Abstract
A third of lung recipients have pre-existing antibodies against non-human leukocyte self-antigens (nHAbs) present in the lung tissue. These nHAbs also form de novo in about 70% of patients within 3-years following transplantation. Both pre-existing and de novo nHAbs can cause murine lung allograft rejection. However, their role in human transplantation remains unclear. We report hyperacute rejection following right lung transplant in a recipient with pre-existing nHAbs. Recipient of left lung from the same donor had uneventful initial course but developed de novo nHAbs at three weeks leading to acute humoral rejection. Both were successfully treated with antibody-directed therapies.
Keywords: Antibody/antigen, Rejection, Lung
Damage attributable to the underlying lung disease can expose “sequestered” non-human leukocyte tissue-restricted self-antigens (sAgs) resulting in autoantibody development in the host [1]. We have previously shown that up to one-third of lung recipients with end-stage lung disease have such pre-existing non-human leukocyte antibodies (nHAbs) [2]. Self-antigens, unlike HLA antigens, are non-polymorphic and do not differ between individuals within a species [3]. Self-antigens are normally hidden but ischemia-reperfusion to the allograft can reveal them to the recipient’s immune system. Hence, pre-existing nHAbs in a recipient can bind to sAgs in the allograft following transplantation. The current cross-match technique utilizes donor lymphocytes that do not express tissue-restricted sAgs and, therefore, does not detect pre-existing autoantibodies. Therefore, hyperacute rejection may occur due to pre-existing nHAbs, despite a negative crossmatch.
In patients that do not have pre-existing nHAbs, de novo nHAbs can develop following lung transplantation [4]. The development of these new antibodies which are of the IgG class takes about 2–3 weeks after antigenic exposure. The de novo nHAbs can mediate acute antibody-mediated rejection (AMR) following lung transplantation. Accordingly, we demonstrate, for the first time, development of hyperacute rejection and AMR in two recipients with pre-existing and de novo nHAbs, respectively.
Donor
The donor was a brain dead 26yo non-smoker male with a gunshot wound to the head. Chest imaging, bronchoscopy, and lung function were normal. Procurement was performed by two attending thoracic surgeons from Northwestern University and Cleveland Clinic Foundation.
Recipient 1
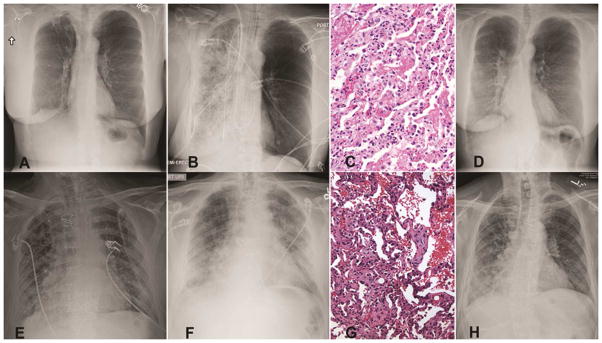
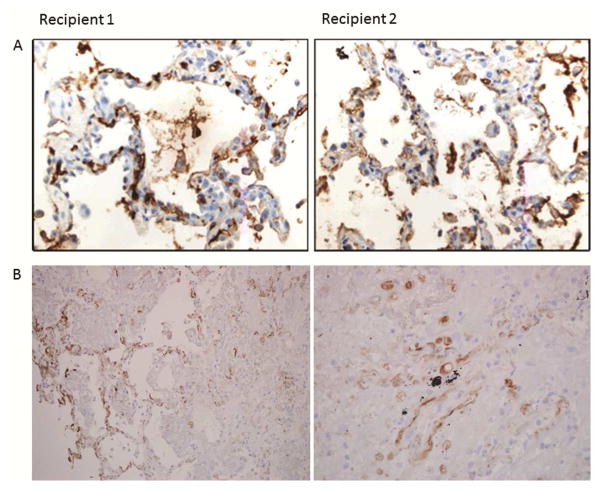
The right lung recipient was a 53yo female with emphysema and normal pulmonary pressures whom underwent transplantation without cardiopulmonary bypass. Induction immunosuppression consisted of methylprednisolone (500mg) and basiliximab (20mg). Panel reactive HLA antibodies (PRA) were not detected; T- and B-lymphocyte cross-matches were negative. Implantation was uncomplicated with 243minutes of total and 39minutes of warm ischemia. Following reperfusion, the recipient had a PaO2 of 155mmHg on 30% inspired oxygen (FiO2). Trans-esophageal echocardiogram revealed normal flow velocities across vascular anastomoses. Thirty-minutes after reperfusion, the allograft became acutely congested and the patient required 100% FiO2 to maintain a PaO2>70mmHg. Chest radiograph revealed dense infiltrates