Abstract
In this study, we undertook a survey to analyze the distribution and frequency of microsatellites or Simple Sequence Repeats (SSRs) in Spodoptera littoralis multiple nucleopolyhedrovirus (SpliMNPV) genome (isolate AN–1956). Out of the 55 microsatellite motifs, identified in the SpliMNPV-AN1956 genome using in silico analysis (inclusive of mono-, di-, tri- and hexa-nucleotide repeats), 39 were found to be distributed within coding regions (cSSRs), whereas 16 were observed to lie within intergenic or noncoding regions. Among the 39 motifs located in coding regions, 21 were located in annotated functional genes whilst 18 were identified in unknown functional genes (hypothetical proteins). Among the identified motifs, trinucleotide (80%) repeats were found to be the most abundant followed by dinucleotide (13%), mononucleotide (5%) and hexanucleotide (2%) repeats. The 39 motifs located within coding regions were further validated in vitro by using PCR analysis, while the 21 motifs located within known functional genes (15 genes) were characterized using nucleotide sequencing. A comparison of the sequence analysis data of the 21 sequenced cSSRs with the published sequences is presented. Finally, the developed SSR markers of the 39 motifs were further mapped/localized onto the SpliMNPV-AN1956 genome. In conclusion, the SSR markers specific to SpliMNPV, developed in this study, could be a useful tool for the identification of isolates and analysis of genetic diversity and viral evolutionary status.
Baculoviruses, the most common type of insect specific viruses, are extremely diverse with interesting applications and a wide host range (about 600 species of insects worldwide). They are enveloped viruses with a circular double-strand DNA genome that ranges in size from 80 to 200 kb1. Baculoviruses are popularly regarded as pathogens that are specific for invertebrates especially insects of the order Lepidoptera, Hymenoptera and Diptera2. In this respect, baculoviruses have garnered a significant amount of attention as potential agents for biological control of pests belonging to the abovementioned orders. As an added advantage, increasing our insight into baculovirus molecular biology has enabled us to optimally utilize viruses as vectors for the expression of foreign proteins inside insect cells3. As an aid to further augment our understanding of the molecular genetics of these viruses, several baculoviral genomes have been sequenced in the last two decades. The first completely sequenced baculoviral genome belonging to the Autographa californica Multiple Nucleopolyhedrovirus (AcMNPV) was reported by Ayres et al.4. To the best of our knowledge, there are 73 fully sequenced baculoviral genomes available in GenBank (41 genomes of the Alpha-baculovirus genus, 13 of the Beta-baculovirus, 3 of the Gamma-baculovirus and one of the Delta-baculovirus). The number of fully sequenced baculoviral genomes available to us are infinitesimally low when compared to the number of species that exist in nature (about 600 species)5. However, with the development of advanced molecular biology based techniques such as gene cloning and sequencing, DNA restriction analysis and molecular phylogeny, several new and highly useful tools for gene and genome characterization are now accessible to us. The major impediment to the absence of clarity regarding the real diversity of baculoviruses is because of the absence of reliable system for virus identification5. Simple sequence repeats (SSRs), also known as microsatellites, refer to mono-, di-, tri-, tetra-, penta- and hexanucleotide sequence units that are repeated in tandem in a genome6,7. Microsatellites are widely regarded as the most variable type of DNA sequence within the viral genome. SSRs are found in a variety of genomic regions including the 3′ and 5′ untranslated regions as well as exons and introns (protein-coding and non-coding regions)8,9,10. For this reason, SSRs are speculated to play a variety of diverse roles in the eukaryotic, prokaryotic and viral genomes. In spite of their hypermutable nature, SSRs have been widely used as markers for a variety of studies such as genome mapping, ecology and evolutionary genetics. It is also well known that the inherent instability of microsatellites plays a crucial role in the development of frame shift mutations that encode phenotypic changes and confer an adaptive advantage for the evolution of certain mutated viral strains11. Despite the growing number of completely sequenced viral genomes submitted to the public database, little attention has been paid towards surveying SSRs at the genome level for viruses in general and baculoviral genomes in particular. In spite of their abundance and functional relevance in viral genomes, the distribution pattern of microsatellites remains to be fully elucidated7. As a result of this, determining microsatellites distribution in baculoviruses has become crucial for understanding the evolution of baculoviral genomes. Spodoptera littoralis, the Egyptian cotton leafworm, causes significant damage to a wide range of economically important crops in Africa, southern Europe and in Middle East12. Recently, full genome sequencing of the S. littoralis multiple nucleopolyhedrovirus (SpliMNPV) revealed that the viral genome is 137,998 bp in size and is composed of 132 open reading frames and 15 homologous repeat regions13. In the current study, as an attempt to develop specific microsatellites markers to SpliMNPV, we present results from a genome-wide in silico analysis, characterization and identification of microsatellites distribution within the SpliMNPV genome. We propose that the results have the potential to expand our understanding of virus diversity, evolution and isolate identification.
