Abstract
Within EU FP7 project NANOVALID, the (eco)toxicity of 7 well-characterized engineered nanomaterials (NMs) was evaluated by 15 bioassays in 4 laboratories. The highest tested nominal concentration of NMs was 100 mg/l. The panel of the bioassays yielded the following toxicity order: Ag > ZnO > CuO > TiO2 > MWCNTs > SiO2 > Au. Ag, ZnO and CuO proved very toxic in the majority of assays, assumingly due to dissolution. The latter was supported by the parallel analysis of the toxicity of respective soluble metal salts. The most sensitive tests/species were Daphnia magna (towards Ag NMs, 24-h EC50 = 0.003 mg Ag/l), algae Raphidocelis subcapitata (ZnO and CuO, 72-h EC50 = 0.14 mg Zn/l and 0.7 mg Cu/l, respectively) and murine fibroblasts BALB/3T3 (CuO, 48-h EC50 = 0.7 mg Cu/l). MWCNTs showed toxicity only towards rat alveolar macrophages (EC50 = 15.3 mg/l) assumingly due to high aspect ratio and TiO2 towards R. subcapitata (EC50 = 6.8 mg Ti/l) due to agglomeration of TiO2 and entrapment of algal cells. Finally, we constructed a decision tree to select the bioassays for hazard ranking of NMs. For NM testing, we recommend a multitrophic suite of 4 in vitro (eco)toxicity assays: 48-h D. magna immobilization (OECD202), 72-h R. subcapitata growth inhibition (OECD201), 30-min Vibrio fischeri bioluminescence inhibition (ISO2010) and 48-h murine fibroblast BALB/3T3 neutral red uptake in vitro (OECD129) representing crustaceans, algae, bacteria and mammalian cells, respectively. Notably, our results showed that these assays, standardized for toxicity evaluation of “regular” chemicals, proved efficient also for shortlisting of hazardous NMs. Additional assays are recommended for immunotoxicity evaluation of high aspect ratio NMs (such as MWCNTs).
Keywords: Metals, multitrophic test battery, physico-chemical characterization, risk assessment, solubilization
Introduction
Taking into account the increasing number of nanomaterials (NMs) entering the market, hazard and risk assessment of each variant of NMs and all their possible derivatives is not realistic. Therefore, the need for the hazard ranking and grouping of NMs is widely acknowledged (Arts et al., 2014,2015; Gebel et al., 2014; Godwin et al., 2015). A comprehensive overview on NM classification methods proposed by different authorities and the initial decision-making framework were recently published (Arts et al., 2015; Godwin et al., 2015). In order to classify NMs, reliable toxicity data and cost-efficient toxicity assays allowing to pinpoint the hazardous NMs are urgently needed. This would allow to focus on the mechanisms of toxicity of hazardous NMs to different organisms, depending on the potential use and production volume of each NM type. It is equally important to denote non-hazardous NMs that are safe to use to promote further development of nanotechnologies. In line with the above-mentioned aspects, there are currently several ongoing discussions on the need of publishing not only these nanosafety studies, where toxic effects have been registered but also no-effect studies as the potential risk from NM exposures is probably exaggerated (Gebel et al., 2014; Krug, 2014; Warheit & Donner, 2015).
Recently, OECD has come to the conclusion that the approaches for the testing of traditional chemicals are in general appropriate for assessing the NM safety, but should be adapted to the specificities of NMs (OECD, 2012). Currently, different consortia are involved in the process of adapting existing OECD toxicity tests for NM testing (Petersen et al., 2015). However, in the absence of standardized NM-specific tests, industry and the whole society would benefit from simple cost-effective in vitro screening assays to serve as a starting point for obtaining preliminary hazard information. The earlier the toxic side-effects of NMs will be discovered, the more time and development costs will be saved. Indeed, Choi et al. (2009) estimated that the costs for the testing of the existing nanoparticles (NPs) could range from $249 million (presuming the NPs are in general safe and require simple in vitro screening assays) to $1.18 billion (presuming the NPs require long-term in vivo testing) and the complete toxicity testing would take 34–53 years. This would not only be a heavy financial burden but also an ethical problem, because a large number of animal experiments were involved.
Alternative methods are increasingly promoted to reduce or replace vertebrate animals in (nano)toxicology experimentation (Hartung, 2010; Kandarova & Letašiova, 2011). Various data sets have already been generated and several testing strategies have been proposed for the in vitro screening of NMs with the emphasis on mechanism-based high-throughput approaches (Farcal et al., 2015; George et al., 2011; Godwin et al., 2015; Nel et al., 2013). Most of these high-throughput mechanistic studies focused mainly on human cells and didn’t consider potential environmental hazard of NMs. Although there is also a relatively large number of nano-ecotoxicity studies available (reviewed by Adam et al., 2015; Bondarenko et al., 2013a; Chen et al., 2015; Coll et al., 2015; Jackson et al., 2013; Juganson et al., 2015; Vale et al., 2016), only a few single studies provided data for a wide range of environmentally relevant organisms and enable to retrieve the most suitable organisms and endpoints for the environmental hazard testing of NMs.
Numerous EU research consortia are currently dedicated to nanosafety. The respective EU projects are consolidated under the ‘Nanosafety Cluster’ that involves around 100 projects including two flagship FP7 projects NANOVALID and MARINA. The main aim of the NANOVALID project (www.nanovalid.eu; 2011–2015) was to develop a set of reliable reference methods and materials for physico-chemical characterization and hazard identification of NMs, whereas the authors of the current paper focused on (eco)toxicological screening of NMs. Altogether 15 different test organisms and cell lines (6 medically important bacterial species, yeast, alga, protozoan, 2 crustacean species, zebrafish and 3 mammalian cell lines in vitro) involving a wide variety of cells and ecologically relevant organisms were used. The selected ecotoxicological organisms represent both, particle-ingesting (protozoa, crustaceans) and presumably non-ingesting (bacteria, algae, fish embryos) species. All these test organisms are abundant in the terrestrial compartment (bacteria, isopods), wastewater treatment plants (bacteria, protozoa) and natural waterbodies (algae, protozoa, aquatic crustaceans, fish). In addition, this selection represents organisms from different trophic levels including consumers (protozoa, crustaceans, fish), primary producers (algae) and decomposers (bacteria). For comparison, we tested the toxicity of NMs to mammalian cell lines in vitro: human bone marrow-derived mesenchymal stem cells, murine fibroblasts and rat alveolar macrophages.
Bacterial growth and bioluminescence inhibition, yeast viability, algal growth, crustacean immobilization, zebrafish embryos’ malformation and several assays detecting the loss of viability of cells (ATP production, propidium iodide staining, neutral red uptake, MTT and WST-1 reduction and acridine orange/ethidium bromide staining) were used as endpoints to determine the (eco)toxicity of NMs. Five of the assays were conducted in accordance to ISO or OECD guidelines (Table 1).
Table 1. Summary of (eco)toxicological methods used in this study.
| Test name | Reference | Modification(s) compared to reference (if any) |
|---|---|---|
| Bacterial growth inhibition assay (all bacteria except Vibrio fischeri) | Bondarenko et al., 2013b | The initial optical density (OD600) of bacterial suspension was OD600nm = 0.07.The 96-well plates were incubated statically at 30 °C during assay and were shaken once before each measurement. |
| V. fischeri bioluminescence inhibition assay | ISO, 2010; Kurvet et al., 2011 | The temperature was 20 °C instead of 15 °C stated in the ISO guideline as most of the luminometers can not be adjusted below room temperature. |
| Yeast viability assay (Saccharomyces cerevisiae) | Suppi et al., 2015 | No modifications. |
| Algal growth inhibition assay (Raphidocelis subcapitata) | OECD, 2011 | No modifications of the guideline. |
| Protozoan viability assay (Tetrahymena termophila) | Jemec et al., 2016 | No modifications. |
| Crustacean acute immobilization assay (Daphnia magna) | Jemec et al., 2016; OECD, 2004 | Differently from OECD guideline, the daphnid neonates used in the assays, were not obtained from the in-house culture but hatched from the daphnia dormant eggs. |
| Isopod (Porcellio scaber) membrane integrity assay (AO/EB staining) | Valant & Drobne, 2012 | The exposure for 3 h at room temperature instead of 18 h at 4 °C was used.The test was performed on 24-well plates instead of glass vials. |
| Zebrafish (Danio rerio) embryo toxicity assay | Jemec et al., 2016; OECD, 2013 | No modifications of the guideline. |
| Human mesenchymal stem cell membrane integrity assay in vitro (PI staining) | Zhang et al.,1999 | Test was initiated 24 h after exposure to NMs. 488 nm excitation/578 nm emission filters were used to read plates. |
| Human mesenchymal stem cell mitochondrial activity assay in vitro (MTT reduction) | Mosmann, 1983 | Test was initiated 24 h after exposure to NMs and incubated with test reagent for 4 h.After cell lysis with sodium dodecyl sulfate the absorbance was read at 570 nm. |
| Murine fibroblast BALB/c 3T3 membrane integrity assay in vitro (NRU) | OECD, 2010 | No modifications of the guideline. |
| Murine fibroblast BALB/c 3T3 mitochondrial activity assay in vitro (WST-1) | Zou et al., 2012 | No modifications. |
| Rat alveolar macrophage NR8383 mitochondrial activity assay in vitro (WST-1) | Zou et al., 2012 | Rat alveolar macrophages NR8383 (ATCC, CRL-2192) instead of BALB/c 3T3 described in the reference were used.Cells were seeded into 96-well plates at density 12 000 cells/well. |
AO/EB, acridine orange/ethidium bromide; MTT, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NRU, neutral red uptake; PI, propidium iodide; WST-1, 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium.
