Abstract
Background
The PAST-BP trial found that using a lower systolic blood pressure target (<130 mmHg or lower versus <140 mmHg) in a primary care population with prevalent cerebrovascular disease was associated with a small additional reduction in blood pressure (2.9 mmHg).
Objectives
To determine the cost effectiveness of an intensive systolic blood pressure target (<130 mmHg or lower) compared with a standard target (<140 mmHg) in people with a history of stroke or transient ischaemic attack on general practice stroke/transient ischaemic attack registers in England.
Methods
A Markov model with a one-year time cycle and a 30-year time horizon was used to estimate the cost per quality-adjusted life year of an intensive target versus a standard target. Individual patient level data were used from the PAST-BP trial with regard to change in blood pressure and numbers of primary care consultations over a 12-month period. Published sources were used to estimate life expectancy and risks of cardiovascular events and their associated costs and utilities.
Results
In the base-case results, aiming for an intensive blood pressure target was dominant, with the incremental lifetime costs being £169 lower per patient than for the standard blood pressure target with a 0.08 quality-adjusted life year gain. This was robust to sensitivity analyses, unless intensive blood pressure lowering reduced quality of life by 2% or more.
Conclusion
Aiming for a systolic blood pressure target of <130 mmHg or lower is cost effective in people who have had a stroke/transient ischaemic attack in the community, but it is difficult to separate out the impact of the lower target from the impact of more active management of blood pressure.
Keywords: Hypertension, stroke, transient ischaemic attack, blood pressure target, cost effectiveness, decision model, decision analysis
Background
Stroke is a major cause of morbidity and mortality in the UK. There are approximately 110,000 strokes per year in England and around 300,000 people living with moderate to severe disabilities as a result of stroke.1 After a first stroke, patients are at high risk of a recurrent event: for every 1000 first strokes, 240 will have a recurrent cardiovascular disease event within five years of the first episode, of which 180 would be a stroke and 29 of these would be fatal.2 In 2008–2009, the direct care cost of stroke was £3 billion annually, within a wider economic cost of about £8 billion. Without preventive action, there is likely to be an increase in strokes as the population ages.1 Therefore, secondary prevention has a major potential role to play in reducing both morbidity and costs of stroke care.
There is controversy over how intensively to lower blood pressure (BP) in people who have had a stroke, with different international guidelines recommending different target BPs,3,4 and uncertainty over the applicability of the current evidence base for BP reduction after stroke to people with a history of transient ischaemic attack (TIA) or stroke in community populations.5,6 A systematic review of the effect of intensive BP lowering in populations including those with a history of stroke found that more intensive BP lowering does lead to reduced risk of major cardiovascular events,6 and the recent SPRINT trial, albeit in a population without a history of stroke, found that intensive BP lowering reduced major cardiovascular events and all-cause mortality.7 Therefore, there is renewed interest in strategies to lower BP intensively in high-risk populations, such as those with a history of stroke or TIA. The Prevention AfTer Stroke – Blood Pressure (PAST-BP) randomised controlled trial compared the impact of an intensive systolic blood pressure (SBP) target (<130 mmHg or 10 mmHg reduction from baseline if this was <140 mmHg) with a standard target (<140 mmHg) in people with a history of stroke or TIA recruited from primary care.8 The trial involved active management in all patients, and found that this led to important reductions in BP in both arms.9 The more intensive target was associated with only a small additional reduction in BP (2.9 mmHg), which raises the question as to whether such an intensive target is cost effective.
Here, we report the results of a model-based cost-utility analysis, which extrapolates the results of the PAST-BP trial9 to estimate the long-term cost effectiveness of intensive BP lowering targets after stroke/TIA in a primary care population, compared to a standard target.
