Abstract
Background
Determining anatomic sites and circumstances under which a fracture may be a consequence of osteoporosis is a topic of ongoing debate and controversy that is important to both clinicians and researchers.
Methods
We conducted a systematic literature review and generated an evidence report on fracture risk based on specific anatomic bone sites as well as fracture diagnosis codes. Using the RAND/UCLA appropriateness process, we convened a multi-disciplinary panel of 11 experts who rated fractures according to their likelihood of being due to osteoporosis based on the evidence report. Fracture sites (as determined by ICD-CM codes) were stratified by four clinical risk factor categories based on age, sex, race/ethnicity (African- American and Caucasian) and presence or absence of trauma.
Results
Consistent with current clinical experience, the fractures rated most likely due to osteoporosis were the femoral neck, pathologic fractures of the vertebrae, and lumbar and thoracic vertebral fractures. The fractures rated least likely due to osteoporosis were open proximal humerus fractures, skull, and facial bones. The expert panel rated open fractures of the arm (except proximal humerus) and fractures of the tibia/fibula, patella, ribs, and sacrum as being highly likely due to osteoporosis in older Caucasian women but a lower likelihood in younger African American men.
Conclusion
Osteoporosis attribution scores for all fracture sites were determined by a multidisciplinary expert panel to provide an evidence-based continuum of the likelihood of a fracture being associated with osteoporosis.
Introduction
Based on current guidelines, a diagnosis of osteoporosis relies on a history of fragility fracture or the result of bone mineral density (BMD) evaluation. Determining anatomic sites and circumstances under which a fracture may be a consequence of osteoporosis has been a topic of ongoing controversy. Without an evidence-based consensus on what constitutes an osteoporosis-related fracture, the epidemiology and public health burden of osteoporosis cannot be accurately determined. In addition, the inconsistent use of the terms “fragility fracture” or “osteoporotic fracture” in clinical trials leads to varying reports of efficacy for osteoporosis therapies [1–3]. Past efforts to define the fractures that are most strongly associated with osteoporosis have utilized formal group processes during which experts reviewed available evidence [4, 5]. However, increasing data on fracture epidemiology and newer approaches to utilizing formal group processes to define consensus motivated a careful reconsideration of the attribution of specific fracture sites to osteoporosis [6, 7]. Most prior efforts to synthesize fracture literature have included only osteoporotic fractures at the typical sites (hip, spine, wrist, and humerus). Our approach sought to broaden the understanding of osteoporosis attribution at all fracture sites and to specify these fractures through the use of the International Classification of Diseases Clinical Modification (ICD-CM) fracture codes that are often used in epidemiologic studies to define events of interest.
There is a growing interest in the U.S. and other countries in using large administrative databases, such as Medicare data, to examine the epidemiology of osteoporosis and fractures [8–12]. These databases identify fractures based on ICD-CM diagnosis codes and Current Procedural Terminology (CPT) procedure codes. The relationship of these codes to osteoporosis is very useful to determine fracture epidemiology at a population level. These databases may be particularly helpful to study populations in which fracture epidemiology has been less well characterized, such as in non-Caucasians and older adults.
To assess the attribution to osteoporosis of fractures at different anatomic sites among persons with different osteoporosis risk factors, we used a modification of the Research and Development/University of California at Los Angeles (RAND/UCLA) Appropriateness Method [13] to assemble the published evidence for the relationship between osteoporosis and fractures at all anatomic sites. To allow our findings to be useful for future investigations using administrative databases, we further grouped fractures based on ICD-9-CM. A multi-disciplinary expert panel used this evidence and their collective expertise to grade the strength of the association between osteoporosis and different fracture sites.
Methods
Overview
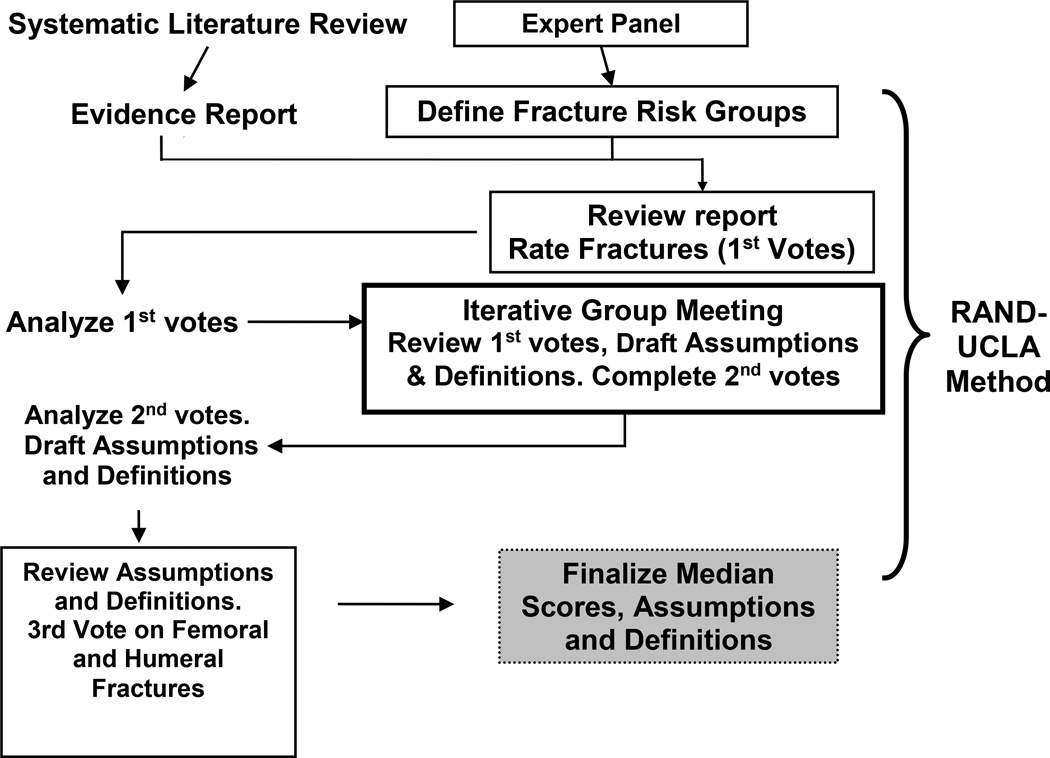
We systematically reviewed the relevant literature to formulate an evidence report containing tables summarizing the association with osteoporosis for each fracture type, stratified by key clinical risk factors (e.g. age, race/ethnicity, sex). We then convened a multi-disciplinary, eleven member panel with expertise in the fields of orthopedics, rheumatology, endocrinology, medical coding, bone pathology, and epidemiology (see Members of the Task Force Panel, listed above). The expert panel utilized the modified RAND/UCLA appropriateness process to provide an osteoporosis attribution grading for each fracture by anatomic site, administrative diagnosis code, and key risk factors, as depicted in Figure 1. The panelists’ fracture scores were based on their interpretation of the relevant literature that was provided to them in the evidence report and their existing knowledge.
