Abstract
Smooth muscle cell proliferation can be inhibited by heparan sulfate proteoglycans whereas the removal or digestion of heparan sulfate from perlecan promotes their proliferation. In this study we characterized the glycosaminoglycan side chains of perlecan isolated from either primary human coronary artery smooth muscle or endothelial cells and determined their roles in mediating cell adhesion and proliferation, and in fibroblast growth factor (FGF) binding and signaling. Smooth muscle cell perlecan was decorated with both heparan sulfate and chondroitin sulfate, whereas endothelial perlecan contained exclusively heparan sulfate chains. Smooth muscle cells bound to the protein core of perlecan only when the glycosaminoglycans were removed, and this binding involved a novel site in domain III as well as domain V/endorepellin and the α2β1 integrin. In contrast, endothelial cells adhered to the protein core of perlecan in the presence of glycosaminoglycans. Smooth muscle cell perlecan bound both FGF1 and FGF2 via its heparan sulfate chains and promoted the signaling of FGF2 but not FGF1. Also endothelial cell perlecan bound both FGF1 and FGF2 via its heparan sulfate chains, but in contrast, promoted the signaling of both growth factors. Based on this differential bioactivity, we propose that perlecan synthesized by smooth muscle cells differs from that synthesized by endothelial cells by possessing different signaling capabilities, primarily, but not exclusively, due to a differential glycanation. The end result is a differential modulation of cell adhesion, proliferation and growth factor signaling in these two key cellular constituents of blood vessels.
Keywords: smooth muscle cell, endothelial cell, extracellular matrix, perlecan, glycosaminoglycan, fibroblast growth factors
1. Introduction
Smooth muscle cell proliferation contributes to arteriopathies including atherosclerosis, restenosis after vascular procedures and in the failure of vein graft bypass (Hedin et al., 2004). Mature blood vessels remain quiescent in the absence of vascular trauma and the SMCs remain unresponsive to mitogens that might be present in their surrounding extracellular matrix. This indicates that the extracellular matrix contains intrinsic growth suppressive capability over SMCs. Individual SMCs are surrounded by a basement membrane containing laminin, type IV collagen, fibronectin and perlecan (Heickendorff, 1989). However, upon SMC activation after arterial injury a loss of laminin is evident and an accumulation of fibronectin around the proliferating cells (Thyberg et al., 1997) from which we deduce that the intact basement membrane provides an important barrier between cells and regulates SMC proliferation (Hedin et al., 1999; Walker et al., 2003).
Perlecan is a multi-domain HSPG with dual function in developmental and experimental angiogenesis (Iozzo et al., 2009). Primarily through its N-terminal HS chains, perlecan is pro-angiogenic (Iozzo, 2005) by functioning as a co-activator of FGF2 and VEGF and for presenting them to their cognate receptors (Aviezer et al., 1997; Chuang et al., 2010; Iozzo and San Antonio, 2001; Iozzo and Sanderson, 2011; Ishijima et al., 2012; Sharma et al., 1998; Zoeller et al., 2009). Notably, genetic ablation of either the whole perlecan gene in mice or block of perlecan expression in early embryogenesis leads to profound cardiovascular defects in mammalians and vertebrates (Costell et al., 2002; González-Iriarte et al., 2003; Gustafsson et al., 2013; Zoeller et al., 2008). In contrast, a recombinantly expressed C-terminal-processed form of perlecan, named endorepellin to signify its anti-endothelial activity (Mongiat et al., 2003), inhibits endothelial cell migration, collagen-induced capillary morphogenesis, and proliferation of blood vessels both in vitro and in tumor xenografts (Bix et al., 2006; Bix et al., 2004; Willis et al., 2012; Woodall et al., 2008).
Perlecan is also present in avascular tissues such as hyaline cartilage (Chuang et al., 2010; Melrose et al., 2006; Wilusz et al., 2012), intervertebral disc (Melrose et al., 2003), meniscus (Melrose et al., 2005) and synovium (Kaneko et al., 2013) which are devoid of a basement membrane. Perlecan influences cell function as it can both suppress and promote cell proliferation, has been associated with quiescent SMCs (Weiser et al., 1996) and its expression is inversely correlated with SMC proliferation and the formation of intimal hyperplasia (Kinsella et al., 2003). Perlecan is down regulated at times of maximal SMC proliferation which is within two weeks after balloon-injury of rat carotid arteries while perlecan deposition is seen in the later stages of lesion development when SMC proliferation has ceased.
The HS chains that decorate perlecan contribute to the growth inhibition of SMCs (Forsten et al., 1997) as heparinase treatment of perlecan abolishes its ability to inhibit SMC proliferation (Bingley et al., 1998; Clowes and Karnowsky, 1977; Tran et al., 2004) and changes SMCs from a quiescent to a contractile phenotype (Campbell et al., 1992; Kinsella et al., 2003). Transgenic mice harboring a deletion of exon 3 (Hspg2Δ3/Δ3), thereby lacking the three HS chains, exhibit increased intimal hyperplasia and vascular lesions enriched in migrated SMCs, thus, implicating HS in SMC growth modulation (Tran et al., 2004). Interestingly, heparin, a highly sulfated form of HS, also inhibits the proliferation of SMCs in bovine (Clowes and Clowes, 1985; Kinsella et al., 2003; Lundmark et al., 2001) but not human (Underwood et al., 1998) serum. The effect of EC proteoglycans on SMC growth is controversial as inconsistent reports in the literature suggest that they can either support or inhibit proliferation. EC conditioned medium and co-cultures of ECs with SMCs can inhibit SMC proliferation (Benitz et al., 1990; Castellot et al., 1981) while the addition of Hep I or III counteracts this inhibitory effect (Nugent et al., 1993). In contrast, human EC proteoglycans do not inhibit the proliferation of SMCs (Whitelock et al., 1997).
Perlecan interacts with a number of growth factors including FGFs 1, 2, 7, 9, 18; hepatocyte growth factor, platelet derived growth factors- AA and -BB, VEGF and connective tissue growth factor which induce cellular proliferation, differentiation and matrix production (Iozzo, 2005; Ishijima et al., 2012; Kirn-Safran et al., 2004; Whitelock et al., 2008). These interactions stabilize and sequester growth factors pericellularly in tissues where they can act as growth factor reservoirs (Whitelock et al., 2008). FGF2 is a mitogen for SMCs (Nugent and Iozzo, 2000) and has been implicated in the proliferation of SMCs during arteriopathies (Lindner et al., 1991). Exogenous FGF2 promotes SMC proliferation when grown in the presence of serum (Berry et al., 2003) while EC perlecan inhibits FGF2-mediated proliferation of SMCs (Nugent et al., 2000). The protein core of perlecan has established roles in SMC growth inhibition (Forsten et al., 1997). SMCs bind to human EC perlecan protein core (Whitelock et al., 1999), while the addition of a perlecan domain III antibody to SMC cultures promotes proliferation (Walker et al., 2003). Moreover, anti-sense perlecan domain III constructs also promote intimal hyperplasia (Nugent et al., 2000). The LG3 C-terminal fragment of perlecan domain V, supports the migration of SMCs and is also anti-apoptotic (Soulez et al., 2012) via β1 integrin interactions which mediate extracellular signal-regulated kinase (ERK)1/2 activation. Perlecan also up-regulates focal adhesion kinase-related nonkinase, an inhibitor of focal adhesion kinase (FAK) in SMCs and this suppresses FAK-mediated ERK 1/2-dependent growth signals (Walker et al., 2003).
Collectively, the studies summarized above offer a quite complex biological scenario for this large and unique gene product. Although it is firmly established that perlecan can play essential roles in the control of SMC function, the respective contributions of its protein core and GAG side chains have yet to be fully been determined. In this study we have characterized perlecan synthesized by both SMCs and ECs and investigated their functional activity. We discovered that, unlike EC, SMC perlecan is a proteoglycan decorated with HS and CS and is pro-adhesive via novel interactions involving domain III and α2β1 integrin-dependent interactions with domain V/endorepellin once its GAG chains are removed. The HS chains of SMC perlecan facilitate FGF2 cell signaling through FGF receptor 1 (FGFR1) and, although they also promote ternary complex formation with FGF1, FGFR1c or 3c, these latter complexes do not participate in cell signaling.
2. Results
2.1 Expression of fibronectin, laminin, perlecan, HS, CS and KS in cultured SMC
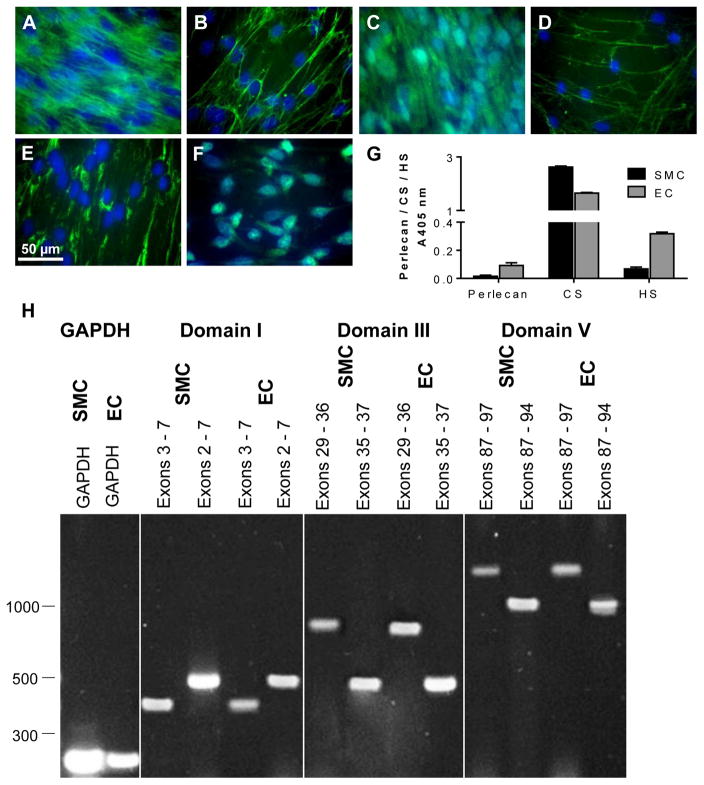
SMCs expressed and secreted fibronectin and perlecan into the pericellular/extracellular space as microfibrillar structures (Fig. 1A and B) while laminin, detected using an antibody that binds to the cell adhesion sites of most laminin isoforms, was only localized intracellularly (Fig. 1C). HS had a micro-fibrillar distribution in the ECM as well as punctate staining that was most likely associated with the cell surface (Fig. 1D). CS and KS were detected in peri-nuclear region in close association with the Golgi apparatus (Fig. 1E and F). The ECM produced by SMCs contained perlecan as well as HS and CS, which was similar in overall composition to the ECM produced by cultured ECs (Fig. 1G). Thus the SMCs secrete all major ECM components.
