Abstract
Importance
The histologic changes associated with acute gastroesophageal reflux disease (GERD) have not been studied prospectively in humans. Recent studies in animals have challenged the traditional notion that reflux esophagitis develops when esophageal surface epithelial cells are exposed to lethal chemical injury from refluxed acid.
Objective
To evaluate histologic features of esophageal inflammation in acute GERD to study its pathogenesis.
Design
Patients with reflux esophagitis healed by proton pump inhibitors (PPIs) had 24-hour esophageal pH/impedance monitoring and esophagoscopy [including confocal laser endomicroscopy (CLE)] with biopsies from non-eroded areas of distal esophagus at baseline (on PPIs), 1 and 2 weeks after stopping PPIs. Enrollment began May 2013, follow-up ended July 2015.
Setting
Single-site, VA hospital.
Participants
Patients with prior reflux esophagitis and esophageal healing documented at baseline esophagoscopy.
Intervention
PPIs stopped for 2 weeks.
Main Outcome and Measures
Twelve patients (11 men, mean age 57.6±13.1 years) completed the study. Primary outcome was change in esophageal inflammation 2 weeks after stopping the PPI, determined by comparing lymphocyte, eosinophil, and neutrophil infiltrates (each scored on a 0–3 scale) in esophageal biopsies. Also evaluated were changes in epithelial basal cell and papillary hyperplasia, surface erosions, intercellular space width, endoscopic grade of esophagitis, esophageal acid exposure and mucosal impedance (an index of mucosal integrity).
Results
At 1 and 2 weeks after discontinuation of the PPI, biopsies showed significant increases in intraepithelial lymphocytes, which were predominantly T cells [median 0 (range 0–2) at baseline to 1 (range 1–2) at both 1 and 2 weeks, p<0.01]; neutrophils and eosinophils were few or absent. Biopsies also showed widening of intercellular spaces (confirmed by CLE), and basal cell and papillary hyperplasia developed without surface erosions. Two weeks after stopping the PPI, esophageal acid exposure increased (median 1.2% to 17.8% of the monitoring period, Δ=16.2%; 95%CI 4.4–26.5%, p=.005), mucosal impedance decreased (mean 2671.3 to 1508.4 Ω, Δ=1162.9; 95%CI 629.9–1695.9, p=.001), and all patients had evidence of esophagitis.
Conclusions and Relevance
In this preliminary study of 12 patients with severe reflux esophagitis responsive to PPI therapy, stopping PPIs was associated with T-lymphocyte-predominant esophageal inflammation, and basal cell and papillary hyperplasia without loss of surface cells. If replicated, these findings suggest that the pathogenesis of reflux esophagitis may be cytokine-mediated rather than the result of chemical injury.
Trial Registration
Clinicaltrials.Gov Identifier: NCT01733810
Approximately 20% of adult Americans have symptoms of gastroesophageal reflux disease (GERD).1 The conceptual framework for GERD pathogenesis emerged from a 1935 JAMA report by Winkelstein that described patients with heartburn and inflammation in the distal esophagus, and proposed that they had “peptic esophagitis…resulting from the irritant action on the mucosa of free hydrochloric acid and pepsin.”2 This concept, that reflux esophagitis develops as an acid-peptic “burn”, has been largely unchallenged. The esophageal histologic abnormalities thought to be typical of GERD (basal cell hyperplasia, elongation of connective tissue papillae, infiltration by neutrophils and eosinophils) have been attributed to refluxed gastric acid-related chemical injury to esophageal epithelial cells starting at the luminal surface. The acid-induced death of surface cells is assumed to stimulate hyperplasia of basal progenitor cells, make papillae appear elongated, and attract granulocytes.3–6
An earlier study in rats found that reflux esophagitis did not develop as a chemical injury starting at the epithelial surface, but rather began with a submucosal infiltration by lymphocytes that later progressed upward to the epithelial surface.7 Basal cell hyperplasia and papillary elongation were observed to precede surface cell damage, and it was noted that brief exposures to acid and bile salts did not kill human esophageal cells in culture, but stimulated them to secrete inflammatory cytokines.7,8 Thus, an alternative concept for GERD pathogenesis was proposed in which refluxed gastric juice did not damage esophageal epithelial cells directly, but stimulated them to secrete cytokines that attracted immune cells, which ultimately damaged the mucosa.7
Patients typically have GERD symptoms for years before seeing a physician,9 and early features of reflux esophagitis have not been evaluated prospectively in humans. Studies have shown that erosive esophagitis healed by proton pump inhibitors (PPIs) usually returns within 6 to 12 months after stopping PPIs,10,11 but the rapidity with which esophagitis redevelops is not clear. We hypothesized that acute reflux esophagitis could be induced by briefly interrupting PPI therapy in patients with severe erosive esophagitis healed by PPIs. The aim of this study was to evaluate the histologic features of esophageal inflammatory changes in acute GERD.
Methods
This study was approved by Dallas VA Medical Center’s Institutional Review Board. Patients provided written informed consent and were compensated for study participation.
Study Population and Design
Dallas VA’s endoscopy database was searched for patients with Los Angeles grade C (LA-C) reflux esophagitis diagnosed between December 2011 and January 2014 (LA Grades: 0=no esophagitis, A=≥1 mucosal break ≤5 mm long not extending between mucosal folds, B=≥1 mucosal break >5 mm long not extending between mucosal folds, C=≥1 mucosal break continuous between the tops of ≥2 mucosal folds, involving <75% of the circumference, D=≥1 mucosal break involving ≥75% of the circumference).12 Two gastroenterologists (KBD, SJS) reviewed endoscopic images, and invited patients with verified LA-C esophagitis to participate. Exclusion criteria included history of esophageal varices, esophageal or gastric surgery, non-GERD esophageal disease, coagulopathy, anticoagulant usage, pregnancy, and comorbidity precluding safe participation. Enrollment began May 2013; follow-up ended July 2015.