Results
Distribution of SSRs in SpliMNPV Genome
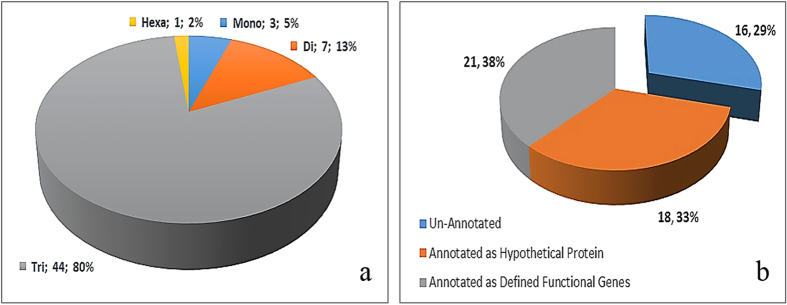
In the present study, we analyzed the distribution of perfect SSRs (1–6 bp long) within the S. littoralis nucleopolyhedrovirus (SpliMNPV-AN1956) genome. Our attempts were successful in identifying 55 different SSR motifs (mono-, di-, tri- and hexa-nucleotide repeats) distributed within the SpliMNPV genome sequence. Interestingly, there were no tetra- and penta-nucleotide repeats observed in the SpliMNPV genome. Among the identified SSR motifs, trinucleotides (44 motifs; 80%) were the most common type of repeats followed by dinucleotides (7 motifs; 13%), mononucleotides (3 motifs; 5%) and lastly, the hexa-nucleotides (1 motif; 2%) motifs (Fig. 1a). Sixteen (29%) microsatellite motifs were found to be distributed within intergenic or noncoding regions, while 39 (71%) were present in Open Reading Frames (ORFs or coding regions; cSSRs). Of these 39 cSSR motifs, 21 were localized within defined functional genes, while 18 were present within Coding DNA Sequence (CDS) regions annotated as hypothetical proteins (with unknown function). As some genes were found to harbor more than one microsatellite repeat region, the 21 different cSSR motifs were found to be localized within 15 defined functional genes (Fig. 1b). Out of the 132 known ORFs in the SpliMNPV-AN1956 genome13, the 39 cSSRs identified in this study successfully covered 33 (25% of the total ORFs). Assessing the relative composition of the repeat types within the covered ORFs revealed that the 38 cSSRs (97.4%) concerned were predominantly composed of trinucleotide repeats with only one mononucleotide motif (2.6%) identified. Of the 33 ORFs covered by cSSRs, ORF–14 was found to have the highest number of cSSR motifs (3 trinucleotide cSSR motifs).
Figure 1.
(a) Types of SSR motifs, number of motifs for each type and their frequency in SpliMNPV genome. (b) Distribution of SSR motifs on SpliMNPV genome components and their frequency.
Frequency of Classified Repeat Types
Frequency analysis of the classified repeat types revealed that the SpliMNPV-AN1956 genome had seven types of trinucleotide repeats: AAC/GTT, AAT/ATT, ACG/CGT, ACT/AGT, AGC/CTG, ATC/ATG, and CCG/CGG. The CCG/CGG repeats were the most prevalent, whereas the AAT/ATT repeats were the least represented. Mono-, di- and hexanucleotide repeats were found to be composed of only one type of each (A/T, AC/GT and AATACT/AGTATT). The distribution of mono-, di-, tri- and hexanucleotide repeats is summarized in Table 1.
Table 1. Characteristics of the repeat types (motifs and their sequence complementary) for the Spodoptera littoralis nucleopolyhedrovirus.
| Type | Motif | Motif repeats |
Total no. of SSR | Frequency % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | 10 | 15 | 16 | ||||
| Mono | A/T | 3 | 3 | 100.0* | |||||||
| Di | AC/GT | 4 | 1 | 1 | 1 | 7 | 100.0** | ||||
| Tri | AAC/GTT | 1 | 1 | 2 | 4.5*** | ||||||
| AAT/ATT | 1 | 1 | 2.3*** | ||||||||
| ACG/CGT | 4 | 3 | 1 | 2 | 2 | 12 | 27.3*** | ||||
| ACT/AGT | 1 | 1 | 1 | 1 | 4 | 9.1*** | |||||
| AGC/CTG | 1 | 2 | 1 | 1 | 5 | 11.4*** | |||||
| ATC/ATG | 3 | 2 | 2 | 7 | 15.9*** | ||||||
| CCG/CGG | 8 | 3 | 2 | 13 | 29.5*** | ||||||
| Hexa | AATACT/AGTATT | 1 | 1 | 100.0**** | |||||||
| Total | 55 | ||||||||||
*Frequency of motifs in total number of mononucleotide SSRs.
**Frequency of motifs in total number of dinucleotide SSRs.
***Frequency of motifs in total number of trinucleotide SSRs.
****Frequency of motifs in total number of hexanucleotide SSRs.
Development and Characterization of cSSR Markers
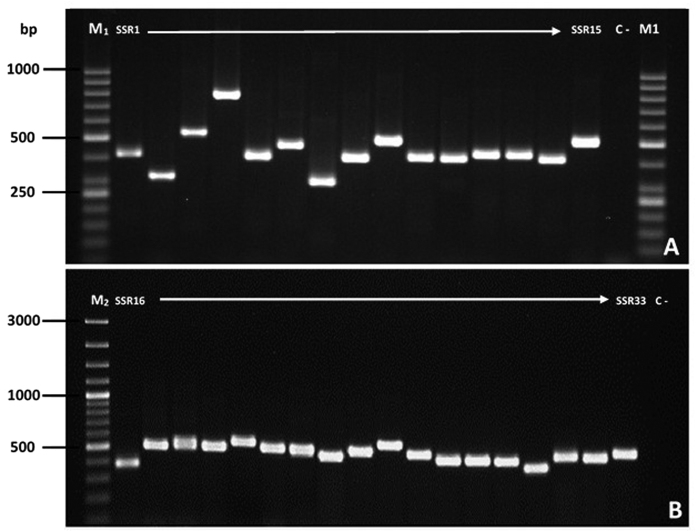
Out of the 55 microsatellite motifs that were identified in the SpliMNPV genome, 39 motifs (located in coding regions) were analyzed in vitro using PCR analysis. We designed 33 SSR-PCR primer pairs to amplify the 39 motifs. The number of cSSR primers designed were less than the targeted amplified motifs due to the fact that some genes harbored more than one SSR motif. The cSSRs name, motif, motif length, motif position, gene, ORF number and protein identification are summarized in Table 2. From the 55 identified SSR motifs, 39 motifs (cSSRs) were selected and 33 primer pairs were designed in order to generate amplicons containing the targeted motifs (Table 2). The 33 cSSR markers produced reliable and reproducible PCR products with the expected molecular size (Fig. 2).