Fig. 1.
Expression of various extracellular matrix constituents by SMCs. The expression of [A] fibronectin (A17), [B] perlecan (A71), [C] laminin (polyclonal anti-laminin), [D] HS (10E4), [E] CS (CS56) and [F] KS (5D4) by SMCs. SMCs were cultured on TCPS and probed for the presence of ECM components (green) and cell nuclei were counterstained with DAPI (blue). The scale bar represents 50 μm. [G] ELISA of SMC and EC ECMs produced by cells and analysed after removal of the cells by ammonium hydroxide using mAbs against perlecan protein core (5D7-2E4), HS (10E4) and CS (CS56). Data are corrected for background absorbance and presented as mean ± S.D. (n = 3). [H] mRNA expression of HSPG2 from SMCs and ECs. mRNA derived from both cell types was isolated and used to generate cDNA that was amplified using domain-specific primers and electrophoresed on 1% (w/v) agarose gels. PCR products from the GAPDH primer set were electrophoresed on each gel. PCR products for domain I primer sets included exons 3 – 7 (403 bp) and 2 – 7 (510 bp), domain III primer sets included exons 29 – 36 (796 bp) and 35 – 37 (454 bp) and domain V primer sets included exons 87 – 97 (1406 bp) and 87 – 94 (1042 bp).
The production of perlecan by SMCs and EC was analyzed by isolating mRNA from each cell type and performing reverse transcriptase PCR (RT-PCR) over 40 cycles. Domain-specific primer sets were designed to span exons 2 – 7 from the N terminus (Domain I), exons 29 – 37 from the laminin-like region of the protein core (Domain III) and exons 87 – 97 from the C-terminus (Domain V) (Table 1). Transcripts generated from mRNA isolated from ECs was used to confirm the presence of transcripts from all three domains and as an indication of successful priming at the expected sizes (Table 1 and Fig. 1H). Transcripts generated from mRNA isolated from the SMC were also at the expected sizes. Together these data indicated that SMCs produced transcripts for the perlecan protein core.
Table 1.
Primers for PCR amplification of HSPG2 cDNA (accession number NM_005529).
| Domain | Exons | Primer sequences (5′ – 3′) | Expected PCR product size (bp) |
|---|---|---|---|
| I | 3 – 7 | F:ACCTGGGCAGTGGGGACCTG R:GCCTCCGTGCAGGCTCTTGG |
403 |
| 2 – 7 | F:CCATGGGCTGAGGGCATACG R:GGCACTGTGCCCAGGCGTCGGAACT |
510 | |
| III | 29 – 36 | F:ACCCCACCTGTGATGCGTGCTC R:GTGGCCCGGATCAGGAGCTCAT |
796 |
| 35 – 37 | F:CGATGTGCAGATCAGGGC R:CTGGCATTGCGAGCAGG |
454 | |
| V | 87 – 97 | F:GTGTGACAGTGACCACCCCCT R:CTACGAGGGGCAGGGGCGT |
1406 |
| 87 – 94 | F:GTGTGACAGTGACCACCCCCT R:CTGAAGACAAGGTGCCCG |
1045 |
2.2 Biochemical characterization of SMC perlecan
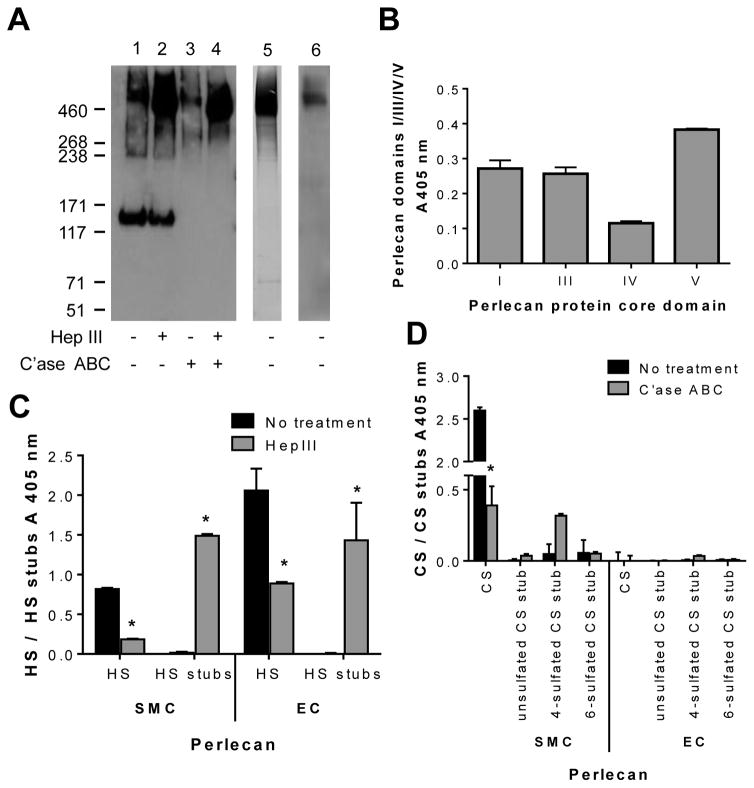
Immunopurified SMC and EC perlecan isolated from medium conditioned by cells in culture was characterized with respect to molecular mass and GAG composition using Western blotting before and after endoglycosidase digestion. The SMC perlecan migrated as a polydisperse product, typical of proteoglycan migration on SDS-PAGE gels, with a median mass of ~640 kDa, which corresponds to the predicted molecular mass of intact perlecan decorated with GAGs (Fig. 2A, lane 1). In addition, we found an immunoreactive band of ~130 kDa. Notably, the 640 kDa band shifted to ~520 kDa after digestion with Hep III, indicating that SMC perlecan contained approximately 120 kDa HS; however, there was no change in relative migration of the 130 kDa band (Fig. 2A, lane 2). Treatment of SMC perlecan with C’ase ABC reduced the molecular mass to ~580 kDa indicating the presence of ~60 kDa of CS (Fig. 2A, lane 3), while treatment with both Hep III and C’ase ABC reduced SMC perlecan to ~ 460 kDa, indicative of the presence of the full-length protein core (Fig. 2A, lane 4). Immunoreactivity of the 130 kDa band was not detected after C’ase ABC digestion indicating that the epitope was sensitive to treatment with C’ase ABC (Fig. 2A, lanes 3 and 4). Immunopurified EC perlecan was reactive with the polyclonal perlecan antibody as a smear centering at ~640 kDa (Fig. 2A, lane 5). However, unlike the SMC perlecan, no band at 130 kDa was detected (Fig. 2A, lane 5). As perlecan preparations were immunopurified with an anti-domain I mAb (clone A71) cross-linked column, we further probed SMC perlecan with a mouse monoclonal domain I antibody (clone A76) by Western blotting and found it to be reactive at a molecular mass greater than 460 kDa (Fig. 2A, lane 6). The perlecan polyclonal antibody reactive band at 130 kDa was excised from an SDS-PAGE gel, trypsin digested and analyzed by mass spectrometry (LC-MS/MS). Notably, no perlecan-specific peptides were identified (data not shown).
Fig. 2.
Biochemical characterization of SMC perlecan. [A] Western blot of immunopurified SMC perlecan (lanes 1 – 4) and EC perlecan (lane 5) probed for the presence of perlecan using a rabbit polyclonal perlecan antibody (CCN-1). Western blot of immunopurified SMC perlecan (lane 6) probed for the presence of perlecan using a mouse monoclonal perlecan domain I antibody (A76). Samples were analyzed either without (−) or with (+) heparinase III (Hep III) and chondroitinase ABC (C’ase ABC) digestion. [B] ELISA of immunopurified SMC perlecan for the presence of perlecan protein core domains using monoclonal antibodies against perlecan domains I (A71), III (7B5), IV (A7L6) and V (A74). Data are corrected for background absorbance and presented as mean ± S. D. (n = 3). ELISA of the immunopurified SMC and EC perlecan for the presence of [C] HS side chains using mAbs against HS chains (10E4) and HS stub epitopes generated after Hep III digestion (3G10) and [D] CS side chains using mAbs against CS chains (CS56) and CS stubs (1B5, 2B6 and 3B3) epitopes generated after no treatment or C’ase ABC digestion. Data are corrected for background absorbance and presented as mean ± S. D. (n=3). * indicates significant differences compared to untreated control probed with the same antibody.
Notably, the SMC perlecan reacted with mAbs against perlecan domains I, III, IV and V (Fig. 2B) further confirming that SMC perlecan was expressed as the full-length protein core. The sub-structure of the HS and CS GAGs that decorated immunopurified SMC perlecan was further investigated by ELISA using antibodies against specific HS and CS epitopes and compared to immunopurified EC perlecan (Fig. 2C and D). Immunopurified SMC and EC perlecan were both reactive with the HS chain mAb 10E4, indicating the presence of N-sulfated glucosamine residues, while digestion with Hep III significantly reduced (p < 0.05) the reactivity of both products with this antibody (Fig. 2C). Hep III digestion of each of the perlecan species also significantly increased (p < 0.05) their reactivity with an unsaturated HS stub antibody (3G10) confirming the presence of HS. CS chains were detected on SMC perlecan as shown by reactivity with the antibody, CS56, which reacts with both C-4-S and C-6-S, however CS was not detected on EC perlecan (Fig. 2D). Digestion of the immunopurified SMC and EC perlecan with C’ase B confirmed that dermatan sulfate was not present as there was no change in reactivity of the CS antibodies (data not shown). Digestion of the SMC perlecan with C’ase ABC generated significant reactivity (p < 0.05) with mAb 2B6 indicating that this perlecan was decorated with 4-sulfated CS stub structures. Collectively, these data demonstrate for the first time a cell-specific and differential glycosylation of a key vascular proteoglycan of the pericellular matrix and basement membranes with EC perlecan decorated with HS and SMC perlecan decorated with both HS, 4- and 6-sulfated CS and a 4-sulfated CS stub.