in the allograft (Figure 1A&B). Contrast computed tomography did not show fat or thrombo-embolism. Transbronchial allograft biopsies on day 1 demonstrated septal neutrophils, diffuse alveolar damage, hyaline membrane formation (Figure 1C), and complement (C4D) deposition (Figure 2B), consistent with antibody-mediated rejection (AMR), as proposed by ISHLT Pathology Council [5]. Furthermore, IgG deposition was noted (Figure 2A). There was no growth of bacteria, fungi, or viruses in the bronchoalveolar fluid. Due to histological features consistent with AMR, despite negative HLA antibodies, we tested for lung tissue-restricted nHAbs on serum collected on the day of transplant, as previously described [2]. The recipient was positive for antibodies to collagen type-V (214μg/ml, normal <106 μg/ml), Kα1-tubulin (160.8μg/ml, normal <145μg/ml) and collagen type-I (14μg/ml, normal <7.3μg/ml) but not non-lung antigens collagen type-II, and IV. The patient was treated with intravenous immunoglobulin (IVIG, 1g/kg), rituximab (375mg/m2) and bortezomib (1.3mg/m2). Allograft function improved with resolution of infiltrates (Figure 1D) within 72-hours. Maintenance immunosuppression included tacrolimus (target trough level, 8–12 ng/ml), mycophenolate mofetil (1000 mg twice daily), and prednisone (0.5 mg/kg). At 6-months, the forced expiratory volume in 1-sec (FEV1) was 65% predicted and she remained on room air. Treatment with antibody-directed therapy for 6-months cleared nHAbs.
Figure 1.
Clinical course of study patients. A) Pre-transplant chest radiograph of Recipient 1. B) Post-operative imaging showing opacification of the transplanted right lung in Recipient 1. C) Lung allograft biopsy of Recipient 1 with signs of humoral rejection (hyaline membranes, septal neutrophils and alveolar damage). D) Resolution of lung infiltrates in Recipient 1 following treatment. E) Post-operative chest radiograph of Recipient 2. F) Imaging on day 24 showing new left-sided infiltrates in Recipient 2. G) Allograft biopsy of Recipient 2 from day 24 showing antibody mediated rejection. H) Follow-up chest radiograph of Recipient 2 at three months.
Figure 2.
Evidence supporting the diagnosis of AMR in both recipients. A) IgG deposition. B) Complement deposition.
Recipient 2
The left lung was transplanted using cardiopulmonary bypass support into a 66yo male with idiopathic pulmonary fibrosis, pulmonary hypertension and left internal mammary artery bypass graft. Cross-match was negative and there were no HLA antibodies. After an uneventful recovery, he was discharged breathing room air on day 18 (Figure 1E). However, on day 24, he presented with hypoxemic respiratory failure and new allograft infiltrates (Figure 1F). Microbial cultures were negative and allograft biopsy revealed AMR (Figure 1G) with IgG and C4D deposition. (Figure 2A). Repeat cross-match and PRA screen for HLA were negative. IgG-nHAbs against collagen type V, I and Kα1-tubulin were negative on transplant. However, de novo antibodies to collagen type-V (264μg/ml), Kα1-tubulin (182.6μg/ml) and collagen type-I (19μg/ml), but not collagen type-II, and IV, were identified on day 24. The patient received plasmapheresis, IVIG and rituximab. Allograft function recovered in 72-hours and at 6-months the chest radiograph was normal (Figure 1H); FEV1 was 78% of predicted.