The main goals of this research were (i) to generate toxicity data for 7 physico-chemically well-characterized NMs using a suite of 15 bioassays, (ii) to pinpoint the most sensitive bioassays and (iii) to suggest (on the basis of a decision-tree) a refined in vitro test battery, comprising of selected in vitro bioassays, for (eco)toxicity screening and initial hazard ranking of NMs.
Materials and methods
Study design and participating institutions
Four partner institutions of the EU FP7 project NANOVALID participated in the study: National Institute of Chemical Physics and Biophysics (NICPB, Estonia), University of Ljubljana (UL, Slovenia), The Center for Cellular & Molecular Biology (CCMB, India) and University of Tampere (UTA, Finland). Experimental set-up is schematically presented in Figure S1. The partners were provided with NMs of the same batch. All the partners followed the same procedure for the storage and preparation of the test suspensions, if not stated otherwise in the “Nanomaterials” section.
Chemicals
All the chemicals were at least of analytical grade. AgNO3 was from J.T. Baker; ZnSO4, 3,5-dichlorophenol, ATP assay reagent and dilution buffer, neutral red solution (3.3 g/l in phosphate buffered saline), propidium iodide and acetic acid were from Sigma-Aldrich; CuSO4*5H2O from Alfa Aesar; Vibrio fischeri Reagent from Aboatox (Turku, Finland); Daphnia magna’s dormant eggs were from MicroBioTests Inc (Belgium), ATP standard from BioOrbit; Dulbecco’s Modified Eagle’s Medium (DMEM) and Kaighn’s Modification of Ham’s F-12 from ATCC (UTA) or Invitrogen (CCMB), fetal bovine serum (FBS) from Gibco Invitrogen, Newborn Calf Serum from Biochrom Ag, WST-1 Cell Proliferation Reagent from Roche or Life Technologies and acridine orange and ethidium bromide from Merck.
NMs and their characterization
Seven NMs were characterized and tested: SiO2 (NNV-002), Ag (NNV-003), Au (NNV-004), MWCNTs (NNV-006), CuO (NNV-011), ZnO (NNV-012) and TiO2 (NNV-013). The Ag NMs and MWCNTs tested in the current study were also used in the EU FP7 project MARINA.
NMs were provided centrally as aqueous suspensions (Au and Ag NMs) or as dry powders (SiO2, TiO2, MWCNTs, CuO and ZnO). All the NMs were at least 99% pure according to X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and inductively coupled plasma mass spectrometry (ICP-MS). Suspensions of Au and Ag NMs were provided in nominal concentrations of 60 and 40 000 mg/l, respectively. The shape, specific surface area (according to BET methodology) and primary size (according to transmission electron microscopy, TEM) were measured by NM providers (Table 2).
Table 2. Physico-chemical properties of the studied nanomaterials (NMs were ≥99% pure).
| NMs | Provider | Coating | Shape | Specific surface area [m2/g] | Primary size (TEM) [nm] | Hydrodynamic size in DI (DLS) [nm] | pdi in DI | ζ -potential in DI [mV] |
|---|---|---|---|---|---|---|---|---|
| SiO2* | Nanologica | No | Spherical | 910 ± 42 | 212 ± 52 | 854 ± 38 | 0.6 | −37.9 |
| Ag | Colorobia | PVP-stabilized | Spherical | n.d. | 20.4 ± 6.8 | 132 ± 0.5 | 0.2 | −10.6 |
| Au** | Inmetro | Citrate | Spherical | n.d. | 13.3 ± 0.8 | 23 ± 4 | 0.2 | −48 |
| MWCNT | Nanocyl | No | Tubular | 265 ± 18 | diameter: 10.2 ± 0.5length: >1000*** | n.a. | n.a. | n.a. |
| CuO | Intrinsiq Materials | No | Spherical | 23 ± 3.7 | 24.5 ± 2.3 | 152 ± 2 | 0.2 | 45.4 |
| ZnO | Nanogate | No | Spherical | 58 ± 5 | 13.6 ± 1.7 | 102 ± 1 | 0.2 | 32.1 |
| TiO2**** | CCMB | No | Spherical | 257 ± 47 | <5 | 367 ± 60 | 0.4 | 14.2 |
*Mesoporous; **Acidic suspension (pH = 5.8); ***Measured by SEM; ****Anatase crystal structure with some rutile present according to XRD analysis. DI, deionized water; DLS, hydrodynamic light scattering; n.a., not applicable; PVP, polyvinyl-pyrrolidone; pdi, polydispersity index; SEM, scanning electron microscopy; TEM, transmission electron microscopy.
Preparation of NM suspensions
All the stock suspensions of NMs and the respective soluble metal salts (ionic controls) were prepared on metal basis to facilitate comparison of their toxicities. The stock suspensions of NMs (except MWCNTs, Ag and Au NMs) were prepared at nominal concentrations of 5000 mg metal/l in sterile deionized (DI) water (pH = 5.8), homogenized using ultrasonic probe immediately after the preparation of the suspensions (40 W, 4 minutes; 450 Ultrasonifier, Branson Ultrasonics Corporation, Danbury, CT) at continuous mode without temperature adjustment and left for two days to equlibrate. Stock suspensions were stored in the dark at room temperature for up to 2 weeks. To obtain a stable suspension of MWCNTs, 0.01% Triton X-100 was used as a dispergant.
Generally, all partners followed the same protocols for the dispersion of NMs (40 W, 4 minutes) with the following exceptions: (i) CCMB prepared the NM stock suspensions at 1000 mg metal/l in DI water and dispersed using ultrasonic probe (30 W, 10 minutes; SONICS Vibracell); (ii) UTA prepared stock suspension of MWCNTs at 200 mg/l freshly before use and ultrasonicated in a water bath sonicator (40 kHz, 2 hours; B3510, Branson Ultrasonics); (iii) UTA prepared the stock suspension of SiO2 NMs at 2000 mg/l freshly before use and ultrasonicated in a water bath sonicator (40 kHz, 5 minutes; B3510, Branson Ultrasonics). As Au and Ag NMs were provided as stable suspensions, they were used for testing without prior sonication.
Characterization of NMs in suspension
Hydrodynamic size (measured by dynamic light scattering, DLS), polydispersity index (pdi) and ζ-potential were measured at a concentration of 10 mg metal/l for Au and 100 mg metal/l for the other NMs using Malvern Zetasizer (Nano-ZS, Malvern Instruments, UK). Dissolution of Ag, CuO and ZnO NMs was quantified using atomic absorption spectroscopy (AAS). For that, NMs at 10 mg/l were incubated in DI water or selected toxicity test media for 30 min, 4 h, 24 h or 72 h, ultracentrifuged (390 000g, 30–60 min) and the supernatant was analyzed by GF-AAS in accredited laboratory of the Institute of Chemistry of Tallinn University of Technology (Estonia) using EVS-EN ISO/IEC 17025:2005. The ratio of determined dissolved metal to total nominal metal in NMs was designated as dissolution (%) (Table 3).
Table 3. Dissolution (share of dissolved metal to total metal) of Ag, CuO and ZnO nanomaterials was determined by atomic absorption spectroscopy in supernatants of ultracentrifuged 10 mg metal/l NM suspensions after 30-min, 24-h, 48-h and 72-h incubation in deionized water (DI water) or test media.
| DI water (yeast, protozoan), 24-h dissolution, % | 2% NaCl (Vibrio fischeri), 30-min dissolution, % | OECD202 (Daphnia magna), 24-h dissolution, % | OECD129 (BALB/c 3T3 cells), 48-h dissolution, % | OECD201 (alga), 72-h dissolution, % | |
|---|---|---|---|---|---|
| Ag | 49.8 | 0.12 | 0.5 | 4.7 | 0.35 |
| CuO | 9.2 | 8.3 | 1 | 61.1 | 0.7 |
| ZnO | 34 | 17.7 | 28.7 | 100 | 13.2 |
Toxicity assays
The references to and the description of the used toxicity assays are consolidated in Table 1. Due to the large number of implemented different toxicity assays and to avoid redundancy, the details of the toxicity testing procedures can be found in the referred articles and/or the respective guideline documents. All the modifications of these previously published methods and/or guidelines are listed in Table 1.