2.3 SMC adhesion and proliferation on perlecan
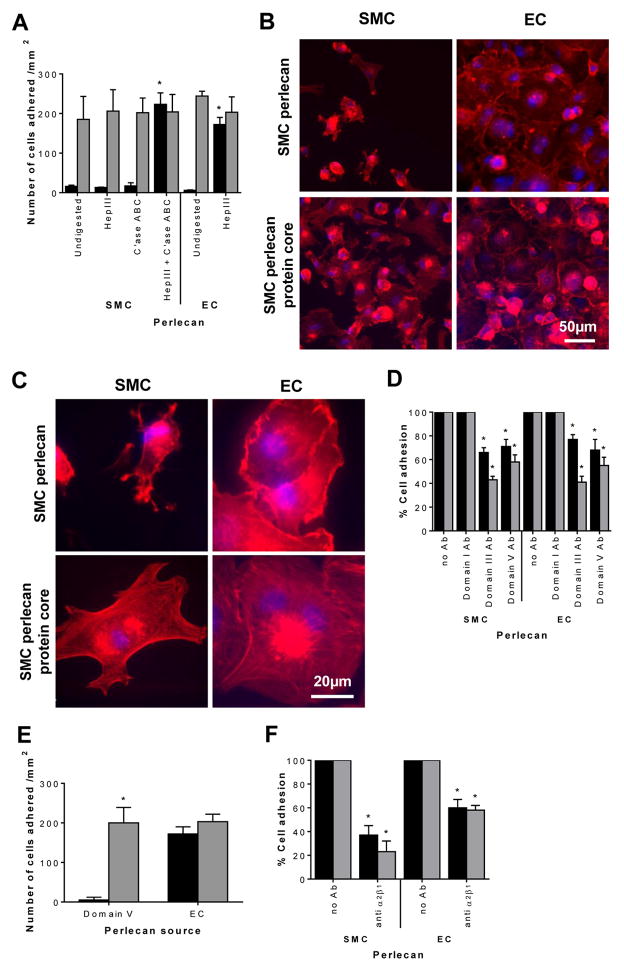
Next, we tested the effect of the two different species of perlecan on SMC and EC adhesion. SMCs adhered to both differentially-glycanated perlecan species once the GAG chains were removed as shown by the significant (p < 0.05) increase in the number of SMCs adhered to both proteoglycan species following removal of the GAG chains with both Hep III and C’ase ABC or Hep III alone (Fig. 3A). EC perlecan was only treated with HepIII to remove its HS as it lacked CS chains (cfr. Fig. 2D). Notably, ECs adhered to both perlecan species in either the presence or absence of GAG chains with there being no significant differences (p < 0.05) in the level of adhesion when the perlecan was treated with endoglycosidases to remove the GAGs (Fig. 3A and B). SMCs adhered to glycanated SMC-derived perlecan and exhibited a rounded morphology with radial protrusions containing actin that was not filamentous (Fig. 3B and C). In contrast, the cells that adhered to SMC perlecan core protein exhibited a spread morphology with well-developed actin fibers at the leading edge of the cell membrane (Fig. 3 B and C). ECs adhered to SMC-derived perlecan both with and without GAG chains and exhibited a well-spread morphology with polymerized actin fibers and actin-rich membrane ruffles (Fig. 3C).
Fig. 3.
SMC and EC adhesion on perlecan. [A] SMC (black bars) or EC (light grey bars) adhesion to SMC perlecan untreated or treated with Hep III, C’ase ABC or both or EC perlecan untreated or treated with Hep III. Data are presented as mean ± S. D. (n=3) * denotes significant differences compared to cell adhesion in the absence of endoglycosidase treatment for each cell type. [B and C] Morphology of SMCs and ECs adhered to SMC perlecan and SMC perlecan treated with Hep III and C’ase ABC. [D] SMC (black bars) or EC (light grey bars) adhesion to perlecan pre-treated with monoclonal antibodies against perlecan domains I (A76), III (7B5) or V (anti-endorepellin) before the addition of cells compared to cell adhesion in the absence of antibodies. Data presented as percent cell adhesion compared to cell adhesion to perlecan in the absence of antibodies as shown in panel A after treatment of SMC perlecan with Hep III and C’ase ABC and EC perlecan with HepIII. * denotes significant differences compared to cell adhesion for each of the respective perlecan shown in panel A. [E] SMC (black bars) or EC (light grey bars) adhesion to recombinant perlecan domain V treated with Hep III and C’ase ABC or EC perlecan treated with Hep III. Data are presented as mean ± S. D.; * denotes significant differences compared to the level of SMC adhesion. [F] SMC (black bars) or EC (light grey bars) adhesion to SMC perlecan treated with Hep III and C’ase ABC and EC perlecan treated with HepIII when cells were pre-treated with antibodies against the integrin α2β1 (BHA2.1) compared to cell adhesion in the absence of antibodies. Data are presented as percent cell adhesion compared to cell adhesion to perlecan species in the absence of antibodies as shown in panel A. * denotes significant differences compared to cell adhesion for each of the respective perlecan species shown in panel A.
Adhesion of both cell types to the protein core of perlecan was analyzed after treatment of the various perlecan coatings with antibodies against perlecan domains I, III or V prior to the addition of cells. Both perlecan domains III and V were found to be involved in the adhesion of both cell types as antibodies specific for these domains significantly reduced (p < 0.05) cell adhesion (Fig. 3D). Interestingly, the ECs were inhibited to a greater degree with the domain III antibody than the SMCs (Fig. 3D). The anti-domain I antibody had no effect on either SMC or EC adhesion to the protein core of perlecan (Fig. 3D). Adhesion of both SMCs to purified recombinantly expressed perlecan domain V devoid of GAG chains indicated that domain V alone supported a low level of SMC adhesion while it still supported EC adhesion to a similar extent as full length EC perlecan devoid of GAG chains (Fig. 3E). Adhesion of both cells to the protein core of the two different forms of perlecan was analyzed after treatment of the cells with a function blocking anti-α2β1 integrin antibody. Inhibition of the α2β1 integrin significantly (p < 0.05) reduced adhesion of both cell types to the protein core of both perlecan species (Fig. 3F). These data support the hypothesis that this integrin is directly involved in both SMC and EC adhesion to perlecan.
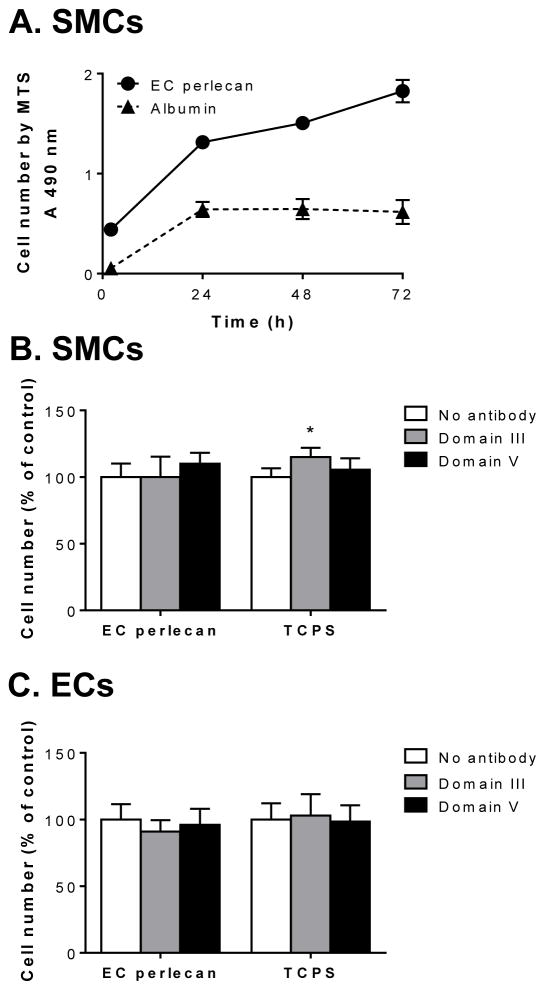
The number of viable SMCs on EC perlecan protein core was analyzed over a period of 72 h by the MTS assay and revealed that SMCs plated on EC-derived perlecan proliferated to an extent greater than cells plated on albumin (Fig. 4A). To investigate the effect of perlecan protein core domains on cell proliferation, SMCs and ECs were plated onto EC-perlecan protein core or on uncoated tissue culture polystyrene (TCPS) plates for 24 h prior to the addition of antibodies against perlecan protein core domains III or V. The number of cells present was analyzed after a further 48 h of incubation (Fig. 4B and C). The perlecan domain III antibody caused a small, but significant (p < 0.05), increase in the number of SMCs both on EC-perlecan and TCPS compared to cells in the absence of antibodies (Fig. 4B). Interestingly, the same antibody had no effect on the number of ECs on either surface (Fig. 4C).
Fig. 4.
Cell proliferation studies using various experimental conditions. [A] The number of SMCs cultured on EC-derived perlecan treated with Hep III or albumin as over 72 h determined by the MTS assay. Data presented as mean ±S. D. (n=3). [B] SMC or [C] EC cell number after 48 h measured by MTS with the addition of antibodies in solution against perlecan domain III (7B5) or domain V (anti-endorepellin) after the cells had been cultured on EC perlecan treated with Hep III or TCPS for 24 h. Data are presented as mean ± S. D. (n=3) as a percentage of the number of cells adhered to each of the surfaces in the absence of antibody. * denotes significant differences compared to cell adhesion in the absence of antibody treatment on each surface.
