIGMA consortium has already launched a research project studying such associations. The Osaka site supplied its own data set of T1-weighted images and clinical information to this ENIGMA project. The results of their study are expected to be published in the near future.
In conclusion, we demonstrated reduced bilateral hippocampus, amygdala, thalamus, accumbens and ICVs, but enlarged bilateral caudate, putamen, pallidum and lateral ventricle volumes in schizophrenia. The rank order of effect sizes for subcortical regional volumetric differences between patients with schizophrenia and healthy subjects reported by van Erp et al.44 was successfully replicated and confirmed. Further, we revealed leftward asymmetry for thalamus, lateral ventricle, caudate and putamen volumes, and rightward asymmetry for amygdala and hippocampal volumes both in controls and in patients with schizophrenia. Also, we demonstrated schizophrenia-specific leftward asymmetry in globus pallidus volume. These findings suggest the possibility of aberrant laterality in neural pathways and connectivity patterns related to the pallidum in schizophrenia.
Acknowledgments
We thank all of the participants in this study. A part of this work was carried out under Brain/MINDS and SRPBS by AMED, KAKENHI and CBSN by MEXT, the Brain Science Program by NINS. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Part of computations were performed using Research Center for Computational Science, Okazaki, Japan.
Author contributions
RH supervised the entire project. NOk, MFuk, KK and RH designed the study. NOk, MFuk, DK, HYamam, KO, YY, MFuj, YoshiyW, NY, HF, MI, SI, TN, HN, NH, JM, SK, TT, HYamas, KM, TO, TI, YK, RY, YoshifW, MS, MT, NOz, KK and RH contributed to data collection. NOk, Fuk, FY, DK, KN and RH analyzed the data. NOk, MFuk, FY, DK, HYamam, KO, YY, MFuj, YoshiyW, NY, KN, DPH, TGMvE, HF, MI, SI, TN, HN, NH, JM, SK, TT, HYamas, KM, TO, TI, YK, RY, YoshifW, MS, JAT, MT, PMT, NOz, KK and RH interpreted the results. NOk, MFuk and RH wrote the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Modinos G, Costafreda SG, van Tol MJ, McGuire PK, Aleman A, Allen P. Neuroanatomy of auditory verbal hallucinations in schizophrenia: a quantitative meta-analysis of voxel-based morphometry studies. Cortex 2013; 49: 1046–1055. [DOI] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Schneider F, Pagel AD, Laird AR, Fox PT, Eickhoff SB. Progressive pathology is functionally linked to the domains of language and emotion: meta-analysis of brain structure changes in schizophrenia patients. Eur Arch Psychiatry Clin Neurosci 2011; 261(Suppl 2): S166–S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Sun D, Cannon TD. Structural and functional brain abnormalities in schizophrenia. Curr Dir Psychol Sci 2010; 19: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol 2002; 12: 169–177. [DOI] [PubMed] [Google Scholar]
- van Schouwenburg MR, den Ouden HE, Cools R. The human basal ganglia modulate frontal-posterior connectivity during attention shifting. J Neurosci 2010; 30: 9910–9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisman G, Braun-Benjamin O, Melillo R. Cognitive-motor interactions of the basal ganglia in development. Front Syst Neurosci 2014; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg M, Aarts E, Cools R. Dopaminergic modulation of cognitive control: distinct roles for the prefrontal cortex and the basal ganglia. Curr Pharm Des 2010; 16: 2026–2032. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJ Jr., Pung CJ et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry 2010; 68: 41–50. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 2000; 157: 16–25. [DOI] [PubMed] [Google Scholar]
- Hartberg CB, Sundet K, Rimol LM, Haukvik UK, Lange EH, Nesvag R et al. Subcortical brain volumes relate to neurocognition in schizophrenia and bipolar disorder and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1122–1130. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Gaser C, Jager M, Bottlender R, Frodl T, Holzinger S et al. Structural correlates of psychopathological symptom dimensions in schizophrenia: a voxel-based morphometric study. Neuroimage 2008; 39: 1600–1612. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res 2011; 127: 46–57. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Schindler MK, Splinter NR, Wang L, Gado M, Selemon LD et al. Abnormalities of thalamic volume and shape in schizophrenia. Am J Psychiatry 2004; 161: 896–902. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Koutsouleris N, Bottlender R, Scheuerecker J, Jager M, Teipel SJ et al. Structural brain alterations at different stages of schizophrenia: a voxel-based morphometric study. Schizophr Res 2008; 104: 44–60. [DOI] [PubMed] [Google Scholar]
