To the Editor
Fatty acid hydroxylase-associated neurodegeneration (FAHN) is caused by mutations in FA2H (OMIM 612319) and encompasses a spectrum ranging from complicated spastic paraplegia (SPG35) [1, 2], to leukodystrophy with spastic paraparesis and dystonia [3], to neurodegeneration with brain iron accumulation (NBIA) [4] [5]. FA2H encodes fatty acid 2-hydroxylase, an important enzyme in galactolipid synthesis that is essential for neuronal myelin sheath maintenance. To date, only a few patients with FA2H mutations have been reported [1, 2, 4, 6, 7].
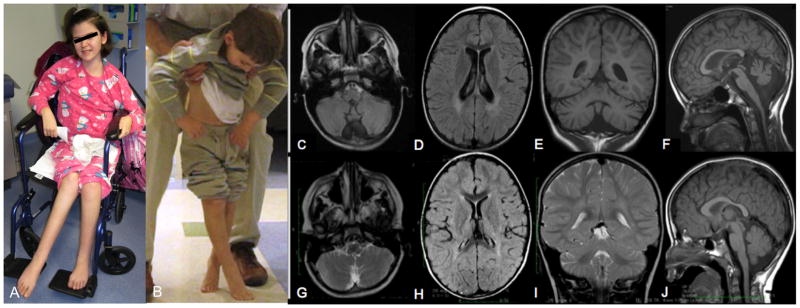
We describe 2 siblings born to non-consanguineous parents from Montenegro with SPG35 caused by mutation in FA2H (figure 1a–b). Patient 1 is a 13 year-old girl who developed normally until 3 years when she started toe walking and had frequent falls. By 4, her legs crossed with ambulation, consistent with spastic diplegia. Lower extremity spasticity progressed, and she lost independent ambulation at 9 years, when she also developed difficulty with handwriting. By 10, she developed dysarthic speech and a head and hand tremor. By 11, she developed scoliosis and head drop. When first seen at 13 years, she was non-ambulatory and unable to write, with unintelligible speech and difficulty chewing. Receptive language, cognition and hearing appeared intact. She had no history of seizures. Brain MRI at 8 years demonstrated an atrophic cerebellum, slightly flat pons, mild thinning of the corpus callosum, prominent lateral ventricles, and bilateral posterior periventricular FLAIR hyperintensities. There was no evidence of brain iron accumulation (Figure 1c–f).
Figure 1.
Patient 2 is her 5 year-old brother. He developed normally until 2 years, when a labored gait associated with scissoring and clumsiness was noted. By 3 years, he had lower extremity spasticity and slow speech. At 5 years, he had lost independent ambulation, had difficulty gripping a pencil, and developed a head drop and tremor. His decline seemed quicker than his sister’s. Brain MRI at 5 years revealed a mildly atrophic cerebellum, normal pons and corpus callosum, and subtle T2 hyperintensities near the posterior horns of the lateral ventricles. There was no brain iron accumulation (Figure 1g–j). Table 1 shows clinical examination findings. Oligonucleotide based microarray analysis (Affymetrix 6.0, >1.8 million probes) revealed two large, contiguous genomic segments (16q21-q23.1, including FA2H, and 19q3.12-q13.33) with loss of heterozygosity. Direct sequencing of FA2H in the affected siblings identified a homozygous deletion (c.509_510 delAC) resulting in a frameshift and premature stop codon (p.Y170*). The parents and unaffected sister were heterozygous carriers. This mutation was previously reported in two brothers from Albania with NBIA [4], possibly representing a founder mutation from the Balkan region.
Table 1.
Clinical and examination findings of 2 siblings with SPG35. Ashworth Scale: (0) No increase in muscle tone, (1) Slight increase in tone with a catch and release or minimal resistance at end of range, (2) As 1 but with minimal resistance through range following catch, (3) More marked increase tone through ROM, (4) Considerable increase in tone, passive movement difficult, (5) Affected part rigid.