FIGURE 1.
OSTEOPOROSIS FRACTURE EXPERT PANEL PROCESS
Systematic Literature Review
To conduct the systematic review and develop the evidence report, we used PubMed restricted to English language articles with abstracts indicating the use of human subjects and retrieved articles published in the ten years from January 1, 1999 to February 13, 2008. A meta-analysis published in 2000 was used as reference for earlier studies [14]. To identify articles linking osteoporosis with fractures overall and by particular anatomic site, we limited our search to the exploded Medical Subject Headings (MeSH) ‘osteoporosis’ and ‘fracture’. The search strategy initially yielded 4,016 articles. Two reviewers (AW, NP) independently reviewed the abstracts of these articles and excluded 3,848 articles that were irrelevant to fracture epidemiology. We retrieved full text of the remaining 168 potentially relevant articles and excluded 100 additional articles for the same reason. The remaining 68 articles underwent detailed review and abstraction of data elements for an evidence report. The evidence report described the published estimates of fracture risk associated with osteoporosis for each anatomic bone site. As evidence that a fracture at a given site was most likely due to osteoporosis, we used an association with either low bone mineral density or a subsequent fracture. A summary of the data available in the current literature, which was provided to the expert panel members within an evidence report, is found in Appendix 1. Because of the possibility that some clinical risk factors might modify the relationship between having osteoporosis and sustaining a fracture, the evidence report also included estimates of the association of fractures with age, sex, race, trauma, glucocorticoid use, and chronic kidney disease.
Expert Panel Process using the RAND/UCLA appropriateness method
Our expert panel used the RAND/UCLA appropriateness method [13], which includes elements of the nominal and Delphi methods [15], to score the likelihood of fractures being due to osteoporosis. The expert panel voting process consisted of three separate meetings, including two internet-based conference calls and one face-to-face meeting.
The initial expert panel meeting included discussions of clinical risk factor strata for osteoporosis fractures among an older population, i.e. persons over the age of 65. The panel excluded fractures in persons with a concurrent diagnosis of Paget disease of bone, hyperparathyroidism, osteogenesis imperfecta, or osteomalacia. Following a presentation of the available evidence linking each osteoporosis risk factor with fracture risk, the expert panel ranked risk factors they wished to consider further in their deliberations. These clinical risk factors formed the voting strata for attributing fractures to osteoporosis.
Following the initial teleconference, the panel received a revised evidence report along with voting tables. The voting tables listed fractures by anatomic site and associated ICD-9-CM codes (Appendix 2). Use of the ICD-CM codes allowed for further specification regarding the site and type of fracture, i.e. open or closed, pathologic or traumatic. For each fracture site, and stratified by the 4 risk factor groups pre-specified by the panel (sex, age, race/ethnicity, trauma), the panelists indicated fractures most likely to be osteoporosis-related voting along a 9-point Likert scale. On the Likert scale, a score of 1 indicated a fracture least likely to be osteoporosis-related and a score of 9 indicated a fracture most likely to be osteoporosis-related.
At a face-to-face expert panel meeting convened at the University of Alabama at Birmingham, and consistent with the RAND/UCLA method, the panel members reviewed a comparison of their first round votes with the panel’s median scores and the range of the panelists’ scores. Discussion focused on the fractures where there was a lack of agreement during the first round of voting. Lack of agreement was defined as more than two panelists voting 1, 2, or 3 for a fracture for which the panel’s median score occurred in the 7, 8, or 9 range, or vice versa, as defined in the RAND/UCLA method [13]. To assist in further discussion, the panel defined key assumptions and operational definitions that could be applied to administrative data to identify fractures associated with severe trauma and “Malignancy-Related Fractures”. In a third expert panel teleconference, panelists adjudicated areas of dissent and amended definitions and assumptions.
Source of Funding
This research was supported by a contract between the University of Alabama at Birmingham and Amgen, Inc. The analysis, presentation and interpretation of the results were solely the responsibility of the authors.
Results
The risk factors (and categories) determined by the expert panel included eight strata defined by: age (65–79 and ≥80 years), sex, race/ethnicity, and presence or absence of concurrent trauma. Race/ethnicity categories were limited to African American (AA) and Caucasian because of the relative paucity of literature on osteoporosis fracture risk in other racial/ethnic populations.