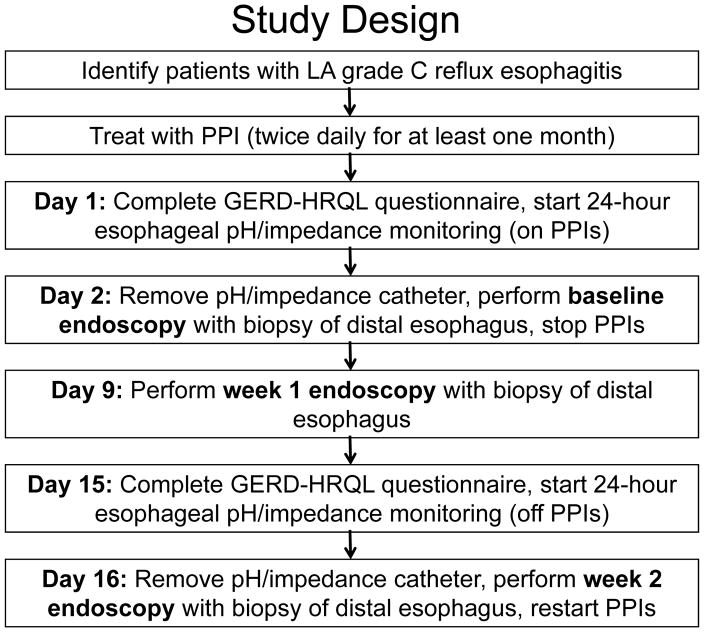
Patients with LA-C esophagitis were treated with PPI BID for ≥1 month (Figure 1). On study day 1, patients took their morning PPI and completed the GERD-Health Related Quality of Life (HRQL) questionnaire [a validated instrument for GERD symptom severity, possible scores 0 (no symptoms) to 50 (worst symptoms)].13 Esophageal manometry and 24-hour pH/impedance monitoring were performed with pH electrode positioned 5 cm above lower esophageal sphincter (LES). Patients took PPI that evening and next morning, when esophagoscopy was performed using both high-definition white light endoscopy (HD-WLE, Olympus Medical) and confocal laser endomicroscopy (CLE, Pentax Medical). Patients with LA-B, C or D esophagitis were not eligible for further study. Patients with no esophagitis or LA-A esophagitis had 4 esophageal biopsies obtained 1–3 cm proximal to squamo-columnar junction (SCJ) for histologic evaluation. PPIs were then stopped; patients were given antacids for heartburn. On day 9, esophagoscopy was performed for LA grading and biopsy. On day 15, patients completed another GERD HRQL questionnaire and had pH/impedance monitoring. The next day, esophagoscopy was performed for LA grading and biopsy, and patients resumed PPI therapy. During the second and third esophagoscopy, care was exercised to avoid taking biopsies from prior biopsy sites or mucosal breaks.
Figure 1.
Study design.
CLE Procedures
During CLE, which provides 1000-fold magnification of esophageal mucosa, patients were given fluorescein sodium (5 ml) intravenously to enhance identification of cells and capillaries. Images were acquired from distal (1–3 cm above SCJ) and proximal esophagus (10 cm above SCJ). After the procedure, one investigator (KBD) who was blinded to procedure time point reviewed all images, and chose two from proximal and distal esophagus that were technically best suited for intercellular space and capillary width measurements by ImageJ 1.48 software (NIH, Bethesda, Maryland) using the mean of 10 measurements of the widest intercellular spaces seen, and of any capillaries seen.
Resting Esophageal Mucosal Impedance
Resting esophageal mucosal impedance reflects electrical conductivity of the esophageal wall, and is an index of mucosal integrity.14–16 Resting impedance was measured at the start of each impedance/pH monitoring period, at a level 5 cm above LES.
Histologic Procedures
Histologic features were assessed by consensus of two study pathologists (ATA, RDO) blinded to endoscopic order and findings. Formalin-fixed, H&E-stained biopsies were scored on a 0–3 scale (0=absent, 1=mild, 2=moderate, 3=severe) for: a) type and degree of epithelial inflammation (lymphocytes, eosinophils, neutrophils), b) basal cell and papillary hyperplasia, and c) spongiosis (dilated intercellular spaces). In the absence of a validated system for scoring inflammation in esophageal biopsies, our study pathologist (RDO) chose to use this scale because he had used a similar, stepwise 4-point grading system for scoring inflammatory activity in the colon in an earlier publication.17 (Note: the study protocol mistakenly specified use of a 0–4 scale, but the study pathologists used a 0–3 scale to score inflammation.) Quantitative assessment of inflammatory cell density was performed by counting peak number of lymphocytes, neutrophils, and eosinophils per high power field (HPF, 40× = 0.238mm2) in each of the three most representative and best-oriented biopsy fragments. Immunoperoxidase studies were performed on a Leica Bond III autostainer, using Polymer Refine Detection Kit (cat# DS9800) and heat-induced epitope retrieval (HIER) using either Bond Epitope Retrieval Solution 1, pH 6.0 (cat #AR9961) or Solution 2, pH 9.0 (cat# AR9640) for pan-T cell marker CD3 (Dako A0452, HIER 2 for 20 minutes, 1:250) and B cell marker CD20 (Dako M0755, HIER 1 for 30 minutes, 1:500). Slides were incubated in primary antibody for 30 minutes, followed by secondary polymer for 15 minutes. DAB (3,3′-diaminobenzidine) was used for visualization.
Outcomes
The study protocol did not clearly specify primary and secondary outcomes, and cited study purpose as “to elucidate the early histological events in the pathogenesis of reflux esophagitis in patients with GERD, and to correlate those events with esophageal expression of HIF-2α and pro-inflammatory cytokines, and with changes in esophageal proliferation”. ClinicalTrials.Gov cites study purpose as “to determine the role of HIF-2α on the production of inflammatory cytokines that lead to reflux esophagitis,” and lists the primary and secondary outcome measures as “Change in esophageal inflammation from baseline to 14 days” and “Change in HIF-2α levels from baseline to 14 days”, respectively. However, this report describes only clinical study findings. Molecular studies on HIF-2α, pro-inflammatory cytokines, and esophageal proliferation are in progress and will be described in future reports.
