Abstract
Infant survival and wellbeing is dependent upon good parenting skills. In some species of primates, fathers are necessary to ensure both positive developmental and social outcomes for their offspring. Common marmosets and the related cotton-top tamarin monkeys provide extensive paternal care of multiple offspring and are essential for infant survival. However, we have found significant variation in a father’s motivation to respond to infant stimuli. Additionally, marmoset males who are experienced fathers are significantly more motivated to respond to infants and infant stimuli than adult males who have yet to be fathers. Expectant fathers appear to be preparing for their energetic role in infant care by responding with increases in multiple reproductive hormones and showing weight gain during their mate’s pregnancy. Male marmosets have been shown to be hormonally responsive to scent signals. Males show increased testosterone shortly after smelling periovulatory scents and lower levels of testosterone following presentation of their own infant’s scent. These two inverse testosterone responses combined indicate that paternal males have a flexible system of responding to socially relevant odor cues. Thus males can be ready to mate when their mate is fertile while continuing to be responsive to their infants when these two events occur simultaneously. A male’s hormonal and physical responsiveness to parenting may be due to pair bonding between the male and his mate. Examining the variability between males in their behavioral, physical, and hormonal responses to their mate’s pregnancy, and infant stimuli provides the means for determining the mechanisms of good parenting in fathers.
The role of fathers in parenting has varied with societal influences and throughout time. While men in the remote Akma pygmy’s societies will spend ~50% of their time with their infants, many societies have left infant and child rearing to mothers and other females (Hewlett, 1992). However, current societal and economic shifts in Western nations have expanded the roles that fathers play in their families. Recent studies have indicated that father’s involvement is associated with positive cognitive, developmental, and sociobehavioral child outcomes such as improved weight gain in preterm infants, improved breastfeeding rates, higher receptive language skills, and higher academic achievement (Garfield and Isacco, 2006). Therefore, increased father involvement in parenting may greatly benefit offspring.
Clearly there are many factors involved in a father’s relationship with his offspring. Society and role models are extremely important in determining paternal behaviors. However, the considerable variation that occurs between fathers within the same society, and even under similar rearing experiences, indicates that there may be a physiological basis for positive parenting behaviors.
Unfortunately, while the positive outcomes of father involvement are becoming clearer, child neglect and abuse have increased (Sedlack and Broadhurst, 1996). An estimated 1.5 million children are abused or neglected each year in the United States. Furthermore, National Incidence Studies have documented an increase in the incidence of child abuse since these studies began in 1980 (Sedlack and Broadhurst, 1996). Childhood maltreatment strongly predicts poor psychiatric and physical health outcomes in adulthood (Arnow, 2004). Individuals who suffer abuse, neglect, or serious family dysfunction as children are more likely to be depressed, to experience other types of psychiatric illness, to have more physical symptoms (both medically explained and unexplained), and to engage in more health-risk behaviors than their non abused counterparts. While neglect and abuse can come from both parents, fathers or unrelated males are more likely to be involved (Erickson, 2006).
Outside of humans, there are only a few species of non-human primates who display extensive social bonding between a male, his mate, and their offspring. In the family Callitrichidae, common marmosets, Callithrix jacchus, and the closely related cotton-top tamarin monkey, Saguinus oedipus, have been used as models for human family relationships (see Fig. 1). These socially monogamous monkeys live in cooperative groups in which all family members engage in infant care behaviors including carrying, grooming, protecting, and feeding usually twin infants. However, not all callitrichid species show the extensive paternal care immediately after birth that is seen in the common marmoset and the cotton-top tamarin (Tardif et al., 1986; Ziegler and Snowdon, 2000).
Fig. 1.

A father common marmoset, Callithrix jacchus, carries an infant while other family members show interest in the infant. Photo by Judith Sparkles. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
While paternally experienced males from these two species are extensively involved in infant care, there is wide variability in many aspects of their parenting behavior. We have focused on two kinds of parental variation: the variability that occurs between parentally inexperienced males and experienced fathers and the variation in expression of infant care within experienced fathers in order to better understand what promotes positive father—infant bonds. Through multiple studies, we have begun to investigate the behavioral, experiential, hormonal, physical, and genetic differences between males, and how these are associated with positive paternal care in the common marmoset. While all marmosets gain experience with infants through caring for their younger siblings, it is the experience of being a father that provides for the major physical, endocrine, and behavioral changes that appear to be important in ensuring the successful development of the infant. In the studies we describe here, we define parentally experienced males as those who have experienced birth and care of their own infants as opposed to inexperienced males who have not experienced birth of their own infants.