The expert panel discussed fractures most likely due to severe trauma (i.e. high speed motor vehicle collision, injury from a projectile) and those that were “malignancy-related” (Table 1). The panel determined that these categories of fractures should be excluded prior to consideration of the relationship between osteoporosis and fractures. In doing so, the panel agreed that all other fractures they considered were a result of low or moderate trauma and in the absence of malignancy. In defining malignancy-related fractures, the panel recognized that administrative codes for cancer diagnoses do not address the severity or stage of the cancer. Therefore, in order to improve the specificity of the definition used for malignancy, the panel recommended a malignancy-related fracture required a pathologic fracture and a malignancy code as well as a code for treatment of cancer, hospice referral, or death.
TABLE 1.
OPERATIONAL DEFINITION FOR FRACTURES DUE TO MALIGNANCY IN ADMINISTRATIVE DATA
|
Table 2 lists the key assumptions approved by the panelists that influenced subsequent voting.
TABLE 2.
EXPERT PANEL KEY ASSUMPTIONS ON OSTEOPOROSIS FRACTURE ATTRIBUTION USING ADMINISTRATIVE CLAIMS DATA AND CLINICAL RISK FACTORS
|
During the first round of voting, 100 of the total 424 fracture sites within each clinical risk strata satisfied the criteria for agreement as determined by the RAND/UCLA appropriateness method [13]. After the panel discussed severe trauma and defined malignancy-associated fractures, there was much greater consensus on the likelihood of fractures being due to osteoporosis. Subsequently, in the second and third round of voting, all fracture sites met the criteria for agreement.
The expert panel’s final osteoporosis attribution scores for fractures (stratified by ICD coding) are shown in Tables 3a (most likely due to osteoporosis) and 3b (least likely due to osteoporosis). The fractures ranked as most likely due to osteoporosis (Table 3a) included femoral neck, pathologic fractures of the vertebrae, lumbar and thoracic vertebral fractures, pelvis, and closed fractures of the humerus, radius, ulna and femoral shaft. Conversely, open fractures of the proximal humerus and closed fractures of the skull, face, toe, scapula, and finger, atypical fractures (i.e. flail chest, larynx and trachea) and multiple concurrent fractures were scored as least likely due to osteoporosis (Table 3b). The panel members more strongly associated fractures with osteoporosis in persons over the age of 80 and in women. The panel scored open fractures of the proximal humerus as least likely due to osteoporosis in all risk factor groups (Table 3b). Moreover, the panel concluded that fractures receiving the lowest attribution scores (1 to 3) were most likely due to severe trauma or cancer-related fractures. Fractures for which there was limited data to support or refute their linkage with osteoporosis received mid-range scores (4 to 6).
TABLE 3.
(a). Fractures (categorized as listed in ICD-CM codes) more likely to be due to osteoporosis and 3(b) fractures less likely to be due to osteoporosis listed by median score.
| (a). | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Women | Men | Women | Men | ||||
| Race/Ethnicity | C | AA | C | AA | C | AA | C | AA |
| Age | > 80 |
> 80 |
> 80 |
> 80 |
65– 79 |
65– 79 |
65– 79 |
65– 79 |
| Femoral neck | 9 | 9 | 9 | 9 | 9 | 8 | 8 | 8 |
| Pathologic vertebral fractures | 9 | 9 | 8 | 9 | 8 | 8 | 8 | 7 |
| Lumbar | 9 | 9 | 8 | 8 | 8 | 8 | 8 | 8 |
| Thoracic | 9 | 9 | 8 | 8 | 8 | 8 | 8 | 7 |
| Closed distal forearm and radius/ulna (NOS) | 9 | 9 | 8 | 8 | 8 | 8 | 7 | 7 |
| Pelvis | 9 | 8 | 8 | 8 | 8 | 8 | 8 | 7 |
| Closed femur, shaft | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Closed humerus, proximal and distal | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Vertebral (NOS) | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 7 |
| Closed forearm, upper and shaft | 8 | 8 | 8 | 8 | 8 | 8 | 7 | 7 |
| Pathologic fracture of the humerus | 8 | 8 | 8 | 8 | 8 | 8 | 7 | 8 |
| Closed humerus, shaft | 8 | 8 | 8 | 8 | 8 | 8 | 7 | 7 |
| Pathologic fracture of the distal radius/ulna | 8 | 8 | 8 | 8 | 8 | 8 | 7 | 7 |
| Closed femur, distal | 8 | 8 | 8 | 8 | 8 | 7 | 7 | 7 |
| C-spine | 8 | 8 | 8 | 8 | 8 | 7 | 7 | 7 |
| Pathologic fractures of the femur | 8 | 8 | 8 | 8 | 8 | 7 | 7 | 7 |
| Closed ankle/malleolus | 8 | 8 | 8 | 8 | 7 | 7 | 7 | 7 |
| Rib | 8 | 8 | 8 | 8 | 7 | 7 | 7 | 7 |
| Open radius/ulna (NOS) | 8 | 8 | 7 | 7 | 8 | 7 | 6 | 6 |
| Sacrum | 8 | 8 | 7 | 7 | 7 | 7 | 7 | 6 |
| Open distal humerus and open distal forearm | 8 | 8 | 7 | 7 | 7 | 7 | 6 | 6 |
| Closed tibia & fibula (NOS) | 8 | 7 | 7 | 6 | 7 | 7 | 6 | 6 |