Table 2. Characteristics of the 33 microsatellite markers developed for the Spodoptera littoralis nucleopolyhedrovirus.
| Name | (Motif) length | Position | Gene | ORF | Expected Size (bp) |
|---|---|---|---|---|---|
| SSR1 | (A)10, (GCG)5* | 88474–88483, -------------- | LEF–5 | ORF84 | 386 |
| SSR2 | (AAC)5 | 14862–14876 | IE–1 | ORF14 | 316 |
| SSR3 | (AGT)3**, (TGC)5 | 32697–32711, 33052–33066 | PP31 | ORF29 | 537 |
| SSR4 | (TCA)5, (GCG)5, (ATC)7 | 4215–4229, 4414–4428, 4856–4876 | HOAR | ORF4 | 810 |
| SSR5 | (CGA)8 | 79413–79436 | LEF–4 | ORF77 | 409 |
| SSR6 | (CGA)5 | 67543–67557 | DNA polymerase | ORF63 | 464 |
| SSR7 | (CGG)5, (GGC)6 | 1539–1553, 1570–1587 | pp78/81 | ORF2 | 301 |
| SSR8 | (CGG)5**, (GAT)6**, (CGT)5* | 59499–59513, 59531–59548, --------------- | Apoptosis inhibitor | ORF58 | 414 |
| SSR9 | (GCC)5, (GAC)8 | 92590–92604, 92648–92671 | VP80 | ORF89 | 503 |
| SSR10 | (GAC)5 | 46842–46856 | LEF–10 | ORF44 | 418 |
| SSR11 | (GCA)7 | 129403–129423 | pkip | ORF125 | 417 |
| SSR12 | (GGC)5 | 18102–18116 | ODV-E56 | ORF15 | 439 |
| SSR13 | (GTC)7 | 126169–126189 | 38.7 kDa protein | ORF120 | 438 |
| SSR14 | (TCG)9 | 70961–70987 | VLF–1 | ORF69 | 413 |
| SSR15 | (TCG)6 | 38044–38061 | LEF–8 | ORF34 | 501 |
| SSR16 | (TCG)5 | 55421–55435 | HP | hr5 | 376 |
| SSR17 | (TTA)5 | 57045–57059 | HP | hr6 | 494 |
| SSR18 | (TGC)8 | 7005–7028 | HP | ORF6 | 500 |
| SSR19 | (CGA)6 | 19460–19477 | HP | ORF18 | 500 |
| SSR20 | (ATC)6 | 24789–24806 | HP | ORF21 | 539 |
| SSR21 | (GTT)8 | 34341–34364 | HP | ORF31 | 500 |
| SSR22 | (TAG)8 | 46038–46061 | HP | ORF42 | 469 |
| SSR23 | (CCG)7 | 49243–49263 | HP | ORF48 | 412 |
| SSR24 | (TCA)7 | 69808–69828 | HP | ORF66 | 500 |
| SSR25 | (GCC)5 | 72288–72302 | HP | ORF70 | 556 |
| SSR26 | (CAG)6 | 81544–81561 | HP | ORF79 | 491 |
| SSR27 | (ATG)5 | 101095–101109 | HP | ORF96 | 446 |
| SSR28 | (TGC)6 | 104359–104376 | HP | ORF100 | 442 |
| SSR29 | (CCG)6 | 112560–112577 | HP | ORF108 | 439 |
| SSR30 | (GGC)6 | 116277–116294 | HP | ORF113 | 400 |
| SSR31 | (GAC)9 | 119289–119315 | HP | ORF114 | 456 |
| SSR32 | (CGG)7 | 127202–127222 | HP | ORF122 | 448 |
| SSR33 | (TCG)5 | 133751–133765 | HP | ORF128 | 481 |
*New SSR motifs not observed in the SpliMNPV published genome sequence.
**Absent SSR motifs in the tested SpliMNPV genome in comparison with the published genome.
HP: Hypothetical protein.
Figure 2. Representative electrophoresis gel showing the PCR amplicon of the developed cSSR markers in SpliMNPV.
(A) cSSR markers from SSR1 to SSR15 (annotated as defined functional genes). (B) cSSR markers from SSR16 to SSR33 (annotated as hypothetical protein). M1: 50 bp DNA ladder, M2: 100 bp ladder plus, C: Negative controls (PCR using DNA extracted from non-infected S. littoralis larvae).