MALE MOTIVATION TO RESPOND TO INFANT CUES
Fathers are highly responsive to infants but within a family context, it is difficult to determine a father’s interest in parenting separate from the influences of other family members affecting the actual time he shows direct infant care. Infants are attractive to all family members and there can be competition between the older siblings in the family with fathers for infant carrying (Yamamoto and Box, 1997). Therefore, fathers would need to be tested away from family influences to accurately assess their interest (or motivation) in parenting. Additionally, callitrichid males may learn parenting from assisting in rearing their younger siblings as they develop. In some species, such as the cotton-top tamarin, learning to care for siblings is critical to becoming a successful parent with one’s own infants (Snowdon, 1996; Tardif et al., 1984). Both males and female cotton-top tamarins are less able to raise their own offspring if they have not had experience in learning to parent with their younger siblings (Snowdon, 1996).
Monogamous pairs occur when a male establishes a long-term bond with a female. Pair-bonded males have tied their reproductive success to their mate’s and therefore gain a high degree of paternity certainty (Strier, 2007). Phylogenetic comparisons suggest that pair bonding precedes male infant care and that the costs associated with the male’s help may be offset by the advantages he incurs (Dunbar, 1995). Marmosets and tamarins, have a high-reproductive rate consisting of twinning and multiple births within the same year. This reproductive strategy allows the female to raise more offspring than is found in other primate social systems but requires extensive help from males and older offspring. Females will allow fathers to carry their infants on the day of birth.
As is found for humans, we have determined that there is extensive variability in the amount of time and interest a parentally experienced father spends with his infants. Not only do father marmosets show variability in their behaviors towards infants, but they also show differences in motivation. To determine the variability within fathers for responsiveness to their infants (motivation) and to determine if parental experience affects a male’s motivation to engage in infant care, we tested 15 paternally experienced marmoset fathers and 6 parentally inexperienced males (Zahed et al., 2008). All studies described have been reviewed and approved by the University of Wisconsin Graduate School or Letters and Science IACUC review committee. The inexperienced males had not yet been fathers but were sexually mature adults and lived with a nonpregnant female. All males were tested with a live infant and with an infant vocalization (distress infant calls played on an mp3 player). Experienced fathers were tested with both their own infant and an unfamiliar live infant and with their own and an unfamiliar infant vocalization. Vocalizations were taken from infants three weeks of age and live infants were also three weeks of age during testing. Males were removed from their family in their nest box and placed in a testing cage without other marmoset’s olfactory or vocal cues except for the infant stimulus. They were habituated to the test environment for 3 days and then presented with the infant stimuli on the fourth day. At this time, a door was opened, and the male was allowed to enter another cage via a bridge and interact with the stimuli for 10 minutes. Males were tested for their stimulus directed behaviors such as latency to approach the stimulus and behaviors such as look at stimuli, enter the stimulus cage, contact behaviors, and attempt to retrieve the stimulus were recorded.
There was a significant difference in response to infants based on previous parental experience. Fathers were significantly more responsive to the unfamiliar infants and infant vocalizations than the inexperienced males (see Fig. 2). Additionally, experienced fathers showed significantly shorter latencies to enter the stimulus cage than the inexperienced males (see Fig. 3). Fathers also interacted with the unfamiliar infant more than the inexperienced males, but this was not significant and varied dependent on the temperament and behavior of the infant. We found that all experienced males immediately retrieved their own infant as soon as the test began so that the best stimuli for assessing individual responsiveness to infant cues was through the use of infant vocalizations. Fathers showed significant variability in their response scores to both unfamiliar and familiar (own infant) vocalizations. Father’s accumulative behavioral scores ranged from 25 to 250. Father’s behavioral scores were significantly correlated between the familiar and unfamiliar vocalization response (r = 0.73). All parentally experienced males displayed high responsiveness to infant stimuli and were equally motivated to care for an unfamiliar infant as to their own offspring. Despite the evident disparity in parental behavior expressed by the parentally experienced fathers compared to the inexperienced males, there was marked individual variation even within experience levels to respond to the infant vocalizations.