| Closed proximal tibia & fibula | 8 | 8 | 7 | 7 | 6 | 6 | 6 | 6 |
| Pathologic fracture of the tibia and fibula | 8 | 7 | 7 | 7 | 6 | 6 | 6 | 6 |
| Open femur, shaft | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Open humerus shaft; open forearm (upper & shaft); Ill-defined fractures of the upper limb | 7 | 7 | 7 | 7 | 7 | 7 | 6 | 6 |
| Multiple fractures involving both upper limbs and upper limb with rib(s) and sternum | 7 | 7 | 7 | 7 | 7 | 7 | 6 | 5 |
| Closed tibia & fibula shaft | 7 | 7 | 6 | 6 | 6 | 5 | 5 | 5 |
| Patella | 7 | 7 | 7 | 5 | 6 | 6 | 5 | 5 |
| Open ankle/malleolus | 7 | 7 | 6 | 6 | 6 | 6 | 5 | 5 |
| (b). | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Women | Men | Women | Men | ||||
| Race/Ethnicity | C | AA | C | AA | C | AA | C | AA |
| Age | > 80 |
> 80 |
> 80 |
> 80 |
65– 79 |
65– 79 |
65– 79 |
65– 79 |
| Closed clavicle | 6 | 6 | 6 | 6 | 6 | 6 | 5 | 5 |
| Other, multiple and ill-defined fractures of lower limb | 6 | 6 | 6 | 6 | 5 | 5 | 5 | 5 |
| Pathologic fracture, unspecified site | 6 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Open tibia & fibula fracture (NOS) | 6 | 6 | 5 | 4 | 4 | 4 | 3 | 3 |
| Sternum | 6 | 6 | 5 | 5 | 4 | 4 | 4 | 4 |
| Pathologic fracture of other specified site | 5 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Fracture of unspecified bones | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Hand (carpal, metacarpal) | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 |
| Foot (tarsal, metatarsal) | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 |
| Coccyx | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 |
| Open proximal tibia & fibula | 5 | 5 | 4 | 4 | 4 | 3 | 3 | 3 |
| Open shaft tibia & fibula | 5 | 5 | 4 | 4 | 4 | 3 | 3 | 3 |
| Ill-defined bones of trunk | 5 | 5 | 5 | 5 | 4 | 4 | 3 | 3 |
| Open clavicle | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 2 |
| Phalanges--hand | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 |
| Scapula | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 2 |
| Phalanges--foot | 3 | 2 | 3 | 3 | 2 | 2 | 2 | 2 |
| Multiple fractures involving both lower limbs, lower with upper limb, lower limb(s) with rib(s) and sternum | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 |
| Skull/facial; flail chest; larynx and trachea | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Open proximal humerus | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Fractures were scored from 1 to 9 with 1 representing those least likely due to osteoporosis and 9 those most likely due to osteoporosis. Fractures were scored within the clinical fracture risk groups of sex, race (Caucasian {C} or African American {AA}) and age (65–79 years old and 80 years old or older). The likelihood of a fracture being due to osteoporosis increases as the number rises, as indicated by the shading (4a: darker shade indicates fractures more likely due to osteoporosis, 4b: darker shade indicates fractures less likely to be due to osteoporosis). From left to right and top to bottom, the likelihood of a fracture being due to osteoporosis declines.
NOS = not otherwise specified.
Figure 2 depicts the expert panel’s final fracture rankings conveying the strength of the association of fractures with osteoporosis for risk factor groups at opposite ends of the clinical risk factor spectrum defined by the panel: Caucasian women above 80 years of age (left half of skeleton) and African American men between 65 and 79 years (right half of skeleton). Most notably, the panel indicated that, in the absence of major trauma and cancer, open fractures of the arm and fractures of the tibia/fibula, patella, ribs, and sacrum had a high likelihood of being due to osteoporosis in older Caucasian women but a lower likelihood in younger African American men.
FIGURE 2.
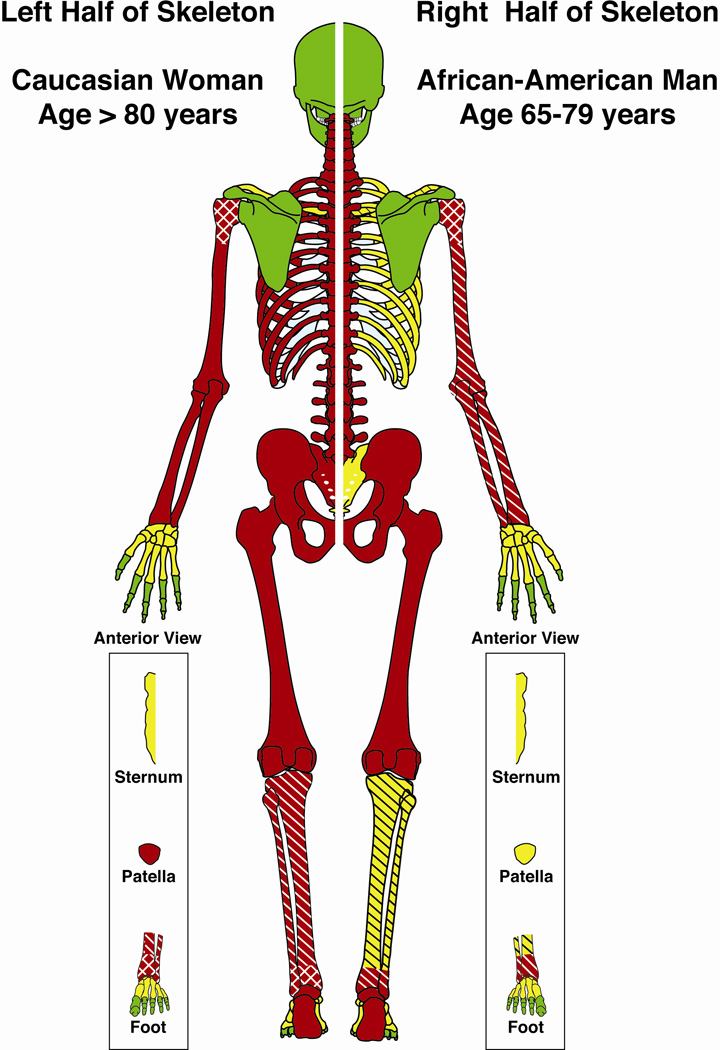
Skeletal depiction of Expert Panel (EP) osteoporosis fracture attribution scores. The two halves of the skeleton depict persons with differing clinical risk factors. Colors indicate three levels of fracture attribution by the EP across the 9- point Likert scale. Red: most likely due to osteoporosis (median Likert scores 7–9), Green: least likely due to osteoporosis (scores 1–3), Yellow: indeterminate likelihood of being due to osteoporosis (scores 4–6). Unless specified, scores were of the same level for closed, open and pathological fractures at that site. A single hatch pattern  indicates that an open fracture score was one level less likely to be osteoporotic than a closed fracture at the same site and a double hatch pattern
indicates that an open fracture score was one level less likely to be osteoporotic than a closed fracture at the same site and a double hatch pattern  indicates a decline of two levels.