To determine change in esophageal inflammation histologically, lymphocytic, eosinophilic, and neutrophilic infiltration in esophageal biopsies (scored on the 0–3 scale described above) at baseline, 1 week and 2 weeks were compared. In addition, changes in GERD-HRQL symptom scores, esophageal acid exposure (% total time esophageal pH<4), endoscopic grade of esophagitis, esophageal mucosal impedance, and changes in epithelial basal cell and papillary hyperplasia, spongiosis, surface erosions, and intercellular space width were evaluated. These were listed as “procedures to be performed” in the study protocol, but were not specified as outcome measures.
Statistical Methods
Continuous parameters are reported as mean ± standard deviation, ordinal parameters as median and range, discrete parameters as N and percent. Continuous dependent variables were tested for normality with Shapiro-Wilk test. Normally distributed continuous parameters were compared with paired samples t tests and repeated measures MANOVA with LSD multiple comparisons. Non-normally distributed continuous parameters and ordinal parameters were compared with Wilcoxon signed-rank and Friedman tests. Binary dependent variables were tested with repeated measures logistic regression (generalized linear models with logit functions). Analyses were performed with SPSS 22.0 for Windows. Study alpha was 0.05, with all tests reflecting two-tailed comparisons.
Sample Size Calculation and Power Analysis
The study protocol specified that a power analysis performed using SAS 9.2 indicated that 12 participants were required to achieve study aims, based on a one-sample repeated measures analysis of inflammation over time with an anticipated effect size of 80%, a study alpha of .05, beta of .10, two-tailed. “Effect size of 80%” meant that no histologic evidence of esophageal inflammation (grade 0) was anticipated in any patient at baseline, and some degree of histologic inflammation (grade 1–3) was expected in 80% of patients by 2 weeks after stopping PPIs. These estimates were based on studies in an animal model of reflux esophagitis, and on studies of GERD patients who redeveloped esophagitis within 6–12 months of stopping PPIs.7,10,11 An attrition rate of 40% (due to dropouts, inability to remain off PPIs, esophagitis on initial endoscopy, etc.) was estimated, and enrollment of up to 30 patients was planned in order to have 12 complete the study.
Results
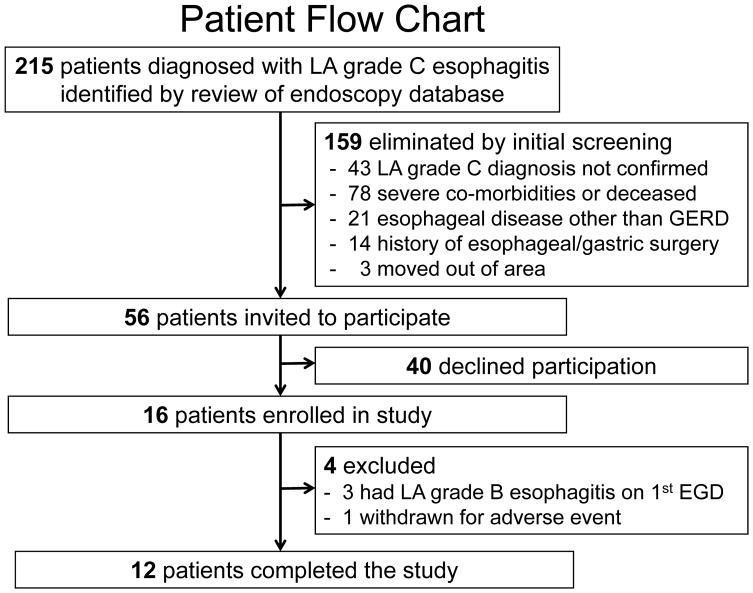
Endoscopy database review identified 215 patients diagnosed with LA-C esophagitis (Figure 2); 159 were eliminated by initial screening and 56 were invited to participate; 40 declined. Attrition rate was lower than estimated; it was necessary to enroll only 16 patients to attain 12 who completed the study; 3 patients were excluded because baseline endoscopy showed LA-B esophagitis, 1 was withdrawn for an adverse event unrelated to study procedures. Enrollment was terminated when the 12th patient completed study procedures. Among the 12 study patients (11 men, mean age 57.6±13.1 years), 8 took pantoprazole 40 mg BID and 4 took omeprazole 40 mg BID during the month before study procedures.
Figure 2.
Patient flow chart. LA=Los Angeles grade of esophagitis, EGD=endoscopy.
GERD Symptoms, Esophageal Acid Exposure, Mucosal Impedance, and Endoscopic Findings
GERD-HRQL symptom scores increased from median of 2 at baseline on PPIs to 11.5 at 2 weeks off PPIs (median Δ=4.5; 95%CI: 2.0–12.0; p=.008) (Table 1). Esophageal pH monitoring data were available both at baseline and 2 weeks off PPIs for 10 of the 12 patients. Acid exposure increased from median 1.2% of the monitoring period at baseline to 17.8% two weeks off PPIs (median Δ=16.2%; 95%CI: 4.4–26.5%, p=.005). Mucosal impedance decreased from mean 2671.3 Ω at baseline to 1508.4 Ω 2 weeks off PPIs (Δ=1162.9; 95%CI: 629.9–1695.9, p=.001).
Table 1.