Fig. 2.
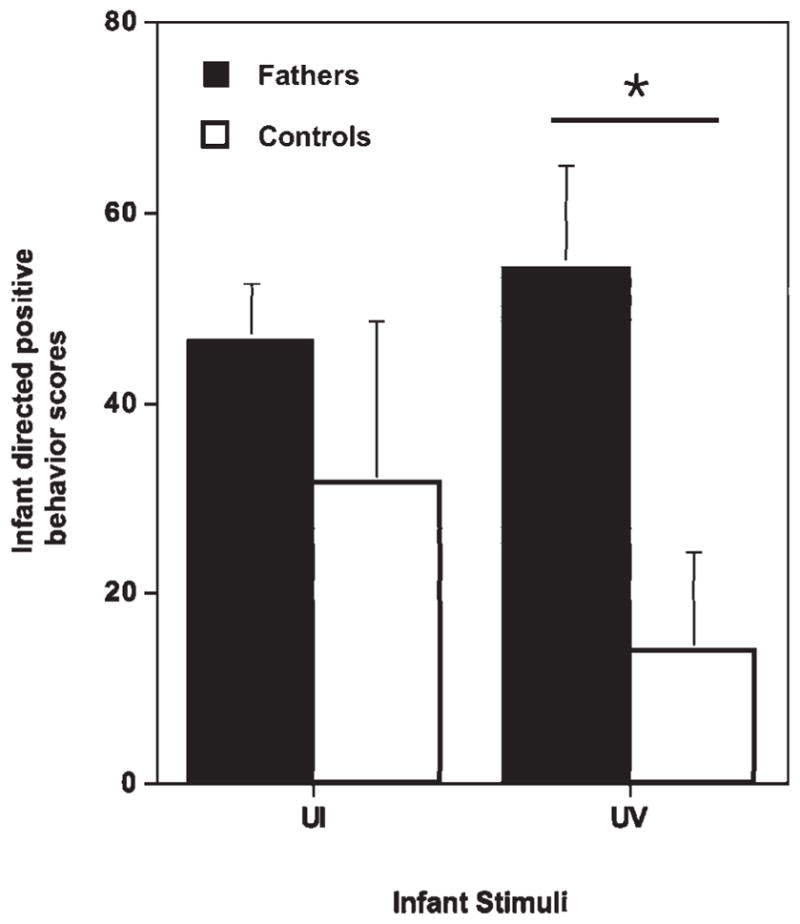
Experienced fathers show higher infant responsive behavioral scores than control males (inexperienced nonfathers). UV indicates the test stimulus unfamiliar infant vocalization and UI indicates the test stimulus of a live unfamiliar infant. (Reprinted from Zahed SR, et al. Male parenting and response to infant stimuli in the common marmoset (Callithrix jacchus). American Journal of Primatology, 70:84–92. © 2008 John Wiley & Sons.).
Fig. 3.