2.4 Ternary complexes of perlecan with FGFs and their receptors
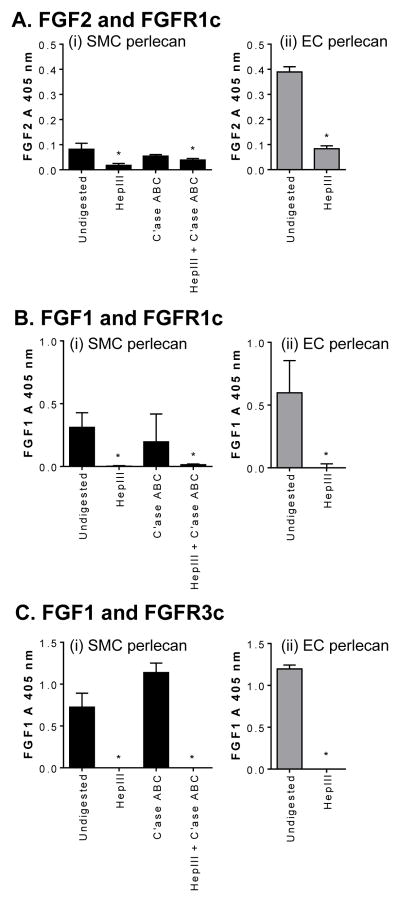
It is well established that various FGFs, including FGF1 and 2, positively regulate SMC cell growth. Thus, we determined whether SMC perlecan supported the binding of these growth factors together with their cognate receptors. EC perlecan, which is known to form ternary complexes with FGF receptors and natural ligands (Ornitz et al., 1992), was used as a positive control for these assays. Both perlecan species formed ternary complexes with FGF2 and FGFR1c that were sensitive to Hep III digestion (Fig. 5A (i) and (ii)). C’ase ABC treatment of SMC-derived perlecan had no effect on the formation of ternary complexes while treatment with both Hep III and C’ase ABC significantly reduced (p < 0.05) the level of ternary complexes formed (Fig. 5A (i)). Moreover, both perlecan species formed ternary complexes with FGF1 and FGFR1c, while pre-treatment of these perlecan preparations with Hep III significantly reduced (p < 0.05) their formation (Fig. 5B (i) and (ii)). C’ase ABC treatment alone of SMC-derived perlecan had no effect on the formation of ternary complexes suggesting that CS was not involved in their formation (Fig. 5B (i)). Both perlecan preparations also promoted the formation of ternary complexes between FGF1 and FGFR3c while pre-treatment with Hep III significantly decreased their formation (p < 0.05) (Fig. 5C (i) and (ii)). C’ase ABC treatment of SMC-derived perlecan had no effect on the formation of ternary complexes with FGF1 and FGFR3c supporting the HS-dependent nature of these interactions (Fig. 5C (i)). Collectively, these results indicate that the ternary complex requires HS chains of either SMC or EC perlecan and that there is no appreciable participation of CS chains in this biological process.
Fig. 5.
Ternary complexes of perlecan with growth factors and receptors. Ternary complexes formed between SMC (black bars) or EC (light grey bars) perlecan, either [A] FGF2 or [B and C] FGF1, and either FGFR [A and B] 1c or [C] 3c. Perlecan species were adsorbed onto wells of a 96-well plate and SMC perlecan was either undigested or digested with Hep III, C’ase ABC or both while EC perlecan was either undigested or digested with Hep III before the LACE assay was performed. Measurements were corrected for absorbance measurements detected in the absence of FGF and are presented as mean ± S.D. (n=3). * denotes significant differences compared to the absorbance for undigested perlecans in the presence of growth factor and receptor.
2.5 FGF1 and 2 growth promoting activities of SMC-derived perlecan
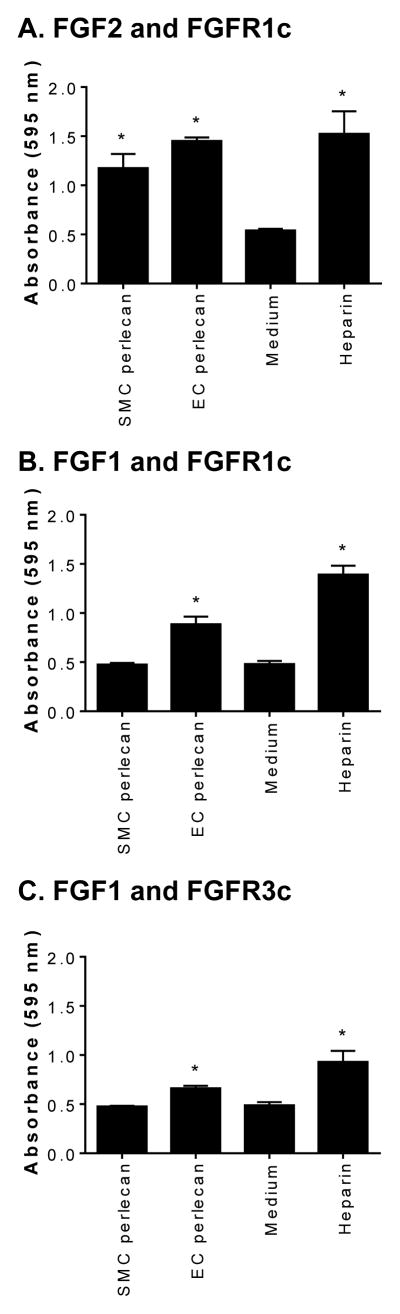
The ability of the ternary complexes formed between FGFs and their cognate receptors to signal was analyzed in BaF-32 cells transfected with either FGFR1c (Fig. 6A and B) or FGFR3c receptors (Fig. 6 C). Heparin and either FGF1 or FGF2 were used as positive controls for the assay while cells in the presence of medium and growth factor alone were used as negative controls. BaF-32 cells expressing FGFR1c responded to FGF2 in the presence of either perlecan species to a level significantly above (p < 0.05) the medium only control and to a similar extent as heparin. SMC-derived perlecan did not appreciably enhance the signal of FGF1 through either FGFR1c or FGFR3c (Fig. 6B and C). In contrast, EC-derived perlecan significantly enhanced the signal of FGF1 through either FGFR1c or FGFR3c (Fig. 6B and C). Collectively, these novel findings demonstrate a differential signaling potential for perlecan synthesized by SMCs and ECs, with the latter enhancing the growth promoting ability of FGF1 through their cognate receptors.
Fig. 6.
FGF1 and 2 growth promoting activities of SMC-derived perlecan. Proliferation of BaF-32 cells expressing either FGFR1c or FGFR3c in the presence of SMC or EC perlecan with either FGF1 or FGF2. [A and B] FGFR1c-expressing or [C] FGFR3c-expressing BaF-32 cells were incubated with SMC or EC perlecan in the presence of either [A] FGF2 or [B and C] FGF1 and compared to cells with heparin in the presence of either FGF2 or FGF1. Cells exposed to medium and the growth factor only was used as a negative control. Data are presented as mean ±S. D. (n=3). * denotes significant differences compared to the negative control.
3. Discussion
In this study we have demonstrated that perlecan synthesized by vascular SMCs is substituted with both CS and HS. Murine SMC perlecan has been shown to be decorated with HS (Tran et al., 2004) while human SMC extracellular matrix has been shown to contain both HS and CS (Yamamoto et al., 2005). Perlecan has previously been immunopurified from many cell types including endothelial cells, colon carcinoma cells, articular chondrocytes and keratinocytes (Farndale et al., 1986; Knox et al., 2005; Knox et al., 2001; Knox et al., 2002; Melrose et al., 2006). EC and colon carcinoma perlecan is predominantly HS substituted (Knox et al., 2001; Knox et al., 2002), chondrocyte perlecan is substituted with both CS and HS (Chuang et al., 2010) while epithelial perlecan is tri-substituted with CS, HS and KS (Knox et al., 2005). The HS side chains of perlecan are involved in matrix stabilization, remodeling and repair, cell signaling and angiogenesis (Bix et al., 2006; Bix and Iozzo, 2008; Iozzo, 2005; Kadenhe-Chiweshe et al., 2008; Melrose et al., 2008; Whitelock and Iozzo, 2005; Whitelock et al., 2008). The specific role(s) of CS chains in vascular biology remain to be established; however, other proteoglycans such as cartilage aggrecan also contain a significant CS content (Plaas et al., 1997) and in this case the CS chains have important water binding and space-filling properties which provide the cartilage with weight bearing properties. Such a role cannot be envisaged for vascular perlecan; however, its CS chains may nevertheless provide hydrophilic properties that may interact with cells to control cellular attachment to the perlecan protein core when denuded of its GAG chains. Moreover, CS chains that decorate cartilage perlecan facilitate collagen fibril assembly (Kvist et al., 2006) which suggests a role for CS in vascular matrix assembly.
Some of the growth inhibitory activity of SMC-derived perlecan resides within the HS chains and it is thought that these chains bind to and inhibit the activity of heparin-binding growth factors (Tran et al., 2004). SMC proliferation is increased in late lesions after heparinase treatment, supporting the idea that the HS chains act as a growth factor reservoir and inhibit growth (Kinsella et al., 2003). The anti-proliferative properties of SMC perlecan can be inhibited by heparinase treatment while Hspg2Δ3/Δ3 mice that lack perlecan decorated with HS also display increased intimal hyperplasia in response to vascular injury (Bingley et al., 1998; Campbell et al., 1992; Clowes and Clowes, 1985; Kinsella et al., 2003; Tran et al., 2004). In contrast, the role of EC proteoglycans in the modulation of SMC proliferation remains controversial as they have been reported to either inhibit or promote SMC proliferation, which may depend on the source and state of the endothelial cells (Benitz et al., 1990; Castellot et al., 1981; Nugent et al., 1993; Whitelock et al., 1997). In this study we have demonstrated that SMCs do not adhere to perlecan derived from either SMCs or ECs that is decorated with GAGs. However, when the GAGs are removed, the cells adhere to the protein core and proliferate. Thus, EC perlecan can either inhibit or support SMC adhesion, depending upon the presence of the GAG chains. We have also discovered in this study that SMCs exhibit reduced levels of polymerized actin fibers when they are plated onto perlecan proteoglycan compared to cells plated onto the protein core. This suggests that SMC perlecan GAG chains inhibit actin polymerization required for proper cell spreading and adhesion. Indeed, endorepellin promotes actin fiber depolymerization in ECs and inhibits angiogenesis (Bix et al., 2004; Mongiat et al., 2003). Notably, adsorbed full length EC perlecan and recombinantly expressed perlecan domain V support endothelial cell adhesion via the α2β1 integrin indicating that endorepellin in solution is acting as a competitive inhibitor of endogenously produced perlecan (Jung et al., 2013).