Clinical Features at Baseline (on PPIs), and at 1 and/or 2 Weeks after Stopping PPIs
| Patient Number | GERDHRQL Baseline |
GERDHRQL 2 weeks |
% Time pH<4 Baseline |
% Time pH<4 2 weeks |
Esophageal Impedance In Ohms Baseline |
Esophageal Impedance In Ohms 2 weeks |
LA Grade Baseline (numerical value) |
LA Grade 1 week (numerical value) |
LA Grade 2 weeks (numerical value) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 9 | 0.3 | 26.8 | NA | 650 | 0 (0) | A (1) | C (3) |
| 2 | 25 | 22 | 10.7 | 15.1 | 2249 | 773 | 0 (0) | B (2) | B (2) |
| 3 | 17 | 29 | 1.2 | 23.6 | 3993 | 1329 | 0 (0) | B (2) | B (2) |
| 4 | 1 | 3 | 0.0 | 21.6 | 1422 | 1113 | 0 (0) | B (2) | B (2) |
| 5 | 19 | 20 | 6.5 | 6.8 | 3676 | 1670 | 0 (0) | B (2) | C (3) |
| 6 | 11 | 16 | 4.8 | 18.6 | 2004 | 1231 | 0 (0) | B (2) | C (3) |
| 7 | 0 | 14 | 0.6 | 16.9 | 3271 | 2660 | 0 (0) | A (1) | C (3) |
| 8 | 2 | 4 | 37.0§ | 88.3§ | 983§ | 1021§ | 0 (0) | 0 (0) | A (1) |
| 9 | 0 | 5 | 1.9 | 37.4 | 2130 | 1784 | A (1) | 0 (0) | A (1) |
| 10 | 2 | 4 | 0.1 | 16.1 | 2947 | 2000 | 0 (0) | A (1) | B (2) |
| 11 | 4 | 18 | 1.2 | NA | 2000 | 850 | 0 (0) | A (1) | C (3) |
| 12 | 0 | 4 | 0.5 | 5.6 | 3021 | 1674 | 0 (0) | B (2) | A (1) |
| Median 2 (range 0–25) | Median 11.5 (range 3–29) | Median 1.2 (range 0–10.7) | Median 17.8 (range 5.6–37.4) | Mean 2671.3 SD 832.8 |
Mean 1508.4 SD 571.3 |
Median 0 (range 0–1) | Median 1.5 (range 0–2) p=.006* vs. baseline |
Median 2 (range 1–3) p=.02* vs. 1 week |
|
| Difference baseline-2 weeks | 4.5 (Median) 95%CI 2.0–12.0 p=.008* |
16.2 (Median) 95%CI 4.4–26.5 p=.005* |
1162.9 (Mean) 95%CI 629.9–1695.9 p=.001+ |
||||||
Baseline values are presented in the shaded columns. NA=data not available for technical reasons.
GERD-HRQL is a validated instrument used to assess the symptomatic response to GERD treatments.13 The patient is asked to rank 10 GERD symptom questions (e.g. How bad is your heartburn? Does heartburn wake you from sleep?) on a scale of 0 (no symptoms) to 5 (incapacitating symptoms); thus, the possible range of scores is 0 to 50. No minimally important difference in GERD-HRQL scores has been defined. In clinical trials, a positive outcome generally is defined as ≥50% improvement in GERD-HRQL scores.35
Esophageal impedance (electrical resistance) was measured at the start of the monitoring period at a level 5 cm above the LES.
LA endoscopic esophagitis grades: 0=no esophagitis, A=≥1 mucosal break ≤5 mm long not extending between the tops of mucosal folds, B=≥1 mucosal break >5 mm long not extending between the tops of mucosal folds, C=≥1 mucosal break continuous between the tops of ≥2 mucosal folds, involving <75% of the circumference. For statistical comparisons, numerical values were assigned to LA grades (0=0, A=1, B=2, C=3).
Due to technical difficulties in identifying the LES in this patient, the pH electrode was inadvertently positioned in the stomach rather than the esophagus. This incorrect positioning also resulted in spurious values for impedance. Consequently, these data were not included in the group analysis of the pH monitoring and impedance results.
Wilcoxon signed-rank test.
Paired samples t-test.
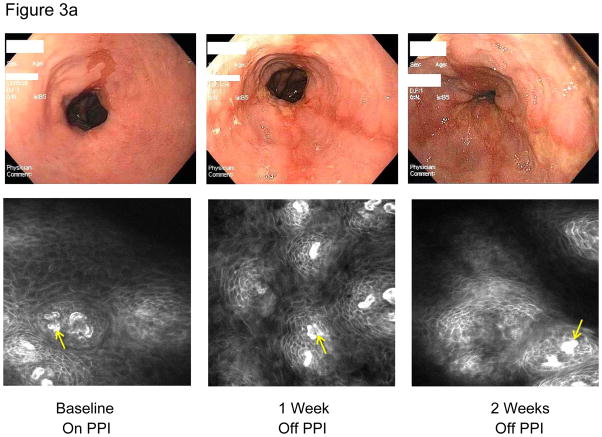
At baseline, 11 of 12 patients had no visible esophagitis; 1 had LA-A esophagitis (Table 1). Off PPIs, esophagitis grade increased in 10 patients by week 1, and in 11 by week 2; 5 developed severe (LA-C) esophagitis (Figure 3a). By assigning numerical values to LA grades (0=0, A=1, B=2, C=3), it was determined that esophagitis increased significantly from baseline (median 0) to week 1 (median 1.5, p=.006), and from week 1 to week 2 (median 2, p=.02).
Figure 3a.