Alignment and Mapping of cSSRs
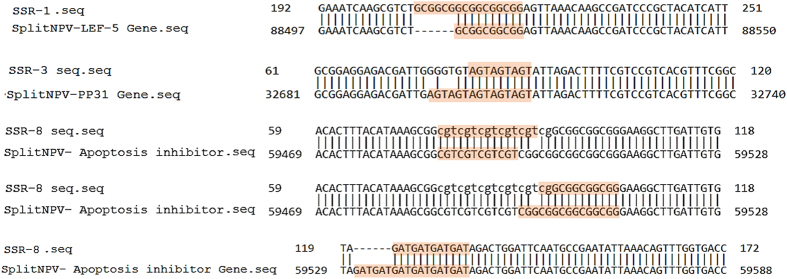
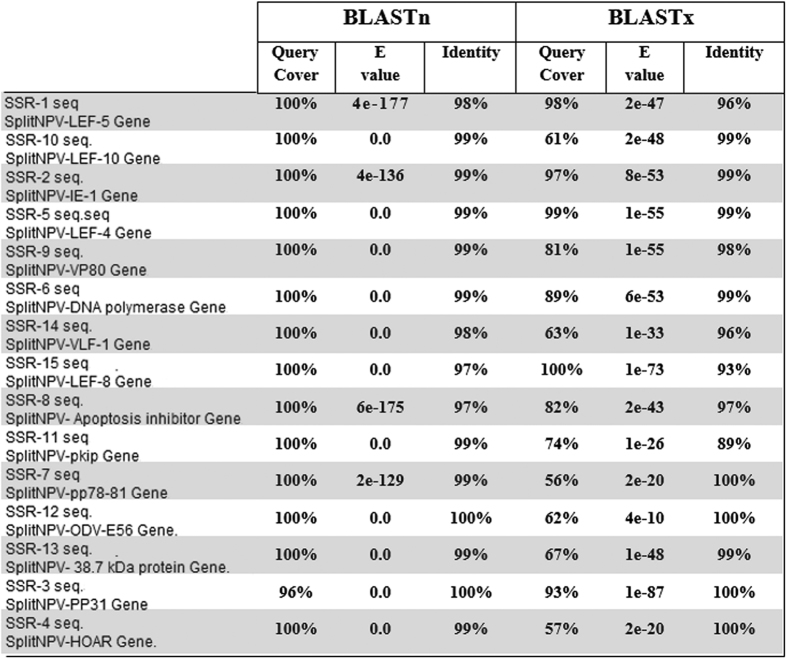
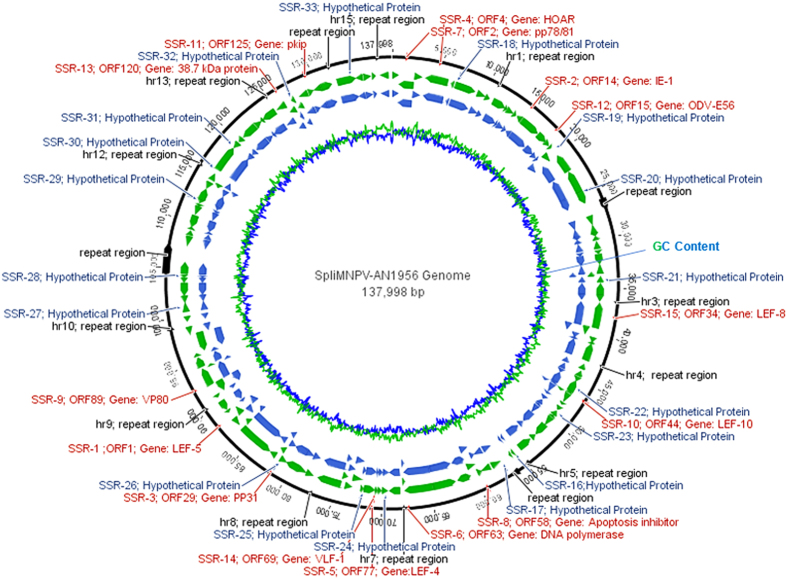
BLASTn and BLASTx were used to align the 15 cSSR sequences with the GenBank database. The results of BLASTn alignment revealed a high degree of query coverage (96–100%) and a high identity percentage (97–100%) between the 15 cSSR sequences and their equivalent genes from the published SpliMNPV-AN1956 isolate genome sequence. Interestingly, BLASTn alignment of SSR1 and SSR8 in the tested SpliMNPV genome revealed the presence of a novel triplet motif which is not observed in the published SpliMNPV genome sequence. In contrast, the sequence data analysis revealed the absences of three triplet motifs (one in SSR3 and two in SSR8) in the tested SpliMNPV genome when it was compared with the published genome (Fig. 3). The results of BLASTx alignment revealed various degrees of query coverage (57–100%) and a high identity percentage (89−100%) with their equivalent amino acid sequences as derived from the published SpliMNPV-AN1956 annotated genome (Fig. 4). Furthermore, the 33 cSSRs markers were mapped/localized on the SpliMNPV-AN1956 genome. Of 33 cSSRs, 15 were mapped within defined functional genes while 18 have been mapped within CDS sequences annotated as hypothetical proteins (Fig. 5).
Figure 3. Diagrammatic representation illustrate the differences in SSR motifs between the tested SpliMNPV genome and the published genome.
Figure 4. Alignment of the 15 sequenced cSSR markers (partial genes) against their original sequences distributed over the SpliMNPV-AN1956 isolate complete genome.
Figure 5. Diagrammatic representation of the SpliMNPV-AN1956 genome showing genes (blue arrows), CDS (green arrows), GC content (blue-green peaks), repeat regions (black arrows), SSRs localized within defined functional gene sequences (SSR–1 to SSR–15; red font), and SSRs localized within CDS sequences annotated as hypothetical protein (SSR–16 to SSR–33; blue font) as localized/distributed on genome.