Testing strategy
Due to the large testing matrix (7 NMs and 15 test species) the maximum nominal NM concentration to be tested was set to 100 mg/l. In the first round of testing the following concentrations of NMs were analyzed: 0.1; 1; 10 and 100 mg metal/l or MWCNTs/l. In case of Au NM (concentration 60 mg Au/l in the stock provided), lower maximum concentration (30 mg/l) was used. Abiotic controls were run in parallel to evaluate the potential interference of the tested NMs with the toxicity endpoints. In addition, ionic controls (respective soluble metal salts) were used in parallel to estimate the role of dissolution of NMs in their toxicity. The tests were performed at least in duplicates and in two independent experiments. If the toxicity of NM was not observed in the first round of testing, the EC50 was assigned >100 mg metal/l (in case of MWCNTs mg compound/l). If toxicity was observed, more thorough testing (i.e., lower concentrations and 2-fold dilutions) was performed to determine precise EC50.
Dose-response curves and calculation of EC50
EC50 and confidence limits were calculated from the concentration-effect curves based on nominal exposure concentrations and using the log-normal model of MS Excel macro Regtox (Vindimian, 2005; NICPB, CCMB), MS Excel Regression analysis (NICPB, UL) or SigmaPlot Regression analysis (UTA).
The dose-response curves were based on at least six concentrations and at least six concentrations were used for the regression. The confidence limits and the number of replicates for each assay are shown in Table S1.
Constructing the decision tree for NMs testing
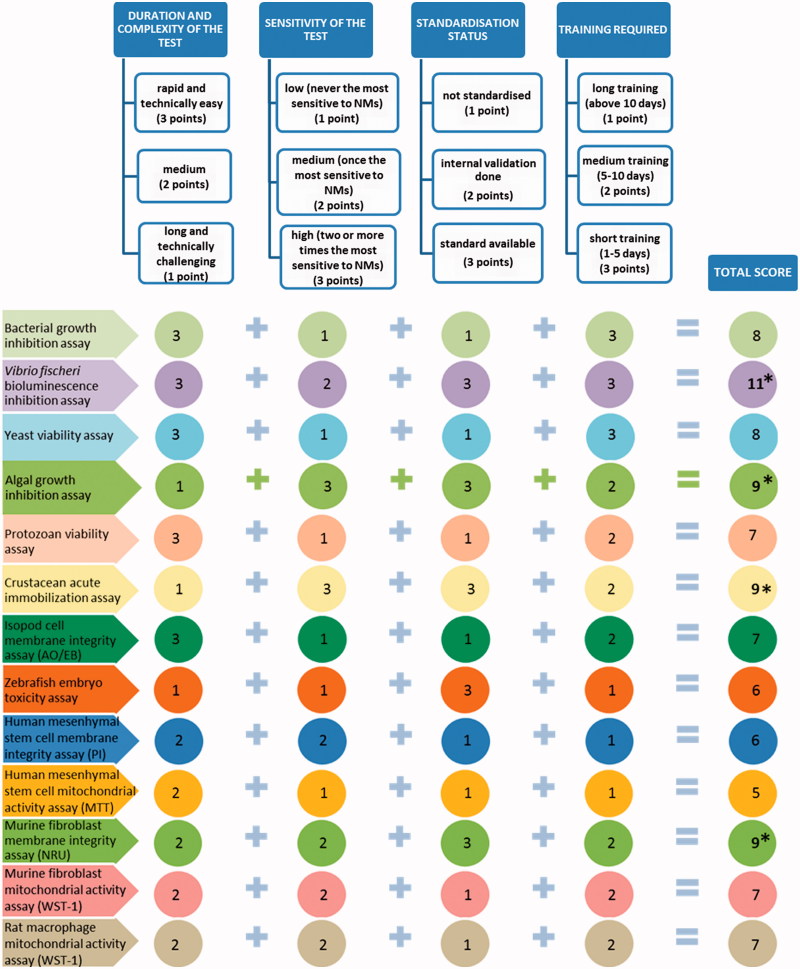
The decision tree was constructed to select the bioassays for hazard ranking of NMs on the basis of four benchmarks: duration and complexity of the assay, sensitivity, standardization status and time needed for training of the technician/researcher. For each of these four benchmarks 1 to 3 points were scored: 1 point to describe low performance and 3 points for high performance. The most suitable tests were selected on the basis of the highest total scores. Rapidity and ease of performance were the criteria for duration and complexity of the test and the scoring was based on the partners’ experience. Toxicity values (EC50) were the criteria for the sensitivity of the test. The highest score (3) was assigned if the test was the most sensitive to two or more NMs and lowest score (1) if the test was never the most sensitive to NMs (Table 4). Standardization of the test was considered a quality criterion and the standardized tests (Table 1) were therefore assigned the highest score (3). Time duration was the criterion for the training required to master execution of the test and was based on the partners’ experience. The tests with the shortest estimated required training time were assigned the highest score (3).
Table 4. Toxicity (EC50, MBC or LOEC) values of nanomaterials expressed in a heat map.
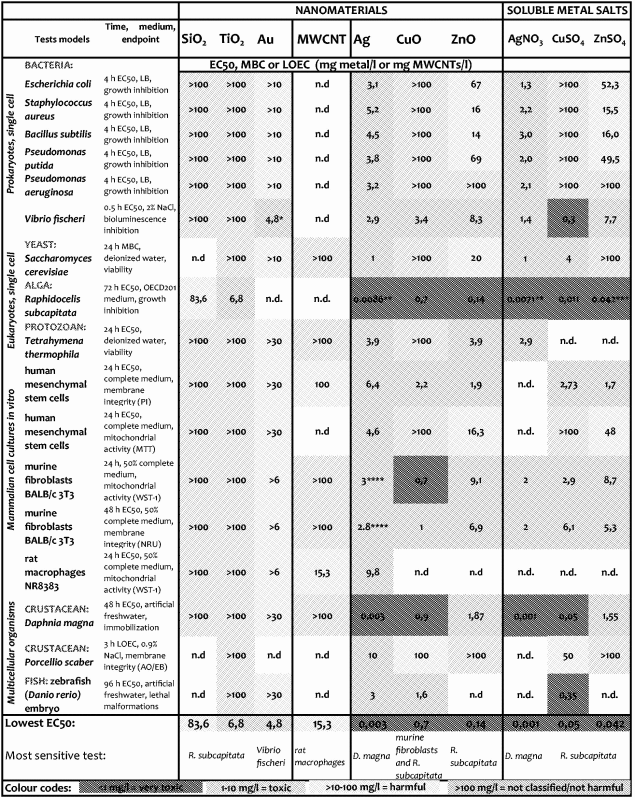 |
*The toxicity of Au NMs to V. fischeri was due to acidic pH; **Jemec et al., 2016; ***Aruoja et al., 2009; ****Zou et al., 2012 . AO/EB, acridine orange/ethidium bromide; LB, Luria–Bertani; LOEC, lowest observed effect concentration; MBC, minimal bactericidal concentration; n.d, not determined; PI, propidium iodide.
Results
Characterization of NMs
The physico-chemical characterization data for analyzed NMs are shown in Table 2. The primary size of NMs (TEM) was in the nano-size range (1–100 nm, according to European Commission recommendation; EC, 2012) with the exception of SiO2 (primary size 212 nm). However, SiO2 had the pores in the nanometer scale that translated into large specific surface area of 910 m2/g (Table 2). As one of the definitions of “nano” refers to the large specific surface area rather than the small primary size (NM is defined as the material with ≥60 m2/cm3 volume-specific surface area according Kreyling et al. 2010), we considered SiO2 as NM. The suspensions of Ag, Au, CuO and ZnO NMs were stable (pdi = 0.2; Table 2) in DI water. Au NMs had the smallest hydrodynamic size in DI water (23 nm), followed by ZnO (102 nm), Ag (132 nm) and CuO (152 nm), whereas SiO2 and TiO2 formed visible agglomerates with hydrodynamic size of 367 nm (TiO2) and 854 (SiO2) and pdi ≥0.4. However, all the NMs agglomerated in the actual test media to various degrees, depending on the test media used (Table S2).
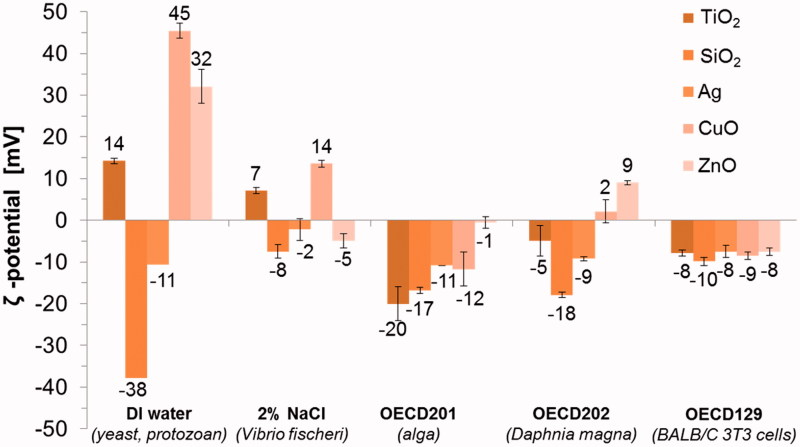
The surface charges (ζ-potential) of NMs in DI water ranged from −37.9 mV (SiO2) to 45.4 mV (CuO) (Table 2). However, in the actual test media all the NMs gained more comparable, mostly slightly negative, ζ-potentials (Figure 1). Similar phenomenon was observed in our previous study, where ζ-potential of 11 different metal oxides varied from −30 mV to 30 mV in DI water but was comparable in serum-supplemented cell culture medium (close to the ζ-potential of this test medium, −8.5 mV) (Ivask et al., 2015). This may explain more prominent agglomeration of NMs in the actual test media compared to DI water (Table S2) since the ζ-potential higher than +30 mV or lower than −30 mV is usually required to maintain the NM dispersed by charge stabilization (Hitchman et al., 2013).