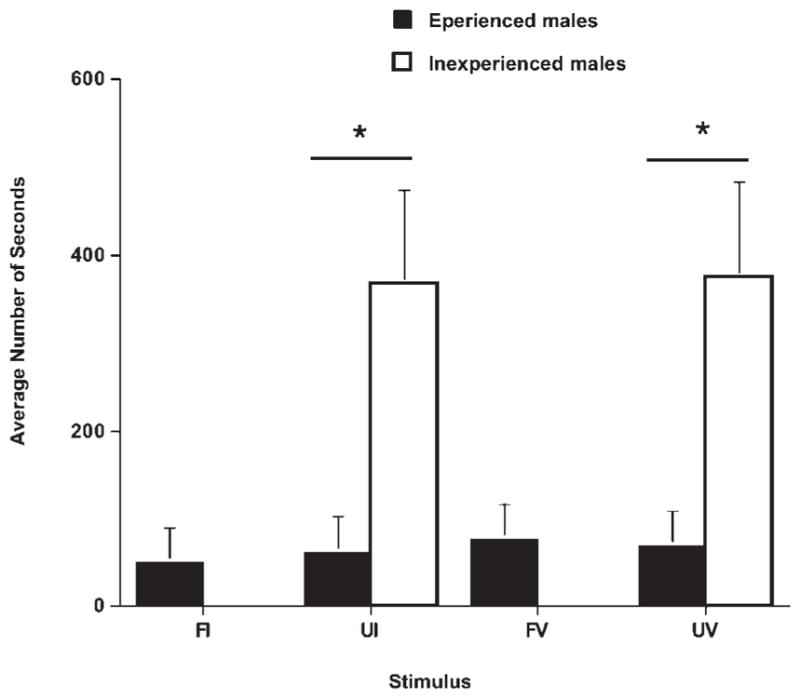
Experienced fathers show a short latency to enter the infant’s cage and approach for all four stimuli: familiar infant (FI), unfamiliar infant (UI), familiar vocalization (FV), and unfamiliar vocalization (UV). Fathers had a significantly shorter latency than control males (inexperienced nonfathers). (Reprinted from Zahed SR, et al. Male parenting and response to infant stimuli in the common marmoset (Callithrix jacchus). American Journal of Primatology, 70:84–92. © 2008 John Wiley & Sons.).
Almost all marmoset fathers show an interest in carrying and caring for their own infants, however, when presented with a motivation task, not all males show the same level of responsiveness. This was also seen when they interacted with an unfamiliar infant or infant stimuli. The variability in responses to the infant stimuli allowed us to begin to determine on an individual level how hormonal and other physical differences influence interest and expression of parental care.
Variability in Physical Changes
Expectant fathers of both common marmosets and cotton-top tamarins are physiologically responsive to their mate’s pregnancy. Males gain weight while their mates are pregnant (Ziegler et al., 2006). The weight gain might be preparing males for the energetic cost of fatherhood. The most significant weight gain occurs during the last 2 months of pregnancy at the time when their mates are showing their biggest weight gain. The weight gains in males during their mate’s pregnancy also demonstrate the variability of males to respond to the pregnancy. We found that weight varied between a gain of 2.4% and 20.4% in 14 experienced male marmosets. It is unknown whether the males who showed the largest gain in weight also showed the most positive parenting behaviors. But since the weight gain helps to prepare the male for his participation in infant care, those who show the most weight increase might be the ones who are responding the strongest to their mate’s chemical cues of pregnancy and might be those who invest most in the care of their offspring.
The only other species of primates who have been reported to gain weight during their mate’s pregnancy are human males. However, there has not been a systematic study of weight gain in men. Symptoms of pregnancy in mates of pregnant females, or sympathetic pregnancy, have been termed couvades, and these symptoms have been documented in men throughout time and in different societies (Klein, 1991). While weight changes in men could be due to the shared communication of the pregnancy, it would be very interesting to find out if men who show the most physical symptoms of pregnancy would be the fathers who are most involved with their infants following birth. Physical changes, such as male weight gain, may be incidental to the hormonal changes that occur to prepare males for their role in infant care and may be a part of an evolutionary process that occurs in biparental species (Daly, 1979).
Testosterone Responsiveness to Odor Cues in Male Marmosets
Parentally experienced common marmosets show different hormonal responses to odor cues than parentally inexperienced male marmosets. We have tested male marmoset responses in processing both infant and female ovulatory scent secretions. Variability in testosterone response occurred between males due to paternal experience. To study the effect of scents on male hormones, we removed 15 males from their natal cage and placed them alone in a small box. We collected anogenital scents from an ovulatory female on a glass stopper and stored the scents at −80°C until testing. They were then presented to the male on a wooden dowel held in the box for 10 min near his nose. Males were left to process the scent for an additional 10 min, and then a blood sample was taken for hormonal analyses. When males were tested for responses to the unfamiliar female’s ovulatory scents, we found that five males who were paternally experienced showed little change in testosterone or cortisol levels, and their response was similar to their hormonal changes after processing from a control scent. However, 10 males who had not been fathers were either paired with nonpregnant females (5) or lived singly (5) showed significant increases in testosterone levels in response to the periovulatory scent versus the control scent (Ziegler et al., 2005). Single males showed the greatest change in testosterone levels while family males showed the lowest change (see Fig. 4).