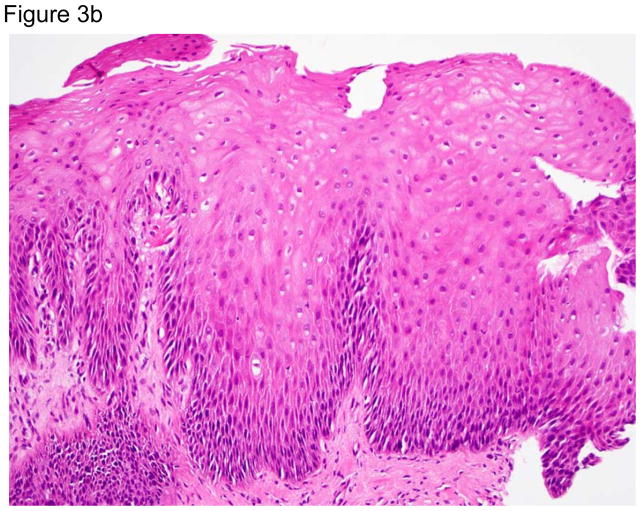
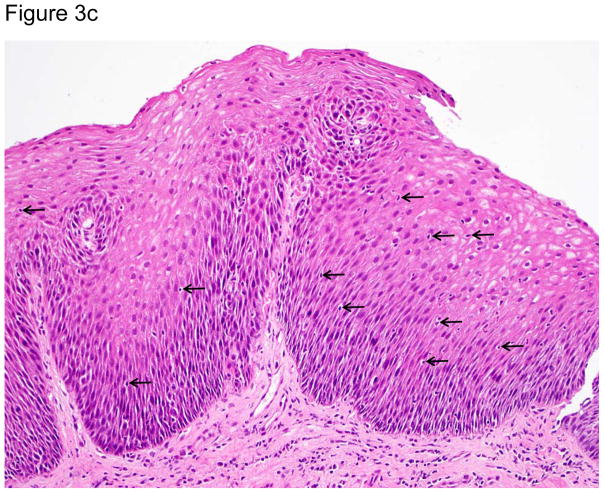
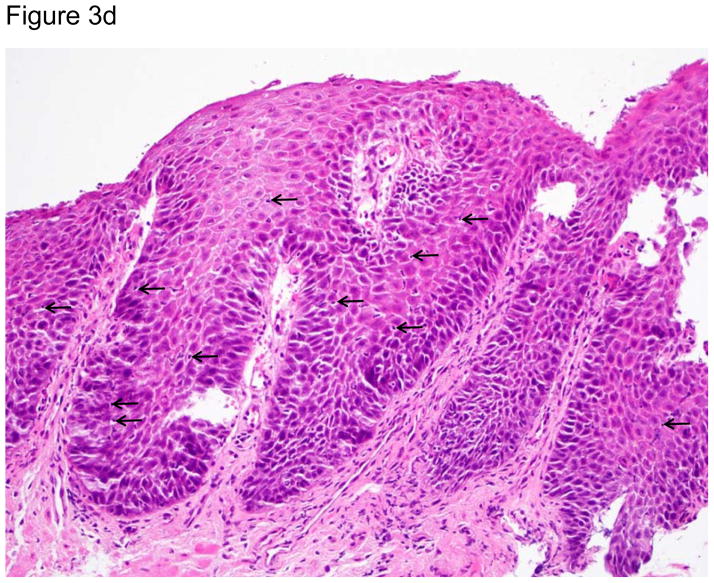
Representative images of the distal esophagus at baseline, and at 1 and 2 weeks off PPI therapy. All images are from the same patient. Top row: high definition white light endoscopy (HD-WLE), bottom row: confocal laser endomicroscopy (CLE) images. At baseline, HD-WLE reveals an irregular tongue of columnar mucosa (Barrett’s esophagus) in the 12 o’clock position, but no esophagitis. At week 1, HD-WLE shows long linear mucosal breaks (4 and 6 o’clock positions) extending up the esophagus from the gastroesophageal junction (LA-B esophagitis). At week 2, HD-WLE shows long mucosal breaks continuous between the tops of mucosal folds (LA-C esophagitis). CLE reveals fluorescein within bright intraepithelial capillaries (yellow arrows), with fluorescein that leaked from blood vessels into intercellular spaces surrounding individual cells, creating a reticular appearance characteristic of squamous epithelium. CLE measurements revealed widened intercellular spaces with increased intercellular fluorescein at weeks 1 and 2. 3b–d. Photomicrographs of biopsies of the distal esophagus in the same patient: 3b) at baseline, 3c) at one week after stopping PPIs; arrows point to some of the numerous intraepithelial lymphocytes, and 3d) at two weeks after stopping PPIs; note the prominent lymphocytosis, basal cell hyperplasia and papillary elongation (all photomicrographs H&E, original magnification ×20). All images were manipulated in Photoshop to remove patient identification data, and to enhance clarity. Any adjustments in contrast, color balance, brightness or sharpness were applied to the entire image.
Histologic Findings and CLE Measurements
At baseline, there was minimal histologic inflammation (Table 2, Figure 3b–d). One and two weeks off PPIs, significant increases were noted in ordinal (0–3) histologic scores for intraepithelial lymphocytic infiltration [median 0 (range 0–2) to 1 (range 1–2), p<0.01], basal cell and papillary hyperplasia [median 0.5 (range 0–1) to 2 (range 1–3), p<0.01], and spongiosis [median 0.5 (range 0–1) to 2 (range 1–3), p<0.01]. Neutrophils were found only in infrequent areas of micro-erosion; the median maximum number of neutrophils/HPF in any biopsy specimen at any time was 0 (range 0–31). Eosinophils also were few in number in most patients at all time points; the median maximum number of intraepithelial eosinophils/HPF in any biopsy specimen at any time was 1.5 (range 0–9). Lymphocytes were the predominant inflammatory cell type at all time points; the median maximum number of intraepithelial lymphocytes/HPF in any biopsy at any time was 51.5 (range 26–163). Immunostaining showed that these lymphocytes were almost exclusively CD3+ T cells (eFigure 1).
Table 2.
Histologic Findings and Confocal Laser Endomicroscopy (CLE) Measurements
| Histologic Findinga | Baseline On PPIs Median (Range) |
Week 1 Off PPIs Median (Range) |
Absolute Difference Baseline-Week 1 | Week 2 Off PPIs Median (Range) |
Absolute Difference Baseline-Week 2 |
|---|---|---|---|---|---|
| Intraepithelial lymphocytes | 0 (0–2) | 1 (1–2) | .67 95%CI .25 − 1.08 p=.005 |
1 (1–2) | .58 95%CI .26 − .91 p=.002 |
| Intraepithelial neutrophils | 0 (0) | 0 (0–2) | .17 95%CI −.20 − .53 p=.32 |
0 (0–2) | .25 95%CI −.14 − .64 p=.18 |
| Intraepithelial eosinophils | 0 (0–1) | 0 (0–1) | .08 95%CI −.10 − .27 p=.32 |
0 (0–1) | .08 95%CI −.10 − .27 p=.32 |
| Basal cell and papillary hyperplasia | .5 (0–1) | 2 (1–3) | 1.25 95%CI .86 − 1.64 p=.002 |
2 (1–3) | 1.42 95%CI .91 − 1.92 p=.003 |
| Spongiosis (dilated intercellular spaces) | .5 (0–1) | 2 (1–3) | 1.17 95%CI .80 − 1.53 p<.0001 |
2 (1–3) | 1.25 95%CI .86 − 1.65 p<.0001 |
| CLE Measurementsb | Mean ± SD (n) | Mean ± SD (n) | Mean ± SD (n) | ||
| Intercellular space distal esophagus (μm) | 3.2 ± 0.6 (n=8) | 4.0 ± 0.5 (n=8) | .82 95%CI .10 − 1.54 p=.031 |
5.1 ± 1.1 (n=8) | 1.91 95%CI 1.0 − 2.81 p=.002 |
| Intercellular space proximal esophagus (μm) | 3.5 ± 0.5 (n=8) | 4.7 ± 1.3 (n=7) | 1.32 95%CI .05 − 2.60 p=.044 |
5.7 ± 1.2 (n=7) | .2.53 95%CI 1.62 − 3.45 p=.001 |
| Capillary width distal esophagus (μm) | 11.0 ± 1.6 (n=7) | 14.1 ± 3.0 (n=7) | 3.03 95%CI .76 − 5.3 p=.017 |
15.0 ± 2.6 (n=6) | 3.87 95%CI .86 − 6.88 p=.021 |
| Capillary width proximal esophagus (μm) | 10.9 ± 3.5 (n=6) | 13.8 ± 1.6 (n=6) | 3.30 95%CI −.55 − 7.14 p=.08 |
13.1 ± 2.5 (n=6) | 3.03 95%CI .78 − 5.28 p=.018 |
Histologic findings are scored on a scale of 0–3 (0=absent, 1=mild, 2=moderate, 3=severe). Comparisons were made using Wilcoxon signed-rank tests.