Figure 1.
Surface charge (ζ-potential) of five nanomaterials in five different test media analyzed at concentration 100 mg metal/l using Malvern Zetasizer.
Our dissolution analysis (i.e., potential of metal-based NMs to release soluble metal ions) showed that three NMs (Ag, CuO and ZnO) were partly dissolving in DI water. The dissolution of CuO, ZnO and Ag at concentration 10 mg metal/l after 24-h incubation in DI water was 9.2%, 34% and 49.8%, respectively (Table 3). In addition to DI water, dissolution was analyzed in four test media that were used in toxicity assays which proved the most sensitive (lowest EC50, Table 4). Remarkably, the dissolution of Ag NMs was very low in all the test media compared to DI water (Table 3). At the same time, the dissolution of CuO and ZnO NMs was enhanced in BALB/c 3T3 cell culture medium (serum-supplemented DMEM) compared to the other test media (mineral media without serum), probably due to serum content.
Toxicity values and criteria for hazard ranking of NMs
The toxicity of 7 NMs (SiO2, TiO2, Au, Ag, CuO, ZnO and MWCNTs) was analyzed using 15 bioassays. The highest tested nominal concentration was 100 mg/l. Representative dose-response curves on the example of CuO NMs are shown in Figure S2. EC50 values (expressed in nominal concentrations on metal basis) were calculated from the dose-response curves and are color-coded in Table 4 to form a toxicity heat map. For the assays with yeast S. cerevisiae and crustacean P. scaber the minimal biocidal concentration (MBC) and the lowest observed effect concentrations (LOEC), respectively, were used instead of EC50. In addition, Table 4 contains toxicity data for the soluble salts of Ag, Cu and Zn to estimate the role of dissolution in toxicity of NMs that are prone to solubilization (Ag, CuO and ZnO, respectively). Parallel analysis of solubilization products of NMs is also recommended by the European research network (Handy et al., 2012). Hazard ranking of NMs and soluble metal salts was performed as in our previous studies (Bondarenko et al., 2013a; Kahru & Dubourguier, 2010): EC50 value <1 mg/l ranked NMs as very toxic; 1–10 mg/l = toxic; >10–100 mg/l = harmful; >100 mg/L = not classified/not harmful. MBC (yeast viability assay) and LOEC (isopod membrane integrity assay) values were ranked on similar basis as EC50 values.
Very toxic NMs: Ag, CuO and ZnO
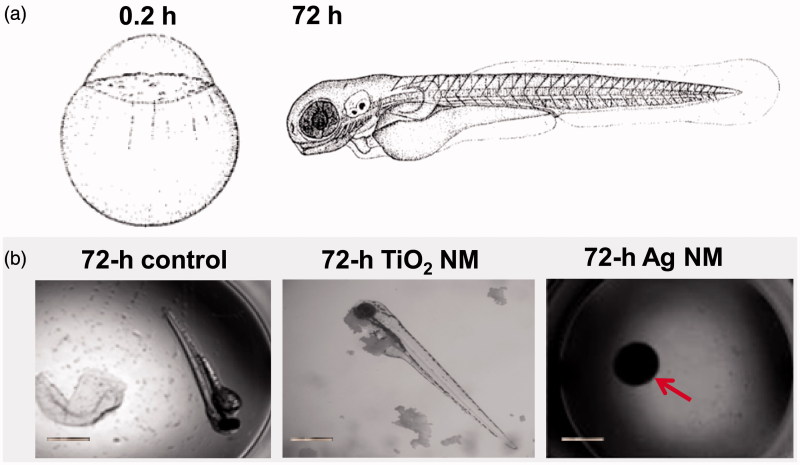
Ag, CuO and ZnO were ranked as very toxic (EC50 value <1 mg/l). Thus, these three NMs belong to the Category Acute 1 according to EU’s regulation on classification, labeling and packaging (CLP) of substances and mixtures (EC, 2008) based on their toxicity to fish (96 h), daphnids (48 h) and algae (72 or 96 h). Indeed, Ag was the most toxic NM tested. Crustacean D. magna and alga R. subcapitata (formerly known as Selenastrum capricornutum and Pseudokirchneriella subcapita) were most sensitive to Ag NMs (24-h EC50 = 0.003 and 72-h EC50 = 0.0086 mg Ag/l, respectively). Thus, the toxicity tests with D. magna and R. subcapitata flagged the toxicity of Ag NMs already at μg/l levels, being up to three orders of magnitude more sensitive than all the other assays used. This demonstrates the hazard of Ag NMs to aquatic life, if leaching from the consumer products to the waterbodies even at very low concentrations. In addition to high toxicity of Ag NMs to aquatic crustaceans and alga, the hazardous nature of Ag NMs was also demonstrated by zebrafish embryo toxicity assay (96-h EC50 = 3 mg Ag/l). At 10 mg Ag/l, Ag NMs completely inhibited the hatching of fish embryos (Figure 2). Interestingly, the toxicity of Ag NMs to both mammalian cells in vitro and various bacterial cells was observed at the same concentration range (3–10 mg/l, Table 4), indicating the higher toxicity of Ag NMs to non-target organisms such as crustaceans than to target organisms (microbes) (Bondarenko et al., 2013a). In line with our results there are several recent multispecies studies and numerous reviews showing potential ecotoxicity of Ag NMs (Coll et al., 2015; Garner et al., 2015; Kwak et al., 2016; Vale et al., 2016). All these studies support the commentary that “the European Commission should be regulating nanosilver, not asking for yet another report on its impact on health and the environment” (Hansen & Baun, 2012).
Figure 2.
Shematic representation of embryonic development (0.2 h and 72 h are shown) of zebrafish (Kimmel et al., 1995, reprinted with the permission of John Wiley and Sons) (a). Representative images of development of unexposed zebrafish embryo (control) or exposed to 100 mg/l TiO2 NMs or to 10 mg/l Ag NMs after 72 h (b). Red arrow indicate undeveloped embryo exposed to Ag NMs. Scale bar = 1 mm.
ZnO was the second most toxic NM being especially toxic in acute assays with the aquatic organisms (R. subcapitata, D. magna, T. thermophila and V. fischeri). As in case of Ag NMs, D. magna and especially R. subcapitata, were the most susceptible to ZnO NMs (48-h EC50 = 1.87 and 72-h EC50 = 0.14 mg Zn/l, respectively, Table 4). This is in agreement with the recent comprehensive study of Adam et al. (2015) showing that algae and crustaceans were more sensitive to ZnO NMs than other aquatic organisms. The toxicity of ZnO NMs to mammalian cell lines in vitro was slightly lower (1.9–16.3 mg Zn/l) depending on the cells used. These results are in agreement with previous reports by Farcal et al. (2015) and Ivask et al. (2015) who showed using various endpoints that for most of the mammalian cell lines EC50 of ZnO was in the range of 10–30 mg/l.
The toxicity of CuO NMs ranged from 0.7 mg Cu/l (murine fibroblasts and R. subcapitata) to >100 mg Cu/l (various bacteria) depending on test organism, toxicity endpoint and test medium used (Table 4). It has been previously shown that the test medium and especially the presence of organic compounds significantly modifies the toxicity of CuO and speciation of copper (Blinova et al., 2010; Käkinen et al., 2011). However, in some tests with unicellular organisms (e.g., protozoa and yeast) CuO was not toxic despite of the use of DI water (that does not complex Cu ions nor induces agglomeration of NMs) as an exposure environment. This suggests that some organisms migh be inherently more resistant to CuO. Alarmingly, assays with murine fibroblasts BALB/3T3 showed the lowest EC50 values for CuO, demonstrating that human cell lines in vitro are highly susceptible to this NM. We have previously suggested that differently from most of the other cell types and organisms, mammalian cells might be susceptible also to particle-specific adverse effects of CuO NMs (Bondarenko et al., 2013b) that is probably related to intracellular dissolution of internalized CuO particles in the acidic environment of lysosomes (Trojan horse mechanism; Cronholm et al., 2013). High sensitivity of mammalian cells to CuO NMs observed in this study supports this hypothesis.
Notably, the toxicity patterns (toxicity to different organisms) of dissolution-prone NMs (Ag, CuO and ZnO) and the respective metal salts were almost identical (Table 4), suggesting that the toxicity of these NMs was due to dissolution.