Methods
A Markov model was constructed to estimate the long-term cost effectiveness, in terms of the cost per quality-adjusted life year (QALY) gained, of an intensive target strategy versus a standard target strategy for BP lowering in people with a history of stroke or TIA. The model was developed using TreeAge Pro Suite 2012 software (TreeAge Software Inc., Williamstown, MA, USA). The analysis was conducted from a UK National Health Service (NHS) and personal social services perspective.10
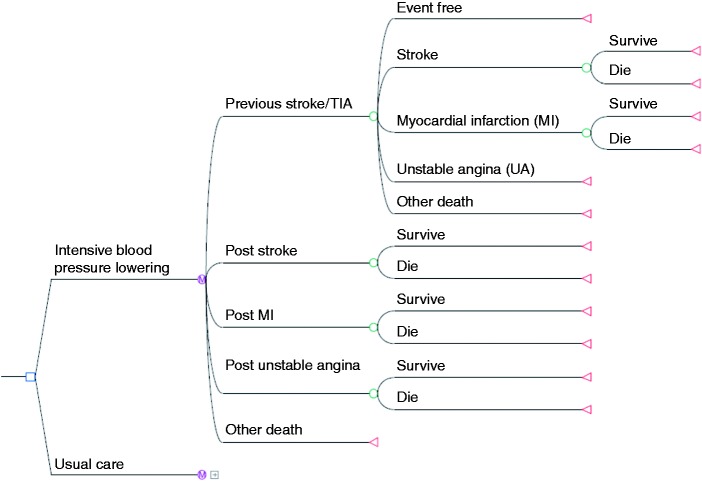
The model had a time cycle of one year with a 30-year time horizon (i.e. lifetime). The base-case analysis considered a cohort similar to that recruited to the PAST-BP trial (aged 70 years, 41% female). Baseline characteristics for important potential confounders were similar in both arms.9 Movements between model health states were defined by transition probabilities, which represented the risk of experiencing an event within a year time cycle. Long-term costs and health outcomes were assessed by attaching estimates of costs and utilities to the model health states. QALYs were calculated by multiplying life expectancy by the health state utility. Cost effectiveness was expressed as cost per additional QALY gained. The structure of the Markov model is shown in Figure 1.
Figure 1.
Markov model.
Note: The Markov model in this figure is only being displayed for the ‘intensive blood pressure lowering’ strategy. The standard target strategy is identical. Similarly, the model is identical at every node ending with green circles. Final outcomes (shown as red triangles) are survival and death.
Individual patient level data were used from the PAST-BP trial9 supplemented by parameter estimates from published studies (Table 1). In the PAST-BP trial9 participants were recruited from stroke/TIA registers in English general practices during 2009–2011 and were randomly assigned to an intensive BP target (<130 mmHg or a 10 mmHg reduction if baseline pressure was <140 mmHg) or a standard SBP target (<140 mmHg). Over one year, mean SBP dropped by 16.1 mmHg in the intensive target arm and by 12.8 mmHg in the standard arm (adjusted difference between groups 2.9 mmHg, P = 0.03). For extrapolation beyond one year, we assumed that this difference in BP was maintained.
Table 1.
Model parameters.
| Parameter | Value | Distribution | Source |
|---|---|---|---|
| Reduction in systolic blood pressure at 12 months (mmHg) | |||
| Intensive BP lowering | 16.1 | PAST-BP trial9 | |
| Standard target | 12.8 | ||
| 12 Months’ difference between groups (95% CI) | –2.9 (–5.7, −0.2) | ||
| Annual event probabilities | |||
| Stroke | |||
| 60–69 years old | 0.0348 | PROGRESS & NICE, Lipid Modification Guidelines12,18 | |
| 70–79 years old | 0.0589 | ||
| 80–89 years old | 0.0713 | ||
| MI and UA | |||
| 60–69 years old | 0.0139 | PROGRESS & NICE, Lipid Modification Guidelines12,18 | |
| 70–79 years old | 0.0232 | ||
| 80–89 years old | 0.0232 | ||
| Age-related relative risks at 12 months for intensive and standard BP lowering a | |||
| MI and UA – intensive BP lowering | |||
| 60–69 years old | 0.62 [0.59, 0.65] | PAST-BP trial & Law et al.9,13 | |
| 70–79 years old | 0.68 [0.63, 0.70] | ||
| 80–89 years old | 0.74 [0.69, 0.77] | ||
| Stroke – intensive BP lowering | |||
| 60–69 years old | 0.52 [0.47, 0.56] | PAST-BP trial & Law et al.9,13 | |
| 70–79 years old | 0.58 [0.54, 0.63] | ||
| 80–89 years old | 0.74 [0.68, 0.78] | ||
| MI and UA – standard target | |||
| 60–69 years old | 0.68 [0.65, 0.70] | PAST-BP trial & Law et al.9,13 | |