Fig. 4.
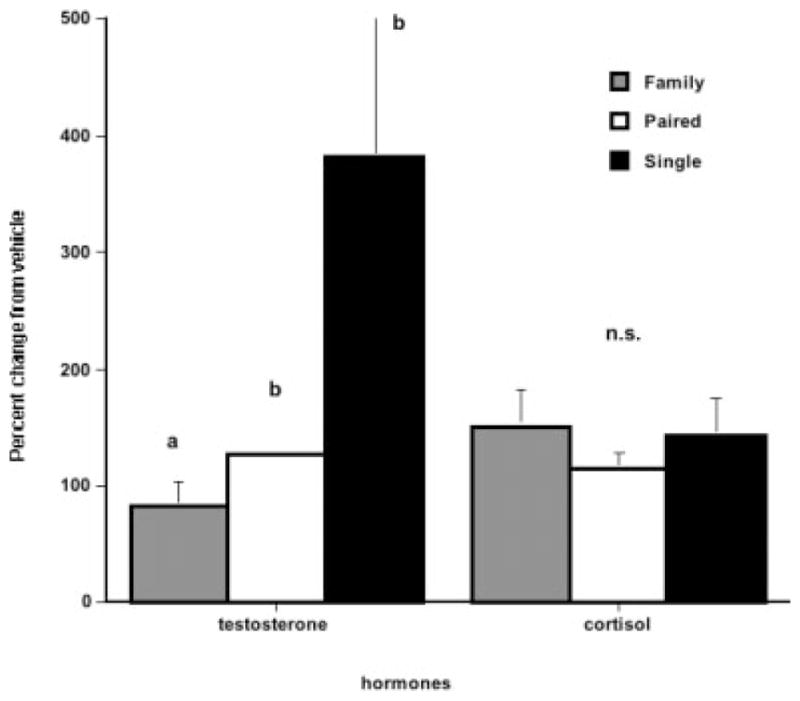
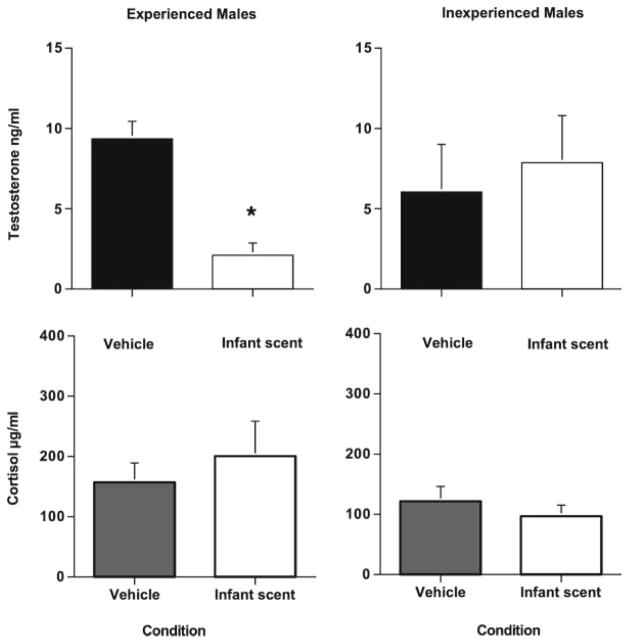
Fathers showed significantly lower levels of testosterone in response to the scent of an unfamiliar ovulating female than either males who were paired but not fathers and males who were housed alone. Levels are expressed in percent change from their testosterone levels after being presented with a control vehicle scent. There were no changes in cortisol from control vehicle scent for any of the males. (Reprinted from Ziegler TE, et al. Neuroendocrine response to female ovulatory odors depends upon social condition in male common marmosets, Callithrix jacchus. Hormones & Behavior, 47:56–64. © 2005 with permission Elsevier.).