- Stegmayer K, Horn H, Federspiel A, Razavi N, Bracht T, Laimbock K et al. Ventral striatum gray matter density reduction in patients with schizophrenia and psychotic emotional dysregulation. Neuroimage Clin 2014; 4: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TH, Youn T, Ha KS, Rho KS, Lee JM, Kim IY et al. Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res 2004; 132: 251–260. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci 2003; 4: 37–48. [DOI] [PubMed] [Google Scholar]
- Corballis MC. Left brain, right brain: facts and fantasies. PLoS Biol 2014; 12: e1001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Joshi SC, Miller MI, Csernansky JG. Statistical analysis of hippocampal asymmetry in schizophrenia. Neuroimage 2001; 14: 531–545. [DOI] [PubMed] [Google Scholar]
- Pedraza O, Bowers D, Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J Int Neuropsychol Soc 2004; 10: 664–678. [DOI] [PubMed] [Google Scholar]
- Qiu A, Wang L, Younes L, Harms MP, Ratnanather JT, Miller MI et al. Neuroanatomical asymmetry patterns in individuals with schizophrenia and their non-psychotic siblings. Neuroimage 2009; 47: 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallai J, Csatho A, Kover F, Makany T, Nemes J, Horvath K et al. MRI-assessed volume of left and right hippocampi in females correlates with the relative length of the second and fourth fingers (the 2D:4D ratio). Psychiatry Res 2005; 140: 199–210. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Eliaz Y, Chosiad L, Feiwell R, Rogers L. Magnetic resonance imaging of the thalamus in male patients with schizophrenia. Schizophr Res 2002; 58: 135–144. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, McCarley RW, Metcalf D, Tieman J, Jolesz FA. Application of automated MRI volumetric measurement techniques to the ventricular system in schizophrenics and normal controls. Schizophr Res 1991; 5: 103–113. [DOI] [PubMed] [Google Scholar]
- Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br J Psychiatry 2001; 178: 344–351. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Yoshiura T, Hiwatashi A, Noguchi T, Togao O, Takayama Y et al. Volumetric asymmetry and differential aging effect of the human caudate nucleus in normal individuals: a prospective MR imaging study. J Neuroimaging 2011; 21: 34–37. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P et al. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex 2001; 11: 868–877. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Acker JD. Age, gender, and hemispheric differences in human striatum: a quantitative review and new data from in vivo MRI morphometry. Neurobiol Learn Mem 1995; 63: 133–142. [DOI] [PubMed] [Google Scholar]
- Ifthikharuddin SF, Shrier DA, Numaguchi Y, Tang X, Ning R, Shibata DK et al. MR volumetric analysis of the human basal ganglia: normative data. Acad Radiol 2000; 7: 627–634. [DOI] [PubMed] [Google Scholar]
- Wyciszkiewicz A, Pawlak MA. Basal ganglia volumes: MR-derived reference ranges and lateralization indices for children and young adults. Neuroradiol J 2014; 27: 595–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Head D, McQuain J, Acker JD, Raz N. Differential aging of the human striatum: a prospective MR imaging study. AJNR Am J Neuroradiol 1998; 19: 1501–1507. [PMC free article] [PubMed] [Google Scholar]
- Ribolsi M, Daskalakis ZJ, Siracusano A, Koch G. Abnormal asymmetry of brain connectivity in schizophrenia. Front Hum Neurosci 2014; 8: 1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry 2008; 165: 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Balain V, Liddle PF. The neuroanatomy of psychotic diathesis: a meta-analytic review. J Psychiatr Res 2012; 46: 1249–1256. [DOI] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull 2013; 39: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan B, Newell J, Soares JC, Brambilla P, Strakowski SM, Fleck DE et al. Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biol Psychiatry 2011; 69: 326–335. [DOI] [PubMed] [Google Scholar]
- Novak NM, Stein JL, Medland SE, Hibar DP, Thompson PM, Toga AW. EnigmaVis: online interactive visualization of genome-wide association studies of the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) consortium. Twin Res Hum Genet 2012; 15: 414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L et al. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage 2013; 81: 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet 2012; 44: 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Jahanshad N, Sprooten E, Nichols TE, Mandl RC, Almasy L et al. Multi-site study of additive genetic effects on fractional anisotropy of cerebral white matter: comparing meta and megaanalytical approaches for data pooling. Neuroimage 2014; 95: 136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbrook DG, Williams RW, Lu L, Stein JL, Hibar DP, Nichols TE et al. Joint genetic analysis of hippocampal size in mouse and human identifies a novel gene linked to neurodegenerative disease. BMC Genomics 2014; 15: 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivieres S, Jahanshad N et al. Common genetic variants influence human subcortical brain structures. Nature 2015; 520: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry (e-pub ahead of print 30 June 2015; doi: 10.1038/mp.2015.69). [DOI] [PMC free article] [PubMed]