| 70–79 years old | 0.72 [0.69, 0.75] | ||
| 80–89 years old | 0.78 [0.74, 0.81] | ||
| Stroke – standard target | |||
| 60–69 years old | 0.59 [0.55, 0.63] | PAST-BP trial & Law et al.9,13 | |
| 70–79 years old | 0.65 [0.61, 0.68] | ||
| 80–89 years old | 0.78 [0.73, 0.82] | ||
| Utilities for the initial health state | |||
| Intensive BP lowering and standard target | |||
| 60–69 years old | 0.7241 | Beta | PAST-BP trial9 |
| 70–79 years old | 0.6631 | Beta | |
| 80–89 years old | 0.6362 | Beta | |
| Utilities for acute disease b | |||
| UA | 0.77 | Beta | NICE, Lipid Modification Guidelines18 |
| MI | 0.76 | Beta | |
| Stroke | 0.63 | Beta | |
| Dead | 0.00 | By definition | |
| Utilities for long-term (chronic) disease b | |||
| UA | 0.88 | Beta | NICE, Lipid Modification Guidelines18 |
| MI | 0.88 | Beta | |
| Stroke | 0.63 | Beta | |
| Probability of death from an event | |||
| Fatal stroke | 0.23 | Beta | Bamford et al.29 |
| Fatal MI | |||
| 60–69 years old | 0.23 | ONS, Deaths Registry 2011 & Kerr et al.11,30 | |
| 70–79 years old | 0.39 | ||
| 80–89 years old | 0.52 | ||
| Annual cost of consultation per patient (UK£) – intensive BP lowering | |||
| GP consultations | 86 | PAST-BP trial & Curtis9,16 | |
| PN consultations | 35 | ||
| Annual cost of consultation per patient (UK£) – standard target | |||
| GP consultations | 50 | PAST-BP trial & Curtis9,16 | |
| PN consultations | 29 | ||
| Average cost of hypertensive drugs per patient £per year c | |||
| Intensive BP lowering | 23 | BNF 20122,8 | |
| Standard target | 20 | ||
| Cost for the initial state £per year | |||
| Intensive BP lowering | 144 | Gamma | PAST-BP trial, Curtis, BNF 20129,16,28 |
| Standard target | 100 | Gamma | |
| Costs of acute disease £one-off cost | |||
| Stroke | 11020 | Gamma | Youman et al.19 |
| MI | 5487 | Gamma | Palmer et al.20 |
| UA | 3292 | Gamma | Assumed 60% of MI |
| Costs for long-term (chronic) disease £per year | |||
| Stroke | 2721 | Gamma | Youman et al.19 |
| MI | 572 | Gamma | NICE, Lipid Modification Guidelines18 |
| UA | 572 | Gamma | NICE, Lipid Modification Guidelines18 |
MI: myocardial infarction; UA: unstable angina; BP: blood pressure; GP: general practitioner; PN: practice nurse.
Relative risk comparing blood pressure after treatment with baseline blood pressure.
These figures are multiplied by initial health state utility to estimate new health state utility.
Annual cost of drugs was calculated on the basis of commonest drug and dose per drug group per arm at 6 and 12 months.
Model structure and inputs
The cohort started in the initial health state ‘previous stroke/TIA’, and a patient could remain in the ‘previous stroke/TIA’ health state if they did not have a recurrent event or died. If a cardiovascular event or death occurred the patient moved to one of four possible health states: new stroke, myocardial infarction (MI), unstable angina (UA), or dead (see Figure 1). Life tables were used to determine overall mortality dependent on age and gender, adjusted by cardiovascular disease mortality.11 Death was attributed to either stroke, MI or other causes. After a cardiovascular event, individuals could survive from the event or die, with death from an event occurring within a year. Individuals who survived a cardiovascular event moved to the chronic health state for that event, in which annual costs were incurred and quality of life was lower than in the ‘previous stroke/TIA’ state (Table 1). Individuals in a chronic health state were assumed to remain in that state for the rest of their lives unless they died from other causes.