We suggest that male marmosets have a natural behavioral and neuroendocrine response to sexually relevant cues such as ovulatory scent marks from novel females. However, under stable family conditions, there may be an inhibitory process that prevents fathers from exhibiting a full response. This could be part of their cooperative social system. The family males in this study were directly involved in infant care at the time of testing. Single males, such as those in this experiment, would have the best opportunity to show a high-testosterone response to sexually relevant cues from a novel female. While single housing is not considered a normal condition for male marmosets, there are times when nonparental wild marmosets are assessing other groups to determine the reproductive state of females as potential pair mates (Lazaro-Perea, 2001). A male’s testosterone response to a novel sexually relevant stimulus might be a method to test a male’s pair-bond strength to his family.
Fathers are hormonally responsive to the scent of their infants. Males were tested for their response to their infant’s scent and to a control scent in a crossover design (vehicle of water and ethanol). Six fathers of 3-week-old infants had significantly lower levels of testosterone following the odor presentation with their own infant’s scent than a control scent however, five inexperienced paternal males (who had never been fathers) did not show a change between a novel infant scent and control (see Fig. 5, Prudom et al., 2008). Cortisol levels did not differ by experience or by scent (infant or vehicle). This study demonstrates how odor cues from infants can have an acute effect on lowering their father’s testosterone levels. Parentally naive males showed no changes in testosterone from a novel infant scent to the control. This could be due to a lack of paternal experience or that only one’s own infant can affect the testosterone levels in males. This trigger of one’s own infant may explain why within the cooperative system of care repeated exposure to sibling infants throughout development to maturity, hormonal changes do not occur until the male becomes a father.
Fig. 5.
Experienced fathers show a significant decrease in testosterone in response to the scent of their infant while inexperienced males do not. No difference was found in cortisol levels. (Reprinted from Prudom SL, et al. Exposure to infant scent lowers serum testosterone in father common marmosets (Callithrix jacchus). Biology Letters, 4:603–605. Licensed 2008 by Royal Society Publishing.).
These two studies combined indicate that paternal males have a flexible system of responding to socially relevant odor cues. Males are responsive to the odors of females and infants by adjusting their hormones to prepare them for appropriate behavioral responses. Thus, males can be ready to mate when their mate is fertile while continuing to be responsive to their infants when these two events occur simultaneously as they do in marmosets and tamarins.
Variation of Hormones with Parenting
Changes in several reproductive hormones, such as estradiol, testosterone, and prolactin, may be involved in the behavioral stimulus to parent. However, only prolactin has been studied extensively. Elevated prolactin levels have been found to be associated with parenting in marmosets and tamarins. Prolactin levels are elevated in males at the end of their mate’s pregnancy in the common marmoset, cotton-top tamarin, and the Goeldi’s monkey, Callimico goeldii (Schradin et al., 2003; Ziegler et al., 2004; Ziegler and Snowdon, 2000). Prolactin levels peak in the cotton-top tamarin father during his mate’s mid pregnancy shortly before the male begins to gain weight and may be involved in the metabolic changes that occur in fathers to gain weight. Prolactin is considered to be a metabolic hormone, and elevated levels of prolactin are associated with increased food intake and body weight gain in mice and humans (Ben-Jonathan et al., 2006).
Prolactin levels also vary with the level of experience in both marmosets and tamarins. During their mate’s pregnancy, prolactin levels are significantly higher in experienced fathers than in first-time fathers, and these fathers have levels higher than nonexpectant males for both species (Ziegler et al., 2004). Thus, becoming a father appears to promote prolactin activation in males. Prolactin levels are also elevated postpartum in experienced common marmosets, and this appears to be associated with infant contact (Dixson and George, 1982; Mota and Sousa, 2000; Torii et al., 1998). Infant carrying, or infant contact, elevates prolactin in fathers and other group members (Dixson and George, 1982; Mota and Sousa, 2000; Roberts et al., 2001). Human fathers also have been reported to exhibit increased prolactin levels before and after infant birth (Storey et al., 2000), and fathers are more responsive to infant cries when they have higher prolactin levels (Fleming et al., 2002).