indicates a decline of two levels.
Discussion
Using a systematic literature review and formal group process involving a multi-disciplinary expert panel, we developed osteoporosis attribution scores for all fracture sites and found that the most agreed upon sites for “osteoporosis-related fractures” were the vertebrae, femoral neck, and distal radius-ulna, as expected. However, we were also able to evaluate other fracture sites less commonly associated with osteoporosis and found that most fractures, even in the presence of moderate trauma, were attributed to osteoporosis, as corroborated by recent literature. In contrast, ethnicity/race and sex played a lesser role in determining if a fracture was osteoporosis-related. The likelihood of osteoporosis contributing to fractures of the humerus and femur increased as the fracture site became more distal in these bones and was greater in closed than in open fractures. Although closed fractures of the humerus were attributed to osteoporosis, the panel determined that open fractures of the proximal humerus were the fracture type least likely to be due to osteoporosis. This finding was consistent in all eight risk subpopulations and similar to closed fractures of the skull and face. The panel concluded that while certain open fractures of the long bones could occur with minimal to no trauma in older persons, an open fracture of the proximal humerus was very unlikely to occur without concurrent trauma.
This expert panel process differed in a number of important ways from a previous osteoporosis fracture attribution assessment by Melton and colleagues in 1997 [4]. We created an evidence report based on a systematic literature review of current fracture and osteoporosis data as the initial step in our RAND/UCLA appropriateness process. We identified 68 relevant papers published after a meta-analysis on the topic [14], which was published in 1999. Within the evidence report, panel members had access to a summation of the available data linking fractures to osteoporosis through association with both BMD changes and a history of prior fracture. The evidence report also enabled our multidisciplinary panel to objectively assess literature relevant to osteoporosis demographic groups and other clinical risk factors most strongly associated with osteoporosis-related fractures at all fracture sites. The RAND/UCLA method is a validated technique that has diagnostic properties similar to many routine tests [16]. This method was initially developed in the 1980’s to evaluate the “appropriateness” of various procedures used in medical practice [17–19] and has been used extensively to design guidelines and as support for decision making [16]. While different expert panels may reach slightly different sets of recommendations, the subsequent outcomes associated with this process varied minimally from group to group [20, 21]. The prior group process also used fracture incidence rates to guide their osteoporosis attribution probabilities [4] whereas our expert panel concluded that the absolute fracture incidence rate should have minimal influence on the determination of whether a fracture occurred due to osteoporosis. Because our results represent a continuum of fracture risk, we presented the RAND/UCLA score on an ordinal scale from 1 to 9. In contrast, the prior expert panel [4] used an attribution probability score for each risk group from which they developed mean attribution probabilities.
Administrative claims data are an important and growing resource to assess fracture burden at a population level and our administrative data based fracture definition provides a unique resource to assist in analyzing fracture epidemiology and outcomes. We also propose a standardized approach to group fracture types using administrative data and ICD-9-CM fracture codes. The fractures and the populations that were discussed in our panel meetings differed from the prior panel experience because we included fractures at all sites and our population was limited to persons over the age of 65.
Since the time that Melton and colleagues conducted their fracture attribution panel [4], a significant amount of new data about osteoporosis and fracture risk has emerged. Recent data indicate that older adults who experience a fracture, regardless of the degree of trauma, have an increased risk of future fracture [22]. Major trauma fractures typically have been defined as fractures either due to events such motor vehicle crashes, injury due to fast-moving projectiles, or falls from greater than standing height (not including stairs). Lower-trauma fractures have been classically defined as fractures due to falls from standing height or less; falls on stairs, steps, or curbs; moderate trauma other than a fall (i.e., collisions with objects during normal activities); and minimal trauma other than a fall [22–26]. However, motor vehicle accidents, in particular, may vary greatly in speed, impact, and degree of injury. The expert panel recognized and discussed these findings in relation to fractures at all sites during the fracture attribution process.
The panel also addressed the controversy of how to identify fractures due to malignancy (e.g. pathologic fractures). Current medical record diagnostic coding practices do not provide a direct method for identifying malignancy-related fractures. Currently, ICD-9-CM coding guidelines define pathologic fractures as any fracture that occurs as the result of an event that conventionally would not lead to fracture in a healthy subject [27]. Based on this definition, all fractures considered osteoporotic could be coded as pathologic fractures and thus ICD-9-CM codes for pathologic fractures may not be particularly discriminating. In an effort to better define fractures due to malignancy, the expert panel created an operational definition. The malignancy-related fracture definition requires both a cancer diagnosis and a pathologic fracture code along with a code for a treatment, a hospice referral, or death. The expert panel determined that the requirements for this cancer definition would lead to greater specificity in the identification of true cancer-related fractures than would a simple requirement of any concurrent or past cancer diagnosis, because a history of cancer is common among the elderly.