It has been reported that heparin or HS alone do not elicit the same level of SMC growth inhibition as intact perlecan (Garl et al., 2004), suggesting a role for the protein core in inhibiting proliferation in concert with the HS chains. Here, we have shown that both domains III and V of perlecan are involved in SMC and EC adhesion and that with the addition of the perlecan domain III antibody (mAb 7B5) the proliferation of SMCs is stimulated, in agreement with an earlier report (Walker et al., 2003). Additionally, ECs transfected with an antisense vector against perlecan domain III have a reduced capacity to limit intimal hyperplasia (Nugent et al., 2000). The integrin α2β1 is involved in the adhesion of SMCs and ECs to adsorbed perlecan and also supports the adhesion of vascular SMCs to collagen (Skinner et al., 1994) as well as platelets and endothelial cells to perlecan (Bix et al., 2004; Jung et al., 2013; Lord et al., 2011). ECs bind to perlecan domain V/endorepellin through integrin α2β1 (Jung et al., 2013), and specifically to the LG3 region (Bix et al., 2004). Notably, osteosarcoma MG63 cells bind to perlecan domain IV through the β1 integrin and this interaction triggers cytoskeletal organization and FAK activation (Farach-Carson et al., 2008), suggesting a role for other domains of perlecan in cell adhesion. In our study, SMCs did not bind to domain V/endorepellin alone, however the domain V antibody was able to reduce SMC adhesion to the full length protein core. This suggests that SMCs bind to domain V when present in the full protein core and that other sites in perlecan may be involved in SMC adhesion. SMC adhesion to the perlecan protein core was also found to involve the α2β1 integrin whose binding site is in domain V. Given that the antibodies against domain III were able to interfere with cell adhesion, it is plausible that specific sequences that promote vascular SMC adhesion reside within domains III and V.
Both SMC and EC perlecan form HS-dependent ternary complexes with FGF2 and FGFR1c and these complexes enhance FGF signaling by promoting the growth of BaF-32 cells. FGF2 is a potent mitogen for the proliferation of medial SMCs after vascular injury; however, FGF2 does not regulate the proliferation of SMCs that have migrated into the intima (Koyama and Reidy, 1997; Olson et al., 1992), indicating that other growth factors may be involved. Bovine EC proteoglycans inhibit FGF2-mediated proliferation of bovine SMCs (Nugent et al., 1993; Nugent et al., 2000). Interestingly, bovine SMCs synthesize FGF2 and not FGF1, while human SMCs synthesize both FGF1 and 2 (Weich et al., 1990) indicating that different growth factor activities may occur between species. Human vascular SMCs express FGFRs 1 – 4 in both normal and diseased arteries while FGF2 is also constitutively expressed by contractile SMCs in normal arteries, suggesting an important role for this growth factor family in vascular homeostasis (Hughes, 1996). ECs and SMCs in the quiescent adult aortic intima express low levels of FGF1 and FGF2 while both SMCs and ECs in culture express much higher levels of these growth factors (Speir et al., 1991), suggesting that growth factor expression is up-regulated in proliferating SMCs and ECs and this may contribute in vivo to SMC proliferation. While FGF2 is reported to be the major mitogen for SMCs (Lindner and Reidy, 1991), FGF1 is also known to stimulate the proliferation of SMCs in vitro (Ghiselli et al., 2003; Weich et al., 1990; Winkles et al., 1987). In this study, SMC perlecan did not enable FGF1 signaling through either FGFR1c or FGFR3c. Together these data suggest that FGF1 may also have a mitogenic role for SMCs and that HS structures other than those decorating SMC perlecan support the activity of this growth factor. It is possible that proliferation of SMCs during vascular injury, which is mediated by FGF1, may occur through direct contact of the SMCs with the EC basement membrane. During endothelial injury, damage to the EC basement membrane may occur exposing the SMCs to EC perlecan, which supports FGF1 signaling through FGFR1c and 3c. This suggests that FGF1 may also be an important growth factor for SMC proliferation that is sequestered in the EC basement membrane (Castellot et al., 1981; Whitelock et al., 1997).
In summary, we report here for the first time a differential glycosylation for a central proteoglycan simultaneously expressed by the two main cells of the vasculature, ECs and SMCs. We hypothesize that this differential glycosylation, with EC perlecan covalently linked to HS chains and the SMC perlecan covalently linked to both HS and CS chains, may represent an additional level of fine tuning and cell-specific control of potent angiogenic signals such as FGF family members as well as cell adhesion and proliferation. Our results are of broad significance insofar as the HS-enriched perlecan could not only contribute to the well-established EC inhibitory activity towards SMCs but could also play direct roles in modulating the bioactivity of other pro-angiogenic factors such as VEGF and PDGF family members.
4. Experimental Procedures
4.1 Antibodies, enzymes and cells
The monoclonal anti-perlecan domain III (clone 7B5) was provided by Prof Renato Iozzo, while the monoclonal antibodies against perlecan domain I (clones A71 and A76), domain IV (clone A7L6) and domain V (clone A74) and fibronectin (clone A17) were purchased from Abcam, Cambridge, MA, USA. The mouse monoclonal antibody against perlecan (clone 5D7-2E4) and the mouse monoclonal antibody against the integrin α2β1 (clone BHA2.1) were purchased from Merck-Millipore, Billerica, MA, USA. A rabbit polyclonal anti-domain V antibody (anti-endorepellin) was as previously described (Bix et al., 2004), while a rabbit polyclonal anti-perlecan antibody (clone CCN-1) was raised in house against immunopurified primary human coronary artery endothelial cell (EC) perlecan. The rabbit polyclonal anti-laminin antibody was purchased from Rockland, Gilbertsville, PA, USA. Antibodies against HS (clone 10E4) and heparinase (Hep) III generated HS-stubs (clone 3G10) were from Seikagaku Corp., Tokyo, Japan. Antibodies reactive against keratan sulfate (KS, clone 5D4) and the chondroitinase ABC (C’ase ABC) generated unsulfated (clone 1B5), 4-sulfated (clone 2B6) and 6-sulfated (clone 3B3) CS stubs were provided by Prof. Bruce Caterson, Cardiff University, Cardiff, Wales, UK. The biotinylated anti-mouse immunoglobulin (Ig) or anti-rabbit IgG secondary antibodies, streptavidin-horse radish peroxidase (SA-HRP) and streptavidin-fluorescein (SA-FITC) were purchased from GE Healthcare, Little Chalfont Buckinghamshire, UK. HRP conjugated anti-mouse Ig raised in sheep was purchased from Merck-Millipore, Billerica, MA, USA. Endoglycosidase enzymes, chondroitinase ABC (C’ase ABC), heparinase I (Hep I) (EC 4.2.2.7) and heparinase III (Hep III) (EC 4.2.2.8) were purchased from Seikagaku Corp., Tokyo, Japan. Primary human coronary artery smooth muscle cells (SMC) and EC were purchased from Cell Applications (San Diego, CA, USA). All other chemicals were purchased from Sigma Aldrich (Castle Hill, Australia) unless stated otherwise.
4.2 Immunocytochemistry
SMC (passage 4) were cultured to confluence on microscope slides (Ultrafrost, Lomb Scientific), fixed with ice cold acetone for 3 min and rinsed with 50 mM Tris-HCl, 0.15 M NaCl, pH 7.6 (TBS). Slides were then blocked with 0.1% (w/v) casein in TBS for 1 h at 25 °C followed by incubation with the primary antibodies at a final concentration of 2 μg/ml for 16 h at 4 °C. Primary antibodies were used against perlecan (clone A71, 2 μg/ml), fibronectin (1 μg/ml), laminin (2 μg/ml), HS (clone 10E4, 1 μg/ml), CS (clone CS56, ascites 1:500) and KS (clone 5D4, conditioned medium 1:500). Slides were rinsed twice with TBS containing 0.1 % (w/v) Tween-20 (TBST) and incubated with the biotinylated anti-mouse or anti-rabbit secondary antibodies (1:500) for 1 h at 25 °C before rinsing twice with TBST and incubation with SA-FITC (1:250) for 30 min at 25 °C followed by 4 TBST washes. The slides were then counterstained with 1 μg/ml of 4′, 6-diamidino-2-phenylindole, dilactate (DAPI, Invitrogen, Carlsbad, CA, USA) in PBS for 10 min in the dark and rinsed 4 times with the deionized water before imaging using fluorescence microscopy (Zeiss Axioskop Mot Mat 2, Sydney, Australia).
4.3 Isolation of perlecan from SMCs and ECs
Perlecan was isolated from the conditioned medium produced by cultured SMC and ECs by anion exchange and monoclonal anti-perlecan domain I antibody (clone A71) affinity chromatography, as previously described (Knox et al., 2001; Whitelock et al., 1999).
4.4 ELISA
Immunopurified perlecan (10 μg/ml) was coated onto 96 – well high – binding ELISA plates for 2 h at 25°C. Wells were rinsed twice with Dulbecco’s phosphate buffered saline (DPBS), pH 7.4, and blocked with 0.1% (w/v) casein in DPBS for 1 h at 25 °C and were then either digested or not with C’ase ABC (0.05 U/ml) and/ or Hep III (0.01 U/ml) in PBS, pH 7.2 for 16 h at 37 °C and rinsed with DPBS containing 1% (w/v) Tween-20 (PBST). Primary antibodies diluted in 0.1% (w/v) casein in DPBS were then added to the wells for 2 h at 25 °C. These included perlecan antibody clones A76 (2 μg/ml), 7B5 (2 μg/ml), A7L6 (4 μg/ml) and A74 (2 μg/ml), mouse monoclonal anti - CS (clone CS56, ascites 1:1000), un-sulfated CS stub (clone 1B5, 1:500), anti-4-sulfated CS stub (clone 2B6, 1:500) and anti-6-sulfated CS stub (clone 3B3, 1:500), mouse monoclonal anti-HS (clone 10E4,1 μg/ml) and mouse monoclonal anti-HS stub antibody (clone 3G10, 1:500 dilution), bound primary antibody was detected by incubation with biotinylated mouse or rat specific secondary antibodies (GE Biosciences, Sydney, Australia, 1:1000) diluted in 0.1 % (w/v) casein in DPBS for 1 h at RT. The bound biotin was detected with SA-HRP (GE Biosciences, Sydney, Australia, 1:500) for 30 min at 25 °C using 2 mM 2,2′-azino-di-3-ethylbenzthiazoline sulfonic acid (ABTS) and H2O2 as chromogen in 50 mM sodium citrate, pH 4.6. Absorbance values were measured at 405 nm.
Analysis of the ECM produced by SMCs and ECs was performed by ELISA. SMC or ECs seeded into well plates at a density of 1×104 cells/well in either EC medium, MesoEndo cell, or SMC growth medium (Cell Applications, San Diego, CA, USA) were cultured for 7 days in an atmosphere of 5% CO2 in air at 37°C with 95% humidity. The media was replaced every 3 days. After day 7 the cultures were terminated by cell lysis using 0.01 M ammonium hydroxide and the plates rinsed in DPBS prior to measurement of the cell elaborated ECM by ELISA analysis as indicated above.