Annual transition probabilities determining the risk of a cardiovascular event were based on the results of the PROGRESS trial.12 Age-related risk reductions for coronary heart disease (CHD) and stroke associated with subsequent reductions in SBP observed in the PAST-BP trial were obtained from Law et al. (Table 1).13 The risk reduction for CHD was applied to both MI and UA. This approach has previously been used by other studies to convert a decrease in SBP to reductions in CHD and stroke risk.14,15 The probability of each cardiovascular event occurring, the risks of dying from stroke or MI and the increased risk of death once in a chronic health state incorporated in the model are shown in Table 1. Outcomes and costs were discounted at the standard annual rate of 3.5%.10
Resource use and costs
Costs are reported in UK pounds at 2011–2012 unit prices, and are discounted at 3.5% per annum.10
Costs were derived from a combination of standard unit costs, NHS reference costs and previously published literature and were adjusted using the Hospital and Community Health Service index to the 2011/2012 price year.16 Resource use and costs per patient were obtained from the PAST-BP trial and applied to the initial health state in the model.9 Costs for acute and chronic states were obtained from published sources.17–20 Costs considered over the lifetime of the model included the cost of antihypertensive drugs, consultation costs and subsequent cardiovascular events (Table 1).
Utility values
The primary outcome measure was QALYs (Table 1). The utility value for the starting ‘previous stroke/TIA’ health state in the model was obtained from the PAST-BP trial using the overall mean EQ-5D score at baseline. The EQ-5D is a widely used generic instrument that has been validated in many patient populations, and is recommended by the National Institute for Health and Care Excellence (NICE).10 This was adjusted for age group using weights calculated from Ara and Brazier,21 which allowed a reduction in quality of life with increasing age to be incorporated in the model. Acute events were assumed to happen six months into a one-year cycle. Individuals stayed in that acute state for six months before moving into a chronic health state. Utilities for the acute state were applied mid-way through the one-year cycle and those for the chronic state at the start of the next cycle following an acute event. Future health state utilities were estimated by multiplying the starting quality of life with that of the new health state. In the base-case analysis it was assumed that different intensities of BP management had no effect on quality of life.22
Analysis
An incremental cost-utility analysis was undertaken. Probabilistic sensitivity analysis was based on 10,000 Monte Carlo simulations. A gamma distribution was fitted to the costs obtained from the PAST-BP trial. Beta distributions were used to model the probability of dying from any of the cardiovascular events as well as the uncertainty around the utility values. A cost-effectiveness plane23 and a cost-effectiveness acceptability curve (CEAC) were constructed, the latter to depict the probability of intensive BP lowering being more cost effective compared to standard target at different cost per QALY willingness-to-pay thresholds.
Uncertainty in the results of the model was assessed through sensitivity analyses. These involved: varying the time horizon for the model; changing costs of disease and the initial cost for the intensive BP lowering arm by 30%; varying the effect size in the intensive BP lowering arm according to the 95% confidence interval of the BP reduction difference achieved at 12 months; incorporating a quality of life decrement due to antihypertensive medication by reducing utility values (multiplicatively) for the initial health state in the intensive BP lowering arm by up to 10%.21
Results
The base-case lifetime costs and QALYs are presented in Table 2. Compared to a standard BP target of 140 mmHg SBP, intensive BP lowering was in a position of dominance, being cheaper and more effective. Intensive BP lowering was associated with average cost savings per patient of £169 and an additional 0.08 QALYs over 30 years.
Table 2.
Base-case result: lifetime costs and outcomes per patient.
| Costs (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER (£per QALY) | |
|---|---|---|---|---|---|
| Standard target | 9889 | 7.4719 | |||
| Intensive blood pressure lowering | 9720 | 7.5539 | –169 | 0.082 | Dominant |
QALY: quality-adjusted life year; ICER: incremental cost-effectiveness ratio.
