Abstract
An important proportion of patients with aortic stenosis (AS) have a ‘low-gradient’ AS, i.e. a small aortic valve area (AVA <1.0 cm2) consistent with severe AS but a low mean transvalvular gradient (<40 mmHg) consistent with non-severe AS. The management of this subset of patients is particularly challenging because the AVA-gradient discrepancy raises uncertainty about the actual stenosis severity and thus about the indication for aortic valve replacement (AVR) if the patient has symptoms and/or left ventricular (LV) systolic dysfunction. The most frequent cause of low-gradient (LG) AS is the presence of a low LV outflow state, which may occur with reduced left ventricular ejection fraction (LVEF), i.e. classical low-flow, low-gradient (LF-LG), or preserved LVEF, i.e. paradoxical LF-LG. Furthermore, a substantial proportion of patients with AS may have a normal-flow, low-gradient (NF-LG) AS: i.e. a small AVA—low-gradient combination but with a normal flow. One of the most important clinical challenges in these three categories of patients with LG AS (classical LF-LG, paradoxical LF-LG, and NF-LG) is to differentiate a true-severe AS that generally benefits from AVR vs. a pseudo-severe AS that should be managed conservatively. A low-dose dobutamine stress echocardiography may be used for this purpose in patients with classical LF-LG AS, whereas aortic valve calcium scoring by multi-detector computed tomography is the preferred modality in those with paradoxical LF-LG or NF-LG AS. Although patients with LF-LG severe AS have worse outcomes than those with high-gradient AS following AVR, they nonetheless display an important survival benefit with this intervention. Some studies suggest that transcatheter AVR may be superior to surgical AVR in patients with LF-LG AS.
Keywords: Aortic stenosis, Low flow, Low gradient, Echocardiography, Computed tomography, Aortic valve replacement, Transcatheter aortic valve replacement
Introduction
Aortic stenosis (AS) is the most frequent valvular heart disease and the most frequent cause of valve procedure. There is currently no pharmacological therapy available to reduce the progression of AS and aortic valve replacement (AVR) is thus the only available treatment for this disease. During the past decade, transcatheter aortic valve replacement (TAVR) as emerged as an alternative to surgical aortic valve replacement (SAVR).
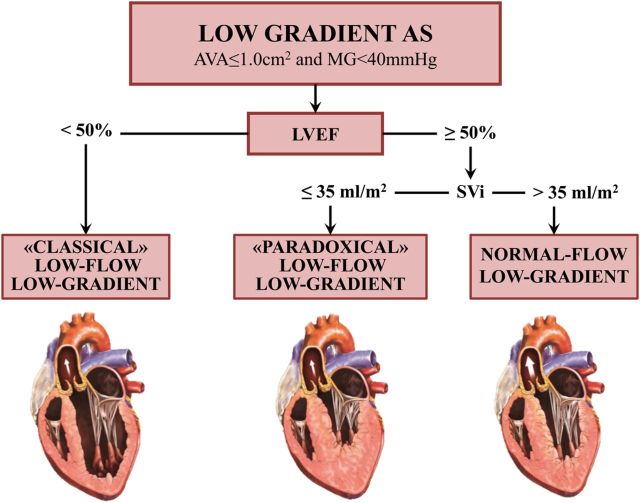
The therapeutic management of AS is essentially determined by: (i) the severity of the stenosis; (ii) the patient's symptomatic status, and (iii) the status of LV systolic function. Severe AS is defined as a peak aortic jet velocity ≥4 m/s, a mean transvalvular pressure gradient ≥40 mmHg, and/or an aortic valve area (AVA) <1.0 cm2. Doppler-echocardiography is the primary modality for the assessment and follow-up of stenosis severity and LV function. The 2012 ESC-EACTS guidelines1 and 2014 ACC-AHA guidelines2 recommend AVR (Class I indication) in patients with high-gradient (mean gradient ≥40 mmHg) severe AS who have symptoms, LV systolic dysfunction defined as a left ventricular ejection fraction (LVEF) <50%, and/or undergo another cardiac surgery. However, an important proportion (up to 50%) of patients with AS have a ‘low-gradient’ AS, i.e. a small AVA (<1.0 cm2) consistent with severe AS but a low-gradient (<40 mmHg) consistent with non-severe AS. The management of this subset of patients is particularly challenging because the AVA-gradient discrepancy raises uncertainty about the actual stenosis severity and thus about the indication of AVR if the patient has symptoms or LVEF <50%. The most frequent cause of low-gradient (LG) AS is the presence of a low LV outflow state, which may occur with reduced LVEF, i.e. classical low flow, or preserved LVEF, i.e. paradoxical low flow. Low flow is defined in the guidelines as a stroke volume index <35 mL/m2 and is present in up to 35% of patients with AS (Figure 1).3–10 The transvalvular pressure gradient is highly flow-dependent (i.e. a squared function of flow) and may thus be ‘pseudo-normalized’ and underestimate stenosis severity in presence of low flow. On the other hand, the AVA may be pseudo-severe due to incomplete opening of the valve orifice and may thus overestimate stenosis severity. Of note, a low-flow state does not necessarily imply the presence of low gradient and some patients with very severe AS may have low flow and still a high gradient (mean gradient >40 mmHg). Furthermore, a substantial proportion of patients with AS may have a low gradient but with a normal flow (stroke volume index >35 mL/m2) (Figure 1). This entity is often referred as to normal-flow, LG AS. Hence, three main subtypes of LG AS can be identified depending on the values of LVEF and flow:5,11 (i) classical (low LVEF) low-flow, low-gradient (LF-LG) AS, (ii) paradoxical (preserved LVEF) LF-LG AS, and (iii) normal flow-LG (NF-LG) AS (Figure 1). These three entities are all characterized by the conjunction of a small AVA and low gradient and pose major challenges for the diagnosis and therapeutic decision making. The purpose of this article is to provide a state-of-the art review of the clinical management for these three subtypes of LG AS.
Figure 1.
Subtypes of low-gradient aortic stenosis. AS, aortic stenosis; AVA, aortic valve area; LVEF, left ventricular ejection fraction; MG, mean transvalvular gradient; SVi, stroke volume index.
Technical pitfalls and measurement errors
The finding of a LG AS at echocardiographic exam may be related to an error in the echocardiographic measurement of the AVA a or gradient. The first step when confronted to the combination of a small AVA with a low gradient at Doppler-echocardiographic exam is thus to rule-out measurement errors. In order to obtain accurate measurement of peak aortic jet velocity and mean gradient, it is important to perform a multi-window interrogation and align the continuous-wave Doppler beam with the direction of aortic flow jet (see Supplementary material online).12 The AVA is even more prone than the mean gradient to measurement errors, particularly in the elderly patient with a calcified aortic valve. The AVA is determined by the continuity equation method where the numerator is the stroke volume measured in the LV outflow tract (LVOT) and the denominator is the time–velocity integral of the aortic flow.13 Given that the LVOT diameter is squared in the continuity equation, a small error in this measure may result in an important error in the calculation of the AVA (see Supplementary material online).
Classical (Reduced Left Ventricular Ejection Fraction) Low-Flow, Low-Gradient Aortic Stenosis
Definition and pathophysiology
Classical LF-LG AS is defined in the guidelines as an AVA <1.0 cm2, a mean gradient <40 mmHg, and an LVEF <50% (Figure 1).1,2 Although this is not always the case, this definition assumes that a low LVEF is associated with low LV outflow. In these patients, the low-flow state is predominantly due to depressed LV systolic dysfunction, which may be related to the presence of severe AS and ensuing LV afterload mismatch and/or to the presence of concomitant cardiomyopathy, most frequently from ischaemic origin. Classical LF-LG AS shares several pathophysiological and clinical similarities with reduced-LVEF heart failure. This entity is found in ∼5–10% of the AS population and is associated with worse outcomes compared with patients with high-gradient AS and/or preserved LVEF.14–17
Stenosis severity
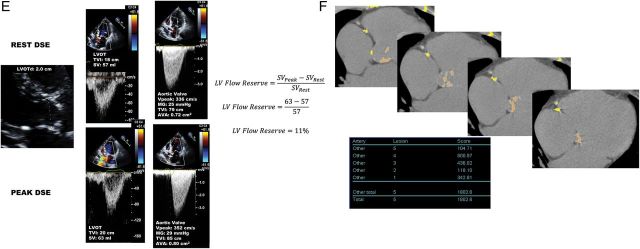
One of the main diagnostic challenges in LF-LG AS is to differentiate a true-severe AS that generally benefits from AVR vs. a pseudo-severe AS (i.e. non-severe AS with incomplete valve opening) that may not benefit from this intervention. A low-dose dobutamine stress echocardiography (DSE) is recommended to confirm stenosis severity and assess LV flow reserve (Figures 2 and 3).1,2,18–21 The DSE protocol differs from that used for the detection of ischaemic heart disease in the sense that the maximum target dose is lower: 20 μg/kg/min (vs. 40 μg/kg/min) and the duration of each dose stage (5, 10, 15, and 20 μg/kg/min) is longer: 5–8 min (vs. 3 min) to allow measurements of stroke volume, gradient, and AVA in a stable, steady-state, haemodynamic condition. It is recommended to hold β-blocker medication, if any, on the day of DSE, when physician considers it safe.22 The stenosis is considered true-severe when the mean gradient is ≥40 mmHg with an AVA being generally <1.0 cm2 at any of the DSE stages (Figures 2 and 3A).1,2 On the other hand, pseudo-severe AS is characterized by a stress mean gradient <40 mmHg and a stress AVA >1.0 cm2 (Figures 2 and 3B). The inter-individual flow response to DSE varies extensively depending on the degree of stenosis severity and the presence of LV afterload mismatch and/or concomitant cardiomyopathy. About one-third of patients with classical LF-LG AS have no LV flow reserve, which is defined as a per cent increase in stroke volume <20% during DSE.20,21 These patients have high surgical risk19,23,24 and, because of the absence of flow increase, the stenosis severity often remains indeterminate with DSE (Figure 3E).18 Among the patients with significant flow reserve on DSE, some exhibit complete normalization of transvalvular flow, whereas others only have modest increase in flow and thus remain below the normal flow range. In the latter subset, the small AVA-low-gradient pattern and thus the uncertainty about stenosis severity may persist at DSE. In such patients, it is useful to calculate the projected AVA at normal flow rate (Figures 2 and 3C and D).20,21 This parameter provides an estimate of what would be the AVA had the patient reached normal flow rate with DSE (Figure 3D). A projected AVA <1.0 cm2 suggests the presence of true-severe AS. However, a minimum of 15% increase in mean transvalvular flow rate (stroke volume/LV ejection time) is required to ensure reliable assessment of the projected AVA. Some studies suggest that larger cut-point value (i.e. <1.2 cm2 instead of <1.0 cm2) of stress AVA and projected AVA and/or lower cut-point value of stress mean gradient (>30 mmHg instead of >40 mmHg) should be used to identify true-severe stenosis and consider AVR.21,25 This suggestion is based on the rationale that a patient with a depressed systolic LV function may be more vulnerable to the increased LV afterload related to AS and that a moderate AS may thus represent a severe haemodynamic burden for a patient with decompensated LV function.