Comment
HLA antibody-mediated hyperacute rejection has significantly decreased following allo-transplantation due to the lymphocytic cross-match. Nevertheless, primary graft dysfunction (PGD) remains a frequent occurrence following lung transplantation [6]. In our series, Recipient 2 had idiopathic pulmonary fibrosis, pulmonary hypertension and required cardiopulmonary bypass for transplantation, all risk factors for PGD [6]. Despite that, Recipient 1 developed PGD but not Recipient 2. We have previously shown that pre-existing nHAbs are present in about a third of lung recipients and these can increase the risk of PGD 7-folds [7]. The time course, histological features, presence of pre-existing nHAbs but not HLA antibodies, lack of other etiologies, and prompt improvement with antibody-mediated therapy are highly suggestive of nHAb-mediated hyperacute rejection that manifested as PGD in Recipient 1. Therefore, it is possible that nHAb-mediated lung rejection is one possible etiology for PGD. The native lung was unaffected likely because sAgs remain sequestered and, therefore, nHAbs cannot bind to the native lung.
The development of de novo nHAbs in Recipient 2 is possibly from memory B-cells against sAgs resulting from the end-stage lung disease [8]. These memory B-cells can form nHAbs upon re-exposure to sAgs in the allograft after ischemia-reperfusion. Lung-restricted sAgs can be expressed for over a month following transplantation [9] and development of nHAbs during this time can cause AMR as seen in Recipient 2. Hence, we postulate that Recipient 2 had AMR from de novo nHAbs.
Our data suggest that antibodies against lung sAgs can lead to hyperacute or acute humoral rejection that can be treated upon early recognition. This is in agreement with recent murine studies [10]. Testing for these nHAbs should be considered during transplant workup and in patients presenting with either unexplained PGD or post-transplant AMR in the absence of HLA antibodies.
Acknowledgments
Dr Bharat is supported by NHLBI K08 HL125940. TM is supported by NIH HL 056643. Dr Chiu is supported by NIDDK T32 DK077662. The authors wish to thank Ms. Elena Susan for administrative assistance in the preparation and submission of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kheradmand F, Shan M, Xu C, Corry DB. Autoimmunity in chronic obstructive pulmonary disease: clinical and experimental evidence. Expert Rev Clin Immunol. 2012;8(3):285–92. doi: 10.1586/eci.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharat A, Kuo E, Saini D, et al. Respiratory virus-induced dysregulation of T-regulatory cells leads to chronic rejection. Ann Thorac Surg. 2010;90(5):1637–44. doi: 10.1016/j.athoracsur.2010.06.048. discussion 1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerder S, Viret C, Luche H, Ardouin L, Malissen B. Differential processing of self-antigens by subsets of thymic stromal cells. Curr Opin Immunol. 2012;24(1):99–104. doi: 10.1016/j.coi.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Hachem RR, Tiriveedhi V, Patterson GA, et al. Antibodies to K-alpha 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12(8):2164–71. doi: 10.1111/j.1600-6143.2012.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry G, Burke M, Andersen C, et al. Pathology of pulmonary antibody-mediated rejection: 2012 update from the Pathology Council of the ISHLT. J Heart Lung Transplant. 2013;32(1):14–21. doi: 10.1016/j.healun.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187(5):527–34. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharat A, Saini D, Steward N, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90(4):1094–101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stegall MD, Raghavaiah S, Gloor JM. The (re)emergence of B cells in organ transplantation. Curr Opin Organ Transplant. 2010;15(4):451–5. doi: 10.1097/MOT.0b013e32833b9c11. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida S, Haque A, Mizobuchi T, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6(4):724–35. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian V, Ramachandran S, Banan B, et al. Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation. Am J Transplant. 2014;14(10):2359–66. doi: 10.1111/ajt.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]