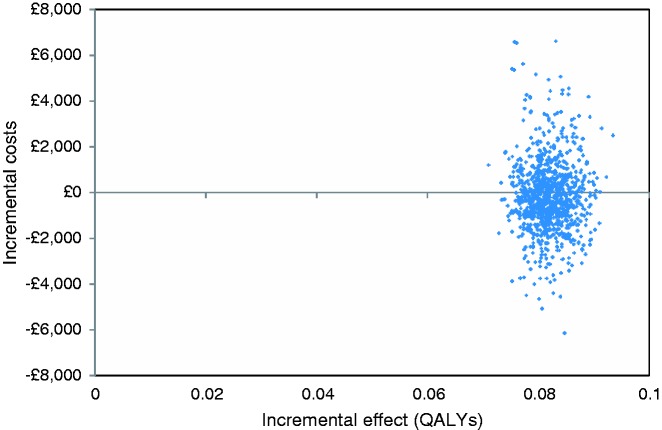
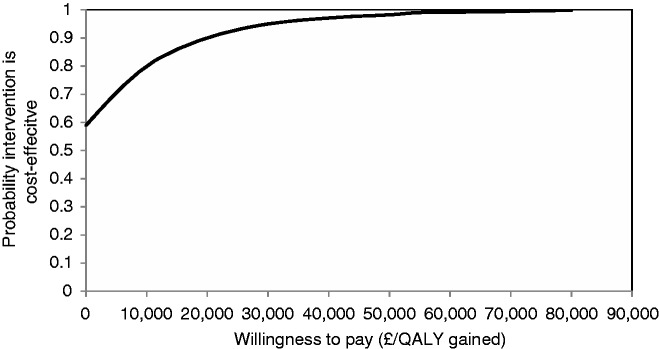
Figure 2 presents the cost-effectiveness plane comparing intensive BP lowering to standard target incorporating parameter uncertainty. The mean incremental costs and incremental effects (QALY gains) mostly lie in the north-east and south-east quadrants, indicating that intensive BP lowering is highly likely to be effective but with a large amount of uncertainty around its cost impact. The CEAC shows that if a decision-maker has a willingness to pay of £20,000 per QALY gained, the likelihood of cost effectiveness was 90% (Figure 3).
Figure 2.
Incremental cost-effectiveness plane comparing the intensive blood pressure lowering strategy with standard target strategy or usual care.
Figure 3.
Cost-effectiveness acceptability curve for the intensive blood pressure lowering model showing the probability that the intervention is cost effective.
Sensitivity analysis
Intensive BP lowering was cost effective at £20,000 per QALY provided at least two years of treatment was given, and became the dominant strategy after six years (Supplementary Table 1). Varying costs had little impact on the overall conclusion, but if the effect size was reduced to the lower bound of the 95% confidence interval for BP reduction, intensive targets were no longer cost effective. If intensive BP lowering is associated with a 2% or more reduction in quality of life, it is no longer effective, but remains the less expensive strategy because of the reduction in cardiovascular events. In this circumstance, the incremental cost-effectiveness ratio (ICER) suggests that standard targets are more cost effective (Supplementary Table 1).