The Role of Chemical Communication in Paternal Care
The studies described earlier indicate that male common marmosets and cotton-top tamarins are responsive to scent signals from their mates and their infants. Chemical communication is a proximate mechanism that appears to stimulate the hormonal and physical changes in experienced and first-time fathers. The communication between the pair-bonded male and his mate works to ensure that the male will be available and ready for his important role in infant care. The pair bond itself may ensure that the male is present to receive signals from the female. Additionally, close proximity to his infants may ensure that fathers remain to provide proper positive care. Infants provide a stimulus to influence their own care.
In the ultimate sense, parental experience as a father is the mechanism that turns on the hormonal and physical changes. Males do not appear to be as responsive to social and olfactory signals from a pregnant female or from an infant until they become fathers. The experience of fatherhood may alter gene expression to turn on the hormonal and physical changes. Studies in the common marmoset have shown that fathers have enhanced changes in the prefrontal cortex area of the brain with fatherhood. There is an increased density of dendritic spines on the neurons that synthesize the neurohormones, vasopressin, and oxytocin and express the specific receptor for vasopressin (Kozorovitskiy et al., 2006). These changes may be associated with the reward system of the brain that would encourage fathers to bond with their infant and care for them. Both oxytocin and vasopressin may be involved in the expression of paternal care behaviors and may interact with other maternal-like hormones (i.e., estradiol and prolactin).
The variation between fathers in their motivation to interact with their infants may be one way to begin to understand how the parental system works. Further information is needed to understand how the brain interacts with the hormones to “switch on fatherhood.” However, the intensity of the pair bond between the male and his mate may partly determine his responsiveness. Males who show the most responsiveness to infants may be the males who are the most pair-bonded and keep in close proximity to their mate to process the chemical signals and to ensure success in raising offspring.
Understanding the underlying physical and hormonal aspects of male paternal care behaviors may allow us to identify means to encourage more fathers to remain close to their infants and create the strong social bonds needed between fathers and their infants for promoting positive parenting and developmental experiences for the offspring. Human offspring, similar to most nonhuman primates, have a long dependency on parental care, since they are slow to mature, and this requires a heavy investment in care behaviors. Sharing the cost of infant care with a mate allows for the social support needed to ensure proper care.
Acknowledgments
Contract grant sponsor: Wisconsin National Primate Research Center, NIH; Contract grant numbers: NCRR000167, MH070423, MH35215, and MH58700.
LITERATURE CITED
- Arnow BA. Relationships between childhood maltreatment, adult health and psychiatric outcomes, and medical utilization. J Clin Psychiatry. 2004;65(Suppl):10–15. [PubMed] [Google Scholar]
- Ben-Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab. 2006;17:110–116. doi: 10.1016/j.tem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Daly M. Why don’t male mammals lactate? J Theor Biol. 1979;78:325–345. doi: 10.1016/0022-5193(79)90334-5. [DOI] [PubMed] [Google Scholar]
- Dixson AF, George L. Prolactin and parental behaviour in a male New World primate. Nature. 1982;299:551–553. doi: 10.1038/299551a0. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. The mating system of callitrichid primates: I. Conditions for the co-evolution of paired bonding and twinning. Anim Behav. 1995;50:1057–1070. [Google Scholar]
- Erickson M. Nature disrupted: evolution, kinship and child sexual abuse. Clin Neuropsychiatry. 2006;3:110–120. [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steriner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Garfield GF, Isacco A. Fathers and the well-child visit. Pediatrics. 2006;117:e637–e645. doi: 10.1542/peds.2005-1612. [DOI] [PubMed] [Google Scholar]