Toxic NMs: TiO2
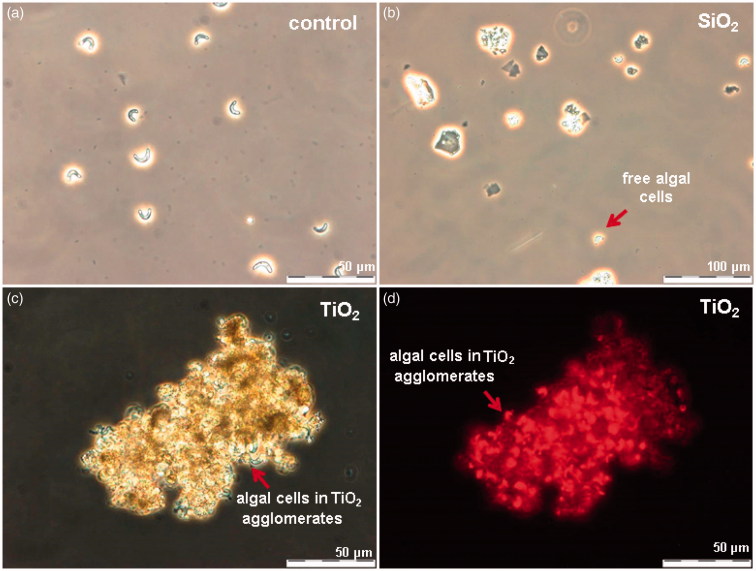
TiO2 exhibited toxicity only in the assay with algae R. subcapitata (72-h EC50 = 6.8 mg Ti/l). The visual observation of TiO2-exposed algae showed that most likely the inhibition of algal growth was due “entrapment” of the algal cells into TiO2 NM agglomerates (Figure 3c,d). No such effect was observed in case of SiO2 NMs-exposed algae (Figure 3a,b), although the surface charges of these two NMs (−17 mV for SiO2 and −20 mV for TiO2, Figure 1) as well as hydrodynamic sizes (575 nm and 478 nm, respectively, Table S2) in the algal test medium were similar. Notably, differently from all the other bioassays used for toxicity testing, the algal growth inhibition assay is performed under illumination. In our previous study covering the toxicity of 12 metal oxides to algae, we showed that TiO2 NMs are photoactivated upon illumination and generate reactive oxygen species (ROS) (Aruoja et al., 2015). We suggested that the entrapment of algae into TiO2 NM agglomerates enhanced the contact of algal cells with TiO2 and thus, susceptibility of algae to ROS generated by TiO2. Thus, the toxicity of TiO2 used in our study was algae-specific and most probably due to photoactivation-induced ROS. In addition, the shedding of light by TiO2 agglomerates cannot be excluded.
Figure 3.
Algae Raphidocelis subcapitata after 72-h exposure in OECD medium (a), with 100 mg/l of nano-SiO2 (b) and 100 mg/l of TiO2, photographed in parallel using phase contrast (c) and fluorescence microscopy (d).
Harmful NMs: MWCNTs and SiO2
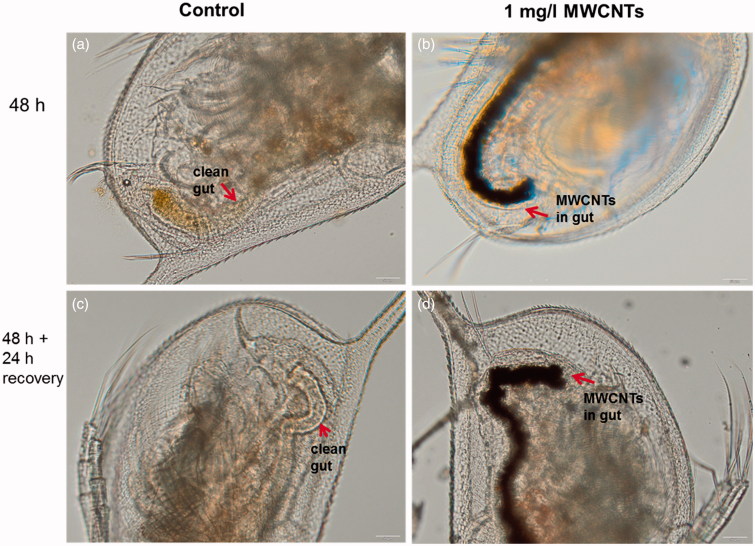
MWCNTs exhibited the toxicity only in one assay – the bioassay with rat alveolar macrophages (Table 4), indicating that all the other 14 assays were not able to pinpoint the adverse effects of these NMs. Indeed, analogously to asbestos fibers, CNTs elicit toxicity via high aspect ratio and pose adverse effects mainly on immune cells (Bhattacharya et al., 2013). Furthermore, the wide range of immune effects triggered by CNTs (e.g., induction of chemokine and cytokine secretion, generation of reactive oxygen species, interference with cellular response to pathogens) occur already at sub-toxic levels (Kodali et al., 2013) and might not be captured by traditional cytotoxicity/viability assays. Therefore, additional functional assays with immune cells could be recommended for the hazard identification of these biopersistant high aspect ratio NMs. Interestingly, we observed some sub-toxic effects of MWCNTs in the acute toxicity assay with D. magna. Namely, even upon exposure at low concentration (1 mg/l of MWCNTs), D. magna gut was filled with MWCNTs (Figure 4b). Furthermore, when MWCNT-exposed crustaceans were further incubated in artificial freshwater without MWCNTs for 24 h after the termination of the exposure, MWCNTs were not eliminated (egested) from the gut (Figure 4d). This observation suggests that although MWCNTs did not exhibit any direct acute effects on this organism in the used test design (OECD202), the long-term effects (e.g., possible interference with the feeding of the organism, trophic transfer) should be studied in more detail.
Figure 4.
Images of Daphnia magna: not exposed (a, c) and after 24-h incubation with MWCNTs (b, d). Red arrows indicate the gut of Daphnia. Upper panels depict the gut of Daphnia after 24-h incubation and lower panels after transfer of daphnids to artificial freshwater. Note that even after the 24-h recovery in the clean artificial freshwater the gut remained filled with MWCNTs (d). Images were taken with Olympus BX61. Scale bar = 50 μm.
SiO2 NMs showed moderate toxicity only in the assay with algae R. subcapitata (72-h EC50 = 83.6 mg Si/l. Although we did not determine the dissolution of SiO2 in this study, the adverse effects of SiO2 to algae may be related to the dissolved fraction as discussed previously (Aruoja et al., 2015). The absence of toxic effect of mesoporous SiO2 in the rest of the 14 assays, including mammalian cells in vitro (Table 4), is in agreement with various reports on the safety of mesoporous silica and is very encouraging given the perspective of this type of SiO2 in drug delivery and other biomedical applications (Fadeel & Garcia-Bennett, 2010; Wang et al., 2015; Witasp et al., 2009). Notably, another variety of silica particles – crystalline SiO2 – has been shown to be toxic both in vitro and in vivo conditions (Napierska et al., 2010) as crystalline phase is an important parameter in the toxicity of SiO2.
Not classified/non-hazardous NMs: Au
Au NM was toxic only in the V. fischeri bioluminescence inhibition assay. However, once the acidic suspension of Au NMs (pH = 5.8) was neutralized, no toxicity was observed. Thus, the toxicity of Au NMs to V. fischeri was most likely due to acidic pH of the Au NM suspension. However, the toxicity of Au NMs cannot be fully certified in this study since the maximum available test concentration was too low (30 mg Au/l). On the basis of the literature, the toxicity of Au NMs cannot be excluded. For example, Nam et al. (2015) conducted a comprehensive ecotoxicity evaluation of Au NMs and found these NMs hazardous (with the 48-h EC50 = 0.6 × 1010 particles/ml to the most sensitive test organism cladoceran Moina macrocopa) and suggested the guideline values for the protection of the aquatic ecosystems.
Discussion
Seven representative NMs were tested with 15 different test species and/or cell lines to find the refined suite of bioassays for identification of hazardous NMs both, for the ecosystem as well as for human. Various toxicity endpoints were applied to cover the different modes of action of NMs. On the basis of the gained experience, we outlined the advantages and limitations of each assay (Table 5). In addition, we selected four benchmarks and constructed a decision tree to select a refined toxicity test battery for NM screening (Figure 5).