Discussion
We found that a strategy of intensive BP lowering in primary care, as tested by the PAST-BP trial, is likely to be cost effective. The extra initial costs of the intensive strategy are offset by subsequent cost savings in terms of reduced cardiovascular events, such that the strategy is less expensive after six years, although there was much greater uncertainty around the impact on costs as compared to the impact on benefits (Figure 2). The intensive strategy is not cost effective if it is associated with a 2% or more reduction in quality of life. However, we have found in a previous trial that reductions in BP of the order of magnitude seen in PAST-BP were not associated with any effect on quality of life,24 and there were no significant differences in adverse effects during the trial.9 This analysis assumes that the difference in BP between the arms is maintained over time: the sensitivity analysis suggested that the ICER remains below £20,000/QALY provided the time horizon is at least two years. Furthermore, there is evidence from the SPS3 trial, which involved different targets for BP in people with a history of lacunar stroke, that differences between arms were maintained up to eight years after randomisation.25
PAST-BP was not powered to detect differences in cardiovascular events between arms, and so the impact of observed BP reductions was estimated by applying these to the results of a systematic literature review.13 Recent evidence reinforces the likelihood that BP reductions are indeed likely to lead to a reduction in the risk of cardiovascular events.6 While this evidence was not restricted to people with previous stroke, the relative reductions in cardiovascular risk associated with reduction in BP appear to be similar in people with and without existing cerebrovascular disease.26
Our results are consistent with the results of a cost-effectiveness analysis based on the PROGRESS trial, which found treating people with cerebrovascular disease was cost effective, with a cost per QALY of £6927 over four years.27 Whereas our analysis found long-term treatment to be dominant, the PROGRESS trial found long-term treatment remained more expensive than standard care. It is likely that this difference in costs reflects changes in drug prices since the PROGRESS economic analysis was performed. Our sensitivity analysis (see Supplementary Table 1) showed that a 30% increase in the initial cost of intensive BP lowering resulted in the intensive target arm becoming more expensive than the standard care arm. A change of this magnitude is plausible given that, for example, perindopril now costs £1.72 per month, as opposed to £10.95, as applied in 2005.27,28
Strengths and limitations
This study used cost and outcome data from a primary care based pragmatic randomised controlled trial in patients with a past history of stroke or TIA.9 The use of a Markov model overcame limitations associated with within-trial analyses, specifically allowing the modelling of effects and costs on long-term events and the assessment of the long-term cost effectiveness beyond the trial period.
The model did not include the recurrence of cardiovascular events beyond the first event. However, as the intensive lowering strategy was more effective and therefore likely to reduce cardiovascular risk, then this model simplification is likely to have produced more conservative model results.
Linked to this, an additional limitation derives from the nature of Markov models. These assume that the probability of an individual moving to any given health state in one time period depends only on their current health state. Therefore a patient’s outcomes and costs are assumed to depend only on the current health state, and this may underestimate overall costs and overestimate health outcomes for those who have suffered more than one event. Again, this is likely to have reduced the apparent cost effectiveness of intensive BP lowering.
The PAST-BP trial did not have a ‘usual care’ arm – rather it compared two active management strategies, one to an intensive target, one to a standard target. As a result, the cost-effectiveness analysis can only compare these two active strategies – it cannot examine the cost effectiveness of moving from usual care to active management.
Clinical implications
This analysis suggests that intensive BP lowering in a post-stroke population in primary care is likely to be cost effective, despite the relatively small reduction in SBP with which it is associated. However, comparison of achieved BP in the control group with less active BP management suggests that it is also likely that active management of BP is more important than the target that is used.9,24 Therefore, it is difficult to determine from this economic analysis whether the priority should be to promote systolic targets less than 130 mmHg, or to promote more active management of BP. The overall conclusion from this work is that interventions lowering BP post-stroke are likely to be cost effective provided that they can be achieved without excessive additional cost or impact on quality of life. Intensive lowering of BP in primary care appears to be one such option.
Supplementary Material
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This report is independent research funded by the National Institute for Health Research (Stroke Prevention in Primary Care, Programme Grant for Applied Research, RP-PG-0606-1153), and by an National Institute for Health Research (NIHR) professorship (RJM). FDRH is part funded by the NIHR School for Primary Care Research, the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) Oxford, the NIHR Biomedical Research Centre (BRC) Oxford, and as a professorial fellow, Harris Manchester College, Oxford. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS.
References
- 1.Morse A. Progress in Improving Stroke Care. London: The Stationery Office, 2010.
