Abstract
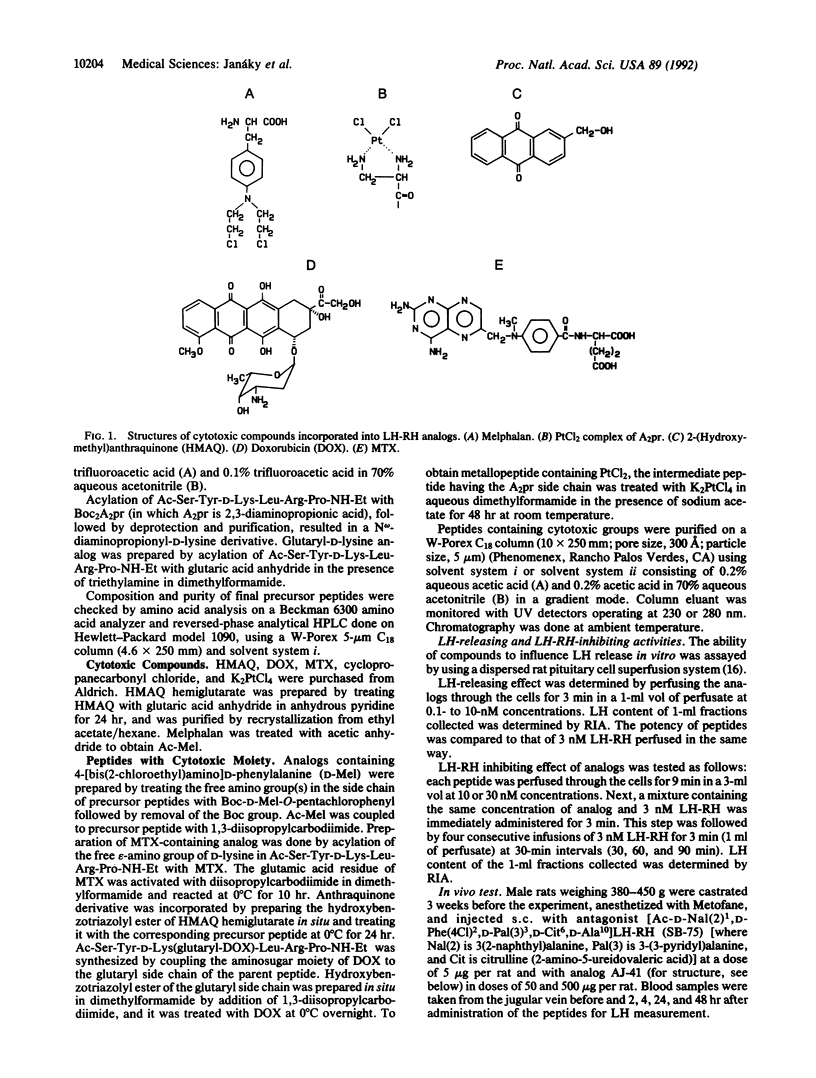
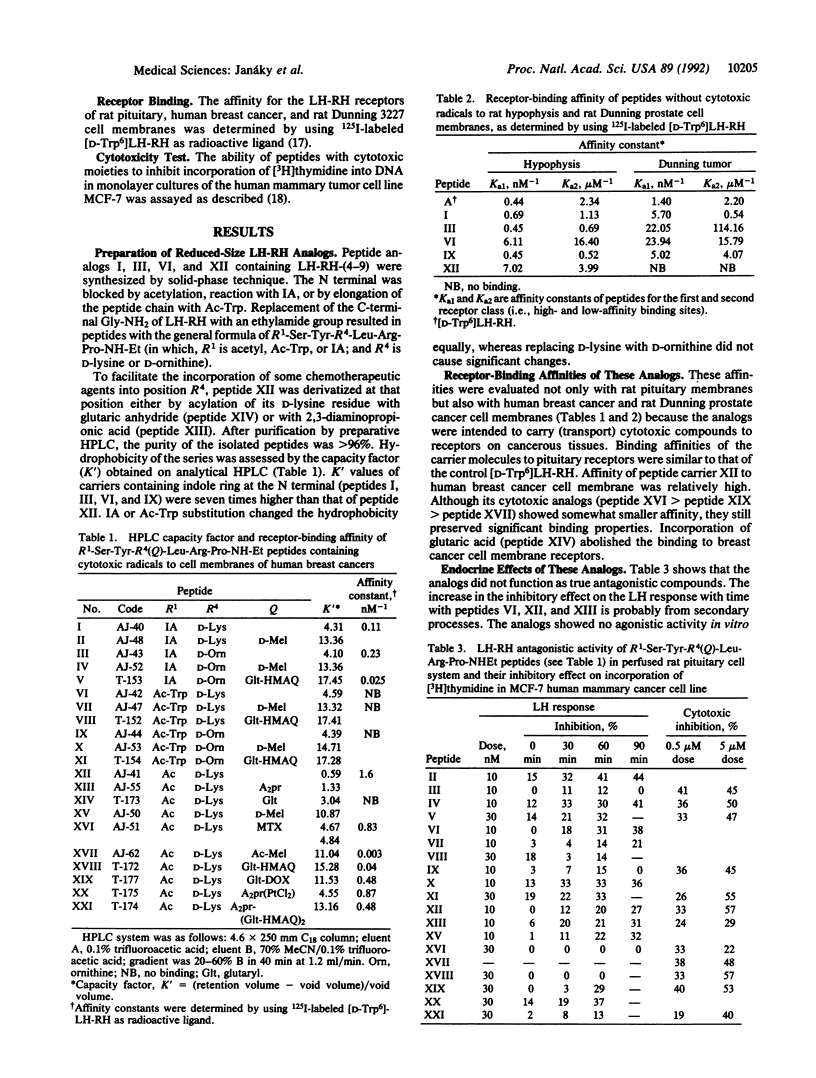
Five hexapeptide and heptapeptide analogs of luteinizing hormone-releasing hormone (LH-RH) were synthesized for use as carriers for cytotoxic compounds. These short analogs were expected to enhance target selectivity of the antineoplastic agents linked to them. Native LH-RH-(3-9) and LH-RH-(4-9) containing D-lysine and D-ornithine at position 6 were amidated with ethylamine and acylated on the N terminus. The receptor-binding affinity of one hexapeptide carrier AJ-41 (Ac-Ser-Tyr-D-Lys-Leu-Arg-Pro-NH-Et) to human breast cancer cell membranes was similar to that of [D-Trp6]LH-RH. Alkylating nitrogen mustards (melphalan, Ac-melphalan), anthraquinone derivatives including anticancer antibiotic doxorubicin, antimetabolite (methotrexate), and cisplatin-like platinum complex were linked to these peptides through their omega-amino group at position 6. The hybrid molecules showed no LH-RH agonistic activity in vitro and in vivo but had nontypical antagonistic effects on pituitary cells in vitro at the doses tested. These analogs showed a wide range of receptor-binding affinities to rat pituitaries and cell membranes of human breast cancer and rat Dunning prostate cancer. Several of these conjugates exerted some cytotoxic effects on MCF-7 breast cancer cell line.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajusz S., Janaky T., Csernus V. J., Bokser L., Fekete M., Srkalovic G., Redding T. W., Schally A. V. Highly potent analogues of luteinizing hormone-releasing hormone containing D-phenylalanine nitrogen mustard in position 6. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6318–6322. doi: 10.1073/pnas.86.16.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajusz S., Janaky T., Csernus V. J., Bokser L., Fekete M., Srkalovic G., Redding T. W., Schally A. V. Highly potent metallopeptide analogues of luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6313–6317. doi: 10.1073/pnas.86.16.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajusz S., Kovacs M., Gazdag M., Bokser L., Karashima T., Csernus V. J., Janaky T., Guoth J., Schally A. V. Highly potent antagonists of luteinizing hormone-releasing hormone free of edematogenic effects. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1637–1641. doi: 10.1073/pnas.85.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenstein M. A., Henkelman M. S., Klijn J. G. Direct inhibitory effect of a luteinizing hormone-releasing hormone agonist on MCF-7 human breast cancer cells. Eur J Cancer Clin Oncol. 1985 Dec;21(12):1493–1499. doi: 10.1016/0277-5379(85)90244-5. [DOI] [PubMed] [Google Scholar]