4.5 PCR
Total RNA was isolated from SMCs and ECs using TRI Reagent (1 ml per 107 cells) and then treated with DNase using the RQ1 RNase-free DNase kit (Promega) to remove contaminating DNA. Subsequently, 1 μg RNA was transcribed into cDNA using oligo d(T)23 priming (ProtoScript® M-MuLV First Strand cDNA synthesis kit, GeneSearch Pty Ltd, Arundel, Australia) and amplified during 40 cycles by PCR utilizing perlecan domain specific primers (Table 1). The reactants were cycled at 95 °C for 1 min, 60 °C for 1 min and 72 °C for 90 s to enable denaturation, annealing and extension respectively. PCR reaction products were then separated on a 1 % (w/w) agarose gel at 60 V for 1 h in TBE buffer (89 mM Tris base, 89 mM boric acid and 2 mM EDTA, pH 8.0). The gels were stained with GelRed (Jomar Diagnostics, Stepney, Australia) for 30 min and DNA bands visualized under UV light.
4.6 SDS-PAGE and immunoblotting of perlecan samples
Purified perlecan samples (10 μg/lane) were predigested with Hep III (0.01 U/ml) and/or C’ase ABC (0.05U/ml) in DPBS pH 7.2 at 37 °C for 16 h, prior to electrophoresis in 3–8% Tris-Acetate NuPAGE® SDS-PAGE gels (Invitrogen, Carlsbad, CA, USA) in Tris-tricine running buffer (50 mM tricine, 50 mM Tris base, 0.1% w/v SDS, pH 8.3) at 200 V for 1 h. Molecular weight markers (HiMark®, Invitrogen, Carlsbad, CA, USA) were electrophoresed on each gel. The gels were immunoblotted to polyvinylidene difluoride (PVDF) membranes (Immobilon-P, Millipore, Billerica, MA, USA) in transfer buffer (5 mM bicine, 5 mM Bis-Tris, 0.2 mM EDTA, 0.005% SDS, 1% v/v methanol, pH 7.2) using a semi-dry blotter (Invitrogen, Carlsbad, CA, USA) using constant power (300 mA and 20 V) for 1 h. The membranes were blocked with 1% w/v BSA in 20 mM Tris-HCL, 136 mM NaCl, pH 7.6 containing 0.1% (w/v) Tween-20 (TBST) for 1 h at RT. The transferred perlecan samples were incubated with primary antibodies diluted in 1% w/v BSA/TBST for 16 h at 4°C. Membranes were then rinsed with TBST, incubated with anti-rabbit or anti-mouse IgG HRP conjugated secondary antibody (1:50,000) for 45 min at RT, rinsed with TBST and TBS before being imaging by chemiluminescence (Femto reagent kit, Pierce Biotechnology, Rockford, IL, USA) and the images recorded on X-ray film (Australian Imaging Distributors, North Ryde, NSW, Australia).
4.7 Mass spectrometry
Purified perlecan samples (10 μg/lane) were reduced (10 mM DTT for 10 min at 95°C), alkylated (25 mM IAA for 20 min at 25 °C) and electrophoresed on 3–8% Tris-Acetate NuPAGE® SDS-PAGE gels as described above. The gels were stained with 0.1% (w/v) Coomassie Blue G250 in 2 % (v/v) phosphoric acid, 10 % (w/v) ammonium sulfate, and 20 % (v/v) methanol at 25 °C for 16 h followed by destaining with 50 % (v/v) methanol. Gels were imaged prior to excising bands of interest for liquid chromatography tandem mass spectrometry (LC-MS2) analysis. Excised bands were destained with 50 % (v/v) acetonitrile in 50 mM NH4HCO3, washed with 100 % (v/v) acetonitrile, and dried in an oven at 50 °C. Trypsin (sequencing grade, Promega, Sydney, Australia) at a final concentration of 20 μg/ml in 50 mM NH4HCO3 was added to the dried bands and incubated at 30 °C for 16 h. Ten microlitres of 50 mM NH4HCO3 was then added to each band prior to sonication for 5 min. Twenty microlitres of each sample was subjected to peptide analysis by LC-MS2.
4.8 Cell adhesion assays
96 well tissue culture polystyrene (TCPS) plates were coated with SMC perlecan (10 μg/ml), EC perlecan (10 μg/ml) or recombinant perlecan domain V (10 μg/ml), expressed and purified as previously described (Jung et al., 2013) for 16 h at 4 °C. Wells were then blocked with 3% (w/v) BSA in PBS for 1 h at 37 °C. Selected wells were treated with 0.01 U/ml Hep III, 0.05 U/ml C’ase ABC or both endoglycosidases for 16 h at 37 °C. The wells were then rinsed twice with PBS. Selected wells were then incubated with antibodies against perlecan, either the rabbit polyclonal antibody against endorepellin (1:1000 dilution) or a mouse monoclonal perlecan protein core domain III (clone 7B5, 2 μg/ml) for 1 h at 37 °C. ECs (passage 4) and SMCs (passage 4) were seeded at a density of 5×104 cells/ml for 2 h at 37 °C in 200 μl serum-free M199 culture medium containing 1% (w/v) BSA. To investigate the role of integrin α2β1 in EC adhesion to perlecan, cells were pre-treated with a mouse monoclonal antibody against α2β1 (clone BHA2.1, Millipore, Sydney, Australia, 2 μg/ml) for 20 min at 37 °C prior to seeding into perlecan coated wells for 2 h at 37 °C. Cells were rinsed with PBS and fixed with 4 % (w/v) paraformaldehyde in PBS for 15 min at 37 °C. Wells were then blocked with 1% (w/v) BSA in PBS for 5 min at 37 °C and then incubated with rhodamine-phalloidin (1:200, Life Technologies, Sydney, Australia) in 1 % (w/v) BSA in PBS for 15 min at 25 °C. Cells were rinsed three times with 0.5 % (w/v) Tween-20 in PBS for 5 min. Cells were then counterstained with DAPI (1:1000 in 1 % (w/v) BSA in PBS) for 15 min at 25 °C. Cells were imaged using a fluorescence microscope (Zeiss Axioskop Mot Mat 2, Sydney Australia) and cells were counted using morphometric software (ImageJ version 1.43u).
4.9 Cell proliferation assays
96 well flat bottom TCPS plates were coated with SMC perlecan (10 μg/ml) or EC perlecan (10 μg/ml) for 16 h at 4 °C. Wells were then blocked with 3% (w/v) BSA in PBS for 1 h at 37 °C and selected wells predigested with 0.01 U/mL Hep III, 0.05 U/ml C’ase ABC or with both endoglycosidases for 16 h at 37 °C and rinsed twice with PBS. Selected wells were incubated with rabbit polyclonal antibody against endorepellin (1:1000 dilution), or mouse monoclonal against perlecan domain III (clone 7B5, 2 μg/ml) for 1 h at 37 °C. Cells were seeded on to the wells at a density of 5×104 cells/ml for 2 h at 37 °C in 200 μl serum-free M199 culture medium containing 1% (w/v) BSA. To investigate the role of integrin α2β1 in EC adhesion to perlecan, cells were pre-treated with a blocking mouse monoclonal antibody against α2β1 (clone BHA2.1, Millipore, Sydney, Australia, 2 μg/ml) for 20 min at 37 °C prior to seeding on to perlecan coated wells for 2 h at 37 °C. The cells were then rinsed with PBS and fixed with 4 % (w/v) paraformaldehyde in PBS for 15 min at 37 °C and non-specific binding was blocked with 1% (w/v) BSA in PBS for 5 min at 37 °C. Cellular morphology was assessed using rhodamine-phalloidin which was added to the wells (1:200, Life Technologies, Sydney, Australia) in 1 % (w/v) BSA in PBS for 15 min at RT. The cells were then rinsed three times with 0.5 % (w/v) Tween-20 in PBS for 5 min. Cell nuclei were stained with DAPI (1:1000 in 1 % (w/v) BSA in PBS) for 15 min at RT. The cells were imaged using fluorescence microscopy (Zeiss Axioskop Mot Mat 2, Sydney Australia) and cells counted using morphometric software (ImageJ version 1.43u).
4.10 Ligand and Carbohydrate Engagement assay (LACE)
Perlecan (5 μg/ml) was adsorbed onto 96-well high-binding ELISA plates for 16 h at 4 °C. Wells were washed with DPBS and selected wells were digested with 0.01 U/ml Hep III, 0.05 U/mL C’ase ABC, or both, in 0.01% (w/v) BSA with DPBS pH 7.2 for 3 h at 37 °C. Wells were blocked with 3% (w/v) BSA in PBS for 1 h at 25 °C and washed twice with PBST. Either recombinant human FGF1 or 2 (5 nM, Life Technologies, Carlsbad, CA, USA) and either soluble recombinant human FGFR 1c or 3c Fc chimeric proteins (5 nM, R&D Systems, Minneapolis, MN, USA) were incubated in PBST for 10 min before transferring into each well and incubated for 1 h at 37 °C. Controls were incubated with FGFR1c or 3c only. Wells were then washed twice with PBST and incubated with HRP conjugated anti-human IgG (FC specific) secondary antibody (1:1000) in PBST for 1 h at 37 °C. Wells were washed 4 times with PBST before the addition of ABTS and the absorbance measured at 405 nm.
4.11 BaF-32 cell proliferation assays
BaF-32 cells are an IL-3 dependent and HSPG deficient myeloid B cell line that have been stably transfected with either FGFR1c or FGFR3c (Ornitz et al., 1996; Ornitz et al., 1992). BaF-32 cells are a model system developed to identify HS/heparin structures that interact with FGFs and their receptors. The readout of this assay is cell proliferation that indicates the formation of ternary complexes in situ. BaF-32 cells were maintained in RPMI 1640 medium containing 10 % (v/v) FBS, 10 % (v/v) WEHI-3BD conditioned medium and 1 % (v/v) penicillin/streptomycin. WEHI-3BD cells were maintained in RPMI 1640 medium supplemented with 2 g/l sodium bicarbonate, 10 % (v/v) FBS and 1 % (v/v) penicillin/streptomycin and the conditioned medium was collected 3 times per week and stored at −20 °C until required. For the mitogenic assays, the BaF-32 cells were transferred into IL-3 depleted medium for 24 h prior to experimentation and seeded into 96-well plates at a density of 2×104 cells/well in the presence of either FGF1 (0.03 nM) or FGF2 (0.03 nM), heparin (30 nM) and either EC perlecan (2 μg/ml) or SMC perlecan (2 μg/ml). Cells were incubated for 96 h in 5% CO2 at 37 °C and the number of cells present was assessed using the MTS reagent (Promega, Madison, Wisconsin, USA) by adding to the cell cultures for 6 h prior to measuring the absorbance at 490 nm.