Figure 2.
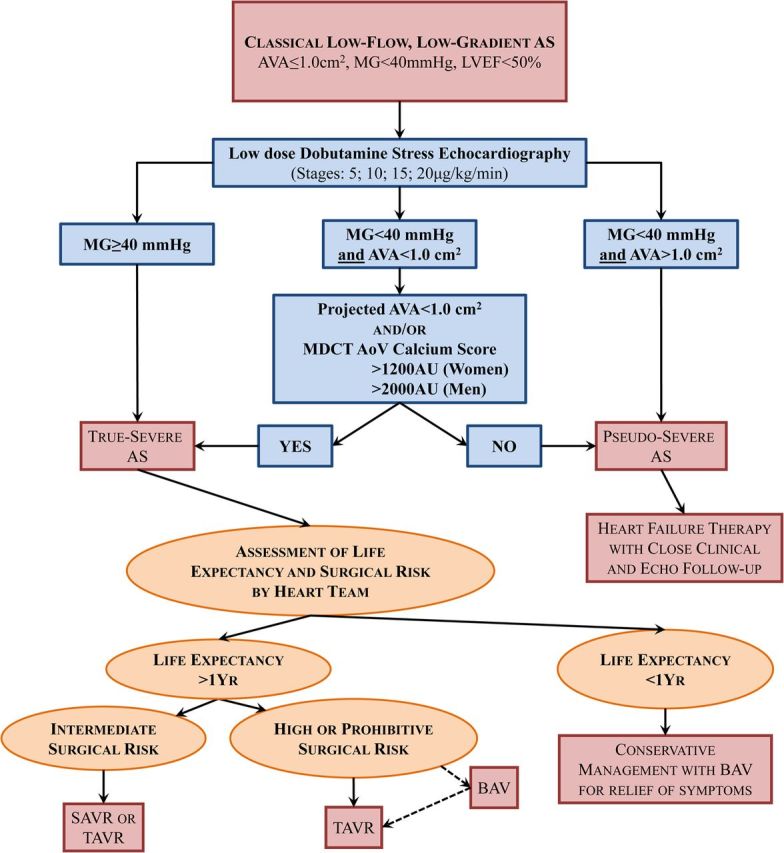
Algorithm for the management of classical (reduced left ventricular ejection fraction) low-flow, low-gradient aortic stenosis. AoV, aortic valve; BAV, balloon aortic valvuloplasty; MDCT, multi-detector computed tomography; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement. Other abbreviations as in Figure 1.
Figure 3.
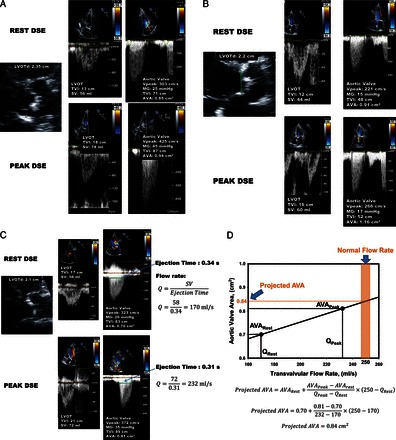
Dobutamine stress echocardiography for the assessment of stenosis severity in classical (reduced left ventricular ejection fraction), low-flow, low-gradient aortic stenosis. (A) Patient with true-severe aortic stenosis; (B) patient with pseudo-severe aortic stenosis; (C and D) patient with persisting discordant grading (i.e. small aortic valve area with low mean transvalvular gradient) at dobutamine stress echocardiography (C) and calculation of the projected aortic valve area at normal flow rate (0.84 cm2) to confirm the presence of severe stenosis in this patient (D). (E and F) Patient with no left ventricular flow reserve on dobutamine stress echocardiography and measurement of aortic valve calcification score by multi-detector computed tomography to confirm stenosis severity. This patient is a woman and has a calcium score of 1802 AU, which is consistent with true-severe aortic stenosis (see Figure 4). DSE, dobutamine stress echocardiography; LVOT, LV outflow tract; LVOTd, LVOT diameter; TVI, time-velocity integral; SV, stroke volume; SVRest, SV at rest; SVPeak, SV at peak DSE; Q, mean transvalvular flow rate (SV/ LV ejection time); VPeak, peak aortic jet velocity. Other abbreviations as in Figures 1 and 2.
In the patients with no significant increase in flow rate (<15%), it is often impossible to differentiate true- vs. pseudo-severe AS by DSE (Figure 3E) and, in these patients, the quantitation of the degree of aortic valve calcification by multi-detector computed tomography (MDCT) may be used to corroborate stenosis severity (Figures 2, 3F, and 4). The amount of aortic valve calcification may indeed accurately be quantitated by MDCT using the modified Agatston method (Figure 4). The aortic valve calcification score has been shown to predict AS haemodynamic severity as well as the rate of stenosis progression and the occurrence of adverse events.26–28 Lower cut-point values of aortic valve calcium score should be used in women (≥1200 AU) vs. in men (≥2000 AU) to distinguish true-severe from pseudo-severe AS (Figure 4).26–28 In patients with small or large aortic annuli, the aortic valve calcium score should be indexed for the cross-sectional area of the aortic annulus to calculate the ‘aortic valve calcium density’. Values of valve calcium density ≥300 AU/cm2 and ≥500 AU/cm2 are consistent with true-severe AS in women and men, respectively.26–28 One limitation of MDCT is that it only measures the valvular calcification but not the valvular fibrosis, which may also contribute to the stenosis. For that reason, this modality may yield to false-negative results in younger patients with bicuspid AS. However, such patients are rare in the LF-LG AS population.
Figure 4.
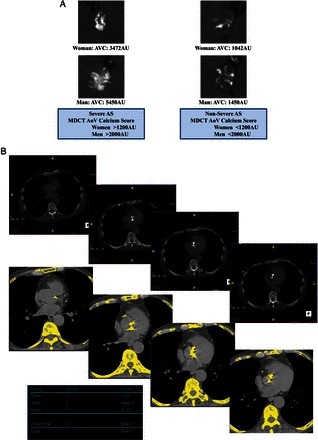
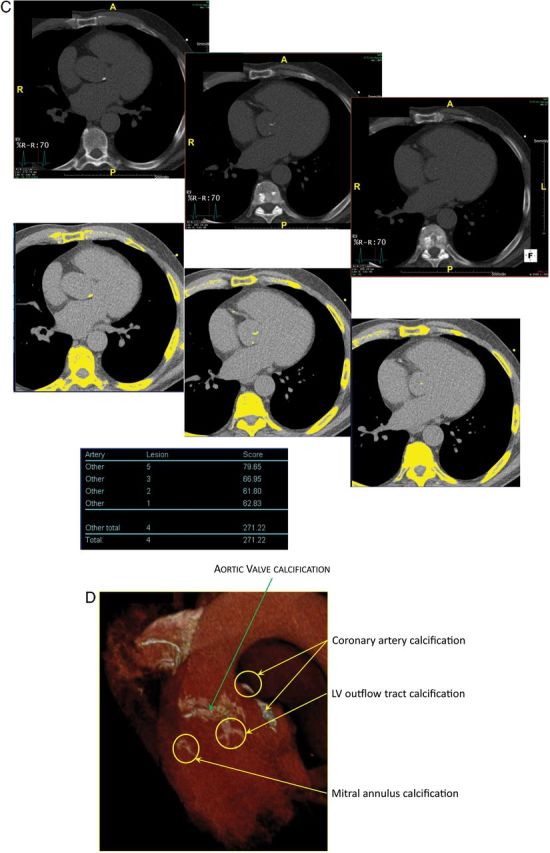
Quantitation of aortic valve calcium by multi-detector computed tomography for the assessment of stenosis severity in low-gradient aortic stenosis. (A) Multi-detector computed tomography can be used to quantitate aortic valve calcification by the modified Agatston method. With this method, calcification is defined as four adjacent pixels with density >130 Hounsfield units. Different cut-point values of valve calcium score should be used in women (>1200 AU) vs. men (>2000 AU) to differentiate true-severe vs. pseudo-severe stenosis in low-flow, low-gradient aortic stenosis. (B) Serial multi-detector computed tomography slices at the level of the aortic valve showing a severely calcified valve with a calcium score of 5040 AU consistent with true-severe aortic stenosis. Calcified areas are displayed in yellow in the bottom images. (C) Mild calcification (score 271 AU) consistent with pseudo-severe aortic stenosis. (D) Pitfalls in the assessment of aortic valve calcification by multi-detector computed tomography. For the calculation of calcium score, it is important to only include aortic valve calcification and exclude calcification of aorta, coronary arteries, LVOT, and mitral annulus. Abbreviations as in Figures 1 and 2.