| Patient 1: 13 year old sister | Patient 2: 5 year old brother | |
|---|---|---|
| Physical Findings | No dysmorphic features. Near constant drooling. Thoracolumbar scoliosis. | No dysmorphic features. Slight ptosis. |
| Mental status | Pleasant and appears normal but speech is difficult to understand. | Pleasant and appears normal but speech is difficult to understand. |
| Cranial nerve | Spastic dysarthria, bilateral optic atrophy, intermittent exotropia, broken saccades and supranuclear gaze palsy. | Spastic dysarthria, bilateral optic atrophy, intermittent exotropia, broken saccades and supranuclear gaze palsy. |
| ROM/Tone | Slow tongue movements. Spastic tetraparesis Ashworth 2. Nondystonic tendency to preferentially keep head laterally flexed to the right. | Slow tongue movements. Mild-moderate contractures in the ankles, knees, and hips. Truncal hypotonia. Spastic tetraparesis Ashworth 1. Nondystonic tendency to preferentially keep head laterally flexed to the right |
| Gait | Non-ambulatory. | Ambulates only with assistance and demonstrates a spastic diplegic gait. |
| Cerebellar | Bradykinesia and moderate-to-severe appendicular dysmetria along with an intention tremor. Titubation at rest. | Bradykinesia and moderate-to-severe appendicular dysmetria along with an intention tremor. |
| Muscle bulk | Atrophy below knees bilaterally. | Atrophy below knees bilaterally. |
| Strength | Appeared full; testing limited by spasticity. | Appeared full; testing limited by spasticity. |
| Reflexes | +4/4 throughout with a crossed adductor response and sustained ankle clonus. Babinski sign present bilaterally. | +4/4 throughout with a crossed adductor response and sustained ankle clonus. Babinski sign preset bilaterally. + Jaw jerk. |
| Electromyography and nerve conduction | Normal | - not performed - |
| Ophthalmologic exam | Optic nerve head pallor. | Bilateral +1 temporal optic nerve pallor. |
Review of the few published cases does not reveal a clear genotype-phenotype correlation, though putative null mutations or deletions causing absence of FA2H may result in a more severe phenotype than missense mutations [1, 3, 7]. Within the spectrum of FAHN, MRI can be unremarkable or show leukodystrophy or subcortical and periventricular T2 white matter hyperintensities, atrophy of the cerebellum, brainstem or cervical spinal cord, thinning of the corpus callosum, or iron accumulation in the globus pallidus. A family with the same c.509_510 delAC mutation and NBIA has been reported [4]. In contrast, the siblings presented here showed no brain iron deposition, indicating that this mutation may not always result in NBIA. Thus, additional factors may play a role in FAHN phenotypic expression. Imaging studies also indicate that FAHN represents a clinical spectrum rather than a distinct syndrome [5]. The significant supranuclear upgaze palsy seen here has only been reported in a Pakistani patient [1] and adds to oculomotor abnormalities (strabismus and ocular apraxia) reported in other FAHN patients. The clinical spectrum and pathogenic mechanisms of FAHN are still unfolding.
Acknowledgments
The authors wish to thank the family for participating. The authors thank Elizabeth Hartnett and Keonna Harrison for their coordinating efforts. This work was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Footnotes
There are no conflicts to disclose.
References
- 1.Dick KJ, et al. Mutation of FA2H underlies a complicated form of hereditary spastic paraplegia (SPG35) Hum Mutat. 2010;31(4):E1251–60. doi: 10.1002/humu.21205. [DOI] [PubMed] [Google Scholar]
- 2.Pierson TM, et al. Exome sequencing and SNP analysis detect novel compound heterozygosity in fatty acid hydroxylase-associated neurodegeneration. Eur J Hum Genet. 2012;20(4):476–9. doi: 10.1038/ejhg.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edvardson S, et al. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am J Hum Genet. 2008;83(5):643–8. doi: 10.1016/j.ajhg.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruer MC, et al. Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA) Ann Neurol. 2010;68(5):611–8. doi: 10.1002/ana.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider SA, Bhatia KP. Three faces of the same gene: FA2H links neurodegeneration with brain iron accumulation, leukodystrophies, and hereditary spastic paraplegias. Ann Neurol. 2010;68(5):575–7. doi: 10.1002/ana.22211. [DOI] [PubMed] [Google Scholar]
- 6.Rupps R, et al. Novel Mutations in FA2H-Associated Neurodegeneration: An Underrecognized Condition? J Child Neurol. 2012 doi: 10.1177/0883073812458538. [DOI] [PubMed] [Google Scholar]
- 7.Tonelli A, et al. Atypical adult onset complicated spastic paraparesis with thin corpus callosum in two patients carrying a novel FA2H mutation. Eur J Neurol. 2012 doi: 10.1111/j.1468-1331.2012.03838.x. [DOI] [PubMed] [Google Scholar]