Discussion
With next-gen DNA sequencing technologies becoming increasingly efficient, fast, and cheap, a large number of baculoviral genome sequences are now being generated and made publicly available. These genome sequences represent a potentially valuable resource for mining SSR markers. In the present study, we have identified and characterized 39 cSSRs from a total of 55 SSRs motifs distributed within the SpliMNPV genome (isolate AN–1956). It was observed that the relative abundance of SSR motifs in the SpliMNPV genome (~138 Kb) was 0.39 motif/kb. When compared with the herpes simplex virus type 1 (HSV–1) which has relatively bigger genome size (152 Kb), it was observed that the relative abundance of SSR motifs in the SpliMNPV genome was comparatively lower (0.39 motif/kb for SpliMNPV vs. 0.52 motif/kb for HSV–1). Interestingly, alpha virus, which has small genome size (~11.5 kb), has relative abundance values ranging between 2.32–5.05 motif/kb14. These observed variations in the relative abundance of SSR motifs between different viral genome may be attributed to differences in genome sizes or virus type. In the specific case of the SpliMNPV genome, results revealed that the trinucleotide motif was the most abundant type of repeat (80%) followed by the dinucleotide (13%). In partial agreement with our results, dinucleotide and trinucleotide SSRs were reported as the most frequently observed repeat types in the Human Immunodeficiency Virus Type 1 (HIV–1) genomes while tetra-, penta- and hexanucleotide SSRs were almost non-existent15. A similar survey of microsatellites in the hepatitis C virus (HCV) revealed that mono-, di- and trinucleotide repeat types were dominant while other types of repeats were observed to occur very rarely7. In a sharp contrast, an exploration of 30 alphavirus genomes revealed that mononucleotide repeats were the most prevalent followed by dinucleotide and trinucleotide repeats14. A study on the HSV–1 genome reported that mononucleotide repeats occurred with the maximum frequency followed by trinucleotide and dinucleotide repeats16. The exploration of microsatellites in diverse Gemini virus genomes showed that among the analyzed genomes dinucleotide repeats were the most abundant followed by the trinucleotide ones; the relative abundance of tetra−, penta−, and hexanucleotide repeats was seen to be very low17. Also, a genome wide survey of microsatellite distribution in ssDNA viruses that infect vertebrates revealed that mononucleotide repeats were the most dominant followed by dinucleotide and trinucleotide repeats18.
Comparative distribution across coding and non-coding regions
The distribution of SSRs motifs among coding/non-coding region in the SpliMNPV genome revealed a high incidence (71%) of repeats within coding regions as compared to the non-coding regions (29%). Furthermore, an assessment of the relative composition of repeat motifs revealed that the coding regions predominantly contained trinucleotide repeats (97.4%) with only a solitary mononucleotide repeat sequence (2.6%). In contrast, within non-coding regions it was seen that the di-nucleotide motifs (43.7%) were the most prevalent followed by trinucleotide motifs (37.5%). In agreement with our results, Chen et al.7 have found that coding regions of the HCV genomes are significantly richer in microsatellite composition as compared to non-coding regions. In Escherichia coli, which serve as a prokaryotic model, coding regions are richer in microsatellites as compared to non-coding regions; this can be attributed to the fact that the bulk of the genome is composed of open reading frames19,20.
Tri-nucleotide repeats
It was recently reported that coding regions of eukaryotic and prokaryotic genomes have a higher density of trinucleotide repeats as compared to any other repeat type8,21,22. Interestingly, dynamic mutations in trinucleotide repeats have occasionally been associated with the development of some diseases23, as well as in other important functions24. Microsatellite mutations are more frequently observed in trinucleotide repeats than in any other type of repeats; it is also known that microsatellites can alter their overall length by deletion (contraction) or insertion (expansion) of a small number of repeat units25. Interestingly, in the current study, variations between the published genome sequence and the tested SpliMNPV genome have been observed for SSR1, SSR3 and SSR8. These variations can be attributed to contraction of the template strand via loop formation or expansion via replication slippage; the latter is considered as the most likely mutational process in case of the trinucleotide repeats type26. In eukaryotes, triplet repeats are more common than non-triplet ones as changes in non-triplet repeats lead to frameshift mutations within coding sequences8,27. Studies with the alphavirus genomes14 have demonstrated that tri-nucleotide repeats are the third most abundant SSRs.
Di-nucleotide repeats
Dinucleotide repeats are reported to increase the number of expected slippage events per unit length of DNA as they have the highest slippage rate as compared to any other type of repeat28. Among 257 viral genomes examined in a published study, it was found that dinucleotide SSRs account for the largest proportion of repeats with the others occurring in significantly lesser proportions29. In case of SpliMNPV, dinucleotide repeats were found to be the second most abundant type of repeats following the trinucleotide. It is noteworthy that the exact opposite findings were observed in geminivirus genome. In that case, dinucleotide repeats were significantly more common that the trinucleotides17. This can be attributed to the elevated instability rate of dinucleotide repeats due to their higher slippage rate30. Additionally, a genome wide survey of microsatellites in ssDNA viruses that infect vertebrates revealed that dinucleotide repeats are the second most frequently occurring type followed by the trinucleotide repeats18. Dinucleotide repeats are also speculated to be recombination hot spots31,32,33. This function rapidly adjusts to the evolutionary demands through recovery of genetic variation lost by genetic drift34,35.
Mononucleotide repeats
The results obtained in this study clearly demonstrate that mononucleotide repeats have a rare occurrence in the SpliMNPV-AN1956 genome. Poly (A/T) repeats occur more frequently as compared to poly (G/C) repeats (Table 1). The SpliMNPV genome is known to have a relatively high GC content of 44.68% and it is generally assumed that the higher poly (G/C) frequencies in the genome are attributable to the high GC content of the genome36. In this context it was interesting to find that poly (G/C) repeats were entirely absent in the SpliMNPV genome. Hence it can be concluded that GC content of genome has negligible or no influence on the occurrence of mononucleotide repeats; this is particularly true for poly (G/C) repeats in the SpliMNPV genome. In general, in eukaryotic or prokaryotic genomes, it has been observed that poly (A/T) tracts are more abundant than poly (G/C) tracts10,20,36,37. In the same context, in baculoviruses the frequency of A/T mononucleotide repeats was found to be significantly higher than that of the G/C mononucleotide repeats38. In yeast and E. coli, mononucleotide repeats were found to strongly affect protein expression by virtue of higher error rates of transcription and translation36,39,40,41.