Cardiac catheterization for haemodynamic assessment of AS is only recommended in symptomatic patients when there are uncertainties or discrepancies among the non-invasive parameters of stenosis severity.1,2 However, in low-flow state conditions, rest cardiac catheterization does not provide any additional information on stenosis severity or LV function besides that obtained by rest echocardiography. Dobutamine stress catheterization can be used to differentiate TS from PS AS and assess the presence of LV flow reserve29 but it is more invasive than DSE and is also subject to technical pitfalls and measurement errors (see Supplementary material online).
Outcomes and risk stratification
The classical LF-LG AS is one of the subsets of the AS population that has the highest risk of mortality and adverse events. The survival rate of these patients is low (40–60% at 2-year) with conservative management but the 30-day mortality with SAVR is high (8–33%).15,19,23–25,29–36 Nevertheless, despite the high surgical risk, SAVR is associated with major survival benefit compared with conservative management in patients with classical LF-LG severe AS.24,32,35 Patients with pseudo-severe AS have better survival than patients with true-severe AS or those with no LV flow reserve treated conservatively.37
Once the presence of severe AS and thus the indication for AVR are confirmed, careful assessment of surgical risk and post-AVR estimated life expectancy by the Heart Team is crucial to select the most appropriate modality of treatment, i.e. SAVR vs. TAVR vs. conservative management, for the given patient (Figure 2).1,2 The main factors that have been associated with increased risk of mortality under conservative management as well as after AVR in patients with classical LF-LG AS include: (i) severe functional impairment defined as NYHA class ≥ III, 6-min walk test distance <320 m, and/or Duke activity score index <20, (ii) the presence of multi-vessel coronary artery disease; (iii) very low LVEF: i.e. <35% at rest or at DSE; (iv) severe impairment of LV longitudinal systolic function at rest and at DSE: i.e. LV global longitudinal strain measured by speckle tracking imaging <9% and <10%, respectively; (v) the absence of LV flow reserve on DSE; (vi) moderate-to-severe stenosis defined as a projected AVA <1.2 cm2; (vii) Moderate/severe tricuspid regurgitation; (viii) marked elevation of circulating levels of B-type natriuretic peptides (BNP >550 pg/mL or NT-pro BNP >3950 pg/mL); and (viii) higher degree of myocardial fibrosis at cardiac magnetic resonance imaging.15,19,24,25,29,30,35,38–40
In the subset of patients undergoing AVR, pre-operative NYHA class ≥III, mean gradient <20 mmHg, LVEF <35%, global longitudinal strain <9%, the presence of multi-vessel coronary disease, the absence of flow reserve on DSE, and higher logistic EuroSCORE or STS score are associated with increased risk of perioperative mortality.15,19,24,25,30,35,39
The surgical risk of patients with AS having an indication for AVR should be estimated using the integrative approach recommended in the guidelines and classified as: low, intermediate, high, and prohibitive. Given that patients with classical LF-LG AS have, by definition, a reduced LVEF, their surgical risk is never low and ranges from intermediate to prohibitive depending on the other co-morbidities. The approach proposed in the guidelines combines EuroSCORE or STS risk estimate, criteria of frailty, the presence of major organ system dysfunction, and procedure-specific impediments. However, in the patients with LF-LG AS, the surgical risk stratification process should also integrate specific risk factors that are not included in the risk assessment algorithm proposed in the guidelines.1,2 In particular, patients with very low pre-operative gradient, the absence of flow reserve, markedly reduced global longitudinal strain should be considered at high surgical risk, even if the guidelines risk assessment suggests intermediate risk.
Therapeutic management
Among patients with classical LF-LG AS, those with evidence of true-severe AS on DSE (i.e. progression Stage D2 in ACC-AHA guidelines) have a class IIa indication for AVR (Figure 2).1,2 However, according to ESC-EACTS guidelines, the patients with no LV flow reserve on DSE have a weaker indication (IIb) for AVR because their stenosis severity often remains indeterminate with DSE and, in addition, they have a high operative risk with SAVR.1 Despite the high surgical risk, these patients with no LV flow reserve nonetheless have much better survival with AVR compared with conservative management.19,24 Furthermore, patients who survive operation have similar recovery of LV function and functional status and similar long-term survival compared with those with flow reserve.23 Hence, AVR should be considered even in patients with no LV flow reserve or with other LF-LG-specific operative risk markers (e.g. severely reduced LVEF or global longitudinal strain) if the presence of severe AS is corroborated by the assessment of the projected AVA and/or of MDCT valve calcium score (Figures 2 and 3D–F). However, the presence of these markers of high surgical risk may influence the selection of TAVR vs. SAVR.
Some studies suggest that TAVR may be associated with better and faster recovery of LV function and with improved survival compared with SAVR.41,42 Furthermore, TAVR is associated with less prosthesis-patient mismatch,43–45 which has been shown to be highly detrimental in patients with reduced LVEF including those with classical LF-LG AS.46–49 The survival benefit of TAVR vs. SAVR may be more important in the subset of patients with no LV flow reserve. In the PARTNER-I Cohort B (inoperable patients) trial, TAVR was associated with a major survival benefit compared with conservative management in patients with classical LF-LG AS.50 In the Cohort A (high surgical risk), survival was similar in the TAVR vs. SAVR arms.50 However, patients with no LV flow reserve as well as those with very low LVEF were excluded from the PARTNER-I trial. Additional studies are necessary to confirm the potential superiority of TAVR over SAVR in patients with classical LF-LG severe AS (Stage D2). In the meantime, a comprehensive assessment of surgical risk and post-AVR life expectancy should be performed by the Heart Team. In patients with expected life expectancy <1 year, conservative therapy may be considered with the utilization of palliative balloon valvuloplasty for relief of cardiovascular symptoms and improvement of quality of life (Figure 2). In patients with classical LF-LG severe AS, prohibitive surgical risk, and life expectancy >1 year, TAVR is recommended. In patients with high surgical risk, TAVR may be preferable to SAVR, whereas in those with intermediate risk, SAVR or TAVR may be considered depending on the evaluation by the Heart Team (Figure 2).
Coronary artery disease is frequent in patients with classical LF-LG AS and it contributes to the LV dysfunction, low-flow sate, symptoms, and adverse cardiac events. Hence, percutaneous or surgical coronary revascularization should be considered whenever it is necessary.51 This may be performed as a staged approach (percutaneous revascularization first and then AVR) or as a concomitant procedure at the time of surgical or transcatheter AVR.
Patients with pseudo-severe AS should, a priori, be managed conservatively (Figure 2).37 However, they require optimized heart failure therapy, percutaneous coronary revascularization if indicated, and close echocardiographic and clinical follow-up. These patients may eventually necessitate AVR if the stenosis progresses during follow-up and/or if they do not improve despite optimized medical therapy. Given that even a moderate AS may be highly detrimental in the context of severely depressed LV function, it is possible that some patients with pseudo-severe AS and persistent heart failure symptoms despite optimal medical therapy may benefit from AVR. This provocative hypothesis would merit to be tested in a randomized trial.
Up to 70% of patients with classical low-flow AS have concomitant LV dyssynchrony,52 which may contribute to the low LVEF, the LF-LG AS pattern, and to the lack of LV flow reserve on DSE. In these patients, cardiac resynchronization therapy has been shown to improve LV function and patient clinical status. Hence, in patients with classical LF-LG AS and LV dyssynchrony, cardiac resynchronization therapy should be considered first and the echocardiographic parameters, symptomatic status, and indication for AVR then be re-assessed.52,53
Paradoxical (Preserved Left Ventricular Ejection Fraction) Low-Flow, Low-Gradient Aortic Stenosis
Definition and pathophysiology
Paradoxical LF-LG AS is defined as an AVA <1.0 cm2, indexed AVA <0.6 cm2/m2, mean gradient <40 mmHg, LVEF ≥50%, and presence of low flow (stroke volume index <35 mL/m2) (Figures 1, 5, and 6).1,2 Given that the transvalvular flow rate is not only dependent on stroke volume but also on LV ejection duration, some investigators proposed to define low flow as a mean transvalvular flow rate <200 mL/s.54,55
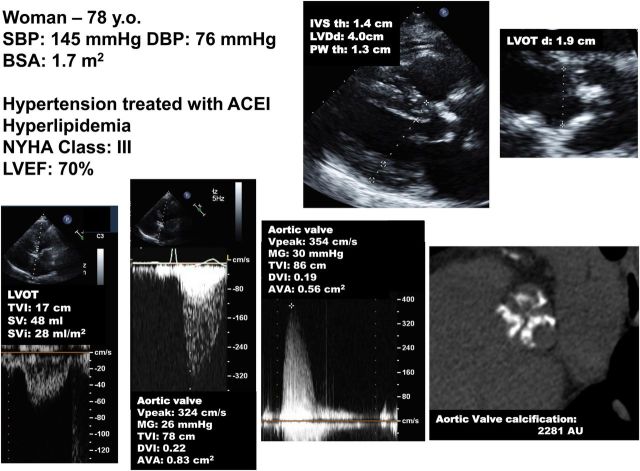
Figure 5.