Table 5. Advantages and limitations of the selected test assays based on the experience gained by the partners of the NANOVALID project.
| Test, organism | Model for | Advantages | Limitations |
|---|---|---|---|
| Bacterial growth inhibition assay (Escherichia coli, Staphylococcus aureus, Pseudomonas sp. Bacillus subtilis) | Medically important pathogenic bacteria;Environmentally relevant bacteria | Simple and rapid tests showing whether the tested NMs exhibit general toxicity to the given bacterial strains. | Growth inhibition assays are conducted in organics-rich media that may sorb or interfere with NMs (agglomeration/dissolution etc). |
| Vibrio fischeri bioluminescence inhibition assay | Marine bacteria | The assay with V. fischeri is rapid and reliable standardized assay (ISO, 2010). | Test is sensitive to pH (the test medium is not buffered). High salinity (2% NaCl). |
| Yeast (Saccharomyces cerevisiae) viability assay | The simpliest model for eukaryotic cell | The use of DI water as the test environemnt eliminates the speciation-related effects of metallic NMs. | DI water is not a natural environment and may trigger osmotic stress in cells. This may change the susceptibility of cells to NMs. |
| Algal (Raphidocelis subcapitata) growth inhibition assay | Environmentally relevant freshwater primary producer | Algal growth inhibition assay (OECD, 2011) is among the most sensitive ecotoxicological tests. | NMs may interfere with optical density measurements and shade the light necessary for the growth of algae. |
| Protozoan (Tetrahymena thermophila) viability assay | Environmentally relevant eukaryotic organism | T. thermophila can internalize NMs by phagocytosis. | DI water is not a natural environment and may trigger osmotic stress in cells. This may change the susceptibility of cells to NMs. |
| Crustacean (Daphnia magna) acute immobilization assay | Environmentally relevant freshwater organism | One of the most sensitive organisms used in ecotoxicity assessment of chemicals and D. magna test (OECD, 2004) is among the assays that are required by the REACH guidelines for the ecotoxicity assessment.Particle-feeding organism and thus, very relevant model for NMs. | Test medium may induce agglomeration and precipitation of NMs.High sample volume (50 ml per concentration in one experiment) is required. |
| Isopod (Porcellio scaber) cell membrane integrity assay (AO/EB) | Environmentally relevant terrestrial organism | Isolated digestive gland is the model for tissue cell membrane permeability. The test provides very specific data on the cell membrane stability. | Accurate training of staff is required. The method is not robust. The physiological state of the gland need to be precisely described. |
| Zebrafish (Danio rerio) embryo toxicity assay | Environmentally relevant freshwater organism | The test is robust and highly valuable for NM testing in aquatic environment. Fish embryos are transparent allowing direct observation of malformations. | The set-up of rearing the zebrafish embryos is technically challenging. |
| Human mesenchymal stem cell membrane integrity (PI) and mitochondrial activity (MTT) assays | Non-cancerous primary derived human cells | Test endpoints are based on fundamental cellular processes, i.e., mitochondrial activity (MTT, WST-1) and membrane integrity (NRU and PI).NRU assay with murine fibroblasts is standardized toxicological assay for chemical testing (OECD, 2010). | Some NMs may interfere with the fluorescence and absorbance-based viability assays. |
| Murine fibroblast BALB/c 3T3 membrane integrity (NRU) and mitochondrial activity (WST-1) assays | Non-cancerous mammalian cell line | ||
| Rat alveolar macrophage NR8383 mitochondrial activity assay (WST-1) | Non-cancerous mammalian macrophages | Does not require washing after exposure prior to the assay, and is therefore suitable for suspension cells. Very fast and easy to perform. | Some NMs may interfere with the assays. |
AO/EB: acridine orange/ethidium bromide; NRU: neutral red uptake; PI: propidium iodide; WST-1: 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium.
Figure 5.
Decision tree for the selection of optimal toxicity assays for screening and hazard ranking of NMs. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NRU, neutral red uptake; PI, propidium iodide; WST-1, 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium.
Test battery of toxicity screening assays
Test battery of toxicity screening assays was selected on the basis of decision tree (Figure 5) as described in Materials and Methods. Taking into account the technical complexity, standardization status, sensitivity of the test and required training, we selected the following four acute assays for the nanotoxicity screening: 48-h D. magna immobilization (OECD202), 72-h R. subcapitata growth inhibition (OECD201), 30-min Vibrio fischeri bioluminescence inhibition (ISO21338:2010) and 48-h murine fibroblast BALB/3T3 neutral red uptake in vitro (OECD129) (Figure 5). All these four assays were efficient in revealing the adverse effects of toxic NMs (Table 4), are standardized and their combination provides a diversity in terms of biological organization of the test species, involving bacteria, alga, crustacea and a model for human cells.
Physico-chemical properties versus toxicity of NMs
As highlighted by Godwin et al. (2015), the “holy grail” of nanosafety field would be the ability to rank the NMs into hazard categories on the basis of physico-chemical properties. In our study we observed that the dissolution (a function of chemical composition of NM and the test conditions) was the main physico-chemical property explaining the toxicity of the most toxic NMs (Ag, ZnO and CuO) to a wide range of ecotoxicological organisms and cells lines. This was concluded on the basis of the comparison of toxicity patterns of NMs and the respective metal salts (Table 4). Indeed, numerous papers have previously identified dissolution as the main factor contributing to the (eco)toxicity of NMs (Adam et al., 2015; Bondarenko et al., 2013a; Heinlaan et al., 2008; Ivask et al., 2010; Notter et al., 2014; von Moos & Slaveykova, 2014). This is also in line with one of the few successful QSAR models developed for NMs by Puzyn et al. (2011), who described the relationship between the structures of 17 metal oxides and their cytotoxicity to E. coli cells and showed that the formation of a cation (dissolution) of the NMs was the main determinant of toxicity. The same study found no influence of NM particle size on toxicity. Similar conclusion was drawn by Horie et al. (2012) for 24 metal oxide NMs, showing that the primary particle size and specific surface area did not affect the toxicity of NMs to two mammalian cell lines in vitro and that dissolution was the most important cytotoxicity factor. Recently, Notter et al. (2014) performed a meta-analysis of published ecotoxicity data of Ag, ZnO and CuO NMs and respective salts and found that as the NM form is mostly less toxic than the dissolved metal, the existing regulations on soluble metals can be easily adjusted to the respective NMs. All these previous studies and our comprehensive data set on the toxicity of Ag, ZnO and CuO NMs and the respective metal salts to 15 test organisms and cells lines show that (i) dissolution is the key factor in the toxicity of these NMs and (ii) the regulations and guidelines that are applied for the toxicity testing of metal salts can be potentially adapted for the respective NMs in standardized (laboratory) conditions.
There are numerous articles suggesting that in addition to the dissolution, many other physico-chemical properties such as primary and hydrodynamic size, shape, aspect ratio, degree of agglomeration, surface coating, surface defects, surface reactivity and charge may contibute to toxicity (Djurišić et al., 2015; Jiang et al., 2009; Nel, 2013; Oomen et al., 2015; Suresh et al., 2013). On the basis of our toxicity screening results and the literature data, we suggest that these properties are more relevant for the non-dissolving NMs (that are mostly less toxic). For example, Li et al. (2012) showed that in addition to dissolution, the antibacterial activity of seven types of UV-irradiated metal-oxide NPs correlated quantitatively with their ability to induce ROS and suggested that large specific surface area of NMs contributed to this process.
No apparent correlation between the toxicity of NMs (Table 4) and the hydrodynamic size or ζ-potential (Table 2) was found in our study using simple correlation models (Figure S3). Nevertheless, as highlighted by Chen et al. (2015), the toxicity data, obtained by following the widely applied guidelines and backed up with solid physico-chemical data, are especially valuable and can be used as a part in further modeling applications and nano-QSAR development. Finally, our comprehensive matrix of the toxicity data (7 × 15 across-species quantitative toxicity values) can be reused in other EU projects (e.g., eNanoMapper, NANoREG), uploaded into comprehensive databases and used for the systems biology analysis to understand the potential adverse effects of different type of NMs on cell and organism functions and the ecosystem at large.
Conclusions and outlook
Four partner laboratories working together in FP7 project NANOVALID analyzed the toxicity of seven well-characterized NMs by 15 test organisms and cells lines in vitro. Overall, crustacean D. magna and algae R. subcapitata were the most sensitive to the toxicity of the tested NMs (e.g., R. subcapitata was the most sensitive to four out of seven NMs tested: SiO2, TiO2, CuO and ZnO). This is very encouraging given the fact that both, D. magna immobilization assay (OECD202) and R. subcapitata growth inhibition assay (OECD201) are standardized and can be supposedly easily adapted for the testing of NMs.
The dissolution-prone NMs (Ag, CuO and ZnO) were ranked very toxic by most of the assays. By comparing the toxicity patterns of NMs and the respective metal salts, we suggest that the toxicity of dissolution-prone NMs was due to shedding of toxic metal ions. TiO2 was ranked toxic by algal growth inhibition assay, most probably due to entrapping of algae by TiO2 agglomerates. MWCNTs proved harmful only in the assay with rat alveolar macrophages (immune cell model), suggesting that for these NMs, specific immunotoxicological assays for the toxicity screening should be applied. Thus, dissolution-prone NMs were toxic due to the shedding of toxic ions, whereas other NM- and test organism-specific physico-chemical properties came into play in case of less toxic non-dissolving NMs (e.g., aspect ratio of MWCNTs in the assay with alveolar macrophages and entrapment of algae by TiO2 agglomerates in the algal growth inhibition assay). Finally, SiO2 NMs were ranked as harmful only in the algal growth inhibition assay. These findings support the recent opinion of Maynard (2016) that “the prefix ‘nano’ cannot effecively be used as an actual risk predictor”.