Technical issues (excessive motion artifact, equipment malfunction, patient inability to tolerate the large CLE endoscope) precluded obtaining CLE measurements in all patients at all time points. CLE measurements were made using ImageJ 1.48 software (National Institutes of Health, Bethesda, Maryland). Comparisons were made using paired samples t-tests.
After stopping PPIs, CLE revealed dilated intercellular spaces containing increased amounts of fluorescein (Figure 3a). At weeks 1 and 2, intercellular space width in proximal and distal esophagus, and capillary width in distal esophagus had increased significantly from baseline values (Table 2).
Discussion
In patients with severe erosive reflux esophagitis healed by PPIs, this study showed that interrupting PPI therapy was followed by rapid development of acute GERD associated with a significant increase in esophageal acid exposure and significant decrease in mucosal integrity. Within one week of stopping PPIs, most patients redeveloped erosive esophagitis associated with dilation of esophageal intercellular spaces and capillaries. Histologically, this acute GERD was a T-lymphocyte predominant form of inflammation, with minimal involvement by neutrophils and eosinophils. Furthermore, esophageal basal cell and papillary hyperplasia developed in areas without surface erosions. If the traditional notion were true, that acute GERD is caused by refluxed acid directly inflicting lethal, chemical injury to surface epithelial cells, then basal cell and papillary hyperplasia would have been expected only in areas with surface erosions, and the infiltrating inflammatory cells would have been granulocytes primarily.6
Apical membranes of esophageal squamous epithelial cells are highly impermeable to hydrogen ions, unlike their basolateral membranes, which are highly acid-permeable.18 According to the traditional notion of GERD pathogenesis, esophagitis starts when refluxed acid and pepsin initiate damage to proteins of junctional structures binding esophageal epithelial cells to one another.19,20 These structures normally form a barrier to paracellular diffusion of acid and, when damaged, refluxed acid can diffuse into intercellular spaces to enter epithelial cells through their vulnerable basolateral membranes. Once inside esophageal cells, acid was thought to kill them by denaturing vital proteins, by activating phospholipases and endonucleases, and by interfering with cell respiration.21,22 This lethal acid injury was assumed to start at the esophageal luminal surface, inducing an acute inflammatory response characterized by epithelial infiltration with granulocytes. The acid-induced death of surface cells was assumed to stimulate hyperplasia of squamous basal progenitor cells and to be associated with elongated and hyperplastic papillae.3,5 With persistent reflux inducing more epithelial cell death, inflammation was thought to progress into lamina propria and, with ulceration, into submucosa.6 Thus, acute reflux esophagitis was assumed to develop as an acid-peptic burn progressing from luminal surface through to submucosa. However, this pattern of injury was not observed in the present study.
The results of this study in GERD patients are consistent with those described in our report on a rat model of reflux esophagitis in which we proposed that refluxed gastric juice might not damage the esophagus directly, but rather incited a cytokine-mediated inflammatory response that ultimately caused esophageal damage.7 Elevated esophageal levels of pro-inflammatory cytokines have been found in GERD patients, although it remains unclear whether those cytokines are a cause or effect of esophageal inflammation.23–27 In our earlier studies, we found that acidic bile salts stimulated human esophageal squamous epithelial cells in culture to secrete potent pro-inflammatory cytokines [interleukin (IL)-8 and IL-1β], and that conditioned media from those cells increased migration of inflammatory cells in a transwell assay system.7,8 Cytokines like IL-8 and IL-1β also have pro-proliferative effects,28,29 which might have contributed to esophageal basal cell and papillary hyperplasia observed in the absence of surface erosions. In esophageal epithelial cells in culture, moreover, PPIs inhibit secretion of IL-8 through acid-independent mechanisms.8,30 This observation raises the interesting possibility that anti-inflammatory PPI effects, independent of their effects on acid inhibition, might contribute to GERD healing by PPIs.
In the present study, biopsies were taken purposely from areas of distal esophagus that had no visible erosions. Biopsies of eroded areas undoubtedly would have revealed prominent neutrophilic infiltrates, which develop in erosions of virtually any etiology.31 The finding of neutrophils in such biopsies would provide no useful information about the pathogenesis of the erosions. Biopsies of non-eroded esophageal epithelium after PPI interruption revealed infiltration by T-lymphocytes with basal cell hyperplasia and papillary elongation, consistent with the proposal that acute GERD is primarily a cytokine-mediated process.7 Further studies are needed to establish that cytokines are indeed the cause of the histologic changes observed, but our findings suggest that those changes do not appear to be caused by acid-induced death of surface cells.