Miscoding, which is a recurring problem in analyses of administrative data, is a potential limitation of our work, since we used fracture categories identified by standard diagnostic codes. Previous studies that evaluated coding practices showed that fractures were identified accurately compared with a gold standard of medical record review in 94% of cases [28]. However, fractures such as the ankle (misclassified as tibia/fibula) and femoral shaft (misclassified as hip) are sometimes miscoded [28]. The panel recognized the paucity of clinical information on the extent of trauma and the possibility that a fracture due to a malignancy would not be identified using administrative data and accounted for these uncertainties during the voting process. Additionally, the panel recognized that their administrative data definition for malignancy likely erred on the side of greater specificity at the expense of some sensitivity. The validity of this empiric definition will require testing in actual data sets.
In conclusion, we conducted a systematic review of the medical literature and convened a multi-disciplinary expert panel to attribute fracture sites with osteoporosis, specific to age, sex, and ethnic/racial groups. We used administrative codes to group the sites of fracture and included all fracture sites, rather than limiting the evaluation to a small number of fractures presumed to be related to osteoporosis. Our expert panel concluded that femoral neck, pathologic fractures of the vertebrae, lumbar and thoracic vertebral fractures, and distal radius fractures were most strongly attributable to osteoporosis in all risk factor groups. Conversely, the panel rated open fractures of the proximal humerus and closed fractures of the skull and facial bones as least likely due to osteoporosis. A revised consensus on the likelihood of fractures that are most strongly and least strongly associated with osteoporosis will help clinicians in weighing the likelihood of osteoporosis and the potential need for further testing and treatment in a patient with a recent fracture. These attribution ratings, in addition to the panel’s assumptions and operational definition of fractures due to malignancy, will guide future epidemiologic studies of osteoporosis.
Supplementary Material
Members of the Expert Panel
David W. Dempster, PhD (Columbia University, New York), Gillian Hawker, MD, MSc, FRCPC (University of Toronto, Toronto, Ontario, Canada), Rebecca Jackson, MD (The Ohio State University), Joseph M. Lane, MD (Hospital of Special Surgery, New York, New York), Cora Elizabeth Lewis, MD, MSPH (University of Alabama at Birmingham, Birmingham, Alabama), Midge N. Ray, MSN, Med (University of Alabama at Birmingham, Birmingham, Alabama), Stuart L. Silverman, MD, FACP, FACR (University of California, Los Angeles, Los Angeles, California), Katie Stone, PhD (California Pacific Medical Center-Research Institute, San Francisco, California), David Volgas, MD (University of Alabama at Birmingham, Birmingham, Alabama). Rachel Wagman, MD (Amgen, South San Francisco, California; Stanford University, Stanford, California), Nelson Watts, MD (University of Cincinnati, Cincinnati, Ohio)
Footnotes
APPENDIX 1. SUMMARY LISTING OF LITERATURE RELATING RISK OF OSTEOPOROSIS FOR EACH FRACTURE SITE, AS DESCRIBED IN FULL DETAIL IN AN EVIDENCE REPORT PROVIDED TO THE EXPERT PANEL.
APPENDIX 2. LISTING OF FRACTURES BY ICD-9-CM CODES.
Contributor Information
Amy H. Warriner, Email: warriner@uab.edu.
Nivedita M. Patkar, Email: Nivedita.Patkar@ccc.uab.edu.
Jeffrey R. Curtis, Email: Jeffrey.Curtis@ccc.uab.edu.
Elizabeth Delzell, Email: EDelzell2@ms.soph.uab.edu.
Lisa Gary, Email: LGary@ms.soph.uab.edu.
Meredith Kilgore, Email: mkilgore@uab.edu.
Kenneth G. Saag, Email: ksaag@uab.edu.
References
- 1.Thomas-John M, et al. Risk factors for the development of osteoporosis and osteoporotic fractures among older men. J Rheumatol. 2009;36(9):1947–1952. doi: 10.3899/jrheum.080527. [DOI] [PubMed] [Google Scholar]
- 2.Hippisley-Cox J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. Bmj. 2009;339:b4229. doi: 10.1136/bmj.b4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings SR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 4.Melton LJ, 3rd, et al. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12(1):16–23. doi: 10.1359/jbmr.1997.12.1.16. [DOI] [PubMed] [Google Scholar]
- 5.Phillips S, et al. The direct medical costs of osteoporosis for American women aged 45 and older, 1986. Bone. 1988;9(5):271–279. doi: 10.1016/8756-3282(88)90009-9. [DOI] [PubMed] [Google Scholar]
- 6.Shekelle P. The appropriateness method. Med Decis Making. 2004;24(2):228–231. doi: 10.1177/0272989X04264212. [DOI] [PubMed] [Google Scholar]
- 7.Shekelle PG, et al. Sensitivity and specificity of the RAND/UCLA Appropriateness Method to identify the overuse and underuse of coronary revascularization and hysterectomy. J Clin Epidemiol. 2001;54(10):1004–1010. doi: 10.1016/s0895-4356(01)00365-1. [DOI] [PubMed] [Google Scholar]
- 8.Incidence and costs to Medicare of fractures among Medicare beneficiaries aged > or = 65 years--United States, July 1991–June 1992. MMWR Morb Mortal Wkly Rep. 1996;45(41):877–883. [PubMed] [Google Scholar]
- 9.Mamdani M, Kopp A, Hawker G. Hip fractures in users of first- vs. second-generation bisphosphonates. Osteoporos Int. 2007;18(12):1595–1600. doi: 10.1007/s00198-007-0446-5. [DOI] [PubMed] [Google Scholar]