Using the duration, complexity, sensitivity (EC50) and standardization status of the toxicity assays as the benchmarks, we constructed a decision tree to suggest a refined suite of bioassays for cost-effective toxicity screening of NMs. On the basis of the decision tree, we recommend a multitrophic suite of four in vitro (eco)toxicity assays: 48-h D. magna immobilization (OECD202), 72-h R. subcapitata growth inhibition (OECD201), 30-min V. fischeri bioluminescence inhibition (ISO21338:2010) and 48-h murine fibroblast BALB/3T3 neutral red uptake in vitro (OECD129). These four assays efficiently pinpointed the toxic NMs (Table 4). Also, their combination involves bacteria (decomposers), algae (primary producers), crustaceans (multicellular particle-ingesting organisms) and model for human cells (mammalian cell lines in vitro). It is noteworthy that although the selected most sensitive assays have been standardized for the ‘regular soluble’ chemicals, they also proved the most sensitive in pinpointing the hazardous NMs, showing the potency of the existing regulatory test formats to be used for NM testing.
Supplementary Material
Acknowledgements
We thank Heiki Vija, Kai Künnis-Beres, Katre Juganson, Kaja Kasemets, Aleksandr Käkinen, Liina Kanarbik, Sandra Käosaar, Monika Mortimer, Mirja Hyppönen, Sara Novak and Barbara Drašler for their contribution.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The research was funded by EU FP7 project NANOVALID (Development of reference methods for hazard identification, risk assessment and LCA of engineered nanomaterials; grant no. 263147), IUT23-5 and PUT1015 and ETF9347 grant of the Estonian Ministry of Education and Research.
Glossary
Abbreviations
- 3,5-DCP
3,5-dichlorophenol
- AAS
atomic absorption spectroscopy
- AFW
artificial freshwater
- AO/EB
acridine orange/ethidium bromide
- BET
Brunauer, Emmett and Teller
- CNTs
carbon nanotubes
- DI water
deionized water
- DLS
dynamic light scattering
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- CLP
regulation on Classification, Labeling and Packaging
- hMSC
human bone marrow-derived mesenchymal stem cells
- ICP-MS
inductively coupled plasma mass spectrometry
- QSAR
quantitative structure–activity relationship
- LB
Luria–Bertani medium
- LOEC
lowest observed effect concentration
- MBC
minimal biocidal concentration
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MWCNTs
multi-walled carbon nanotubes
- n.a.
not applicable
- n.d.
not determined
- NPs
nanoparticles
- NMs
nanomaterials
- NRU
neutral red uptake
- OECD
Organization for Economic Co-operation and Development
- pdi
polydispersity index
- PI
propidium iodide
- PVP
polyvinylpyrrolidone
- REACH
European Union regulation on Registration, Evaluation, Authorization and Restriction of Chemicals
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulfate
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
- SSA
specific surface area
- WST-1
2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
- XRD
X-ray diffraction
- XPS
X-ray photoelectron spectroscopy
Supplementary material available online
Supplementary figures S1-S3 and Tables S1-S2
References
- Adam N, Schmitt C, De Bruyn L, Knapen D, Blust R. Aquatic acute species sensitivity distributions of ZnO and CuO nanoparticles. Sci Total Environ. 2015;526:233–42. doi: 10.1016/j.scitotenv.2015.04.064. [DOI] [PubMed] [Google Scholar]
- Arts JH, Hadi M, Keene AM, Kreiling R, Lyon D, Maier M, et al. A critical appraisal of existing concepts for the grouping of nanomaterials. Regul Toxicol Pharm. 2014;70:492–506. doi: 10.1016/j.yrtph.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Arts JH, Hadi M, Irfan MA, Keene AM, Kreiling R, Lyon D, et al. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul Toxicol Pharmacol. 2015;71:S1–27. doi: 10.1016/j.yrtph.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Aruoja V, Dubourguier HC, Kasemets K, Kahru A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. . Sci Total Environ. 2009;407:1461–8. doi: 10.1016/j.scitotenv.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Aruoja V, Pokhrel S, Sihtmäe M, Mortimer M, Mädler L, Kahru A. Toxicity of 12 metal based nanoparticles to algae, bacteria and protozoa. Environ Sci Nano. 2015;2:630–44. [Google Scholar]
- Bhattacharya K, Andón FT, El-Sayed R, Fadeel B. Mechanisms of carbon nanotube-induced toxicity: focus on pulmonary inflammation. Adv Drug Deliv Rev. 2013;65:2087–97. doi: 10.1016/j.addr.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Blinova I, Ivask A, Heinlaan M, Mortimer M, Kahru A. Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ Pollut. 2010;158:41–7. doi: 10.1016/j.envpol.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol. 2013a;87:1181–200. doi: 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko O, Ivask A, Käkinen A, Kurvet I, Kahru A. Particle-cell contact enhances antibacterial activity of silver nanoparticles. PLoS One. 2013b;8:e64060. doi: 10.1371/journal.pone.0064060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Vijver MG, Peijnenburg WJ. Summary and analysis of the currently existing literature data on metal-based nanoparticles published for selected aquatic organisms: applicability for toxicity prediction by (Q)SARs. Altern Lab Anim. 2015;43:221–40. doi: 10.1177/026119291504300404. [DOI] [PubMed] [Google Scholar]
- Choi JY, Ramachandrran G, Kandlikar M. The impact of toxicity testing costs on nanomaterial regulation. Environ Sci Technol. 2009;43:3030–4. doi: 10.1021/es802388s. [DOI] [PubMed] [Google Scholar]
- Coll C, Notter D, Gottschalk F, Sun T, Som C, Nowack B. Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes). Nanotoxicology. 2015;10:1–9. doi: 10.3109/17435390.2015.1073812. [DOI] [PubMed] [Google Scholar]
- Cronholm P, Karlsson HL, Hedberg J, Lowe TA, Winnberg L, Elihn K, et al. Intracellular uptake and toxicity of Ag and CuO nanoparticles: a comparison between nanoparticles and their corresponding metal ions. Small. 2013;9:970–82. doi: 10.1002/smll.201201069. [DOI] [PubMed] [Google Scholar]
- Djurišić AB, Leung YH, Ng AM, Xu XY, Lee PK, Degger N, Wu RS. Toxicity of metal oxide nanoparticles: mechanisms, characterization, and avoiding experimental artefacts. Small. 2015;11:26–44. doi: 10.1002/smll.201303947. [DOI] [PubMed] [Google Scholar]
- 2008. [Google Scholar]
- 2012. [Google Scholar]
- Fadeel B, Garcia-Bennett AE. Better safe than sorry: understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv Drug Deliv Rev. 2010;62:362–74. doi: 10.1016/j.addr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Farcal L, Torres Andón F, Di Cristo L, Rotoli BM, Bussolati O, Bergamaschi E, et al. Comprehensive in vitro toxicity testing of a panel of representative oxide nanomaterials: first steps towards an intelligent testing strategy. PLoS One. 2015;10:e0127174. doi: 10.1371/journal.pone.0127174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner KL, Suh S, Lenihan HS, Keller AA. Species sensitivity distributions for engineered nanomaterials. Environ Sci Technol. 2015;49:5753–9. doi: 10.1021/acs.est.5b00081. [DOI] [PubMed] [Google Scholar]
- Gebel T, Foth H, Damm G, Freyberger A, Kramer PJ, Lilienblum W, et al. Manufactured nanomaterials: categorization and approaches to hazard assessment. Arch Toxicol. 2014;88:2191–211. doi: 10.1007/s00204-014-1383-7. [DOI] [PubMed] [Google Scholar]
- George S, Xia T, Rallo R, Zhao Y, Ji Z, Lin S, et al. Use of a high-throughput screening approach coupled with in vivo zebrafish embryo screening to develop hazard ranking for engineered nanomaterials. ACS Nano. 2011;5:1805–17. doi: 10.1021/nn102734s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin H, Nameth C, Avery D, Bergeson LL, Bernard D, Beryt E, et al. Nanomaterial categorization for assessing risk potential to facilitate regulatory decision-making. ACS Nano. 2015;9:3409–17. doi: 10.1021/acsnano.5b00941. [DOI] [PubMed] [Google Scholar]
- Handy RD, van den Brink N, Chappell M, Mühling M, Behra R, Dušinská M, et al. Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: what have we learnt so far? Ecotoxicology. 2012;21:933–72. doi: 10.1007/s10646-012-0862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SF, Baun A. When enough is enough. Nat Nanotechnol. 2012;7:409–11. doi: 10.1038/nnano.2012.115. [DOI] [PubMed] [Google Scholar]
- Hartung T. Food for thought … on alternative methods for nanoparticle safety testing. Altex. 2010;27:87–95. doi: 10.14573/altex.2010.2.87. [DOI] [PubMed] [Google Scholar]
- Heinlaan M, Ivask A, Blinova I, Dubourguier H-C, Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. . Chemosphere. 2008;71:1308–16. doi: 10.1016/j.chemosphere.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Hitchman A, Sambrook Smith GH, Ju-Nam Y, Sterling M, Lead JR. The effect of environmentally relevant conditions on PVP stabilised gold nanoparticles. Chemosphere. 2013;90:410–16. doi: 10.1016/j.chemosphere.2012.07.041. [DOI] [PubMed] [Google Scholar]