- Fekete M., Bajusz S., Groot K., Csernus V. J., Schally A. V. Comparison of different agonists and antagonists of luteinizing hormone-releasing hormone for receptor-binding ability to rat pituitary and human breast cancer membranes. Endocrinology. 1989 Feb;124(2):946–955. doi: 10.1210/endo-124-2-946. [DOI] [PubMed] [Google Scholar]
- Fujino M., Lobayashi S., Obayashi M., Shinagawa S., Fukuda T. Structure-activity relationships in the C-terminal part of luteinizing hormone releasing hormone(LH-RH). Biochem Biophys Res Commun. 1972 Nov 1;49(3):863–869. doi: 10.1016/0006-291x(72)90490-1. [DOI] [PubMed] [Google Scholar]
- Harris N., Dutlow C., Eidne K., Dong K. W., Roberts J., Millar R. Gonadotropin-releasing hormone gene expression in MDA-MB-231 and ZR-75-1 breast carcinoma cell lines. Cancer Res. 1991 May 15;51(10):2577–2581. [PubMed] [Google Scholar]
- Haviv F., Palabrica C. A., Bush E. N., Diaz G., Johnson E. S., Love S., Greer J. Active reduced-size hexapeptide analogues of luteinizing hormone-releasing hormone. J Med Chem. 1989 Oct;32(10):2340–2344. doi: 10.1021/jm00130a019. [DOI] [PubMed] [Google Scholar]
- Janáky T., Juhász A., Bajusz S., Csernus V., Srkalovic G., Bokser L., Milovanovic S., Redding T. W., Rékási Z., Nagy A. Analogues of luteinizing hormone-releasing hormone containing cytotoxic groups. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):972–976. doi: 10.1073/pnas.89.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten M. J., Rivier J. E. Gonadotropin-releasing hormone analog design. Structure-function studies toward the development of agonists and antagonists: rationale and perspective. Endocr Rev. 1986 Feb;7(1):44–66. doi: 10.1210/edrv-7-1-44. [DOI] [PubMed] [Google Scholar]
- König W., Geiger R. Eine neue Methode zur Synthese von Peptiden: Aktivierung der Carboxylgruppe mit Dicyclohexycarbodiimid unter Zusatz von 1-Hydroxy-benzotriazolen. Chem Ber. 1970;103(3):788–798. doi: 10.1002/cber.19701030319. [DOI] [PubMed] [Google Scholar]