4.12 Statistical analysis
A one-way analysis of variance (ANOVA) was performed to compare multiple conditions. Results of p < 0.05 were considered significant. Experiments were performed in triplicate and experiments were repeated three times.
Highlights.
SMC adhesion to perlecan core, but not EC adhesion, is inhibited by its GAGs.
SMCs and ECs adhere to perlecan domain III in addition to domain V.
SMC perlecan has distinct FGF signaling activities from EC perlecan.
Acknowledgments
The authors acknowledge the technical assistance of Dr. Bonny Tsoi, Graduate School of Biomedical Engineering, The University of New South Wales and thank Prof. Bruce Caterson (Cardiff University, Wales, UK) for the kind gift of the 1B5, 2B6 and 3B3 hybridoma culture supernatants. These studies were supported by the National Health and Medical Research Council, Australia (Project Grant 352562), the National Heart Foundation funding, Australia, and in part by the National Institutes of Health grant R01 CA47282.
Abbreviations
- C’ase ABC
chondroitinase ABC
- CS
chondroitin sulfate
- EC
human coronary artery endothelial cell
- FGF
fibroblast growth factor
- FGFR
FGF receptor
- GAG
glycosaminoglycan
- Hep
heparinase
- HS
heparan sulfate
- HSPG
heparan sulfate proteoglycan
- SMC
human coronary artery smooth muscle cell
- TCPS
tissue culture polystyrene
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aviezer D, Iozzo RV, Noonan DM, Yayon A. Suppression of autocrine and paracrine functions of basic fibroblast growth factor by stable expression of perlecan antisense cDNA. Mol Cell Biol. 1997;17:1938–1946. doi: 10.1128/mcb.17.4.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitz WE, Kelley RT, Anderson CM, Lorant DE, Bernfield M. Endothelial Heparan Sulfate Proteoglycan. I. Inhibitory Effects on Smooth Muscle Cell Proliferation. Am J Respir Cell Mol Biol. 1990;2:13–24. doi: 10.1165/ajrcmb/2.1.13. [DOI] [PubMed] [Google Scholar]
- Berry D, Shriver Z, Natke B, Kwan CP, Venkataraman G. Heparan sulphate glycosaminoglycans derived from endothelial cells and smooth muscle cells differentially modulate fibroblast growth factor-2 biological activity through fibroblast growth factor receptor-1. Biochem J. 2003;373:241–249. doi: 10.1042/BJ20021760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingley JA, Hayward IP, Campbell JH, Campbell GR. Arterial heparan sulfate proteoglycans inhibit vascular smooth muscle cell proliferation and phenotype change in vitro and neointimal formation in vivo. J Vasc Res. 1998;28:308–318. doi: 10.1016/s0741-5214(98)70167-3. [DOI] [PubMed] [Google Scholar]
- Bix G, Castello R, Burrows M, Zoeller JJ, Weech M, Iozzo RA, Cardi C, Thakur ML, Barker CA, Camphausen K, Iozzo RV. Endorepellin in vivo: targeting the tumor vasculature and retarding cancer growth and metabolism. J Natl Cancer Inst. 2006;98:1634–1646. doi: 10.1093/jnci/djj441. [DOI] [PubMed] [Google Scholar]
- Bix G, Fu J, Gonzalez EM, Macro L, Barker A, Campbell S, Zutter MM, Santoro SA, Kim JK, Höök M, Reed CC, Iozzo RV. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through α2β1 integrin. J Cell Biol. 2004;166:97–107. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix G, Iozzo RV. Novel interactions of perlecan: Unraveling perlecan’s role in angiogenesis. Microsc Res Tech. 2008;71:339–348. doi: 10.1002/jemt.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JH, Rennick RE, Kalevitch SG, Campbell GR. Heparan sulfate-degrading enzymes induce modulation of smooth muscle phenotype. Exp Cell Res. 1992;200:156–167. doi: 10.1016/s0014-4827(05)80084-9. [DOI] [PubMed] [Google Scholar]
- Castellot JJ, Addonizio ML, Rosenberg R, Karnowsky MJ. Cultured endothelial cells produced a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981;90:372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CY, Lord MS, Melrose J, Rees MD, Knox SM, Freeman C, Iozzo RV, Whitelock JM. Heparan sulfate-dependent signaling of fibroblast growth factor 18 by chondrocyte-derived perlecan. Biochemistry. 2010;49:5524–5532. doi: 10.1021/bi1005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes AW, Clowes MM. Kinetics of cellular proliferation after arterial injury. II. Inhibition of smooth muscle growth by heparin. Lab Invest. 1985;52:611–616. [PubMed] [Google Scholar]
- Clowes AW, Karnowsky MJ. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977;265:625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Costell M, Carmona R, Gustafsson E, Gonzalez-Iriarte M, Fässler R, Munoz-Chápuli R. Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circ Res. 2002;91:158–164. doi: 10.1161/01.res.0000026056.81424.da. [DOI] [PubMed] [Google Scholar]
- Farach-Carson MC, Brown AJ, Lynam M, Safran JB, Carson DD. A novel peptide sequence in perlecan domain IV supports cell adhesion, spreading and FAK activation. Matrix Biol. 2008;27:150–160. doi: 10.1016/j.matbio.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Forsten KE, Courant NA, Nugent MA. Endothelial proteoglycans inhibit bFGF binding and mitogenesis. J Cell Physiol. 1997;172:209–220. doi: 10.1002/(SICI)1097-4652(199708)172:2<209::AID-JCP8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Garl PJ, Wenzlau JM, Walker HA, Whitelock JM, Costell M, Weiser-Evans MC. Perlecan-induced suppression of smooth muscle cell proliferation is mediated through increased activity of the tumor suppressor PTEN. Circ Res. 2004;94:175–183. doi: 10.1161/01.RES.0000109791.69181.B6. [DOI] [PubMed] [Google Scholar]
- Ghiselli G, Chen J, Kaou M, Hallak H, Rubin R. Ethanol inhibits fibroblast growth factor-induced proliferation of aortic smooth muscle cells. Arterioscl Throm Vas. 2003;23:1808–1813. doi: 10.1161/01.ATV.0000090140.20291.CE. [DOI] [PubMed] [Google Scholar]
- González-Iriarte M, Carmona R, Pérez-Pomares JM, Macías D, Costell M, Munoz-Chápuli R. Development of the coronary arteries in a murine model of transposition of great arteries. J Mol Cell Cardio. 2003;35:795–802. doi: 10.1016/s0022-2828(03)00134-2. [DOI] [PubMed] [Google Scholar]
- Gustafsson E, Almonte-Becerril M, Bloch W, Costell M. Perlecan maintains microvessel integrity in vivo and modulates their formation in vitro. PLoS One. 2013;8:e53715. doi: 10.1371/journal.pone.0053715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedin U, Roy J, Tran PK. Control of smooth muscle cell proliferation in vascular disease. Curr Opin Lipidol. 2004;15:559–565. doi: 10.1097/00041433-200410000-00010. [DOI] [PubMed] [Google Scholar]
- Hedin U, Roy J, Tran PK, Lundmark K, Rahman A. Control of smooth muscle cell proliferation - The role of the basement membrane. Thromb Haemostasis. 1999;82:23–26. [PubMed] [Google Scholar]
- Heickendorff L. The basement membrane of arterial smooth muscle cells. APMIS. 1989;S9:23–26. [PubMed] [Google Scholar]
- Hughes SE. Localisation and differential expression of the fibroblast growth factor (FGFR) multigene family in normal and atherosclerotic human arteries. Cardiovasc Res. 1996;32:557–569. [PubMed] [Google Scholar]
- Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108:349–355. doi: 10.1172/JCI13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Zoeller JJ, Nyström A. Basement membrane proteoglycans: modulators par excellence of cancer growth and angiogenesis. Mol Cells. 2009;27:503–513. doi: 10.1007/s10059-009-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishijima M, Suzuki N, Hozumi K, Matsunobu T, Kosaki K, Kaneko H, Hassell JR, Arikawa-Hirasawa E, Yamada Y. Perlecan modulates VEGF signaling and is essential for vascularization in endochondral bone formation. Matrix Biol. 2012;31:234–245. doi: 10.1016/j.matbio.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Lord MS, Cheng B, Lyons JG, Alkhouri H, Hughes JM, McCarthy SJ, Iozzo RV, Whitelock JM. Mast cells produce novel shorter forms of perlecan that contain functional endorepellin: A role in angiogenesis and wound healing. J Biol Chem. 2013;288:3289–3304. doi: 10.1074/jbc.M112.387811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadenhe-Chiweshe A, Papa J, McCrudden KW, Frischer J, Bae JO, Huang J, Fisher J, Lefkowitch JH, Feirt N, Rudge J, Holash J, Yancopoulos GD, Kandel JJ, Yamashiro DJ. Sustained VEGF blockade results in microenvironmental sequestration of VEGF by tumors and persistent VEGF receptor-2 activation. Mol Cancer Res. 2008;6:1–9. doi: 10.1158/1541-7786.MCR-07-0101. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Ishijima M, Futami I, Tomikawa-Ichikawa N, Kosaki K, Sadasuki R, Yamada Y, Kurosawa H, Kaneko H, Arikawa-Hirasawa E. Synovial perlecan is required for osteophyte formation in knee osteoarthritis. Matrix Biol. 2013;32:178–187. doi: 10.1016/j.matbio.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella MG, Tran PK, Weiser-Evans MCM, Reidy M, Majack RA, Wight TN. Changes in perlecan expression during vascular injury: Role in the inhibition of smooth muscle cell proliferation in the late lesion. Arterioscl Throm Vas. 2003;23:608–614. doi: 10.1161/01.ATV.0000063109.94810.EE. [DOI] [PubMed] [Google Scholar]
- Kirn-Safran CB, Gomes RR, Brown AJ, Carson DD. Heparan sulfate proteoglycans: coordinators of multiple signaling pathways during chondrogenesis. Birth Defects Res C Embryo Today. 2004;72:69–88. doi: 10.1002/bdrc.20005. [DOI] [PubMed] [Google Scholar]
- Knox S, Fosang AJ, Last K, Melrose J, Whitelock J. Perlecan from human epithelial cells is a hybrid heparan/chondroitin/keratan sulfate proteoglycan. FEBS Lett. 2005;579:5019–5023. doi: 10.1016/j.febslet.2005.07.090. [DOI] [PubMed] [Google Scholar]