Dilation of esophageal intercellular spaces is a characteristic GERD feature, and intercellular space dilation was observed (by CLE and histology) as reflux esophagitis progressed in study patients. It has been proposed that this dilation results from an acid-induced increase in epithelial permeability that enables Cl− and water in the esophageal lumen to enter and expand the intercellular space.32 The CLE observation that GERD is associated with an increase in blood-borne fluorescein in intercellular spaces raises the possibility that reflux-induced esophageal inflammation might increase esophageal vascular permeability. If so, then leakage of fluid from inflammation-damaged esophageal blood vessels also might contribute to dilation of intercellular spaces in GERD.
There are limitations to this study. Most of the eligible patients declined to participate in this rigorous protocol. The study included only patients with severe (LA-C) reflux esophagitis whose esophagitis had been healed by PPIs; 11 of the 12 patients were men, and all had hiatal hernias. Among individuals with typical GERD symptoms, <50% have endoscopic evidence of reflux esophagitis, and <20% of those have esophagitis of LA-C grade severity.33,34 Thus, it is not clear that findings in the study patients are applicable to the general population of patients with GERD of lesser severity. Study patients had recurrent, acute GERD induced by stopping PPIs, and it is not clear that findings in those patients are applicable to new-onset GERD occurring spontaneously in untreated individuals. In addition, there was subjectivity involved in choosing CLE images for analysis, which might have introduced bias in interpretation of the CLE data. Finally, it is possible that the drop in mucosal impedance after stopping PPIs was caused by liquid in the esophageal lumen rather than by impairment of mucosal integrity.
Conclusions
In this preliminary study of 12 patients with severe reflux esophagitis responsive to PPI therapy, stopping PPIs was associated with T-lymphocyte-predominant esophageal inflammation, and basal cell and papillary hyperplasia without loss of surface cells. If replicated, these findings suggest that the pathogenesis of reflux esophagitis may be cytokine-mediated rather than the result of chemical injury.
Acknowledgments
This work was supported by Merit Review Award #BX002666 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research Program (SJS), the National Institutes of Health (R01-DK63621 to RFS and SJS), and the American Gastroenterological Association June and Donald O. Castell Esophageal Clinical Research Award (KBD).
Stuart J Spechler, M.D. is a consultant for Interpace Diagnostics, Ironwood Pharmaceuticals and Takeda Pharmaceuticals
Rhonda F. Souza, M.D. is a consultant for Interpace Diagnostics and Ironwood Pharmaceuticals
Access to Data and Data Analysis: Drs. Stuart Spechler and Rhonda Souza had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Support: This work was supported by Merit Review Award #BX002666 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research Program (SJS), the National Institutes of Health (R01-DK63621 to RFS and SJS), and the American Gastroenterological Association June and Donald O. Castell Esophageal Clinical Research Award (KBD).
Role of Funder/Sponsor: The funding organizations listed above reviewed grant proposals related to the studies described in this report and provided reviewer feedback. However, these organizations had no direct role in design and conduct of the study. The organizations also had no role in collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112(5):1448–56. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 2.Winkelstein A. Peptic esophagitis: a new clinical entity. JAMA. 1935;104(11):906–9. [Google Scholar]
- 3.Ismail-Beigi F, Horton PF, Pope CE., 2nd Histological consequences of gastroesophageal reflux in man. Gastroenterology. 1970;58(2):163–74. [PubMed] [Google Scholar]
- 4.Fiocca R, Mastracci L, Riddell R, Takubo K, Vieth M, Yerian L, Sharma P, Fernström P, Ruth M. Development of consensus guidelines for the histologic recognition of microscopic esophagitis in patients with gastroesophageal reflux disease: the Esohisto project. Hum Pathol. 2010;41(2):223–31. doi: 10.1016/j.humpath.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Eastwood GL. Histologic changes in gastroesophageal reflux. J Clin Gastroenterol. 1986;8(Suppl 1):45–51. doi: 10.1097/00004836-198606001-00007. [DOI] [PubMed] [Google Scholar]
- 6.Frierson HF., Jr Histology in the diagnosis of reflux esophagitis. Gastroenterol Clin North Am. 1990;19(3):631–44. [PubMed] [Google Scholar]
- 7.Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, Zhang X, Yu C, Hormi-Carver K, Genta RM, Spechler SJ. Gastroesophageal reflux may cause esophagitis through a cytokine-mediated mechanism, not by caustic (acid) injury. Gastroenterology. 2009;137(5):1776–84. doi: 10.1053/j.gastro.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 8.Huo X, Zhang X, Yu C, Zhang Q, Cheng E, Wang DH, Pham TH, Spechler SJ, Souza RF. In oesophageal squamous cells exposed to acidic bile salt medium, omeprazole inhibits IL-8 expression through effects on nuclear factor-κB and activator protein-1. Gut. 2014;63(7):1042–52. doi: 10.1136/gutjnl-2013-305533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spechler SJ. Epidemiology and natural history of gastro-oesophageal reflux disease. Digestion. 1992;51(suppl 1):24–9. doi: 10.1159/000200911. [DOI] [PubMed] [Google Scholar]
- 10.Hetzel DJ, Dent J, Reed WD, Narielvala FM, Mackinnon M, McCarthy JH, Mitchell B, Beveridge BR, Laurence BH, Gibson GG, et al. Healing and relapse of severe peptic esophagitis after treatment with omeprazole. Gastroenterology. 1988;95(4):903–12. doi: 10.1016/0016-5085(88)90162-x. [DOI] [PubMed] [Google Scholar]
- 11.Chiba N. Proton pump inhibitors in acute healing and maintenance of erosive or worse esophagitis: a systematic overview. Can J Gastroenterol. 1997;11(Suppl B):66B–73B. [PubMed] [Google Scholar]