- Hewlett BS. Father-child relations: cultural and Biosocial Contexts. In: Hrdy SB, editor. Foundations of human behavior. New York: Aldine E Gruyter; 1992. pp. 153–176. [Google Scholar]
- Klein H. Couvade syndrome: male counterpart to pregnancy. Int J Psychiatry Med. 1991;21:57–69. doi: 10.2190/FLE0-92JM-C4CN-J83T. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nature Neurosci. 2006;9:1094–1095. doi: 10.1038/nnl1753. [DOI] [PubMed] [Google Scholar]
- Lazaro-Perea C. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defense and assessment of neighbors. Anim Behav. 2001;62:11–21. [Google Scholar]
- Mota MT, Sousa MBC. Prolactin levels of fathers and helpers related to alloparental care in common marmosets, Callithrix jacchus. Folia Primatol. 2000;71:22–26. doi: 10.1159/000021727. [DOI] [PubMed] [Google Scholar]
- Prudom SL, Broz CA, Schultz-Darken NJ, Ferris CT, Snowdon CT, Ziegler TE. Exposure to infant scent lowers serum testosterone in father common marmosets (Callithrix jacchus) R Soc Biol Lett. 2008;4:603–605. doi: 10.1098/rsbl.2008.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RL, Lawler TL, Wegner FH, Norcross JL, Newman JD. Prolactin levels are increased after infant retrieval and carrying in parentally inexperienced common marmosets. Phys Behav. 2001;72:713–720. doi: 10.1016/s0031-9384(01)00430-9. [DOI] [PubMed] [Google Scholar]
- Sedlak AF, Broadhurst DD. National Data Archive on Child Abuse and Neglect (NDACAN) 1996 http://www.childwelfare.gov/systemwide/statistics/nis.cfm.
- Schradin C, Reeder DM, Mendoza SP, Anzenberger G. Prolactin and paternal care: comparison of three species of monogamous New World monkeys. J Comp Psychol. 2003;117:166–175. doi: 10.1037/0735-7036.117.2.166. [DOI] [PubMed] [Google Scholar]
- Snowdon CT. Infant care in cooperatively breeding species. In: Rosenblatt JS, Snowdon CT, editors. Parental care: evolution, mechanisms and adaptive significance. San Diego: Academic Press; 1996. pp. 643–689. [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol Human Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Strier KB. Primate behavioral ecology. 3. Allyn and Bacon; Boston: 2007. [Google Scholar]
- Tardif SD, Carson RL, Gangaware BL. Comparisons of infant care in family groups of the common marmoset (Callithrix jacchus) and the cotton-top tamarin (Saguinus Oedipus) Am J Primatol. 1986;11:103–111. doi: 10.1002/ajp.1350110202. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Richter CB, Carson RL. Effects of sibling-rearing experience on future reproductive success in two species of Callitrichidae. Am J Primatol. 1984;6:377–380. doi: 10.1002/ajp.1350060408. [DOI] [PubMed] [Google Scholar]
- Torii R, Moro M, Abbott DH, Nigi H. Urine collection in the common marmoset, Callithrix jacchus, and its applicability to endocrinological studies. Primates. 1998;39:407–417. [Google Scholar]
- Yamamoto EM, Box HO. The role of non-reproductive helpers in infant care in captive Callithrix jacchus. Ethology. 1997;103:760–771. [Google Scholar]
- Zahed SR, Prudom SL, Snowdon CT, Ziegler TE. Male parenting and response to infant stimuli in the common marmoset (Callithrix jacchus) Am J Primatol. 2008;70:84–92. doi: 10.1002/ajp.20460. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Prudom SL, Schultz-Darken NJ, Kurian AV, Snowdon CT. Pregnancy weight gain: marmoset and tamarin dads show it too. Biol Lett. 2006;2:181–183. doi: 10.1098/rsbl.2005.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Schultz-Darken NJ, Scott JJ, Snowdon CT, Ferris CF. Neuroendocrine response to female ovulatory odors depends upon social condition in male common marmosets, Callithrix jacchus. Horm Behav. 2005;47:56–64. doi: 10.1016/j.yhbeh.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Snowdon CT. Preparental hormone levels and parenting experience in male cotton-top tamarins, Saguinus oedipus. Horm Behav. 2000;38:159–167. doi: 10.1006/hbeh.2000.1617. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Washabaugh KF, Snowdon CT. Responsiveness of expectant male cotton-top tamarins, Saguinus oedipus, to mate’s pregnancy. Horm Behav. 2004;45:84–92. doi: 10.1016/j.yhbeh.2003.09.003. [DOI] [PubMed] [Google Scholar]