Patient with paradoxical (preserved left ventricular ejection fraction) low-flow, low-gradient severe aortic stenosis. This case underlines the importance of multi-window continuous-wave Doppler interrogation for the measurement of the aortic velocity and gradient. In this patient, the gradient was higher at the right parasternal window than at the apical window. It is also important to rule-out error in the measurement of stroke volume. In this patient, the stroke volume measured by pulsed wave Doppler in the LVOT (48 mL) is corroborated by the stroke volume obtained with the modified Teichholz method: LV end-diastolic volume by Teichholz (70 mL) × left ventricular ejection fraction by biplane Simpson (70%) = 49 mL. The patient is in low flow (SVi: 28 mL/m2). Aortic valve calcium score by multi-detector computed tomography corroborates presence of true-severe stenosis. ACEI, angiotensin conversion enzyme inhibitor; BSA, body surface area; DVI, Doppler velocity index; LVDd, LV end-diastolic diameter; IVS th, interventricular septum thickness; PWth, posterior wall thickness; SBP/DBP, systolic and diastolic blood pressure. Other abbreviations as in Figures 1, 2, and 3.
Figure 6.
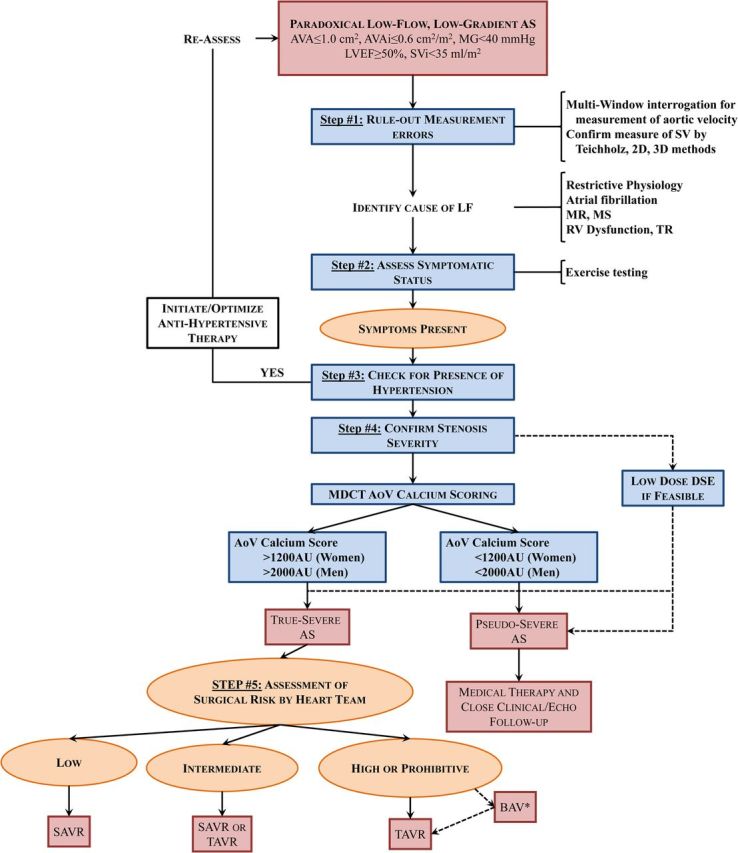
Algorithm for the management of paradoxical (preserved left ventricular ejection fraction) low-flow, low-gradient aortic stenosis. AVAi, indexed aortic valve area; MR, mitral regurgitation; MS, mitral stenosis; TR, tricuspid regurgitation; other abbreviations as in Figures 1, 2, and 3.
An important proportion (25–35% depending on institution/country) of patients with AS and preserved LVEF have a low-flow state.3–5,8–10,17,36,50,56–58 In these patients, the reduced stroke volume is generally related to pronounced LV concentric remodelling with small LV cavity, impaired LV diastolic filling, and reduced LV systolic longitudinal function (Figure 5).3,6,11,32,58 Paradoxical LF-LG AS does, in fact, share many pathophysiological and clinical similarities with preserved-LVEF heart failure.3,11,59 Indeed, these entities are often associated with older age, female sex, and systemic hypertension. Furthermore, the mechanisms (impaired diastolic filling and systolic longitudinal function) underlying the reduction in stroke volume is similar in both entities.
Atrial fibrillation is also a frequent and important factor contributing to the decrease in LV outflow in AS patients with preserved LVEF.8 Other factors may lead to a decrease in LV stroke volume and transvalvular flow rate including significant mitral regurgitation, mitral stenosis, tricuspid regurgitation, or right ventricular dysfunction (Figure 6).8,60 Hence, a large proportion of patients with AS have a low-flow state despite a preserved LVEF and these patients have worse outcomes.3,36,50,61,62 Furthermore, because of the low-flow state, patients often have a low-gradient AS despite a small AVA (Figure 1), which makes difficult the assessment of stenosis severity. The measurement of stroke volume index should therefore be systematically integrated in the Doppler-echocardiographic evaluation of the AS patient and the presence of low flow, i.e. stroke volume index <35 mL/m2, should be reported.1,2
Stenosis severity
If the echocardiographic exam reveals the presence of a low-flow state, one should identify the underlying cause of the reduced stroke volume (Figure 6). If no obvious factor can be identified to explain the low flow, one should then re-assess the accuracy of the echocardiographic measure of stroke volume and/or consider other modalities to measure flow.
The Doppler velocity index (ratio of LVOT to aortic velocity–time integrals) may also be used to corroborate the accuracy of AVA measurements and enhance prognostication in patients with LG AS.63,64 The finding of a small AVA with a Doppler velocity index >0.25 should raise the possibility of LVOT diameter or velocity underestimation. A hybrid method where LVOT area is measured by MDCT and flow velocities by Doppler-echocardiography has also been proposed to calculate the AVA and grade AS severity.65,66 However, given that the values of AVA obtained with this hybrid method are systematically larger than those obtained by echocardiography, one cannot directly apply the same cut-point value (i.e. <1.0 cm2) to define severe AS. In this regard, a recent study reported that the hybrid AVA does not improve the correlation with the transvalvular gradient or the prediction of mortality compared with the echocardiographic AVA.65 Moreover, the best cut-point value to predict outcomes was close to 1.0 cm2 for the echocardiographic AVA (which is consistent with the guidelines criteria) but it was larger for the hybrid AVA: 1.2 cm2.
A small AVA may in fact correspond to a moderate AS and thus a low gradient in a patient with a small body size. Hence, in patients with paradoxical LF-LG AS and small body size, it is important to calculated the indexed AVA and a value >0.6 cm2/m2 would indicate a moderate AS.2 On the other hand, the indexed AVA may overestimate the stenosis severity in obese patients.
Once the presence of bona fide paradoxical LF-LG AS is confirmed, it is then important to differentiate true- vs. pseudo-severe AS (Figure 6). Some studies suggest that DSE may be used for this purpose in patients with paradoxical LF-LG AS.67 A low-dose protocol (up to 20 μg/kg/min) starting at 2.5 μg/kg/min with careful monitoring of ECG, blood pressure, and LVOT velocity should be used in these patients. The same DSE parameters and criteria described for classical LF-LG AS can be applied to paradoxical LF-LG AS in order to identify true-severe stenosis. Approximately, one-third of patients with paradoxical LF-LG AS has pseudo-severe AS, which is similar to that observed in classical LF-LG AS.67 Dobutamine stress echocardiography should not be performed in patients with restrictive LV physiology pattern, which is frequently found in the paradoxical LF-LG AS population. Exercise-stress echocardiography may be useful in patients with paradoxical LF-LG who claim to be asymptomatic or have equivocal symptoms to: (i) ascertain the symptomatic status and (ii) to differentiate true- vs. pseudo-severe AS.67 Hence, the preferred approach to corroborate stenosis severity in these patients is rather to assess the morphology of the valve and the degree of aortic valve calcification by echocardiography and/or MDCT (Figures 4 and 5). The same cut-points of aortic calcium score and density used for classical LF-LG AS can be applied to paradoxical LF-LG AS.27
Outcomes and risk stratification
Patients with paradoxical LF-LG AS generally have worse outcomes compared with moderate AS, high-gradient AS, and NF-LG AS but better outcomes compared with classical LF-LG AS.3,5,8–10,56,58,68,69 Given the higher proportion of women, the older age, the higher prevalence of hypertension, restrictive LV physiology, atrial fibrillation, low LV outflow, and small aortic annulus/root, these patients are at higher surgical risk compared with those with high-gradient AS.9,17,36,50,62,70,71 However, several studies reported that, in patients with paradoxical LF-LG AS, survival is markedly improved by AVR compared with conservative management.8,50,56,57,69,72,73 Except the randomized PARTNER I cohort B trial that reports better survival after TAVR compared with conservative management in paradoxical LF-LG patients,50 all studies that evaluate survival in paradoxical LF-LG patients according to type of treatment were observational and residual confounding factors cannot be excluded. Nevertheless, a recent meta-analysis reported that (i) patients with paradoxical LF-LG AS have 67% higher risk of mortality compared with high-gradient AS and (ii) AVR reduces mortality by 57% in patients with paradoxical LF-LG AS.69
More advanced LV myocardial impairment as documented by higher degree of myocardial fibrosis measured by cardiac resonance imaging, moderate/severe LV diastolic dysfunction, reduced global LV systolic longitudinal strain, and very low stroke volume index are associated with increased risk of mortality in patients with paradoxical LF-LG AS and with worse outcomes following AVR.32,36,58,70,74 In some studies, basal longitudinal strain has been shown to be more sensitive than global longitudinal strain to predict outcomes.6,75 However, regional strain is more susceptible to measurement variability compared with global strain.