- Matsuo H., Baba Y., Nair R. M., Arimura A., Schally A. V. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1334–1339. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- Miller W. R., Scott W. N., Morris R., Fraser H. M., Sharpe R. M. Growth of human breast cancer cells inhibited by a luteinizing hormone-releasing hormone agonist. Nature. 1985 Jan 17;313(5999):231–233. doi: 10.1038/313231a0. [DOI] [PubMed] [Google Scholar]
- Nicholson R. I., Walker K. J., Walker R. F., Read G. F., Turkes A., Robertson J. F., Blamey R. W. Review of the endocrine actions of luteinising hormone-releasing hormone analogues in premenopausal women with breast cancer. Horm Res. 1989;32 (Suppl 1):198–201. doi: 10.1159/000181345. [DOI] [PubMed] [Google Scholar]
- Qayum A., Gullick W., Clayton R. C., Sikora K., Waxman J. The effects of gonadotrophin releasing hormone analogues in prostate cancer are mediated through specific tumour receptors. Br J Cancer. 1990 Jul;62(1):96–99. doi: 10.1038/bjc.1990.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J., Amoss M., Rivier C., Vale W. Synthetic luteinizing hormone releasing factor. Short chain analogs. J Med Chem. 1974 Feb;17(2):230–233. doi: 10.1021/jm00248a019. [DOI] [PubMed] [Google Scholar]
- Rivier J., Vale W., Burgus R., Ling N., Amoss M., Blackwell R., Guillemin R. Synthetic luteinizing hormone-releasing factor analogs. Series of short-chain amide LRF homologs converging to the amino terminus. J Med Chem. 1973 May;16(5):545–549. doi: 10.1021/jm00263a031. [DOI] [PubMed] [Google Scholar]
- Sandow J., König W. Studies with fragments of a highly active analogue of luteinizing hormone releasing hormone. J Endocrinol. 1979 May;81(2):175–182. [PubMed] [Google Scholar]
- Sievertsson H., Chang J. K., Bogentoft C., Currie B. L., Folkers K. Synthesis of the luteinizing releasing hormone of the hypothalamus and its hormonal activity. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1566–1571. doi: 10.1016/s0006-291x(71)80265-6. [DOI] [PubMed] [Google Scholar]
- Sondak V. K., Bertelsen C. A., Tanigawa N., Hildebrand-Zanki S. U., Morton D. L., Korn E. L., Kern D. H. Clinical correlations with chemosensitivities measured in a rapid thymidine incorporation assay. Cancer Res. 1984 Apr;44(4):1725–1728. [PubMed] [Google Scholar]
- Szepeshazi K., Schally A. V., Juhasz A., Nagy A., Janaky T. Effect of luteinizing hormone-releasing hormone analogs containing cytotoxic radicals on growth of estrogen-independent MXT mouse mammary carcinoma in vivo. Anticancer Drugs. 1992 Apr;3(2):109–116. doi: 10.1097/00001813-199204000-00006. [DOI] [PubMed] [Google Scholar]