- 10.Rothberg AD, Matshidze PK. Monitoring and management of bone status in patients on chronic glucocorticoid treatment--the Medscheme experience. S Afr Med J. 2000;90(11):1125–1129. [PubMed] [Google Scholar]
- 11.Watts NB, et al. Comparison of risedronate to alendronate and calcitonin for early reduction of nonvertebral fracture risk: results from a managed care administrative claims database. J Manag Care Pharm. 2004;10(2):142–151. doi: 10.18553/jmcp.2004.10.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang NP, et al. Estimated prevalence of osteoporosis from a Nationwide Health Insurance database in Taiwan. Health Policy. 2006;75(3):329–337. doi: 10.1016/j.healthpol.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Fitch K, et al. The RAND/UCLA Appropriateness Method User's Manual. RAND Corporation; 2001. xiii, 109. [Google Scholar]
- 14.Klotzbuecher CM, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15(4):721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2005;64(5):669–681. doi: 10.1136/ard.2004.028886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez N, et al. Review of the utilization of the RAND appropriateness method in the biomedical literature (1999–2004) Gac Sanit. 2009;23(3):232–237. doi: 10.1016/j.gaceta.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Brook RH, et al. Diagnosis and treatment of coronary disease: comparison of doctors' attitudes in the USA and the UK. Lancet. 1988;1(8588):750–753. doi: 10.1016/s0140-6736(88)91550-4. [DOI] [PubMed] [Google Scholar]
- 18.Siu AL, et al. Inappropriate use of hospitals in a randomized trial of health insurance plans. N Engl J Med. 1986;315(20):1259–1266. doi: 10.1056/NEJM198611133152005. [DOI] [PubMed] [Google Scholar]
- 19.Winslow CM, et al. The appropriateness of performing coronary artery bypass surgery. Jama. 1988;260(4):505–509. [PubMed] [Google Scholar]
- 20.Edelen MO, et al. Obtaining utility estimates of the health value of commonly prescribed treatments for asthma and depression. Med Decis Making. 2008;28(5):732–750. doi: 10.1177/0272989X08315251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shekelle PG, et al. The reproducibility of a method to identify the overuse and underuse of medical procedures. N Engl J Med. 1998;338(26):1888–1895. doi: 10.1056/NEJM199806253382607. [DOI] [PubMed] [Google Scholar]
- 22.Mackey DC, et al. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298(20):2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 23.Seeley DG, et al. Which fractures are associated with low appendicular bone mass in elderly women? The Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1991;115(11):837–842. doi: 10.7326/0003-4819-115-11-837. [DOI] [PubMed] [Google Scholar]
- 24.Stone KL, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18(11):1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 25.Center JR, et al. Risk of subsequent fracture after low-trauma fracture in men and women. Jama. 2007;297(4):387–394. doi: 10.1001/jama.297.4.387. [DOI] [PubMed] [Google Scholar]
- 26.Cummings SR, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 27.ICD-9-CM Official Guidelines for Coding and Reporting. [cited accessed Feb. 22, 2008];effective. 2007 Oct 1; http://www.eicd.com/contact.htm. [Google Scholar]
- 28.Ray WA, et al. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45(7):703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- 29.Pande I, et al. Bone mineral density, hip axis length and risk of hip fracture in men: results from the Cornwall Hip Fracture Study. Osteoporos Int. 2000;11(10):866–870. doi: 10.1007/s001980070046. [DOI] [PubMed] [Google Scholar]
- 30.Cauley JA, et al. Bone mineral density and prevalent vertebral fractures in men and women. Osteoporos Int. 2004;15(1):32–37. doi: 10.1007/s00198-003-1462-8. [DOI] [PubMed] [Google Scholar]
- 31.Legrand E, et al. Bone mineral density and vertebral fractures in men. Osteoporos Int. 1999;10(4):265–270. doi: 10.1007/s001980050225. [DOI] [PubMed] [Google Scholar]
- 32.Leslie WD, et al. Effectiveness of bone density measurement for predicting osteoporotic fractures in clinical practice. J Clin Endocrinol Metab. 2007;92(1):77–81. doi: 10.1210/jc.2006-1415. [DOI] [PubMed] [Google Scholar]
- 33.Cauley JA, et al. Long-term risk of incident vertebral fractures. JAMA. 2007;298(23):2761–2767. doi: 10.1001/jama.298.23.2761. [DOI] [PubMed] [Google Scholar]
- 34.Ling X, et al. Vertebral fractures in Beijing, China: the Beijing Osteoporosis Project. J Bone Miner Res. 2000;15(10):2019–2025. doi: 10.1359/jbmr.2000.15.10.2019. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen TV, Center JR, Eisman JA. Femoral neck bone loss predicts fracture risk independent of baseline BMD. J Bone Miner Res. 2005;20(7):1195–1201. doi: 10.1359/JBMR.050215. [DOI] [PubMed] [Google Scholar]
- 36.Bagger YZ, et al. The long-term predictive value of bone mineral density measurements for fracture risk is independent of the site of measurement and the age at diagnosis: results from the Prospective Epidemiological Risk Factors study. Osteoporos Int. 2006;17(3):471–477. doi: 10.1007/s00198-005-0009-6. [DOI] [PubMed] [Google Scholar]