- Horie M, Fujita K, Kato H, Endoh S, Nishio K, Komaba LK, et al. Association of the physical and chemical properties and the cytotoxicity of metal oxide nanoparticles: metal ion release, adsorption ability and specific surface area. Metallomics. 2012;4:350–60. doi: 10.1039/c2mt20016c. [DOI] [PubMed] [Google Scholar]
- 2010. [Google Scholar]
- Ivask A, Bondarenko O, Jepihhina N, Kahru A. Profiling of the reactive oxygen species related ecotoxicity of CuO, ZnO, TiO2, silver and fullerene nanoparticles using a set of recombinant luminescent Escherichia coli strains: differentiating the impact of particles and solubilised metals. Anal Bioanal Chem. 2010;398:701–16. doi: 10.1007/s00216-010-3962-7. [DOI] [PubMed] [Google Scholar]
- Ivask A, Titma T, Visnapuu M, Vija H, Kakinen A, Sihtmäe M, et al. Toxicity of 11 metal oxide nanoparticles to three mammalian cell types in vitro. . Curr Top Med Chem. 2015;15:1914–29. doi: 10.2174/1568026615666150506150109. [DOI] [PubMed] [Google Scholar]
- Jackson P, Jacobsen NR, Baun A, Birkedal R, Kühnel D, Jensen KA, et al. Bioaccumulation and ecotoxicity of carbon nanotubes. Chem Cent J. 2013;7:154. doi: 10.1186/1752-153X-7-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemec A, Kahru A, Potthoff A, Drobne D, Heinlaan M, Böhme S, et al. An interlaboratory comparison of nanosilver characterisation and hazard identification: harmonising techniques for high quality data. Environ Int. 2016;87:20–32. doi: 10.1016/j.envint.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Jiang J, Oberdörster G, Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J.Nanopart Res. 2009;11:77–89. [Google Scholar]
- Juganson K, Ivask A, Blinova I, Mortimer M, Kahru A. NanoE-Tox: new and in-depth database concerning ecotoxicity of nanomaterials. Beilstein J Nanotechnol. 2015;6:1788–804. doi: 10.3762/bjnano.6.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahru A, Dubourguier HC. From ecotoxicology to nanoecotoxicology. Toxicology. 2010;269:105–19. doi: 10.1016/j.tox.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Käkinen A, Bondarenko O, Ivask A, Kahru A. The effect of composition of different ecotoxicological test media on free and bioavailable copper from CuSO4 and CuO nanoparticles: comparative evidence from a Cu-selective electrode and a Cu-biosensor. Sensors. 2011;11:10502–21. doi: 10.3390/s111110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandarova H, Letašiova S. Alternative methods in toxicology: pre-validated and validated methods. Interdiscip Toxicol. 2011;4:107–13. doi: 10.2478/v10102-011-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dynam. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kodali V, Littke MH, Tilton SC, Teeguarden JG, Shi L, Frevert CW, et al. Dysregulation of macrophage activation profiles by engineered nanoparticles. ACS Nano. 2013;7:6997–7010. doi: 10.1021/nn402145t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyling WG, Semmler-Behnkea M, Chaudhry Q. A complementary definition of nanomaterial. Nano Today. 2010;5:165–8. [Google Scholar]
- Krug HF. Nanosafety research-are we on the right track? Angew Chem Int Ed Engl. 2014;53:12304–19. doi: 10.1002/anie.201403367. [DOI] [PubMed] [Google Scholar]
- Kurvet I, Ivask A, Bondarenko O, Sihtmäe M, Kahru A. LuxCDABE-transformed constitutively bioluminescent Escherichia coli for toxicity screening: comparison with naturally luminous Vibrio fischeri. . Sensors (Basel) 2011;11:7865–78. doi: 10.3390/s110807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JI, Cui R, Nam SH, Kim SW, Chae Y, An YJ. Multispecies toxicity test for silver nanoparticles to derive hazardous concentration based on species sensitivity distribution for the protection of aquatic ecosystems. Nanotoxicology. 2016;10:521–30. doi: 10.3109/17435390.2015.1090028. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Niu J, Chen Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano. 2012;6:5164–73. doi: 10.1021/nn300934k. [DOI] [PubMed] [Google Scholar]
- Maynard AD. Navigating the risk landscape. Nat Nanotechnol. 2016;11:211–12. doi: 10.1038/nnano.2016.28. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nam SH, Shin YJ, Lee WM, Kim SW, Kwak JI, Yoon SJ, An YJ. Conducting a battery of bioassays for gold nanoparticles to derive guideline value for the protection of aquatic ecosystems. Nanotoxicology. 2015;9:326–35. doi: 10.3109/17435390.2014.930531. [DOI] [PubMed] [Google Scholar]
- Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH. The nanosilica hazard: another variable entity. Part Fibre Toxicol. 2010;7:39. doi: 10.1186/1743-8977-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A, Xia T, Meng H, Wang X, Lin S, Ji Z, Zhang H. Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening. Acc Chem Res. 2013;46:607–21. doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel AE. Implementation of alternative test strategies for the safety assessment of engineered nanomaterials. J Intern Med. 2013;274:561–77. doi: 10.1111/joim.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter DA, Mitrano DM, Nowack B. Are nanosized or dissolved metals more toxic in the environment? A meta-analysis. Environ Toxicol Chem. 2014;33:2733–9. doi: 10.1002/etc.2732. [DOI] [PubMed] [Google Scholar]
- OECD. 2004. [Google Scholar]
- OECD. 2010. [Google Scholar]
- OECD. 2011. [Google Scholar]
- OECD. 2012. http://www.oecd.org/science/nanosafety/ [Google Scholar]
- OECD . 2013. [Google Scholar]
- Oomen AG, Bleeker EA, Bos PM, van Broekhuizen F, Gottardo S, Groenewold M, et al. Grouping and read-across approaches for risk assessment of nanomaterials. Int J Environ Res Public Health. 2015;12:13415–34. doi: 10.3390/ijerph121013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen EJ, Diamond SA, Kennedy AJ, Goss GG, Ho K, Lead J, et al. Adapting OECD aquatic toxicity tests for use with manufactured nanomaterials: key issues and consensus recommendations. Environ Sci Technol. 2015;49:9532–47. doi: 10.1021/acs.est.5b00997. [DOI] [PubMed] [Google Scholar]
- Puzyn T, Rasulev B, Gajewicz A, Hu X, Dasari TP, Michalkova A, et al. Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles. Nat Nanotechnol. 2011;6:175–8. doi: 10.1038/nnano.2011.10. [DOI] [PubMed] [Google Scholar]
- Suppi S, Kasemets K, Ivask A, Künnis-Beres K, Sihtmäe M, Kurvet I, et al. A novel method for comparison of biocidal properties of nanomaterials to bacteria, yeasts and algae. J Hazard Mater. 2015;286:75–84. doi: 10.1016/j.jhazmat.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Suresh AK, Pelletier DA, Doktycz MJ. Relating nanomaterial properties and microbial toxicity. Nanoscale. 2013;5:463–74. doi: 10.1039/c2nr32447d. [DOI] [PubMed] [Google Scholar]
- Valant J, Drobne D. Biological reactivity of TiO2 nanoparticles assessed by ex vivo testing. Protoplasma. 2012;249:835–42. doi: 10.1007/s00709-011-0298-x. [DOI] [PubMed] [Google Scholar]
- Vale G, Mehennaoui K, Cambier S, Libralato G, Jomini S, Domingos RF. Manufactured nanoparticles in the aquatic environment-biochemical responses on freshwater organisms: a critical overview. Aquat Toxicol. 2016;170:162–74. doi: 10.1016/j.aquatox.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Vindimian E. 2005. http://eric.vindimian.9online.fr/ [Google Scholar]
- von Moos N, Slaveykova VI. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae-state of the art and knowledge gaps. Nanotoxicology. 2014;8:605–30. doi: 10.3109/17435390.2013.809810. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine. 2015;11:313–27. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Donner M. How meaningful are risk determinations in the absence of a complete dataset? Making the case for publishing standardized test guideline and ‘no effect’ studies for evaluating the safety of nanoparticulates versus spurious ‘high effect’ results from single investigative studies. Sci Technol Adv Mater. 2015;16:034603. doi: 10.1088/1468-6996/16/3/034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witasp E, Kupferschmidt N, Bengtsson L, Hultenby K, Smedman C, Paulie S, et al. Efficient internalization of mesoporous silica particles of different sizes by primary human macrophages without impairment of macrophage clearance of apoptotic or antibody-opsonized target cells. Toxicol Appl Pharmacol. 2009;239:306–19. doi: 10.1016/j.taap.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Zhang L, Mizumoto K, Sato N, Ogawa T, Kusumoto M, Niiyama H, Tanaka M. Quantitative determination of apoptotic death in cultured human pancreatic cancer cells by propidium iodide and digitonin. Cancer Lett. 1999;142:129–37. doi: 10.1016/s0304-3835(99)00107-x. [DOI] [PubMed] [Google Scholar]
- Zou J, Feng H, Mannerström M, Heinonen T, Pyykkö I. Toxicity of silver nanoparticle in rat ear and BALB/c 3T3 cell line. J Nanobiotechnol. 2012;12:52. doi: 10.1186/s12951-014-0052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.