Although plasma levels of BNP have been shown to be useful for risk stratification in patients with classical LF-LG AS,38 their role is unclear for those with paradoxical LF-LG AS.5,76,77 In contrast to patients with classical LF-LG AS, those with paradoxical LF-LG AS have pronounced LV concentric remodelling and small LV cavities. The extent of myocardial stretch and ensuing release of natriuretic peptides may thus not accurately reflect the severity of impairment of myocardial structure/function in these patients.
Therapeutic management
Aortic valve replacement should be considered in symptomatic patients with bona fide paradoxical LF-LG and true-severe AS (Stage D3 in the ACC/AHA guidelines) confirmed by echocardiography, DSE, and/or MDCT (Figures 5 and 6).1,2 Given that systemic arterial hypertension is frequent in these patients and may contribute to the LF-LG pattern as well as to the symptoms and cardiac events, it is first important to institute/optimize anti-hypertensive therapy if hypertension is present and then reassess the echocardiographic parameters, symptoms, and indication of AVR once blood pressure is normalized (Figure 6).2,3,78,79 Patient with paradoxical LF-LG AS and pseudo-severe AS should be managed conservatively with optimization of anti-hypertensive therapy (Figure 6).
Recent studies suggest that TAVR may be superior to SAVR in the subset of patients with paradoxical LF-LG AS.50 Indeed, these patients have some features (restrictive LV physiology, small aortic annulus/root) that may substantially increase the risk of perioperative morbidity/mortality as well as that of prosthesis-patient mismatch with SAVR.17,80 The combination of paradoxical LF-LG AS and prosthesis-patient mismatch is associated with markedly increased risk of mortality following SAVR.80 In the PARTNER-I Cohort A trial, TAVR was associated with better 1-year survival compared with SAVR in patients with paradoxical LF-LG AS.50 Further studies are necessary to confirm the potential superiority of TAVR vs. SAVR in this subset of patients. In the meantime, TAVR may be preferable to SAVR in patients with paradoxical LF-LG AS, particularly if they are considered at high surgical risk (Figure 6). In patients in whom it is uncertain that the symptoms are solely related to AS and/or will improve following AVR, a staged approach with balloon valvuloplasty first and then TAVR 6 months later if symptoms improve may be considered (Figures 2 and 6).
Normal-Flow, Low-Gradient Aortic Stenosis
Definition and pathophysiology
Normal-flow, LG AS is defined as an AVA <1.0 cm2, indexed AVA <0.6 cm2/m2, mean gradient <40 mmHg, LVEF ≥50%, but normal flow, i.e. stroke volume index >35 mL/m2 (Figures 1 and 7). This entity is relatively frequent (15–40%) and may be related to inherent discrepancies in the guidelines criteria for severe AS.4,5,10,11,56,58 From a haemodynamic stand-point, the AVA cut-point value of 1.0 cm2, as proposed in the guideline to define severe AS, does not correspond to a mean gradient of 40 mmHg but rather to a gradient of 30–35 mmHg.4 To reconcile this inherent discrepancy, some investigators suggested lowering the severity cut-point value of AVA from 1.0 to 0.8 cm2.4,81 However, several studies showed that the optimal cut-point value of AVA to predict mortality is 1.0 cm2, whereas the one for the gradient is 30 mmHg.82–84 Hence, it seems preferable to maintain the status quo with regards to the cut-point values of AVA and gradient and use the AVA cut-point (1.0 cm2) as a sensitive marker of severe AS and the gradient cut-point (40 mmHg) as a specific marker. Some studies also suggest that reduced aortic compliance and associated systolic hypertension may lead to a substantial decrease in gradient and thus to a NF-LG pattern in patients with severe AS (Figure 7).78,85,86
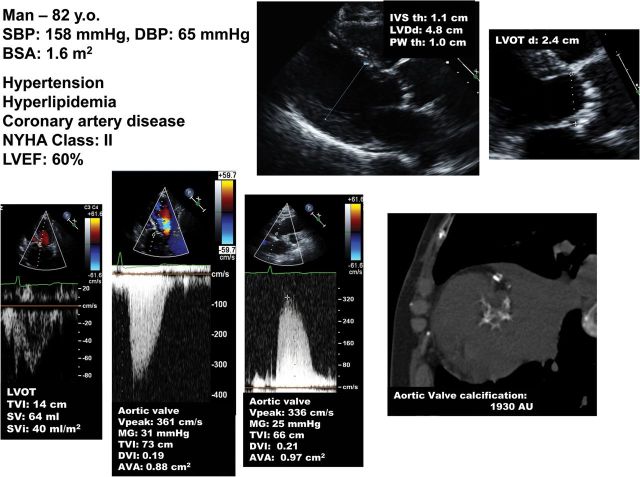
Figure 7.
Patient with normal-flow, low-gradient aortic stenosis. This patient has a normal flow (stroke volume index >35 mL/m2), small aortic valve area, small indexed aortic valve area, and low mean transvalvular gradient at echocardiography. As in the patient with paradoxical low-flow, low-gradient (Figure 5), it is important first to rule-out measurement errors and then confirm stenosis severity, especially if the patient is symptomatic. In this patient, aortic valve calcium score by multi-detector computed tomography suggests the presence of a moderate to severe aortic stenosis.
Outcomes and risk stratification
In patients with LG AS, lower stroke volume index is a powerful predictor of outcomes both before and after AVR.17,36,50,61,74,87 Patients with NF-LG AS are generally at a less advanced stage of the disease and they have better survival compared with paradoxical LF-LG or high-gradient AS.5,58,69 Accordingly, a recent study found no difference in survival between patients with NF-LG AS treated by early surgery vs. by conservative management with wait-for-symptoms strategy.88 On the other hand, other studies, including a recent meta-analysis, revealed that AVR may improve survival in these patients.9,56,69,72 These findings may be explained by the fact that this subset of patients is highly heterogeneous and that a substantial proportion (40–50%) of these patients may nonetheless have severe stenosis.27 Hence, it is important to perform additional tests to confirm stenosis severity in patients with NF-LG AS who are symptomatic.
Stenosis severity
As in patients with paradoxical LF-LG AS, the first step is to rule-out measurement errors (Figure 6). Given that the flow rate is already normal in patients with NF-LG AS, DSE has limited utility in these patients, and aortic valve calcium scoring by MDCT is likely the preferred approach to differentiate severe from non-severe AS.27
Therapeutic management
There is no specific definition and recommendation in the guidelines for the patients with NF-LG AS. A similar algorithm as the one proposed for paradoxical LF-LG AS (Figure 6) may be applied for NF-LG AS and should include the following steps: Step #1: Rule-out measurement errors, Step #2: Assess symptomatic status; Step #3: Check for presence of hypertension, and, if any, institute/optimize anti-hypertensive therapy and re-assess parameters of stenosis severity and symptomatic status; Step #4: Confirm stenosis severity; Step #5: If stenosis is severe and AVR is indicated; assess surgical risk to select the type of AVR: SAVR vs. TAVR. Patients with NF-LG AS who have no symptoms or no evidence of severe AS should be treated conservatively. In light of the results of some recent studies,69 AVR should probably be considered in symptomatic patients with NF-LG AS in whom MDCT or other imaging modalities support the presence of severe AS. However, further studies are needed to confirm the benefit of AVR in symptomatic patients with NF-LG severe AS.
Conclusion
Low-gradient AS is one of the most challenging entities in valvular heart disease. It includes three main subtypes: (i) classical (reduced LVEF) LF-LG, (ii) paradoxical (preserved LVEF) LF-LG, and (iii) NF-LG AS. One of the most important clinical challenges in these patients with LG AS is to differentiate a true-severe AS that generally benefits from AVR vs. a pseudo-severe AS that should be managed conservatively. A low-dose DSE may be used for this purpose in patients with classical LF-LG AS, whereas aortic calcium scoring by MDCT is the preferred modality in those with paradoxical LF-LG or NF-LG AS. Although patients with LF-LG severe AS have worse outcomes than those with high-gradient AS following AVR, they nonetheless get an important survival benefit with this intervention. Some studies suggest that TAVR may be superior to SAVR in patients with LF-LG AS, and particularly in those with classical LF-LG AS and no LV flow reserve or those with paradoxical LF-LG AS.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
P.P. handled funding and supervision; M.A.C. and P.P. drafted the manuscript; M.A.C., J.M., P.P.: made critical revision of the manuscript for key intellectual content.
Funding
P.P. is the Canada Research Chair in Valvular Heart Diseases, Canadian Institutes of Health Research (CIHR), Ottawa, Ontario, Canada. His research program is funded by research grants # FDN-143225 and MOP 126072, 114997, 102737 from CIHR.