- 37.Hongsdusit N, von Muhlen D, Barrett-Connor E. A comparison between peripheral BMD and central BMD measurements in the prediction of spine fractures in men. Osteoporos Int. 2006;17(6):872–877. doi: 10.1007/s00198-005-0061-2. [DOI] [PubMed] [Google Scholar]
- 38.Wainwright SA, et al. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab. 2005;90(5):2787–2793. doi: 10.1210/jc.2004-1568. [DOI] [PubMed] [Google Scholar]
- 39.Johnell O, et al. Acute and long-term increase in fracture risk after hospitalization for vertebral fracture. Osteoporos Int. 2001;12(3):207–214. doi: 10.1007/s001980170131. [DOI] [PubMed] [Google Scholar]
- 40.Naves M, et al. The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporos Int. 2003;14(6):520–524. doi: 10.1007/s00198-003-1405-4. [DOI] [PubMed] [Google Scholar]
- 41.van Staa TP, Leufkens HG, Cooper C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int. 2002;13(8):624–629. doi: 10.1007/s001980200084. [DOI] [PubMed] [Google Scholar]
- 42.Johnell O, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15(3):175–179. doi: 10.1007/s00198-003-1514-0. [DOI] [PubMed] [Google Scholar]
- 43.Kaptoge S, et al. Whom to treat? The contribution of vertebral X-rays to risk-based algorithms for fracture prediction. Results from the European Prospective Osteoporosis Study. Osteoporos Int. 2006;17(9):1369–1381. doi: 10.1007/s00198-005-0067-9. [DOI] [PubMed] [Google Scholar]
- 44.Lindsay R, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285(3):320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 45.Schuit SC, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34(1):195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Ismail AA, et al. Rib fractures predict incident limb fractures: results from the European prospective osteoporosis study. Osteoporos Int. 2006;17(1):41–45. doi: 10.1007/s00198-005-1887-3. [DOI] [PubMed] [Google Scholar]
- 47.Lee SH, Dargent-Molina P, Breart G. Risk factors for fractures of the proximal humerus: results from the EPIDOS prospective study. J Bone Miner Res. 2002;17(5):817–825. doi: 10.1359/jbmr.2002.17.5.817. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen TV, et al. Risk factors for proximal humerus, forearm, and wrist fractures in elderly men and women: the Dubbo Osteoporosis Epidemiology Study. Am J Epidemiol. 2001;153(6):587–595. doi: 10.1093/aje/153.6.587. [DOI] [PubMed] [Google Scholar]
- 49.Ettinger B, et al. Limb fractures in elderly men as indicators of subsequent fracture risk. Arch Intern Med. 2003;163(22):2741–2747. doi: 10.1001/archinte.163.22.2741. [DOI] [PubMed] [Google Scholar]
- 50.Olsson C, Nordqvist A, Petersson CJ. Increased fragility in patients with fracture of the proximal humerus: a case control study. Bone. 2004;34(6):1072–1077. doi: 10.1016/j.bone.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen ND, et al. Risk factors for fracture in nonosteoporotic men and women. J Clin Endocrinol Metab. 2007;92(3):955–962. doi: 10.1210/jc.2006-1476. [DOI] [PubMed] [Google Scholar]
- 52.Hosmer WD, Genant HK, Browner WS. Fractures before menopause: a red flag for physicians. Osteoporos Int. 2002;13(4):337–341. doi: 10.1007/s001980200035. [DOI] [PubMed] [Google Scholar]
- 53.Vogt MT, et al. Distal radius fractures in older women: a 10-year follow-up study of descriptive characteristics and risk factors. The study of osteoporotic fractures. J Am Geriatr Soc. 2002;50(1):97–103. doi: 10.1046/j.1532-5415.2002.50014.x. [DOI] [PubMed] [Google Scholar]
- 54.Cuddihy MT, et al. Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int. 1999;9(6):469–475. doi: 10.1007/s001980050172. [DOI] [PubMed] [Google Scholar]
- 55.Wei TS, et al. Fall characteristics, functional mobility and bone mineral density as risk factors of hip fracture in the community-dwelling ambulatory elderly. Osteoporos Int. 2001;12(12):1050–1055. doi: 10.1007/pl00004184. [DOI] [PubMed] [Google Scholar]
- 56.Johnell O, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 57.Stewart A, et al. Predicting a second hip fracture. J Clin Densitom. 1999;2(4):363–370. doi: 10.1016/s1094-6950(06)60401-0. [DOI] [PubMed] [Google Scholar]
- 58.Kanis JA, et al. The use of multiple sites for the diagnosis of osteoporosis. Osteoporos Int. 2006;17(4):527–534. doi: 10.1007/s00198-005-0014-9. [DOI] [PubMed] [Google Scholar]
- 59.Center JR, et al. Volumetric bone density at the femoral neck as a common measure of hip fracture risk for men and women. J Clin Endocrinol Metab. 2004;89(6):2776–2782. doi: 10.1210/jc.2003-030551. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen ND, et al. Identification of high-risk individuals for hip fracture: a 14-year prospective study. J Bone Miner Res. 2005;20(11):1921–1928. doi: 10.1359/JBMR.050520. [DOI] [PubMed] [Google Scholar]
- 61.Ojo F, et al. History of fractures as predictor of subsequent hip and nonhip fractures among older Mexican Americans. J Natl Med Assoc. 2007;99(4):412–418. [PMC free article] [PubMed] [Google Scholar]
- 62.Lau EM, et al. Risk factors for hip fracture in Asian men and women: the Asian osteoporosis study. J Bone Miner Res. 2001;16(3):572–580. doi: 10.1359/jbmr.2001.16.3.572. [DOI] [PubMed] [Google Scholar]
- 63.Hasselman CT, et al. Foot and ankle fractures in elderly white women. Incidence and risk factors. J Bone Joint Surg Am. 2003;85-A(5):820–824. doi: 10.2106/00004623-200305000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.