- 2.Lee S, Shafe ACE, Cowie MR. UK stroke incidence, mortality and cardiovascular risk management 1999–2008: time-trend analysis from the General Practice Research Database. BMJ Open 2011; 1(2): e000269–e000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mancia G, Fagard R, Narkiewicz K, et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Blood Pressure 2013; 22(4): 193–278. [DOI] [PubMed]
- 4.Intercollegiate Stroke Working Party. National Clinical Guidelines for Stroke, 4th ed London: Royal College of Physicians, 2012. [Google Scholar]
- 5.Mant J, McManus R, Hare R. Applicability to primary care of national clinical guidelines on blood pressure lowering for people with stroke: Cross sectional study. BMJ 2006; 332: 635–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: Updated systematic review and meta-analysis. Lancet 2015; 387: 435–443. [DOI] [PubMed] [Google Scholar]
- 7.The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22): 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher K, Mant J, McManus R, et al. Protocol for PAST-BP: a randomised controlled trial of different blood pressure targets for people with a history of stroke or transient ischaemic attack (TIA) in primary care. BMC Cardiovasc Disord 2010; 10(37), doi:10.1186/1471-2261-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mant J, McManus RJ, Roalfe A, et al. Different systolic blood pressure targets for people with history of stroke or transient ischaemic attack: PAST-BP (Prevention After Stroke – Blood Pressure) randomised controlled trial. BMJ 2016; 352: i708–i708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NICE. Guide to the methods of technology appraisal, London: National Institute for Health and Care Excellence, 2013. [PubMed] [Google Scholar]
- 11.Office for National Statistics. Interim Life Tables for England. Secondary Interim Life Tables for England 2012, London: Office for National Statistics, 2012. [Google Scholar]
- 12.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 13.Law M, Morris J, Wald N. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemilogical studies. BMJ 2009; 338: b1665–b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaambwa B, Bryan S, Jowett S, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a cost-effectiveness analysis. Eur J Prev Cardiol 2013; 21(12): 1517–1530. [DOI] [PubMed] [Google Scholar]
- 15.Penaloza-Ramos MC, Jowett S, Mant J, et al. Cost-effectiveness of self-management of blood pressure in hypertensive patients over 70 years with suboptimal control and established cardiovascular disease or additional cardiovascular risk diseases (TASMIN-SR). Eur J Prev Cardiol 2016; 23(9): 902–912. [DOI] [PubMed] [Google Scholar]
- 16.Curtis L. Unit Costs of Health and Social Care, Kent: Personal Social Services Research Unit, University of Kent, 2012. [Google Scholar]
- 17.Department of Health. NHS Reference Costs Schedule 2011–12, London: Department of Health, 2012. [Google Scholar]
- 18.NICE. Lipid modification: Cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. NICE guidelines CG67, London: National Institute for Health and Care Excellence, 2008. [PubMed] [Google Scholar]
- 19.Youman P, Wilson K, Harraf F, et al. The economic burden of stroke in the United Kingdom. Pharmacoeconomics 2003; 21: 43–50. [DOI] [PubMed] [Google Scholar]
- 20.Palmer S, Sculpher M, Philips Z, et al. A cost effectiveness model comparing alternative management strategies for the use of glycoprotein IIB/IIIA antagonists in Non-ST-Elevation Acute Coronary Syndrome. York, UK: York Centre for Health Economics, 2004.
- 21.Ara R and Brazier J. Using health state utility values from the general population to approximate baselines in decision analytic models when condition specific data are not available. HEDS Discussion Paper 10/11. Sheffield: University of Sheffield, 2011. [DOI] [PubMed]
- 22.McManus R, Mant J, Bray E, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet 2010; 376: 163–172. [DOI] [PubMed] [Google Scholar]
- 23.Gray AM, Clarke PM, Wolstenholme JL, et al. Applied Methods of Cost-Effectiveness Analysis in Health Care, Oxford: Oxford University Press, 2011. [Google Scholar]
- 24.McManus RJ, Mant J, Haque M, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA 2014; 312(8): 799–808. [DOI] [PubMed] [Google Scholar]
- 25.Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382(9891): 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until 1 March 2003. J Hypertens 2003; 21(6): 1055–1076. [DOI] [PubMed] [Google Scholar]
- 27.Tavakoli M, Pumford N, Woodward M, et al. An economic evaluation of a perindopril-based blood pressure lowering regimen for patients who have suffered a cerebrovascular event. Eur J Health Econ 2009; 10(1): 111–119. [DOI] [PubMed] [Google Scholar]
- 28.Joint Formulary Committee. British National Formulary. London: BMJ Publishing Group and RPS Publishing, 2012.
- 29.Bamford J, Sandercock P, Dennis M, et al. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project – 1981–86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol, Neurosurg Psychiatry 1990; 53(1): 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerr M, Bray B, Medcalf J, et al. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012; 27(Suppl 3): iii73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.