- van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry (e-pub ahead of print 18 August 2015; doi: 10.1038/mp.2015.118). [DOI] [PMC free article] [PubMed]
- Han B, Eskin E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am J Hum Genet 2011; 88: 586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedelahi A, Hasanzadeh H, Hadizadeh H, Joghataie MT. Morphometric and volumetric study of caudate and putamen nuclei in normal individuals by MRI: Effect of normal aging, gender and hemispheric differences. Pol J Radiol 2013; 78: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- Guadalupe T, Zwiers MP, Teumer A, Wittfeld K, Vasquez AA, Hoogman M et al. Measurement and genetics of human subcortical and hippocampal asymmetries in large datasets. Hum Brain Mapp 2014; 35: 3277–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D et al. Caudate, putamen, and globus pallidus volume in schizophrenia: a quantitative MRI study. Psychiatry Res 1995; 61: 209–229. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry 2008; 64: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laywer G, Nyman H, Agartz I, Arnborg S, Jonsson EG, Sedvall GC et al. Morphological correlates to cognitive dysfunction in schizophrenia as studied with Bayesian regression. BMC Psychiatry 2006; 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D, Wang L, Barch D, de Erausquin GA, Gado M, Csernansky JG. Structural analysis of the basal ganglia in schizophrenia. Schizophr Res 2007; 89: 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt GN, Bonelli RM. Structural neuroimaging of the basal ganglia in schizophrenic patients: a review. Wien Med Wochenschr 2008; 158: 84–90. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RC, van Haren NE, Koning H, Collins DL et al. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry 2001; 58: 1118–1125. [DOI] [PubMed] [Google Scholar]
- Early TS, Reiman EM, Raichle ME, Spitznagel EL. Left globus pallidus abnormality in never-medicated patients with schizophrenia. Proc Natl Acad Sci USA 1987; 84: 561–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeno R, Molina M, Guirao M, Isoardi R. Severity of negative symptoms in schizophrenia correlated to hyperactivity of the left globus pallidus and the right claustrum. A PET study. World J Biol Psychiatry 2004; 5: 20–25. [DOI] [PubMed] [Google Scholar]
- Zedkova L, Woodward ND, Harding I, Tibbo PG, Purdon SE. Procedural learning in schizophrenia investigated with functional magnetic resonance imaging. Schizophr Res 2006; 88: 198–207. [DOI] [PubMed] [Google Scholar]
- Mwansisya TE, Wang Z, Tao H, Zhang H, Hu A, Guo S et al. The diminished interhemispheric connectivity correlates with negative symptoms and cognitive impairment in first-episode schizophrenia. Schizophr Res 2013; 150: 144–150. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Mori T, Nemoto K, Moriguchi Y, Noguchi H, Nakabayashi T et al. Abnormal microstructures of the basal ganglia in schizophrenia revealed by diffusion tensor imaging. World J Biol Psychiatry 2009; 10: 65–69. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Delong MR. Deep-brain stimulation for basal ganglia disorders. Basal Ganglia 2011; 1: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger D, Kita H. Functional connectivity and integrative properties of globus pallidus neurons. Neuroscience 2011; 198: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll H, Hamker FH. Computational models of basal-ganglia pathway functions: focus on functional neuroanatomy. Front Syst Neurosci 2013; 7: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Oldenburg IA, Berezovskii VK, Johnson CA, Kingery ND, Elliott HL et al. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 2015; 521: 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6: 312–324. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry 1986; 43: 114–124. [DOI] [PubMed] [Google Scholar]
- Panenka WJ, Khorram B, Barr AM, Smith GN, Lang DJ, Kopala LC et al. A longitudinal study on the effects of typical versus atypical antipsychotic drugs on hippocampal volume in schizophrenia. Schizophr Res 2007; 94: 288–292. [DOI] [PubMed] [Google Scholar]
- Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med 2009; 39: 1763–1777. [DOI] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J et al. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia?—a systematic review. Curr Pharm Des 2009; 15: 2535–2549. [DOI] [PubMed] [Google Scholar]
- Ebdrup BH, Norbak H, Borgwardt S, Glenthoj B. Volumetric changes in the basal ganglia after antipsychotic monotherapy: a systematic review. Curr Med Chem 2013; 20: 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev 2013; 37: 1680–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker G, Schumacher M. Simpson's paradox visualized: the example of the rosiglitazone meta-analysis. BMC Med Res Methodol 2008; 8: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.