Conflict of interest: P.P. received research grant from Edwards Lifesciences for echocardiography core laboratory analyses in transcatheter heart valves.
Supplementary Material
References
- 1. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De BM, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol 2014;63:2438–2488. [DOI] [PubMed] [Google Scholar]
- 3. Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low flow, low gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007;115:2856–2864. [DOI] [PubMed] [Google Scholar]
- 4. Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart 2010;96:1463–1468. [DOI] [PubMed] [Google Scholar]
- 5. Lancellotti P, Magne J, Donal E, Davin L, O'Connor K, Rosca M, Szymanski C, Cosyns B, Piérard LA. Clinical outcome in asymptomatic severe aortic stenosis. Insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol 2012;59:235–243. [DOI] [PubMed] [Google Scholar]
- 6. Adda J, Mielot C, Giorgi R, Cransac F, Zirphile X, Donal E, Sportouch-Dukhan C, Réant P, Laffitte S, Cade S, Le Dolley Y, Thuny F, Avierinos JF, Lancellotti P, Habib G. Low-flow, low-gradient severe aortic stenosis despite normal ejection fraction is associated with severe left ventricular dysfunction as assessed by speckle tracking echocardiography: a multicenter study. Circ Cardiovasc Imaging 2012;5:27–35. [DOI] [PubMed] [Google Scholar]
- 7. Ozkan A. Low gradient ‘severe’ aortic stenosis with preserved left ventricular ejection fraction. Cardiovasc Diagn and Ther 2012;2:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation 2013;128:1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohty D, Magne J, Deltreuil M, Aboyans V, Echahidi N, Cassat C, Pibarot P, Laskar M, Virot P. Outcome and impact of surgery in paradoxical low-flow, low-gradient severe aortic stenosis and preserved left ventricular ejection fraction: A cardiac catheterization study. Circulation 2013;128(26 Suppl. 1):S235-S242. [DOI] [PubMed] [Google Scholar]
- 10. Tribouilloy C, Rusinaru D, Marechaux S, Castel AL, Debry N, Maizel J, Mentaverri R, Kamel S, Slama M, Levy F. Low-gradient, low-flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications for surgery. J Am Coll Cardiol 2015;65:55–66. [DOI] [PubMed] [Google Scholar]
- 11. Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J 2010;31:281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thaden JJ, Nkomo VT, Lee KJ, Oh JK. Doppler imaging in aortic stenosis: the importance of the nonapical imaging windows to determine severity in a contemporary cohort. J Am Soc Echocardiogr 2015;28:780–785. [DOI] [PubMed] [Google Scholar]
- 13. Otto CM, Pearlman AS, Comess KA, Reamer RP, Janko CL, Huntsman LL. Determination of the stenotic aortic valve area in adults using Doppler echocardiography. J Am Coll Cardiol 1986;7:509–517. [DOI] [PubMed] [Google Scholar]
- 14. Connolly HM, Oh JK, Orszulak TA, Osborn SL, Roger VL, Hodge DO, Bailey KR, Seward JB, Tajik AJ. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction: prognostic indicators. Circulation 1997;95:2395–2400. [DOI] [PubMed] [Google Scholar]
- 15. Connolly HM, Oh JK, Schaff HV, Roger VL, Osborn SL, Hodge DO, Tajik AJ. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction. Result of aortic valve replacement in 52 patients. Circulation 2000;101:1940–1946. [DOI] [PubMed] [Google Scholar]
- 16. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, Tornos P, Vanoverschelde J-L, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular heart Disease. Eur Heart J 2003;24:1231–1243. [DOI] [PubMed] [Google Scholar]
- 17. Clavel MA, Berthelot-Richer M, Le VF, Capoulade R, Dahou A, Dumesnil JG, Mathieu P, Pibarot P. Impact of classic and paradoxical low flow on survival after aortic valve replacement for severe aortic stenosis. J Am Coll Cardiol 2015;65:645–653. [DOI] [PubMed] [Google Scholar]
- 18. deFilippi CR, Willett DL, Brickner E, Appleton CP, Yancy CW, Eichhorn EJ, Grayburn PA. Usefulness of dobutamine echocardiography in distinguishing severe from nonsevere valvular aortic stenosis in patients with depressed left ventricular function and low transvalvular gradients. Am J Cardiol 1995;75:191–194. [DOI] [PubMed] [Google Scholar]
- 19. Monin JL, Quere JP, Monchi M, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Tribouilloy C, Gueret P. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003;108:319–324. [DOI] [PubMed] [Google Scholar]
- 20. Blais C, Burwash IG, Mundigler G, Dumesnil JG, Loho N, Rader F, Baumgartner H, Beanlands RS, Chayer B, Kadem L, Garcia D, Durand LG, Pibarot P. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low flow, low-gradient aortic stenosis: the multicenter TOPAS (Truly or Pseudo Severe Aortic Stenosis) study. Circulation 2006;113:711–721. [DOI] [PubMed] [Google Scholar]
- 21. Clavel MA, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, Bergler-Klein J, Senechal M, Mathieu P, Couture C, Beanlands R, Pibarot P. Validation of conventional and simplified methods to calculate projected valve area at normal flow rate in patients with low flow, low gradient aortic stenosis: the multicenter TOP (True or Pseudo Severe Aortic Stenosis) study. J Am Soc Echocardiogr 2010;23:380–386. [DOI] [PubMed] [Google Scholar]
- 22. Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. ASE recommendations for performance, interpretation and application of stress echocardiography. J Am Soc Echocardiography 2007;20:1021–1041. [DOI] [PubMed] [Google Scholar]
- 23. Quere JP, Monin JL, Levy F, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Gueret P, Tribouilloy C. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation 2006;113:1738–1744. [DOI] [PubMed] [Google Scholar]
- 24. Tribouilloy C, Levy F, Rusinaru D, Gueret P, Petit-Eisenmann H, Baleynaud S, Jobic Y, Adams C, Lelong B, Pasquet A, Chauvel C, Metz D, Quere JP, Monin JL. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009;53:1865–1873. [DOI] [PubMed] [Google Scholar]
- 25. Clavel MA, Fuchs C, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, Bergler-Klein J, Beanlands RS, Mathieu P, Magne J, Pibarot P. Predictors of outcomes in low-flow, low-gradient aortic stenosis: results of the multicenter TOPAS study. Circulation 2008;118(14 Suppl.):S234-S242. [DOI] [PubMed] [Google Scholar]
- 26. Cueff C, Serfaty JM, Cimadevilla C, Laissy JP, Himbert D, Tubach F, Duval X, Iung B, Enriquez-Sarano M, Vahanian A, Messika-Zeitoun D. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart 2011;97:721–726. [DOI] [PubMed] [Google Scholar]
- 27. Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal S, Malouf J, Araoz P, Michelena H, Cueff C, Larose É, Capoulade R, Vahanian A, Enriquez-Sarano M. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined doppler-echocardiographic and computed tomographic study. J Am Coll Cardiol 2013;62:2329–2338. [DOI] [PubMed] [Google Scholar]
- 28. Clavel MA, Pibarot P, Messika-Zeitoun D, Capoulade R, Malouf J, Aggarval S, Araoz PA, Michelena HI, Cueff C, Larose E, Miller JD, Vahanian A, Enriquez-Sarano M. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol 2014;64:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishimura RA, Grantham JA, Connolly HM, Schaff HV, Higano ST, Holmes DR Jr. Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: the clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation 2002;106:809–813. [DOI] [PubMed] [Google Scholar]
- 30. Levy F, Laurent M, Monin JL, Maillet JM, Pasquet A, Le Tourneau T, Petit-Eisenmann H, Gori M, Jobic Y, Bauer F, Chauvel C, Leguerrier A, Tribouilloy C. Aortic valve replacement for low-flow/low-gradient aortic stenosis: operative risk stratification and long-term outcome: a European multicenter study. Journal of the American College of Cardiology 2008;51:1466–1472. [DOI] [PubMed] [Google Scholar]
- 31. Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008;86:1781–1789. [DOI] [PubMed] [Google Scholar]
- 32. Herrmann S, Stork S, Niemann M, Lange V, Strotmann JM, Frantz S, Beer M, Gattenlöhner S, Voelker W, Ertl G, Weidemann F. Low-gradient aortic valve stenosis: myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol 2011;58:402–412. [DOI] [PubMed] [Google Scholar]
- 33. Gotzmann M, Lindstaedt M, Bojara W, Ewers A, Mugge A. Clinical outcome of transcatheter aortic valve implantation in patients with low-flow, low gradient aortic stenosis. Catheter Cardiovasc Interv 2012;79:693–701. [DOI] [PubMed] [Google Scholar]