Microsatellites as a component of viral genomes
In this study, a variety of simple sequence repeats were identified and characterized in the SpliMNPV genome. It was observed that some microsatellite types were significantly over represented which is suggestive of the fact that they may play an important role in SpliMNPV genome organization. In viruses, microsatellites are known as the most hypermutable regions42. Mutation rates of SSRs have been reported to be affected by a variety of parameters such as motif composition, motif length, and purity of repetition27. The functional and evolutionary role of microsatellites in baculoviruses is poorly understood and further studies are needed in order to explore their distribution and frequency within these genomes. Variations in their complexity and frequency across species and also within coding and non-coding sequences is suggestive of the fact that they may be involved in the recombination process occurring within hot spots and consequently be important from a gene regulation point of view14. Also, microsatellites have been reported to be involved in different processes such as replication, recombination, and repair mechanisms, which in turn results in sequence diversity that drives adaptive forces24. Some pathogens are found to have the ability to utilize SSRs to frustrate the host immune system by using it to enhance their antigenic variability29. It has been reported in literature that errors in high fidelity polymerase activity are not the only reason for any evolutionary event to occur within virus genomes, but that it may also be governed by replication speed and genomic architecture of the virus43. In conclusion, the study of microsatellites in SpliMNPV genome is the first step towards a better understanding of the nature, function and evolutionary biology of baculoviruses. Additionally, microsatellites are also known to provide a molecular basis for virus persistence and adaptation to environmental stresses. Our preliminary results can be considered as a useful tool in the study of viral genetic diversity, virus evolution and strain demarcation. Our group is in the process of conducting similar studies on all completely sequenced baculoviral genomes in order to elucidate the functional significance and evolutionary dynamics of microsatellites.
Methods
SpliMNPV Genome sequence
The publicly available whole genome sequence of SpliMNPV isolate AN1956 (Accession no. JX454574), as obtained from the NCBI database (http:// http://www.ncbi.nlm.nih.gov/nuccore/449139050), was used for genome-wide in silico microsatellites analysis. More information on this genome can be obtained from data published by Breitenbacha et al.13. Both the genomes, i.e., the publicly available genome used for the in silico analysis as well as the isolate used for SSR in vitro identification, are Egyptian in origin.
Genome-wide Microsatellites Identification
The following criteria were used to configure the MIcroSAtellite (MISA) identification tool software to identify SSRs: mono-nucleotide (×10), di- (×6), tri- (×5), tetra- (×5), penta- (×5) and hexa-nucleotide (×5). This tool facilitates the identification and localization of perfect and compound microsatellites. Identified SSRs were classified as coding (cSSRs) and non-coding based upon their presence within coding or non-coding regions of the SpliMNPV genome. The maximum distance permitted between two different SSR in a compound sequence was 100 bp. Subsequently, Primer3Plus web tool (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi/) was used to design primer pairs flanking each identified SSR motif located within the coding regions.
Insect and virus
Cotton leafworm, S. littoralis (Boised.) used for virus propagation, was obtained from the Insect Rearing Unit (IRU), Agricultural Genetic Engineering Research Institute, Agricultural Research Center, Giza, Egypt. Larvae were reared on a semi-artificial diet comprising of dry beans, yeast, agar and ascorbic acid sterilized for 20 min at 120 °C44. The SpliMNPV, a local Egyptian isolate (AN1956), was used in this study. Viral Occluded Bodies (OBs) were propagated by individually orally inoculating each early fourth instar larvae (L4) of S. littoralis with 1000 OB/larva on a small piece of medium for 24 h. Five to seven days post infection (p.i), cadavers were collected and subjected to purification of OBs by homogenizing them in 0.5% sodium dodecyl sulfate (SDS). The homogenate was filtered through two layers of cheesecloth and cotton prior to being washed with additional volumes of 0.5% SDS. Suspended OBs were collected after centrifugation at 12,000 rpm for 10 min. Pellets were washed twice with 0.1% SDS and once with 0.5 M NaCl. OBs were resuspended in deionized distilled H2O and viral genomic DNA was extracted as described previously by O’Reilly45.
Microsatellite PCR Analysis and Sequencing
Motifs located within defined functional gene sequences were PCR amplified using especially designed SSR-PCR primer pairs. All PCR reactions were performed in 25 μL reaction mixtures containing 1X PCR buffer, 1.5 mM MgCl2, 0.2 μM of each deoxynucleoside triphosphate (dNTPs), 1 μM of forward and reverse primers, 1 U of proofreading Taq polymerase (Platinum, Invitrogen) and 25 ng SpliMNPV genomic DNA. PCR amplification was performed in a Thermal Cycler system 2720 (Applied Biosystems, Inc.). The thermo-cycling profile used was as follows: 1 cycle of denaturation (2 min at 94 °C), 35 cycles (30 s at 94 °C, 30 s at Tm of primer, 60 s at 68 °C), and a final elongation step (10 min at 68 °C). The amplified products were resolved by electrophoresis in a 2% agarose gel at 100 volts. Ethidium bromide was used for detection of amplified DNA. The PCR amplified SSR products were visualized and photographed using a Gel Doc™ XR+ System with Image Lab™ Software (Bio-Rad®). Subsequently, the PCR products were purified using QIAquick® PCR Purification Kit (QIAGEN, Santa Clarita, CA) and the purified fragments were subjected to nucleotide sequencing using 3100 ABI sequencer (Applied Biosystems, Inc.) as described by Sanger et al.46. All sequences obtained were analyzed twice in each direction.