- Knox S, Melrose J, Whitelock J. Electrophoretic, biosensor, and bioactivity analyses of perlecans of different cellular origins. Proteomics. 2001;1:1534–1541. doi: 10.1002/1615-9861(200111)1:12<1534::aid-prot1534>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Knox S, Merry C, Stringer S, Melrose J, Whitelock J. Not all perlecans are created equal: interactions with fibroblast growth factor (FGF) 2 and FGF receptors. J Biol Chem. 2002;277:14657–14665. doi: 10.1074/jbc.M111826200. [DOI] [PubMed] [Google Scholar]
- Koyama H, Reidy M. reinjury of arterial lesions induces intimal smooth muscle cell replication that is not controlled by fibroblast growth factor 2. Circ Res. 1997;80:408–417. [PubMed] [Google Scholar]
- Kvist AJ, Johnson AE, Mörgelin M, Gustafsson E, Bengtsson E, Lindblom K, Aszódi A, Fässler R, Sasaki T, Timpl R, Aspberg A. Chondroitin sulfate perelcan enhances collagen fibril formation. Implications for perlecan chondrodysplasias. J Biol Chem. 2006;281:33127–33139. doi: 10.1074/jbc.M607892200. [DOI] [PubMed] [Google Scholar]
- Lindner V, Lappi DA, Baird A, Majack RA, Reidy MA. Role of fibroblast growth factor in vascular lesion formation. Circ Res. 1991;68:106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Lindner V, Reidy M. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991;88:3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord MS, Cheng B, McCarthy SJ, Jung M, Whitelock JM. The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials. 2011;32:6655–6662. doi: 10.1016/j.biomaterials.2011.05.062. [DOI] [PubMed] [Google Scholar]
- Lundmark K, Tran PK, Kinsella MG, Clowes AW, Wight TN, Hedin U. Perlecan inhibits smooth muscle cell adhesion to fibronectin: Role of heparan sulfate. J Cell Physiol. 2001;188:67–74. doi: 10.1002/jcp.1094. [DOI] [PubMed] [Google Scholar]
- Melrose J, Hayes AJ, Whitelock JM, Little CB. Perlecan, the “jack of all trades” proteoglycan of cartilaginous weight-bearing connective tissues. Bioessays. 2008;30:457–469. doi: 10.1002/bies.20748. [DOI] [PubMed] [Google Scholar]
- Melrose J, Roughley P, Knox S, Smith S, Lord M, Whitelock J. The structure, location, and function of perlecan, a prominent pericellular proteoglycan of fetal, postnatal, and mature hyaline cartilages. J Biol Chem. 2006;281:36905–36914. doi: 10.1074/jbc.M608462200. [DOI] [PubMed] [Google Scholar]
- Melrose J, Smith S, Cake M, Read R, Whitelock J. Spatial and temporal immunolocalisation of perlecan in the ovine meniscus. Histochem Cell Biol. 2005;124:225–235. doi: 10.1007/s00418-005-0005-0. [DOI] [PubMed] [Google Scholar]
- Melrose J, Smith S, Ghosh P, Whitelock J. Perlecan, the multidomain heparan sulfate proteoglycan of basement membranes, is also a prominent component of the cartilaginous primordia in the developing human fetal spine. J Histochem Cytochem. 2003;51:1331–1341. doi: 10.1177/002215540305101010. [DOI] [PubMed] [Google Scholar]
- Mongiat M, Sweeney S, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32:115–120. doi: 10.1016/s1357-2725(99)00123-5. [DOI] [PubMed] [Google Scholar]
- Nugent MA, Karnowsky MJ, Edelman ER. Vascular cell-derived heparan sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circ Res. 1993;73:1051–1060. doi: 10.1161/01.res.73.6.1051. [DOI] [PubMed] [Google Scholar]
- Nugent MA, Nugent HM, Iozzo RV, Sanchack K, Edelman ER. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc Natl Acad Sci U S A. 2000;97:6722–6727. doi: 10.1073/pnas.97.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson NE, Chao S, Lindner V, Reidy MA. Intimal smooth muscle cell proliferation after balloon catheter injury: The role of basic fibroblast growth factor. Am J Pathol. 1992;140:1017–1023. [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Yayon A, Flanagan JG, Svahn CM, Levi E, Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992;12:240–247. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaas AHK, Wong-Palms S, Roughley PJ, Midura RJ, Hascall VC. Chemical and immunological assay of the nonreducing terminal residues of chondroitin sulfate from human aggrecan. J Biol Chem. 1997;272:20603–20610. doi: 10.1074/jbc.272.33.20603. [DOI] [PubMed] [Google Scholar]
- Sharma B, Handler M, Eichstetter I, Whitelock J, Nugent MA, Iozzo RV. Antisense targeting of perlecan blocks tumor growth and angiogenesis in vivo. J Clin Invest. 1998;102:1599–1608. doi: 10.1172/JCI3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MP, Raines EW, Ross P. Dynamic expression of alpha 1 beta 1 and alpha 2 beta 1 integrin receptors by human vascular smooth muscle cells. Alpha 2 beta 1 integrin is required for chemotaxis across type I collagen-coated membranes. Am J Pathol. 1994;145:1070–1081. [PMC free article] [PubMed] [Google Scholar]
- Soulez M, Pilon EA, Dieudé M, Cardinal H, Brassard N, Qi S, Wu SJ, Durocher Y, Madore F, Perreault C, Hébert MJ. The perlecan fragment LG3 is a novel regulator of obliterative remodeling associated with allograft vascular rejection. Circ Res. 2012;110:94–104. doi: 10.1161/CIRCRESAHA.111.250431. [DOI] [PubMed] [Google Scholar]
- Speir E, Sasse J, Shtivastav S, Casscells W. Culture-induced increase of acidic and basic fibroblast growth factor activities and their association with the nuclei of vascular endothelial and smooth muscle cells. J Cell Physiol. 1991;147:362–373. doi: 10.1002/jcp.1041470223. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Blomgren K, Roy J, Tran PK, Hedin U. Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. J Histochem Cytochem. 1997;45:837–846. doi: 10.1177/002215549704500608. [DOI] [PubMed] [Google Scholar]
- Tran PK, Tran-Lundmark K, Soininen R, Tryggvason K, Thyberg J, Hedin U. Increased Intimal Hyperplasia and Smooth Muscle Cell Proliferation in Transgenic Mice with Heparan Sulfate-Deficient Perlecan. Circ Res. 2004;94:550–558. doi: 10.1161/01.RES.0000117772.86853.34. [DOI] [PubMed] [Google Scholar]
- Underwood PA, Mitchell S, Whitelock JM. Heparin fails to inhibit the proliferation of human vascular smooth muscle cells in the presence of human serum. J Vasc Res. 1998;35:449–460. doi: 10.1159/000025616. [DOI] [PubMed] [Google Scholar]
- Walker HA, Whitelock JM, Garl PJ, Nemenoff RA, Stenmark KR, Weiser-Evans MCM. Perlecan up-regulation of FRNK suppresses smooth muscle cell proliferation via inhibition of FAK signaling. Mol Biol Cell. 2003;14:1941–1952. doi: 10.1091/mbc.E02-08-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weich HA, Iberg N, Klagsbrun M, Folkman J. Expression of acidic and basic fibroblast growth factors in human and bovine vascular smooth muscle cells. Growth Factors. 1990;2:313–320. doi: 10.3109/08977199009167026. [DOI] [PubMed] [Google Scholar]
- Weiser MCM, Belknap JK, Grieshaber SS, Kinsella MG, Majack RA. Developmental regulation of perlecan gene expression in aortic smooth muscle cells. Matrix Biol. 1996;15:331–340. doi: 10.1016/s0945-053x(96)90136-5. [DOI] [PubMed] [Google Scholar]
- Whitelock J, Mitchell S, Underwood PA. The effect of human endothelial cell-derived proteoglycans on human smooth muscle cell growth. Cell Biol Int. 1997;21:181–189. doi: 10.1006/cbir.1996.0125. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Graham LD, Melrose J, Murdoch AD, Iozzo RV, Underwood PA. Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol. 1999;18:163–178. doi: 10.1016/s0945-053x(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Iozzo RV. Heparan sulfate: a complex polymer charged with biological activity. Chem Rev. 2005;105:2745–2764. doi: 10.1021/cr010213m. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CD, Schaefer L, Iozzo RV. The biology of perlecan and its bioactive modules. In: Karamanos NK, editor. Extracellular Matrix: Pathobiology and Signaling. Berlin: Walter de Gruyter GmbH & Co. KG; 2012. pp. 171–184. [Google Scholar]
- Wilusz RE, DeFrate LE, Guilak F. A biomechanical role for perlecan in the pericellular matrix of articular carrtilage. Matrix Biol. 2012;31:320–327. doi: 10.1016/j.matbio.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkles JA, Friesel R, Burgess WH, Howk R, Mehlman T, Weinstein R, Maciag T. Human vascular smooth muscle cells both express and respond to heparin-binding growth factor I (endothelial cell growth factor) Proc Natl Acad Sci U S A. 1987;84:7124–7128. doi: 10.1073/pnas.84.20.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall BP, Nyström A, Iozzo RA, Eble JA, Niland S, Krieg T, Eckes B, Pozzi A, Iozzo RV. Integrin a2b1 is the required receptor for endorepellin angiostatic activity. J Biol Chem. 2008;283:2335–2343. doi: 10.1074/jbc.M708364200. [DOI] [PubMed] [Google Scholar]
- Yamamoto C, Wakata T, Fujiwara Y, Kaji T. Induction of synthesis of a large heparan sulfate proteoglycan, perlecan, by thrombin in cultured human coronary smooth muscle cells. Biochim Biophys Acta. 2005;1722:92–102. doi: 10.1016/j.bbagen.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Zoeller JJ, McQuillan A, Whitelock J, Ho S-Y, Iozzo RV. A central function for perlecan in skeletal muscle and cardiovascular development. J Cell Biol. 2008;181:381–394. doi: 10.1083/jcb.200708022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller JJ, Whitelock J, Iozzo RV. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 2009;28:284–291. doi: 10.1016/j.matbio.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]