- 34. Lauten A, Zahn R, Horack M, Sievert H, Linke A, Ferrari M, Harnath A, Grube E, Gerckens U, Kuck KH, Sack S, Senges J, Figulla HR. Transcatheter aortic valve implantation in patients with low-flow, low-gradient aortic stenosis. J Am Coll Cardiol Intv 2012;5:552–559. [DOI] [PubMed] [Google Scholar]
- 35. Dahou A, Magne J, Clavel MA, Capoulade R, Bartko PE, Bergler-Klein J, Senechal M, Mundigler G, Burwash I, Ribeiro HB, O'Connor K, Mathieu P, Baumgartner H, Dumesnil JG, Rosenhek R, Larose E, Rodes-Cabau J, Pibarot P. Tricuspid regurgitation is associated with increased risk of mortality in patients with low-flow low-gradient aortic stenosis and reduced ejection fraction: results of the multicenter TOPAS study (True or pseudo-severe aortic stenosis). J Am Coll Cardiol 2015;8:588–596. [DOI] [PubMed] [Google Scholar]
- 36. Le Ven F, Freeman M, Webb J, Clavel MA, Wheeler M, Dumont E, Thompson C, De LR, Moss R, Doyle D, Ribeiro HB, Urena M, Nombela-Franco L, Rodes-Cabau J, Pibarot P. Impact of low flow on the outcome of high risk patients undergoing transcatheter aortic valve replacement. J Am Coll Cardiol 2013;62:782–788. [DOI] [PubMed] [Google Scholar]
- 37. Fougères É, Tribouilloy C, Monchi M, Petit-Eisenmann H, Baleynaud S, Pasquet A, Chauvel C, Metz D, Adams C, Rusinaru D, Guéret P, Monin JL. Outcomes of pseudo-severe aortic stenosis under conservative treatment. Eur Heart J 2012;33:2426–2433. [DOI] [PubMed] [Google Scholar]
- 38. Bergler-Klein J, Mundigler G, Pibarot P, Burwash IG, Dumesnil JG, Blais C, Beanlands R, Hachicha Z, Mohty D, Fuchs C, Loho N, Florian R, Baumgartner H. B-type natriuretic peptide in low-flow, low-gradient aortic stenosis: relationship to hemodynamics and clinical outcome. Circulation 2007;115:2848–2855. [DOI] [PubMed] [Google Scholar]
- 39. Dahou A, Bartko PE, Capoulade R, Clavel MA, Mundigler G, Grondin SL, Bergler-Klein J, Burwash I, Dumesnil JG, Senechal M, O'Connor K, Baumgartner H, Pibarot P. Usefulness of global left ventricular longitudinal strain for risk stratification in low ejection fraction, low-gradient aortic stenosis: results from the multicenter true or pseudo-severe aortic stenosis study. Circ Cardiovasc Imaging 2015;8:e002117. [DOI] [PubMed] [Google Scholar]
- 40. Hayek S, Pibarot P, Harzand A, Cheng JW, Gay H, Chrysohoou C, Ribeiro H, Rodés-Cabau J, Babaliaros V, Lerakis S. Dobutamine stress echocardiography for risk stratification of patients with low-gradient severe aortic stenosis undergoing TAVR. JACC Cardiovasc Imaging 2015;8:380–382. [DOI] [PubMed] [Google Scholar]
- 41. Clavel MA, Webb JG, Rodés-Cabau J, Masson JB, Dumont E, De Larochelliere R, Doyle D, Bergeron S, Baumgartner H, Burwash I, Dumesnil JG, Mundigler G, Moss R, Kempny A, Bagur R, Bergler-Klein J, Gurvitch R, Mathieu P, Pibarot P. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation 2010;122:1928–1936. [DOI] [PubMed] [Google Scholar]
- 42. Ben-Dor I, Maluenda G, Iyasu GD, Laynez-Carnicero A, Hauville C, Torguson R, Okubagzi P, Xue Z, Goldstein SA, Lindsay J, Satler LF, Pichard AD, Waksman R. Comparison of outcome of higher versus lower transvalvular gradients in patients with severe aortic stenosis and low (<40%) left ventricular ejection fraction. Am J Cardiol 2012;109:1031–1037. [DOI] [PubMed] [Google Scholar]
- 43. Clavel MA, Webb JG, Pibarot P, Altwegg L, Dumont E, Thompson C, De Larochelliere R, Doyle D, Masson JB, Bergeron S, Bertrand OF, Rodes-Cabau J. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol 2009;53:1883–1891. [DOI] [PubMed] [Google Scholar]
- 44. Pibarot P, Weissman NJ, Stewart WJ, Hahn RT, Lindman BR, McAndrew T, Kodali SK, Mack MJ, Thourani VH, Miller DC, Svensson LG, Herrmann HC, Smith CR, Rodés-Cabau J, Webb J, Lim S, Xu K, Hueter I, Douglas PS, Leon MB. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis- A PARTNER trial cohort A analysis. J Am Coll Cardio 2014;64:1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reardon MJ, Adams DH, Kleiman NS, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Lee JS, Hermiller JB Jr, Chetcuti S, Heiser J, Merhi W, Zorn GL III, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Maini B, Mumtaz M, Conte JV, Resar JR, Aharonian V, Pfeffer T, Oh JK, Qiao H, Popma JJ. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol 2015;66:113–121. [DOI] [PubMed] [Google Scholar]
- 46. Blais C, Dumesnil JG, Baillot R, Simard S, Doyle D, Pibarot P. Impact of valve prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation 2003;108:983–988. [DOI] [PubMed] [Google Scholar]
- 47. Ruel M, Al-Faleh H, Kulik A, Chan K, Mesana TG, Burwash IG. Prosthesis-patient mismatch after aortic valve replacement predominantly affects patients with pre-existing left ventricular dysfunction: Effect on survival, freedom from heart failure, and left ventricular mass regression. J Thorac Cardiovasc Surg 2006;131:1036–1044. [DOI] [PubMed] [Google Scholar]
- 48. Kulik A, Burwash IG, Kapila V, Mesana TG, Ruel M. Long-term outcomes after valve replacement for low-gradient aortic stenosis: Impact of prosthesis-patient mismatch. Circulation 2006;114(Suppl. 1):I5553-I5558. [DOI] [PubMed] [Google Scholar]
- 49. Mohty D, Dumesnil JG, Echahidi N, Mathieu P, Dagenais F, Voisine P, Pibarot P. Impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: influence of age, obesity, and left ventricular dysfunction. J Am Coll Cardiol 2009;53:39–47. [DOI] [PubMed] [Google Scholar]
- 50. Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, Kapadia S, Tuczu EM, Babaliaros V, Thourani V, Szeto WY, Bavaria JE, Kodali S, Hahn RT, Williams M, Miller DC, Douglas PS, Leon MB. Predictors of mortality and outcomes of therapy in low flow severe aortic stenosis: A PARTNER trial analysis. Circulation 2013;127:2316–2326. [DOI] [PubMed] [Google Scholar]
- 51. Goel SS, Ige M, Tuzcu EM, Ellis SG, Stewart WJ, Svensson LG, Lytle BW, Kapadia SR. Severe aortic stenosis and coronary artery disease – implications for management in the transcatheter aortic valve replacement era: A comprehensive review. J Am Coll Cardiol 2013;62:1–10. [DOI] [PubMed] [Google Scholar]
- 52. Garnier F, Eicher JC, Jazayeri S, Bertaux G, Bouchot O, Aho LS, Wolf JE, Laurent G. Usefulness and limitations of contractile reserve evaluation in patients with low-flow, low-gradient aortic stenosis eligible for cardiac resynchronization therapy. Eur J Heart Fail 2014;16:648–654. [DOI] [PubMed] [Google Scholar]
- 53. Konstantino Y, Zimetbaum PJ, Hsing J, Kramer DB, Chang JD. Cardiac resynchronization therapy for low-flow, low-gradient aortic stenosis. Eur J Heart Fail 2010;12:889–892. [DOI] [PubMed] [Google Scholar]
- 54. Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol 2012;60:1845–1853. [DOI] [PubMed] [Google Scholar]
- 55. Chahal NS, Drakopoulou M, Gonzalez-Gonzalez AM, Manivarmane R, Khattar R, Senior R. Resting aortic valve area at normal transaortic flow rate reflects true valve area in suspected low gradient severe aortic stenosis. JACC Cardiovasc Imaging 2015;8:1133–1139. [DOI] [PubMed] [Google Scholar]
- 56. Ozkan A, Hachamovitch R, Kapadia SR, Tuzcu EM, Marwick TH. Impact of aortic valve replacement on outcome of symptomatic patients with severe aortic stenosis with low gradient and preserved left ventricular ejection fraction. Circulation 2013;128:622–631. [DOI] [PubMed] [Google Scholar]
- 57. Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Sénéchal M, Pibarot P. Outcome of patients with aortic stenosis, small valve area and low-flow, low-gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol 2012;60:1259–1267. [DOI] [PubMed] [Google Scholar]
- 58. Mehrotra P, Jansen K, Flynn AW, Tan TC, Elmariah S, Picard MH, Hung J. Differential left ventricular remodelling and longitudinal function distinguishes low flow from normal-flow preserved ejection fraction low-gradient severe aortic stenosis. Eur Heart J 2013;34:1906–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cramariuc D, Cioffi G, Rieck AE, Devereux RB, Staal EM, Ray S, Wachtell K, Gerdts E. Low-flow aortic stenosis in asymptomatic patients: Valvular arterial impedance and systolic function from the SEAS substudy. J Am Coll Cardiol Img 2009;2:390–399. [DOI] [PubMed] [Google Scholar]