Sequencing Data Analysis
The nucleotide sequence data of the developed SSR markers was aligned against their equivalent genes sequences in the SpliMNPV genome using the MegAlign tool (DNASTAR, Inc.) in accordance with the ClustalW multiple sequence alignment algorithm47. In addition, alignment (MegaBLAST, discontiguous-MegaBLAST) analysis48 was used to identify specific regions among the reads that may not be well aligned with the SpliMNPV genome. Furthermore, the sequences were also subjected to the BLASTx analysis which compares translational products of the nucleotide query sequence to protein databases (http://www.ncbi.nlm.nih.gov).
Additional Information
How to cite this article: Atia, M. A. M. et al. Genome-wide In Silico Analysis, Characterization and Identification of Microsatellites in Spodoptera littoralis Multiple nucleopolyhedrovirus (SpliMNPV). Sci. Rep. 6, 33741; doi: 10.1038/srep33741 (2016).
Acknowledgments
The authors acknowledge the Agricultural Genetic Engineering Research Institute, ARC, Giza, Egypt for funding the activities presented in this study. They also recognize the valuable technical help of both Molecular Genetics & Genome Mapping lab. and Molecular Entomology Lab. staff. They also would like to thank Prof. Said El Salamouny, (Faculty of Agriculture, Cairo University, Giza, Egypt), for providing of virus isolate.
Footnotes
Author Contributions M.A.M.A. designed the study, performed in silico analysis, sequence data analysis, interpreted the data, wrote the manuscript; G.H.O. interpreted data, helped in revision of the manuscript; and W.H.E. prepared the viral DNA, performed PCR analysis, helped in data interpretation manuscript drafting.
References
- Van Oers M. M. & Vlak J. M. Baculovirus genomics. Curr. Drug Targets 8, 1051–1068 (2007). [DOI] [PubMed] [Google Scholar]
- Adams J. R. & McClintock J. T. Nuclear polyhedrosis viruses of insects. In “Atlas of Invertebrate Viruses” , pp. 87–204, Edited by Adams J. R. & Bonami J. R.Boca Raton, FL, CRC Press (1991). [Google Scholar]
- Miller L. K. Baculoviruses as gene expression vectors. Ann. Rev. Microbio . 42, 177–199 (1988). [DOI] [PubMed] [Google Scholar]
- Ayres M. D., Howard S. C. Kuzio J., Lopez-Ferber M. & Possee R. D. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virol. 202, 586–605 (1994). [DOI] [PubMed] [Google Scholar]
- Lange M., Wang H., Zhihong H. & Jehle J. A. Towards a molecular identification and classification system of lepidopteran-specific baculoviruses. Virol . 325, 36–47 (2004). [DOI] [PubMed] [Google Scholar]
- Chen M., Tan Z., Zeng G. & Peng J. Comprehensive Analysis of Simple Sequence Repeats in Pre-miRNAs. Mol. Biol. Evol. 27, 2227–2232 (2010). [DOI] [PubMed] [Google Scholar]
- Chen M., Tan Z. & Zeng G. Microsatellite is an important component of complete Hepatitis C virus genomes. Infect. Genet. Evol. 11, 1646–1654 (2011). [DOI] [PubMed] [Google Scholar]
- Li Y. C., Korol A. B., Fahima T. & Nevo E. Microsatellites within genes: structure, function, and evolution. Mol. Biol. Evol. 21, 991–1007 (2004). [DOI] [PubMed] [Google Scholar]
- Rajendrakumar P., Biswal A. K., Balachandran S. M., Srinivasarao K. & Sundaram R. M. Simple sequence repeats in organellar genomes of rice: frequency and distribution in genic and intercoding regions. Bioinformatics 23, 1–4 (2007). [DOI] [PubMed] [Google Scholar]
- Toth G., Gaspari Z. & Jurka J. Microsatellites in different eukaryotic genomes: survey and analysis. Genome Res. 10, 967–981 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Barreno B., Delgado T. & Melero J. A. Oligo(A) sequences of human respiratory syncytial virus G protein gene: assessment of their genetic stability in frameshift mutants. J. Virol. 68, 5460–5468 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S. E. New Pest Response Guidelines: Spodoptera, USDA/APHIS/PPQ/PDMP http://www.aphis.usda.gov/ppq/manuals/.(2004).