- 12.Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GNJ, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45(2):172–180. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20(2):130–4. doi: 10.1111/j.1442-2050.2007.00658.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhong C, Duan L, Wang K, Xu Z, Ge Y, Yang C, Han Y. Esophageal intraluminal baseline impedance is associated with severity of acid reflux and epithelial structural abnormalities in patients with gastroesophageal reflux disease. J Gastroenterol. 2013;48(5):601–10. doi: 10.1007/s00535-012-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinucci I, de Bortoli N, Savarino E, Piaggi P, Bellini M, Antonelli A, Savarino V, Frazzoni M, Marchi S. Esophageal baseline impedance levels in patients with pathophysiological characteristics of functional heartburn. Neurogastroenterol Motil. 2014;26(4):546–55. doi: 10.1111/nmo.12299. [DOI] [PubMed] [Google Scholar]
- 16.Kandulski A, Weigt J, Caro C, Jechorek D, Wex T, Malfertheiner P. Esophageal intraluminal baseline impedance differentiates gastroesophageal reflux disease from functional heartburn. Clin Gastroenterol Hepatol. 2015;13(6):1075–81. doi: 10.1016/j.cgh.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Odze R, Antonioli D, Peppercorn M, Goldman H. Effect of topical 5-aminosalicylic acid (5-ASA) therapy on rectal mucosal biopsy morphology in chronic ulcerative colitis. Am J Surg Pathol. 1993;17(9):869–75. doi: 10.1097/00000478-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Khalbuss WE, Marousis CG, Subramanyam M, Orlando RC. Effect of HCl on transmembrane potentials and intracellular pH in rabbit esophageal epithelium. Gastroenterology. 1995;108(3):662–72. doi: 10.1016/0016-5085(95)90437-9. [DOI] [PubMed] [Google Scholar]
- 19.Tobey NA, Hosseini SS, Caymaz-Bor C, Wyatt HR, Orlando GS, Orlando RC. The role of pepsin in acid injury to esophageal epithelium. Am J Gastroenterol. 2001;96(11):3062–70. doi: 10.1111/j.1572-0241.2001.05260.x. [DOI] [PubMed] [Google Scholar]
- 20.Jovov B, Que J, Tobey NA, Djukic Z, Hogan BL, Orlando RC. Role of E-cadherin in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106(6):1039–47. doi: 10.1038/ajg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg HI, Dodds WJ, Gee S, Montgomery C, Zboralske FF. Role of acid and pepsin in acute experimental esophagitis. Gastroenterology. 1969;56(2):223–30. [PubMed] [Google Scholar]
- 22.Orlando RC. Pathogenesis of reflux esophagitis and Barrett’s esophagus. Med Clin North Am. 2005;89(2):219–41. doi: 10.1016/j.mcna.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. 2002;50(4):451–459. doi: 10.1136/gut.50.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, Miyazaki M, Murase K, Hayashi T, Inoue K, Murata I, Kohno S. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol. 2003;98(3):551–556. doi: 10.1111/j.1572-0241.2003.07303.x. [DOI] [PubMed] [Google Scholar]
- 25.Oh DS, DeMeester SR, Vallbohmer D, Mori R, Kuramochi H, Hagen JA, Lipham J, Danenberg KD, Danenberg PV, Chandrasoma P, DeMeester TR. Reduction of interleukin 8 gene expression in reflux esophagitis and Barrett’s esophagus with antireflux surgery. Arch Surg. 2007;142(6):554–559. doi: 10.1001/archsurg.142.6.554. [DOI] [PubMed] [Google Scholar]
- 26.Isomoto H, Saenko VA, Kanazawa Y, Nishi Y, Ohtsuru A, Inoue K, Akazawa Y, Takeshima F, Omagari K, Miyazaki M, Mizuta Y, Murata I, Yamashita S, Kohno S. Enhanced expression of interleukin-8 and activation of nuclear factor kappa-B in endoscopy-negative gastroesophageal reflux disease. Am J Gastroenterol. 2004;99(4):589–597. doi: 10.1111/j.1572-0241.2004.04110.x. [DOI] [PubMed] [Google Scholar]
- 27.Altomare A, Ma J, Guarino MP, Cheng L, Rieder F, Ribolsi M, Fiocchi C, Biancani P, Harnett K, Cicala M. Platelet-activating factor and distinct chemokines are elevated in mucosal biopsies of erosive compared with non-erosive reflux disease patients and controls. Neurogastroenterol Motil. 2012;24(10):943–e463. doi: 10.1111/j.1365-2982.2012.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L, Fidler IJ. Interleukin 8: an autocrine growth factor for human ovarian cancer. Oncol Res. 2000;12(2):97–106. doi: 10.3727/096504001108747567. [DOI] [PubMed] [Google Scholar]
- 29.Bigildeev AE, Zezina EA, Shipounova IN, Drize NJ. Interleukin-1 beta enhances human multipotent mesenchymal stromal cell proliferative potential and their ability to maintain hematopoietic precursor cells. Cytokine. 2015;71(2):246–54. doi: 10.1016/j.cyto.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of the proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54(11):2312–7. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leoni G, Neumann PA, Sumagin R, Denning TL, Nusrat A. Wound repair: role of immune-epithelial interactions. Mucosal Immunol. 2015;8(5):959–68. doi: 10.1038/mi.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobey NA, Gambling TM, Vanegas XC, Carson JL, Orlando RC. Physicochemical basis for dilated intercellular spaces in non-erosive acid-damaged rabbit esophageal epithelium. Dis Esophagus. 2008;21(8):757–64. doi: 10.1111/j.1442-2050.2008.00841.x. [DOI] [PubMed] [Google Scholar]
- 33.Hershcovici T, Fass R. Nonerosive Reflux Disease (NERD) - An Update. J Neurogastroenterol Motil. 2010;16(1):8–21. doi: 10.5056/jnm.2010.16.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malfertheiner P, Nocon M, Vieth M, Stolte M, Jaspersen D, Koelz HR, Labenz J, Leodolter A, Lind T, Richter K, Willich SN. Evolution of gastro-oesophageal reflux disease over 5 years under routine medical care - the ProGERD study. Aliment Pharmacol Ther. 2012;35(1):154–64. doi: 10.1111/j.1365-2036.2011.04901.x. [DOI] [PubMed] [Google Scholar]
- 35.Ganz RA, Peters JH, Horgan S, Bemelman WA, Dunst CM, Edmundowicz SA, Lipham JC, Luketich JD, Melvin WS, Oelschlager BK, Schlack-Haerer SC, Smith CD, Smith CC, Dunn D, Taiganides PA. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med. 2013;368(8):719–27. doi: 10.1056/NEJMoa1205544. [DOI] [PubMed] [Google Scholar]