- 60. Itabashi Y, Shibayama K, Mihara H, Utsunomiya H, Berdejo J, Arsanjani R, Siegel R, Chakravarty T, Jilaihawi H, Makkar RR, Shiota T. Significant reduction in mitral regurgitation volume is the main contributor for increase in systolic forward flow in patients with functional mitral regurgitation after transcatheter aortic valve replacement: hemodynamic analysis using echocardiography. Echocardiography 2015;32:1621–1627. [DOI] [PubMed] [Google Scholar]
- 61. Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Survival by stroke volume index in patients with low-gradient normal EF severe aortic stenosis. Heart 2015;101:23–29. [DOI] [PubMed] [Google Scholar]
- 62. Parikh R, Goodman AL, Barr T, Sabik JF, Svensson LG, Rodriguez LL, Lytle BW, Grimm RA, Griffin BP, Desai MY. Outcomes of surgical aortic valve replacement for severe aortic stenosis: incorporation of left ventricular systolic function and stroke volume index. J Thorac Cardiovasc Surg 2015;149:1558–1566. [DOI] [PubMed] [Google Scholar]
- 63. Jander N, Hochholzer W, Kaufmann BA, Bahlmann E, Gerdts E, Boman K, Chambers JB, Nienaber CA, Ray S, Rossebo A, Pedersen TR, Wachtell K, Gohlke-Barwolf C, Neumann FJ, Minners J. Velocity ratio predicts outcomes in patients with low gradient severe aortic stenosis and preserved EF. Heart 2014;100:1946–1953. [DOI] [PubMed] [Google Scholar]
- 64. Michelena HI, Margaryan E, Miller FA, Eleid M, Maalouf J, Suri R, Messika-Zeitoun D, Pellikka PA, Enriquez-Sarano M. Inconsistent echocardiographic grading of aortic stenosis: is the left ventricular outflow tract important? Heart 2013;99:921–931. [DOI] [PubMed] [Google Scholar]
- 65. Clavel MA, Malouf J, Messika-Zeitoun D, Araoz PA, Michelena HI, Enriquez-Sarano M. Aortic valve area calculation in aortic stenosis by CT and Doppler echocardiography. JACC Cardiovasc Imaging 2015;8:248–257. [DOI] [PubMed] [Google Scholar]
- 66. Kamperidis V, van Rosendael PJ, Katsanos S, van der Kley F, Regeer M, Al Amri I, Sianos G, Marsan NA, Delgado V, Bax JJ. Low gradient severe aortic stenosis with preserved ejection fraction: reclassification of severity by fusion of Doppler and computed tomographic data. Eur Heart J 2015;36:2087–2096. [DOI] [PubMed] [Google Scholar]
- 67. Clavel MA, Ennezat PV, Maréchaux S, Dumesnil JG, Capoulade R, Hachicha Z, Mathieu P, Bellouin A, Bergeron S, Meimoun P, Arsenault M, Le Tourneau T, Pasquet A, Couture C, Pibarot P. Stress echocardiography to assess stenosis severity and predict outcome in patients with paradoxical low-flow, low-gradient aortic stenosis and preserved LVEF. J Am Coll Cardiol Img 2013;6:175–183. [DOI] [PubMed] [Google Scholar]
- 68. Maes F, Boulif J, Pierard S, de Meester C, Melchior J, Gerber B, Vancraeynest D, Pouleur AC, Lazam S, Pasquet A, Vanoverschelde JL. Natural history of paradoxical low gradient ‘severe’ aortic stenosis. Circ Cardiovasc Imaging 2014;7:714–722. [DOI] [PubMed] [Google Scholar]
- 69. Dayan V, Vignolo G, Magne J, Clavel MA, Mohty D, Pibarot P. Outcome and impact of aortic valve replacement in patients with preserved LV ejection fraction and low gradient aortic stenosis: a meta-analysis. J Am Coll Cardiol 2015;66:2594–2603. [DOI] [PubMed] [Google Scholar]
- 70. Eleid MF, Michelena HI, Nkomo VT, Nishimura RA, Malouf JF, Scott CG, Pellikka PA. Causes of death and predictors of survival after aortic valve replacement in low flow vs. normal flow severe aortic stenosis with preserved ejection fraction. Eur Heart J Cardiovasc Imaging 2015;16:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grupper A, Beigel R, Maor E, Kuperstein R, Hai I, Perelshtein O, Goldenberg I, Feinberg M, Ben ZS. Survival after intervention in patients with low gradient severe aortic stenosis and preserved left ventricular function. J Thorac Cardiovasc Surg 2014;148:2823–2827. [DOI] [PubMed] [Google Scholar]
- 72. Tarantini G, Covolo E, Razzolini R, Bilato C, Frigo AC, Napodano M, Favaretto E, Fraccaro C, Isabella G, Gerosa G, Iliceto S, Cribier A. Valve replacement for severe aortic stenosis with low transvalvular gradient and left ventricular ejection fraction exceeding 0.50. Ann Thorac Surg 2011;91:1808–1815. [DOI] [PubMed] [Google Scholar]
- 73. Belkin RN, Khalique O, Aronow WS, Ahn C, Sharma M. Outcomes and survival with aortic valve replacement compared with medical therapy in patients with low, moderate, and severe-gradient severe aortic stenosis and normal left ventricular ejection fraction. Echocardiography 2011;28:378–387. [DOI] [PubMed] [Google Scholar]
- 74. Maor E, Beigel R, Grupper A, Kuperstein R, Hai I, Medvedofsky D, Perelstein O, Mazin I, Ziv A, Goldenberg I, Feinberg MS, Ben ZS. Relation between stroke volume index to risk of death in patients with low-gradient severe aortic stenosis and preserved left ventricular function. Am J Cardiol 2014;114:449–455. [DOI] [PubMed] [Google Scholar]
- 75. Attias D, Macron L, Dreyfus J, Monin JL, Brochet E, Lepage L, Hekimian G, Iung B, Vahanian A, Messika-Zeitoun D. Relationship between longitudinal strain and symptomatic status in aortic stenosis. J Am Soc Echocardiogr 2013;26:868–874. [DOI] [PubMed] [Google Scholar]
- 76. Lancellotti P, Donal E, Magne J, O'Connor K, Moonen ML, Cosyns B, Pierard LA. Impact of global left ventricular afterload on left ventricular function in asymptomatic severe aortic stenosis: a two-dimensional speckle-tracking study. Eur J Echocardiogr 2010;11:537–543. [DOI] [PubMed] [Google Scholar]
- 77. Mizia-Stec K, Adamczyk T, Mizia M, Haberka M, Gasior Z, Trusz-Gluza M, Tendera M. Low-flow severe aortic stenosis with preserved ejection fraction, N-terminal pro-brain natriuretic peptide (NT-proBNP) and cardiovascular remodeling. J Heart Valve Dis 2011;20:301–310. [PubMed] [Google Scholar]
- 78. Eleid MF, Nishimura RA, Sorajja P, Borlaug BA. Systemic hypertension in low gradient severe aortic stenosis with preserved ejection fraction. Circulation 2013;128:1349–1353. [DOI] [PubMed] [Google Scholar]
- 79. Eleid MF, Nishimura RA, Borlaug BA, Sorajja P. Invasive measures of afterload in low gradient severe aortic stenosis with preserved ejection fraction. Circ Heart Fail 2013;6:703–710. [DOI] [PubMed] [Google Scholar]
- 80. Mohty D, Boulogne C, Magne J, Pibarot P, Echahidi N, Cornu E, Dumesnil J, Laskar M, Virot P, Aboyans V. Prevalence and long-term outcome of aortic prosthesis-patient mismatch in patients with paradoxical low-flow severe aortic stenosis. Circulation 2014;130(11 Suppl. 1):S25-S31. [DOI] [PubMed] [Google Scholar]
- 81. Zoghbi WA. Low-gradient ‘severe’ aortic stenosis with normal systolic function: time to refine the guidelines? Circulation 2011;123:838–840. [DOI] [PubMed] [Google Scholar]
- 82. Malouf J, Le TT, Pellikka P, Sundt TM, Scott C, Schaff HV, Enriquez-Sarano M. Aortic valve stenosis in community medical practice: determinants of outcome and implications for aortic valve replacement. J Thorac Cardiovasc Surg 2012;144:1421–1427. [DOI] [PubMed] [Google Scholar]
- 83. Bahlmann E, Gerdts E, Cramariuc D, Gohlke-Baerwolf C, Nienaber CA, Wachtell K, Seifert R, Chambers JB, Kuck KH, Ray S. Prognostic value of energy loss index in asymptomatic aortic stenosis. Circulation 2013;127:1149–1156. [DOI] [PubMed] [Google Scholar]
- 84. Berthelot-Richer M, Pibarot P, Capoulade R, Dumesnil JG, Dahou A, Thebault C, Le VF, Clavel MA. Discordant grading of aortic stenosis severity: echocardiographic predictors of survival benefit associated with aortic valve replacement. J Am Coll Cardiol 2016. (in press). [DOI] [PubMed] [Google Scholar]
- 85. Kadem L, Dumesnil JG, Rieu R, Durand LG, Garcia D, Pibarot P. Impact of systemic hypertension on the assessment of aortic stenosis. Heart 2005;91:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Little SH, Chan KL, Burwash IG. Impact of blood pressure on the doppler echocardiographic assessment of aortic stenosis severity. Heart 2007;93:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Le VF, Thebault C, Dahou A, Ribeiro HB, Capoulade R, Mahjoub H, Urena M, Nombela-Franco L, Allende CR, Clavel MA, Dumont E, Dumesnil J, De LR, Rodes-Cabau J, Pibarot P. Evolution and prognostic impact of low flow after transcatheter aortic valve replacement. Heart 2015;101:1196–1203. [DOI] [PubMed] [Google Scholar]
- 88. Kang DH, Jang JY, Park SJ, Kim DH, Yun SC, Song JM, Park SW, Chung CH, Song JK, Lee JW. Watchful observation versus early aortic valve replacement for symptomatic patients with normal flow, low-gradient severe aortic stenosis. Heart 2015;101:1375–1381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.