- Breitenbacha J. E. et al. Determination and analysis of the genome sequence of Spodoptera littoralis multiple nucleopolyhedrovirus. Virus Res . 17, 194–208 (2012). [DOI] [PubMed] [Google Scholar]
- Alam C. M., Singh A. K., Sharfuddin C. & Ali S. In- silico exploration of thirty alphavirus genomes for analysis of the simple sequence repeats. Meta Gene . 2, 694–705 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. et al. Similar distribution of simple sequence repeats in diverse completed Human Immunodeficiency Virus Type 1 genomes. FEBS Lett. 583, 2959–2963 (2009). [DOI] [PubMed] [Google Scholar]
- Deback C. et al. Utilization of Microsatellite Polymorphism for Differentiating Herpes Simplex Virus Type 1 Strains. J. Clinc. Microb. 47, 533–540 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- George B., Mashhood Alam Ch., Jain S. K., Sharfuddin Ch. & Chakraborty S. Differential distribution and occurrence of simple sequence repeats in diverse geminivirus genomes. Virus Genes 45, 556–566 (2012). [DOI] [PubMed] [Google Scholar]
- Jain A., Mittal N. & Sharma P. C. Genome wide survey of microsatellites in ssDNA viruses infecting vertebrates. Gene 552, 209–218 (2014). [DOI] [PubMed] [Google Scholar]
- Chen M. et al. Compound microsatellites in complete Escherichia coli genomes. FEBS Lett. 585, 1072–1076 (2011b). [DOI] [PubMed] [Google Scholar]
- Gur-Arie R. et al. Simple sequence repeats in Escherichia coli: abundance, distribution, composition, and polymorphism. Genome Res. 10, 62–71 (2000). [PMC free article] [PubMed] [Google Scholar]
- Kim T. S. et al. Simple sequence repeats in Neurospora crassa: distribution, polymorphism and evolutionary inference. BMC Genomics 9, 31–49 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgante M., Hanafey M. & Powell W. Microsatellites are preferentially associated with nonrepetitive DNA in plant genomes. Nat. Genet. 30, 194–200 (2002). [DOI] [PubMed] [Google Scholar]
- Usdin K. The biological effects of simple tandem repeats: lessons from the repeat expansion diseases. Genome Res . 18, 1011–1019 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashi Y. & King D. G. Simple sequence repeats as advantageous mutators in evolution. Trends Genet . 22, 253–259 (2006). [DOI] [PubMed] [Google Scholar]
- Xu X., Peng M. & Fang Z. The direction of microsatellite mutations is dependent upon allele length. Nat. Genet. 24, 396–399 (2000). [DOI] [PubMed] [Google Scholar]
- Lin Y., Dent S. Y., Wilson J. H., Wells R. D. & Napierala M. R. loops stimulate genetic instability of CTG. CAG repeats. Proc. Natl. Acad. Sci. 107, 692–697 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat. Rev. Genet. 5, 435–445 (2004). [DOI] [PubMed] [Google Scholar]
- Di Rienzo A. et al. Mutational processes of simple-sequence repeat loci in human populations. Proc. Natl. Acad. Sci . 91, 3166–3170 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. et al. Co evolution between simple sequence repeats (SSRs) and virus genome size. BMC Genomics 13, 435–446 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katti M. V., Ranjekar P. K. & Gupta V. S. Differential distribution of simple sequence repeats in eukaryotic genome sequences. Mol. Biol. Evol. 18, 1161–1167 (2001). [DOI] [PubMed] [Google Scholar]
- Treco D. & Arnheim N. The evolutionarily conserved repetitive sequence d(TG.AC)n promotes reciprocal exchange and generates unusual recombinant tetrads during yeast meiosis. Mol. Cell Biol. 6, 3934–3947 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahls W., Wallace L. J. & Moore D. The Z-DNA motif d(TG)30 promotes reception of information during gene conversion events while stimulating homologous recombination in human cells in culture. Mol. Cell Biol. 10, 785–793 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A. D., Pavelitz T. & Weiner A. M. The microsatellite sequence (CT)n × (GA)n promotes stable chromosomal integration of large tandem arrays of functional human U2 small nuclear RNA genes. Mol Cell Biol . 18, 2226−2271 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L. & Trimarchi J. M. Adaptive reversion of a frame shift mutation in Escherichia coli by simple base deletions in homopolymeric runs. Science 265, 407–409 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. M., Longrich S., Gee P. & Harris R. S. Adaptive mutation by deletions in small mononucleotide repeats. Science 265, 405–407 (1994). [DOI] [PubMed] [Google Scholar]
- Karaoglu H., Lee C. M. & Meyer W. Survey of simple sequence repeats in completed fungal genomes. Mol. Biol. Evol. 22, 639–649 (2005). [DOI] [PubMed] [Google Scholar]
- Coenye T. & Vandamme P. Characterization of mononucleotide repeats in sequenced prokaryotic genomes. DNA Res . 12, 221–233 (2005). [DOI] [PubMed] [Google Scholar]
- Ream D. C., Murakami S. T., Schmidt E. E., Huang G. H., Liang C., Friedberg I. & Cheng X. W. Comparative analysis of error-prone replication mononucleotide repeats across baculovirus genomes. Virus Res . 178, 217–225 (2013). [DOI] [PubMed] [Google Scholar]
- Tran H. T., Keen J. D., Kricker M., Resnick M. A. & Gordenin D. A. Hypermutability of homonucleotide runs in mismatch repair and DNA polymerase proofreading yeast mutants. Mol. Cell Biol. 17, 2859–2865 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner L. A., Weiss R. B., Driscoll R., Dunn D. S. & Gesteland R. F. Transcriptional slippage occurs during elongation at runs of adenine or thymine in Escherichia Coli. Nucleic Acids Res . 18, 3529–3535 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthzel A. M. K., Stancek M. & Isaksson L. A. Growth phase dependent stop codon read through and shift of translation reading frame in Escherichia coli. FEBS Lett. 421, 237–242 (1998). [DOI] [PubMed] [Google Scholar]
- Chen M., Tan Z., Zeng G. & Zeng Z. Differential distribution of compound microsatellites in various human immunodeficiency virus type 1 complete genomes. Infect. Genet. Evol. 12, 1452–1457 (2012) [DOI] [PubMed] [Google Scholar]
- Duffy S., Shackelton L. A. & Holmes E. C. Rates of evolutionary change in viruses: patterns and determinants. Nat. Rev. Genet. 9, 267–276 (2008). [DOI] [PubMed] [Google Scholar]
- Khalifa A., Salama H. S. & El-Sharaby A. F. Rearing the Cotton Leafworm Spodoptera littoralis Boisd. on a Semi artificial Diet. J. Appl. Entomol. 73, 129–132 (1973). [Google Scholar]
- O’Reilly D. R., Miller L. & Luckow V. A. Baculovirus Expression Vectors: A Laboratory Manual . Vol. xiii, Freeman W. H. , New York. p. 347 (1992). [Google Scholar]
- Sanger F., Nicklen S. & Coulson A. R. DNA sequencing with chain terminating inhibitors. Proc. Nat. Acad. Sci. 74, 5463–5467 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M. A. et al. Clustal W and ClustalX version 2.0. Bioinformatics 23, 2947–2948 (2007). [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W. & Lipman D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990). [DOI] [PubMed] [Google Scholar]